Abstract
Objective:
To construct a model for depression in Parkinson disease (PD) and to study the relative contribution of PD-specific and nonspecific risk factors to this model.
Methods:
Structural equation modeling of direct and indirect associations of risk factors with the latent depression outcome using a cross-sectional dataset of 342 patients with PD.
Results:
A model with acceptable fit was generated that explained 41% of the variance in depression. In the final model, 3 PD-specific variables (increased disease duration, more severe motor symptoms, the use of levodopa) and 6 nonspecific variables (female sex, history of anxiety and/or depression, family history of depression, worse functioning on activities of daily living, and worse cognitive status) were maintained and significantly associated with depression. Nonspecific risk factors had a 3-times-higher influence in the model than PD-specific risk factors.
Conclusion:
In this cross-sectional study, we showed that nonspecific factors may be more prominent markers of depression than PD-specific factors. Accordingly, research on depression in PD should focus not only on factors associated with or specific for PD, but should also examine a wider scope of factors including general risk factors for depression, not specific for PD.
Clinically relevant depressive symptoms are present in 35% of patients with Parkinson disease (PD), and depression has been identified as the most important determinant of quality of life in these patients.1–3 Depressive disorders, in the general population as well as in patients with PD, develop in the context of multiple interacting risk and protective factors. These factors may or may not be related to PD. In the general population, longitudinal studies have shown that risk factors for depression include female sex, older age, being single, a low level of education, physical disease, a personal or family history of depression, cognitive impairment, smoking, and alcohol. Moreover, personal circumstances, such as early childhood adversity, personality traits and coping, and recent positive and negative life events, also have a role.4–7 In cross-sectional studies, several PD-specific risk factors for depression have been identified, including more severe motor symptoms, longer disease duration, more advanced disease stage, greater limitations in disease-related activities of daily living (ADL), higher daily levodopa equivalent dose, and the presence of nonmotor symptoms such as hallucinations, sleep disturbances, and dysautonomia.8–11 Few studies have examined the role of general risk factors for depression in PD.
The objective of this study was to construct a model for depression in PD, and to compare the relative contribution of PD-specific and nonspecific markers to this model.
METHODS
The present study was performed from the database of a cross-sectional multicenter study on anxiety disorders in PD that was conducted in 2008 and 2009, the results of which have been described in 2 reports published earlier.12,13
Population.
The database includes 342 patients with PD, diagnosed according to the Queen Square Brain Bank clinical criteria.14 Subjects, recruited from the movement disorders clinics and the neurology and psychiatry clinics of 6 centers in the United States, Europe, and Australia, underwent a comprehensive neurologic and neuropsychiatric assessment. Patients with neurodegenerative disorders other than PD were excluded. Patients with clinically relevant cognitive symptoms were also excluded. This was operationalized as a Mini-Mental State Examination (MMSE) score <26, following the recommendation of a Movement Disorders Society (MDS) Task Force.15,16 All types of neurologic and psychopharmacologic medication were allowed. Patients undergoing deep brain stimulation were excluded.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the local Medical Ethics Committees of all participating institutions. Patients gave written informed consent before inclusion in the study.
Assessment.
Demographic and disease-related variables were assessed during an unstructured clinical interview. Motor function, disease-related decline in ADL, and complications of therapy were assessed with the Unified Parkinson's Disease Rating Scale (UPDRS).17 Disease stage was assessed with the Hoehn & Yahr staging system.18 Cognitive functions and instrumental ADL were assessed with the MMSE and Lawton Instrumental ADL (IADL) Scale.15,19 Depressive symptom severity was quantified with the 17-item Hamilton Depression Rating Scale (HAMD)20; anxiety was assessed with the clinician-rated Hamilton Anxiety Rating Scale.21 Patients with “on/off” fluctuations were assessed only during “on” states, as advised by an MDS Task Force.22 Also in accordance with MDS Task Force recommendations, we followed an “inclusive” approach to rating symptoms, meaning that symptoms were scored as observed or reported, irrespective of their assumed etiology.22 The presence of DSM-IV–defined depressive and anxiety disorders was determined using the Mini International Neuropsychiatric Inventory (a structured interview for DSM-IV disorders) sections for depression (A, B) and anxiety (D, E, F, H).23
Statistical analysis.
Structural equation modeling was performed in Mplus 7 (Muthén & Muthén, Los Angeles, CA) with the aim of identifying the most parsimonious model (for model fit and number of included parameters) that still accounted for a substantial part of the variance in the depression outcome.
Independent variables were chosen on the basis of their known contribution to depression in the general population and in PD from the available literature. An exploratory correlation analysis was conducted between all potential parameters to discover collinearity. If the Pearson correlation coefficient “r” between 2 potential parameters was both significant (p < 0.005 after correction for multiple testing) and >0.40, a decision was made to include only one of these parameters in the model, where general markers would have preference over PD-specific markers. An initial theoretical Multiple Indicators Multiple Causes model was constructed with the remaining parameters. Because the Multiple Indicators Multiple Causes model included continuous, binary, and ordered categorical variables, a mean- and variance-corrected weighted least-squares estimator was used.
