Abstract
Objective
The western borderland between Yunnan Province, China, and Myanmar is characterized by a climate that facilitates year-round production of mosquitoes. Numerous mosquito-transmitted viruses, including Japanese encephalitis virus circulate in this area. This project was to describe seasonal patterns in mosquito species abundance and arbovirus activity in the mosquito populations.
Methods
Mosquitoes were collected in Mangshi and Ruili cities of Dehong Prefecture near the border of China and Burma in Yunnan Province, the Peoples Republic of China in 2010. We monitored mosquito species abundance for a 12-month period using ultraviolet light, carbon dioxide baited CDC light and gravid traps; and tested the captured mosquitoes for the presence of virus to evaluate mosquito-virus associations in rural/agricultural settings in the area.
Results
A total of 43 species of mosquitoes from seven genera were collected, including 15 Culex species, 15 Anopheles spp., four Aedes spp., three Armigeres spp., one Mimomyia spp., two Uranotaenia spp. and three Mansonia spp.. Species richness and diversity varied between Mangshi and Ruili. Culex tritaeniorhynchus, Culex quinquefasciatus, Anopheles sinensis and Anopheles peditaeniatus were the most abundant species in both sampling sites. Ultraviolet light traps collected more specimens than CDC light traps baited with dry ice, though both collected the same variety of mosquito species. The CDC gravid trap was the most effective trap for capture of Culex quinquefasciatus, a species underrepresented in light trap collections. A total of 26 virus strains were isolated, which included 13 strains of Japanese encephalitis virus, four strains of Getah virus, one strain of Oya virus, one strain from the orbivirus genus, and seven strains of Culex pipien pallens densovirus.
Conclusions
The present study illustrates the value of monitoring mosquito populations and mosquito-transmitted viruses year-round in areas where the climate supports year-round adult mosquito activity.
Introduction
Yunnan Province is located in Southwest China, adjacent to Burma, Laos and Vietnam. Ecologically, Yunnan Province supports year-round production of mosquitoes and transmission of mosquito-borne viruses because of its low altitude and climate, which is borderline tropical - South sub-tropical, characterized by high temperature, humidity and rainfall during half of the year, and moderate temperature and rainfall conditions during the winter months. Human cases of Japanese encephalitis (JE) occur commonly in Yunnan Province [1] and Japanese encephalitis virus (JEV) (Genus Flavivirus, Family Flaviviridae), Dengue virus (DENV) (Genus Flavivirus, Family Flaviviridae), Chikungunya virus (CHIKV) (Genus Alphavirus, Family Togaviridae), Sindbis virus (SINV) (Genus Alphavirus, Family Togaviridae), Getah virus (GETV) (Genus Alphavirus, Family Togaviridae), Batai virus (BATV) (Genus Orthobunyavirus, Family Bunyaviridae), Banna virus (BAV) (Genus Seadornavirus, Family Reoviridae), Yunnan orbivirus (YUOV) (Genus Obivirus, Family Reoviridae) and several other viruses have been isolated from human patients and from mosquitoes collected in Yunnan Province [2-18]. Previous investigations describing mosquitoes and their association with arboviruses [3-8,10,14,16-21], have been limited to summer months and do not provide information about the ecology of vectors and viruses during periods when mosquito and virus activity is low. This information is critical for preventative vector-control interventions aimed at reducing virus amplification in subsequent seasons.
The primary objective of this project is to describe 12-month seasonal patterns in mosquito species abundance and arbovirus transmission activity in western Yunnan Province and secondarily, evaluate the performance of three different mosquito collecting traps in sampling different mosquito species at different times of year.
Methods
Ethics Statement
During mosquito collections, the local residents allowed us to access to their residences and land.
Collection Sites
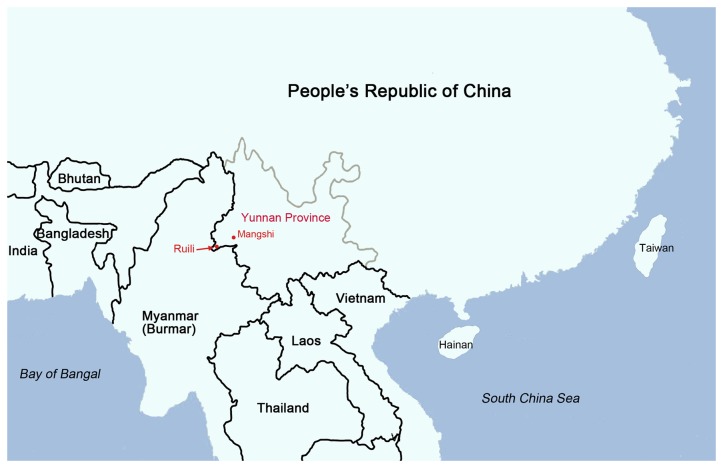
Mosquitoes were collected in Yunnan Province, the Peoples Republic of China, in 2 villages each near the cities of Mangshi (previously known as Luxi city) and Ruili of Dehong Prefecture located near the Myanmar border (Table 1, Figure 1). The villages where collections were carried out are similar and characterized as agricultural with residential farms, supporting irrigated rice fields, row crops, and vegetable gardens, and scattered fish ponds. Pigpens were usually located adjacent to the residential houses. Weather data (monthly average temperature and relative humidity, and total precipitation) were obtained from weather stations at local airports located near each city. In general, Ruili, located approximately 80 km to the NW is warmer and wetter than Mangshi.
Table 1. Geographic location and elevation of villages where mosquito collections occurred from Jan-Dec 2010.
| City | Average Temp. °C | Average Rainfall | Villages/site | Longitude | Latitude | Elevation |
|---|---|---|---|---|---|---|
| Mangshi | 20.9/9.8 (high/low) | 960 mm | Lameng/1 | 98.565175 | 24.39278 | 849 m |
| Lameng/2 | 98.567766 | 24.39231 | 850 m | |||
| Lahui/1 | 98.58739 | 24.47483 | 869 m | |||
| Lahui/2 | 98.542197 | 24.47391 | 872 m | |||
| Ruili | 27.4/15.8 (high/low) | 1,454 mm | Hexing/1 | 97.80429 | 23.98538 | 729 m |
| Hexing/2 | 97.81492 | 23.99796 | 718 m | |||
| Qidui/1 | 97.88141 | 24.03757 | 740 m | |||
| Qidui/2 | 97.88329 | 24.03808 | 729 m |
Figure 1. Location of villages in Mangshi and Ruili, Yunnan Province, China, where mosquito collection occurred.
Mosquito Sampling Procedures
Two residences in each of the 4 rural villages, were selected as sampling sites. Each of the selected residences had pigs or cows on the property that were housed adjacent to farmland or wooded areas. At each site mosquito sampling was carried out with three types of mosquito traps.
Traditional Trap (TT)
Kongfu Xiaoshuai trap (Wuhan Jixing Environmental Protection Scientific and Technological Limited-liability Company) is designed to collect host-seeking female mosquitoes and has been used extensively in previous surveys carried out in Yunnan Province [5-7,16]. The trap contains an ultraviolet (UV) light source and a fan, and utilizes 220V power source. For this study, this type of trap was placed in close proximity to each residence (often next to the pigpen) where a power supply was available.
Light Trap (LT)
The CDC miniature light trap (BioQuip Products, Rancho Dominguez, CA, USA) also traps host-seeking mosquitoes, has a light source and uses dry ice as a carbon dioxide bait [22]. Because these traps are powered by D-cell batteries, they were placed in wooded areas adjacent to the houses.
Gravid Trap (GT)
The CDC Gravid Trap [23] (BioQuip Products, Rancho Dominguez, CA, USA), was designed with a plastic basin (550 mm × 400 mm × 200 mm) in which a fresh mixture of pig manure and water was made each night to attract gravid mosquitoes. Gravid traps were placed within 15 m of pigpens or cow sheds near the residence.
Mosquito collections were conducted for two consecutive nights each month, at each site, for a total of 16 trap nights/trap type/month. Traps were set at 1700-1900 h on each collecting day and mosquitoes were removed from the trap at 0700-0800 the following morning. Collected mosquitoes were immediately transported from the field sites to the laboratory and killed with cold by placing them at -20 °C for 30 min. Specimens were then identified to species and sex, and counted on a chill table. Following identification to species, females were sorted into pools of up to 100 specimens according to species, date, location, and trap type. Pools of mosquitoes were stored on liquid nitrogen until processed for arboviruses at the laboratory of the Yunnan Institute of Endemic Control and Prevention in Dali, Yunnan Province.
Identification of Mosquito Species and Estimation of Abundance, Species Richness and Diversity
Results were tabulated by species, sex, date, location, and trap type in order to evaluate efficiency of the different trap types and differences in mosquito community structure and relative abundance by location. Since trap effort was consistent among cities (i.e., the same number of traps of each type run each month), only two trap failures were recorded during the entire project, and additional trap days were added at the affected sites to compensate for the trap failures, overall measures of species abundance are given as the sum of mosquitoes of a given species from all three trap types during a given collecting month.
