Abstract
Objective:
To determine the consistency and facilitating cofactors of postictal generalized EEG suppression (PGES) of >20 seconds after convulsive seizures (CS), a suggested predictor of sudden unexpected death in epilepsy risk.
Methods:
We retrospectively reviewed video-EEG data of people with ≥2 recorded CS. Presence and duration of PGES were assessed by 2 independent observers blinded to patient status. Intraindividual consistency of PGES >20 seconds was determined and correlations with clinical characteristics were analyzed after correction for individual effects and the varying number of seizures.
Results:
One hundred fifty-four seizures in 59 people were analyzed. PGES >20 seconds was found in 37 individuals (63%) and 57 (37%) of CS. The proportion of persons in whom PGES occurred consistently (presence or absence of PGES >20 seconds in all CS) was lower in those with more CS. PGES of >20 seconds was more frequent in seizures arising from sleep (odds ratio 3.29, 95% confidence interval 1.21–8.96) and when antiepileptic medication was tapered (odds ratio 4.80, 95% confidence interval 1.27–18.14).
Conclusion:
Apparent PGES consistency was less frequent in people with more CS recorded, suggesting that PGES is an inconsistent finding in any one individual. Thus, we believe that PGES >20 seconds is not a reliable predictor of sudden unexpected death in epilepsy. Sleep and antiepileptic drug reduction appear to facilitate the occurrence of PGES.
Sudden unexpected death in epilepsy (SUDEP) is the most important epilepsy-related cause of premature death.1–3 In most ictal recordings of SUDEP, postictal generalized EEG suppression (PGES), followed by apnea and asystole, appears to be an EEG hallmark.2
The mechanism of PGES is not well understood.2 PGES occurs more frequently after convulsive seizures (CS),4,5 and is associated with more severe peri-ictal hypoxemia and postictal coma.6–9 Seizure duration is not related to PGES,4,5,7,9–11 but a longer duration of the tonic phase has been suggested to facilitate PGES.9 We found that PGES occurs more frequently after CS arising from sleep.11 Nocturnal CS increase SUDEP risk,12 thus adding to the evidence that PGES may be a biomarker for SUDEP. PGES was associated with older age at seizure onset11; this might be explained by differences in epilepsy etiology, but requires further study.13 Antiepileptic drug (AED) changes may affect occurrence of PGES: introduction of levetiracetam after AED withdrawal reduced severity of postictal EEG suppression and coma.14
It remains unclear whether PGES is a predictor of SUDEP. PGES >20 seconds after CS was associated with higher SUDEP risk, which increased proportionally with PGES duration.4 These findings, however, could not be replicated in a larger study.5 High intraindividual variability of PGES >20 seconds may explain these conflicting results. We addressed PGES consistency in people with multiple CS and analyzed cofactors that facilitate occurrence of PGES >20 seconds, including state of wakefulness, age of seizure onset, and AED tapering.
METHODS
Sample selection.
We reviewed reports of digital video-EEGs from 2 tertiary epilepsy referral centers (Bonn and Heemstede) from the period 2003 to 2011 and selected people 15 years and older who had 2 or more CS recorded on long-term monitoring. The study size was determined by the number of available video-EEGs. We identified 170 CS in 64 individuals. EEG recordings with less than 1-minute postictal recording time (n = 13) and those of insufficient quality because of lead disconnection (n = 3) were discarded, leaving 59 people with 154 CS (19 with 47 seizures from Heemstede and 40 with 107 seizures from Bonn). The median number of monitoring days was 5 (range 1–13).
Collection of variables.
We collected the following data: sex, age, presence of mental retardation (yes/no), epilepsy classification (symptomatic or cryptogenic/idiopathic), lesion on MRI (yes/no), age at onset, duration of epilepsy, frequency of CS, AED regimen (before admission and for each subsequent monitoring day), state of wakefulness before seizure onset (awake/asleep), sleep stage before seizure onset (rapid eye movement, non–rapid eye movement 1–3), localization of EEG seizure onset (temporal/extratemporal), and duration of the tonic and tonic-clonic phase as well as entire seizure duration. Tapering was defined as any reduction of the total AED load during the recording period, compared with the regimen before admission. The overall drug load (before admission and each subsequent monitoring day) was estimated as the sum of the prescribed daily dose/defined daily dose ratios for each AED.15 Heart rate (HR) was measured during the last minute before seizure onset and the first minute after seizure end.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the ethics committees at both sites; because of its observational nature, informed consent was not required at either site.
