Abstract
Objective:
To investigate prospective, population-based long-term outcomes concerning seizures and antiepileptic drug (AED) treatment after resective epilepsy surgery in Sweden.
Methods:
Ten- and 5-year follow-ups were performed in 2005 to 2007 for 278/327 patients after resective epilepsy surgery from 1995 to 1997 and 2000 to 2002, respectively. All patients had been prospectively followed in the Swedish National Epilepsy Surgery Register. Ninety-three patients, who were presurgically evaluated but not operated, served as controls.
Results:
In the long term (mean 7.6 years), 62% of operated adults and 50% of operated children were seizure-free, compared to 14% of nonoperated adults (p < 0.001) and 38% of nonoperated children (not significant). Forty-one percent of operated adults and 44% of operated children had sustained seizure freedom since surgery, compared to none of the controls (p < 0.0005). Multivariate analysis identified ≥30 seizures/month at baseline and long epilepsy duration as negative predictors and positive MRI to be a positive predictor of long-term seizure-free outcome. Ten years after surgery, 86% of seizure-free children and 43% of seizure-free adults had stopped AEDs in the surgery groups compared to none of the controls (p < 0.0005).
Conclusions:
This population-based, prospective study shows good long-term seizure outcomes after resective epilepsy surgery. The majority of the patients who are seizure-free after 5 and 10 years have sustained seizure freedom since surgery. Many patients who gain seizure freedom can successfully discontinue AEDs, more often children than adults.
Classification of evidence:
This study provides Class III evidence that more patients are seizure-free and have stopped AED treatment in the long term after resective epilepsy surgery than nonoperated epilepsy patients.
There is Class I evidence of short-term efficacy of epilepsy surgery from 2 randomized controlled trials (RCT).1,2 In the largest of these, 64% of the operated patients were seizure-free after 12 months compared to 8% of medically treated patients.1 Many cohort studies have shown similar short-term outcomes.3
Increasing numbers of epilepsy surgery centers have reported long-term outcomes in cohorts of patients following a variety of interventions. Since RCT for long-term follow-up would be ethically difficult to implement, prospective cohort studies are important. In one single-center study of long-term outcome after temporal lobe resection (TLR) in 325 patients, 48% were continuously seizure-free after 5 years and 41% after 10 years.4 In another single-center follow-up of 615 adults, 52% remained seizure-free 5 years after surgery and 47% at 10 years.5
Side effects of antiepileptic drugs (AEDs) contribute to poor quality of life,6 and many patients have expectations to withdraw AEDs after successful surgery.7 There are no systematic studies of the optimal timing of postoperative drug withdrawal, but in a recent study, early AED withdrawal did not affect long-term seizure outcome in children.8
The Swedish National Epilepsy Surgery Register (SNESUR) provides a possibility to study outcomes of epilepsy surgery prospectively and population-based at a national level.9–11
The aim of this study was to investigate long-term outcomes concerning seizures and AED treatment 5 and 10 years after resective epilepsy surgery in adults and children, compared to the outcomes of controls who were evaluated for epilepsy surgery but not operated.
METHODS
In Sweden, all epilepsy surgery procedures are reported to the SNESUR, which was initiated in 1990. Information is collected longitudinally for each patient and the register is population-based. An internal control system rejects certain impossible combinations of data and regular external quality controls are performed by an independent controller. Since 2005, the follow-up has been extended from 2 years to 5, 10, and 15 years postoperatively. SNESUR contains baseline information on patient's epilepsy history, preoperative seizure types and syndromes, mean monthly seizure frequency during the year preceding the presurgical investigation, AEDs, preoperative investigations, psychosocial data, surgical data (type and location of surgery), histopathologic diagnoses, and postoperative complications. Two-year follow-up data cover seizure situation, AEDs, and psychosocial data. The 5-, 10-, and 15-year follow-ups are structured telephone interviews regarding seizure situation, AEDs, psychosocial aspects, and driving.
In this study, we analyzed seizure outcome and AED medication 5 and 10 years after resective epilepsy surgery in patients who had 5- and 10-year follow-ups in 2005 to 2007 (and hence were operated on in 2000 to 2002 and 1995 to 1997). The cohort comprises the 327 patients who had resective surgery during these time periods.
In 2005 to 2007, 144/176 patients operated on in 1995 to 1997 (98/116 adults and 46/60 children ≤18 years) had a 10-year follow-up, and 134/151 patients operated on in 2000 to 2002 (92/103 adults and 42/48 children ≤18 years) had a 5-year follow-up. Seventeen patients were reoperated before long-term follow-up and there were 11 deaths. Twenty-one patients (6.4%) were lost to follow-up (for details, see figure e-1 on the Neurology® Web site at www.neurology.org).
As a control group, consecutive patients who underwent presurgical investigations during the same time periods but were not operated were identified at 3 of the 6 operating centers (Göteborg, Uppsala, and Lund). Baseline data for these patients were ascertained during the presurgical assessment. Eighty adults and 13 children out of 94 adults and 13 children underwent a cross-sectional long-term follow-up in 2008 after a mean of 9.3 years (adults) and 8.8 years (children) using the same structured telephone interview as for the surgical group. Thirteen adult patients had died (4 epilepsy-related deaths) and 1 was lost to follow-up (1%). Reasons for not having surgery were nonconclusive workup (n = 41), multifocality (n = 27), patient declined surgery (n = 12), seizure onset within eloquent cortex (n = 11), and neuropsychological reasons (n = 2).