Species richness is represented by the number of species collected at each location. Species diversity for each location was estimated using the Simpson Diversity Index (SDI), which is calculated as 1-(Σ n(n-1) / N(N-1)) where n=the total number of mosquitoes of a species and N = the total number of mosquitoes of all species in each location [24]. The SDI represents the probability that two randomly-selected individuals in a community are of different species. An index of 0 indicates no species diversity (i.e. each sampled member of the community belongs to a same species) whereas an index of 1 indicates maximum diversity. SDI gives more weight to the more abundant species and rare species only cause small changes in the value.
Statistical Analysis
Due to the difference of latitude, altitude, humidity, and rainfall between Mangshi and Ruili, statistical analysis was conducted to compare species richness and the total number collected between these two sampling locations. Different trap types were also statistically compared for their abilities in colleting mosquitoes.
The t-Test was performed to compare SDI and species richness between Mangshi and Ruili.
The Z-Test was used to compare means of populations characterized by a Poisson distribution. In this study, Z-test was performed to compare mean number of specimens and number of species collected among different trap types, and to compare the abundance of the four most commonly collected species in Ruili and Mangshi.
The Mann-Whitney U Test was used to compare number of the four most commonly collected species among different trap types, and to evaluate differences among different trap types.
All statistical tests were conducted using SPSS (version 16.0, IBM).
Canonical Correspondence Analysis (CCA) is a multivariate method widely used to analyze the relationships between species and environmental variables [25-28]. In the present study, CCA (CCA, CANOCO version 4.5) was used to examine the relationship between the abundance of the mosquito species and the meteorological variables including monthly average temperature, precipitation, and relative humidity. In view of the high species richness and many uncommon species being collected in very small number, species whose abundance was less than 1% of the total were excluded from this analysis. We constructed two contingency tables: data on the abundance of mosquito species collected in each month of the year, and data on the three meteorological variables. CCAs then were run separately for Mangshi and Ruili, since the areas differed in meteorological characteristics. Thus, two ordinations were generated, expressing the main relationships between species and each of the climatic variables; and two ordination biplots were constructed to aid interpretation of temporal patterns observed in the selected species.
Virus Isolation and Identification
Mosquito pools were homogenized with minimum essential medium (MEM) in a sterile grinder and centrifuged as descibed [29-31]. To isolate the virus, 150 μL of supernatant from each pool was inoculated onto monolayers of baby hamster kidney-BHK-21, African green monkey kidney- Vero-Osaka, and Aedes albopictus C6/36 cells in 24-well cell culture plates. After seven days, 150-μL supernatant from the previous passage was inoculated onto a new passage in cells as above. Inoculated cells were maintained at 37 °C or 28 °C in an incubator with 5% CO2, and examined daily for cytopathic effect (CPE) from 1-7 days post-inoculation. A specimen was considered to be positive if it caused CPE in three successive cell passages. Infected cell supernatants were harvested and stored at -80°C until further identification.
Reverse-transcription polymerase chain reaction (RT-PCR) was employed to identify the isolates. The following primers were chosen according to the distribution of arboviruses in Southeast Asia: flavivirus-specific primers [32], alphavirus-specific primers [33], bunyavirus-specific primers [6], and primers to detect the JEV envelope gene [34], GETV E2 gene [35], VP6 gene of Orbivirus (Family Reoviridae) [18], VP7 gene of YUOV [18], partial NS1 gene of densovirus (Genus Densovirus, Family Densoviridae) [30,36], and S gene of Oya virus (OYV) (Simbu serogroup, Genus bunyavirus, Family Bunyaviridae) [31] (Table 2). Viral RNA was extracted by using the QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA), and cDNA was synthesized by using Ready-To-Go You-Prime First Strand Beads (American Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer’s instructions. With the above-mentioned primers in order of descending taxon from genus to species, each of the positive isolates was tested until PCR products were visible in gel electrophoresis.
Table 2. Primers used to identify and sequence viruses isolated from mosquitoes in this study.
| Primers | Primers sequence (5′ to 3′) | Size, base pairs |
|---|---|---|
| Alphavirus | ||
| M2W | YAGAGCDTTTTCGCAYSTRGCHW | |
| cM3W | ACATRAANKGNGTNGTRTCRAANCCDAYCC | 434 |
| M2W2 | TGYCCNVTGMDNWSYVCNGARGAYCC | |
| GETVCaF | TGGTCCCGCATCGGATTGAG | |
| GETVCaR | CTTTCATGTCATGCCCGGCGATG | 1450 |
| GETVE2F | GTAACAATAGTGCACGCCACC | |
| GETVE2R | GGCAGCAGCAAAGCAGGTTC | 1400 |
| Flavivirus | ||
| FU1 | TACCACATGATGGGAAAGAGAGAGAA | |
| cFD2 | GTGTCCCAGCCGGCGGTGTCATCAGC | 310 |
| JEPrM251F | CGTTCTTCAAGTTTACAGCATTAGC | |
| JEPrM743R | CGYTTGGAATGYCTRGTCCG | 492 |
| JEPrM925R | CCYRTGTTYCTGCCAAGCATCCAMCC | |
| JE955F | TGYTGGTCGCTCCGGCTTA | |
| JE2536R | AAGATGCCACTTCCACAYCTC | 1581 |
| Bunyavirus | ||
| BUP | ATGACTGAGTTGGAGTTTGATGTCGC | |
| BDW | TGTTCCTGTTGCCAGGAAAAT | 250 |
| Densovirus | ||
| DNV-3F | TGTCTCTTTCTCTTGGTATTTCTTC | |
| DNV-3R | CATACTACACATTCGTCCTCCAC | 903 |
| Yunnan obivirus | ||
| YUOVSeg7S1 | AGCATTCGGTACGCAGTATCTCG | |
| YUOVSeg7R1 | GCCGAGCCGATCATGTCACG | 453 |
| YUOVSeg7S2 | GAACGACAACGCATTTTGA | |
| YUOVSeg7R2 | CACGTTGTTCCTGCACTTGGTCTG | 369 |
| Orbivirus | ||
| 6-1-2F/230F | ATGCGTATGGAGGAAGAG | |
| 6-1-2R/1202R | AAAGTTGAGGCACCAGTA | 972 |
| Oya virus | ||
| Oya-S-21f | GACGCCGAGGCCCAATGTTA | |
| Oya-S-334r | CAAGCGGGAAGAAGGTGAATGTC | 313 |
Note: F means forward primer; R means reverse primer. M: C/A; W: A/T; Y: C/T; K: G/T; R: G/A; V: G/A/C; D: T/
Sequencing was performed by Beijing BioMed Biotechnology Co., Ltd. Initial sequence assembly and analysis were conducted by using the ATGC software package Version 4.0 (GENETYX Corp, Tokyo, Japan). Clustal X (Version 1.83) and MegAlign (DNASTAR, Madison, USA) were used for homology and alignment analysis, and MEGA 3.1 for phylogenetic analysis and tree construction, based on the neighbor-joining assay. The bootstrap value (the number of replications) was 1,000.
Results
Species Richness and Diversity of Mosquitoes
Over the course of the 12-month study, a total of 85,307 female mosquitoes comprised of 43 species representing seven genera were collected using the three types of mosquito traps in villages near the cities of Mangshi and Ruili (Table 3). Of the total number, 82% were Culex (15 spp.) and 16% were Anopheles (15 spp.). Representatives of Aedes (four spp.), Armigeres (three spp.), Mansonia (three spp.), Uranotaenia (two spp.), and Mimomyia (one spp.) constituted the remaining 2% of the collections.
Table 3. Summary of mosquito species collected in Mangshi and Ruili during Jan-Dec, 2010.