For the sake of the analysis, on the measurement side of the model, a latent variable of “depression” was constructed by regressing the 17 items of the HAMD on a single continuous latent variable. A “latent” variable in structural equation modeling is a hypothetical construct that is not measured directly, but estimated in the model from several measured variables, in this case from the individual HAMD items. Compared with using observed HAMD total scores, this approach has the advantage that only shared variances among the items contribute to the depression factor, whereas nonshared (unique) variance is regarded as measurement error, resulting in a purer operationalization of the latent (“true”) depression construct.
For the structural part of the model, direct and indirect paths of the variables theorized to influence the depression outcome were specified in an initial model (model 1). Model fit was primarily assessed by inspecting the root mean square error of approximation (RMSEA) and the Comparative Fit Index (CFI). For the RMSEA, scores ≤0.05 indicate good fit, and scores ≤0.08 indicate acceptable fit. The CFI ranges from 0 to 1 with scores ≥0.95 indicating good fit, and scores ≥0.90 indicating acceptable fit.24 Because the χ2 test is known to become positively biased with increasing sample size, this measure was not used to assess model fit.24
Based on model fit, the model was respecified via a number of consecutive steps. First, paths that did not contribute substantially (p > 0.10) to the model were removed in a backward 1-to-1 fashion, starting with the model with the path with the highest p value, resulting in a second model (model 2). Next, modification indices (MIs) were inspected to explore whether the model could be improved by specifying additional paths among the remaining variables. Additional paths were included based on the MIs and substantive interpretation. Finally, MIs were inspected for correlated errors (residual correlation) among variables whose specification might improve the model. This resulted in the final model.
In a last step, we examined whether PD-specific variables and PD-unrelated variables contributed equally to the depression outcome. Because a direct comparison of the joint effect of the manifest variables was not possible because of the correlated nature of individual items, 2 continuous latent variables (factors) were generated and regressed on depression. Their standardized regression coefficients were compared using a Wald test. To have both factors on the same scale, their factor variances were fixed to 1.
RESULTS
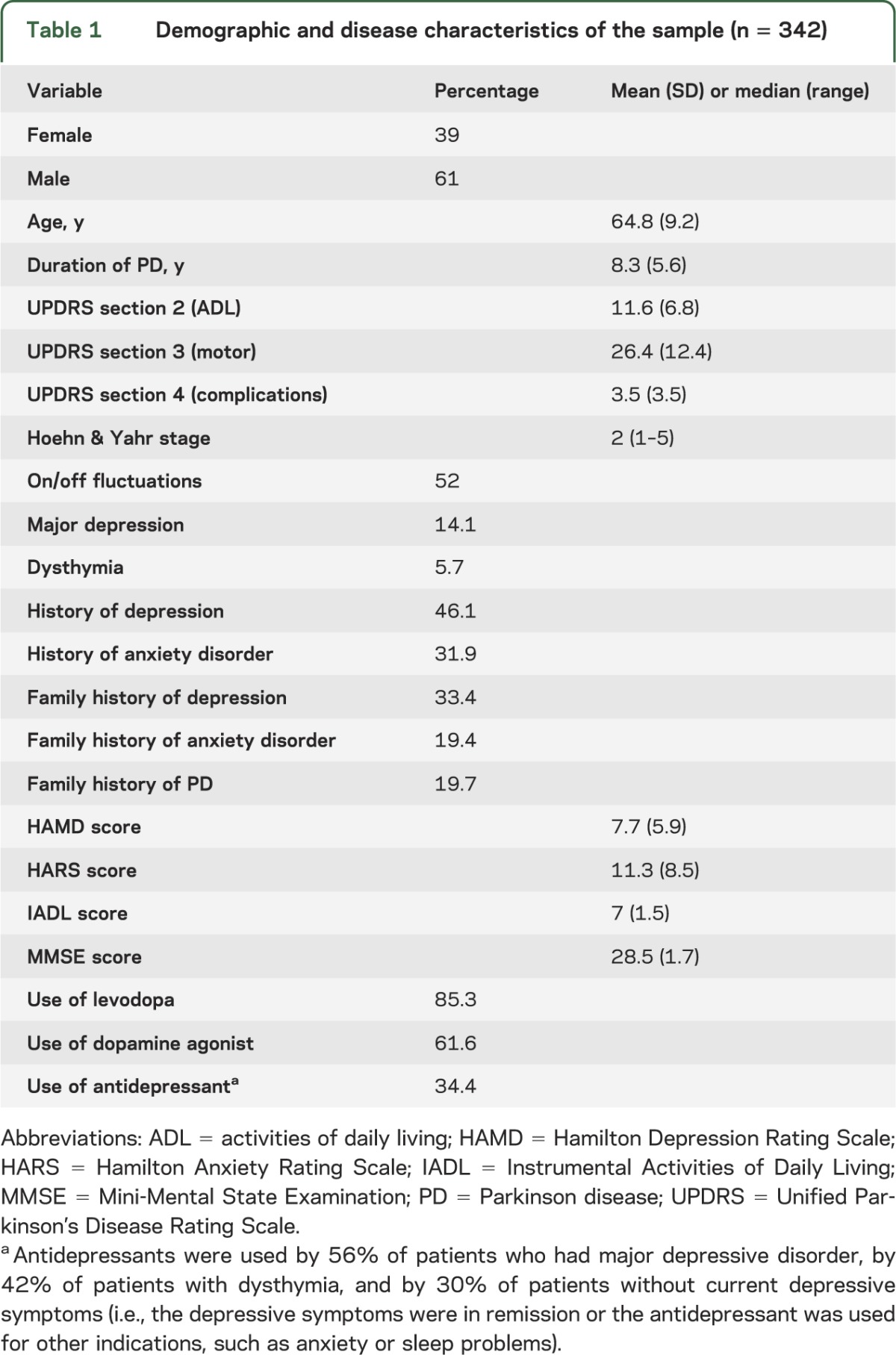
