Abstract
The Priorities in Pediatric Epilepsy Research workshop was held in the spirit of patient-centered and patient-driven mandates for developing best practices in care, particularly for epilepsy beginning under age 3 years. The workshop brought together parents, representatives of voluntary advocacy organizations, physicians, allied health professionals, researchers, and administrators to identify priority areas for pediatric epilepsy care and research including implementation and testing of interventions designed to improve care processes and outcomes. Priorities highlighted were 1) patient outcomes, especially seizure control but also behavioral, academic, and social functioning; 2) early and accurate diagnosis and optimal treatment; 3) role and involvement of parents (communication and shared decision-making); and 4) integration of school and community organizations with epilepsy care delivery. Key factors influencing pediatric epilepsy care included the child's impairments and seizure presentation, parents, providers, the health care system, and community systems. Care was represented as a sequential process from initial onset of seizures to referral for comprehensive evaluation when needed. We considered an alternative model in which comprehensive care would be utilized from onset, proactively, rather than reactively after pharmacoresistance became obvious. Barriers, including limited levels of evidence about many aspects of diagnosis and management, access to care—particularly epilepsy specialty and behavioral health care—and implementation, were identified. Progress hinges on coordinated research efforts that systematically address gaps in knowledge and overcoming barriers to access and implementation. The stakes are considerable, and the potential benefits for reduced burden of refractory epilepsy and lifelong disabilities may be enormous.
Epilepsy affects as many as 1 in 26 people.1 Up to one-tenth of the lifetime risk of epilepsy is realized in the first 3 years of life.2–4 In contrast to the majority of epilepsies occurring in older children and adults, early-onset epilepsies represent numerous, distinct, and rare disorders; many are devastating and associated with severe lifelong disability, dependence, and significant economic and personal costs.5–7 Currently there is little to guide specific practice in diagnosing and treating these epilepsies. Relatively little is known that can improve long-term outcomes. The “Priorities in Pediatric Epilepsy Research: Improving Children's Futures Today” workshop (October 23–24, 2012, Ann & Robert H. Lurie Children's Hospital of Chicago) brought together parents of children with epilepsy, voluntary advocacy organizations, pharmaceutical industry representatives, health services, clinical, and translational science researchers, educators, adult and pediatric neurologists and epileptologists, nurses, and neuropsychologists to address needs in pediatric epilepsy research and care. A goal of the meeting was to identify the greatest problems and current gaps in knowledge and practice—areas that could become the focus of targeted research efforts to improve practice and patient outcomes. The discussion focused on patient-centered issues (barriers to care, parents' needs for information and support), parental perspectives on the highest priorities in care, how to overcome barriers, and utilization of current knowledge and resources to optimize care and outcomes. All workshop participants were invited to read and provide input on the workshop summary report.
WORKSHOP THEMES
The concerns and questions raised during the meeting were grouped into 4 driving themes as outlined below.
Patient outcomes.
The single most important outcome for parents of children with epilepsy is seizure freedom. Seizure control is paramount for the following main reasons:
The possibility of irreversible deleterious impact of seizures, particularly early in life, on the child's development, which can result in lifelong disability and dependency.
The disruption that seizures cause in the lives of both the child and the family. This can result from the seizures themselves, postictal periods, and associated health care and treatment (e.g., emergency department visits, hospitalizations, and medication burden). Side effects of medications were also of concern as complete seizure freedom cannot come at the price of unacceptable medication effects.
The risk of medical complications from seizures, including injury and death: seizure control reduces the risk of seizure-related death including sudden unexpected death in epilepsy and related injury and disability from seizures.
Early seizure control without significant side effects was viewed as necessary, although not always sufficient, to optimize behavioral, cognitive, and social outcomes.
Diagnosis.
To achieve the primary goal of early seizure control, the overarching priority identified was early, accurate diagnosis not only of epilepsy, per se, but also of specific forms of epilepsy, seizure types, and underlying causes. This was considered the top priority because of its relevance in selecting the most appropriate treatment for each individual child.
Role of parents and impact on families.
The parent is the critical link between the child (patient), the physician, the medical care system, and the services beyond the care system such as insurance companies, voluntary organizations, and parent and patient support/advocacy groups. From the first contact with the medical care system, engagement of the parents in the care process and the information conveyed to the parents as well as when, how, and by whom it is conveyed were identified as key components of the family's interaction with the care system. Issues raised included poor communication about the diagnosis of epilepsy and different seizure types. Some therapies for epilepsy can be invasive (surgery), time-demanding and complicated (ketogenic diet), or relatively new (immunomodulatory). Inadequate information and communication about options and how to make the best decision for a child were seen as significant impediments to care. Furthermore, with information easily available through the internet, parents often do their own research and bring keen insights into their child's disorder to the attention of the physician. Physicians need to consider this information.
Resources outside the medical system.
Voluntary and community agencies and services such as the birth-to-three and special education systems provide information and additional services including early educational and other therapeutic interventions (e.g., speech therapy).8 Although parents are typically the primary caretakers, daycare and school personnel spend considerable time with children. They need to be aware of the child’s epilepsy, what the seizures look like, the impact that seizures and medications have on behavior and function, and any chronic and emergency treatment plans. They also have an important role in assessing whether seizures are controlled and whether developmental or behavioral difficulties emerge or worsen.
