Abstract
Objective:
To evaluate the association between migraine without aura (MO) and migraine with aura (MA) and 3 types of structural brain abnormalities detected by MRI: white matter abnormalities (WMAs), infarct-like lesions (ILLs), and volumetric changes in gray and white matter (GM, WM) regions.
Methods:
PubMed as well as the reference lists of identified studies and reviews were used to identify potentially eligible studies through January 2013. Candidate studies were reviewed and eligible studies were abstracted. Pooled odds ratios (OR) and 95% confidence intervals (CI) were calculated for WMAs and ILLs.
Results:
Six population-based and 13 clinic-based studies were identified. The studies suggested that structural brain changes, including WMAs, silent ILLs, and volumetric changes in GM and WM regions, were more common in migraineurs than in control groups. The results were strongest for MA. The meta-analysis of WMAs showed an association for MA (OR 1.68; 95% CI 1.07–2.65; p = 0.03) but not for MO (OR 1.34; 95% CI 0.96–1.87; p = 0.08). The association of ILLs was greater for MA (OR 1.44; 95% CI 1.02–2.03; p = 0.04) than for MO, but no association was found for MA (p = 0.52) and MO (p = 0.08) compared to controls.
Conclusion:
These data suggest that migraine may be a risk factor for structural changes in the brain. Additional longitudinal studies are needed to determine the differential influence of migraine without and with aura, to better characterize the effects of attack frequency, and to assess longitudinal changes in brain structure and function.
Migraine is a common neurologic disorder, characterized by paroxysmal attacks of unilateral throbbing headache and autonomic nervous system dysfunction. About one-third of migraineurs experience transient neurologic symptoms known as auras, which characterize a variant known as migraine with aura (MA).1 Migraine affects about 10%–15% of the general population and is associated with a substantial personal and social burden.2–4 Migraine and other headache disorders account for about 20% of outpatient visits to neurologists.5
Traditionally, migraine has been considered a benign disorder without long-term consequences for the brain. Neurologists usually image patients with migraine to exclude secondary causes of headache. These imaging procedures often reveal white matter abnormalities (WMAs) and not uncommonly reveal infarct-like lesions (ILLs), which may be a source of concern for both neurologists and patients.6 Emerging data report that migraineurs are at increased risk for clinically silent brain lesions such as WMAs,7–14 ILLs,10,13–16 and volumetric changes in gray and white matter (GM, WM) regions,17–24 detected on MRI. The pathogenesis and clinical significance of these abnormalities is unclear. These abnormalities are reported to increase with migraine frequency, which may represent a form of anatomic progression of the disorder.10,25,26
We summarize data on the association of MA and migraine without aura (MO) with 3 forms of structural brain abnormalities, WMAs, ILLs, and volumetric changes, and conduct meta-analysis comparing imaging findings in MA and MO. This review will help neurologists provide a context for interpreting these abnormalities in clinical practice.
METHODS
Search strategy and selection criteria.
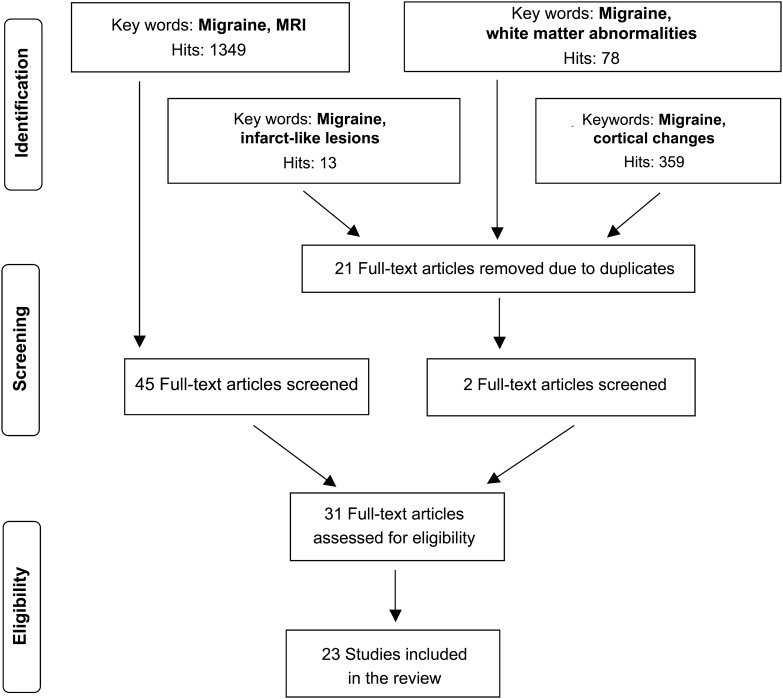
We searched on PubMed for eligible articles investigating MRI abnormalities in migraineurs, based on title, in the period 1989–2013. The search terms were “white matter abnormalities,” “infarct-like lesions,” “cortical changes,” and “MRI” in combination with “migraine” (figure 1). The search was limited to English-language publications and studies of humans. We also reviewed the reference lists of relevant primary articles and reviews to identify studies that may have been missed in the search. All articles were screened for content, methodology, and design. Eligible studies included original studies with a case-control, cross-sectional, and cohort design with collected structural data using MRI on a migraine sample and a contemporaneous control group. Diagnostic criteria for migraine were carefully reviewed. Most studies that we included used the International Classification of Headache Disorders (ICHD-I and ICHD-II) for MO and MA. One study16 had less specific headache case definition of MA. We included it because the definition was clear and misclassification would likely, if anything, attenuate measured associations. We included the following imaging techniques: T1- and T2-weighted and fluid-attenuated inversion recovery MRI, diffusion tensor imaging (DTI), and voxel-based morphometry (VBM). Studies performed at 1.0 to 3.0 T were included. We excluded studies performed at 0.5 T as well as functional MRI and PET.
