Abstract
Objective:
To inform whether the Alzheimer's Disease Neuroimaging Initiative (ADNI) should change its policy of not returning research results to ADNI participants, we surveyed investigators and research staff about disclosing ADNI biomarker information to research participants, with particular emphasis on amyloid imaging results.
Methods:
In April 2012, just before Food and Drug Administration approval of the amyloid-binding radiotracer, florbetapir, all ADNI investigators and personnel were recruited to complete an anonymous online survey that contained fixed choice and free-text questions.
Results:
Although ADNI participants often requested amyloid imaging results (the proportions of investigators who reported requests from more than half of their participants with normal cognition or mild cognitive impairment were 20% and 22%, respectively), across all diagnostic groups, the majority of ADNI investigators (approximately 90%) did not return amyloid imaging results to ADNI participants. However, the majority of investigators reported that, if the Food and Drug Administration approved florbetapir, they would support the return of amyloid imaging results to participants with mild cognitive impairment and normal cognition, but they emphasized the need for guidance on how to provide these results to participants and for research to assess the value of returning results as well as how returning results will affect study validity and participant well-being.
Conclusions:
A majority of ADNI investigators support returning amyloid imaging results to ADNI participants. The findings that they want guidance on how to do this and research on the impact of disclosure suggest how to develop and monitor a disclosure process.
Since 2004, the Alzheimer's Disease Neuroimaging Initiative (ADNI) has gathered extensive medical data; neuropsychometrics; and blood, CSF, and MRI/PET imaging biomarkers from participants with normal cognition, mild cognitive impairment (MCI), and probable Alzheimer disease (AD) dementia.1,2 To date, ADNI has had a policy that researchers will not disclose individual research results to participants.3 Similar “nondisclosure” policies have, until recently, prevailed in genetics research.4–6 These policies reflect guidelines that separate research from clinical care based on strict criteria for analytic and clinical validity as well as clinical utility.4 The goals of these policies are to protect research participants from harm and to share only results with clinical utility.
The Food and Drug Administration (FDA) April 2012 approval of the amyloid-binding radiotracer florbetapir7 provided a unique opportunity to explore the tension over the return of research results at the moment when a research tool transitions from research to clinical practice and to review the ADNI disclosure policy. Should ADNI investigators tell participants clinically relevant research results, or continue to adhere to the requirements of a natural history study not to disclose research data?
To address this question, we surveyed ADNI investigators to identify whether they disclosed biomarker results and, assuming FDA approval of florbetapir, their attitudes about disclosure of amyloid imaging results. Surveying investigators well familiar with the strengths and limitations of AD biomarker data can help to inform policies on returning research results to ADNI participants, and more generally, whether PET amyloid imaging, as well as other biomarker tests, should be used in clinical practice.
METHODS
Eligibility criteria.
All ADNI investigators, study physicians, clinicians, and study coordinators (categories designated by ADNI) were eligible to complete the survey. All respondents, regardless of whether they had direct contact with research participants, answered questions to understand attitudes about returning research results to ADNI participants and the clinical value of ADNI results. Those who reported direct contact with research participants as part of ADNI study procedures were eligible to answer questions about their practices for returning research results. Those who were physicians who had contact with ADNI participants were asked questions to understand their perceptions about the clinical value of the biomarker, cognitive, and psychological measures collected in ADNI.
Survey development.
We applied Dillman's tailored design method to develop and administer the survey.8 Questions were developed by the investigators, reviewed by ADNI leadership, and pilot-tested among a convenience sample of ADNI personnel for clarity and ease of completion and revised based on their feedback. Request a copy of the survey from J.K. The survey had 5 parts, outlined below.
Practices about returning research results to ADNI participants.
Respondents who reported direct contact with research participants as part of ADNI answered for each ADNI participant type (normal, MCI, and AD dementia) whether the respondent “always,” “sometimes,” or “never” returned amyloid imaging (Pittsburgh compound B [PiB] or florbetapir) results to ADNI participants, and for those who replied “always” or “sometimes,” the reasons they did so. Next, respondents answered the proportion of participants who have asked for their results, and for persons with normal cognition and MCI, whether they have used ADNI results to inform a participant “whether he or she is at ‘increased’ or ‘decreased’ risk for developing Alzheimer's disease” and, if they did, the kinds of results they used.
Attitudes about returning amyloid imaging results to ADNI participants assuming FDA approval.
All respondents were asked regarding ADNI participants with normal cognition and MCI, “Suppose the FDA approves PET amyloid imaging with florbetapir. Please indicate whether you support a policy that allows you to tell ADNI participants their amyloid imaging result” with answer choices “definitely support,” “probably support,” “unsure,” “probably do not support,” and “definitely do not support.”