The demographic and disease characteristics of the included sample are listed in table 1. The study population comprised 207 men and 134 women with an average age of 64.8 years (SD 9.2 years). Based on the Mini International Neuropsychiatric Inventory, 48 participants (14.1%) met diagnostic criteria for a current major depressive episode, 19 (5.6%) met the diagnostic criteria for dysthymia, and 64 (18.8%) had a clinically relevant depressive symptom (defined here as a score ≥12), but did not meet the criteria for major depressive episode or dysthymia.
Table 1.
Demographic and disease characteristics of the sample (n = 342)
Results of the exploratory correlation analysis are shown in table e-1 on the Neurology® Web site at www.neurology.org. Based on the correlations, it was decided to exclude disease stage (Hoehn & Yahr classification) from the model because of a moderately strong correlation with UPDRS sections 2 and 3 and IADL (r = 0.60, r = 0.46, and r = −0.51, respectively; all p < 0.01). UPDRS 4 total score was excluded because of its strong correlation with off-periods (r = 0.66; p < 0.01). In addition, it was considered more informative to include separate parameters for the presence of “off-periods” and dyskinesias in the model instead of the single parameter of the UPDRS section 4. It was decided to keep both the UPDRS section 2 (ADL) and the score on the IADL Scale in the model, despite moderate correlation (r = 0.46; p < 0.01). This was done because it was thought that the UPDRS section 2 scores PD-related ADL, whereas the IADL Scale can be considered a more general measure of personal functioning. It was further decided to keep the UPDRS section 2 score in the model despite its moderate correlation with UPDRS section 3 (r = 0.51; p < 0.01).
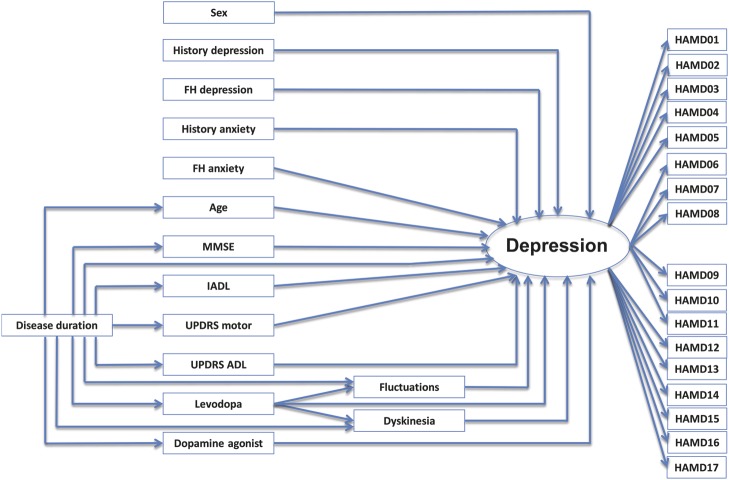
The initial theoretical model is shown in figure 1 and specified in table 2. Nonspecific parameters included in this model were age, sex, cognitive status (MMSE total score), instrumental ADL function (IADL total score), history of depression, history of anxiety, family history of depression, and family history of anxiety. PD-specific parameters in the model were disease duration, motor symptom severity (UPDRS section 3 total score), disease-specific ADL (UPDRS section 2 total score), the presence of motor fluctuations (based on UPDRS section 4), the presence of dyskinesias (based on UPDRS section 4), the use of levodopa, and the use of a dopamine agonist. This model showed acceptable fit for RMSEA, but poor fit for CFI (RMSEA 0.069 with a 90% confidence interval [CI] = 0.064–0.074; CFI 0.644).
Figure 1. Initial model of depression in PD (model 1).