STAKEHOLDERS AND FACTORS IN THE DIAGNOSTIC AND TREATMENT PROCESS
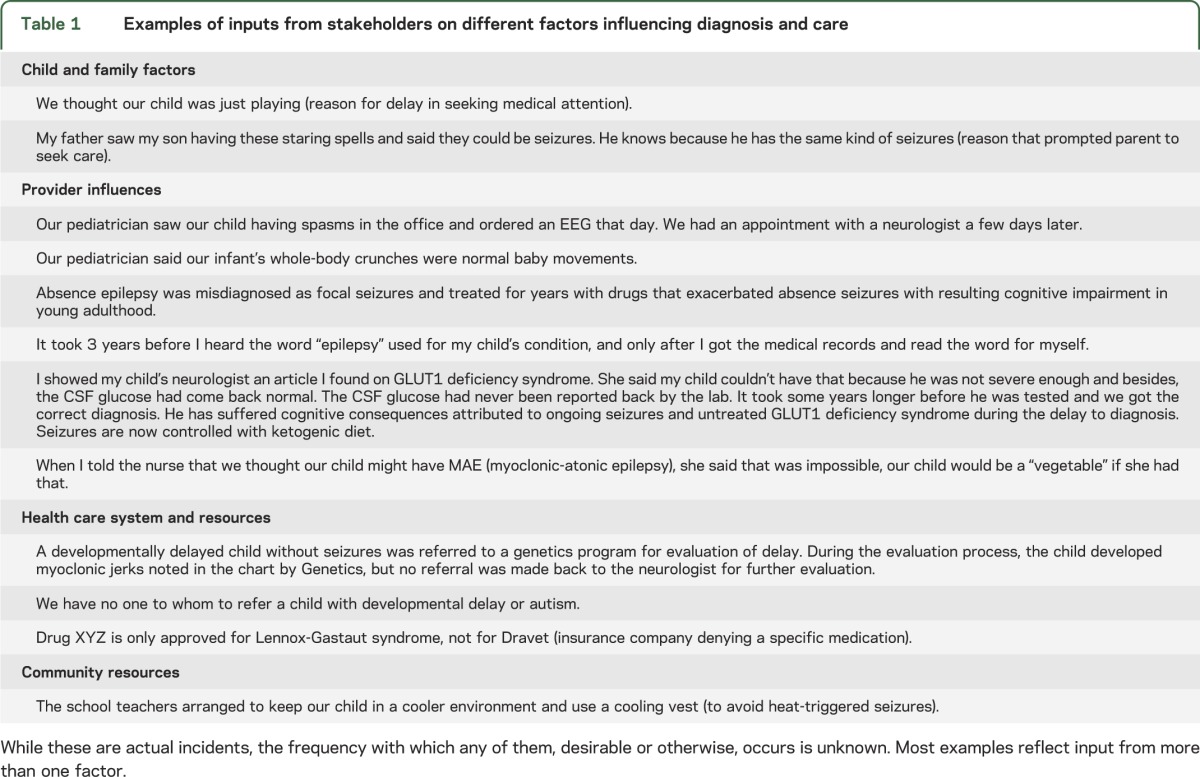
Many different factors have input into the process of pediatric epilepsy care and play different roles at different points in the process (table 1).
Table 1.
Examples of inputs from stakeholders on different factors influencing diagnosis and care
The child and seizure presentation.
Subtle seizures are often not recognized initially and may persist for months or years before being diagnosed.9,10 Seizures are also underrecognized in children who are developmentally impaired11 although overdiagnosed in children with autism.12
Parent and family.
Parental factors include socioeconomic status, race, education, health literacy, language, and cultural beliefs. All of these can influence the understanding of illness and interaction with the medical system.13–17
Medical care providers.
Providers' training, knowledge, attitudes toward race/ethnicity, skills in communication and shared decision-making, cultural competence, and subtle biases can affect health care utilization and contribute to delayed or unmet access to care.18,19 Willingness to refer complicated patients for advanced diagnostic and specialized care may mean loss of practice revenue. Recent trends in epilepsy surgical evaluations raise this concern.20 The model of physician-directed care is changing to one in which the physician is part of a health care team that collaborates with the family to arrive at the best individualized decision and helps the family navigate the health care system and care choices.21
Health care system.
Limited geographic availability of specialty care, financial barriers, waiting times for insurance approval, and appointment availability are ubiquitous throughout health care. All pose obstacles to timely, appropriate care for children with epilepsy.22 Uncoordinated care across primary and specialty providers further impedes the goal of early diagnosis and optimized treatment.23
Community resources.
School systems and voluntary organizations are essential in extending and implementing the care and recommendations of physicians and providing additional services. Parents also need support,8 which can sometimes be provided through community organizations.24
PROCESS OF DIAGNOSIS AND TREATMENT
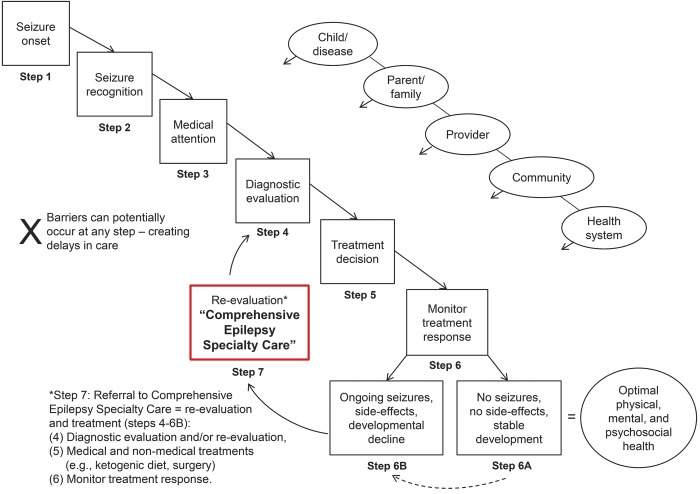
We conceptualized this process as a series of steps from initial seizure to achieving optimal outcomes. Inputs to the system have different degrees of influence at each stage (figure 1).
Figure 1. Current disease/care continuum of pediatric epilepsy.
The typical sequence of steps taken from onset of seizures (step 1) to achieving optimal care of epilepsy (step 6A) in which specialty-comprehensive care is not sought until and unless difficulties occur (step 6B). Even when difficulties occur (failure to control seizures or developmental declines), referral to comprehensive care may not be sought immediately as physicians attempt to find a better treatment. The distinction between standard neurologic care and specialty care is also not clearly defined; there may be overlap and interactions between the two.
Steps 1–3: From seizure onset to first medical contact.