Explanations for attitudes about returning amyloid imaging results.
Participants could enter free-text responses to explain their fixed choice answers about their support or nonsupport of a policy that allows telling ADNI participants their amyloid imaging results, and any general thoughts or comments on returning amyloid imaging results to ADNI participants.
Attitudes about the clinical value of ADNI results.
Physicians who had contact with ADNI participants were asked for each of the 3 ADNI participant types, “regardless of whether you support or do not support returning research results to ADNI participants, for each of the following measures collected or assessed in ADNI, please indicate whether you think the results typically produce meaningful clinical information” with answer choices of “yes” or “no” for Mini-Mental State Examination (MMSE), Geriatric Depression Scale (GDS), Clinical Dementia Rating (CDR), brain MRI for microvascular disease, brain MRI for hippocampal volumes, [18F]-fluorodeoxyglucose (FDG)-PET, amyloid imaging (PiB or florbetapir), and CSF Aβ42 and/or CSF tau.
Respondent characteristics.
Fixed choice questions asked whether respondents cared clinically for patients who are also ADNI participants, educational/training degrees, specialty, and role in ADNI using ADNI defined roles (protocol principal investigator [primary or secondary], study physician, clinician, and study coordinator [primary or backup]).
Data gathering.
Data were collected electronically via Qualtrics survey software. Survey responses were collected anonymously, with no link between identifying information and an individual's responses. All current ADNI investigators, study physicians, clinicians, and study coordinators were first contacted via e-mail on March 22, 2012 by the ADNI principal investigator to inform them of the upcoming survey, and 1 week later they received an e-mail with a link to the online survey. One week later, eligible participants received a short e-mail with the survey link thanking them for their participation and reminding them to complete the survey. Two weeks after the initial survey distribution, nonresponders received a second reminder e-mail including the survey link. Data collection was closed on April 10, 2012.
Data analyses.
Fixed choice responses.
Results were analyzed using descriptive statistics. To determine between group significance, parametric and nonparametric tests were used as appropriate; p values <0.05 were considered significant without adjustment for multiple comparisons.
Free-text responses.