| Species |
Ruili
|
Mangshi
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TT | LT | GT | total | % | TT | LT | GT | total | % | |
| Cx. quinquefasciatus Say | 264 | 207 | 3,410 | 3,881 | 10.47 | 341 | 211 | 5,646 | 6,198 | 12.84 |
| Cx. tritaeniorhynchus Giles | 23,997 | 2,865 | 19 | 26,881 | 72.55 | 29,998 | 1,168 | 106 | 31,272 | 64.81 |
| Cx. mimeticus Noe | 4 | 5 | 2 | 11 | 0.03 | 1 | 2 | 0 | 3 | 0.01 |
| Cx. theileri Theobald | 10 | 6 | 1 | 17 | 0.05 | 26 | 1 | 1 | 28 | 0.06 |
| Cx. bitaeniorhynchus Giles | 12 | 8 | 0 | 20 | 0.05 | 2 | 2 | 1 | 5 | 0.01 |
| Cx..halifaxi Theobald | 0 | 0 | 1 | 1 | 0.00 | 0 | 0 | 2 | 2 | 0.00 |
| Cx. pallidothrax Theobald | 9 | 8 | 15 | 32 | 0.09 | 0 | 2 | 7 | 9 | 0.02 |
| Cx. fuscanus Wiedemann | 9 | 18 | 8 | 35 | 0.09 | 9 | 16 | 6 | 31 | 0.06 |
| Cx. annulus Theobald | 96 | 252 | 1 | 349 | 0.94 | 165 | 65 | 1 | 231 | 0.48 |
| Cx. nigropunctatus Edwards | 20 | 39 | 15 | 74 | 0.20 | 4 | 4 | 4 | 12 | 0.02 |
| Cx. gelidus Theobald | 473 | 26 | 0 | 499 | 1.35 | 5 | 1 | 0 | 6 | 0.01 |
| Cx. jacksoni Edwards | 0 | 1 | 0 | 1 | 0.00 | 0 | 0 | 0 | 0 | 0.00 |
| Cx fuscocephalus Theobald | 853 | 113 | 2 | 968 | 2.61 | 37 | 0 | 1 | 38 | 0.08 |
| Cx. bailyi Barraud | 0 | 10 | 0 | 10 | 0.03 | 0 | 0 | 0 | 0 | 0.00 |
| Cx. pseudovishnui Colless | 0 | 1 | 0 | 1 | 0.00 | 0 | 1 | 0 | 1 | 0.00 |
| An. sinensis Wiedemann | 1,029 | 64 | 1 | 1,094 | 2.95 | 6,404 | 141 | 11 | 6,556 | 13.59 |
| An. maculatus Theobald | 8 | 0 | 0 | 8 | 0.02 | 2 | 1 | 0 | 3 | 0.01 |
| An. minimus Theobald | 7 | 2 | 0 | 9 | 0.02 | 3 | 2 | 0 | 5 | 0.01 |
| An. culicifacies Giles | 1 | 0 | 0 | 1 | 0.01 | 4 | 0 | 0 | 4 | 0.01 |
| An. vagus Donitz | 279 | 41 | 2 | 322 | 0.87 | 536 | 17 | 45 | 598 | 1.24 |
| An. peditaeniatus Leicester | 1,065 | 322 | 0 | 1,387 | 3.74 | 2,487 | 421 | 11 | 2,919 | 6.05 |
| An. tessellatus Theobald | 512 | 111 | 1 | 624 | 1.68 | 67 | 4 | 4 | 75 | 0.16 |
| An.barbirostris Van der Mulp | 33 | 2 | 0 | 35 | 0.09 | 1 | 2 | 0 | 3 | 0.01 |
| An. kochi Donitz | 19 | 4 | 0 | 23 | 0.06 | 1 | 0 | 0 | 1 | 0.00 |
| An. splendidus Koidzumi | 7 | 0 | 0 | 7 | 0.02 | 0 | 0 | 0 | 0 | 0.00 |
| An. annularis Van der Mulp | 1 | 0 | 0 | 1 | 0.00 | 0 | 0 | 0 | 0 | 0.00 |
| An. crawfordi Reid | 6 | 3 | 0 | 9 | 0.02 | 1 | 0 | 0 | 1 | 0.00 |
| An. aconitus Donitz | 0 | 1 | 0 | 1 | 0.00 | 0 | 0 | 0 | 0 | 0.00 |
| An.gigasbaileyi Edwards | 2 | 0 | 0 | 2 | 0.01 | 4 | 0 | 0 | 4 | 0.01 |
| Ae. elsiae Barraud | 341 | 26 | 0 | 367 | 0.99 | 173 | 25 | 0 | 198 | 0.41 |
| Ae. lineatopennis Ludlow | 3 | 2 | 0 | 5 | 0.01 | 0 | 0 | 0 | 0 | 0.00 |
| Ae. albopictus Skuse | 3 | 1 | 1 | 5 | 0.01 | 0 | 1 | 0 | 1 | 0.00 |
| Ae. annandalei Theobald | 0 | 2 | 0 | 2 | 0.01 | 0 | 0 | 0 | 0 | 0.00 |
| Ar. subalbatus Coquillett | 89 | 35 | 60 | 184 | 0.50 | 23 | 7 | 14 | 44 | 0.09 |
| Ar. inchoatus Barraud | 1 | 2 | 0 | 3 | 0.01 | 0 | 0 | 0 | 0 | 0.00 |
| Ar. omissus Edwards | 0 | 1 | 0 | 1 | 0.00 | 0 | 0 | 0 | 0 | 0.00 |
| Mm. luzonensis Ludlow | 20 | 13 | 1 | 34 | 0.09 | 0 | 1 | 0 | 1 | 0.00 |
| Ur. maxima Leicester | 1 | 3 | 0 | 4 | 0.01 | 0 | 0 | 0 | 0 | 0.00 |
| Ur. novobscura Barraud | 0 | 5 | 0 | 5 | 0.01 | 0 | 0 | 0 | 0 | 0.00 |
| Ma. uniformis Theobald | 6 | 2 | 0 | 8 | 0.02 | 0 | 0 | 0 | 0 | 0.00 |
| Ma. annulifera Theobald | 123 | 4 | 0 | 127 | 0.34 | 5 | 1 | 0 | 6 | 0.01 |
| Ma. dives Schiner | 1 | 0 | 0 | 1 | 0.00 | 0 | 0 | 0 | 0 | 0.00 |
| Totals | 29,307 | 4,205 | 3,540 | 37,052 | 40,299 | 2,096 | 5,860 | 48,255 | ||
| Number of Species | 35 | 35 | 16 | 43 | 24 | 23 | 15 | 29 | ||
| Simpson Diversity Index | 0.54 | 0.46 | ||||||||
Notes: TT: Traditional light traps; LT: CDC light traps (with dry ice); GT: CDC gravid traps; Cx: Culex; An: Anopheles; Ae: Aedes; Ar: Armigeres; Mm: Mimomyia; Ur: Uranotaenia; Ma: Mansonia.
The species richness (number of species) was significantly greater in Ruili than in Mangshi (43 and 29, respectively, t=4.19, P<0.0001) (Table S1 and Table 3). The Simpson Diversity Index of Mangshi differed significantly from that of Ruili (Ruili=0.54; Mangshi=0.46; t =2.66, P=0.014) (Table S2 and Table 3). Figure 2 shows the seasonal pattern in species richness, with more species collected in Ruili than in Mangshi during each month of the year.
Figure 2. Comparison of species richness (the number of species) between Manshi and Ruili for each month , 2010.
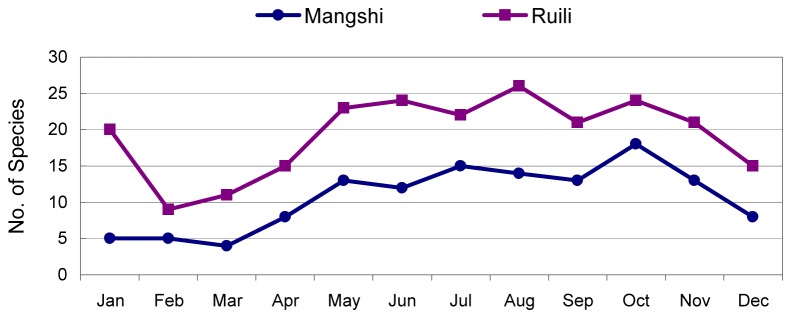
Species richness was generally higher during the summer and fall, with 26 species being collected during August in Ruili and 18 species collected during October in Mangshi (Table 4 and Figure 2). Only Culex tritaeniorhynchus Giles and Culex quinquefasciatus Say were collected in moderate to high numbers every month of the year (Figures 3 and 4, and Figure S1).
Table 4. Species richness (number of mosquito species) each month during 2010 in Mangshi and Ruili.
| City |
Species Richness (Number of species)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | |
| Mangshi | 5 | 5 | 4 | 8 | 13 | 12 | 15 | 14 | 13 | 18 | 13 | 8 |
| Ruili | 20 | 9 | 11 | 15 | 23 | 24 | 22 | 26 | 21 | 24 | 21 | 15 |
Figure 3. Monthly average temperature, total precipitation and abundance of the four predominant mosquito species collected in Mangshi, 2010.
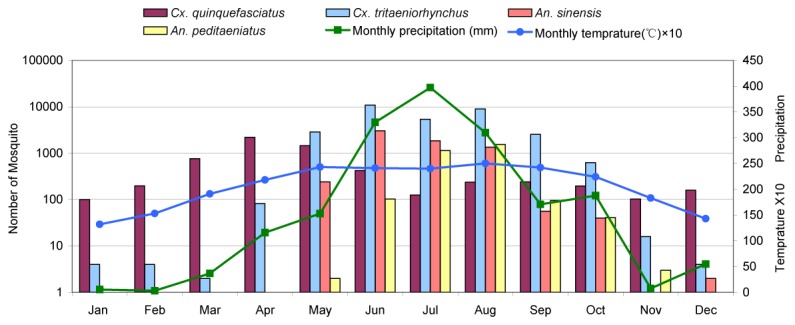
Figure 4. Monthly average temperature , total precipitation and abundance of the four predominant mosquito species collected in Ruili, 2010.
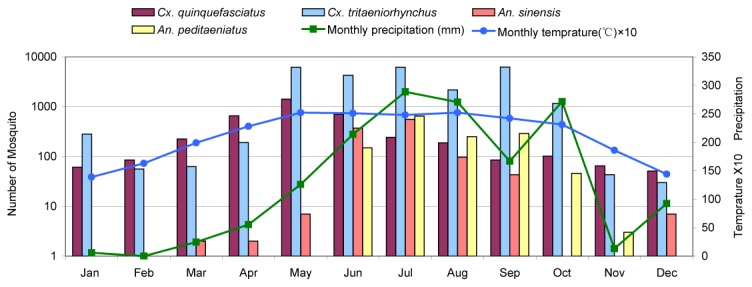
In both sampling locations, Cx. tritaeniorhynchus was by far the most frequently collected species, comprising 72.5% of the total in Ruili and 64.8% in Mangshi. In Ruili, this was followed by Cx. quinquefasciatus (10.5% of total), Anopheles peditaeniatus Leicester (3.7%) and Anopheles sinensis Wiedamann (2.9%), In Mangshi, An. sinensis was the second-most abundant species (13.5% of total) followed by Cx. quinquefasciatus (12.8%) and An. peditaeniatus (6.0%). These four species were referred to as the common species.
