Abstract
Introduction
The dichlorofluorescein (DCF) assay is a popular method for measuring cellular reactive oxidant species (ROS). Although caveats have been reported with the DCF assay and other compounds, the potential for artifactual results due to cell-free interactions between the DCF compound and toxicants has hardly been explored. We evaluated the utility of the DCF assay for measuring ROS generation by the toxicants mono-(2-ethylhexyl) phthalate (MEHP), and tetrabromobisphenol A (TBBPA).
Methods
DCF fluorescence was measured spectrofluorometrically after a 1-h incubation of toxicants with 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA). MEHP was incubated with carboxy-H2DCFDA in cell-free solutions of Hank’s buffered salt solution (HBSS), or in Royal Park Memorial Institute (RPMI) medium with or without fetal bovine serum. TBBPA was incubated with carboxy-H2DCFDA in cell-free HBSS and with human trophoblast cells (HTR8/SVneo cells).
Results
MEHP did not increase fluorescence in solutions of carboxy-H2DCFDA in HBSS or RPMI medium without serum. However, MEHP (90 and 180 μM) increased DCF fluorescence in cell-free RPMI medium containing serum. Furthermore, serum-free and cell-free HBSS solutions containing 25 μM TBBPA exhibited concentration-dependent increased fluorescence with 5–100 μM carboxy-H2DCFDA (p<0.05), but not 1 μM carboxy-H2DCFDA. In addition, we observed increased fluorescence in HTR8/SVneo cell cultures exposed to TBBPA (0.5–25 μM) (p<0.05), as we had observed in cell-free buffer.
Discussion
MEHP demonstrated an interaction with serum in cell-free generation of DCF fluorescence, whereas TBBPA facilitated conversion of carboxy-H2DCFDA to the fluorescent DCF moiety in the absence of serum. Because TBBPA increased fluorescence in the absence of cells, the increased DCF fluorescence observed with TBBPA in the presence of cells cannot be attributed to cellular ROS and may, instead, be the result of chemical activation of carboxy-H2DCFDA to the fluorescent DCF moiety. These data illustrate the importance of including cell-free controls when using the DCF assay to study toxicant-stimulated cellular production of ROS.
Keywords: brominated flame retardant, 6-carboxy-2′, 7′-dichlorodihydrofluorescein diacetate, dichlorofluorescein assay, methods, mono-(2-ethylhexyl) phthalate, reactive oxidant species, tetrabromobisphenol A
1. Introduction
The DCF assay utilizes a cell-permeant acetylated form of fluorescein that diffuses into the cytoplasm where cellular esterases remove the acetate groups from the compound to form a non-fluorescent moiety that can be oxidized by intracellular ROS to form the fluorescent product. The DCF assay can be conducted with the 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) parent compound or with more recently introduced derivatives such as 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate 2′,7′-dichlorofluorescein (carboxy-H2DCFDA). After deacetylation of H2DCFDA to H2DCF or carboxy-H2DCFDA to carboxy-H2DCF, cellular ROS oxidize H2DCF and carboxy-H2DCF to generate 2′,7′-dichlorofluorescein and 2′,7′-carboxydichlorofluorescein, respectively. The 2′,7′-carboxydichlorofluorescein has improved cellular retention compared to the non-carboxylated form due to the additional two negative charges (Invitrogen, 2006). Both the parent compounds and the deacetylated forms are non-fluorescent whereas DCF fluorescence is measurable by spectrofluorometry (Kim, et al., 2005) or flow cytometry (Epling, et al., 1992; Fruhwirth, et al., 1998; Hafer, et al., 2008), with the magnitude of DCF fluorescence proportional to the formation of ROS such as peroxynitrite and OH•, and molecules that include peroxyl, alkoxyl, carbonate (CO3•−) and NO2• groups (Halliwell & Whiteman, 2004).
Various caveats have been identified that may hinder the accurate measurement of ROS production in cells with the DCF assay. For example, conversion of H2DCFDA to DCF increases with serum concentration (Korystov, et al., 2007), in the presence of heme, heme proteins, and metalloporphyrins (Ohashi, et al., 2002), and in DMEM cell culture medium (Boulton, et al., 2011). Furthermore, peroxidase catalyzes superoxide radical formation as a byproduct of the conversion of H2DCF to DCF (Rota, et al., 1999), and conversion of carboxy-H2DCFDA to carboxy-DCF increases in the presence of native bovine serum albumin (Subramaniam, et al., 2002). Despite these caveats, few reports of toxicant chemical-stimulated ROS as assessed by the DCF assay discuss evaluation of experimental conditions for potential confounding of resultant experimental data.