Figure 1. PubMed search for studies of migraine and structural brain changes (1989–2013).
Definition of structural changes and statistics.
The sections on definition of structural changes in the brain and statistics (meta-analysis) are available online as supplemental files (appendices e-1 and e-2 on the Neurology® Web site at www.neurology.org).
RESULTS
The PubMed search identified 13 clinic-based and 6 population-based studies (4 studies were based on the same cohort). We present the results focusing first on the clinic-based and then population-based studies by type of structural brain changes in tables 1–3 and table e-1.
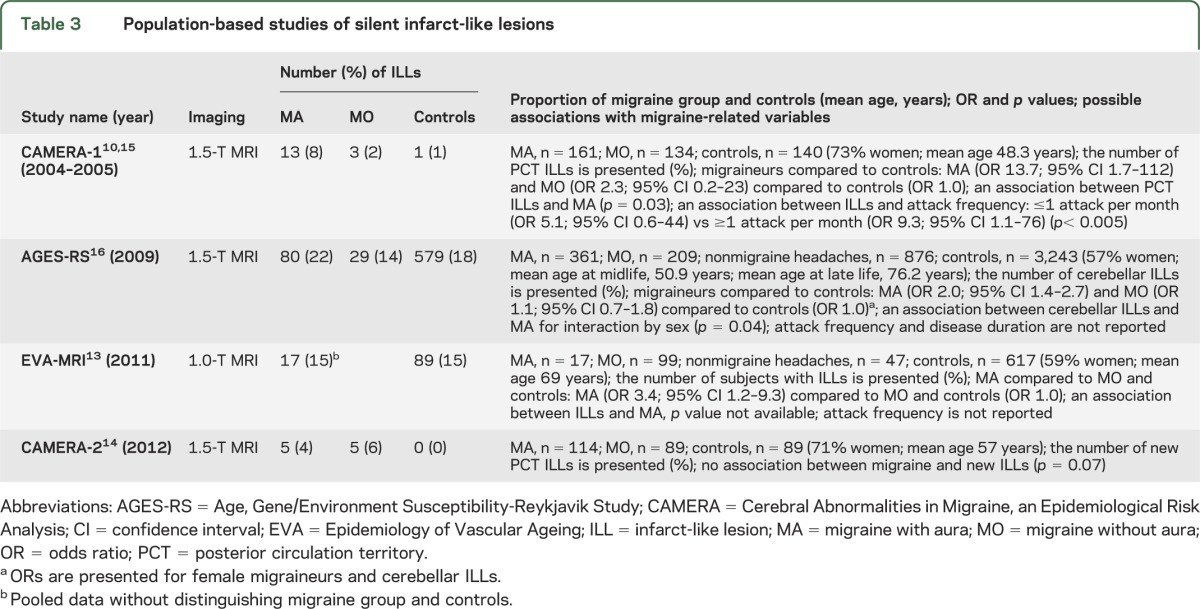
Table 1.
Clinic-based studies of white matter abnormalities
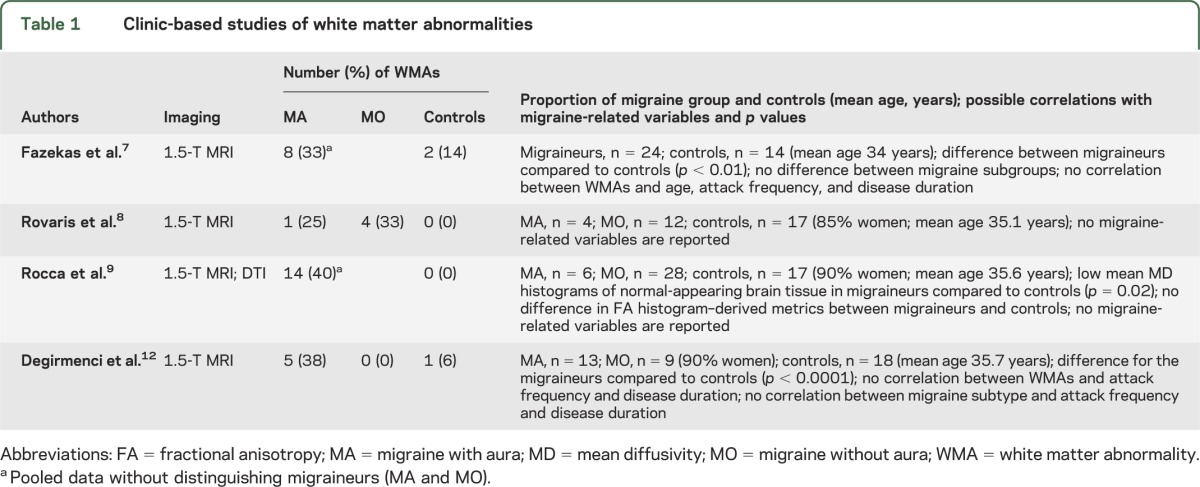
Table 2.