The total number of female mosquitoes collected was significantly greater in Mangshi (n=48,255) than in Ruili (n=37,025) (Z=38.36, P<0.00001). The number collected for each of the common species was significant higher in Manhsi than in Ruili (Z= 23.08, 18.21, 60.68, and 29.37, respectively for Cx. tritaeniorhynchus, Cx. quinquefasciatus, An. sinensis and An. peditaeniatus, P<0.00001) (Table S3).
Seasonal Abundance of Mosquitoes, Temperature, Precipitation and Humidity
Mosquitoes were collected during each month of the year in both cities of Mangshi and Ruili. Abundance of the four most common mosquito species, temperature, and rainfall patterns occurring in Mangshi and Ruili are shown in Figures 3 and 4, respectively. Rainfall and temperature patterns were similar in both sampling sites. January through March were relatively dry, rainfall increased each month until July, stayed relatively high through the rainy season until September-October, then declined during November and December. Average monthly temperatures were at their minimum (approx 15 °C) in January and climbed gradually to approximately 24 °C in May and stayed at approximately that level through September, then gradually declined during October through December. In Mangshi (Figure 3), Cx. quinquefasciatus was the most abundant species collected from January through April. Subsequently Cx. tritaeniorhynchus abundance exceeded that of Cx. quinquefasciatus and remained that way until November (Figure 5 and Figure S1). This seasonal change in abundance of these two species was also observed in Ruili, where An. sinensis and An. pediateniatus were not collected until May. Their abundance increased through the course of the rainy-hot season and declined as temperatures and rainfall decreased in September through December. The temperature and precipitation patterns in Ruili were similar (Figure 4), however An. sinensis were first collected in March (three months earlier than observed in Mangshi), increased gradually until the population peaked in June and July, and then gradually decreased until December. The pattern of An. peditaeniatus in Ruili was similar to that seen in Mangshi, with this species first being collected during June and persisting until November.
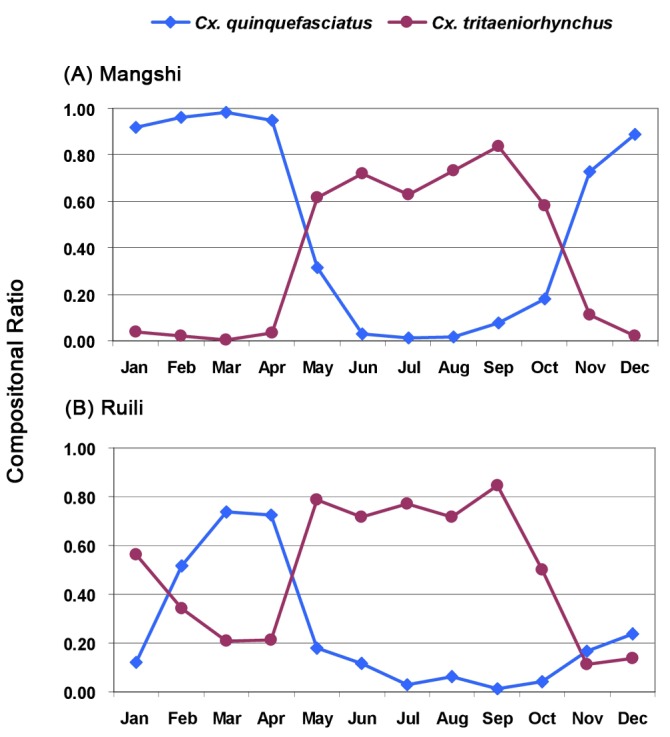
Figure 5. Compositional changes of Cx. quinquefasciatus and Cx. tritaeniorhynchus in total mosquitoes collected in Mangshi and Ruili, 2010.
Temporal trends in the abundance of each species were also illustrated by the ordination bioplots constructed by CCA. In both Manshi and Ruili (Figures S2 and S3), Cx. tritaeniorhynchus, An. sinensis and An. pediateniatus, as well as Anopheles vagus Donitz in Mangshi, displayed considerable associations with the three meteorological variables, whereas Cx. quinquefasciatus showed the poorest relationship with temperature, humidity and rainfall. Meanwhile, for the four common species collected from either of sampling sites, the three meteorological factors, which appeared to exert similar effect upon each species, tended to be identified as one “common factor”, largely due to their co-variation in seasonal change.
In Ruili (Figure S3), Culex fuscocephalus Theobald, Culex gelidus Theobald, and Anopheles tessellatus Theobald did not have strong associations with temperature. The two Cx. spp. were trapped in greatest abundance in October, while the latter An. tessellatus sustained its peak from June through October. These results suggested that local mosquito species, even those belonging to the same genus, did not display the same temporal trend in seasonal abundance variation.
Comparison of Trap Types
By using different trap types at the same locations, we were able to compare their ability to sample the local mosquito populations. In both Ruili and Mangshi sampling sites, the TT collected significantly more host seeking mosquitoes than the LT (Z=229.7, P<0.0001) and GT (Z=214.2, P<0.0001) (Table 3). In Ruili and Mangshi respectively, the TT collected approximately 7X and 19X more total mosquitoes than the LT, primarily resulting from the very large numbers of Cx. tritaeniorhynchus collected by the TT. However, both the TT and LT collections indicated similar species richness in the two sites; despite collecting many fewer total specimens, the LT collected 35 species in Ruili and 23 in Mangshi, compared to 35 and 24 by the TT in Ruili and Mangshi, respectively. The GT collected significantly more Cx. quinquefasciatus than the TT (Mann-Whitney U=42, P=0.0001) or LT (Mann-Whitney U=27.5, P=0.0001) (Figure S4) with differences ranging from 12X to 16X the number of Cx. quinquefasciatus than the TT, and from 16X to 27X the number of Cx. quinquefasciatus than the LT in Ruli and Mangshi, respectively. GT (n=15 in Mangshi; n=16 in Ruili) collected fewer species than the TT (n=24 in Mangshi; n=35 in Ruili) or LT (n=23 in Mangshi; n=35 in Ruili) (P<0.05) (Table 3). In addition, TT collected significantly more An. sinensis and An. peditaeniatus than LT (Mann-Whitney U=135.0, P=0.0001) or GT (Mann-Whitney U=83.5, P=0.0001) (Figure S4).
Virus Isolation and Identification
Of the 85,307 mosquitoes collected in Ruili and Mangshi during 2010, 69,209 specimens were tested for virus isolation in 1,430 pools (Table 5). From these, 26 virus isolates were obtained (Table 6). Of these, 13 strains were identified as JEV, five of which were from mosquitoes collected in Ruili (four from Cx. tritaeniorhynchus and one from Culex pallidothorax Theobald) and eight were from mosquitoes collected in Mangshi (seven from Cx. tritaeniorhynchus and one from Cx. gelidus). Four strains of GETV were isolated from mosquitoes collected in Ruili, one isolate from each of Cx. tritaeriorhynchus, An. sinensis, Cx. fuscocephalus and Culex annulus Theobald. Seven strains of Culex pipiens pallens densovirus (CppDNV) (Genus Densovirus, Family Densoviridae) were isolated; three from mosquitoes collected in Ruili (one from each of Cx. quinquefasciatus, Cx. tritaeriorhynchus, and Armigeres subalbatus Coquillett) and four from mosquitoes collected in Mangshi ( three from An. sinensis and one from Cx. quinquefasciatus). One strain of OYV was isolated from Cx. quinquefasciatus and one strain of Orbivirus genus (unidentified into species) was isolated from An. sinensis collected in Ruili.