Our objective was to evaluate the utility of the DCF assay for measuring toxicant-mediated ROS production, with particular attention to potential artifacts of the DCF assay due to cell-free interactions between carboxy-H2DCFDA and toxicants. For these experiments, we used the environmental contaminants mono-(2-ethylhexyl) phthalate (MEHP) and tetrabromobisphenol A (TBBPA) as example toxicants. MEHP is an active metabolite of the plasticizer di-(2-ethylhexyl) phthalate (DEHP) (Koch, et al., 2006) and TBBPA is a brominated flame retardant (Talsness, et al., 2009). Several reports indicate the use of H2DCFDA or carboxy- H2DCFDA in either PBS or culture medium to measure MEHP-stimulated ROS production in cells (Bolling, et al., 2012; Fan, et al., 2010; Zhao, et al., 2012). However, these MEHP publications do not indicate whether proper cell-free controls were run to determine potential experimental confounders due to the assay solution. Similarly, a study demonstrating TBBPA-mediated ROS formation in human neutrophil granulocytes did not report whether the appropriate cell-free controls were run (Reistad, et al., 2005). We discovered that MEHP and TBBPA increased DCF fluorescence in the absence of cells depending on the solutions used in the assay. These results are discussed with respect to the potential for misinterpretation of results of the DCF assay when assessing cellular ROS production in response to toxicant exposure.
2. Materials and Methods
2.1 Chemicals and reagents
Dimethyl sulfoxide (DMSO; sterile filtered and >99.7% pure) and 3,3′, 5, 5′-tetrabromobisphenol A (TBBPA) were purchased from Sigma Aldrich (St. Louis, MO). Mono-(2-ethylhexyl) phthalate (MEHP) was purchased from Accustandard (New Haven, CT). The probe 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA, catalog # C-400), RPMI medium 1640, fetal bovine serum (FBS), Hank’s Balanced Salt Solution (HBSS, with Ca+2 and Mg+2), 0.25% trypsin/EDTA and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer were purchased from Invitrogen (Carlsbad, CA).
2.2 Preparation of solutions
A stock solution of 362 mM MEHP was prepared in DMSO, which was diluted in assay solution just prior to experiments to 180 μM MEHP. The 180 μM MEHP solution was briefly sonicated and then serially diluted to 90 and 45 μM solutions. MEHP stock solutions were kept at −20°C for longer-term storage and at 4°C for short-term storage. TBBPA was dissolved in DMSO with brief vortexing and a 15-min sonication (at room temperature) to give a final concentration of 50 mM, which was then diluted serially in DMSO to generate 25, 10, 5, 2, 1 and 0.5 mM TBBPA stock solutions. All TBBPA solutions were stored at 4°C when not in use, and were returned to room temperature and sonicated just prior to use in experiments. The carboxy-H2DCFDA was dissolved in DMSO to generate a stock solution of 10 mg/ml. The carboxy-H2DCFDA stock solution was stored in 60-μl aliquots at −20°C; each aliquoted sample was thawed once for use. For experiments, the stock solution was brought to room temperature in the dark, and then diluted in HBSS or culture medium to final concentrations.
2.3 Cell culture
The human first trimester extravillous trophoblast cell line HTR8/SVneo (HTR8) was kindly provided by Dr. Charles S. Graham (Queens University, Ontario). This cell line was immortalized by transfecting a plasmid containing the gene for the simian virus 40 large T antigen (SV40). Similar to primary trophoblasts, HTR8 cells express the epithelial marker cytokeratin and share similar morphology, growth patterns and serum requirements in vitro (Graham, et al., 1993). The HTR8 cells were maintained at 37°C and 5% CO2 in 75 cm2 or 175 cm2 flasks containing RPMI medium supplemented with 10% FBS. Cells were subcultured with 0.25% trypsin/EDTA solution when approximately 80% confluent. For the DCF assay experiments, cells were seeded at 5 × 104 cells/well or 1–2 × 104 cells/well in 24- or 96-well plates, respectively.
2.4. Measurement of MEHP-stimulated fluorescence with the DCF assay
Because culture media with or without serum and physiologic salt solutions have been used in reports of the DCF assay (Bolling, et al., 2012; Fan, et al., 2010; Zhao, et al., 2012) we evaluated interactions between MEHP and DCF in RPMI medium and HBSS buffer. Furthermore, we evaluated the effects of serum on MEHP-stimulated DCF fluorescence in RPMI medium but not in HBSS because serum is a common supplement of medium but not salt solutions like HBSS. Aliquots of the MEHP stock solution were added to either HBSS buffer or to RPMI medium with or without 10% fetal bovine serum (FBS) to obtain final concentrations of 45, 90, and 180 μM MEHP. An appropriate volume of carboxy-H2DCFDA reagent was mixed with each MEHP or solvent control (0.1 % DMSO) solution of HBSS or RPMI medium to obtain a final concentration of 10 μM carboxy-H2DCFDA. Aliquots of 200 μl of the solvent control or the different MEHP concentrations with carboxy-H2DCFDA reagent were then added to wells of a black-sided, clear-bottomed 96-well plate in replicates of six. Fluorescence readings were taken using a plate spectrofluorometer every 10 min for 1 h. Because the pattern of cell-free DCF fluorescence at 1 h was indicative of the response over the 1-h observation period (data not shown), we present results at the 1-h time point.