Seizure freedom (without or with aura, International League Against Epilepsy [ILAE] Class I and II12) is reported for the year preceding the follow-up except for patients with sustained seizure freedom since surgery, which is separately reported. For patients with continuing seizures or seizure relapse postoperatively, the mean monthly seizure frequency in the last year of follow-up is categorized as follows: ≥75% reduction in seizure frequency; 50%–74% reduction in seizure frequency; 0%–49% reduction in seizure frequency; and increased seizure frequency.
For comparison between 2 groups, Fisher exact test was used for dichotomous variables, Mann-Whitney U test for continuous variables, and Mantel-Haenszel χ2 test for ordered categorical variables. All tests were 2-tailed and conducted at the 5% significance level. Logistic regression analysis was performed for each independent variable to predict seizure-free outcome. A forward stepwise multiple logistic regression was used to select independent predictors to outcome. Only univariate predictors attaining a p value of <0.10 were included in the forward stepwise multivariate logistic regression. For model discrimination, the area under receiver operating characteristic curve (AUC) was calculated. Statistical analysis was performed using IBM SPSS Statistics 19 and SAS 9.2.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Regional Board of Medical Ethics at the University of Gothenburg. Consent for research was obtained from all controls. For operated patients, the Board considered long-term follow-up as a quality control measure not necessitating individual consent.
Classification of evidence.
Primary research questions were as follows: are more patients seizure-free and without AED in the long term after resective epilepsy surgery compared to nonoperated patients? This longitudinal observational study provides Class III evidence that 41% of adults and 44% of children have sustained seizure freedom in the long term after surgery compared to none of the nonoperated patients (p < 0.0005). Also, 43% of seizure-free adults and 86% of seizure-free children had stopped AEDs after 10 years compared to none of the nonoperated patients (p < 0.0005).
RESULTS
Baseline characteristics.
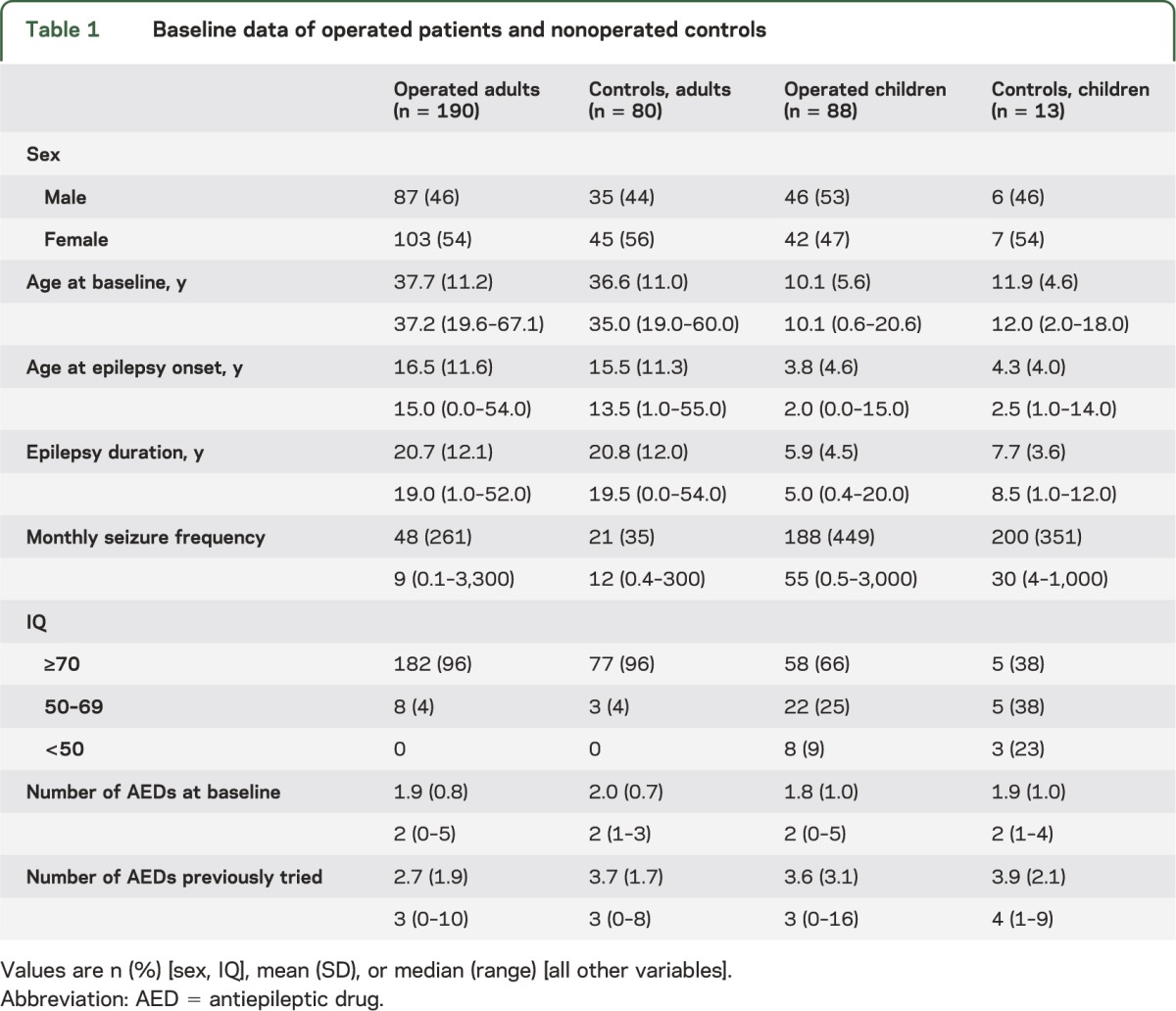
Table 1 shows baseline characteristics of the operated patients and the controls, children (≤18 years) and adults. The only adult who underwent hemispherectomy at age 20 was added to the pediatric hemispherectomy group. None of the baseline characteristics differed between operated and nonoperated patients, except for number of previously tried AEDs in adults (p < 0.001) and IQ in children (p = 0.042).