Occurrence of a seizure, recognition of the event by a caregiver, and the subsequent decision to obtain medical attention (figure 1) occur before medical system involvement unless the child is already hospitalized (e.g., in the neonatal intensive care unit) when seizures first occur. Epidemiologic evidence demonstrates that relatively subtle seizure types may go undetected for long periods of time, even years.9 A quarter (27%) of children with infantile spasms were not brought to their physician for >2 months in one study.10 Longer delays to initial diagnosis were associated with poorer developmental outcomes in early childhood.
Step 4: Diagnosis of epilepsy.
Once a child is brought to medical attention, the diagnostic process involves 2 interconnected stages,25 as discussed below.
Recognition that a child has epileptic seizures.
Over- and underrecognition of seizures and epilepsy can occur. Both errors carry consequences. In the first instance, children with nonepileptic events are mistakenly diagnosed as having epilepsy26–29 and may be treated while the true underlying condition is not addressed. In the second case, the epilepsy diagnosis is missed. Seizures (and sometimes treatable causes) are untreated.11
Diagnosis of the specific seizure type(s), epilepsy, and underlying cause.
Although the first contact medical provider may not be qualified to perform a full epilepsy evaluation, that provider must be able to recognize possible seizures and seizure mimics and then seek the necessary expertise and capabilities for rapid, accurate diagnosis.
Step 5: Epilepsy treatment selection.
Ideally, treatment selection should be guided by seizure types, epilepsy syndrome, cause, or all of these. For example, evidence regarding infantile spasms is sufficient for guidelines targeted at both physicians and parents.30–33 Most early-onset epilepsies, however, are individually rare, thus precluding the feasibility of robust randomized clinical trials needed to develop such guidelines. Recent treatment recommendations concluded that rigorous evidence-based guidelines for selecting treatments based on syndromes were not possible at this time.34
Step 6: Evaluation and monitoring of epilepsy treatment response.
Parents have an essential role in assessing the effect of treatment. Close collaboration among care providers, parents, and others is needed to monitor seizure occurrence, medication adherence, and side effects.
Successful seizure control.
Children with fully controlled seizures and no side effects may need no intervention beyond the care they are already receiving. Often, the epilepsy appears to resolve and remit permanently, and medications can be stopped. Behavioral, cognitive, and social concerns must still be addressed because these may be present and persist regardless of complete seizure control.
Pharmacoresistant seizures.
This determination must be considered in 2 parts35:
Deciding that a drug has truly failed to control a patient's seizures assumes knowledge of how to use and assess medications including when to increase doses and when to recognize that seizures have not responded adequately.
Deciding that sufficient medications have been unsuccessfully tried and that the next phase of diagnostic evaluations and other therapies should be considered.
There is no single definition of pharmacoresistance. A recent proposal suggested failure of 2 appropriate medications used in informative trials.35 The National Association of Epilepsy Centers (NAEC) recommends referral for specialty care if seizures are not controlled within a year.36 The National Institute for Health and Care Excellence guideline recommends referral after 2 years of uncontrolled seizures.37
Step 7: Referral to comprehensive care.
At the comprehensive care level, results of some earlier tests are reviewed, some tests are repeated, and others done for the first time. The diagnosis, beginning with whether a patient has epilepsy, the type of epilepsy and seizures, and the underlying cause are all reconsidered in an effort to arrive at a more precise diagnosis of all of these and identify the most appropriate treatment options. Even for patients diagnosed and treated since onset at a comprehensive center, recognition of pharmacoresistance should trigger reconsideration of the diagnosis and treatment. In reality, however, comprehensive epilepsy care is not well defined and definitions and expectations likely vary considerably across centers, individual providers, and parents. How comprehensive care interacts with, repeats, and enhances standard neurologic care vs replaces it is not well understood. In addition, the timing of referral and seeking of comprehensive care is highly variable with such care often considered as a last resort or at least after a substantial and unnecessarily long delay.
PATIENT-EXPRESSED PRIORITIES VS THE STANDARD APPROACH: DO WE NEED A NEW MODEL?
This 7-step model is typical of epilepsy care in the United States and is reflected in the NAEC recommendations.36 There is inherent tension between this model and the priorities of parents whose children have early-onset epilepsy. Waiting until pharmacoresistance is evident to make a specialty referral seems counterproductive, especially when wasted time may result in lost opportunity to prevent severe, lifelong consequences. It is also inefficient for society and for insurers if upfront savings result in long-term costs. There is typically little emphasis on comorbidities (who should screen for them, when and how often), who provides services, school involvement, or parent engagement, all key issues identified by parents and clinicians alike.
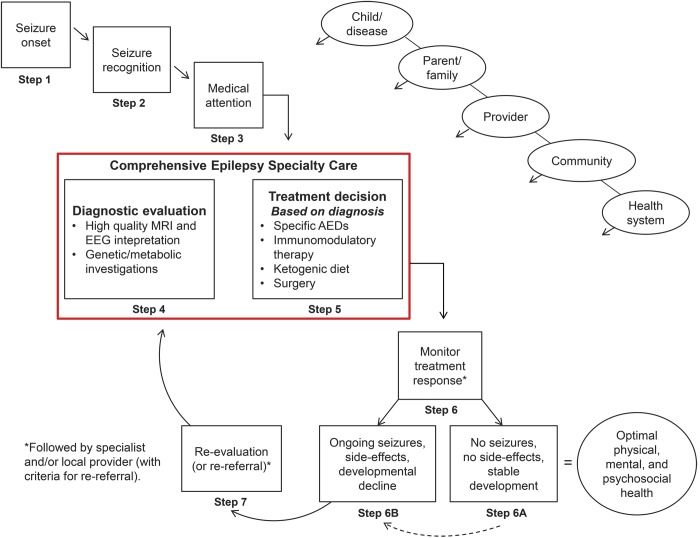
A model was proposed in which specialty care, rather than a reactive measure, could be implemented at onset proactively (figure 2), particularly for children younger than 3 years. Such a model would entail a systematic process in which pediatric epileptologists and other specialists optimally utilize available imaging, genetic, metabolic, and other diagnostic technologies to maximize the chance of obtaining a specific diagnosis for the causes and the epilepsy. They would then use optimal medication strategies and consider more advanced therapies including the ketogenic diet, surgery, and immunomodulatory agents right from the start.38,39 Depending on the child's clinical condition, management might be referred back to a less-specialized, perhaps local, provider with specialists serving in a consultative mode. Alternatively, some children may be kept in specialty care for longer periods of time. This was proposed as part of a triaging system for epilepsy care in general40 and is partially reflected in the National Institute for Health and Care Excellence guidelines.37,41
Figure 2. Comprehensive epilepsy specialty care first.