2.5. Measurement of TBBPA-stimulated fluorescence with the DCF assay
Because fluorescence background levels were lower for controls in HBSS buffer compared to RPMI culture medium in cell-free solutions (Figure 1), we assessed cell-free TBBPA effects on DCF fluorescence in HBSS buffer only. Serial dilutions of the carboxy-H2DCFDA stock solution (100 μM) were added to HBSS to obtain 25 mM TBBPA stock solution final concentrations of 1, 5, 10, 50, and 100 μM carboxy-H2DCFDA. Aliquots from a 25 mM TBBPA stock solution were then added to the carboxy-H2DCFDA solutions to yield a final concentration of 25 μM TBBPA. Immediately thereafter, 200 μl aliquots of these solutions were added to wells of a black-sided, clear-bottomed 96-well plate in replicates of six. Concurrent controls included one group incubated with 0.1% DMSO only (0 μM TBBPA) and one group incubated with HBSS buffer only; both control groups included carboxy-H2DCFDA in the solution.
Figure 1. MEHP effects on DCF fluorescence in HBSS and RPMI medium solutions.
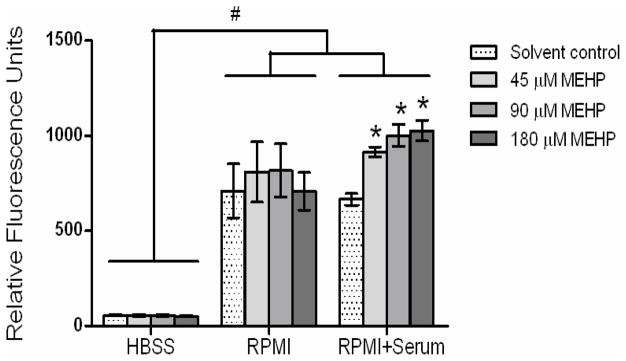
MEHP effects on DCF fluorescence were determined after a 1-h incubation in cell-free solutions containing 10 μM carboxy-DCFH2DA in HBSS or in RPMI medium with or without serum. Columns represent the mean ± SE of 3 independent experiments containing 6 replicates each. #, Statistically significant difference between solution groups (ANOVA, p<0.001). *, Statistically significant increase compared with solvent controls within the RPMI+serum group (p<0.05).
TBBPA-stimulated DCF fluorescence after incubation with cells was assessed in HBSS only in order to minimize background fluorescence and avoid the potential for interaction with serum (Figure 1). To assess cellular response to TBBPA, HTR8 cells were seeded in RPMI medium containing 10% FBS at a density of 2 × 104 cells per well in a black-sided, clear-bottomed 96-well plate. Twenty-four hours later, cell culture medium was removed by aspiration. After rinsing twice with warm (37 °C) HBSS, the cells were incubated in 100 μM carboxy-H2DCFDA in HBSS at 37°C for 1 h. Following the incubation period, the carboxy-H2DCFDA medium was replaced with HBSS containing either 0.1% DMSO (solvent control) or TBBPA. For both the cell-free and cell culture experiments, fluorescence readings (492 nm excitation and 515 nm emission) were taken at 5-min intervals for 1 h at 37°C. Because the pattern of DCF fluorescence at 1 h was representative of the response over the 1-h observation period (data not shown), we present results at the 1-h time point.
2.6. Statistical Analysis
Means from each independent experiment were analyzed using SigmaPlot software (version 11.2, SYSTAT Software, Inc., San Jose, CA) by either a one-way or two-way repeated measures analysis of variance (ANOVA), as appropriate, followed by the Holms-Sidak or Tukey’s posthoc test for comparison of means. In the analysis of cell-free MEHP-stimulated DCF fluorescence, a hierarchical linear mixed effects model was used to test differences between buffer solution and treatment groups simultaneously, as well as the interaction between the two, when accounting for replicates nested within repeated experiments for each treatment group and buffer solution. P < 0.05 was considered statistically significant.
3. Results
3.1. Serum effects on MEHP-stimulated DCF fluorescence in cell-free solutions
In cell-free and serum-free HBSS, 1 h incubation with 45, 90 or 180 μM MEHP had no significant effects on DCF fluorescence compared with controls in HBSS (Figure 1, not significant). DCF fluorescence was higher in all treatment groups in RPMI with or without serum compared to HBSS alone (Figure 1; Assay solution comparison, p<0.001). In mixed effects models there was a significant interaction (p<0.05) between MEHP treatment and serum in the stimulation of DCF fluorescence, meaning there was a difference in response to MEHP when comparing RPMI to RPMI + serum. Although RPMI stimulation was independent of MEHP treatment in the absence of serum, in cell-free RPMI medium containing 10% serum, 1-h incubation with MEHP significantly stimulated DCF fluorescence (Figure 1; ANOVA p<0.001; posthoc comparison of means p<0.01). Because DCF fluorescence was higher in all MEHP treatment groups (including controls) in RPMI with or without serum compared to HBSS alone, RPMI clearly contributes to significant generation of DCF fluorescence in the absence of cells.