This initial model is the hypothesized model and includes all nonspecific and PD-specific variables. Model specifics and regression coefficients are given in table 2. ADL = activities of daily living; FH = family history; HAMD = Hamilton Depression Rating Scale; IADL = Instrumental Activities of Daily Living; MMSE = Mini-Mental State Examination; PD = Parkinson disease; UPDRS = Unified Parkinson's Disease Rating Scale.
Table 2.
Standardized regression coefficients of the initial (theoretical) model including 15 variables (model 1)
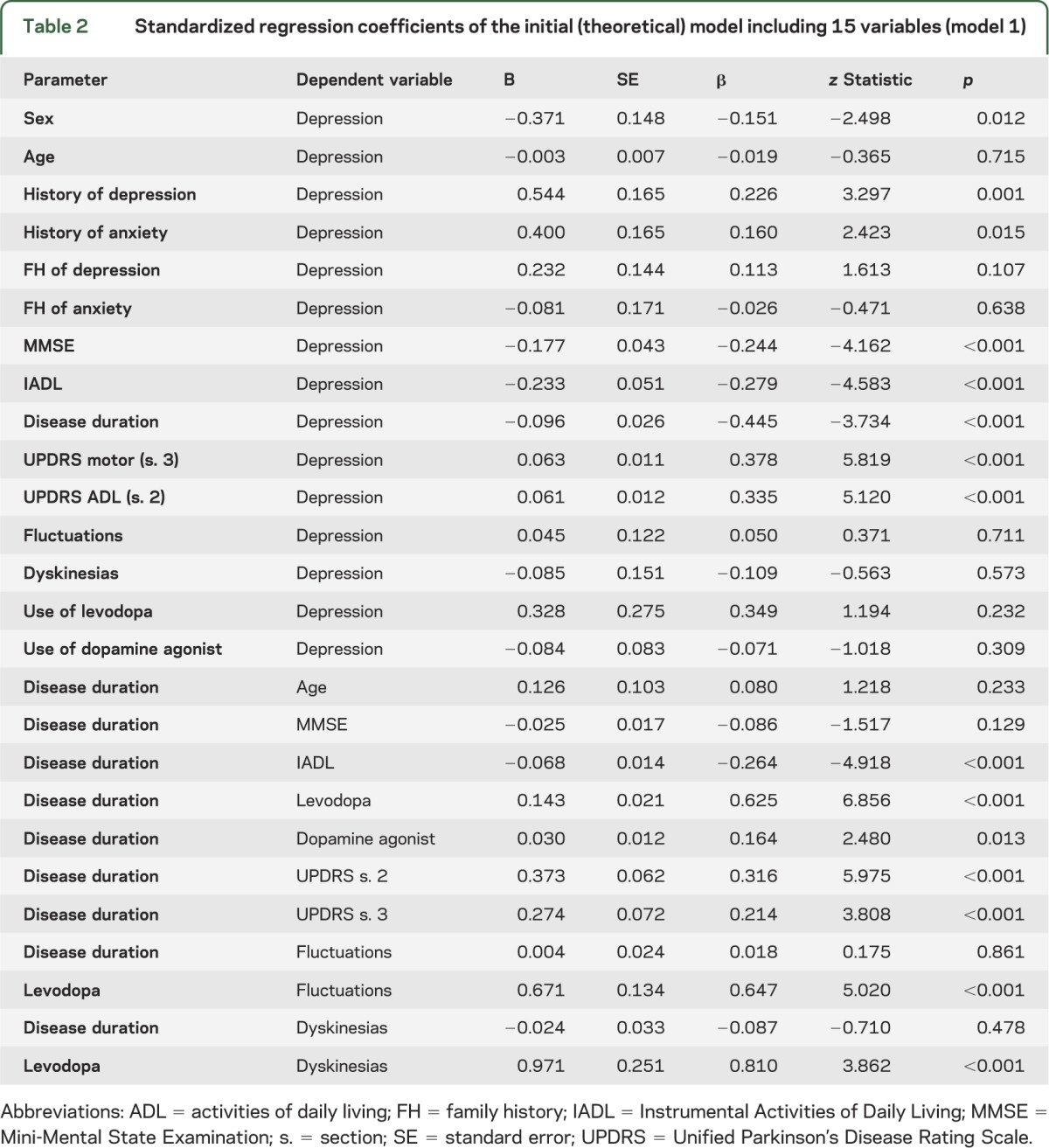
In a first revision, paths that did not contribute substantially to the model were deleted: age, family history of anxiety, the use of a dopamine agonist, the presence of dyskinesias, and the indirect effects of levodopa. This resulted in a second, simpler model showing similar fit (RMSEA 0.074 with a 90% CI = 0.069–0.080; CFI 0.675). This intermediate model is shown in figure e-1, and specified in table e-2.