Evaluation of PGES >20 seconds and duration of the tonic phase.
Conventional scalp EEG recordings (International 10–20 System) (Stellate Harmonie, Stellate Systems, Montreal, Canada; Schwarzer GmbH/Natus, Germany) were performed at a sampling rate of 200 Hz. A modified lead-I ECG (Stellate Harmonie; Schwarzer GmbH/Natus) (adhesive electrode[s] placed below the clavicle) was simultaneously recorded. Random numbers were assigned to all individual seizures to blind patient status. Two board-certified clinical neurophysiologists (A.G., R.D.T.) independently analyzed the presence or absence of PGES >20 seconds and duration of the tonic phase in all CS.
PGES was defined as the immediate postictal (within 30 seconds), generalized absence of EEG activity >10 µV in amplitude, allowing for muscle, movement, breathing, and electrode artifacts.4 Only PGES of >20 seconds after CS were scored because these were previously associated with increased SUDEP risk.4 Because of privacy rules, video-recordings from Bonn could not be evaluated off-site; thus, PGES was only assessed using EEG recordings in this site, but in Heemstede, EEG and video were assessed. In 2 CS from Bonn, PGES based on EEG alone could not be scored because of uncertainty about the presence of movement artifacts, so a third clinical neurophysiologist (R.S.) analyzed both EEG and video for a final decision. In the remaining 152 CS, PGES was evaluated by 2 examiners; in case of disagreement, the examiners discussed to reach consensus. Interobserver agreement on PGES >20 seconds was good: Cohen κ = 0.77.
The onset of the tonic phase was defined as the occurrence of bilateral, symmetrical or asymmetrical, continuous muscle activity obscuring EEG background activity. Muscle activity was evaluated in the frontotemporal regions using a bipolar montage. The end of the tonic and onset of the clonic phase was defined by a sustained pattern of bilateral and synchronous bursts of muscle artifact with burst intervals ≥150 milliseconds, and absence of muscle activity between bursts.
Statistical analysis.
The proportion of people in whom PGES consistently occurred (presence or absence of PGES >20 seconds in all CS) was calculated and related to the number of recorded CS. Associations between PGES >20 seconds and person- or seizure-specific variables were assessed with Fisher exact probability test, χ2, or Mann-Whitney U tests, where appropriate. A mixed linear regression model was used to determine which variables were independently associated with PGES >20 seconds after correction for individual effects and the varying number of seizures contributed by each person. Only those variables with p < 0.05 at univariate analysis were entered, and adjustments for multiple comparisons were made using the Holms-Bonferroni method. Statistical analysis was performed with SPSS version 17 software (SPSS Inc., Chicago, IL) and STATA 12 software (StataCorp LP, College Station, TX).
RESULTS
Prevalence and intraindividual consistency of PGES >20 seconds.
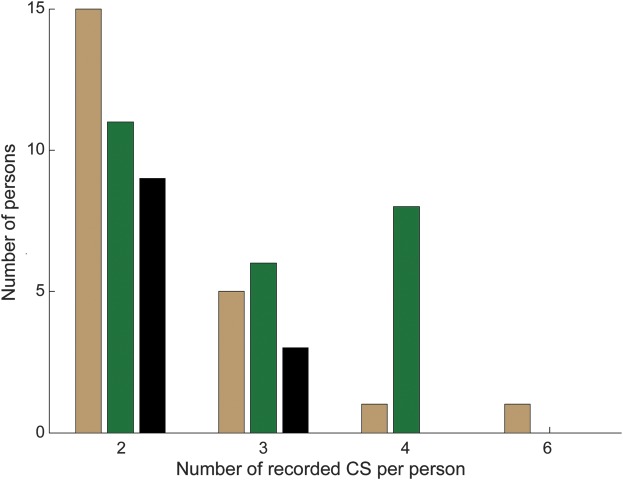
PGES >20 seconds occurred in 63% of individuals (37/59) and in 37% of CS (57/154) (tables 1 and 2). Presence or absence of PGES >20 seconds was a consistent finding in 34 of 59 individuals (presence 12/59; absence 22/59), whereas 25 of 59 individuals had a mixture of CS with and without PGES >20 seconds (figure 1). The number of people with “consistent” results decreased as the number of CS recorded increased (table 1, figure 1).