3.2. TBBPA effects on DCF fluorescence in cell-free buffer
Cell-free experiments with TBBPA were conducted in HBSS only because fluorescence background levels were lower in HBSS buffer compared to RPMI culture medium (Figure 1). In cell-free solutions of HBSS and 25 μM TBBPA, 1 h incubation with carboxy-H2DCFDA increased fluorescence relative to solvent controls in a concentration-dependent manner (Figure 2; TBBPA treatment * carboxy-H2DCFDA concentration interaction, ANOVA p=0.03). Specifically, we observed significantly increased fluorescence with 5, 10, 50 or 100 μM carboxy-H2DCFDA in the presence of TBBPA compared with no TBBPA (solvent controls) for each concentration of carboxy-H2DCFDA (Figure 2; p <0.05). Moreover, fluorescence was elevated at 10, 50 and 100 μM carboxy-H2DCFDA compared with lower carboxy-H2DCFDA concentrations in the presence of TBBPA (p<0.01). Within the solvent controls, the fluorescence increase was significant only at 100 μM carboxy-H2DCFDA compared with 1 μM carboxy-H2DCFDA (p=0.02). Because this experiment was conducted in the absence of cells and serum, our results strongly suggest that TBBPA facilitated conversion of carboxy-H2DCF to the DCF fluorescent moiety.
Figure 2. TBBPA effect on DCF fluorescence in HBSS solution.
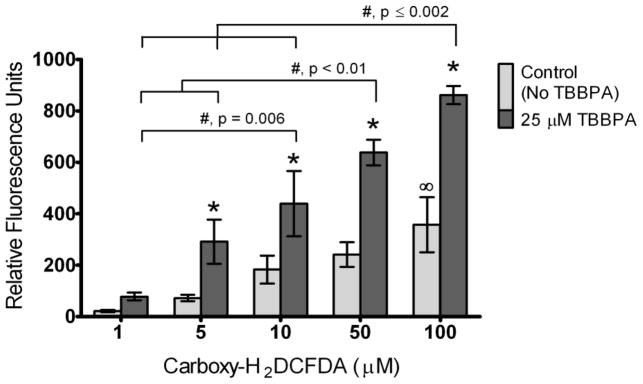
DCF fluorescence was assessed in cell-free and serum-free HBSS solutions containing 1, 5, 10, 50 or 100 μM DCFH2DA in either the absence (controls) or presence of 25 μM TBBPA. Controls were incubated with solvent only (0.1% DMSO). Columns represent means ± SE of 3 independent experiments containing 6 replicates each. There was a significant TBBPA * Carboxy-DCFH2DA interaction (ANOVA, p=0.03). *, Statistically significant increase compared to controls within a particular concentration of carboxy- H2DCFDA (p<0.05). ∞, Statistically significant increase in controls at 100 μM carboxy-H2DCFDA compared to controls at 1 μM carboxy-H2DCFDA. #, Statistically significant differences between samples incubated with TBBPA at different concentrations of carboxy-H2DCFDA (p-values shown on graph).
3.3. TBBPA effects on DCF fluorescence in the presence of cells
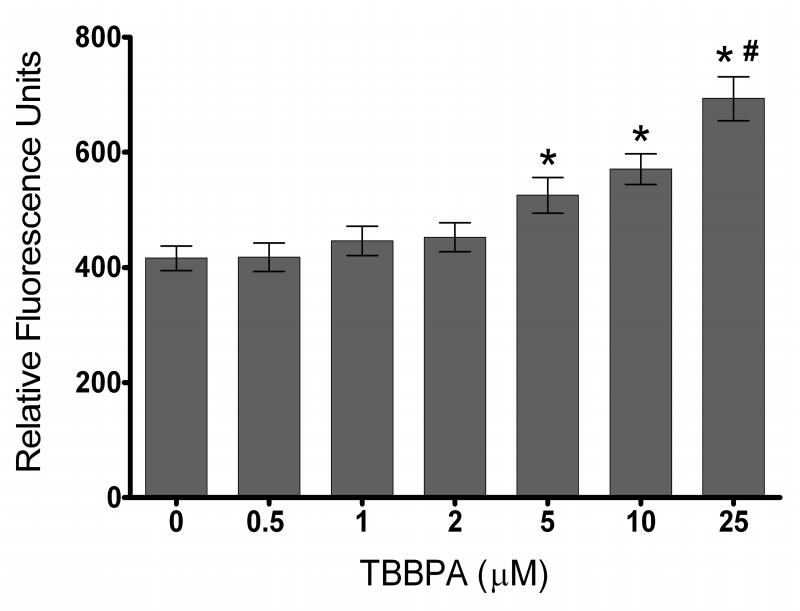
To illustrate the potential for misinterpretation of toxicant-stimulated cellular generation of ROS using the DCF assay, HTR8 cell cultures were exposed to 0.5, 1, 2, 5, 10 or 25 μM TBBPA for 1 h in HBSS. To minimize background fluorescence and avoid potential interaction with serum, as was observed for MEHP (Figure 1), the DCF assay was conducted in HBSS only for this experiment. The DCF fluorescence increased at 5, 10 and 25 μM TBBPA compared with solvent control (0 μM TBBPA) (Figure 3; p<0.05), similar to results observed in cell-free HBSS (compare to Figure 2). In the absence of knowledge about cell-free stimulation of DCF fluorescence by TBBPA, the cell culture results would have been erroneously attributed to cellular generation of reactive chemical species.