Table 1.
Baseline data of operated patients and nonoperated controls
Seizure outcome.
There was no significant difference in seizure outcome between the operated patients who had follow-up after 5 years compared to 10 years. In order to enable comparison with the nonoperated group, who had a mean long-term follow-up of 9.1 years (range 5–14), the results from 5- and 10-year follow-ups in the operated patients were merged (figure 1).
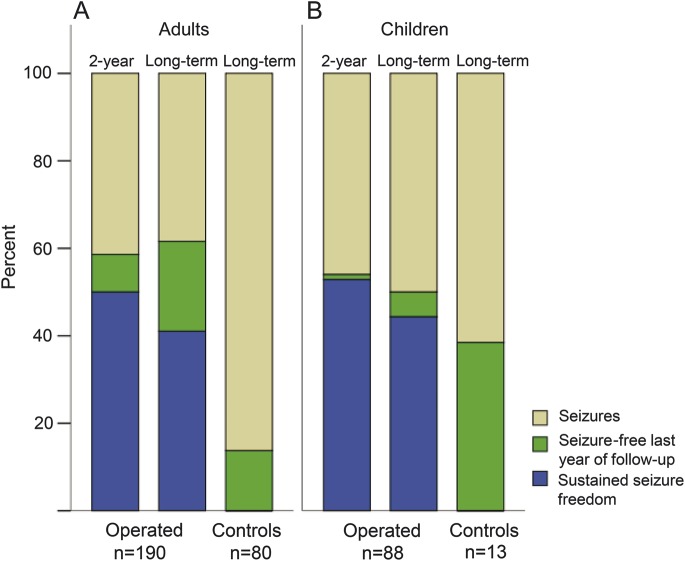
Figure 1. Seizure outcome at 2-year and long-term follow-up after surgery compared to controls.
Two-year and long-term (mean follow-up time 7.6 years, range 5–10) seizure situation for patients after resective epilepsy surgery compared to long-term follow-up of nonoperated controls (mean follow-up time 9.2 years, range 5–14). Seizure-free patients include those with sustained seizure freedom with or without aura since surgery (blue) and patients seizure-free at least the last year before follow-up (green). (A) Adults (>18 years). (B) Children (≤18 years).
Overall, 117 (62%) of the operated adults were seizure-free at long-term follow-up compared to 11 (14%) of the controls (p < 0.001). Fifty percent (n = 93) of the operated adults had sustained seizure freedom at the 2-year and 41% (n = 78) at the long-term follow-up. None of the controls was seizure-free for the whole time period. For the children, 44 (50%) in the operated group were seizure-free the year before long-term follow-up, compared to 5 (38%) in the control group (not significant). Fifty-three percent (n = 46) of the operated children had sustained seizure freedom since surgery at the 2-year and 44% (n = 39) at the long-term follow-up, compared to none in the control group. For both adults and children, the proportion with sustained seizure freedom at long term was significantly higher compared to the nonoperated patients (p < 0.0005). Details on seizure outcome for the operated patients at 2, 5, and 10 years after epilepsy surgery are shown in table e-1.
Eighty-seven percent of the adults as well as of the children who were seizure-free after 2 years were seizure-free at the 5- or 10-year follow-ups (95% confidence interval [CI] 77–94 and 80–95, respectively).
Seizure outcomes related to resection type and histopathology.