Table 5. Summary of mosquito species collected and tested for virus in Mangshi and Ruili during Jan-Dec, 2010.
| Mosquito species |
Mangshi
|
Ruili
|
||||
|---|---|---|---|---|---|---|
| No.collected | No. tested | pools | No. collected | No. tested | pools | |
| Cx. quinquefasciatus | 6,198 | 6,198 | 113 | 3,881 | 3,881 | 82 |
| Cx. tritaeniorhynchus | 31,272 | 21,772 | 248 | 26,881 | 21,702 | 233 |
| Cx. mimeticus | 3 | 3 | 3 | 11 | 11 | 9 |
| Cx. theileri | 28 | 28 | 13 | 17 | 17 | 8 |
| Cx. bitaeniorhynchus | 5 | 5 | 4 | 20 | 20 | 12 |
| Cx. .halifaxi | 2 | 2 | 2 | 1 | 1 | 1 |
| Cx. pallidothrax | 9 | 9 | 4 | 32 | 32 | 20 |
| Cx. fuscanus | 31 | 31 | 17 | 35 | 35 | 19 |
| Cx. annulus | 231 | 231 | 14 | 349 | 349 | 18 |
| Cx. nigropunctatus | 12 | 12 | 6 | 74 | 74 | 21 |
| Cx. gelidus | 6 | 6 | 3 | 499 | 499 | 27 |
| Cx. jacksoni | 1 | 1 | 1 | |||
| Cx. fuscocephala | 38 | 38 | 11 | 968 | 968 | 42 |
| Cx. bailyi | 10 | 10 | 3 | |||
| Cx. pseudovishnui | 1 | 1 | 1 | 1 | 1 | 1 |
| An. sinensis | 6,556 | 5,396 | 71 | 1,094 | 1,094 | 35 |
| An. maculatus | 3 | 3 | 3 | 8 | 8 | 5 |
| An. minimus | 5 | 5 | 4 | 9 | 9 | 6 |
| An. culicifacies | 4 | 4 | 3 | 2 | 2 | 2 |
| An. vagas | 598 | 598 | 20 | 322 | 322 | 22 |
| An. peditaeniatus | 2,919 | 2,660 | 40 | 1,387 | 1,387 | 28 |
| An. tessellatus | 75 | 75 | 15 | 624 | 624 | 35 |
| An. barbirostris | 3 | 3 | 3 | 35 | 35 | 18 |
| An. kochi | 1 | 1 | 1 | 23 | 23 | 10 |
| An. splendidus | 7 | 7 | 4 | |||
| An. annularis | 1 | 1 | 1 | |||
| An. crawfordi | 1 | 1 | 1 | 9 | 9 | 6 |
| An. aconitus | 1 | 1 | 1 | |||
| An.gigas baileyi | 4 | 4 | 3 | 2 | 2 | 2 |
| Ae. elsiae | 198 | 198 | 22 | 367 | 367 | 29 |
| Ae. lineatopennis | 5 | 5 | 4 | |||
| Ae. albopictus | 1 | 1 | 1 | 5 | 5 | 5 |
| Ae. annandalei | 2 | 2 | 2 | |||
| Ar. subalbatus | 44 | 44 | 14 | 184 | 184 | 29 |
| Ar. inchoatus | 3 | 3 | 3 | |||
| Ar. omissus | 1 | 1 | 1 | |||
| Mm. luzonensis | 1 | 1 | 1 | 34 | 34 | 17 |
| Ur. maxima | 4 | 4 | 4 | |||
| Ur. novobscura | 5 | 5 | 3 | |||
| Ma. uniformis | 8 | 8 | 5 | |||
| Ma. Annulifera | 6 | 6 | 1 | 127 | 127 | 11 |
| Ma. dives | 1 | 1 | 1 | |||
| total | 48,255 | 37,336 | 642 | 37,052 | 31,873 | 788 |
Table 6. Summary of viruses isolated from mosquitoes collected in Ruili and Mangshi, Yunnan Province 2010.
| No. | Isolate identifier | Species of mosquito | Collection date | Trap type | Sites | Virus |
|---|---|---|---|---|---|---|
| 1 | DH10M107 | Cx. quinquefasciatus | March 16, 2010 | GT | Mangshi | CppDNV |
| 2 | DH10M390 | Cx. tritaeriorhynchus | May 14, 2010 | TT | Ruili | GETV |
| 3 | DH10M397 | Cx. quinquefasciatus | May 13-14, 2110 | TT, LT and GT | Ruili | CppDNV |
| 4 | DH10M429 | An. sinensis | June 16-17, 2010 | TT, LT and GT | Mangshi | CppDNV |
| 5 | DH10M500 | An. sinensis | June 16, 2010 | TT | Mangshi | CppDNV |
| 6 | DH10M530 | An. sinensis | June 16, 2010 | TT | Mangshi | CppDNV |
| 7 | DH10M585 | Cx. tritaeriorhynchus | June 15, 2010 | TT | Ruili | JEV |
| 8 | DH10M609 | Cx. tritaeriorhynchus | June 15, 2010 | TT | Ruili | JEV |
| 9 | DH10M619 | Cx. tritaeriorhynchus | June 14, 2010 | TT and LT | Ruili | CppDNV |
| 10 | DH10M635 | Cx. tritaeriorhynchus | June 15, 2010 | TT | Ruili | JEV |
| 11 | DH10M648 | Cx. tritaeriorhynchus | July 14-15, 2010 | TT and LT | Mangshi | JEV |
| 12 | DH10M662 | Cx. gelidus | July 15, 2010 | LT | Mangshi | JEV |
| 13 | DH10M663 | Cx. tritaeriorhynchus | July 15, 2010 | TT | Mangshi | JEV |
| 14 | DH10M701 | Cx. tritaeriorhynchus | July 15, 2010 | TT | Mangshi | JEV |
| 15 | DH10M742 | Cx. tritaeriorhynchus | July 15, 2010 | TT | Mangshi | JEV |
| 16 | DH10M865 | Cx. tritaeriorhynchus | August 17, 2010 | TT | Mangshi | JEV |
| 17 | DH10M872 | Cx. tritaeriorhynchus | August 16, 2010 | TT and LT | Mangshi | JEV |
| 18 | DH10M919 | Cx. tritaeriorhynchus | August 17, 2010 | TT | Mangshi | JEV |
| 19 | DH10M978 | Cx. tritaeriorhynchus | August 15, 2010 | TT | Ruili | JEV |
| 20 | DH10M984 | Cx. pallidothrax | August 14, 2010 | GT | Ruili | JEV |
| 21 | DH10M1013 | Cx. quinquefasciatus | August 14-15, 2010 | TT and GT | Ruili | OYV |
| 22 | DH10M1019 | An. sinensis | August 14-15, 2010 | TT | Ruili | orbivirus |
| 23 | DH10M1102 | Cx. fuscocephala | September 15-16, 2010 | TT | Ruili | GETV |
| 24 | DH10M1105 | An. sinensis | September 15-16, 2010 | TT | Ruili | GETV |
| 25 | DH10M1106 | Cx. annulus | September 15-16, 2010 | TT, LT and GT | Ruili | GETV |
| 26 | DH10M1170 | Ar. subalbatus | September 15-16, 2010 | TT, LT and GT | Ruili | CppDNV |
All 26 strains of viruses were isolated from the mosquitoes collected from March through September, with JEV in June, July and August; GETV in May and September; OYV and Orbivirus in August; CppDNV in March, May, July and September (Table 6).
We calculated the minimum infection rate (MIR) of JEV in Cx. tritaeniorhynchus populations in Mangshi and Ruili during June, July and August, the months during which JEV was detected in mosquitoes (Table 7). In Ruili, the MIR ranged from 0 to 0.7/1000 and in Mangshi the MIR ranged from 0 to 0.9/1000 mosquitoes.
Table 7. MIR by month of JEV in Culex tritaeniorhynchus collected in Ruili and Mangshi, Jun-Aug, 2010.
| June | July | August | |
|---|---|---|---|
| Ruili | |||
| No. of specimens | 4,281 | 5,179 | 2,171 |
| No. of positive pools | 3 | 0 | 1 |
| MIR | 0.70 | 0 | 0.46 |
| Mangshi | |||
| No. of specimens | 6,784 | 4,387 | 4,465 |
| No. of positive pools | 0 | 4 | 3 |
| MIR | 0 | 0.91 | 0.67 |
Note: Minimum Infection Rate expressed as number infected/1000 tested.
Discussion
Over a 12-month period in 2010, mosquito collections using 3 distinct traps demonstrated that mosquitoes are active year round and documented significantly more species diversity, 29 species from six genera in Mangshi and 43 species from seven genera in Ruili than the 25 species previously reported from surveys limited to July through August in this part of China [37-40].
Four mosquito species accounted for the majority of the mosquitoes collected in both areas. Cx. tritaeniorhynchus was, by far, the most abundant mosquito, consistent with previous studies in 15 counties near the Yunnan Province border with Myanmar, Laos and Vietnam [19,41]. Like Cx. tritaeniorhynchus, An. sinensis and An. pediateniatus were also abundant in the summer months. However, in previous studies [19-21], An. sinensis was reported as a very common species, whereas An. pediateniatus was collected only in very low numbers. In our study another species, Cx. quinquefasciatus was identified as one of the predominant species in the area, and was shown to be active at moderate to high levels throughout the year, contrary to reports in previous studies. This finding is due to the inclusion of the GT in the sampling strategy. The GT accounted for 87.9% and 91.1% of Cx. quinquefasciatus collected in Mangshi and Ruili, respectively. In previous studies only using the TT, no Cx. quinquefasciatus were collected in Mangshi [37] and very few were collected in Ruili [38] and numerous other sites in the region [19,39,40]. Our study also supported observations that UV light (TT) or incandescent light and CO2 (LT) were virtually unattractive to host-seeking Cx. quinquefasciatus.
The greater species richness found in Ruili (43 species) compared to Mangshi (29 species) is mainly the result of Culex, Anopheles, Aedes, Armigeres, Uranotaenia, and Mansonia species in Ruili. As a rule, species diversity is proportional to altitude, but not to the number of specimens collected [42]. Lower altitude and higher temperature, rainfall and humidity, together with the more southerly geographic location (Table 1) may result in Ruili supporting appropriate conditions for more diverse mosquito species breeding than Mangshi. Conversely, the greater abundance of the four most common species in Mangshi suggests that more habitat for these species exists in Mangshi than in Ruili However, these results may be explained by other environmental factors associated with the different sampling sites, natural and artificial habitat structures, land use patterns, and host availability. These environmental factors are the subject of future research projects.