Table 1.
Characteristics of people with a mixture of CS with and without PGES >20 seconds, and those in whom PGES >20 seconds was either consistently present or absent
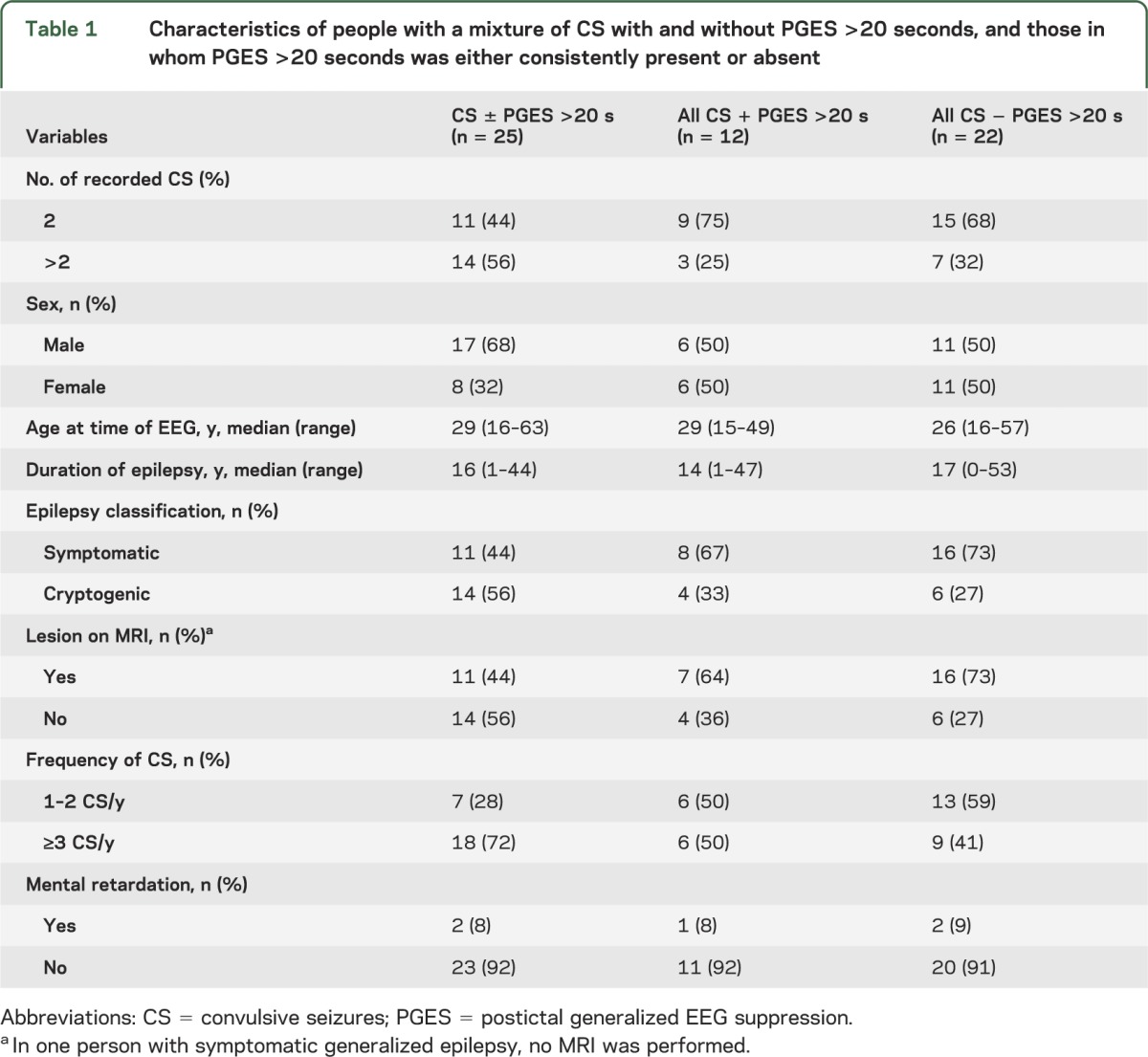
Table 2.