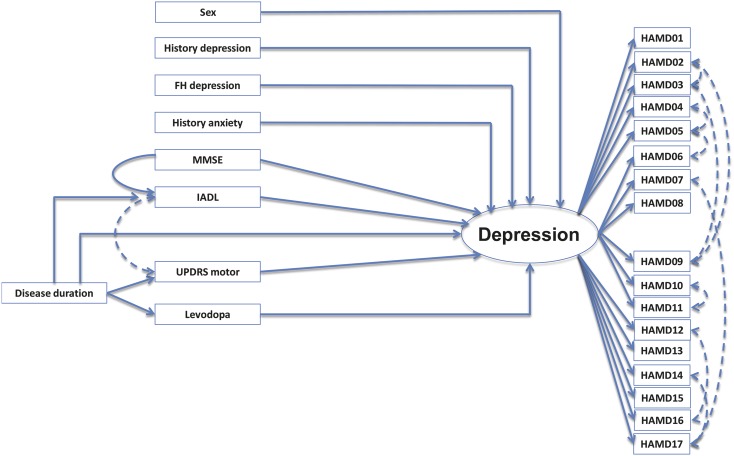
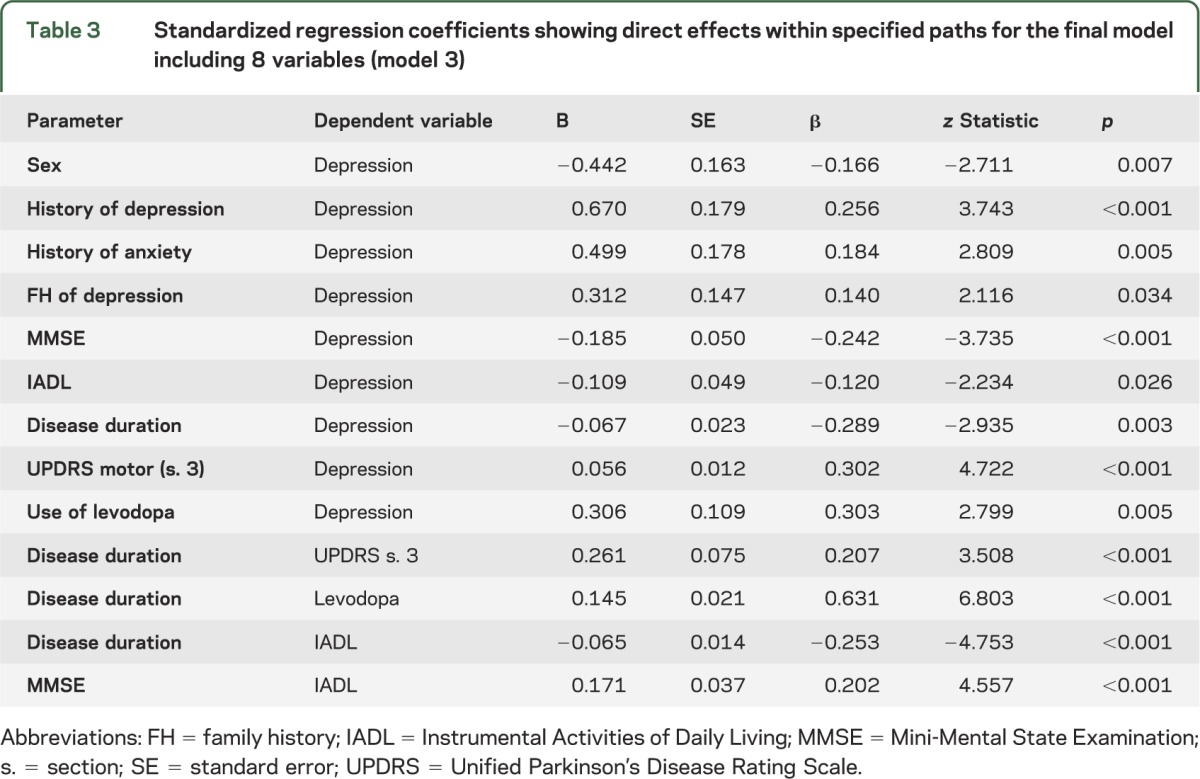
A second revision, which allowed for correlated residuals among variables and extra paths, resulted in a model with better fit (RMSEA 0.051 with a 90% CI = 0.044–0.058; CFI 0.854). In this last model, the disease-specific ADL parameter was removed, and an indirect effect of the MMSE on depression through IADL function was added, as well as a correction for interaction of IADL and UPDRS section 3. This model is shown in figure 2 and specified in table 3. The model explains 41% of the observed variance in the depression outcome.
Figure 2. Final model of depression in PD (model 3).
This final model allows for correlated residuals (dashed lines) among variables and extra paths. Model specifics and regression coefficients are given in table 3. FH = family history; HAMD = Hamilton Depression Rating Scale; IADL = Instrumental Activities of Daily Living; MMSE = Mini-Mental State Examination; PD = Parkinson disease; UPDRS = Unified Parkinson's Disease Rating Scale.