Population-based studies of white matter abnormalities
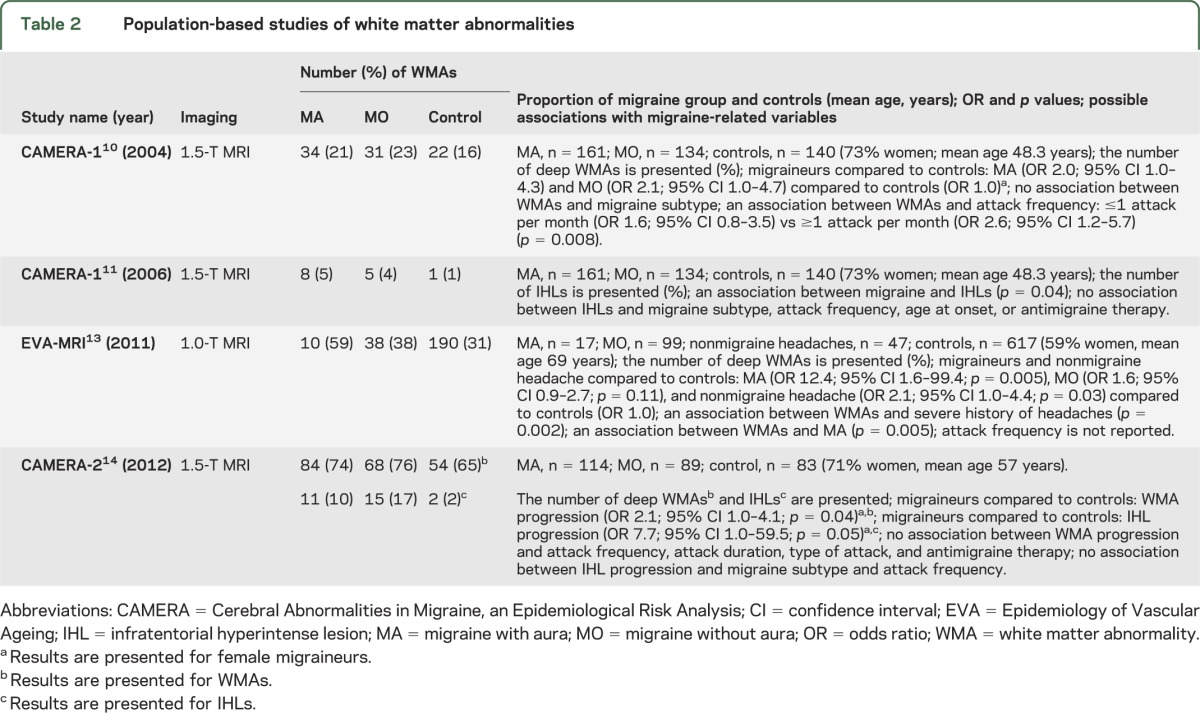
Table 3.
Population-based studies of silent infarct-like lesions
Studies of WMAs.
Only 4 clinic-based studies of WMAs7–9,12 met criteria for inclusion (table 1). All investigated the association between migraine and WMAs on MRI and reported that WMAs were more common in migraineurs than in controls. Two studies examined the influence of migraine-related variables (i.e., age, migraine subtype, attack frequency, disease duration) and did not find any association with the frequency of WMAs.7,12
Of the population-based studies (table 2), the Cerebral Abnormalities in Migraine, an Epidemiological Risk Analysis 1 (CAMERA-1) study was the first.10 The authors investigated the prevalence of WMAs in 295 migraineurs and 140 age- and sex-matched controls, who were randomly selected from the Genetic Epidemiology of Migraine study.27 In women, the prevalence of deep WMAs was higher in migraineurs than controls. The association was independent of the presence or absence of aura and the risk increased with attack frequency (p = 0.008). In men, deep WMAs were not influenced by the presence, subtype, or frequency of migraine. In a subsequent analysis of the same cohort,11 the authors found hyperintense lesions in the cerebellum and brainstem, mostly in the dorsal pons, referred to as infratentorial hyperintense lesions (IHLs). IHLs often were associated with supratentorial WMAs and were not associated with migraine subtype, attack frequency, or cerebellar infarcts. In a 9-year follow-up study, CAMERA-2,14 the authors investigated the association between attack frequency and progression of brain lesions. They obtained follow-up scans in 203 of 295 migraineurs and 83 of 140 controls. The study found that women with migraine, especially MO, had a higher incidence of deep WMA progression than controls. Progression of WMAs was not associated with attack frequency, duration, or severity, or antimigraine therapy. In addition, the association between WMAs and cognitive function was not found (p = 0.07). Furthermore, they did not demonstrate higher progression of IHLs. The risk of brain lesions was independent of cardiovascular risk factors in these studies.10,11,14
The cross-sectional French population-based study confirmed the association of migraine with WMAs.13 The association with deep WMAs here was stronger for MA than MO. The association was not specific to migraine headaches but extended to nonmigraine headaches, especially tension-type headaches. The authors found an interaction of the association between nonmigraine headache and WMAs by age (p = 0.03), indicating an association among participants aged 70 and older. No link was found between overall headache status, WMAs, and cognitive impairment by the Mini-Mental State Examination (p = 0.17). The possible association with the attack frequency with WMAs was not investigated.