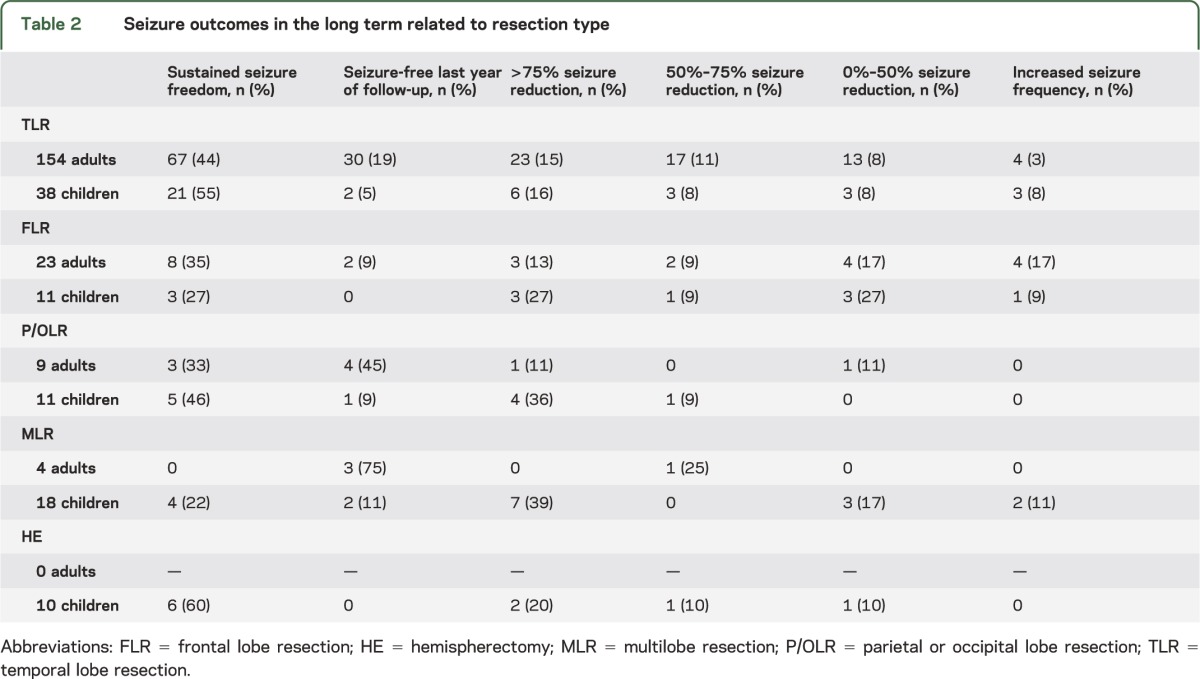
Seizure outcomes for adults and children after different resection types are illustrated in table 2. The most common resection type was TLR (81% in adults, 43% in children) followed by frontal lobe resection (FLR) in adults (12%) and multilobe resection in children (20%). In adults, the best long-term seizure outcome was after TLR (63% seizure-free, sustained seizure freedom since surgery in 44%). In children the TLR outcome (60% seizure-free, 55% with sustained seizure freedom) was matched by the long-term hemispherectomy results (60% seizure-free, all with sustained seizure freedom). If the extratemporal procedures are summed up, 31% of adults had sustained seizure freedom at long-term follow-up, compared to 36% of the children.
Table 2.
Seizure outcomes in the long term related to resection type
The main histopathologic diagnoses were categorized as follows: hippocampal sclerosis (HS, n = 45), neurodevelopmental tumors (n = 34), low-grade astrocytomas (n = 21), cavernous hemangiomas (n = 21), malformations of cortical development (n = 52), gliosis (n = 65), and other (n = 40). Highest seizure freedom rates were seen in HS (60%, 47% sustained since surgery) and epileptogenic lesions (72%, 57% sustained since surgery) (table e-2).
Predictors of seizure outcome.
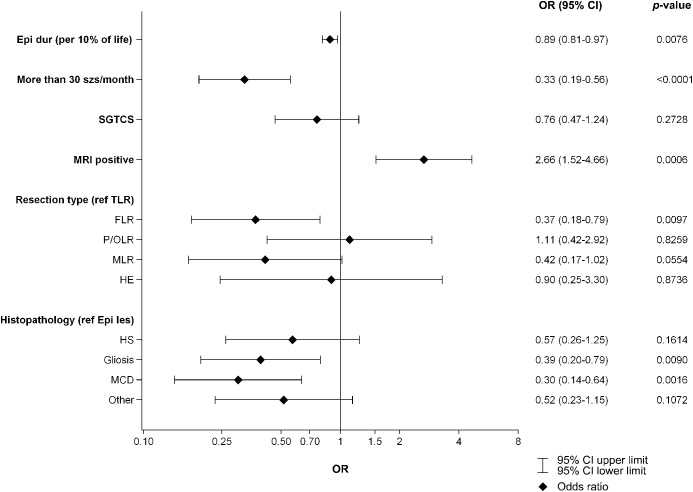
The univariate relationship between long-term seizure freedom and the following baseline variables was explored for both operated patients and controls: epilepsy duration in percent of life length (relative epilepsy duration, in odds ratio [OR] increment units of 10%), ≥30 seizures/month, and presence of secondarily generalized tonic-clonic seizures (SGTCS). For the operated patients, additional variables were positive MRI, resection types, and histopathology (figure 2). For the controls, the univariate associations were nonsignificant although in the same direction as for the operated patients (relative epilepsy duration, OR 0.89 [95% CI 0.73–1.09], ≥30 seizures/month, OR 0.88 [95% CI 0.22–3.47], and SGTCS, OR 0.56 [95% CI 0.18–1.68]). In the surgical group, multivariate analysis was performed, where ≥30 seizures/month, OR 0.40 (95% CI 0.23–0.69), and relative epilepsy duration, OR 0.91 (95% CI 0.83–1.00), were negative predictors, while positive MRI, OR 1.96 (95% CI 1.08–3.55), was a positive predictor of seizure-free long-term outcome. The AUC value (goodness of fit) for the final multiple model was 0.66.
Figure 2. Univariate logistic regression analysis of predictors for seizure freedom at long-term follow-up.