Table 3.
Standardized regression coefficients showing direct effects within specified paths for the final model including 8 variables (model 3)
We finally compared the effects of the PD-specific and PD-unrelated variables in the model after both types of variables had been regressed on 2 latent variables. This showed that both factors were significantly associated with depression and together accounted for 69% of its variance. The PD-unrelated factor was significantly more strongly related to depression (β = 0.742, standard error = 0.078, p < 0.001) than the PD-specific factor (β = 0.247, standard error = 0.082, p = 0.003). This was confirmed in a Wald test comparing the effects of the 2 factors (χ2 = 12.93, df = 1, p < 0.001).
DISCUSSION
In this study, we showed that 41% of the variance in depressive symptoms in patients with PD was explained by 9 variables: 6 variables not specific for PD, and 3 specific for PD. When both types of factors were regressed on 2 latent variables, they together explained 69% of variance. In this model, the latent variable representing the nonspecific factors had a 3-times-higher β, i.e., a 3-times-larger influence, than the latent variable of all PD-specific factors. However, the manifest variables most strongly associated with depression in our final model were all PD-specific markers: the UPDRS motor score, use of levodopa, and disease duration. Dopamine agonists have often been associated with mood-improving effects in patients with PD.25 In our study, however, the use of dopamine agonists did not have a significant negative (nor positive) association with the depression outcome.
Taken together, both nonspecific and PD-specific variables appear to contribute to depression in PD, with PD-specific factors showing strong individual associations, while nonspecific factors seem to have a larger net effect on the depression outcome.
Published research on the relative contribution of PD-specific and nonspecific factors to the risk of depression in PD is scant. In one cross-sectional study involving 161 patients with PD, a logistic regression model consisting of 5 general, PD-nonspecific risk factors for depression (age, sex, history of depression, family history of depression, and somatic comorbidity) correctly predicted whether a patient was depressed or not in 75% of cases. Adding PD-specific risk factors into the logistic model did not increase the discriminative performance.26 Although other studies have included some general risk factors for depression, the influence of these factors has not been directly compared with that of PD-specific factors. In line with our findings, Riedel et al.11 and Becker et al.9 report female sex to be associated with depression. Older age, cognitive decline, the use of nonsteroidal anti-inflammatory drugs, and physical comorbidity other than PD are also related to depression in PD.8,11 Research seems to have moved away from the study of personality characteristics and coping in the etiology of depression in PD. Older studies report a premorbid personality in PD, characterized by inflexibility and obsessiveness, which may predispose for depression.27 Maladaptive cognitive coping is also associated with increased feelings of depression and anxiety.28,29 Finally, life events have been shown to have an important role in the development of major depression in PD, although its effect seems to be modified by social support and coping mechanisms.30 These factors should receive more consideration in future studies.
Our findings have important conceptual implications for our understanding of depression in PD. They show that several PD-specific factors are indeed important markers for depression, but that their true relevance is only understood by adopting a broad multifactorial approach to depression that also includes nonspecific markers: PD-specific factors are associated with depression, but their net effect is smaller than that of a number of general risk factors for depression, not specific for PD. Based on these findings, one could hypothesize that those patients with PD who develop depression are likely to have a preexisting vulnerability because of their exposure to common risk factors for depression that are unrelated to PD. Our findings also imply that a broader approach should be followed in future studies into the etiology of depression in PD, including not only PD-specific, but also general, nonspecific risk factors.
Our study has several limitations. First, the analysis was performed on an existing database of a cross-sectional study into anxiety in patients with PD. Parameters selected to be included in the model were based on available data, which implies that other important parameters that may be considered markers of depression, such as marital status, the availability of a caregiver, personality, coping style, and past or recent life events were not included in the study. Also, protective factors, such as the use of antidepressants and recent positive life events were not included in the analysis. This may also elucidate why our final model explains 41% of the total variance in depression. Although this may seem a low percentage, it underscores that depressive syndromes are complex and etiologically multifactorial. Inclusion of more variables in the model would provide a more complete psychosomatic overview of all factors associated with depressive disturbances in PD. Notable psychological variables, such as the ones listed just above, warrant additional study given the fact that they are known risk factors for depression in the general population as well as in PD. Next, it is difficult to separate markers that are related to PD and those that are not directly related to PD. In patients with PD, cognitive decline and decline in ADL functions may be due to PD, but these are also known risk factors for depression in the general population, and hence not specific for PD. This is why the authors used the terms “PD-specific” and “nonspecific” rather than “PD-related” and “PD-unrelated” factors. Another limitation is the fact that our dataset is cross-sectional, and hence no causal interferences can be drawn. Despite these shortcomings, the database was large enough to allow structural equation modeling analysis with a substantial number of parameters. Finally, model fit for all models was acceptable to good for the RMSEA, although not for CFI. Hence, our reliance on the RMSEA might seem arbitrary. However, CFI tends to worsen as the number of variables increases.31 In addition, use of the CFI (and other incremental measures of fit) has been discouraged if the baseline model has an RMSEA <0.158,32 which is the case in our data (RMSEA of the baseline model is 0.125). Again, these analyses are exploratory and limited as outlined above. Finally, the model requires confirmation in a longitudinal design that includes more psychological and contextual variables.