That mosquito breeding depends largely on appropriate climatic conditions is reflected in the enormous difference in mosquito numbers obtained during winter and summer. However, as Figures 3, 4 (and Figure S1) indicated, the Cx. quinquefasciatus population peaked in April and May, respectively for Mangshi and Ruili, and then began to decrease, while three other most abundant species attained their peaks two-three months later, or maintained this peak value for longer period (e.g. Cx. tritaeniorhynchus, Ruili). This may be explained by the interaction of the properties inherent in GT (i.e., attractiveness to Cx. quinquefasciatus) and the environmental factors associated with seasonal changes and human farming activity. At the time of year when the rainy season is approaching, temperatures are increasing and paddy fields have not been irrigated, GT appears to be a quite attractive device to trap Cx. quinquefasciatus. Once the paddy fields around human residences are filled with water during the rainy season, the GT, a device designed primarily to attract ovipositing gravid Cx. quinquefasciatus, may be outcompeted by other numerous oviposition sites. The ubiquity of flooded paddy fields during the wet growing season supports other mosquitoes, such as Cx. tritaeniorhynchus, which are collected in large numbers in the TT. As a consequence of the mid-April rice planting, our collecting indicates that the proportion of Cx. quinquefasciatus relative to Cx. tritaeniorhynchus decreases sharply in both cities (Figure 5 and Figure S1).
The results demonstrate that the TT, LT and GT, each have advantages in collecting different mosquito species. The TT was the optimal mosquito trap for collecting large numbers of Cx. tritaeriorhynchus, An. sinensis, and An. peditaeniatus. The diversity of mosquitoes sampled collected by the LT was similar to that sampled by TT, however, the numbers collected by the LT were markedly less than those collected by TT. This is likely related to the relatively weak light source of LT, despite the presence of CO2 bait. The disadvantage of the TT is that it requires 220V power and is limited to use around residential areas where power is available. LT can be used in remote locations and to sample habitats where electrical outlets are not available. The GT was by far the optimal mosquito trap for Cx. quinquefasciatus.
We used three trap types to better characterize the structure of local mosquito communities, resulting in new indications that Cx. quinquefasciatus and An. pediateniatus were abundant in the area, Our study represents, to our knowledge, the first time GT have been used in China, and we recommend that it be incorporated into mosquito surveillance programs to provide a more thorough and complete insight to the mosquito community structure wherever surveillance programs are conducted. Only when more sampling tools with distinct and well-defined properties are applied in mosquito collecting, can we better define mosquito community structure in the field.
Knowledge of the year-round activity of Cx. quinquefasciatus in this area is important since this species is not only the well-known vector transmitting JEV [20,43], but one of the most important vectors of West Nile virus (WNV) [44,45]. Though not previously found in Yunnan Province, WNV global distribution has been expanding and WNV and other zoonotic flaviviruses have been found in southeast Asia [46,47] and could establish transmission foci in areas where competent vectors like Cx. quinquefasciatus exist.
The western borderland of Yunnan Province is a known epidemic area for JE [1,48], so detecting JEV in mosquito species that have been previously associated with JEV transmission was not surprising [2,3,8,19,20,41]. The 13 strains of JEV isolated in the present study were all determined to be genotype 1, which is consistent with the recent isolation of genotype 1 JEV in the cerebrospinal fluid samples from JE patients in this area [49]. This is in contrast to the JEV isolated in the same area in the 1980’s which were all genotype 3 [9,34]. These observations suggest that genotype 1 has replaced genotype 3 and has become the prevalent genotype of JEV in the area, consistent with other results demonstrating the emergence of genotype 1 JEV as the dominant genotype found in Asia [50,51]. Some authors proposed that genotype 1 JEV originated in Southeast Asia [52-54]. In that case, this genotype was likely introduced into Yunnan Province and other areas of China in the past decade. However, many explanations as to its transmission pattern and mechanism in Asia remained speculative. A total of 80 human JE cases were reported during 2010 through 2012 in our study area, which occurred between May (two cases) and November (one), and peaked in July (34) [48]. In our study, JEVs were isolated in June through August, consistent with the peak of human cases. Although human cases were not reported in December through April of the next year, and no JEV was isolated other than June-August in the present study, the year-round activity of Cx. tritaeriorhynchus and Cx. quinquefasciatus was confirmed (Figure 3 and 4), implying that JEV could circulate in local mosquitoes all year round.
GETV was first isolated from Cx. gelidus in Malaysia [55], and is widely distributed in Southeast Asia and east Asia. GETV is pathogenic in horses, pigs, and other domestic animals [56,57], but has not been associated with disease in humans. GETV has been previously isolated from An. sinensis and Ar. subalbatus in northwest areas of Yunnan Province [6].The current results represent the first association of GETV with Cx. tritaeriorhynchus, Cx. fuscocephalus and Cx. annulus in Yunnan Province and indicates that Culex species in the China-Myanmar border area may be important GETV vectors. We have detected GETV antibody by hemagglutination inhibition test in human serum samples in areas near the Lancang River in the middle and south areas of Yunnan Province [58], suggesting human infection and a wide distribution in Yunnan Province.
Previously, CppDNV has been isolated in China [30,36]. The seven strains of CppDNV isolated in the present study were from An. sinensis, Cx. quinquefasciatus, Cx. tritaeriorhynchus and Ar. subalbatus, indicating that CppDNV can infect many mosquito species in different genera, but this virus seems to exist only as a mosquito-carried virus, without causing human or animal infection. The orbivirus isolated from An. sinensis in the present study caused CPE in BHK21 cells, while the YNOV previously isolated from the south area of Yunnan Province produced CPE only in C6/36 cells [4,18]. The biological characteristics of these orbivirus isolates are apparently different and need further investigation. In the 1980s, orbiviruses were isolated in pigs and cattle [59,60], indicating that it can infect these animals, but whether the strains of orbivirus obtained in this study were pathogenic to humans and other animals remains unclear. One strain of OYV isolated in the present study was from Cx. quinquefasciatus. OYV, commonly infects pigs [61] and has been isolated from other mosquito species collected in northern Vietnam [62]. This is the first demonstrated association of OYV with Cx. quinquefasciatus. There are no reports about this virus in China. No human cases caused by this virus have been reported. Further investigations of OYV transmission dynamics should be carried out in areas where this virus has been isolated.
In the past, mosquito-borne viruses were isolated only in July and August. In the current study, the time of arboviruses isolation spanned from March through September, illustrating more detailed data on the seasonal pattern of virus cycling. Failure to recover viruses in the rest of months may be related to low number of mosquitoes that had been sampled in the relative cold season. However, the presence of some mosquito species in winter suggested that the adult mosquitoes could carry the associated viruses throughout the entire year. This information will provide a better understanding of the ecology of vector and other mosquitoes in this region, and improve understanding of factors that may influence tran-seasonal virus maintenance. In addition data derived from comprehensive, year-long studies such as this will be useful as a baseline to monitor the effects of changes in land use and climate on vector abundance and virus transmission.
For local public health agencies, our study also presented the fact that well-timed interventions to reduce human cases associated with JEV and other mosquito-borne pathogens should be carried out in April, with efforts focusing upon larval mosquito control during the period when the number of larval habitats is still low. In June and July, vector control should emphasize reducing adult mosquitoes. The abundance and the diversity of mosquitoes highlight the possibility that some exotic viruses, such as WNV, may establish in this area; routine monitoring therefore is needed.
Supporting Information
Seasonal peaks of the four most common mosquito species collected in Mangshi and Ruili cities, Yunnan Province, 2010.
(TIF)
Mangshi CCA biplot: locations of species (>1%) relative to the first two axes derived in CCA. Species are showed as triangles. The abbreviations represented Cx. tritaeniorhynchus, Cx. quinquefasciatus, An. sinensis, An. peditaeniatus, and An. vagas. Each vector represents a given meteorological variable with its mean lying at the origin. Above average values lie alone their corresponding vectors, and below average values project in opposite direction of the vector.
(TIF)
Ruili CCA biplots: locations of species (>1%) relative to the first two axes derived in CCA. Species are showed as triangles. The abbreviations represent Cx. tritaeniorhynchus, Cx. quinquefasciatus, An. sinensis, An. peditaeniatus, Cx. fuscocephalus, Cx. gelidus, and An. tessellates. Each vector represents a given meteorological variable with its mean lying at the origin. Above average values lie alone their corresponding vectors, and below average values project in opposite direction of the vector.
(TIF)
Comparison of sampling ability of different trap types by using Mann-Whitney U test.
(TIF)
The results of t-test for comparing species richness between Mangshi and Ruili.
(DOC)
The results of t-test for comparing SDI between Mangshi and Ruili.
(DOC)
The results of Z-test for comparing the collected number of each common species between Mangshi and Ruili.