Characteristics of CS with PGES >20 seconds vs CS without PGES >20 seconds
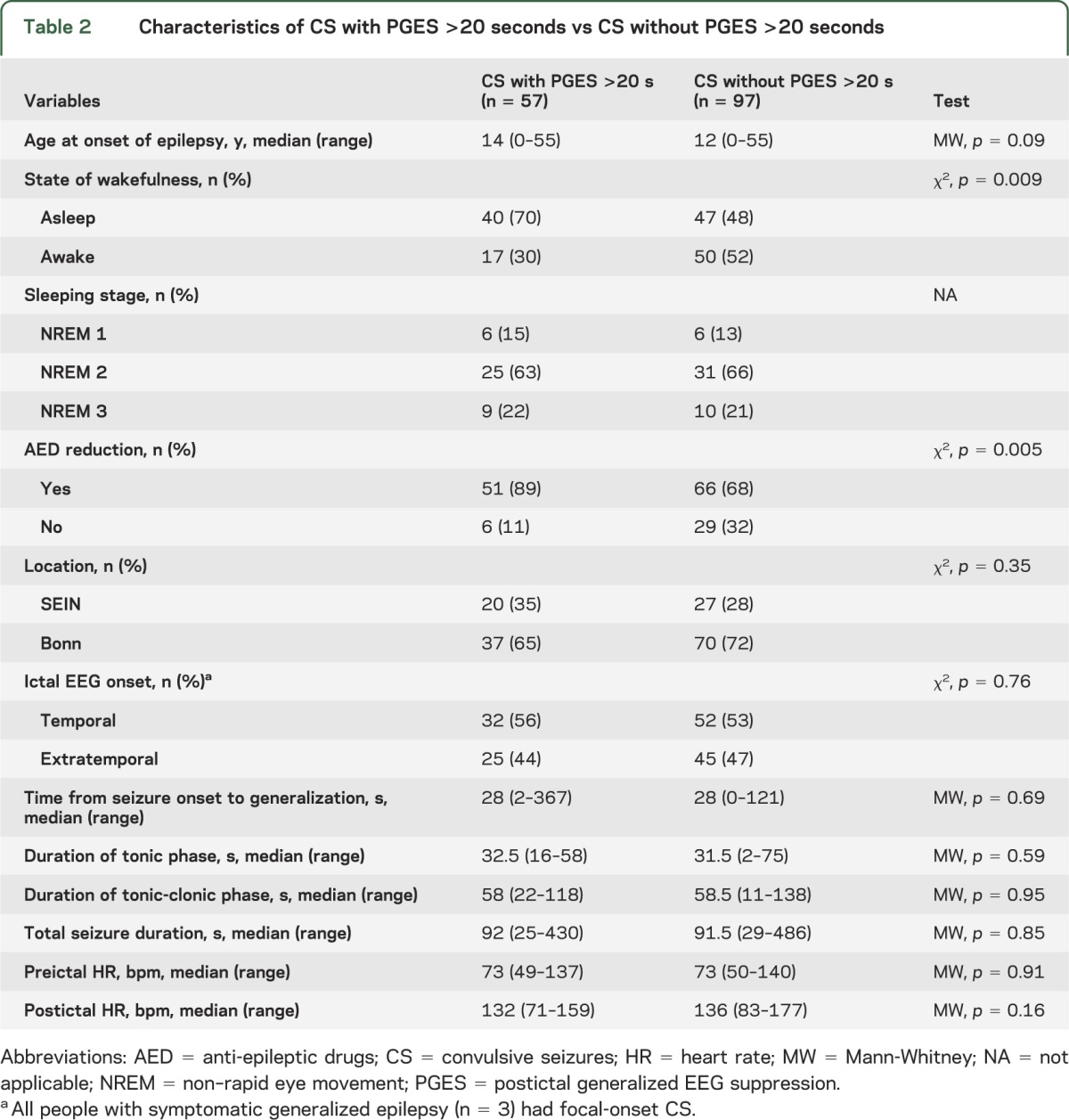
Figure 1. Intraindividual variability of PGES >20 seconds in people with multiple recorded CS.