In this study, we showed that individual PD-specific factors are strongly associated with depression, but that nonspecific factors, as compared with PD-specific factors, contributed to a substantially larger degree to the presence of depressive disturbances. These data underscore the importance of understanding depression in PD within a complex multifactorial framework. Accordingly, it is critical that future studies into the etiology of depression in PD (and most likely studies addressing other psychopathologic syndromes in PD as well) have a wider scope and be designed to include general risk factors for depression that are not specific for PD, while including psychological factors and factors associated with PD. A restricted approach, limited to PD-specific factors, obscures the complex nature of psychopathologic comorbidities encountered in PD, and may subsequently lead to wrong conclusions about what might be salient targets for prevention and treatment.
Supplementary Material
GLOSSARY
- ADL
activities of daily living
- CFI
Comparative Fit Index
- CI
confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- HAMD
Hamilton Depression Rating Scale
- IADL
Instrumental Activities of Daily Living
- MDS
Movement Disorders Society
- MI
modification index
- MMSE
Mini-Mental State Examination
- PD
Parkinson disease
- RMSEA
root mean square error of approximation
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
A.F.G. Leentjens: study concept and design, acquisition of data, analysis and interpretation, writing the manuscript, study supervision. A.J.H. Moonen: acquisition of data, analysis and interpretations, critical revision of the manuscript for intellectual content. K. Dujardin, L. Marsh, P. Martinez-Martin, I.H. Richard, and S.E. Starkstein: data acquisition, interpretation of results, critical revision of the manuscript for intellectual content. S. Köhler: analysis and interpretation, critical revision of manuscript for intellectual content.
STUDY FUNDING
This study did not receive any funding. The study on which database this analysis was performed received funding from the Michael J. Fox Foundation for Parkinson’s Research.
DISCLOSURE
A. Leentjens is Editor-in-Chief of the Journal of Psychosomatic Research. He has received research grants from the Michael J. Fox Foundation, the Stichting Internationaal Parkinson Fonds, and the Internationale Stichting Alzheimer Onderzoek, as well as royalties from Reed-Elsevier, de Tijdstroom, and Van Gorcum publishers. A. Moonen has nothing to disclose. K. Dujardin served on the scientific advisory board on Parkinson disease dementia for Novartis Pharma France SAS and received research support from the Michael J. Fox Foundation for Parkinson's Research. L. Marsh currently serves as an editorial board member of Journal of Parkinson's Disease, receives grant support from NIH, the Dystonia Foundation, American Psychiatric Association, and Veterans Health Administration. During the execution of this proposal, she received royalties from Taylor & Francis/Informa, grant support from the NIH, Michael J. Fox Foundation, Boehringer Ingelheim, Forest Research Institute, and Eli Lilly and Company, and an honorarium from Merck Serono. P. Martinez-Martin has received honoraria for lectures or participation in scientific meetings from Novartis, Britannia, Orion Pharma, and Abbott/AbbVie. He is current holder of grants for research from Michael J. Fox Foundation, Parkinson UK, and the Spanish Official Agencies FIS and IMSERSO. I. Richard serves on a scientific advisory board for the Michael J. Fox Foundation. She receives grant support from the Michael J. Fox Foundation and Institute for Neurodegenerative Disorders and has received research support from NIH/NINDS, Neurologix, and Cornell University. S. Starkstein is funded by grants from the National Health Medical and Research Centre (Australia) and the Michael J. Fox Foundation. He serves as an editorial board member of International Psychogeriatrics. S. Köhler has nothing to disclose. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Reijnders JSAM, Ehrt U, Weber WEJ, Aarsland D, Leentjens AFG. A systematic review of prevalence studies of depression in Parkinson's disease. Mov Disord 2008;23:183–189 [DOI] [PubMed] [Google Scholar]
- 2.Global Parkinson's Disease Survey Steering Committee Factors impacting on quality of life in Parkinson's disease: results from an international survey. Mov Disord 2002;17:60–67 [DOI] [PubMed] [Google Scholar]
- 3.Schrag A. Quality of life and depression in Parkinson's disease. J Neurol Sci 2006;248:151–157 [DOI] [PubMed] [Google Scholar]
- 4.Sonnenberg CM, Beekman ATF, Deeg DJH, Van Tilburg W. Sex differences in late life depression. Acta Psychiatr Scand 2000;101:286–292 [PubMed] [Google Scholar]