For the categorical variables with more than 2 categories (resection type and histopathology), temporal lobe resections (TLR) and epileptogenic lesions (Epi les) were chosen as reference categories. CI = confidence interval; Epi dur (per 10% of life) = epilepsy duration in percentage of life length, odds ratio units per 10% of life; epileptogenic lesions = gangliogliomas, dysembryoplastic neuroepithelial tumors, cavernomas, and low-grade astrocytomas; FLR = frontal lobe resection; HE = hemispherectomy; HS = hippocampal sclerosis; MCD = malformations of cortical development; MLR = multilobe resection; OR = odds ratio; other = vascular malformations other than cavernomas, cysts, tuberous sclerosis, unknown histopathology; P/OLR = parietal or occipital lobe resection; SGTCS = secondarily generalized tonic-clonic seizures.
Medication outcome.
At baseline, 183 (66%) of the patients had ≥2 AEDs, 85 (31%) had 1 AED, and 8 (3%) had no AEDs. The number of AEDs used by the operated seizure-free patients decreased over time (figure 3). Overall, 45 (54%) patients who were seizure-free at 10-year follow-up had stopped AEDs, compared to none of the seizure-free controls (p < 0.0005). More children than adults had stopped AEDs (86% of the children and 43% of the adults, p = 0.002). There was no significant association between the proportion of seizure-free patients who stopped AEDs and resection type. Among patients with seizures, the proportion with ≥2 AEDs had increased from 77% preoperatively to 82% 10 years after surgery.
Figure 3. Antiepileptic drug treatment preoperatively and postoperatively for patients who become seizure-free.
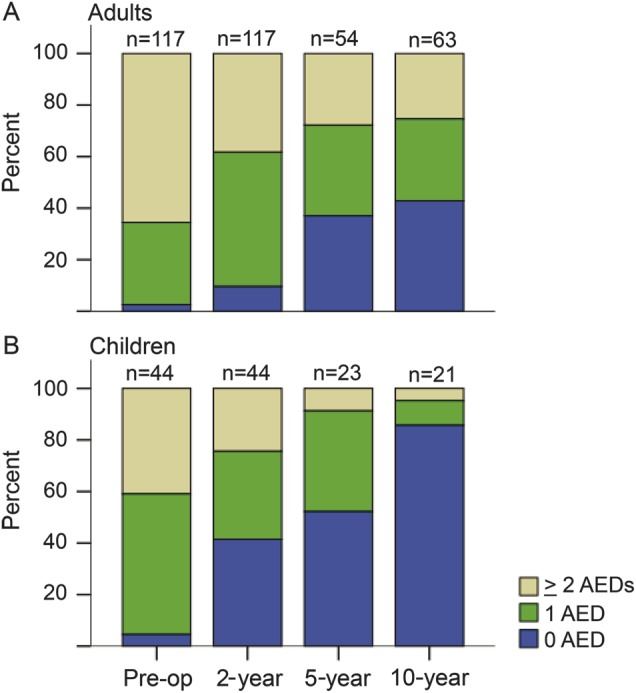
Number of antiepileptic drugs at the start of preoperative investigations, and after 2, 5, and 10 years for patients who were seizure-free at 5- or 10-year follow-up. AED = antiepileptic drug.
DISCUSSION
Since long-term results of epilepsy surgery cannot be obtained from RCTs, observational studies with defined quality criteria are needed. A number of requirements for studies on the prognosis after epilepsy surgery have been suggested.13 Studies based on SNESUR meet many of these requirements, with a prospective design, representative study populations, a standard protocol for outcome measures, and prolonged longitudinal follow-up with few patients lost to follow-up.
We found 63% of adults and 60% of children to be seizure-free after TLR at the long-term follow-up and most had continuous seizure freedom since surgery (adults 41%, children 44%). As expected, the results were not as good after FLR, with 44% of adults seizure-free (sustained in 35%), and in children 27% seizure-free (all sustained). This is still substantially better than for the patients who could not be operated (14% seizure-free adults, none sustained and 38% seizure-free children, none sustained). The adults who underwent other types of resections (posterior or multilobar) were few, and only 3 had sustained long-term seizure freedom. Children who had undergone posterior resections or hemispherectomies had long-term seizure freedom in 55% and 60%, respectively (sustained in 46% vs 60%).
Long-term outcome after resective epilepsy surgery is often reported cross-sectionally. In a meta-analysis from 2005 based on 78 studies, 66% of TLR patients, 46% of parietal or occipital lobe resection patients, and 27% of FLR patients were seizure-free at follow-up ≥5 years postsurgery, but the authors point out that few studies reported sustained seizure freedom from surgery. Almost all studies described patient cohorts without controls.3
A number of recent longitudinal long-term outcome studies report sustained seizure freedom after TLR. Most are retrospective single-center series; only a few are prospective. Sustained seizure freedom is reported as Engel 1,4,14–17 Engel 1a,18,19 or Engel 1a+b20 and in a few studies as ILAE Class I and II.5,21 Sustained seizure freedom around 5 years postoperatively varies between 46% and 55%4,5,18,21 and 60% to 80%.15–17,19,20,22 Among the studies with moderate rates of sustained seizure freedom, 2 of 4 are prospective,5,21 and our prospective study with 41% sustained seizure freedom at long-term follow-up after TLR in adults and 44% in children supports these results. All studies reporting higher rates of sustained seizure freedom were retrospective. A number of studies report cross-sectional long-term follow-up of pediatric epilepsy surgery, with seizure freedom in 50% to 82% of patients 5 to 12 years after TLR.23–28 Only one study is longitudinal and reports sustained seizure freedom (Engel 1) in 54% of patients 5 years after TLR.29
There are few retrospective reports of long-term outcomes in patients after FLR or other extratemporal resections. Sustained seizure freedom 5 years after surgery varies in these studies from 15%30 and 27%31 to 52%,32 which can be compared to 31% (adults) vs 36% (children) in our cohort of 86 patients with varying extratemporal resections.