(DOC)
Acknowledgments
We are grateful to Dr. Jian-Meng Feng from College of Agriculture and Life Science, Dali University for assisting in CCA in this article. We also thank Wei Jiang, Tian-Qiao Guo and Shi-Chao Yang from the Dehong Prefecture CDC, and Xiao-Xiong Yin from Ruili City CDC and other staff who assisted in the field work of this project.
Funding Statement
This study was supported by from China Centers for Disease Control and Prevention - U.S. Centers for Disease Control and Prevention Cooperative Agreement U-19-GH000004; The Ministry of Science and Technology, China (2011CB504702); and Development grant of the State Key Laboratory for Infectious Disease Prevention and Control (2008SKLID105). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Deng SZ, Zhang HL, Liu XQ (2009) Analysis of epidemiological characteristics of Japanese encephalitis in Yunnan Province from 1976 to 2007. Difangbing Tongbao 24: 1-7. [Google Scholar]
- 2. Zhang HL, Zi DY, Gong ZD (2000) Host and vector of Japanese encephalitis virus in Yunnan Province. Chin J Prevent Veterinary Med 22:81-83
- 3. Zhang HL, Zi DY, Shi HF, Mi ZQ, Gong ZD et al. (1990) The nature infection rate of mosquitoes by Japanese encephalitis B virus in Yunnan Province. Chin J Prevent Med 24: 265-267. [PubMed] [Google Scholar]
- 4. Tao SJ, Zhang HL, Yang DR, Wang HQ, Liu QZ et al. (2003) Investigation of arboviruses in Lancang River downstream area in Yunnan Province. Chin J Exp Clin Virol 17:322-326 [PubMed]
- 5. Wang JL, Zhang HL, Sun XH, Fu SH, Wang HQ (2011) Distribution of mosquitoes and mosquito-borne arboviruses in Yunnan Province near the China-Myanmar-Laos border. Am J Trop Med Hyg 84: 738-746. doi: 10.4269/ajtmh.2011.10-0294. PubMed: 21540383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun XH, Fu SH, Gong ZD, Ge JQ, Meng WS et al. (2009) Distribution of arboviruses and mosquitoes in Northwestern Yunnan Province, China. Vector Borne Zoonotic Dis 9: 623-630. doi: 10.1089/vbz.2008.0145. PubMed: 19196130. [DOI] [PubMed] [Google Scholar]
- 7. Yang DJ, Fu SH, Zhang HL, Yang WH, Feng Y et al. (2011) The survey of distribution characteristics of mosquitoes and mosquito-borne arboviruses in northeast and some other areas of Yunnan Province. Chin J Vector Biol Control 22:304-308
- 8. Liu H, Lu HJ, Liu ZJ, Jing J, Ren JQ et al. (2013) Japanese encephalitis virus in mosquitoes and swine in Yunnan Province, China 2009-2010. Vector Borne Zoonotic Dis 13: 41-49. doi: 10.1089/vbz.2012.1016. PubMed: 23199264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang JL, Zhang HL, Zhou JH, Liang GD (2008) The genotype of Japanese encephalitis viruses isolated in Yunnan. Chin J Exp Clin Virol 22:87-90 [PubMed]
- 10. Zhang HL, Zi DY, Gong ZD (1999) Epidemiological survey of dengue fever in Yunnan Province, China. Difangbing Tongbao 14: 50-54. [Google Scholar]
- 11. Wang JL, Zhang HL,Sun XH, Fu SH, Mi ZQ et al. (2008) Identification and sequence analysis of NS1 and NS2a of two strains of dengue type-4 virus from mosquitoes in Yunnan Province. Chin J Zoonoses 24: 636-640. [Google Scholar]
- 12. Zhang HL, Shi HF, Liu LH, Yu YX, Zi DY et al. (1989) Isolationg of Chikungunya virus from bat in Yunnan Province and serological investigations. Chin J Virol 5:31-36
- 13. Zhang HL, Tao SJ, Yang DR, Zhang YZ, Yang WH et al. (2005) Isolation of Sindbis, Batai and Coltivirus in Yunnan Province. Chin J Zoonoses 21: 548-557. [Google Scholar]
- 14. Wang JL,Zhang HL,Sun XH, Fu SH, Feng Y et al. (2008) The first isolated Sindbis virus from mosquito pools in Yunnan Provence. Chin J Zoonoses 24: 1-4. [Google Scholar]
- 15. Wang JJ, Zhang HL,Che YC, Wang LC,Ma SH et al. (2008) Isolation and complete genomic sequence analysis of a new Sindbis-like virus. Virol Sin 23: 31-36. doi: 10.1007/s12250-008-2891-5. [DOI] [Google Scholar]
- 16. Feng Y, Fu SH, Zhang HL, Li MH, Zhou T, Wang JL et al. (2012) Distribution of mosquitoes and mosquito-borne viruses in Yunnan Province along the border of China and Myanmar. Jpn J Infect Dis 65: 215-221. doi: 10.7883/yoken.65.215. PubMed: 22627302. [DOI] [PubMed] [Google Scholar]
- 17. Liu H, Li MH, Zhai YG, Meng WS, Sun XH et al. (2010) Banna virus, China, 1987-2007. Emer. Infect Dis 16: 514-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Attoui H, Jaafar FM, Belhouchet M, Aldrovandi N, Tao SJ et al. (2005) Yunnan orbivirus, a new orbivirus species isolated from Culex tritaeniorhynchus mosquitoes in China. J Gen Virol 86: 1-9. doi: 10.1099/vir.0.80466-0. PubMed: 15604425. [DOI] [PubMed] [Google Scholar]
- 19. Zhang HL, Mi ZQ, Zhang YZ, Gong ZD, Zi DY et al. (2002) Studies on mosquito natural infection with Japanese encephalitis virus in border area, Yunnan Province. Chin J Vector Biol Control 13:101-104
- 20. Deng SZ, Zhang HL, Li JM (2009) Distribution characteristics of mosquito and their natural infection with Japanese encephalitis virus in Yunnan Province. Chin J Vector Biol Control 20:344-348
- 21. Zhang YZ, Zhang HL, Gong ZD, Yang WH, Zhang YZ (2001) Studies on the mosquito communities and the geographical niche of border area in Yunnan Province. Chin J Vector Biol Control 12:28-32
- 22. Carestia RR, Savage LB (1967) Effectiveness of carbon dioxide as a mosquito attractant in the CDC miniature light trap. Mosq News 27: 90-92. [Google Scholar]
- 23. Reiter P (1983) A portable, battery operated trap for collecting gravid Culex mosquitoes. Mosq News 43: 496-498. [Google Scholar]
- 24. Simpson EH (1949) Measurement of diversity. Nature 163: 688. doi: 10.1038/163688a0. [DOI] [Google Scholar]
- 25. Terbraak CJF (1986) Canonical correspondence-analysis – a new elgenvector technique for multivariate direct gradient analysis. Ecology 67: 1167-1179. doi: 10.2307/1938672. [DOI] [Google Scholar]
- 26. Kavazos CRJ, Wallman JF (2012) Community composition of carrion-breeding blowflies (Diptera: Calliphoridae) along an urban gradient in south-eastern Australia. Landscape Urban Plann 106: 183-190. doi: 10.1016/j.landurbplan.2012.03.002. [DOI] [Google Scholar]
- 27. Heino J, Mykra H (2008) Control of stream insect assemblages: roles of spatial configuration and local environmental factors. Ecol Entomol 33: 614-622. doi: 10.1111/j.1365-2311.2008.01012.x. [DOI] [Google Scholar]
- 28. Spitzer K, Bezdek A, Jaros J (1999) Ecological succession of a relict Central European peat bog and variability of its insect biodiversity. J Insect Conserv 3: 97-106. doi: 10.1023/A:1009634611130. [DOI] [Google Scholar]
- 29. Fu SH (2012) Strategy of isolation and identification for Arboviruses. Chin J Vector Biol Control 23:432-435
- 30. Zhai YG, Lv XJ, Sun XH, Fu SH, Gong ZD (2008) Isolation and characterization of the full coding sequence of a novel densovirus from the mosquito Culex pipiens pallens . J Gen Virol 89: 195-199. doi: 10.1099/vir.0.83221-0. PubMed: 18089743. [DOI] [PubMed] [Google Scholar]
- 31. Bryant JE, Crabtree MB, Nam VS, Yen NT, Duc HM et al. (2005) Isolation of arboviruses from mosquitoes collected in northern Vietnam. Am J Trop Med Hyg 73: 470-473. PubMed: 16103625. [PubMed] [Google Scholar]
- 32. Kuno G (1998) Universal diagnostic RT-PCR protocol for arboviruses. J Virol Methods 72: 27-41. doi: 10.1016/S0166-0934(98)00003-2. PubMed: 9672130. [DOI] [PubMed] [Google Scholar]
- 33. Pfeffer M, Proebster B, Kinney RM, Kaaden OR (1997) Genus-specific detection of alphaviruses by a semi-nested reverse transcription-polymerase chain reaction. Am J Trop Med Hyg 57: 709-718. PubMed: 9430533. [DOI] [PubMed] [Google Scholar]
- 34. Wang HY, Takasaki T, Fu SH, Sun XH, Zhang HL et al. (2007) Molecular epidemiological analysis of Japanese encephalitis virus in China. J Gen Virol 88: 885-894. doi: 10.1099/vir.0.82185-0. PubMed: 17325361. [DOI] [PubMed] [Google Scholar]