Most people (25 of 59) had a mixture of convulsive seizures (CS) with and without postictal generalized EEG suppression (PGES) >20 seconds (green bars). In 22 of 59 persons, PGES >20 seconds was consistently absent (tan bars), whereas in 12 of 59 persons, it was consistently present (black bars). Consistency of PGES >20 seconds seemed dependent on the number of recorded CS.
Factors that facilitate the occurrence of PGES >20 seconds.
In univariate analysis, PGES >20 seconds was more frequently found after CS that started from sleep (odds ratio [OR] 2.5, 95% confidence interval [CI] 1.3–5.0) and in CS where medication was tapered (OR 3.7, 95% CI 1.4–9.7). Other person- or seizure-related variables did not differ between groups (tables 1 and 2). In multivariate analysis, the variables “sleep” and “AED reduction” remained independent risk factors for PGES >20 seconds after introduction into a mixed linear regression model: sleep OR 3.29, 95% CI 1.21–8.96; AED reduction OR 4.80, 95% CI 1.27–18.14.
DISCUSSION
We addressed the intraindividual variability of PGES >20 seconds in people with multiple CS and found that PGES consistency depended on the number of seizures recorded per person. Prevalence of PGES was higher in those CS that started from sleep or when medication had been reduced. PGES >20 seconds had limited consistency in people with multiple recorded CS. High intraindividual variability may explain the discrepancy between studies as to the value of PGES as a risk marker for SUDEP.4,5 Interestingly, intraindividual consistency was lower in the study where no link between PGES and SUDEP was found.5 Overall, these findings emphasize that the occurrence of PGES is critically dependent on the number of seizures recorded and therefore unlikely to be a reliable factor to assess SUDEP risk. A link between duration of the tonic phase and PGES has recently been suggested,9 but this was not seen in our study. These conflicting results may be explained by differing seizure selection. In the former study, 18.5% of CS lacked a tonic phase compared with none in our sample.9 Presence of a tonic phase was strongly associated with PGES: OR 180.9 Therefore, the reported link between tonic duration and PGES may predominantly rely on the contrast between seizures with and without a tonic phase. Therefore, we believe that a difference in seizure types rather than a variation in length of the tonic phase may be responsible for the reported association between tonic duration and PGES. We did not find significant differences in peri-ictal HR change between CS with and without PGES >20 seconds, confirming our previous finding.11 In contrast, others reported a lower postictal HR in CS with PGES (119 vs 140 bpm).9 Unfortunately, these cannot be easily compared because in their study no exact definition of HR measurement periods was given and all CS with PGES were included, even those of shorter duration.9 If PGES is associated with postictal HR deceleration, more pronounced HR changes would be expected in our study as we focused on CS with prolonged PGES. It therefore seems unlikely that PGES reflects a primary pathomechanism leading to asystole and SUDEP. Given the high intraindividual variation of PGES, especially when assessing multiple seizures per person, it is unlikely that a person-specific variable will predict PGES occurrence. This may explain why we did not find an association between age of seizure onset and PGES, unlike in our previous study where only one CS per person was analyzed.11 PGES was scored differently in CS from Heemstede (EEG and video) and those from Bonn (EEG only), which may have introduced bias; we consider this unlikely because the prevalence of PGES >20 seconds was similar in both centers (table 2). All EEG studies were scored independently by 2 observers with good interobserver agreement. The effect of peri-ictal hypoxemia on PGES could not be analyzed because we lacked concurrent oximetry measurements. Underlying peri-ictal hypoxemia may possibly explain the link between sleep and PGES. Currently, it remains unclear whether hypoxemia is more severe during CS arising from sleep. An earlier study of seizure-related respiratory dysfunction did not find a higher prevalence of ictal hypoxemia in nocturnal seizures, but