Responses were coded using an iterative process. First, the research team created a set of preliminary codes that covered the categories and kinds of answers expected based on the questions asked. Two coders (M.S. and S.B.) reviewed a sample of 10 responses and applied preliminary codes, while also identifying additional emergent codes. The 2 coders then reviewed their coding and resolved disagreements to produce a revised list of codes. The coders then independently applied the revised codes to a second sample of 10 responses and again met to review and revise the list of codes. They used the further revised code list to independently code a third sample of responses. At this point, it was determined that no new themes had emerged and that the relevant concepts had been adequately represented. The 2 coders used the final code list to independently code all responses, with disagreements resolved by a third rater (K.H.). The research team then met and grouped the codes into broader themes, based on similarity of content.
Standard protocol approvals, registrations, and patient consents.
The University of Pennsylvania IRB approved this project with informed consent modified so that it was indicated by a participant's completion of this anonymous survey.
RESULTS
Participant characteristics and response rate.
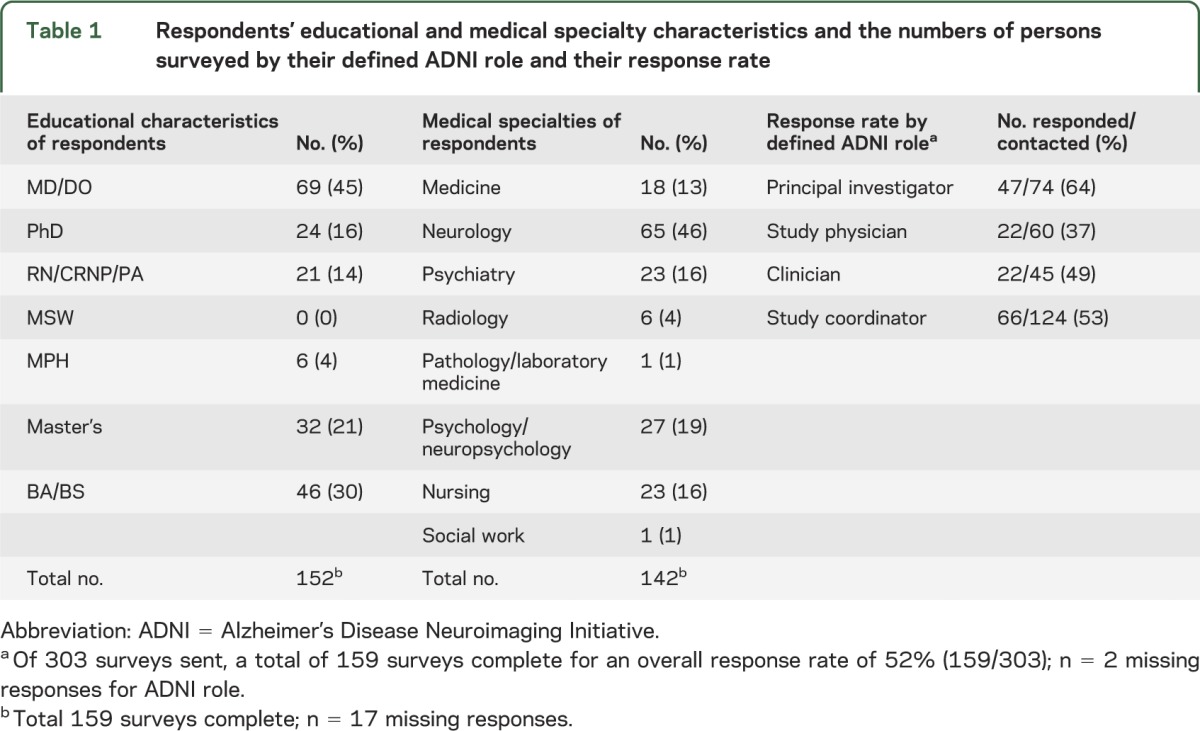
The overall response rate was 52% (159/303). For some questions, the denominator is lower, as some individuals did not provide a response. Table 1 shows the respondents' educational and medical specialty characteristics and the numbers of persons surveyed by their defined ADNI role and their response rate. Most respondents (139/159, 87%) reported direct contact with research participants as part of ADNI study procedures, and among these, 62 were physicians.
Table 1.
Respondents' educational and medical specialty characteristics and the numbers of persons surveyed by their defined ADNI role and their response rate
ADNI participant requests for amyloid imaging results.
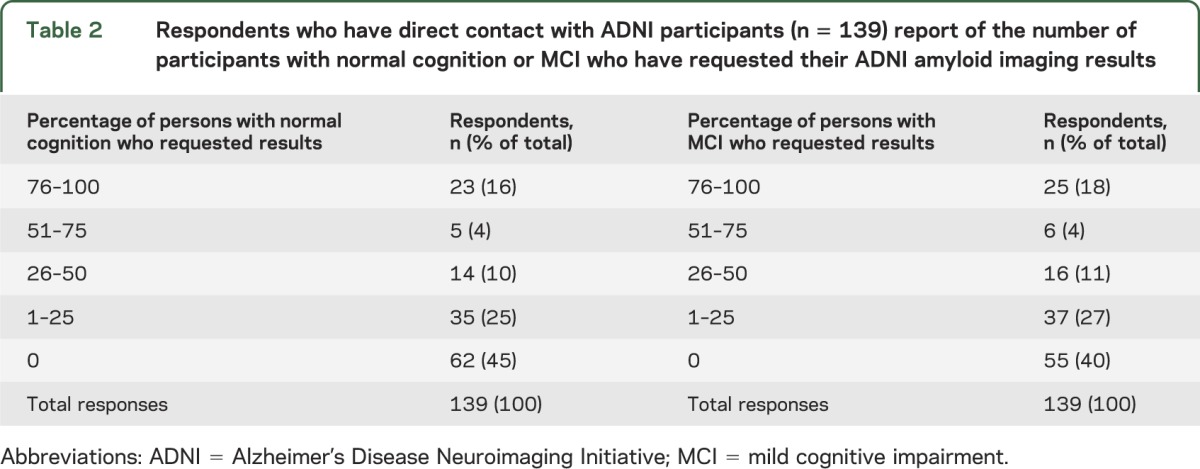
Among the 139 respondents who reported direct contact with ADNI participants, 40% (55/139) reported that no participants with MCI had requested their amyloid imaging results. Similarly, 45% (62/139) reported that no participants with normal cognition had requested their amyloid imaging results. Table 2 shows the proportions of respondents who reported >1% of persons with normal cognition or MCI who have requested their results.
Table 2.
Respondents who have direct contact with ADNI participants (n = 139) report of the number of participants with normal cognition or MCI who have requested their ADNI amyloid imaging results
Practices about returning research results to ADNI participants.