- 35. Zhai YG, Wang HY, Sun XH, Fu SH, Wang HQ et al. (2008) Complete sequence characterization of isolates of Getah virus (genus Alphavirus, family Togaviridae) from China. J Gen Virol 89: 1446-1456. doi: 10.1099/vir.0.83607-0. PubMed: 18474561. [DOI] [PubMed] [Google Scholar]
- 36. Feng Y, Fu SH, Zhang HL, Li MH, Zhou T et al. (2011) Molecular characterization of genome of mosquito densovirus newly isolated in Yunnan Province. Acta Parasitol Med Entomol Sin 18: 15-20. [Google Scholar]
- 37. Mi ZQ, Zi DY, Zhang HL, Gong ZD, Yang LP et al. (2002) Investigation of mosquitoes and isolation of Japanese encephalitis virus in Luxi City, Yunnan Province. Chin J Pests Control 18: 367-369. [Google Scholar]
- 38. Zhang HL, Gong ZD, Mi ZQ, Zi DY, Shi HF et al. (1999) Investigation of mosquitoes and isolation of Japanese encephalitis virus in Ruili County, Yunnan Province. Chin J Pests Control 15: 59-61. [Google Scholar]
- 39. Zhang HL, Mi ZQ, Zi DY, Gong ZD, Kang YR et al. (1999) Investigation of mosquitoes and isolation of Japanese encephalitis virus in Yingjiang County, Yunnan Province. Chin J Pests Control 15: 23-25. [Google Scholar]
- 40. Zhang HL, Mi ZQ, Gong ZD, Zi DY, Shi HF et al. (1998) Characteristic of mosquitoes distributed and isolation of Japanese encephalitis virus in Dehong Prefecture, Yunnan Province. Difangbing Tongbao 13: 78-80. [Google Scholar]
- 41. Zhang HL, Zi DY, Shi HF, Mi ZQ, Gong ZD et al. (1999) Characterized distribution of Culex tritaeniorhynchus and their natural infection with Japanese encephalitis virus in Yunnan Province. Chin J Vector Biol Control 10:192-194
- 42. Pemola Devi N, Jauhari RK (2004) Altitudinal distribution of mosquitoes in mountainous area of Garhwal region: Part–I. J Vect Borne Dis 41: 17-26. [PubMed] [Google Scholar]
- 43. Nitatpattana N, Apiwathnasorn C, Barbazan P, Leemingsawat S, Yoksan S et al. (2005) First isolation of Japanese encephalitis virus from Culex quinquefasciatus in Thailand. Southeast Asian J Trop Med Public Health 36: 875-878. PubMed: 16295539. [PubMed] [Google Scholar]
- 44. Molaei G, Andreadis TG, Armstrong PM Jr RB, Dennett JA, et al (2007) Host Feeding Pattern of Culex quinquefasciatus (Diptera: Culicidae) and Its Role in transmission of West Nile virus in Harris County, Texas. Am J Trop Med Hyg 77: 73-81. PubMed: 17620633. [PubMed] [Google Scholar]
- 45. Kent RJ, Crabtree MB, Miller BR (2010) Transmission of West Nile virus by Culex quinquefasciatus Say infected with Culex Flavivirus Izabal. PLoS Negl Trop. Drosophila Inf Serv 4: e671. doi: 10.1371/journal.pntd.0000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mackenzie JS,Williams DT (2009) The zoonotic flaviviruses of Southern, South-Eastern and Eastern Asia, and Australasia: the potential for emergent viruses. Zoonoses Public Health 56: 338-356. doi: 10.1111/j.1863-2378.2008.01208.x. PubMed: 19486319. [DOI] [PubMed] [Google Scholar]
- 47. Sun J, Zhang HL (2011) Prevalence of Arboviral infection in Southeast Asia. Guoji Bingduxue Zazhi 18: 125-128. [Google Scholar]
- 48. Feng Y, Fu SH, Zhang HL, Petersen LR, Zhang BS et al. (2013) High incidence of Japanese encephalitis, Southern China. Emerg Infect Dis 19: 672-673. doi: 10.3201/eid1904.120137. PubMed: 23750863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang LH, Fu SH, Zhang HL, Ye XF, Yu DS et al. (2010) Identification and isolation of genotype-I Japanese encephalitis virus from encephalitis patients. Virol J 7: 345-348. doi: 10.1186/1743-422X-7-345. PubMed: 21108846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pan XL, Liu H, Wang HY, Fu SH, Liu HZ et al. (2011) Emergence of genotypeⅠof Japanese encephalitis virus as the dominant genotype in Asia. J Virol 85: 9847-9853. doi: 10.1128/JVI.00825-11. PubMed: 21697481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sun YJ, Zhang HL (2012) Distribution and gene-typing of Japanese encephalitis virus in China. Chin J Vector Biol Control 23:436-438
- 52. Solomon T, Ni H, Beasley DWC, Ekkelenkamp M, Cardosa MJ et al. (2003) Origin and evolution of Japanese encephalitis virus in southeast Asia. J Virol 77: 3091-3098. doi: 10.1128/JVI.77.5.3091-3098.2003. PubMed: 12584335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nitatpattana N, Dubot-Pérès A, Gouilh MA, Souris M, Barbazan P et al. (2008) Change in Japanese encephalitis virus distribution, Thailand. Emerg Infect Dis 14: 1762-1765. doi: 10.3201/eid1411.080542. PubMed: 18976565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kuwata R, Nga PT, Yen NT, Hoshino K, Isawa H et al. (2013) Surveillance of Japanese encephalitis virus infection in mosquitoes in Vietnam from 2006 to 2008. Am J Trop Med Hyg 88: 681-688. doi: 10.4269/ajtmh.12-0407. PubMed: 23358634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Berge T (1975) Getah. International Catalog of Arboviruses. 2nd ed. Washington DC: US Department of Health, Education, and Welfare; pp. 278-279. [Google Scholar]
- 56. Kamada M, Ando Y, Fukunaga Y, Kumanomido T, Imagawa H et al. (1980) Equine Getah virus infection: isolation of the virus from racehorses during an enzootic in Japan. Am J Trop Med Hyg 29: 984-988. PubMed: 6254385. [DOI] [PubMed] [Google Scholar]
- 57. Brown CM, Timoney PJ (1998) Getah virus infection of Indian horses. Trop Anim Health Prod 30: 241-252. doi: 10.1023/A:1005079229232. PubMed: 9760716. [DOI] [PubMed] [Google Scholar]
- 58. Zhang HL,Zhang YZ, Yang WH, Zhang YZ, Mi ZQ et al. (2000) Investigation on the antibodies against arboviruses in sera of human being and animal in the lower reaches area of Lancang River in Yunnan Province. Chin J Pests Control 16: 113-115. [Google Scholar]
- 59. Xu PT, Wang YM, Zuo JM (1990) New orbivirus isolated from patients with encephalitis or with unknown fever in Yunnan province. Chin J Virol 6:27-33
- 60. Xu PT, Wang YM, Zhao ZJ (1988) Isolation and identification of two orbivirus from Yunnan Province. Chin J Virol 4:218-222
- 61. Kono Y, Yusnita Y, Mohd AR, Maizan M, Sharifah SH et al. (2002) Characterization and identification of Oya virus, a Simbu serogroup virus of the genus Bunyavirus, isolated from a pig suspected of Nipah virus infection. Arch Virol 147: 1623-1630. doi: 10.1007/s00705-002-0838-y. PubMed: 12181680. [DOI] [PubMed] [Google Scholar]
- 62. Bryant JE, Crabtree MB, Nam VS, Yen NT, Duc HM et al. (2005) Isolation of arboviruses from mosquitoes collected in northern Vietnam. Am J Trop Med Hyg 73: 470-473. PubMed: 16103625. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Seasonal peaks of the four most common mosquito species collected in Mangshi and Ruili cities, Yunnan Province, 2010.
(TIF)
Mangshi CCA biplot: locations of species (>1%) relative to the first two axes derived in CCA. Species are showed as triangles. The abbreviations represented Cx. tritaeniorhynchus, Cx. quinquefasciatus, An. sinensis, An. peditaeniatus, and An. vagas. Each vector represents a given meteorological variable with its mean lying at the origin. Above average values lie alone their corresponding vectors, and below average values project in opposite direction of the vector.
(TIF)
Ruili CCA biplots: locations of species (>1%) relative to the first two axes derived in CCA. Species are showed as triangles. The abbreviations represent Cx. tritaeniorhynchus, Cx. quinquefasciatus, An. sinensis, An. peditaeniatus, Cx. fuscocephalus, Cx. gelidus, and An. tessellates. Each vector represents a given meteorological variable with its mean lying at the origin. Above average values lie alone their corresponding vectors, and below average values project in opposite direction of the vector.
(TIF)
Comparison of sampling ability of different trap types by using Mann-Whitney U test.
(TIF)
The results of t-test for comparing species richness between Mangshi and Ruili.
(DOC)
The results of t-test for comparing SDI between Mangshi and Ruili.
(DOC)
The results of Z-test for comparing the collected number of each common species between Mangshi and Ruili.
(DOC)