Abstract
Objective:
To describe the clinical features and outcome of anti–NMDA receptor (NMDAR) encephalitis in patients ≥45 years old.
Method:
Observational cohort study.
Results:
In a cohort of 661 patients with anti-NMDAR encephalitis, we identified 31 patients ≥45 years old. Compared with younger adults (18–44 years), older patients were more often male (45% vs 12%, p < 0.0001), had lower frequency of tumors (23% vs 51%, p = 0.002; rarely teratomas), had longer median time to diagnosis (8 vs 4 weeks, p = 0.009) and treatment (7 vs 4 weeks, p = 0.039), and had less favorable outcome (modified Rankin Scale score 0–2 at 2 years, 60% vs 80%, p < 0.026). In multivariable analysis, younger age (odds ratio [OR] 0.15, confidence interval [CI] 0.05–0.39, p = 0.0001), early treatment (OR 0.60, CI 0.47–0.78, p < 0.0001), no need for intensive care (OR 0.09, CI 0.04–0.22, p < 0.0001), and longer follow-up (p < 0.0001) were associated with good outcome. Rituximab and cyclophosphamide were effective when first-line immunotherapies failed (OR 2.93, CI 1.10–7.76, p = 0.031). Overall, 60% of patients older than 45 years had full or substantial recovery at 24 months follow-up.
Conclusions:
Anti-NMDAR encephalitis is less severe in patients ≥45 years old than in young adults, but the outcome is poorer in older patients. In this age group, delays in diagnosis and treatment are more frequent than in younger patients. The frequency of underlying tumors is low, but if present they are usually carcinomas instead of teratomas in younger patients. Early and aggressive immunotherapy will likely improve the clinical outcome.
Anti–NMDA receptor (NMDAR) encephalitis is an autoimmune disorder that usually affects children and young adults, resulting in severe neuropsychiatric symptoms that often respond to treatment.1–4 Experience with older patients is limited to a single case report5 and series comprising patients of all ages, but no further information is available. We report a detailed clinical analysis of 31 patients ≥45 years old and describe several novel features associated with this age group.
METHODS
Patients with immunoglobulin G antibodies against the NR1 subunit of NMDAR were identified from a series of 661 cases with anti-NMDAR encephalitis.2 Detailed information on patients ≥45 years old, either as individual cases or age group, has not been reported previously. We used 45 years as the cutoff age because a similar threshold has been used in other autoimmune neurologic disorders, like myasthenia gravis. Clinical information was obtained by the authors or referring physicians at the acute stage of the disease.2 Follow-up information was obtained at regular intervals after symptom onset; neurologic status was assessed with the modified Rankin Scale (mRS) score.6 Initial treatment was considered a failure if no sustained improvement occurred within 4 weeks after initiation of immunotherapy or tumor removal, and if the mRS score remained ≥4.2 Serum and CSF antibody studies were conducted as reported.3
Demographic information and symptoms were analyzed with the Fisher exact test, Fisher-Freeman-Halton test, or Mann-Whitney U test when appropriate, comparing these 31 patients with 338 recently reported patients (aged 18–44 years).2 Because of a skewed distribution, log-transformation was used for age at symptom onset, duration of follow-up, and delay until initiation of treatment. Factors influencing outcome were assessed by univariable binary logistic regression (good outcome defined as mRS 0–2) independently of the treatments given. Factors associated with a good outcome (p < 0.1) were included in generalized linear mixed models with binary distribution using SAS, procedure GLIMMIX, version 9.3 (SAS Institute Inc., Cary, NC), as we recently described.2 All p values provided are uncorrected p values.
Standard protocol approvals, registrations, and patient consents.
Studies were approved by the institutional review boards of the Universities of Pennsylvania and Barcelona.