Long-term reports from population-based cohorts are sparse. In a retrospective cross-sectional comprehensive audit from Ireland, national long-term seizure outcome data were reported. Forty-four percent of patients were seizure-free 10 years after resective surgery, but the total number of operated patients could not be accounted for.33 A recent Norwegian retrospective questionnaire study in children with a response rate of 70% reported 58% seizure-free after a mean of 7 years.23 However, sustained seizure freedom was not reported in these studies.
In a recent systematic review, 20 studies comparing surgical results to those of a nonsurgical control group were identified; 13 had a follow-up of at least 4 years.34 The controls were in general patients evaluated for surgery who for various reasons were not operated. Overall 44% of surgical patients were seizure-free at follow-up (in 90% of the studies, seizure freedom was reported at last follow-up or last year) compared to 12% of medically treated patients. This is well in line with our findings of 58% seizure-free at last year of follow-up in the surgical group and 17% in the nonsurgical group, children and adults together. It must be remembered that patients who after preoperative investigations are considered ineligible for epilepsy surgery are not comparable to surgically treated patients, and they may have another disease course. It is difficult though to identify other reasonable controls for long-term follow-up studies, and from a clinical point of view they match the operated patients in many baseline variables.
Predictors for seizure freedom in the long term (≥4 years) have been sought by several investigators. While some found no remaining predictors in multivariate analysis,21 the most commonly identified predictors are SGTCS at baseline4,35 and epilepsy duration36,37 or age at surgery.5,32 We found high baseline seizure frequency and relative epilepsy duration to be negatively and positive MRI to be positively related with long-term seizure freedom. The one factor possible to influence—epilepsy duration before undertaking presurgical investigation—has repeatedly been shown not to have shortened significantly over the years.2,11 These results from long-term outcome studies underline the importance of earlier identification of good candidates for resective epilepsy surgery.
There is no common Swedish protocol for discontinuation of AEDs in seizure-free patients. Most adults continue AEDs the first 2 postoperative years; further decisions about withdrawal are taken individually. We found that 43% of seizure-free adults and as many as 86% of seizure-free children had discontinued AEDs after 10 years. The reasons for this difference are unknown; possibly, social consequences of seizure relapse are of greater importance to adults (e.g., driving or occupation), who therefore may choose to continue AEDs. By contrast, more concern about adverse effects of AEDs on the developing brain in children may lead to earlier decisions about withdrawal.
The proportion of seizure-free adults and children in whom AEDs have been withdrawn after successful epilepsy surgery varies widely across studies. In a meta-analysis from 2007, 9 studies were identified and a pooled analysis showed that 27% of seizure-free children and 19% of seizure-free adults had discontinued AEDs at a mean follow-up of 7 years.38 However, in an Indian study, AED withdrawal was systematically planned for all seizure-free patients after TLR and was successful in 63% of 258 patients who were followed for ≥5 years.39 Very similar to our results in children, a Canadian study found that 82% of seizure-free children had stopped AEDs 10 years after surgery.24 Possible reasons for these differences in patient decisions about AED cessation may include socioeconomic and cultural differences, as well as how doctors inform their patients, which partly depends on their own assessment of recurrence risk.40 The TimeToStop study showed that early withdrawal of AED in children did not influence the long-term seizure outcome but could unmask incomplete surgical success sooner, and the authors suggest that it is now time to plan an RCT of AED withdrawal after epilepsy surgery in children.8
Our study had several limitations. Like most, if not all, observational outcome studies after epilepsy surgery, we had no masking in seizure outcome assessment. Our nonoperated control group was recruited from the 3 largest of the Swedish epilepsy surgery centers and is hence not a national sample. On the other hand, we have a regional referral system and it is reasonable to assume that this sample is representative of the population of patients who are evaluated for surgery in Sweden but not operated.
Patients need detailed counseling before deciding to undergo irrevocable brain surgery. Realistic expectations concerning long-term outcomes are part of the information they need to consider. This prospective population-based study contributes to this knowledge and supports seizure outcome data from prospective single-center studies, and also shows that a greater proportion of seizure-free patients than in most studies had stopped AED treatment 10 years after surgery.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Mattias Molin for statistical assistance, epilepsy nurse Gerd Ekstedt for assistance with data, and all the Swedish epilepsy surgery teams (in Lund, Göteborg, Linköping, Stockholm, Uppsala, and Umeå) and the steering committee of the Swedish National Epilepsy Surgery Register.