Among the 139 respondents who reported direct contact with ADNI participants, most (126/139, 91%) answered “never” to the question of whether they have returned amyloid imaging results to research participants with AD dementia. The 13 who answered either “always” (2/139, 1%) or “sometimes” (11/139, 8%) selected as reasons, “to clarify diagnosis,” “to provide risk assessment,” “to guide therapy,” or simply, “because (the volunteer) specifically asked.” Similarly, most respondents (125/139, 90%) reported “never” returning amyloid imaging results to MCI participants. The 14 who answered either “sometimes” (12/139, 9%) or “always” (2/139, 1%) reported similar reasons for providing the information as for the AD dementia participants. Nearly all (130/139, 94%) reported that they “never” disclosed amyloid imaging results to participants with normal cognition.
Most respondents (127/139, 91%) answered that they did not use ADNI results to inform a participant who has MCI whether he or she is at “increased” or “decreased” risk of developing AD dementia. The respondents who did use results (12/139, 9%) typically used neuropsychometrics and brain MRI, but a few reported using PET amyloid imaging, FDG-PET, and CSF amyloid and tau. Similarly, most respondents (132/138, 96%) reported that they did not use ADNI results to inform a participant with normal cognition whether he or she is at “increased” or “decreased” risk of developing AD dementia. The 6 who did inform participants of risk used neuropsychometrics and brain MRI, and occasionally PET amyloid imaging, as well as CSF amyloid and tau.
Attitudes about returning amyloid imaging results.
The survey was distributed 2 weeks before the FDA approved florbetapir for amyloid imaging. All 159 respondents were asked to assume FDA approval and indicate whether they would support a policy that allows them to tell ADNI participants with normal cognition and MCI their amyloid imaging results.
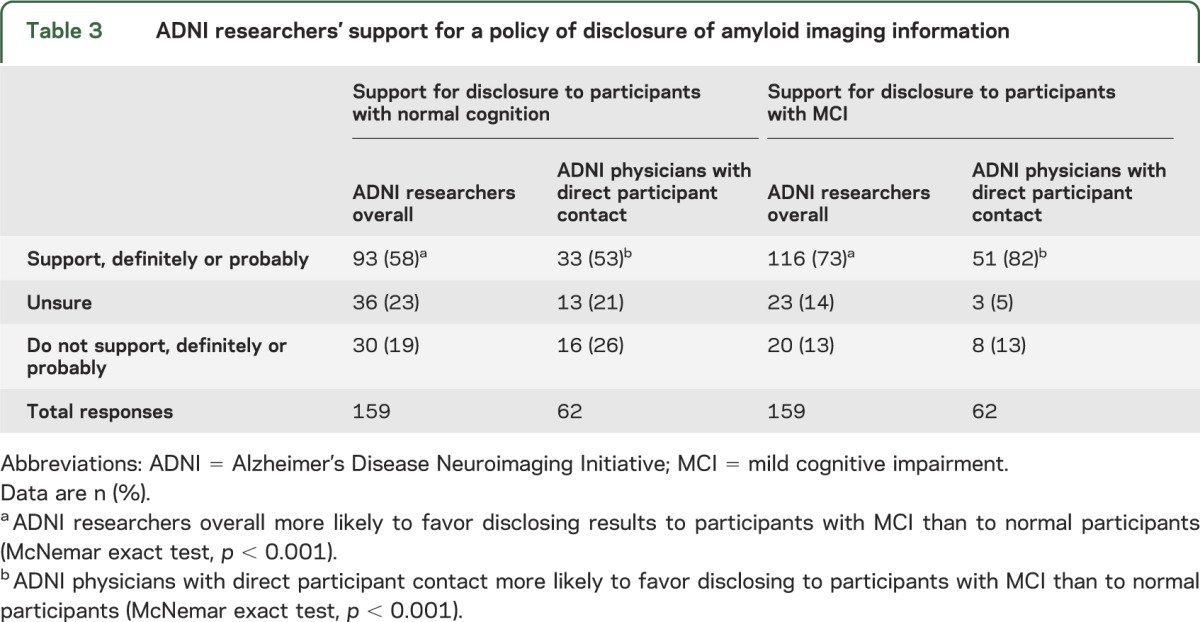
Table 3 shows that a majority of respondents (116/159, 73%) supported disclosing amyloid imaging results to participants with MCI. A majority (93/159, 58%) also supported disclosing results to participants with normal cognition. Respondents were more likely to favor disclosing results to participants with MCI than to those with normal cognition (McNemar exact test, p < 0.001). Respondents who were physicians and reported direct contact with ADNI participants (n = 62) showed similar patterns of support for amyloid imaging disclosure, and they were more likely to favor disclosing to participants with MCI than to those with normal cognition (McNemar exact test, p < 0.001).