RESULTS
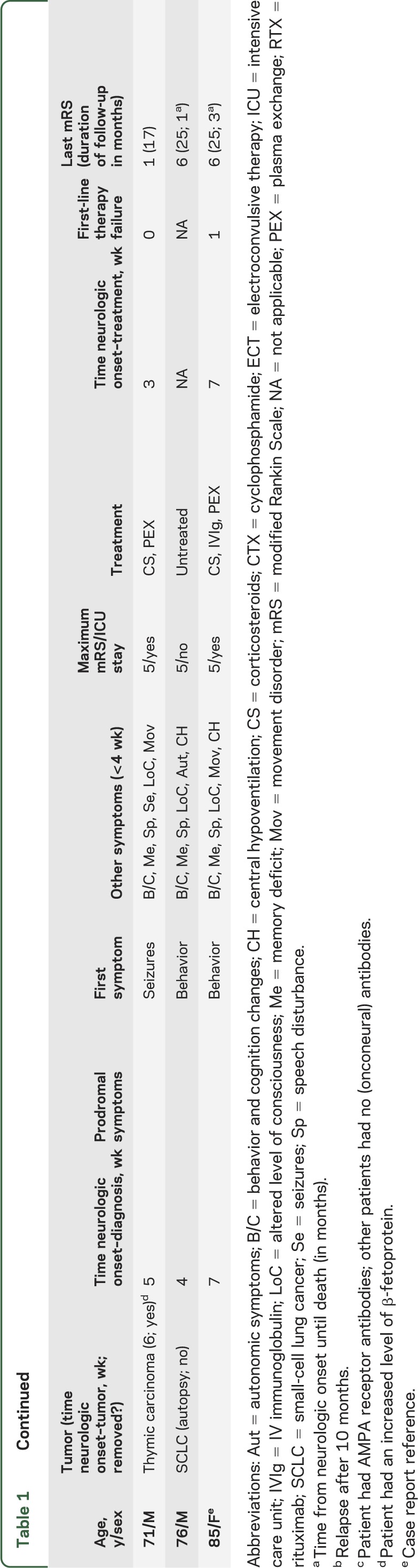
Thirty-one patients were older than 44 years (table 1). The median age was 52 years (range 45–84). Fourteen were male (45%), compared to 41 of 338 (12%) in younger adults (p < 0.0001). Seven patients (23%) had an underlying tumor compared with 173 (51%) younger adults (p = 0.002); only one patient had an ovarian teratoma; other tumors included breast (2), lung (2), thymic (1), and ovarian (1) carcinomas (table 1). All tumors were detected within 6 weeks of neurologic symptom onset.
Table 1.
Characteristics of the patients ≥45 years of age
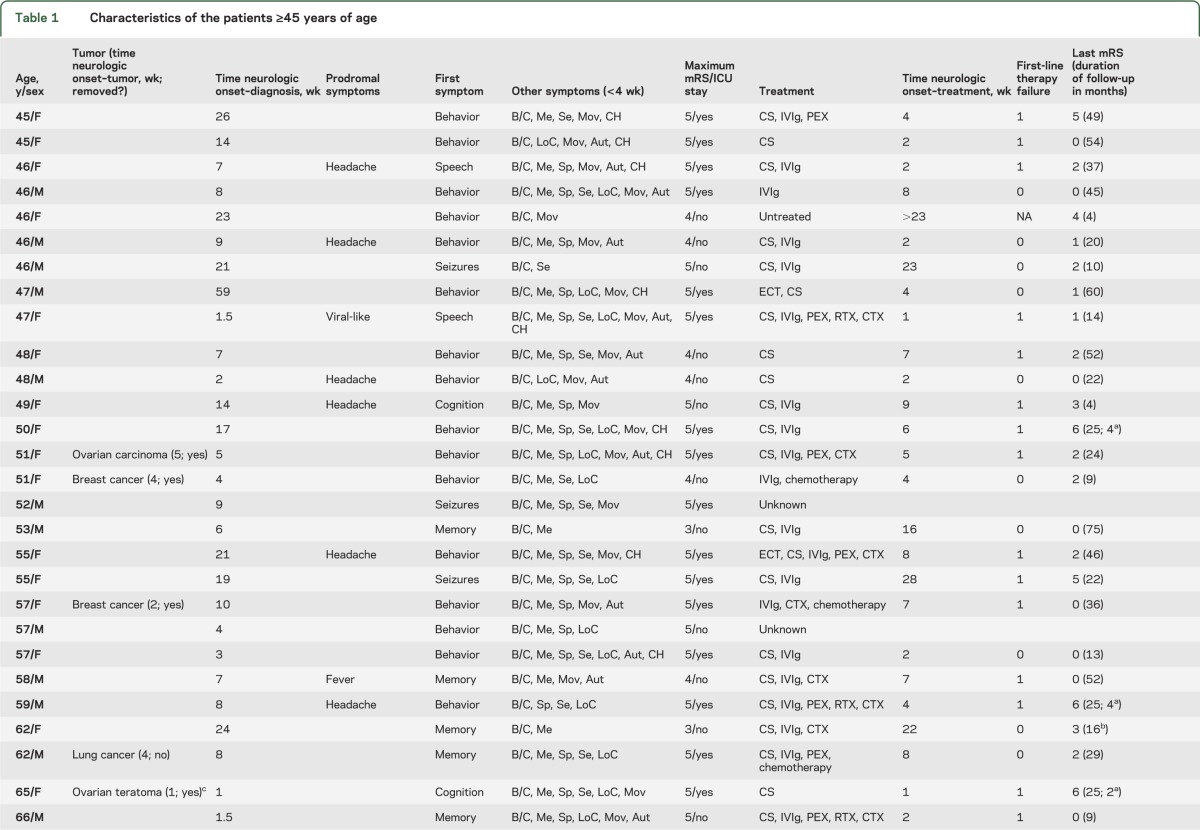
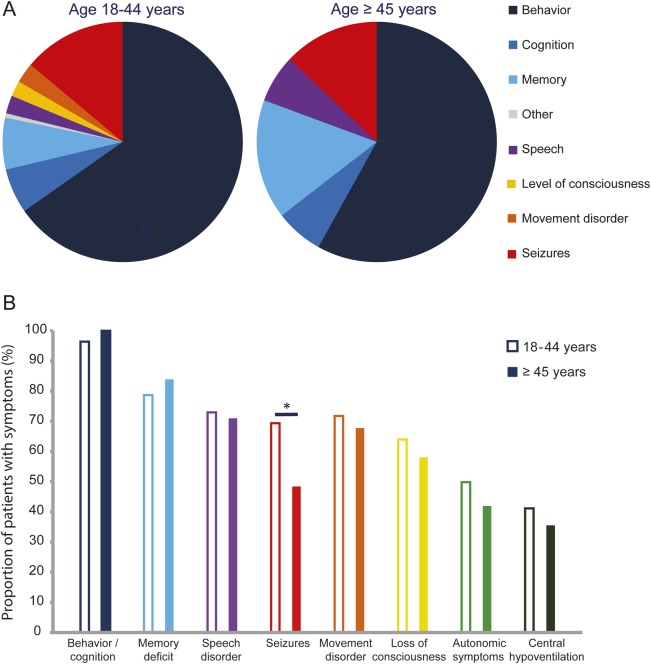
Prodromal symptoms occurred less frequently in patients ≥45 years old (26% vs 54%, p = 0.003). Behavioral problems, seizures, and memory deficits were the most frequent presenting symptoms, with a trend of memory loss being more frequent in patients ≥45 years (16% vs 7%, p = 0.075; figure 1A). Within 1 month of symptom onset, 87% of the patients had developed ≥4 of the typical symptoms of the disease, a finding not different from young adults (85%)2; only seizures occurred less frequently in patients ≥45 years of age (48% vs 69%, p = 0.026, figure 1B). Two patients only had memory dysfunction, frontal disinhibition, and mood disorder during the first 3 months of the disease. The maximum severity of symptoms was lower in older adults (74% had a mRS score of 5% vs 88% in younger adults; p = 0.044) and they needed intensive care support less frequently (58% vs 80% in younger adults; p = 0.011).
Figure 1. Clinical symptoms in adults by age distribution (18–44 and ≥45 years) at onset.
