Abstract
We previously reported clinical improvement, increase in putamen [18F]-dopa uptake on PET imaging, and neuropathologic evidence of sprouting of dopaminergic fibers following chronic intraputaminal delivery of glial cell line–derived neurotrophic factor (GDNF) in idiopathic Parkinson disease (PD).1–3 We now provide clinical and PET evidence of persistent efficacy lasting for at least 3 years following cessation of GDNF infusion in a patient with PD. This is a single-case observational study, providing Class IV evidence.
We previously reported clinical improvement, increase in putamen [18F]-dopa uptake on PET imaging, and neuropathologic evidence of sprouting of dopaminergic fibers following chronic intraputaminal delivery of glial cell line–derived neurotrophic factor (GDNF) in idiopathic Parkinson disease (PD).1–3 We now provide clinical and PET evidence of persistent efficacy lasting for at least 3 years following cessation of GDNF infusion in a patient with PD. This is a single-case observational study, providing Class IV evidence.
Case report.
A 56-year-old man was one of 5 patients in a phase 1 study of GDNF (Amgen, CA) infusion into the postero-dorsal putamen, for treatment of idiopathic 27-year-long akinetic predominant PD.1,2 At baseline, symptoms were poorly controlled, with prolonged “off” periods and severe levodopa-induced dyskinesias (figure e-1 on the Neurology® Web site at www.neurology.org). Daily medications included carbidopa-levodopa 600 mg, lisuride 800 μg, and selegiline 10 mg (680 mg levodopa equivalents). Clinical details of the patient have previously been published.1 Intraparenchymal catheters were stereotactically implanted into both putamens and connected to Synchro-Med pumps (Medtronic Inc., Minneapolis, MN). GDNF was infused continuously at 14–43 mg/putamen/day for 39 months.
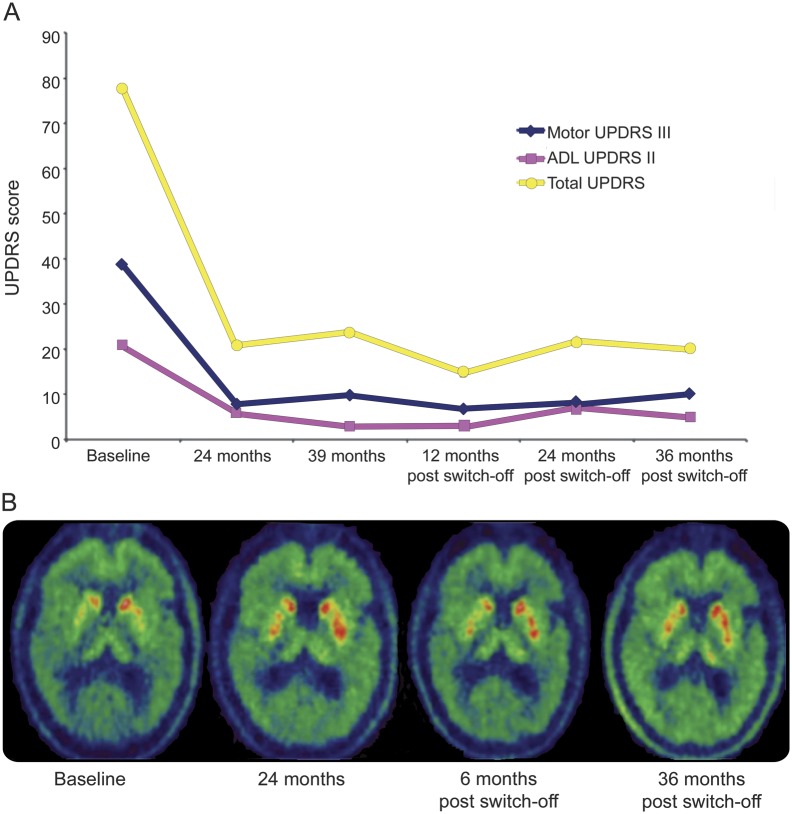
Clinical assessments were based on the Core Assessment Program for Intracerebral Transplantations.4 At 24 and 39 months, the Unified Parkinson's Disease Rating Scale Motor score (UPDRS-III) “off” medication improved by 79% and 74%, respectively, and Activities of Daily Living (UPDRS-II) “off” medication improved by 71% and 86%, respectively (figure 1A). At 24 months, this was accompanied by a 28% increase in whole putamen and 51% increase in posterior dorsal putamen [18F]-dopa uptake2 (figure 1B, table e-1). Resulting reductions in levodopa led to substantial reductions in dyskinesia severity and duration (figure e-1).
Figure 1. Clinical improvement and changes in [18F]-dopa uptake following GDNF infusion.
(A) Changes in Unified Parkinson's Disease Rating Scale (UPDRS) scores (Total, Motor, and Activities of Daily Living [ADL] scores) following 39 months of bilateral glial cell line–derived neurotrophic factor (GDNF) infusion and over the subsequent 36-month withdrawal period. (B) [18F]-dopa uptake constant (Ki) maps at baseline, following 24 months of GDNF infusion, and following 6 and 36 months of GDNF withdrawal, respectively.