Figure 3. TBBPA effect on DCF fluorescence in HTR8 cell cultures.
HTR8 cells preloaded with carboxy-H2DCFDA (100 μM) were exposed to 0, 0.5, 1, 2, 5, 10 or 25 μM TBBPA for 1h in serum-free HBSS. Columns represent the means ± SE of 3 independent experiments containing 6 replicates each. *, Statistically significant increase compared with 0 μM TBBPA (solvent controls exposed to 0.1% DMSO) (p<0.05). #, Statistically significant increase compared with TBBPA concentrations less than 25 μM (p<0.05).
4. Discussion
The DCF assay is a popular method for assessing toxicant-stimulated generation of reactive oxidant species in cells (Bussche & Soares, 2011; Filipič & Hei, 2004; Gong & Han, 2006; Hatcher, et al., 2008; Hu, et al., 2011; Michalowicz, 2010; Naqvi, et al., 2010; Shanker & Aschner, 2003; Shao, et al., 2008; Wan & Winn, 2007; Zhang, et al., 2011; Zhu, et al., 2009). Although numerous caveats have been reported with the DCF assay, including effects of medium composition, serum, heme, heme proteins, metalloporphyrins and bovine serum albumin (Chen, et al., 2010), interactions with chemical toxicants have received nominal attention. Specifically relevant for the present study, reports of MEHP-stimulated and TBBPA-stimulated cellular generation of ROS using the DCF assay did not discuss cell-free assay controls (Bolling, et al., 2012; Fan, et al., 2010; Zhao, et al., 2012, Reistad, et al., 2005)). In contrast, recent reports discuss the impact of various experimental conditions on the accuracy of the DCF assay for assessment of x-radiation-stimulated and UVA-stimulated ROS generation. (Boulton, et al., 2011; Korystov, et al., 2007). In the present study, we used two different toxicants as models to evaluate different conditions under which the DCF assay may be performed, namely: MEHP in cell culture medium with or without serum and TBBPA in the presence or absence of cells.
In cell-free experiments, we observed that MEHP stimulated a concentration-dependent increased fluorescence in cell-free RPMI medium containing carboxy-H2DCFDA, but required the presence of serum. To our knowledge, this report is the first demonstration that a toxicant interacted with serum to stimulate DCF fluorescence. Whereas others have noted stimulation of DCF fluorescence with increasing concentrations of serum in HBSS (Korystov, et al., 2007), we did not see increased fluorescence in RPMI medium with the addition of serum in the absence of MEHP (i.e., comparing control groups of RPMI with RPMI+Serum). Rather, it was the interaction between MEHP and serum that generated increased fluorescence in our experiments. Specifically, MEHP did not significantly affect DCF fluorescence in either HBSS or RPMI assay solutions lacking serum when compared to the solvent control in the same type of assay solution. Additionally, similar to previous research demonstrating that type of assay solution can impact DCF fluorescence (Han, et al., 2008; Han, et al., 2009; Kalinich, et al., 1997; Korystov, et al., 2007), we observed significantly lower DCF fluorescence for all treatment groups when HBSS was used as the buffer compared to RPMI or RPMI + serum.
In contrast to MEHP, TBBPA interacted with carboxy-H2DCFDA in the absence of serum to increase DCF fluorescence in cell-free HBSS. This novel finding suggests that TBBPA facilitated conversion of carboxy-H2DCFDA to the DCF fluorescent moiety. Although we know of no other similar findings with toxicants, Trolox and other antioxidants increase DCF fluorescence in cell-free and serum-free HMCK buffer (Kalinich, et al., 1997). In the latter report, it was suggested that carboxy-H2DCFDA can be deacetylated to the DCFH intermediate in cell-free buffer, and that subsequent oxidation of the antioxidants allows conversion of DCFH to the fluorescent DCF (Kalinich, et al., 1997). TBBPA undergoes photooxidation in the presence of humic acid to form a highly reactive benzoquinone radical intermediate (Han, et al., 2008; Han, et al., 2009) and photodegrades in a pH-dependent manner (Eriksson, et al., 2004), supporting the plausibility that TBBPA chemically converts carboxy-H2DCFDA to the DCF fluorescent moiety. Additionally, others have demonstrated that halogenated compounds with structural similarity to TBBPA are transformed photochemically in the natural environment and under certain experimental conditions (Ohko, et al., 2001; Yao, et al., 1997). Future experiments are needed to determine whether oxidation of TBBPA could explain increased fluorescence in cell-free and serum-free salt solutions containing carboxy-H2DCFDA.