Four studies8,10,12,13 were eligible for meta-analysis according to our selection criteria. An association was found for MA compared to controls (p = 0.03) (figure e-1a), while there was no association for MO compared to controls (p = 0.08) (figure e-1b). In addition, there was no difference between prevalence of WMAs in MA and MO (p = 0.71) (figure e-1c). There was no substantial heterogeneity between studies. Begg and Mazumdar rank correlation test did not indicate publication bias.
Studies of ILLs.
Of the 5 population-based studies of silent ILLs, 3 are based on the CAMERA cohort (table 3). Kruit et al.10,15 reported a high prevalence of ILLs in the posterior circulation territory in subjects with MA and with attack frequency >1 per month, compared to subjects with MO and with attack frequency >1 per month and controls. Most ILLs were located in the cerebellum. They were not associated with supratentorial WMAs.15 Follow-up imaging after 9 years in CAMERA-214 did not find an increased risk of progression of previously identified ILLs in migraineurs. The study showed no association between ILLs and cognitive function (p = 0.984).
Kurth et al.13 reported an increased risk of ILLs only in subjects with MA. Most of the lesions were in deep GM structures such as subcortex and the basal ganglia, and the association with attack frequency was not investigated.
An Icelandic longitudinal study by Scher et al.16 investigated the association between midlife migraine and late-life cerebellar ILLs on MRI. Aura was defined based on the presence of visual disturbances or numbness. In this study, follow-up MRI more than 26 years later revealed that those with midlife MA had an increased risk of late-life ILLs (odds ratio [OR] 1.4; 95% confidence interval [CI] 1.1–1.8). Women with MA had a 2-fold increased risk of cerebellar ILLs (p = 0.04). A separate analysis suggested that the association of cerebellar ILLs with MA in women was stronger for those with visual aura than sensory aura. Results were not significant for men. In subjects aged >50 years with MA, a marginally increased risk for cortical infarcts was also found (p = 0.07). MO and nonmigraine headache were not associated with increased risk of ILLs. ILLs were independent of late-life measures of cardiovascular risk and history of carotid artery disease or TIA/stroke.
Two studies10,16 were eligible for meta-analysis according to our selection criteria. For ILLs, there were no differences for MA compared to controls (p = 0.52) (figure e-2a) or for MO compared to controls (p = 0.08) (figure e-2b). The association of ILLs was greater for MA than for MO (p = 0.04) (figure e-2c). For studies on ILLs, there was no evidence of heterogeneity. Begg and Mazumdar rank correlation test was not performed as there were only 2 studies included (at least 3 studies are needed).
Studies of volumetric changes.
We identified 9 clinic-based studies that used VBM and DTI to assess GM and WM regions in migraineurs and controls (table e-1).17–24,28 Seven studies reported reduced GM density in brain regions in migraineurs compared to controls.17,19–24 In addition, one study17 found increased GM density in the periaqueductal gray (PAG) and the dorsolateral pons, but only in patients with MA. In 5 studies, attack frequency20–22 and disease duration17,21,22,24 were correlated with GM reduction in migraineurs in the frontal,17,20–22,24 temporal,17,20 and parietal lobes,20–22 the limbic system,20–22 the cingulate cortex,20,21,24 the brainstem,22,24 and the cerebellum.22,24 One study20 reported that patients with chronic migraine (≥15 headache days per month) had a volume loss mainly in the anterior cingulate cortex (ACC) and in several other areas, compared to patients with episodic migraine (≤15 headache days per month), indicating an association between attack frequency and GM reduction, especially in the ACC.
DISCUSSION
This review indicates that migraine is a risk factor for WMAs, ILLs, and volumetric changes in the brain. Less clear is the association of these brain changes with migraine subtype and migraine burden, as discussed below.
White matter abnormalities.
Reported prevalence rates of WMAs in migraineurs ranges from 4% to 59% in selected studies (tables 1 and 2). The clinical significance of them remains uncertain. WMAs in some cases have been shown pathologically to have gliosis, demyelination, and loss of axons as their correlates.29 This set of findings has been attributed to microvascular damage. In persons with migraine, WMAs may have similar underpinnings.29,30
It is suggested that WMAs are more common in MA, but results are not consistent across studies. The relationship of migraine subtype, attack frequency, and disease duration to WMAs is of considerable interest. In CAMERA-1,10 the authors examined the relationship between attack frequency and WMAs, and suggested that WMAs could arise due to the cumulative impact of repeated episodes of regional cerebral ischemia during a migraine attack. A longitudinal study from Italy,31 which was not included in our summary tables because it lacked nonmigraine controls, suggested WMA progression in MA at follow-up MRI after about 33 months. The study reported a correlation of the number of new WMAs with attack frequency (p = 0.03) and aura duration (p < 0.0001). In CAMERA-2,14 the incidence of WMAs were higher in women with MO, and it was not associated with attack frequency or duration. This finding challenges the interpretation that WMAs reflect progressive cerebral ischemic changes. In CAMERA-1,10 the median frequency of migraine was 11.05 per year, and headache frequency was not reported in CAMERA-2.14 The lack of association with attack frequency and WMAs in CAMERA-2 may reflect the low attack frequency and the modest sample size. Kurth et al.13 found association between WMAs and MA, but in contrast to CAMERA, the authors did not investigate the association between attack frequency and WMAs.