GDNF infusion was stopped after 39 months following withdrawal of the drug by Amgen (the trial showed that clinical benefit from treatment was not significantly better than placebo; some patients developed anti-GDNF antibodies and cerebellar toxicity was reported in animal studies5). However, clinical assessments were performed for a further 36 months. Based on UPDRS ratings (figure 1A), clinical effects of GDNF were preserved for 36 months post GDNF cessation. The UPDRS-III and UPDRS-II scores “off” medication remained improved by 74% and 76%, respectively. Dyskinesia remained mild at 36 months post GDNF cessation (figure e-1). Levodopa intake ceased at 12 months following GDNF withdrawal. Levodopa equivalent requirement remained reduced by more than 50% at 36 months post GDNF cessation (pramipexole 2.1 mg daily, amantadine 100 mg daily; 310 mg levodopa equivalents).
Six months after GDNF discontinuation, the increases in posterior and whole putamen [18F]-dopa uptake remained essentially unchanged, 58% and 31% higher than preoperatively, respectively. At 36 months post GDNF cessation, [18F]-dopa uptake fell throughout the putamen, with no detectable change in the anterior putamen in comparison to preoperatively, although uptake remained 29% higher in the posterior putamen (figure 1B, table e-1). Neurocognitive tests were within normal range in this patient. The patient also reported improvements in taste, smell, and sexual function (table e-2). No side effects or symptoms related to GDNF withdrawal were observed.
Discussion.
In PD, putaminal [18F]-dopa uptake has been reported to decline annually by 10%. In contrast, we observed a greater than 50% increase in total putamen [18F]-dopa uptake following a 2-year putaminal infusion of GDNF, sustained for 6 months following GDNF withdrawal. The subsequent fall in [18F]-dopa uptake coincides with recurrent disease progression following the potential loss of GDNF's neuroprotective effects. However, there was still a sustained albeit reduced uptake in the posterior putamen 36 months following GDNF withdrawal. This result alongside the sprouting of dopaminergic neurons alludes to the neurorestorative properties of GDNF, providing a possible substrate for the sustained clinical improvement and enhanced posterior putaminal [18F]-dopa uptake seen in this case.
[18F]-dopa reflects density of dopaminergic terminals, activity of AADC (the enzyme that converts l-dopa into dopamine), and dopamine storage capacity, providing in this specific case a very good index of restored dopamine synthesis.
PET and SPECT tracers for the dopamine transporter are also good markers of presynaptic dopaminergic function but provide no information on dopamine synthesis.
Dopamine release measurements with [11C]-raclopride PET and assessment of striatocortical function with H2[15O] PET or fMRI to investigate functional integration of repaired/grafted neurons can be used to further evaluate the effect of restorative surgery in patients with PD. However, this was not possible due to lack of baseline scans.
Among the cohort of patients within the open-label study, this was the only case with akinetic-predominant disease, without significant associated tremor or gait disturbance, to receive chronic bilateral infusion and show sustained benefit beyond 1 year following drug withdrawal. Another open-label study showed significant bilateral benefits following 12 months of unilateral GDNF infusion in 10 patients with PD and reported loss of these improvements within 9 months of drug withdrawal.6 Future work should address the reasons underlying differences in therapeutic response to GDNF.
Supplementary Material
Footnotes
Supplemental data at www.neurology.org
Author contributions: N.K. Patel: research project: conception, organization, execution, including clinical assessments and analysis of clinical data; manuscript: writing of the first draft together with S. Javed. N. Pavese: research project: conception, execution including PET scanning and analysis; manuscript: review and critique of first draft, writing of the revised manuscript together with N.K. Patel and S. Javed. S. Javed: research project: conception, execution including clinical assessments; manuscript: writing of the first draft together with N.K. Patel. G.R. Hotton: research project: conception, execution including PET scanning; manuscript: review and critique of first draft. D.J. Brooks: research project: conception; manuscript: review and critique. S.S. Gill: research project: conception; manuscript: review and critique.
Study funding: No targeted funding reported.
Disclosure: N. Patel, N. Pavese, S. Javed, and G. Hotton report no disclosures. D. Brooks is employed part-time by GE Healthcare. S. Gill is a consultant to Renishaw plc and Medtronic Inc. Go to Neurology.org for full disclosures.
References
- 1.Gill SS, Patel NK, Hotton GR, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 2003;9:589–595 [DOI] [PubMed] [Google Scholar]
- 2.Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS. Intraputaminal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol 2005;57:298–302 [DOI] [PubMed] [Google Scholar]
- 3.Love S, Plaha P, Patel NK, Hotton GR, Brooks DJ, Gill SS. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat Med 2005;11:703–704 [DOI] [PubMed] [Google Scholar]
- 4.Langston JW, Widner H, Goetz CG, et al. Core Assessment Program for Intracerebral Transplantations (CAPIT). Mov Disord 1992;7:2–13 [DOI] [PubMed] [Google Scholar]
- 5.Lang AE, Gill S, Patel NK, et al. Randomized controlled trial of intraputaminal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 2006;59:459–466 [DOI] [PubMed] [Google Scholar]
- 6.Slevin JT, Gash DM, Smith CD, et al. Unilateral intraputaminal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year of treatment and 1 year of withdrawal. J Neurosurg 2007;106:614–620 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.