An alternative model to pediatric epilepsy care in which specialty-comprehensive care is sought right from the outset to optimize patient care and outcomes. Children, particularly the very young, receive a full diagnostic evaluation. Disorders that can be identified and that have specific treatment implications are diagnosed and appropriately treated. More intensive treatments (surgery and diet) are considered early in the course of the epilepsy. AEDs = antiepileptic drugs.
KNOWLEDGE GAPS
Several examples were discussed in which specialty-first care might have avoided years of uncontrolled seizures and developmental disability (see table 1) and provide compelling reasons for a specialty-first model for early-onset epilepsy. Implementing such a model could be resource-intensive. Two large considerations must first be addressed.
Accurate early diagnosis and “optimal” treatment: Impact on outcomes.
There are few data regarding the relative yield of specific diagnoses (brain lesions, metabolic disorders, genetic disorders) in children with newly presenting early-onset epilepsy. Epidemiologic data are out of date and do not reflect the use of advanced neuroimaging, genetic testing, immunologic assays, or high-quality EEG. While there are guidelines recommending the use of EEG42 and neuroimaging43 for evaluating children with epilepsy, details of how these tests should be performed are sometimes lacking. For MRI, guidelines specify a 1.5-tesla MRI with age-appropriate seizure protocol43 whereas 3-tesla is rapidly becoming the standard in epilepsy evaluations.44 EEG is important, but there are different protocols, not addressed by guidelines, for how EEG can be performed. Perhaps most important today is genetic testing. There is no information about the use of genetic testing in patients with new-onset epilepsy and no guidelines regarding use of metabolic and genetic testing or testing for inflammatory markers. There is little information about how these testing modalities are currently used and their impact on patient care and outcome. A set of performance indicators proposed for pediatric epilepsy care did not even mention genetic testing.45
The impact of the diagnosis on the selection of treatment and on patient outcomes has not been studied in the modern context. US guidelines exist for infantile spasms,30,31 but not for other common early-onset epilepsies. Most experts would likely agree on the value of the ketogenic diet for GLUT1 deficiency syndrome.46 Specific treatments for Dravet syndrome, however, while discussed in opinion pieces, do not have a general consensus. A systematic review of treatment for Lennox-Gastaut syndrome concluded that there was inadequate data for strong recommendations.47 This lack of definitive evidence is understandable as early-onset epilepsies comprise numerous rare conditions, and there are 30 or more medications available for use. The typical evidence standard of head-to-head randomized clinical trials has generally been unobtainable given the rarity of each of the many conditions and the large number of available treatments. Consequently, individual providers vary considerably in their practices, and insurers may not always provide access to the expensive diagnostic and therapeutic modalities. The treatment of early-onset epilepsy is, in many regards, an evidence-free practice zone. The schism between hard evidence and the strongly expressed opinion that diagnosis should matter represents an opportunity for research and new methods.48
Despite the lack of formal guidelines, the value of accurate early diagnosis and treatment can be appreciated in a few reports. In one study, both parents and providers reported substantial benefits of SCN1A gene testing in children with suspected Dravet syndrome. Benefits included changing treatment, better seizure control, and, if done early, better developmental outcomes.49 A preliminary report examined the impact of early diagnosis and optimal treatment in children with Doose syndrome.50 Children who initially received certain drugs that experts “feel” are contraindicated for this form of epilepsy did substantially worse in terms of seizure control and development than did children who initially received more optimal treatments. These studies only scratch the surface of the many issues that must be adequately addressed in order to influence practice.
An important role of a comprehensive epilepsy program is to provide nonpharmacologic treatments. Dietary and surgical therapies are key alternatives to traditional pharmacotherapy. They are more difficult either because of the time-intensiveness (ketogenic diet) or the inherent invasiveness (surgery). Optimal use of such therapies requires a team approach and considerable support and expertise that generally can only be provided at a comprehensive center. Notably, these therapies, because of their complexities, are utilized and administered very differently depending on the center; the therapy, as a “package,” is potentially quite different from one center to another. Immunomodulatory therapies are receiving increased attention although there is still much that is not known about when to use them, in whom, and the likely impact. Most studies represent series from individual centers, thereby limiting their value for assisting individuals in making informed decisions for their children. Limited knowledge about effectiveness of these therapies in specific clinical situations adds to the difficulty in developing a solid evidence base for individual decision-making.
Comprehensive/specialty epilepsy care.
Although there was general enthusiasm for the idea of comprehensive or specialty care, there is no single definition of what that constitutes. The NAEC has standards for level 3 and 4 epilepsy centers, which emphasize surgical therapy.36 Little is discussed regarding other therapies (diet, immunomodulatory), the collaborative team environment, or the speed with which diagnosis and interventions must occur. For young children, comprehensive care encompasses diagnostic and therapeutic resources and expertise, but also evaluation, referral, and intervention beyond seizures themselves. It requires a multidisciplinary, collaborative, and coordinated approach. Pediatric epileptologists attending the meeting outlined their own multidisciplinary comprehensive epilepsy programs; all involved a range of specialties including social work, psychiatry, neuropsychology, educational liaisons, nursing staff, advanced practice nurses, ketogenic dieticians, pharmacologists, genetic counselors, neurosurgeons, neuroimaging and nuclear medicine specialists, as well as hospital and community-based parent support groups, schools, and others. Although the models varied, each involved a systematic, coordinated approach and was likely influenced by resources available at each center as well as personal characteristics of the providers.
The role of and the impact on parents is rarely a major focus in “comprehensive” pediatric care. There was great enthusiasm for approaches to create better communication and information for parents and involve parents more explicitly as part of the decision-making and treatment team. There was also no information regarding how best to do this, what the actual impact is, and whether there are different approaches that would be more beneficial to different parents as a function of cultural, linguistic, and socioeconomic characteristics, or individual preference.