WMAs are not specific to migraine, as evidenced by their presence in nonmigraine headaches and advanced age.13 Although there is a reported link between WMAs and cardiovascular risk factors,32,33 the presence of WMAs in migraineurs is independent of these risk factors in selected population-based studies.10,11,13,14 Several medical risk factors are reported to contribute to WMAs on MRI. High incidence of WMAs is found in migraineurs with subclinical hyperthyroidism and hypothyroidism and elevated levels of homocysteine.34 The prevalence of patent foramen ovale35 and mitral valve prolapse36 may also contribute to the WMAs. In some cases, the number, distribution, and location of WMAs may lead to the diagnosis of an underlying disease of which migraine may be a secondary cause, such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy,37 mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes,38 or multiple sclerosis.39 One study investigated relationship between distribution of WMAs and aura symptoms in 185 patients with MA and found that type of aura did not influence the distribution of WMAs.40
A recent prospective study by Rist et al.41 investigated an association between WMAs and cognitive changes in migraineurs, and found no association, which is consistent with results from Kurth et al.13 and CAMERA-2.14 Additionally, there is reported an increased risk of incident stroke with increasing WMA volume.42 However, according to CAMERA-2,14 WMAs are regarded as a benign imaging correlate of migraine, whose functional significance merits further investigation.
Finally, CAMERA-214 did not find any link between WMA progression and antimigraine therapy. In one observational study,43 the use of triptans was not associated with an increased risk of stroke, myocardial infarction, or cardiovascular death. While this result is reassuring, robust conclusions are limited due to confounding by indication. That is, persons who receive prescriptions for triptans are selected in part based on a favorable cardiovascular risk profile, which may offset a hypothetical risk associated with triptans.
The meta-analysis of WMAs supports the hypothesis that MA is associated with an increased risk of WMAs.8,10,12,13 All 4 studies had OR greater than 1 and the overall OR was 1.68. MO was not associated with an increased risk of WMAs. However, our results and the hypothesis that WMAs increase with cumulative exposure to migraine require further investigation.
Silent ILLs.
Silent ILLs are reported in migraineurs, preferentially localized in the cerebellum10,15,16 and deep GM13 (table 3). The association is strongest for persons with MA with frequent attacks.10,15 Scher et al.16 reported a 2-fold increased risk of cerebellar ILLs in late life in women with MA in midlife. In this study, aura was defined loosely. While this definition might have included individuals who would not meet the ICHD definition of aura and might attenuate the measured association between aura and ILLs, the study is valid. The specificity of the finding for women and for cerebellar ILLs cannot be accounted for by misclassification, which would attenuate these results in a nonspecific fashion.
CAMERA-214 only showed a trend for appearance of new and progression of ILLs in migraineurs, which may be attributable to lack of power. It is unclear whether silent ILLs predispose to or are associated with development of clinical stroke. CAMERA-2 reported that migraineurs with ILLs had a less favorable cardiovascular risk profile than those without ILLs and had a higher prevalence of clinically diagnosed stroke.14 The authors suggested that ILLs could represent a combination of episodic focal brain hypoperfusion and embolism.15 Hypertension is reported to be strongly associated with ILLs, indicating that ILLs might be a manifestation of hypertensive small-vessel disease.44,45
Kruit et al.10,15 and Scher et al.16 found most ILLs located in the cerebellum. In contrast, Kurth et al.13 found most lesions in the subcortex and the basal ganglia. Despite the availability of criteria diagnosing ILLs on MRI, the interpretation of these lesions remains controversial. According to Kurth and Tzourio,46 the criteria for ILLs might not identify lesions when applied to the cerebellum. The authors suggested that it was difficult to distinguish ILLs from enlarged perivascular spaces (i.e., the Virchow-Robin space) on MRI, and some lesions might be of a different nature and of no pathology produced by normal structures. There is evidence for subtle cerebellar dysfunction in asymptomatic migraineurs, supporting the interpretation that these lesions are of functional significance.47 If these ILLs are infarcts, it raises concern about long-term consequences of migraine on brain structure and function.
Silent ILLs are reported to be associated with an increased risk of cognitive decline and dementia in elderly.48 However, Rist et al.41 did not find any relationship between ILLs and cognitive decline in persons with migraine or other severe headaches, which is consistent with results from Kurth et al.13 and CAMERA-2.14
The meta-analysis of ILLs is difficult to interpret, in part because it included only 2 studies. Kruit et al.10 demonstrated an association of ILLs with MA compared to controls that was not apparent in the study by Scher et al.16 For MO, Kruit et al.10 reported no effect and Scher et al.16 showed a protective effect with no significance in the meta-analysis. When MA and MO were contrasted, the MA group had a higher relative OR of ILLs.
Volumetric changes in GM and WM.