Table 3.
ADNI researchers' support for a policy of disclosure of amyloid imaging information
Respondents' explanations for attitudes about disclosing amyloid imaging results.
Overall, 92 of 159 respondents provided free-text answers to at least 1 of the 3 questions explaining their support or nonsupport of a policy telling ADNI participants with MCI and normal cognition their amyloid imaging results. There was a trend that respondents who favored a policy of disclosure were more likely than those who did not to provide open-ended answers (Pearson χ2 = 3.2, p = 0.08).
Table 4 shows the 6 major themes regarding disclosure of amyloid imaging results. A notable finding was that respondents who endorsed disclosure often expressed themes that qualified this endorsement, such as the need for developing standardized disclosure procedures and ADNI-endorsed participant education materials to disclose the result, and that the disclosure procedure should be rigorously studied with longitudinal outcomes to assess the effects of the information on a participant's well-being.
Table 4.
Themes identified from respondents' free-text responses on their attitudes about disclosure of amyloid imaging results
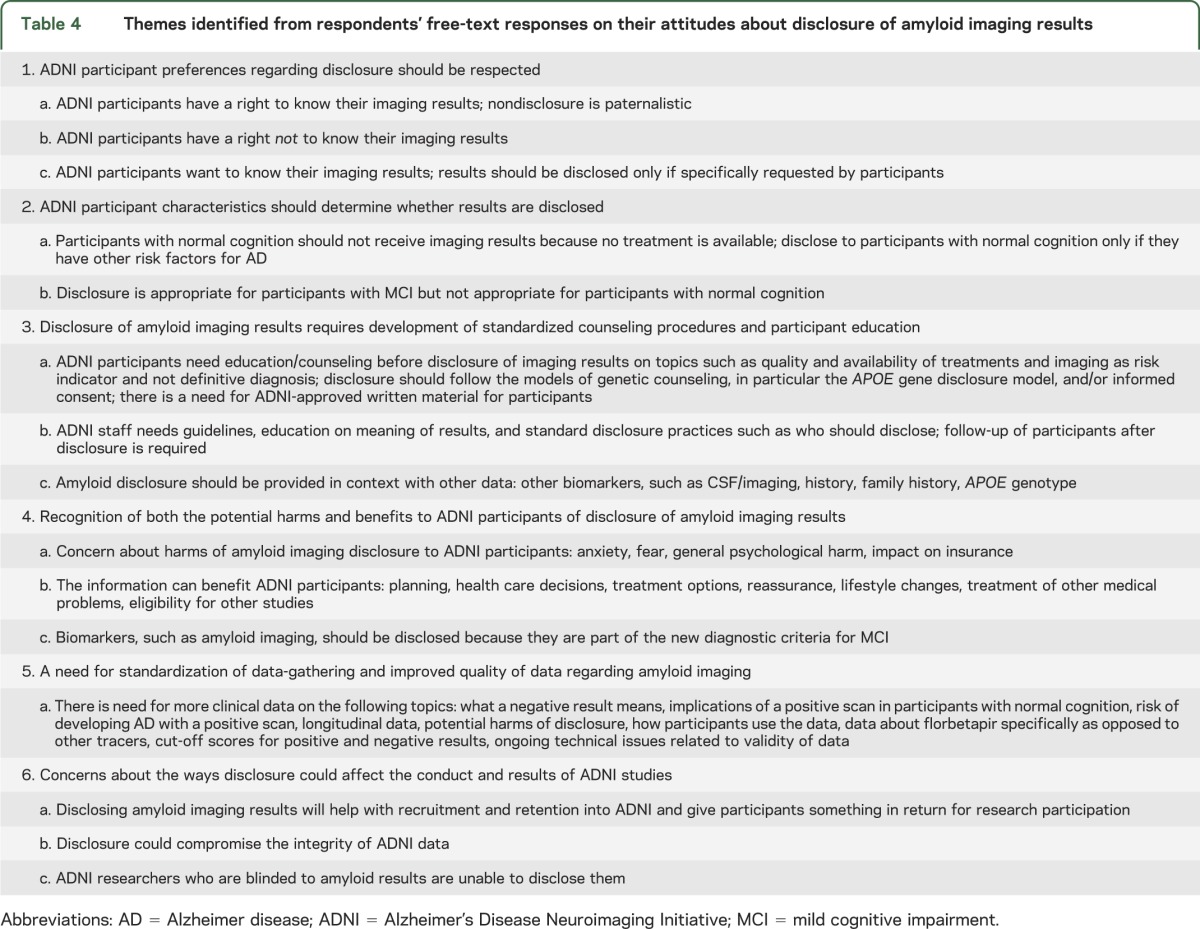
Respondents commented on both the potential risks of disclosure (anxiety, fear, possible impact on acquiring long-term health insurance) as well as potential benefits (implement lifestyle changes, planning for the future, eligibility for intervention trials). Finally, respondents questioned the validity of amyloid imaging results and called for more longitudinal data, especially in cognitively normal individuals, regarding risk of development of AD dementia. Other topics respondents cited in need of research were solving ongoing technical issues related to the validity of amyloid imaging data, determining cut-off scores for positive/negative results, and addressing concerns about the stability of florbetapir vs other amyloid imaging tracers.