mostly partial seizures were analyzed.16 Similarly, underlying ictal hypoxemia may explain the association between AED reduction and PGES. This seems unlikely, however, because no link between AED withdrawal and ictal hypoxemia was found in a systematic analysis of seizure-related respiratory dysfunction.16 Ideally, an analysis of AED withdrawal would take into account the amount and speed of AED reduction. In our study, however, because of its retrospective nature and the great variety of AEDs and withdrawal regimens, such an analysis was not feasible. There are several theories regarding PGES etiology. Neuronal exhaustion has been proposed as an underlying mechanism.17 This explanation seems unlikely because seizure duration is not associated with PGES.4,5,7,9–11 It has also been suggested that PGES triggers respiratory or cardiac dysfunction. However, PGES was not associated with postictal apnea, but with preceding seizure-induced oxygen desaturation.7 Ictal hypoxemia and PGES duration were shorter with earlier intervention after seizure onset.8 CS with PGES are associated with a longer period of postictal immobility.6,8,9 During this period of unconsciousness, the person may not be able to respond appropriately to seizure-induced hypoxemia (e.g., by changing body position), thus exacerbating the cerebral oxygen deficit. Others have proposed that PGES reflects increased activity of inhibitory neuronal networks in response to continuing seizure activity.4 Because sleep is also related to inhibitory neuronal network activation, this may explain the relation between sleep and PGES.18 This association further supports the link between sleep and SUDEP; most victims die during sleep and nocturnal CS are a risk factor for SUDEP.2,12 The use of AEDs may modulate the seizure-related inhibitory neuronal network activation,14 and AED reduction in the epilepsy monitoring unit can lead to an increase in seizure intensity and frequency.19,20 It could be speculated that changes in these seizure characteristics may provoke an exaggerated termination response by inhibitory neuronal networks. A further exploration of the link between different AED withdrawal schedules, changes in seizure characteristics, and PGES would be of interest.
ACKNOWLEDGMENT
The authors are grateful to S.N. Kalitzin for his assistance with peri-ictal heart rate analysis, and Dr. G.S. Bell for critically reviewing the manuscript.
Glossary
- AED
antiepileptic drug
- CI
confidence interval
- CS
convulsive seizures
- HR
heart rate
- OR
odds ratio
- PGES
postictal generalized EEG suppression
- SUDEP
sudden unexpected death in epilepsy
AUTHOR CONTRIBUTIONS
The study was conceived and designed by R.J.L., R.D.T., R.S., and J.W.S. Data were acquired by A.G., R.D.T., and R.J.L. Data analysis and interpretation by R.J.L., J.W.S., R.S., and R.D.T. The manuscript was drafted by R.J.L., J.W.S., R.S., and R.D.T. All revised and approved the submitted version. J.W.S. and C.E.E. are the guarantors.
STUDY FUNDING
Supported by Christelijke Vereniging voor de Verpleging van Lijders aan Epilepsie (Nederland) and Dutch National Epilepsy Fund (project number 10-07).
DISCLOSURE
R. Lamberts and A. Gaitatzis report no disclosures. J. Sander receives research support from the Dr. Marvin Weil Epilepsy Research Fund, Eisai, GSK, UCB Pharma, World Health Organization, and NIH and has been consulted by and received fees for lectures from GSK, Eisai, and UCB. Dr. Sander is a member of PRISM—the Prevention and Risk Identification of SUDEP Mortality Consortium, which is funded by the NIH (NBIH/NINDS 1P20NS076965-01). C. Elger has been consulted by Desitin and Novartis and received fees for lectures from Pfizer and Eisai. R. Surges has received travel support, fees for lectures from Eisai, and had a consultancy agreement with UCB. Dr. Surges is a member of PRISM—the Prevention and Risk Identification of SUDEP Mortality Consortium, which is funded by the NIH (NBIH/NINDS 1P20NS076965-01). R. Thijs receives research support from NUTS Ohra Fund, Medtronic, and AC Thomson Foundation, and has received fees for lectures from Medtronic. Dr. Thijs is a member of PRISM—the Prevention and Risk Identification of SUDEP Mortality Consortium, which is funded by the NIH (NBIH/NINDS 1P20NS076965-01).