GLOSSARY
- AED
antiepileptic drug
- AUC
area under receiver operating characteristic curve
- CI
confidence interval
- FLR
frontal lobe resection
- HS
hippocampal sclerosis
- ILAE
International League Against Epilepsy
- OR
odds ratio
- RCT
randomized controlled trials
- SGTCS
secondarily generalized tonic-clonic seizures
- SNESUR
Swedish National Epilepsy Surgery Register
- TLR
temporal lobe resection
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Edelvik has analyzed and interpreted the data and written the manuscript. Dr. Rydenhag has analyzed and interpreted the data and revised the manuscript. Dr Olsson has interpreted the data and revised the manuscript. Dr. Flink is manager of the Swedish Epilepsy Surgery Register and assisted with data analysis. Dr. Kumlien and Dr. Källén have analyzed and interpreted the data concerning the nonoperated controls. Dr. Malmgren has designed the study, interpreted data, and written the manuscript.
STUDY FUNDING
Funded by grants from the Swedish Research Council (grant 521-2011-169), the Sahlgrenska Academy at Gothenburg University through the LUA/ALF agreement (grant ALFGBG137431), the Gothenburg Foundation for Neurological Research, the Gothenburg Medical Society, the Margaretahem Foundation, and an unconditional research grant from GlaxoSmithKline to Anna Edelvik.
DISCLOSURE
A. Edelvik received speaker honoraria from UCB and Eisai. B. Rydenhag, I. Olsson, and R. Flink report no disclosures. E. Kumlien served on a scientific advisory board for GlaxoSmithKline. K. Källén served on scientific advisory boards for Eisai and GlaxoSmithKline. K. Malmgren served on an educational advisory board for UCB and has received speaker's honoraria from UCB. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311–318 [DOI] [PubMed] [Google Scholar]
- 2.Engel J, Jr, McDermott MP, Wiebe S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 2012;307:922–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain 2005;128:1188–1198 [DOI] [PubMed] [Google Scholar]
- 4.McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, Berkovic SF. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain 2004;127:2018–2030 [DOI] [PubMed] [Google Scholar]
- 5.de Tisi J, Bell GS, Peacock JL, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet 2011;378:1388–1395 [DOI] [PubMed] [Google Scholar]
- 6.Luoni C, Bisulli F, Canevini MP, et al. Determinants of health-related quality of life in pharmacoresistant epilepsy: results from a large multicenter study of consecutively enrolled patients using validated quantitative assessments. Epilepsia 2011;52:2181–2191 [DOI] [PubMed] [Google Scholar]
- 7.Wilson SJ, Saling MM, Kincade P, Bladin PF. Patient expectations of temporal lobe surgery. Epilepsia 1998;39:167–174 [DOI] [PubMed] [Google Scholar]
- 8.Boshuisen K, Arzimanoglou A, Cross JH, et al. Timing of antiepileptic drug withdrawal and long-term seizure outcome after paediatric epilepsy surgery (TimeToStop): a retrospective observational study. Lancet Neurol 2012;11:784–791 [DOI] [PubMed] [Google Scholar]
- 9.Rydenhag B, Silander HC. Complications of epilepsy surgery after 654 procedures in Sweden, September 1990-1995: a multicenter study based on the Swedish National Epilepsy Surgery Register. Neurosurgery 2001;49:51–56; discussion 56–57 [DOI] [PubMed] [Google Scholar]
- 10.Malmgren K, Olsson I, Engman E, Flink R, Rydenhag B. Seizure outcome after resective epilepsy surgery in patients with low IQ. Brain 2008;131:535–542 [DOI] [PubMed] [Google Scholar]
- 11.Rydenhag B, Flink R, Malmgren K. Surgical outcomes in patients with epileptogenic tumours and cavernomas in Sweden: good seizure control but late referrals. J Neurol Neurosurg Psychiatry 2013;84:49–53 [DOI] [PubMed] [Google Scholar]
- 12.Wieser HG, Blume WT, Fish D, et al. ILAE Commission Report: proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia 2001;42:282–286 [PubMed] [Google Scholar]
- 13.Beghi E, Tonini C. Surgery for epilepsy: assessing evidence from observational studies. Epilepsy Res 2006;70:97–102 [DOI] [PubMed] [Google Scholar]
- 14.Engel J. Outcome with respect to epileptic seizures. In: Surgical Treatment of the Epilepsies. New York: Raven Press; 1993:609–621 [Google Scholar]
- 15.Cohen-Gadol AA, Wilhelmi BG, Collignon F, et al. Long-term outcome of epilepsy surgery among 399 patients with nonlesional seizure foci including mesial temporal lobe sclerosis. J Neurosurg 2006;104:513–524 [DOI] [PubMed] [Google Scholar]
- 16.Elsharkawy AE, Alabbasi AH, Pannek H, et al. Long-term outcome after temporal lobe epilepsy surgery in 434 consecutive adult patients. J Neurosurg 2009;110:1135–1146 [DOI] [PubMed] [Google Scholar]