Respondents noted that disclosure of amyloid imaging results might serve as a useful way to help with recruitment and retention in ADNI, providing participants with something in return for their time and effort. Other respondents were concerned that disclosing the results of amyloid imaging, especially in cognitively normal participants, might affect the neuropsychometric or affective data subsequently collected.
Respondents' ethical arguments generally invoked ideas of respect for autonomy conditioned on the participant's clinical status. Most frequently, they argued that disclosure is appropriate for participants with cognitive impairment (MCI or AD dementia) but not appropriate for participants with normal cognition. Some argued that participants have a “right to know” instead of researchers “paternalistically” withholding information from participants, whereas others explained that participants also have a right not to know their imaging results and such information should be disclosed only if specifically requested by participants.
Attitudes about the clinical value of ADNI results.
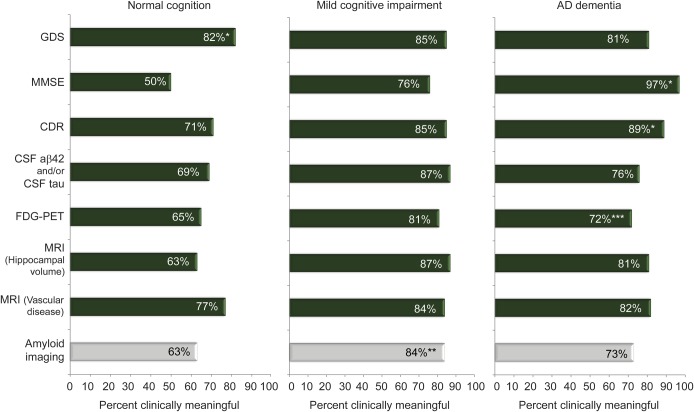
The figure shows that, for all 3 ADNI participant types, respondents who were physicians and had contact with ADNI participants (n = 62) generally answered “yes” that a given measure (MMSE, GDS, CDR, brain MRI for microvascular disease, brain MRI for hippocampal volumes, FDG-PET, amyloid imaging [PiB or florbetapir], CSF Aβ42 and/or CSF tau) typically produces meaningful clinical information. With the exception of the MMSE in cognitively normal persons (endorsed by 50% of respondents), for all measures across all 3 participant groups, most respondents thought each measure produces meaningful clinical information. Amyloid imaging was more likely to be rated as providing clinically meaningful information for persons with MCI (52/62, 84%) than for persons with normal cognition (39/62, 63%, p < 0.001) or AD dementia (45/62, 73%, p = 0.02). Overall, the proportion of physician respondents rating amyloid imaging as clinically meaningful was similar to the proportions for other measures with the exception of greater proportions rating the GDS (51/62, 82%, p = 0.01) as clinically meaningful in persons with normal cognition and the CDR (55/62, 89%, p = 0.04) and MMSE (60/62, 97%, p < 0.001) as clinically meaningful in persons with AD dementia.
Figure. Proportion of physicians with direct participant contact (n = 62) reporting that measures provide clinically meaningful information.
*Proportion of respondents rating GDS as clinically meaningful in persons with normal cognition was significantly different (McNemar exact test, p = 0.01) compared with amyloid imaging. Proportion of respondents rating CDR and MMSE as clinically meaningful in persons with AD dementia was significantly different (McNemar exact test, p = 0.04, p < 0.001) compared with amyloid imaging. **Amyloid imaging was more likely to be rated as providing clinically meaningful information for persons with mild cognitive impairment (52/62, 84%) than for persons with normal cognition (39/62, 63%, McNemar exact test, p < 0.001) or AD dementia (45/62, 73%, McNemar exact test, p = 0.02). ***n = 61 for physicians reporting on clinical value of FDG-PET in persons with AD dementia. AD = Alzheimer disease; CDR = Clinical Dementia Rating; FDG = [18F]-fluorodeoxyglucose; GDS = Geriatric Depression Scale; MMSE = Mini-Mental State Examination.