REFERENCES
- 1.Gaitatzis A, Sander JW. The mortality of epilepsy revisited. Epileptic Disord 2004;6:3–13 [PubMed] [Google Scholar]
- 2.Surges R, Thijs RD, Tan HL, Sander JW. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nat Rev Neurol 2009;5:492–504 [DOI] [PubMed] [Google Scholar]
- 3.Surges R, Sander JW. Sudden unexpected death in epilepsy: mechanisms, prevalence, and prevention. Curr Opin Neurol 2012;25:201–207 [DOI] [PubMed] [Google Scholar]
- 4.Lhatoo SD, Faulkner HJ, Dembny K, Trippick K, Johnson C, Bird JM. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol 2010;68:787–796 [DOI] [PubMed] [Google Scholar]
- 5.Surges R, Strelczyk A, Scott CA, Walker MC, Sander JW. Postictal generalized electric encephalographic suppression is associated with generalized seizures. Epilepsy Behav 2011;21:271–274 [DOI] [PubMed] [Google Scholar]
- 6.Semmelroch M, Elwes RDC, Lozsadi DA, Nashef L. Retrospective audit of postictal generalized EEG suppression in telemetry. Epilepsia 2012;53:e21–e24 [DOI] [PubMed] [Google Scholar]
- 7.Seyal M, Hardin KA, Bateman LM. Postictal generalized EEG suppression is linked to seizure-associated respiratory dysfunction but not postictal apnea. Epilepsia 2012;53:825–831 [DOI] [PubMed] [Google Scholar]
- 8.Seyal M, Bateman LM, Li CS. Impact of periictal interventions on respiratory dysfunction, postictal EEG suppression, and postictal immobility. Epilepsia 2013;54:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao JX, Yung I, Lee A, Rose S, Jacobsen J, Ebersole JS. Tonic phase of a generalized convulsive seizure is an independent predictor of postictal generalized EEG suppression. Epilepsia 2013;54:858–865 [DOI] [PubMed] [Google Scholar]
- 10.Poh MZ, Loddenkemper T, Reinsberger C, et al. Autonomic changes with seizures correlate with postictal EEG suppression. Neurology 2012;78:1868–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamberts RJ, Laranjo S, Kalitzin SN, et al. Postictal generalized EEG suppression is not associated with peri-ictal cardiac autonomic instability in people with convulsive seizures. Epilepsia 2013;54:523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamberts RJ, Thijs RD, Laffan A, Langan Y, Sander JW. Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk. Epilepsia 2012;53:253–257 [DOI] [PubMed] [Google Scholar]
- 13.Kim AJ, Kuroda MM, Nordli DR. Abruptly attenuated terminal ictal pattern in pediatrics. J Clin Neurophysiol 2006;23:532–550 [DOI] [PubMed] [Google Scholar]
- 14.Tilz C, Stefan H, Hopfengaertner R, Kerling F, Genow A, Wang-Tilz Y. Influence of levetiracetam on ictal and postictal EEG in patients with partial seizures. Eur J Neurol 2006;13:1352–1358 [DOI] [PubMed] [Google Scholar]
- 15.Canevini MP, De Sarro G, Galimberti CA, et al. Relationship between adverse effects of antiepileptic drugs, number of coprescribed drugs, and drug load in a large cohort of consecutive patients with drug-refractory epilepsy. Epilepsia 2010;51:797–804 [DOI] [PubMed] [Google Scholar]
- 16.Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain 2008;131:3239–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLean BN, Wimalaratna S. Sudden death in epilepsy recorded in ambulatory EEG. J Neurol Neurosurg Psychiatry 2007;78:1395–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steriade M, McCormick DA, Sejnowski D. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993;262:679–685 [DOI] [PubMed] [Google Scholar]
- 19.Zhou D, Wang Y, Hopp P, et al. Influence on ictal seizure semiology of rapid withdrawal of carbamazepine and valproate in monotherapy. Epilepsia 2002;43:386–393 [DOI] [PubMed] [Google Scholar]
- 20.Wang-Tilz Y, Tilz C, Wang B, Pauli E, Koebnick C, Stefan H. Changes of seizure activity during rapid withdrawal of lamotrigine. Eur J Neurol 2005;12:280–288 [DOI] [PubMed] [Google Scholar]