Seven clinic-based studies have shown predilection sites of volumetric changes in migraine (table e-1). The sites of volume loss include the bilateral insula, the frontal/prefrontal, temporal, parietal, and occipital cortices, the ACC, the basal ganglia, and the cerebellum.17–24 Sites of volume gain include the dorsolateral pons and the PAG.17 Schmidt-Wilcke et al.49 investigated patients with chronic tension-type headache and found GM decrease in the ACC, the temporal and orbitofrontal cortices, and the cerebellum, indicating volumetric changes were not specific to migraine but extended to tension-type headache. Rocca et al.17 found an increased GM density in the PAG, which is known to be involved in pain processing. An abnormal iron homeostasis has been documented in the PAG in migraineurs along with other brain nuclei such as the red nucleus, putamen, and caudate. The authors suggested that iron accumulation might reflect progressive neuronal damage related to recurrent migraine attacks.50,51
Volumetric changes may reflect a complication of frequent migraine attack, but further studies are required to confirm this hypothesis. The clinical significance of these changes is unclear.
Methodologic comments and concerns.
Clinic-based and population-based studies provide complementary perspectives on the potential influence of migraine on brain structure but interpretation should be cautious. The literature on structural MRI in migraine is difficult to interpret for several reasons as discussed below. We will first consider limitations and strengths common to all of these studies and then those that are specific to clinic-based and population-based studies.
All the imaging studies evaluate heterogeneous groups of subjects because migraine itself is a heterogeneous disorder. Studies vary widely in sample size, subject selection (age, sex, vascular risk factors, antimigraine therapy), headache characteristics (migraine subtype, attack frequency, disease duration), test methodology, timing of study, and data interpretation. Inadequately measured or unmeasured confounding is always possible, although the authors measured and adjusted for many potential confounders, particularly in the larger population-based studies. Both the clinic-based and population-based studies are for the most part cross-sectional. They demonstrate associations between migraine and brain structures, but do not reveal the directionality of the association. It is logically possible that some brain changes in migraine are a consequence of illness or treatment but other changes may reflect the vulnerability to migraine.
The clinic-based studies recruit participants from specialty care settings, creating several potential problems. First, since only a minority of patients is treated in specialty care, the sample may not be broadly representative of migraineurs. Specialist samples are likely to have more frequent and severe attacks, more comorbidities, and more exposure to treatment. In addition, the choice of nonmigraine controls can be difficult as specialty centers may have broad and diverse sources of referrals. For MRI changes that arise because of frequent or severe attacks, clinic-based studies may be the best place to identify abnormalities for validation in population-based studies.
The population-based studies minimize the issues of referral bias and make it easy to select controls. Because participation is never 100%, some selection bias is inevitable. When population-based studies focus on adults over 50, there may be underreporting of remitted migraine. This could result in the inclusion in the control group of former migraine cases. If anything, this would bias toward the null by making the control group more similar to the case group. Population-based studies circumvent selection and referral bias and are readily generalizable. Inclusion of many relatively mild migraine cases may make it more difficult to detect abnormalities that are related to attack frequency and severity.
Conclusions and future perspectives.
The present review suggests that migraine may be a risk factor for structural changes in the brain. In comparison with nonmigraine controls, migraineurs have more WMAs, ILLs, and volumetric changes in GM and WM regions. The evidence on relationship to attack frequency and disease duration is equivocal. At present, the clinical and functional significance of these brain lesions is uncertain. Guidelines from the American Academy of Neurology and US Headache Consortium52 suggest that migraineurs with a normal neurologic examination do not require routine MRI. Only patients with atypical headache, a recent change in headache pattern, other symptoms (such as seizures), or focal neurologic symptoms or signs are recommended for MRI of the brain.
Patients with WMAs can be reassured. Patients with ILLs should be evaluated for stroke risk factors. Volumetric MRI remains a research tool.
To improve our knowledge on this topic, additional longitudinal studies with a broad range of disease frequency and severity are needed to fully understand the association between migraine and structural changes in the brain and to clarify the association to attack frequency and disease duration, as well as the influence of these lesions on brain function and prognosis.
Supplementary Material
GLOSSARY
- ACC
anterior cingulate cortex
- CAMERA
Cerebral Abnormalities in Migraine, an Epidemiological Risk Analysis
- CI
confidence interval
- DTI
diffusion tensor imaging
- GM
gray matter
- ICHD
International Classification of Headache Disorders
- IHL
infratentorial hyperintense lesion
- ILL
infarct-like lesion
- MA
migraine with aura
- MO
migraine without aura
- OR
odds ratio
- PAG
periaqueductal gray
- VBM
voxel-based morphometry
- WM
white matter
- WMA
white matter abnormality
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Bashir and Dr. M. Ashina conceived and designed (including search strategies) the review. Dr. Bashir did the literature search and wrote the first and subsequent drafts of the manuscript. Dr. S. Ashina conducted the meta-analysis. Dr. Lipton, Dr. S. Ashina, and Dr. M. Ashina participated in critical revision and writing of the article. All authors have seen and approved the final version.
STUDY FUNDING
Supported by the Lundbeck Foundation and the Novo Nordisk Foundation.