DISCUSSION
This survey of ADNI investigators' practices and attitudes toward amyloid imaging administered just before FDA approval of florbetapir showed that these investigators recognize that the study of AD is experiencing tremendous flux. Revised AD diagnostic criteria include a proposed “preclinical” stage of the disease,9 prevention studies are now under way,10,11 and biomarker assessments such as amyloid imaging are becoming more readily available.7 In addition, the public is increasingly calling for disclosure of research information.12 As a result, ADNI researchers recognize that their role as a clinician-researcher is sometimes in conflict. The free-text responses describe this conflict: deciding not to disclose results may cause participants to become unwilling to participate and researchers to become concerned that persons are not receiving valuable information; however, disclosing results will require researchers to address how this will affect the validity of the data13 and the potential harms to participants.
To address this conflict, the ADNI researchers called for research to provide an evidence base to guide practice, including investigations on the value and impact of disclosure of biomarker information on ADNI participants, the effects on study validity, further refinement of the predictive value of various biomarkers, and clinical practice recommendations. The findings that a majority of ADNI study personnel endorsed disclosure of amyloid imaging results for participants with MCI (73%), that a smaller majority (58%) endorsed disclosing results for participants with normal cognition, and that free-text responses included comments explicitly opposing disclosure to normal participants, suggest that an ADNI policy may want to initially address persons with MCI.
Assessments of the clinical value of amyloid imaging among physicians who reported direct contact with ADNI participants as well as an array of biomarker data collected from participants with normal cognition, MCI, and probable AD begin to explain why respondents might want to return amyloid imaging results to ADNI participants. Results suggested that amyloid imaging and other emerging biomarker measures (FDG-PET, CSF Aβ42 and/or CSF tau) are considered as clinically meaningful as well-validated psychometric and biomarker measures (MMSE, GDS, CDR, brain MRI for microvascular disease, brain MRI for hippocampal volumes) in persons with normal cognition and AD dementia, and especially persons with MCI. These results suggest that a policy allowing disclosure of amyloid imaging will likely lead to disclosure of other research results, and the free-text response that amyloid imaging should be provided in context with other data—such as CSF/imaging, medical and family histories, and APOE genotype—further supports this.
Our findings suggest that the ADNI community favors a growing trend in biomedical research to revise its “no return” of results position held since ADNI's inception in 2004. Current guidelines for return of research results in genomic studies focus on protecting participants from harm using criteria including analytic validity, clinical validity, actionability, and the severity of outcome,4,14 but these criteria are being extensively debated.5,6,15 For example, commentators urge reconsideration of traditionally restrictive disclosure policies that focus exclusively on protecting participants from harm of disclosure and that fail to take into account these persons' own formulation of benefit, harm, and acceptable risk.5,16 They encourage reliance on empiric evidence to help guide conclusions about disclosure policy, citing data that suggest the prevalence of negative consequences is low and that individuals tend to find disclosure of test results beneficial, regardless of the actual result or accompanying psychological distress.17–20 The Risk Evaluation and Education for Alzheimer's Disease project used a randomized and controlled design to compare the effect of disclosing vs not disclosing a genotype that described an increased risk of developing AD (the APOE ε4 allele), and reported that after learning their results, ε4 carriers were no more likely than persons who either did not learn their APOE status or persons who were told they were ε4-negative to experience a negative impact on their health and psychological well-being.20
Limitations of this research include the response rate of 52%, although principal investigators had a higher response rate (64%) and the average response rate for physician surveys is 54%.21 Also, there was a trend that persons who favored amyloid disclosure were more likely than persons who did not support it to provide free-text answers. Still, the notably high proportions of respondents who supported returning amyloid imaging results and who considered that these results—as well as results from most other biomarker and related research measures—typically provide clinically meaningful information suggests that ADNI researchers are ready, with guidance and ongoing research, to return amyloid imaging research results to ADNI participants, and more generally, other biomarker results as well. These findings also suggest that biomarker results can be disclosed as part of clinical trials designed to prevent cognitive decline.10,11
Supplementary Material
ACKNOWLEDGMENT
The authors thank Simon Basseyn for his assistance in analyzing the qualitative data and the staff of ADNI for assistance in distributing the survey.