- 5.Von Korff MR, Scott KM, Gureje O. Global Perspectives on Mental-Physical Comorbidity in the WHO World Mental Health Surveys. New York: Cambridge University Press; 2009 [Google Scholar]
- 6.Beekman ATF, Deeg DJH, Van Tilburg T, Smit JH, Hooijer C, Van Tilburg W. Major and minor depression in later life: a study of prevalence and risk factors. J Affect Disord 1995;36:65–75 [DOI] [PubMed] [Google Scholar]
- 7.Schoevers RA, Beekman ATF, Deeg DJH, Geerlings MI, Jonker C, Van Tilburg W. Risk factors for depression in later life: results of a prospective community based study (AMSTEL). J Affect Disord 2000;59:127–137 [DOI] [PubMed] [Google Scholar]
- 8.Dissanayaka NNW, Sellbach A, Silburn PA, O'Sullivan JD, Marsh R, Mellick GD. Factors associated with depression in Parkinson's disease. J Affect Disord 2011;132:82–88 [DOI] [PubMed] [Google Scholar]
- 9.Becker C, Brobert GP, Johansson S, Jick SS, Meier CR. Risk of incident depression in patients with Parkinson disease in the UK. Eur J Neurol 2011;18:448–453 [DOI] [PubMed] [Google Scholar]
- 10.Gallagher DA, Schrag A. Psychosis, apathy, depression and anxiety in Parkinson's disease. Neurobiol Dis 2012;46:581–589 [DOI] [PubMed] [Google Scholar]
- 11.Riedel O, Heuser I, Klotsche J, Dodel R, Wittchen HU; GEPAD Study Group Occurrence risk and structure of depression in Parkinson disease with and without dementia: results from the GEPAD Study. J Geriatr Psychiatry Neurol 2010;23:27–34 [DOI] [PubMed] [Google Scholar]
- 12.Leentjens AFG, Dujardin K, Marsh L, Richard IH, Starkstein SE, Martinez-Martin P. Anxiety rating scales in Parkinson’s disease: a validation study of the Hamilton Anxiety Rating Scale, the Beck Anxiety Inventory and the Hospital Anxiety and Depression Scale. Mov Disord 2011;26:407–415 [DOI] [PubMed] [Google Scholar]
- 13.Leentjens AFG, Dujardin K, Marsh L, Richard IH, Starkstein SE, Martinez-Martin P. Symptomatology and markers of anxiety disorders in Parkinson’s disease: a cross-sectional study. Mov Disord 2011;26:484–492 [DOI] [PubMed] [Google Scholar]
- 14.De Rijk MC, Rocca WA, Anderson DW, Melcon MO, Breteler MMB, Maraganore DM. A population perspective on diagnostic criteria for Parkinson's disease. Neurology 1997;48:1277–1281 [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 16.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the Movement Disorder Society Task Force. Mov Disord 2007;16:2314–2324 [DOI] [PubMed] [Google Scholar]
- 17.Fahn S, Elton RL; Members of the UPDRS Committee Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinson's Disease. Florham Park, NJ: MacMillan Health Care; 1987:153–163 [Google Scholar]
- 18.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442 [DOI] [PubMed] [Google Scholar]
- 19.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186 [PubMed] [Google Scholar]
- 20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol 1959;32:50–55 [DOI] [PubMed] [Google Scholar]
- 22.Schrag A, Barone P, Brown RG, et al. Depression rating scales in Parkinson's disease: critique and recommendations. Mov Disord 2007;22:1077–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(suppl 20):22–33 [PubMed] [Google Scholar]
- 24.Byrne BM. Structural Equation Modeling with Mplus: Basic Concepts, Applications, and Programming. New York: Taylor & Francis Group; 2011 [Google Scholar]
- 25.Leentjens AFG. The role of dopamine agonists in the treatment of depression in patients with Parkinson's disease: a systematic review. Drugs 2011;71:1–14 [DOI] [PubMed] [Google Scholar]
- 26.Leentjens AFG, Lousberg R, Verhey FRJ. Markers for depression in Parkinson's disease. Acta Psychiatr Scand 2002;106:196–201 [DOI] [PubMed] [Google Scholar]
- 27.Todes CJ, Lees A. The pre-morbid personality of patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 1985;48:97–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allott R, Wells A, Morrison AP, Walker R. Distress in Parkinson's disease: contributions of disease factors and metacognitive style. Br J Psychiatry 2005;187:182–183 [DOI] [PubMed] [Google Scholar]
- 29.MacCarthy B, Brown R. Psychosocial factors in Parkinson's disease. Br J Clin Psychol 1989;28:41–52 [DOI] [PubMed] [Google Scholar]
- 30.Rod NH, Bordelon Y, Thompson A, Marcotte E, Ritz B. Major life events and the development of major depression in Parkinson disease patients. Eur J Neurol 2013;20:663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenny DA, McCoach DB. Effect of the number of variables on measures of fit in structural equation modelling. Struct Equ Modeling 2003;10:333–351 [Google Scholar]
- 32.Kenny DA. Measuring model fit. Available at: http://davidakenny.net/cm/fit.htm. Accessed January 27, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.