CHALLENGES TO PROGRESS
Evidence.
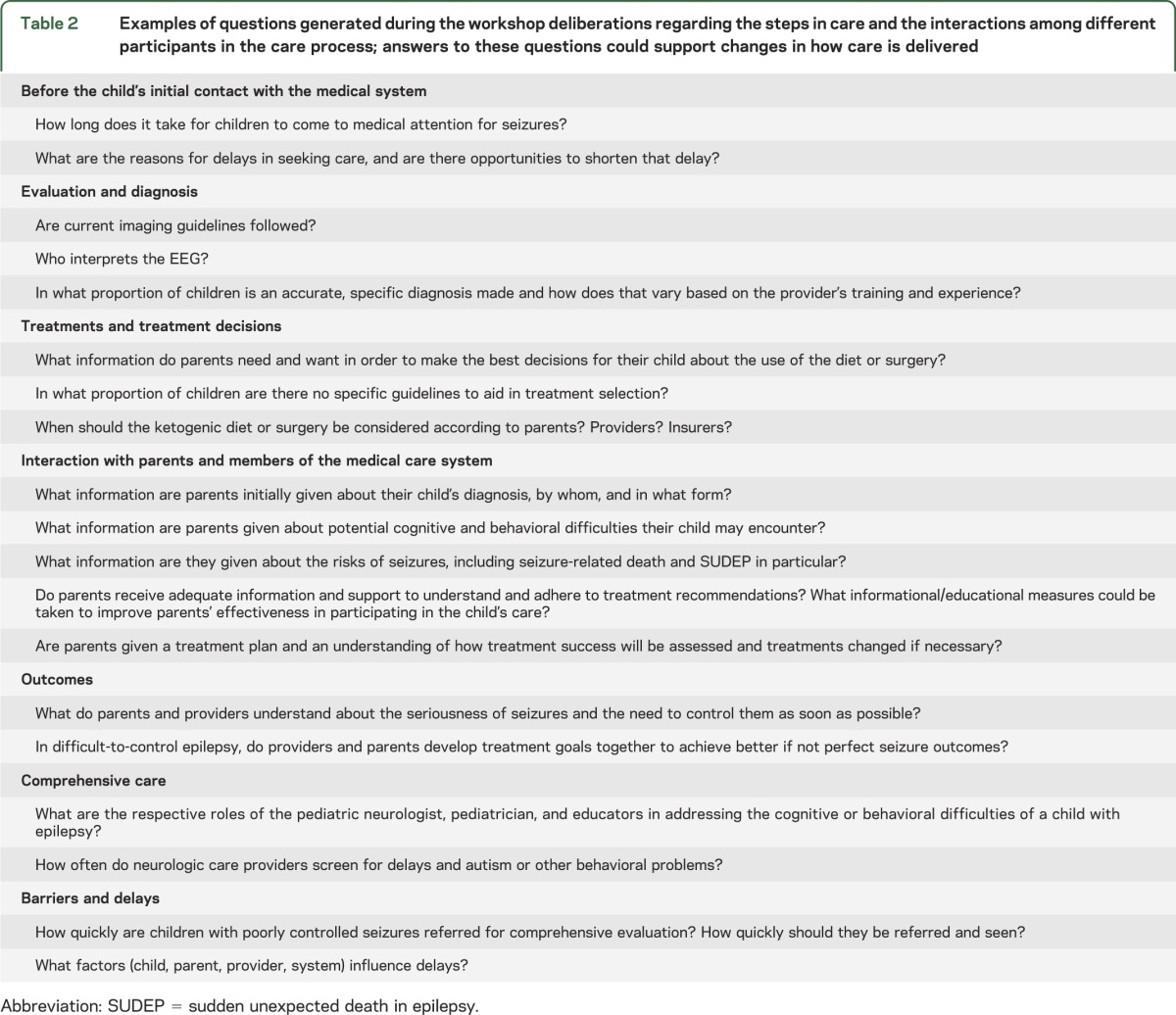
Without good evidence demonstrating the value to patient care and outcomes, it is arguably unjustifiable to recommend time- and resource-intensive care approaches. Overcoming these limitations requires systematic assessment of the value of various diagnostic modalities and treatments, overall and in specific clinical situations, to provide the necessary information to all parties to make informed decisions and develop responsible policies. Questions were generated during discussions that could guide research to improve aspects of pediatric epilepsy care (table 2).
Table 2.
Examples of questions generated during the workshop deliberations regarding the steps in care and the interactions among different participants in the care process; answers to these questions could support changes in how care is delivered
Barriers and access.
Comprehensive care is also not readily available to large segments of the population because of geographic or economic access. A staged approach that prioritizes certain types of patients may need consideration. Other factors such as attitudes and personal preferences of both the parents and providers may influence the decision to seek specialty evaluation and care. Information needs to be communicated effectively to the parent to permit shared decision-making.
Implementation.
Even strong evidence and well-supported guidelines do not always influence practice.51 Basic quality indicators for adult epilepsy care were recently published in a major journal,52 but they have not been fully adopted, especially by nonepileptologists to whom they were particularly targeted.53,54 A guideline on surgery for refractory temporal lobe epilepsy55 did not appear to alter referral practices 7 years after publication.20,56 A recent survey regarding treatment for infantile spasms reported many respondents using non–first line treatments as initial therapies.57 Reasons for and the impact of this variation on patient outcomes are unknown, but there is extensive literature on the role of implementation science in linking evidence to improved population outcomes.58
CONCLUSIONS
Early-onset pediatric epilepsy is a high-stakes condition. Early seizure control is of supreme importance to parents and is an overriding goal for clinicians. While early seizure control is a goal in itself, it also contributes to achieving other critical goals such as better cognitive and behavioral outcomes and decreased mortality. Ultimately, research must provide evidence that directly contributes to changes in care delivery and results in measurable improvements in patient outcomes. Collaboration of all concerned parties including clinical and health services researchers, parents, providers, other allied health professionals, insurers, administrators, health economists, and policy-makers is therefore needed and is consistent with recent announcements from the Patient-Centered Outcomes Research Institute.59,60 The priorities identified in the workshop are also highly consistent with the recommendations in the recent Institute of Medicine report.1 Pediatric epilepsy care is an ideal candidate area for this type of endeavor because there is much at stake and much that could be done, but so little, currently, to guide improvements.