GLOSSARY
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- CDR
Clinical Dementia Rating
- FDA
Food and Drug Administration
- FDG
[18F]-fluorodeoxyglucose
- GDS
Geriatric Depression Scale
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- PiB
Pittsburgh compound B
Footnotes
Editorial, page 1108
AUTHOR CONTRIBUTIONS
Dr. Shulman contributed to study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, and study supervision. Ms. Harkins contributed to study concept and design, acquisition of data, analysis and interpretation, and critical revision of the manuscript for important intellectual content. Dr. Green contributed to study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, and study supervision. Dr. Karlawish contributed to study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, and study supervision.
STUDY FUNDING
Dr. Karlawish was supported by the Marian S. Ware Alzheimer Program and a Robert Wood Johnson Foundation Investigator Award in Health Policy Research, and Dr. Green by NIH grants HG02213 and AG027841.
DISCLOSURE
M. Shulman and K. Harkins report no disclosures. R. Green is funded by NIH grants HG006500, HG002213, HG005092, HG024904, HG005550, and AR047782. J. Karlawish is a coholder of a license of an Integrated Neurodegenerative Disease Database developed at the University of Pennsylvania. He receives royalties for Do We Have a Pill for It?: Treating Dementia, Johns Hopkins University Press. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Alzheimer's Disease Neuroimaging Initiative Clinicaltrials.gov identifier: NCT00106899 [online]. Available at: http://clinicaltrials.gov/show/NCT00106899. Accessed January 16, 2013
- 2.Miller G. Alzheimer’s biomarker initiative hits its stride. Science 2009;326:386–389 [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer's Disease Neuroimaging Initiative ADNI protocol [online]. Available at: http://adni-info.org/PPSB/Pdfs/adni_protocol_03.02.2005_ss.pdf. Accessed January 16, 2013
- 4.Fabsitz RR, McGuire A, Sharp RR, et al. ; National Heart, Lung, and Blood Institute Working Group Ethical and practical guidelines for reporting genetic research results to study participants: updated guidelines from a National Heart, Lung, and Blood Institute Working Group. Circ Cardiovasc Genet 2010;3:574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bredenoord A, Kroes H, Cuppen E, Parker M, van Delden JJ. Disclosure of individual genetic data to research participants: the debate reconsidered. Trends Genet 2011;27:41–47 [DOI] [PubMed] [Google Scholar]
- 6.Dressler LG. Disclosure of research results from cancer genomic studies: state of the science. Clin Cancer Res 2009;15:4270–4276 [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration FDA approves imaging drug Amyvid [online]. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm299678.htm. Accessed April 10, 2012
- 8.Dillman D. Mail and Internet Surveys: The Tailored Design Method. New York: John Wiley; 2000 [Google Scholar]
- 9.Karlawish J. Addressing the ethical, policy, and social challenges of preclinical Alzheimer disease. Neurology 2011;77:1487–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiman EM, Langbaum JBS, Fleisher AS, et al. Alzheimer's Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis 2011;26(suppl 3):321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperling R, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med 2011;3:111cm13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shalowitz DI, Miller FG. Communicating the results of clinical research to participants: attitudes, practices, and future directions. PLoS Med 2008;5:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salmon D, Lineweaver T, Bondi M, Galasko D. Knowledge of APOE genotype affects subjective and objective memory performance in healthy older adults. Alzheimers Dement 2012;8(4 suppl 2):123–124 Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf SM, Crock BN, Van Ness B, et al. Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genet Med 2012;14:361–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levesque E, Joly Y, Simard J. Return of research results: general principles and international perspectives. J Law Med Ethics 2011;39:583–592 [DOI] [PubMed] [Google Scholar]
- 16.Shalowitz DI, Miller FG. Disclosing individual results of clinical research: implications of respect for participants. JAMA 2005;294:737–740 [DOI] [PubMed] [Google Scholar]
- 17.Partridge AH, Wong JS, Knudsen K, et al. Offering participants results of a clinical trial: sharing results of a negative study. Lancet 2005;365:963–964 [DOI] [PubMed] [Google Scholar]
- 18.Smith CO, Lipe HP, Bird TD. Impact of presymptomatic genetic testing for hereditary ataxia and neuromuscular disorders. Arch Neurol 2004;61:875–880 [DOI] [PubMed] [Google Scholar]
- 19.Christensen KD, Roberts JS, Shalowitz DI, et al. Disclosing individual CDKN2A research results to melanoma survivors: interest, impact, and demands on researchers. Cancer Epidemiol Biomarkers Prev 2011;20:522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer's disease. N Engl J Med 2009;361:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellerman SE, Herold J. Physician response to surveys: a review of the literature. Am J Prev Med 2001;20:61–67 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.