Abstract
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is a rare autosomal recessive metabolic disorder caused by a deficiency of thymidine phosphorylase (TP, EC2.4.2.4) due to mutations in the nuclear gene TYMP. TP deficiency leads to plasma and tissue accumulations of thymidine and deoxyuridine which generate imbalances within the mitochondrial nucleotide pools, ultimately leading to mitochondrial dysfunction.1 MNGIE is characterized clinically by leukoencephalopathy, external ophthalmoplegia, peripheral polyneuropathy, cachexia, and enteric neuromyopathy manifesting as gastrointestinal dysmotility. The condition is relentlessly progressive, with patients usually dying from a combination of nutritional and neuromuscular failure at an average age of 37 years.2 Allogeneic hematopoietic stem cell transplantation (AHSCT) offers a permanent cure. Clinical and biochemical improvements following AHSCT have been reported but it carries a high mortality risk and is limited by matched donor availability.3 A consensus proposal for standardizing AHSCT recommends treatment of patients without irreversible end-stage disease and with an optimally matched donor; a majority of patients are ineligible and thus there is a critical requirement for an alternative treatment.4
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is a rare autosomal recessive metabolic disorder caused by a deficiency of thymidine phosphorylase (TP, EC2.4.2.4) due to mutations in the nuclear gene TYMP. TP deficiency leads to plasma and tissue accumulations of thymidine and deoxyuridine which generate imbalances within the mitochondrial nucleotide pools, ultimately leading to mitochondrial dysfunction.1 MNGIE is characterized clinically by leukoencephalopathy, external ophthalmoplegia, peripheral polyneuropathy, cachexia, and enteric neuromyopathy manifesting as gastrointestinal dysmotility. The condition is relentlessly progressive, with patients usually dying from a combination of nutritional and neuromuscular failure at an average age of 37 years.2 Allogeneic hematopoietic stem cell transplantation (AHSCT) offers a permanent cure. Clinical and biochemical improvements following AHSCT have been reported but it carries a high mortality risk and is limited by matched donor availability.3 A consensus proposal for standardizing AHSCT recommends treatment of patients without irreversible end-stage disease and with an optimally matched donor; a majority of patients are ineligible and thus there is a critical requirement for an alternative treatment.4
We report a compassionate use of erythrocyte encapsulated recombinant Escherichia coli TP (EE-TP). In this approach, a predetermined volume of the patient's blood is removed and subjected to reversible hypo-osmotic dialysis to enable encapsulation of TP within the erythrocytes, which are then returned to the patient.5,6 EE-TP has the pharmacologic advantages of prolonging the circulatory half-life of TP and minimizing immunogenic reactions.
Level of evidence.
This is a single observational study without controls and provides Class IV evidence that EE-TP provides clinical benefit.
Case report.
The patient was reported previously by Filosto et al.7 An initial diagnosis of an inflammatory polyneuropathy was made; corticosteroids, immunoglobulins, and cyclophosphamide were administered without clinical benefit. At age 26 years, a compound heterozygous TYMP mutation was demonstrated. Clinical assessment prior to initiating EE-TP confirmed peripheral sensorimotor polyneuropathy, external ophthalmoplegia, minimum intestinal dysmotility, and difficulties in walking a distance of 1 km. At age 28 years, he received his first infusion of EE-TP; a mean thymidine phosphorylase activity of 17 IU/kg was administered and this was gradually escalated to 46 IU/kg in cycle 30 (27 months). Research ethics committee approval and patient informed consent were obtained.
Results.
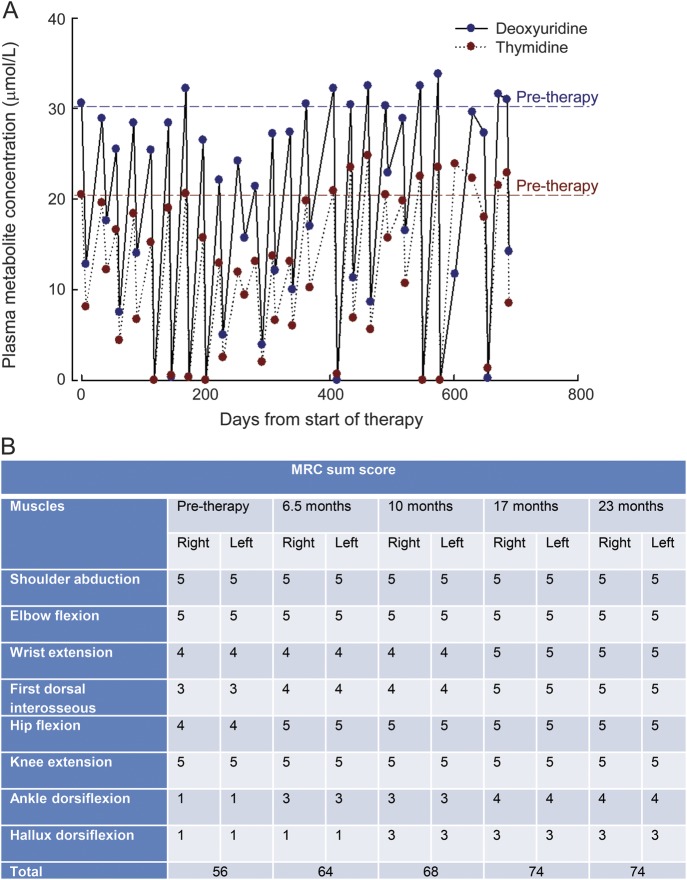
Pretherapy plasma concentrations of thymidine and deoxyuridine were reduced from 20.5 and 30.6 μmol/L, respectively, to intracycle values less than 8.1 and 12.6 μmol/L for thymidine and deoxyuridine, respectively (figure, A). Urinary thymidine and deoxyuridine excretion decreased from pretreatment values of 421 and 324 μmol/24 hours, respectively, to midcycle levels between 192 and 282 μmol/24 hours for thymidine and 0.0–184 μmol/24 hours for deoxyuridine from cycle 21 onwards. Toward the end of the treatment cycles, the metabolites returned to abnormal values, a consequence of a decrease in circulating erythrocyte TP activity.