ACKNOWLEDGMENT
The authors thank Robin Shelton, Clara Samaniego, Eileen Romano, Sana Khan, Diana Umanzor, and Luciano Pedroza for their skillful assistance and cheerful support in organizing this workshop and all of the other members of the Epilepsy Center at Ann & Robert H. Lurie Children's Hospital of Chicago who made accommodations in their schedules to ensure the success of this workshop.
GLOSSARY
- NAEC
National Association of Epilepsy Centers
CONTRIBUTORS
In addition to the authors, the following individuals spoke at and (or) participated in the discussions during the workshop. Their contributions form the basis for the workshop summary. Susan Axelrod (Citizens United for Research in Epilepsy, speaker and participant), Brent Allen (Chicago, participant), Terry Biagi, MD (New York, participant), Bogdan Ewendt (Citizens United for Research in Epilepsy, participant), Tracy Dixon-Salazar, PhD (University of California San Diego, speaker and participant), Leon Epstein, MD (Ann & Robert H. Lurie Children's Hospital of Chicago, speaker and participant), Kathy Evans (Glut1 Deficiency Foundation, participant), William Gaillard, MD (National Children's Medical Center, speaker and participant), Phillip Gattone, MEd (Epilepsy Foundation, speaker and participant), Alicia Goldman, MD (Baylor School of Medicine, speaker and participant), Lisa Gorschels, MA (DeKalb School District, Special Education Coordinator, speaker and participant), Lorie Hamiwka, MD (Nationwide Children's Hospital, speaker and participant), Jouku Isojarvi, MD, PhD (Lundbeck LLC, participant), Sucheta Joshi, MD, MS (University of Michigan, participant), Christine Karam, PharmD (Questcor Pharmaceuticals, participant), Warren Kibbe, PhD (Northwestern University, participant), Sookyong Koh, MD, PhD (Ann & Robert H. Lurie Children's Hospital of Chicago, participant), Kelly Knupp, MD (Colorado Children's Hospital, participant), Patrick M. Magoon (Ann & Robert H. Lurie Children's Hospital of Chicago, speaker), Janna Moore, MPA (Epilepsy Support Network of Orange County, speaker and participant), Brenda Nieme (Chicago, IL, participant), Douglas Nordli, MD (Ann & Robert H. Lurie Children's Hospital of Chicago, speaker and participant), Nan Rothrock, PhD (Northwestern University, speaker and participant), Russel Saneto, MD (Seattle Children's Hospital, speaker and participant), Donald Shields, MD (UCLA, participant), Michael Smith, MD (Rush University Medical School, participant), Cynthia Stack, MD (Ann & Robert H. Lurie Children's Hospital of Chicago, participant), Joseph Sullivan, MD (University of California San Francisco, participant), Steven White, PhD (Citizens United for Research in Epilepsy, participant).
AUTHOR CONTRIBUTIONS
Dr. Berg drafted the original manuscript and participated in revising the manuscript and producing the final draft. Dr. Baca, Dr. Loddenkemper, Dr. Vickrey, and Dr. Dlugos participated in critical review and revision of the manuscript and in producing the final draft.
STUDY FUNDING
Citizens United for Research on Epilepsy (CURE) provided funding for travel and accommodations for many of the speakers and participants and made possible the meeting summarized in this report. Additional support for the meeting was provided by the Foundation of Ann & Robert H. Lurie Children's Hospital of Chicago.
DISCLOSURE
A. Berg has received speaker honoraria and travel support from BIAL and Medical University of South Carolina, and travel support from the ILAE, serves on advisory boards for CURE and Eisai, serves on the editorial boards of Epilepsy & Behavior and Neurology®, and is supported by funding from the NINDS (grant R37-NS31146) and the Pediatric Epilepsy Research Foundation. C. Baca receives support from grant NINDS-R37-NS31146. T. Loddenkemper serves on the Laboratory Accreditation Board for Long Term (Epilepsy and Intensive Care Unit) Monitoring, on the Council of the American Clinical Neurophysiology Society, on the American Board of Clinical Neurophysiology, as an associate editor for Seizure, the European Journal of Epilepsy, as an associate editor for Wyllie's Treatment of Epilepsy 6th edition, as an editorial board member for Biomedical Research International, and performs video EEG long-term monitoring, EEGs, and other electrophysiologic studies at Boston Children's Hospital and bills for these procedures. T. Loddenkemper receives research support from the NIH/NINDS (1R21NS076859-01), a Career Development Fellowship Award from Harvard Medical School and Boston Children's Hospital, the Payer Provider Quality Initiative, the Epilepsy Foundation of America (EF-213882 and EF-213583), the Center for Integration of Medicine and Innovative Technology, the Epilepsy Therapy Project, from an Infrastructure Award by the American Epilepsy Society and Epilepsy Foundation of America, CURE, and from investigator-initiated research grants from Lundbeck and Eisai. B. Vickrey receives support from grant NINDS R37 NS31146. She serves on scientific advisory boards for the Sports Concussion Institute, American Heart Association, and the NIH; serves on the editorial boards of Neurorehabilitation and Neural Repair and is a section editor for Stroke; receives research support from the NIH (NIA RC4AG038804, NINDS U54 NS081764), the US Veterans Administration Health Services Research and Development Service (NRI 11-126-1), and the American Heart Association; and is a consultant to EMD Serono Canada, Imperial Clinical Research Services, Inc., CHDI, and the National Parkinson Foundation. D. Dlugos is funded by NIH grants 1R01NS053998, 2U01NS045911, 1R01LM011124, and U01NS077276. He has also given expert testimony in medicolegal cases. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Institute of Medicine Epilepsy Across the Spectrum: Promoting Health and Understanding. Washington, DC: The National Academies Press; 2012 [PubMed] [Google Scholar]