DISCLOSURE
A. Bashir reports no disclosures. R. Lipton receives research support from the NIH [PO1 AG03949 (Program Director), RO1AG025119 (Investigator), RO1AG022374-06A2 (Investigator), RO1AG034119 (Investigator), RO1AG12101 (Investigator), K23AG030857 (Mentor), K23NS05140901A1 (Mentor), and K23NS47256 (Mentor)], the National Headache Foundation, and the Migraine Research Fund; serves on the editorial board of Neurology®; has reviewed for the NIA and NINDS; holds stock options in eNeura Therapeutics; and serves as consultant, advisory board member, or has received honoraria from Allergan, American Headache Society, Autonomic Technologies, Boston Scientific, Bristol Myers Squibb, Cognimed, Colucid, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, MAP, Merck, Nautilus Neuroscience, Novartis, NuPathe, Pfizer, and Vedanta. Dr. S. Ashina received honoraria for lecturing from Allergan and Neurogesx and is a consultant for Depomed. Dr. M. Ashina is an associate editor of Cephalalgia and is a consultant or scientific adviser for Autonomic Technologies, Inc., Allergan, Amgen, and Alder. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders, 2nd ed. Cephalalgia 2004;24(suppl 1):1–151 [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population: a prevalence study. J Clin Epidemiol 1991;44:1147–1157 [DOI] [PubMed] [Google Scholar]
- 3.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41:646–657 [DOI] [PubMed] [Google Scholar]
- 4.Lyngberg AC, Rasmussen BK, Jørgensen T, Jensen R. Secular changes in health care utilization and work absence for migraine and tension-type headache: a population based study. Eur J Epidemiol 2005;20:1007–1014 [DOI] [PubMed] [Google Scholar]
- 5.IMS Audit, January 1999. Available at: http://www.americanheadachesociety.org/assets/1/7/NAP_for_Web_-_Epidemiology___Impact_of_Headache___Migraine.pdf. Accessed January 5, 2013
- 6.Moschiano F, D’Amico D, Di Stefano M, Rocca N, Bussone G. The role of the clinician in interpreting conventional neuroimaging findings in migraine patients. Neurol Sci 2007;28:S114–S117 [DOI] [PubMed] [Google Scholar]
- 7.Fazekas F, Koch M, Schmidt R, et al. The prevalence of cerebral damage varies with migraine type: an MRI study. Headache 1992;32:287–291 [DOI] [PubMed] [Google Scholar]
- 8.Rovaris M, Bozzali M, Rocca MA, Colombo B, Filippi M. An MR study of tissue damage in the cervical cord of patients with migraine. J Neurol Sci 2001;183:43–46 [DOI] [PubMed] [Google Scholar]
- 9.Rocca MA, Colombo B, Inglese M, Codella M, Comi G, Filippi M. A diffusion tensor magnetic resonance imaging study of brain tissue from patients with migraine. J Neurol Neurosurg Psychiatry 2003;74:501–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruit MC, van Buchem MA, Hofman PA, et al. Migraine as a risk factor for subclinical brain lesions. JAMA 2004;291:427–434 [DOI] [PubMed] [Google Scholar]
- 11.Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Brain Stem and cerebellar hyperintense lesions in migraine. Stroke 2006;37:1109–1112 [DOI] [PubMed] [Google Scholar]
- 12.Degirmenci B, Yaman M, Haktanir A, Albayrak R, Acar M. Cerebral and cerebellar ADC values during a migraine attack. Neuroradiology 2007;49:419–426 [DOI] [PubMed] [Google Scholar]
- 13.Kurth T, Mohamed S, Maillard P, et al. Headache, migraine, and structural brain lesions and function: population based Epidemiology of Vascular Ageing-MRI study. BMJ 2011;342:c7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palm-Meinders IH, Koppen H, Terwindt GM, et al. Structural brain changes in migraine. JAMA 2012;308:1889–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Infarcts in the posterior circulation territory in migraine: the population-based MRI CAMERA study. Brain 2005;128:2068–2077 [DOI] [PubMed] [Google Scholar]
- 16.Scher AI, Gudmundsson LS, Sigurdsson S, et al. Migraine headache in middle age and late-life brain infarcts. JAMA 2009;301:2563–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocca MA, Ceccarelli A, Falini A, et al. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke 2006;37:1765–1770 [DOI] [PubMed] [Google Scholar]
- 18.Dasilva AFM, Granziera C, Tuch DS, Snyder J, Vincent M, Hadjikhani N. Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. Neuroreport 2007;18:301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt-Wilcke T, Gänβbauer S, Neuner T, Bogdahn U, May A. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia 2008;28:1–4 [DOI] [PubMed] [Google Scholar]
- 20.Valfré W, Rainero I, Bergui M, Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache 2008;48:109–117 [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Suh SI, Seol HY, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia 2008;28:598–604 [DOI] [PubMed] [Google Scholar]
- 22.Schmitz N, Admiraal-Behloul F, Arkink EB, et al. Attack frequency and disease duration as indicators for brain damage in migraine. Headache 2008;48:1044–1055 [DOI] [PubMed] [Google Scholar]
- 23.Schmitz N, Arkink EB, Mulder M, et al. Frontal lobe structure and executive function in migraine patients. Neurosci Lett 2008;440:92–96 [DOI] [PubMed] [Google Scholar]