Figure. Plasma nucleosides and MRC sumscore before and during treatment with EE-TP.
(A) Plasma concentrations of thymidine and deoxyuridine during 27 treatment cycles of Escherichia coli thymidine phosphorylase (EE-TP). (B) Medical Research Council (MRC) scale in different muscle groups before and during 27 treatment cycles of EE-TP.
Clinical assessments between 6.5 and 23 months after initiating EE-TP revealed significant improvements. The Medical Research Council sumscore for power increased from 56 to 74 (figure, B). There were improvements in gait and balance, sensory ataxia, and fine finger function; body weight increased from 57.4 kg to 61.2 kg. Coinciding with this clinical improvement and of possible significance, there was a steady fall in plasma creatine kinase activity from 1,200 U/L pretherapy to 254 U/L (normal range 40–320 U/L) at 23 months. The patient-reported outcomes after 23 months included being able to walk 10 km, climb stairs without assistance, tie shoelaces, and feel the sensation of sand on his feet when walking on beach. He has also returned to weight training and resumed playing guitar in a local band. The previously reported numbness in hands and feet also resolved. Nerve conduction and EMG studies at baseline and 27 months did not show any significant changes in spite of the significant clinical improvements.
The only adverse events observed were mild reactions (coughing and erythema of the face and neck) to the enzyme infusion; these were transient, occurring within 5 minutes of infusion, and easily managed by premedication with antihistamine and glucocorticoids. The introduction of a highly purified enzyme largely eliminated these reactions, permitting a gradual withdrawal of the premedication. No circulating anti-TP antibodies were detected.
Discussion.
This report describes a successful enzyme replacement therapy for MNGIE. Although EE-TP strongly reduced plasma nucleosides without eliminating them to undetectable levels, significant clinical improvements were noted, as observed in patients who underwent AHSCT,2 suggesting a greater muscle mitochondrial oxidative function. EE-TP should be considered as a rescue or maintenance therapy prior to the availability of a suitable AHSCT donor or as an alternative therapy for patients who have irreversible end-stage disease and are without an optimally matched donor. Clinical trials with this form of therapy are indicated.
Acknowledgments
Acknowledgment: The authors acknowledge Dr. Lynette Fairbanks, Purine Research Laboratory, St. Thomas' Hospital, London, and Michelle Levene, Clinical Sciences, St. George's University of London, for the biochemical analysis.
Footnotes
Author contributions: Dr. Bax: study concept and design, analysis and interpretation, drafted manuscript, critical revision of the manuscript for important intellectual content, study supervision. Dr. Bain, Dr. Scarpelli, Dr. Filosto, Dr. Tonin, and Dr. Moran: analysis and interpretation and critical revision of the manuscript for important intellectual content.
Disclosure: B. Bax serves on the scientific advisory board for Erytech Pharma, has received a travel grant from Orphan Technologies for the purpose of attending a rare disease conference in Israel, is due to receive license fee payments from Orphan Technologies, is funded by grants from the Purine Metabolic Patients Association, the Medical Research Council, and the Hampshire Primary Care Trust (on behalf of the South of England Specialised Commissioning Group), and received research support from the Medical Research Council, United Mitochondrial Disease Foundation, and the Higher Education Funding Council of England. M. Bain is due to receive license fee payments from Orphan Technologies. M. Scarpelli and M. Filosto report no disclosures. P. Tonin is funded by a grant from Genzyme. N. Moran received sponsorship to attend an international medical conference from GSK and an honorarium for presenting at UCB symposia.
References
- 1.Nishino I, Spinazzola A, Papadimitriou A, et al. Mitochondrial neurogastrointestinal encephalomyopathy: an autosomal recessive disorder due to thymidine phosphorylase mutations. Ann Neurol 2000;47:792–800 [PubMed] [Google Scholar]
- 2.Hirano M, Garone C, Quinzii CM. CoQ10 deficiencies and MNGIE: two treatable mitochondrial disorders. Biochim Biophys Acta 2012;1820:625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filosto M, Scarpelli M, Tonin P, et al. Course and management of allogeneic stem cell transplantation in patients with mitochondrial neurogastrointestinal encephalomyopathy. J Neurol 2012;259:2699–2706 [DOI] [PubMed] [Google Scholar]
- 4.Halter J, Schüpbach WM, Casali C, et al. Allogeneic hematopoietic SCT as treatment option for patients with mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): a consensus conference proposal for a standardized approach. Bone Marrow Transplant 2011;46:330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moran NF, Bain MD, Muqit M, Bax BE. Carrier erythrocyte entrapped thymidine phosphorylase therapy in MNGIE. Neurology 2008;71:686–688 [DOI] [PubMed] [Google Scholar]
- 6.Godfrin Y, Bax BE. Enzyme bioreactors as drugs. Drugs Future 2012;37:263–272 [Google Scholar]
- 7.Filosto M, Scarpelli M, Tonin P, et al. Pitfalls in diagnosing mitochondrial neurogastrointestinal encephalomyopathy. J Inherit Metab Dis 2011;34:1199–1203 [DOI] [PubMed] [Google Scholar]