- 2.Berg AT, Shinnar S, Levy SR, Testa F, Smith-Rapaport S, Beckerman B. Early development of intractable epilepsy in children: a prospective study. Neurology 2001;56:1445–1452 [DOI] [PubMed] [Google Scholar]
- 3.Camfield CS, Camfield PR, Gordon K, Wirrell E, Dooley JM. Incidence of epilepsy in childhood and adolescence: a population-based study in Nova Scotia from 1977 to 1985. Epilepsia 1996;37:19–23 [DOI] [PubMed] [Google Scholar]
- 4.Olafsson E, Hauser WA, Ludvigsson P, Gudmundsson G. Incidence of epilepsy in rural Iceland: a population-based study. Epilepsia 1996;37:951–955 [DOI] [PubMed] [Google Scholar]
- 5.Camfield P, Camfield C. Long-term prognosis for symptomatic (secondarily) generalized epilepsies: a population-based study. Epilepsia 2007;48:1128–1132 [DOI] [PubMed] [Google Scholar]
- 6.Wirrell E, Farrell K, Whiting S. The epileptic encephalopathies of infancy and childhood. Can J Neurol Sci 2005;32:409–418 [DOI] [PubMed] [Google Scholar]
- 7.Berg AT. Epilepsy, cognition, and behavior: the clinical picture. Epilepsia 2011;52(suppl 1):7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood LJ, Sherman EMS, Hamiwka LD, Blackman MA, Wirrell EC. Maternal depression: the cost of caring for a child with intractable epilepsy. Pediatr Neurol 2008;39:418–422 [DOI] [PubMed] [Google Scholar]
- 9.Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE Study. Epilepsia 2001;42:464–475 [DOI] [PubMed] [Google Scholar]
- 10.O'Callaghan FJK, Lux AL, Darke K, et al. The effect of lead time to treatment and of age of onset on developmental outcome at 4 years in infantile spasms: evidence from the United Kingdom Infantile Spasms Study. Epilepsia 2011;52:1359–1364 [DOI] [PubMed] [Google Scholar]
- 11.Chapman M, Iddon P, Atkinson K, et al. The misdiagnosis of epilepsy in people with intellectual disabilities: a systematic review. Seizure 2011;20:101–106 [DOI] [PubMed] [Google Scholar]
- 12.Kim HL, Donnelly JH, Tournay AE, Book TM, Filipek P. Absence of seizures despite high prevalence of epileptiform EEG abnormalities in children with autism monitored in a tertiary care center. Epilepsia 2006;47:394–398 [DOI] [PubMed] [Google Scholar]
- 13.Sirven JI, Lopez RA, Vazquez B, Van Haverbeke P. Que es la Epilepsia? Attitudes and knowledge of epilepsy by Spanish-speaking adults in the United States. Epilepsy Behav 2005;7:259–265 [DOI] [PubMed] [Google Scholar]
- 14.Cooper W, Federspiel CF, Griffin MR, Hickson GB. The use of anticonvulsant medications among children enrolled in the Tennessee medicaid program. Arch Pediatr Adolesc Med 1997;151:1242–1246 [DOI] [PubMed] [Google Scholar]
- 15.Modi AC, Rausch JR, Glauser TA. Patterns of nonadherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA 2011;305:1669–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burneo JG, Black L, Knowlton RC, Faught E, Morawetz R, Kuzniecky RI. Racial disparities in the use of surgical treatment for intractable temporal lobe epilepsy. Neurology 2005;64:50–54 [DOI] [PubMed] [Google Scholar]
- 17.Begley C, Basu R, Lairson D, et al. Socioeconomic status, health care use, and outcomes: persistence of disparities over time. Epilepsia 2011;52:957–964 [DOI] [PubMed] [Google Scholar]
- 18.Smedley BD, Stith AY, Nelson AR, editors; Institute of Medicine Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; 2003 [PubMed] [Google Scholar]
- 19.Swarztrauber K, Dewar S, Engel J. Patient attitudes about treatments for intractable epilepsy. Epilepsy Behav 2003;4:19–25 [DOI] [PubMed] [Google Scholar]
- 20.Englot DJ, Ouyang D, Garcia PA, Barbaro NM, Chang EF. Epilepsy surgery trends in the United States, 1990–2008. Neurology 2012;78:1200–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid R, Friedberg MW, Adams JL, McGlynn EA, Mehrotra A. Associations between physician characteristics and quality of care. Arch Intern Med 2010;170:1442–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baca CB, Vickrey BG, Vassar S, et al. Time to pediatric epilepsy surgery is related to both disease severity and non-clinical factors. Neurology 2013;80:1231–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swarztrauber K, Vickrey BG, Mittman BS. Physicians' preferences for specialty involvement in the care of patients with neurological conditions. Med Care 2002;40:1196–1209 [DOI] [PubMed] [Google Scholar]
- 24.Epilepsy Support Network. Available at: http://www.epilepsysupportnet.org/. Accessed April 1, 2013.