Following a standard protocol for the DCF assay, we observed TBBPA-stimulated DCF fluorescence in HTR8 trophoblast cell cultures. If we had failed to consider possible confounding of the fluorescence results due to interactions between TBBPA and carboxy-H2DCFDA (or its products), we would have concluded that the DCF assay results supported TBBPA-stimulated cellular generation of ROS. However, because TBBPA increased fluorescence in the absence of cells, the increased DCF fluorescence in the presence of cells cannot be attributed to cellular generation of ROS and may instead be the result of chemical activation of carboxy-H2DCFDA to the fluorescent DCF moiety. Nonetheless, it remains possible that TBBPA stimulates ROS formation in HTR8 cells but cannot be measured with the DCF assay, because increased ROS generation has been demonstrated previously using alternative experimental techniques in algae (Liu, et al., 2008) and plants (Sun, et al., 2008).
Both MEHP and TBBPA are toxicants of concern for human health. MEHP is a biologically active metabolite of the plasticizer DEHP (Koch, et al., 2006), to which human exposure is nearly ubiquitous (NHANES 1999–2000) (Silva, et al., 2004). Tetrabromobisphenol A (TBBPA) is a brominated flame retardant suggested as a substitute for polybrominated diphenyl ethers (PBDEs) (Talsness, et al., 2009). Because generation of reactive oxygen species is a common and significant mechanism of intoxication with myriad potential ramifications for the cell and organism (Jones, 2006), it is important to recognize and control for caveats of assays used to assess cellular ROS generation. Our findings demonstrate that preliminary control experiments need to assess potential interactions between a toxicant and carboxy-H2DCFDA prior to utilizing the DCF assay to assess toxicant-stimulated production of ROS. Although our experiments were limited to the more recently available pro-DCF probe carboxy-H2DCFDA, we suggest that the current study nonetheless presents valuable cautionary information for others using the DCF assay to assess toxicant-stimulated ROS production regardless of the parent pro-fluorescence compound used. Our observations further suggest that the DCF assay would not be appropriate to use to determine TBBPA-stimulated cell-mediated ROS production but would be applicable for measuring cell-mediated generation of ROS by MEHP, provided that the experimental solutions were serum free.
Acknowledgments
We thank Dr. Mark Miller for helpful discussions regarding the DCF assay. This research was supported by a Ruth L. Kirschstein NRSA Institutional Training Grant Fellowship from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIEHS, NIH) to P.W. Kamau (T32 ES007062), NIH grant to R. Loch-Caruso (R01 ES014860), and a project supported by the NIEHS Superfund Research Program PROTECT Center to R.L-C (P42 ES017198).
Footnotes
Disclaimer: None
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bolling AK, Ovrevik J, Samuelsen JT, Holme JA, Rakkestad KE, Mathisen GH, Paulsen RE, Korsnes MS, Becher R. Mono-2-ethylhexylphthalate (MEHP) induces TNF-alpha release and macrophage differentiation through different signalling pathways in RAW264.7 cells. Toxicology Letters. 2012;209:43–50. doi: 10.1016/j.toxlet.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Boulton S, Anderson A, Swalwell H, Henderson JR, Manning P, Birch-Machin MA. Implications of using the fluorescent probes, dihydrorhodamine 123 and 2′,7′-dichlorodihydrofluorescein diacetate, for the detection of UVA-induced reactive oxygen species. Free Radical Research. 2011;45:115–122. doi: 10.3109/10715762.2010.517751. [DOI] [PubMed] [Google Scholar]
- Bussche JV, Soares EV. Lead induces oxidative stress and phenotypic markers of apoptosis in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2011;90:679–687. doi: 10.1007/s00253-010-3056-7. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhong Z, Xu Z, Chen L, Wang Y. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radical Research. 2010;44:587–604. doi: 10.3109/10715761003709802. [DOI] [PubMed] [Google Scholar]
- Epling CL, Stites DP, McHugh TM, Chong HO, Blackwood LL, Wara DW. Neutrophil function screening in patients with chronic granulomatous disease by a flow cytometric method. Cytometry. 1992;13:615–620. doi: 10.1002/cyto.990130609. [DOI] [PubMed] [Google Scholar]