(A) First symptom (age 18–44 = 338, and age ≥45 = 31). The frequency of memory dysfunction shows a trend: p = 0.075. (B) Cumulative symptoms during the first month of the disease. For each color, the left column refers to patients 18–44 years (n = 333), and the right solid column to patients ≥45 years (n = 31). *p = 0.026.
The median time until diagnosis was 8 weeks (interquartile range [IQR] 4–17) in patients ≥45 years vs 4 weeks in younger adults (IQR 3–8, p = 0.009). The disorders more frequently suspected at symptom onset are shown in table e-1 on the Neurology® Web site at www.neurology.org. Abnormalities in the first CSF (79%) and EEG (86%) were similar to those reported in young adults and children,1–3 but the first brain MRI more frequently showed disease-related nonspecific abnormalities (53% vs 35% in younger adults, p = 0.049). The median time until treatment was longer (7 weeks, IQR 2.5–8.5) than in young adults (4 weeks, IQR 2–7, p = 0.039). First-line immunotherapy (steroids, IV immunoglobulin, plasma exchange) and second-line immunotherapy (rituximab or cyclophosphamide) were similarly used in older and young adults. Sixteen patients ≥45 years (55%) failed first-line immunotherapy vs 42% of younger adults (p = 0.071). Of these 16 patients, 6/7 had favorable outcome after second-line immunotherapy vs 2/9 who did not receive second-line immunotherapy. Resection of the tumor was performed in 5/7 patients (71% vs 95% in younger adults, p = 0.04).
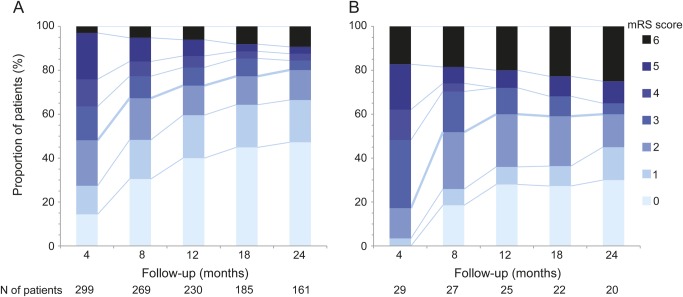
At 24 months follow-up, 60% of the patients had good outcome (mRS 0–2) vs 80% of young adults (p = 0.026; figure 2). In multivariable analysis, independent factors associated with good outcome included younger age (odds ratio [OR] 0.15, 95% confidence interval [CI] 0.05–0.39, p = 0.0001), short time until initiation of treatment (OR 0.60, CI 0.47–0.78, p < 0.0001), no need for admission to intensive care unit (OR 0.09, CI 0.04–0.22, p < 0.0001), and longer follow-up (p < 0.0001, figure 2). In patients not responding to first-line immunotherapy, the use of second-line immunotherapy (rituximab or cyclophosphamide) was an additional predictor of good outcome (OR 2.93, CI 1.10–7.76, p = 0.031).
Figure 2. Clinical outcome after extended follow-up.
(A) Patients 18–44 years old. (B) Patients ≥45 years old. Outcome was measured by modified Rankin Scale (mRS).
DISCUSSION
This study shows that anti-NMDAR encephalitis is less severe in patients ≥45 years old than in young adults, but the outcome is poorer in older patients. Our findings suggest that these differences are due to longer delays in the diagnosis and treatment of the disease in older patients as well as age-related factors. Despite this, 60% of patients ≥45 years old had full or substantial recovery at 24 months follow-up.
Three additional features that are different between older and younger adults with anti-NMDAR encephalitis include sex distribution, frequency and type of tumor association, and symptom presentation. While 80% of young adults with the disorder are women and 51% of them have an underlying teratoma (almost always in the ovary), 45% of older patients are men and only 23% have a tumor, rarely a teratoma. The close temporal association between tumor detection and onset of encephalitis suggests a pathogenic relationship. Tissue available from one patient with breast cancer showed expression of NMDAR (data not shown).