- 24.Jin C, Yuan K, Zhao L, et al. Structural and functional abnormalities in migraine patients without aura. NMR Biomed 2013;26:58–64 [DOI] [PubMed] [Google Scholar]
- 25.Lipton RB, Pan J. Is migraine a progressive brain disease? JAMA 2004;291:493–494 [DOI] [PubMed] [Google Scholar]
- 26.Lipton RB. Tracing transformation: chronic migraine classification, progression, and epidemiology. Neurology 2009;72(suppl 1):S3–S7 [DOI] [PubMed] [Google Scholar]
- 27.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology 1999;53:537–542 [DOI] [PubMed] [Google Scholar]
- 28.Matharu MS, Good CD, May A, Bahra A, Goadsby PJ. No change in the structure of the brain in migraine: a voxel-based morphometric study. Eur J Neurol 2003;10:53–57 [DOI] [PubMed] [Google Scholar]
- 29.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993;43:1683–1689 [DOI] [PubMed] [Google Scholar]
- 30.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology 2008;71:804–811 [DOI] [PubMed] [Google Scholar]
- 31.Dinia L, Bonzano L, Albano B, et al. White matter lesions progression in migraine with aura: a clinical and MRI longitudinal study. J Neuroimaging 2013;23:47–52 [DOI] [PubMed] [Google Scholar]
- 32.Jefferson AL, Tate DF, Poppas A, et al. Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. J Am Geriatr Soc 2007;55:1044–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shrestha I, Takahashi T, Nomura E, et al. Association between central systolic blood pressure, white matter lesions in cerebral MRI and carotid atherosclerosis. Hypertens Res 2009;32:869–874 [DOI] [PubMed] [Google Scholar]
- 34.Trauninger A, Leél-Össy E, Kamson DO, et al. Risk factors of migraine-related brain white matter hyperintensities: an investigation of 186 patients. J Headache Pain 2011;12:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueno Y, Shimada Y, Tanaka R, et al. Patent foramen ovale with atrial septal aneurysm may contribute to white matter lesions in stroke patients. Cerebrovasc Dis 2010;30:15–22 [DOI] [PubMed] [Google Scholar]
- 36.Spence JD, Wong DG, Melendez LJ, Nichol PM, Brown JD. Increased prevalence of mitral valve prolapsed in patients with migraine. Can Med Assoc J 1984;131:1457–1460 [PMC free article] [PubMed] [Google Scholar]
- 37.Vahedi K, Chabriat H, Levy C, Joutel A, Tournier-Lasserve E, Bousser MG. Migraine with aura and brain magnetic resonance imaging abnormalities in patients with CADASIL. Arch Neurol 2004;61:1237–1240 [DOI] [PubMed] [Google Scholar]
- 38.Hirano M, Ricci E, Koenigsberger MR, et al. MELAS: an original case and clinical criteria for diagnosis. Neuromuscul Disord 1992;2:125–135 [DOI] [PubMed] [Google Scholar]
- 39.Filippi M, Rocca MA, De Stefano N, et al. Magnetic resonance techniques in multiple sclerosis. Arch Neurol 2011;68:1514–1520 [DOI] [PubMed] [Google Scholar]
- 40.Rossato G, Adami A, Thijs VN, et al. Cerebral distribution of white matter lesions in migraine with aura patients. Cephalalgia 2010;30:855–859 [DOI] [PubMed] [Google Scholar]
- 41.Rist PM, Dufouil C, Glymour MM, Tzourio C, Kurth T. Migraine and cognitive decline in the population-based EVA study. Cephalalgia 2011;31:1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buyck JF, Dufouil C, Mazoyer B, et al. Cerebral white matter lesions are associated with the risk of stroke but not with other vascular events: The 3-City Dijon study. Stroke 2009;40:2327–2331 [DOI] [PubMed] [Google Scholar]
- 43.Hall GC, Brown MM, Mo J, MacRae KD. Triptans in migraine: the risks of stroke, cardiovascular disease, and death in practice. Neurology 2004;62:563–568 [DOI] [PubMed] [Google Scholar]
- 44.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MMB. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan study. Stroke 2002;33:21–25 [DOI] [PubMed] [Google Scholar]
- 45.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007;6:611–619 [DOI] [PubMed] [Google Scholar]
- 46.Kurth T, Tzourio C. Migraine and cerebral infarct-like lesions on MRI: an observation, not a disease. JAMA 2009;301:2594–2595 [DOI] [PubMed] [Google Scholar]
- 47.Sándor PS, Mascia A, Seidel L, de Pasqua V, Schoenen J. Subclinical cerebellar impairment in the common types of migraine: a three-dimensional analysis of reaching movements. Ann Neurol. 2001;49:668–672 [PubMed] [Google Scholar]
- 48.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222 [DOI] [PubMed] [Google Scholar]
- 49.Schmidt-Wilcke T, Leinisch E, Straube A, et al. Gray matter decrease in patients with chronic tension type headache. Neurology 2005;65:1483–1486 [DOI] [PubMed] [Google Scholar]
- 50.Welch KMA, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache 2001;41:629–637 [DOI] [PubMed] [Google Scholar]
- 51.Kruit MC, Launer LJ, Overbosch J, van Buchem MA, Ferrari MD. Iron accumulation in deep brain nuclei in migraine: a population-based magnetic resonance imaging study. Cephalalgia 2008;29:351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guidelines from the American Academy of Neurology and US Headache Consortium: neuroimaging in patients with nonacute headache. Available at: http://tools.aan.com/professionals/practice/pdfs/gl0088.pdf. Accessed November 28, 2011
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.