- Eriksson J, Rahm S, Green N, Bergman Å, Jakobsson E. Photochemical transformations of tetrabromobisphenol A and related phenols in water. Chemosphere. 2004;54:117–126. doi: 10.1016/S0045-6535(03)00704-5. [DOI] [PubMed] [Google Scholar]
- Fan J, Traore K, Li W, Amri H, Huang H, Wu C, Chen H, Zirkin B, Papadopoulos V. Molecular mechanisms mediating the effect of mono-(2-ethylhexyl) phthalate on hormone-stimulated steroidogenesis in MA-10 mouse tumor Leydig cells. Endocrinology. 2010;151:3348–3362. doi: 10.1210/en.2010-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipič M, Hei TK. Mutagenicity of cadmium in mammalian cells: implication of oxidative DNA damage. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2004;546:81–91. doi: 10.1016/j.mrfmmm.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Fruhwirth M, Ruedl C, Ellemunter H, Bock G, Wolf H. Flow-cytometric evaluation of oxidative burst in phagocytic cells of children with cystic fibrosis. International archives of allergy and immunology. 1998;117:270–275. doi: 10.1159/000024022. [DOI] [PubMed] [Google Scholar]
- Gong Y, Han XD. Nonylphenol-induced oxidative stress and cytotoxicity in testicular Sertoli cells. Reproductive Toxicology. 2006;22:623–630. doi: 10.1016/j.reprotox.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- Hafer K, Iwamoto KS, Schiestl RH. Refinement of the dichlorofluorescein assay for flow cytometric measurement of reactive oxygen species in irradiated and bystander cell populations. Radiation Research. 2008;169:460–468. doi: 10.1667/RR1212.1. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? British Journal of Pharmacology. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Bilski P, Karriker B, Sik RH, Chignell CF. Oxidation of flame retardant tetrabromobisphenol A by singlet oxygen. Environmental Science & Technology. 2008;42:166–172. doi: 10.1021/es071800d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Sik RH, Motten AG, Chignell CF, Bilski PJ. Photosensitized oxidation of tetrabromobisphenol a by humic acid in aqueous solution. Photochemistry and photobiology. 2009;85:1299–1305. doi: 10.1111/j.1751-1097.2009.00608.x. [DOI] [PubMed] [Google Scholar]
- Hatcher JM, Delea KC, Richardson JR, Pennell KD, Miller GW. Disruption of dopamine transport by DDT and its metabolites. Neurotoxicology. 2008;29:682–690. doi: 10.1016/j.neuro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N, Guo R, Han X, Zhu B, Ren J. Cardiac-specific overexpression of metallothionein rescues nicotine-induced cardiac contractile dysfunction and interstitial fibrosis. Toxicology Letters. 2011;202:8–14. doi: 10.1016/j.toxlet.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molecular Probes. Grand Island, NY: 2006. Reactive Oxygen Species (ROS) Detection Reagents; pp. 1–5. Invitrogen. [Google Scholar]
- Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Kalinich JF, Ramakrishnan N, McClain DE. The antioxidant Trolox enhances the oxidation of 2′,7′-dichlorofluorescin to 2′,7′-dichlorofluorescein. Free Radical Research. 1997;26:37–47. doi: 10.3109/10715769709097782. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee BC, Sim GS, Lee DH, Lee KE, Yun YP, Pyo HB. The isolation and antioxidative effects of vitexin from Acer palmatum. Arch Pharm Res. 2005;28:195–202. doi: 10.1007/BF02977715. [DOI] [PubMed] [Google Scholar]
- Koch HM, Preuss R, Angerer J. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure-- an update and latest results. Int J Androl. 2006;29:155–165. doi: 10.1111/j.1365-2605.2005.00607.x. discussion 181–155. [DOI] [PubMed] [Google Scholar]
- Korystov YN, Shaposhnikova VV, Korystova AF, Emel’yanov MO. Detection of reactive oxygen species induced by radiation in cells using the dichlorofluorescein assay. Radiation research. 2007;168:226–232. doi: 10.1667/RR0925.1. [DOI] [PubMed] [Google Scholar]
- Liu H, Yu Y, Kong F, He L, Yu H, Giesy JP, Wang X. Effects of tetrabromobisphenol A on the green alga Chlorella pyrenoidosa. Journal of environmental science and health Part A, Toxic/hazardous substances & environmental engineering. 2008;43:1271–1278. doi: 10.1080/10934520802177821. [DOI] [PubMed] [Google Scholar]
- Michalowicz J. 2,4,5-trichlororophenol and its derivatives induce biochemical and morphological changes in human peripheral blood lymphocytes in vitro. Archives of environmental contamination and toxicology. 2010;59:670–678. doi: 10.1007/s00244-010-9508-3. [DOI] [PubMed] [Google Scholar]