In older patients, there was a tendency to present with memory deficits, leading to a wide differential diagnosis. However, within the first 4 weeks of the disease, most patients developed other symptoms of anti-NMDAR encephalitis, mainly psychosis, dyskinesias, or speech problems. No specific symptom is pathognomonic, but these combined symptoms are highly suggestive of the disease.
The higher frequency of men among patients ≥45 years of age (female:male 1.2 vs 4 in younger adults, and 3.5 in those without teratoma) is similar to the distribution in prepubertal patients, suggesting the influence of hormonal factors in disease development. We do not know why older patients have less symptom severity, but this phenomenon has been reported in other autoimmune disorders. It has been postulated that in older individuals autoantibodies have lower affinity, and the immunologic environment is altered, resulting in weaker autoimmune responses.7,8 Although less severe, the symptoms of our patients were not mild, which along with age-related features (e.g., more limited recovery of the aging brain)9 may explain why patient age was found to be an independent risk factor for less favorable outcome.
Our findings have several implications: 1) in patients ≥45 years old, anti-NMDAR encephalitis occurs with similar frequency in males and females, and the symptom presentation often leads to a wide differential diagnosis; 2) the workup for a tumor should be similar to that recommended for classical paraneoplastic disorders10; and 3) diagnostic and treatment delays are significantly longer than in younger adults. This is important, because “time until treatment” is a known prognostic factor in this disease. It is likely that the outcome will improve if patients are diagnosed and treated earlier.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all physicians, patients, and families of patients who provided clinical information.
GLOSSARY
- CI
confidence interval
- IQR
interquartile range
- mRS
modified Rankin Scale
- NMDAR
NMDA receptor
- OR
odds ratio
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
M.J.T.: study concept and design, acquisition of data, analysis and interpretation of data, statistical analysis, drafting and revision of manuscript, funding. L.M.: acquisition of data, analysis of data, revision of manuscript. I.G.: acquisition of data, revision of manuscript. T.I.: acquisition of data, revision of manuscript. I.K.: acquisition of data, revision of manuscript. L.B.: acquisition of data, revision of manuscript. A.T.: analysis and interpretation of data, statistical analysis, revision of manuscript. M.R.R.: interpretation of data, drafting and revision of manuscript. R.B.-G.: revision of manuscript. F.G.: study concept and design, interpretation of data, revision of manuscript. J.D.: study concept and design, acquisition of data, interpretation of data, drafting and revision of manuscript, study supervision, funding.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
M. Titulaer has been supported by KWF fellowship 2009-4451 of the Dutch Cancer Society, and is currently supported by an ErasmusMC fellowship. L. McCracken, I. Gabilondo, T. Iizuka, I. Kawachi, L. Bataller, A. Torrents, and M. Rosenfeld report no disclosures. R. Balice-Gordon is funded by NIH grant RC1NS068204 and a McKnight Neuroscience of Brain Disorders award. F. Graus is funded by the Fondo de Investigaciones Sanitarias (FIS, Spain) PS09/0193. J. Dalmau receives royalties from Athena Diagnostics for a patent for the use of Ma2 as autoantibody test, and licensing fees from Euroimmun for a patent for the use of NMDAR as autoantibody test. Dr. Dalmau is funded by NIH grants RO1NS077851, RO1CA89054, and RC1NS068204; received a McKnight Neuroscience of Brain Disorders award; and received research support from the Fondo de Investigaciones Sanitarias (FIS, Spain) 11/01780, and Fundació la Marató de TV3. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011;10:63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010;133:1655–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day GS, High SM, Cot B, et al. Anti-NMDA-receptor encephalitis: case report and literature review of an under-recognized condition. J Gen Intern Med 2011;26:811–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607 [DOI] [PubMed] [Google Scholar]
- 7.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens 2007;70:179–189 [DOI] [PubMed] [Google Scholar]
- 8.Johnson SA, Cambier JC. Ageing, autoimmunity and arthritis: senescence of the B cell compartment: implications for humoral immunity. Arthritis Res Ther 2004;6:131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature 2010;464:529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Titulaer MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol 2011;18:19-e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.