- 17.Mihara T, Matsuda K, Tottori T, et al. Long-term seizure outcome following resective surgery at National Epilepsy Center in Shizuoka, Japan. Psychiatry Clin Neurosci 2004;58:S22–S25 [DOI] [PubMed] [Google Scholar]
- 18.Dupont S, Tanguy ML, Clemenceau S, Adam C, Hazemann P, Baulac M. Long-term prognosis and psychosocial outcomes after surgery for MTLE. Epilepsia 2006;47:2115–2124 [DOI] [PubMed] [Google Scholar]
- 19.Sindou M, Guenot M, Isnard J, Ryvlin P, Fischer C, Mauguiere F. Temporo-mesial epilepsy surgery: outcome and complications in 100 consecutive adult patients. Acta Neurochir 2006;148:39–45 [DOI] [PubMed] [Google Scholar]
- 20.Paglioli E, Palmini A, da Costa JC, et al. Survival analysis of the surgical outcome of temporal lobe epilepsy due to hippocampal sclerosis. Epilepsia 2004;45:1383–1391 [DOI] [PubMed] [Google Scholar]
- 21.Aull-Watschinger S, Pataraia E, Czech T, Baumgartner C. Outcome predictors for surgical treatment of temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 2008;49:1308–1316 [DOI] [PubMed] [Google Scholar]
- 22.Jeha LE, Najm IM, Bingaman WE, et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology 2006;66:1938–1940 [DOI] [PubMed] [Google Scholar]
- 23.Aaberg K, Eriksson AS, Ramm-Pettersen J, Nakken K. Long-term outcome of resective epilepsy surgery in Norwegian children. Acta Paediatr 2012;101:e557–e560 [DOI] [PubMed] [Google Scholar]
- 24.Benifla M, Rutka JT, Otsubo H, et al. Long-term seizure and social outcomes following temporal lobe surgery for intractable epilepsy during childhood. Epilepsy Res 2008;82:133–138 [DOI] [PubMed] [Google Scholar]
- 25.Boshuisen K, Braams O, Jennekens-Schinkel A, et al. Medication policy after epilepsy surgery. Pediatr Neurol 2009;41:332–338 [DOI] [PubMed] [Google Scholar]
- 26.Mittal S, Montes JL, Farmer JP, et al. Long-term outcome after surgical treatment of temporal lobe epilepsy in children. J Neurosurg 2005;103:401–412 [DOI] [PubMed] [Google Scholar]
- 27.Sinclair DB, Aronyk KE, Snyder TJ, et al. Pediatric epilepsy surgery at the University of Alberta: 1988-2000. Pediatr Neurol 2003;29:302–311 [DOI] [PubMed] [Google Scholar]
- 28.Van Oijen M, De Waal H, Van Rijen PC, Jennekens-Schinkel A, van Huffelen AC, Van Nieuwenhuizen O. Resective epilepsy surgery in childhood: the Dutch experience 1992-2002. Eur J Paediatr Neurol 2006;10:114–123 [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Gonzalez MA, Gonzalez-Martinez JA, Jehi L, Kotagal P, Warbel A, Bingaman W. Epilepsy surgery of the temporal lobe in pediatric population: a retrospective analysis. Neurosurgery 2012;70:684–692 [DOI] [PubMed] [Google Scholar]
- 30.McIntosh AM, Averill CA, Kalnins RM, et al. Long-term seizure outcome and risk factors for recurrence after extratemporal epilepsy surgery. Epilepsia 2012;53:970–978 [DOI] [PubMed] [Google Scholar]
- 31.Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Luders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain 2007;130:574–584 [DOI] [PubMed] [Google Scholar]
- 32.Elsharkawy AE, Alabbasi AH, Pannek H, et al. Outcome of frontal lobe epilepsy surgery in adults. Epilepsy Res 2008;81:97–106 [DOI] [PubMed] [Google Scholar]
- 33.Dunlea O, Doherty CP, Farrell M, et al. The Irish epilepsy surgery experience: long-term follow-up. Seizure 2010;19:247–252 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt D, Stavem K. Long-term seizure outcome of surgery versus no surgery for drug-resistant partial epilepsy: a review of controlled studies. Epilepsia 2009;50:1301–1309 [DOI] [PubMed] [Google Scholar]
- 35.Schwartz TH, Jeha L, Tanner A, Bingaman W, Sperling MR. Late seizures in patients initially seizure free after epilepsy surgery. Epilepsia 2006;47:567–573 [DOI] [PubMed] [Google Scholar]
- 36.Menon R, Rathore C, Sarma SP, Radhakrishnan K. Feasibility of antiepileptic drug withdrawal following extratemporal resective epilepsy surgery. Neurology 2012;79:770–776 [DOI] [PubMed] [Google Scholar]
- 37.Simasathien T, Vadera S, Najm I, Gupta A, Bingaman W, Jehi L. Improved outcomes with earlier surgery for intractable frontal lobe epilepsy. Ann Neurol 2013;73:646–654 [DOI] [PubMed] [Google Scholar]
- 38.Tellez-Zenteno JF, Dhar R, Hernandez-Ronquillo L, Wiebe S. Long-term outcomes in epilepsy surgery: antiepileptic drugs, mortality, cognitive and psychosocial aspects. Brain 2007;130:334–345 [DOI] [PubMed] [Google Scholar]
- 39.Rathore C, Panda S, Sarma PS, Radhakrishnan K. How safe is it to withdraw antiepileptic drugs following successful surgery for mesial temporal lobe epilepsy? Epilepsia 2011;52:627–635 [DOI] [PubMed] [Google Scholar]
- 40.Jacoby A, Baker G, Chadwick D, Johnson A. The impact of counselling with a practical statistical model on patient's decision-making about treatment for epilepsy: findings from a pilot study. Epilepsy Res 1993;16:207–214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.