- Naqvi S, Samim M, Abdin M, Ahmed FJ, Maitra A, Prashant C, Dinda AK. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. International journal of nanomedicine. 2010;5:983–989. doi: 10.2147/IJN.S13244. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ohashi T, Mizutani A, Murakami A, Kojo S, Ishii T, Taketani S. Rapid oxidation of dichlorodihydrofluorescin with heme and hemoproteins: formation of the fluorescein is independent of the generation of reactive oxygen species. FEBS letters. 2002;511:21–27. doi: 10.1016/s0014-5793(01)03262-8. [DOI] [PubMed] [Google Scholar]
- Ohko Y, Ando I, Niwa C, Tatsuma T, Yamamura T, Nakashima T, Kubota Y, Fujishima A. Degradation of bisphenol A in water by TiO2 photocatalyst. Environmental Science & Technology. 2001;35:2365–2368. doi: 10.1021/es001757t. [DOI] [PubMed] [Google Scholar]
- Reistad T, Mariussen E, Fonnum F. The effect of a brominated flame retardant, tetrabromobisphenol-A, on free radical formation in human neutrophil granulocytes: the involvement of the MAP kinase pathway and protein kinase C. Toxicological sciences: an official journal of the Society of Toxicology. 2005;83:89–100. doi: 10.1093/toxsci/kfh298. [DOI] [PubMed] [Google Scholar]
- Rota C, Chignell CF, Mason RP. Evidence for free radical formation during the oxidation of 2′-7′-dichlorofluorescin to the fluorescent dye 2′-7′-dichlorofluorescein by horseradish peroxidase: possible implications for oxidative stress measurements. Free radical biology & medicine. 1999;27:873–881. doi: 10.1016/s0891-5849(99)00137-9. [DOI] [PubMed] [Google Scholar]
- Shanker G, Aschner M. Methylmercury-induced reactive oxygen species formation in neonatal cerebral astrocytic cultures is attenuated by antioxidants. Brain research Molecular brain research. 2003;110:85–91. doi: 10.1016/s0169-328x(02)00642-3. [DOI] [PubMed] [Google Scholar]
- Shao J, White CC, Dabrowski MJ, Kavanagh TJ, Eckert ML, Gallagher EP. The role of mitochondrial and oxidative injury in BDE 47 toxicity to human fetal liver hematopoietic stem cells. Toxicological sciences: an official journal of the Society of Toxicology. 2008;101:81–90. doi: 10.1093/toxsci/kfm256. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam R, Fan XJ, Scivittaro V, Yang J, Ha CE, Petersen CE, Surewicz WK, Bhagavan NV, Weiss MF, Monnier VM. Cellular oxidant stress and advanced glycation endproducts of albumin: caveats of the dichlorofluorescein assay. Arch Biochem Biophys. 2002;400:15–25. doi: 10.1006/abbi.2002.2776. [DOI] [PubMed] [Google Scholar]
- Sun Y, Guo H, Yu H, Wang X, Wu J, Xue Y. Bioaccumulation and physiological effects of tetrabromobisphenol A in coontail Ceratophyllum demersum L. Chemosphere. 2008;70:1787–1795. doi: 10.1016/j.chemosphere.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Talsness CE, Andrade AJ, Kuriyama SN, Taylor JA, vom Saal FS. Components of plastic: experimental studies in animals and relevance for human health. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2009;364:2079–2096. doi: 10.1098/rstb.2008.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Winn LM. Benzene’s metabolites alter c-MYB activity via reactive oxygen species in HD3 cells. Toxicology and Applied Pharmacology. 2007;222:180–189. doi: 10.1016/j.taap.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Yao Y, Kakimoto K, Ogawa HI, Kato Y, Hanada Y, Shinohara R, Yoshino E. Photodechlorination pathways of non-ortho substituted PCBs by ultraviolet irradiation in alkaline 2-propanol. Bulletin of environmental contamination and toxicology. 1997;59:238–245. doi: 10.1007/s001289900470. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Su L, Huang B, Zhao J, Zhao BX, Zhang SL, Miao JY. N-benzyl-5-phenyl-1H-pyrazole-3-carboxamide promotes vascular endothelial cell angiogenesis and migration in the absence of serum and FGF-2. Acta pharmacologica Sinica. 2011;32:209–216. doi: 10.1038/aps.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ao H, Chen L, Sottas CM, Ge RS, Li L, Zhang Y. Mono-(2-ethylhexyl) phthalate affects the steroidogenesis in rat Leydig cells through provoking ROS perturbation. Toxicology in vitro: an international journal published in association with BIBRA. 2012;26:950–955. doi: 10.1016/j.tiv.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Kalen AL, Li L, Lehmler HJ, Robertson LW, Goswami PC, Spitz DR, Aykin-Burns N. Polychlorinated-biphenyl-induced oxidative stress and cytotoxicity can be mitigated by antioxidants after exposure. Free radical biology & medicine. 2009;47:1762–1771. doi: 10.1016/j.freeradbiomed.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]