- 25.Cross JH. Pitfalls in the diagnosis and differential diagnosis of epilepsy. Paediatr Child Health 2009;19:199–202 [Google Scholar]
- 26.Hamiwka LD, Singh N, Niosi J, Wirrell EC. Diagnostic inaccuracy in children referred with “first seizure”: role for a first seizure clinic. Epilepsia 2007;48:1062–1066 [DOI] [PubMed] [Google Scholar]
- 27.Anand G, McShane T. Is paediatric epilepsy getting less common? Arch Dis Child 2013;98:167. [DOI] [PubMed] [Google Scholar]
- 28.Hindley D, Ali A, Robson C. Diagnoses made in a secondary care “fits, faints, and funny turns” clinic. Arch Dis Child 2006;91:214–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uldall P, Alving J, Hansen LK, Kibæk M, Buchholt J. The misdiagnosis of epilepsy in children admitted to a tertiary epilepsy centre with paroxysmal events. Arch Dis Child 2006;91:219–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKay MT, Weiss SK, Adams-Webber T, et al. Practice parameter: medical treatment of infantile spasms. Neurology 2004;62:1668–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pellock JM, Hrachovy R, Shinnar S, et al. Infantile spasms: a U.S. consensus report. Epilepsia 2010;51:2175–2189 [DOI] [PubMed] [Google Scholar]
- 32.Wheless J, Gibson P, Rosbeck K, et al. Infantile spasms (West syndrome): update and resources for pediatricians and providers to share with parents. BMC Pediatr 2012;12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JC, Jonas R, Fu CM, Ng CY, Douglass L. Quality-of-care indicators for infantile spasms. J Child Neurol 2013;28:13–20 [DOI] [PubMed] [Google Scholar]
- 34.Glauser T, Ben-Menachem E, Bourgeois B, et al. ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2006;47:1094–1120 [DOI] [PubMed] [Google Scholar]
- 35.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069–1077 [DOI] [PubMed] [Google Scholar]
- 36.Labiner DM, Bagic AI, Herman ST, et al. Essential services, personnel, and facilities in specialized epilepsy centers: revised 2010 guidelines. Epilepsia 2010;51:2322–2333 [DOI] [PubMed] [Google Scholar]
- 37.National Institute for Health and Care Excellence The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care; 2012. Available at: guidance.nice.org.uk/cg137. Accessed April 1, 2013
- 38.Wong-Kisiel LC, McKeon A, Wirrell EC. Autoimmune encephalopathies and epilepsies in children and teenagers. Can J Neurol Sci 2012;39:134–144 [DOI] [PubMed] [Google Scholar]
- 39.Lancaster E, Dalmau J. Neuronal autoantigens: pathogenesis, associated disorders and antibody testing. Nat Rev Neurol 2012;8:380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berg AT, Cross JH. Classification of epilepsies and seizures: historical perspective and future directions. In: Stefan H, Theodore WH, editors. Handbook of Clinical Neurology. Amersterdam: Elsevier; 2012:99–111 [DOI] [PubMed] [Google Scholar]
- 41.Nunes VD, Sawyer L, Neilson J, Sarri G, Cross JH. Diagnosis and management of the epilepsies in adults and children: summary of updated NICE guidance. BMJ 2012;344:e281. [DOI] [PubMed] [Google Scholar]
- 42.Hirtz D, Ashwal S, Berg AT, et al. Evaluating a first nonfebrile seizure in children: an evidence-based practice parameter. Neurology 2000;55:616–623 [DOI] [PubMed] [Google Scholar]
- 43.Gaillard WD, Chiron C, Cross JH, et al. Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia 2009;50:2147–2153 [DOI] [PubMed] [Google Scholar]
- 44.Craven IJ, Griffiths PD, Bhattacharyya D, et al. 3.0 T MRI of 2000 consecutive patients with localisation-related epilepsy. Br J Radiol 2012;85:1236–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caplin DA, Rao JK, Filloux F, Bale JF, Van Orman C. Development of performance indicators for the primary care management of pediatric epilepsy: expert consensus recommendations based on the available evidence. Epilepsia 2006;47:2011–2019 [DOI] [PubMed] [Google Scholar]
- 46.Klepper J, Leiendecker B. GLUT1 deficiency syndrome: 2007 update. Dev Med Child Neurol 2007;49:707–716 [DOI] [PubMed] [Google Scholar]
- 47.Hancock EC, Cross JH. Treatment of Lennox-Gastaut syndrome. Cochrane Database Syst Rev 2013;(2): CD003277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vendrame M, Loddenkemper T. Approach to seizures, epilepsies, and epilepsy syndromes. Sleep Med Clin 2012;7:59–73 [Google Scholar]
- 49.Brunklaus A, Dorris L, Ellis R, et al. The clinical utility of an SCN1A genetic diagnosis in infantile-onset epilepsy. Dev Med Child Neurol 2013;55:154–161 [DOI] [PubMed] [Google Scholar]
- 50.Nangia S, Millichap JJ, Berg AT, Nordli DR. Early exposure to carbamazepine, oxcarbazepine, phenytoin, and lamotrigine in epilepsy with myoclonic-atonic seizures is associated with worse outcome. Abstract. Presented at the Annual Meeting of the American Epilepsy Society; November 30–December 4, 2012; San Diego, CA.
- 51.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med 2003;348:2635–2645 [DOI] [PubMed] [Google Scholar]
- 52.Fountain NB, Van Ness PC, Swain-Eng R, et al. Quality improvement in neurology: AAN epilepsy quality measures—report of the Quality Measurement and Reporting Subcommittee of the American Academy of Neurology. Neurology 2011;76:94–99 [DOI] [PubMed] [Google Scholar]
- 53.Wasade VS, Spanaki M, Iyengar R, Barkley GL, Schultz L. AAN epilepsy quality measures in clinical practice: a survey of neurologists. Epilepsy Behav 2012;24:468–473 [DOI] [PubMed] [Google Scholar]
- 54.Wicks P, Fountain NB. Patient assessment of physician performance of epilepsy quality-of-care measures. Neurol Clin Pract 2012;2:335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engel J, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia 2003;44:741–751 [DOI] [PubMed] [Google Scholar]
- 56.Haneef Z, Stern J, Dewar S, Engel J. Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology 2010;75:699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mytinger JR, Joshi S. The current evaluation and treatment of infantile spasms among members of the Child Neurology Society. J Child Neurol 2012;27:1289–1294 [DOI] [PubMed] [Google Scholar]
- 58.Vickrey BG, Hirtz D, Waddy S, Cheng EM, Johnston SC. Comparative effectiveness and implementation research: directions for neurology. Ann Neurol 2012;71:732–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Methodology Committee of the Patient-Centered Outcomes Research Institute (PCORI) Methodological standards and patient-centeredness in comparative effectiveness research: the PCORI perspective. JAMA 2012;307:1636–164022511692 [Google Scholar]
- 60.Gabriel SE, Normand SLT. Getting the methods right—the foundation of patient-centered outcomes research. N Engl J Med 2012;367:787–790 [DOI] [PubMed] [Google Scholar]