Abstract
Although the breast cancer susceptibility gene BRCA1 is one of the most extensively characterized genetic loci, much less is known about its upstream variable number tandem repeat element, the RNU2 locus. RNU2 encodes the U2 small nuclear RNA, an essential splicing element, but this locus is missing from the human genome assembly due to the inherent difficulty in the assembly of repetitive sequences. To fill the gap between RNU2 and BRCA1, we have reconstructed the physical map of this region by re-examining genomic clone sequences of public databases, which allowed us to precisely localize the RNU2 array 124 kb telomeric to BRCA1. We measured by performing FISH analyses on combed DNA for the first time the exact number of repeats carried by each of the two alleles in 41 individuals and found a range of 6-82 copies and a level of heterozygosity of 98%. The precise localisation of the RNU2 locus in the genome reference assembly and the implementation of a new technical tool to study it will make the detailed exploration of this locus possible. This recently neglected macrosatellite could be valuable for evaluating the potential role of structural variations in disease due to its location next to a major cancer susceptibility gene.
Introduction
Structural variation in the human genome has gained considerable attention in the recent years as it accounts for much of the variation between human genomes and may represent the main genetic basis of phenotypic differences. These variations may also provide an explanation for the missing heritability of complex diseases. Indeed large deletions, duplications, translocations and inversions have potentially great effects, including the changing of gene structure and dosage, altering gene regulation and exposing recessive alleles [1]–[2]. CNVs (Copy Number Variations), the most prevalent type of structural variation in the human genome, refer to DNA segments greater than 1 kb in size that are present at variable copy number [2]–[3]. The assessment of CNV phenotypic and pathologic potency has been made easier recently by the great improvement of CNV maps [4]. According to the high-resolution recent maps, most CNVs in the array-accessible regions of the genome are ancient bi-allelic polymorphisms that are in linkage disequilibrium (LD) with SNPs (Single Nucleotide Polymorphisms). This implies that the contribution of most common CNVs to human phenotypic variation was already detectable in genome-wide association studies (GWAS) as associations to nearby SNPs [5]. However, the question of the implication of multi-allelic CNVs in complex traits remains largely open as most of them cannot be genotyped by array technology, especially macrosatellites, the largest variable number tandem repeats (VNTR) [6]. Some, among which long-published and well documented structural variations, are not even present on the reference genome-assemblies, so their sequence is discarded when alternative genotyping technologies such as next generation sequencing are used [7]. One of such missing CNVs is the RNU2 locus, which is all the more detrimental that this highly polymorphic macrosatelllite sits next to a major cancer predisposing gene, BRCA1.
The RNU2 gene is transcribed by RNA polymerase II to give the U2 small nuclear RNA (snRNA), an essential component of the spliceosome. In 1984 it was found to lie within a 6.1 kb unit organised as a nearly perfect tandem array of 10 to 20 copies per haploid genome [8]–[9]. It was subsequently localised on chromosome band 17q21q22 [10] to an adenovirus 12-induced metaphase chromosome fragility site [11], in close proximity to the BRCA1 gene according to FISH (Fluorescent In Situ Hybridization), radiation hybrid, physical and genetic maps [12]–[17]. The sequencing of the 6,132 bp unit (5,834 bp initially because an Alu sequence was missing in the original Genbank submission) failed to reveal any other coding sequence but showed a high content of interspersed repeats (comprising 62.87% of the 6.1-kb unit), including notably five Alu and one LTR (Long Terminal Repeat) sequences, this latter suspected to be involved in the origin or maintenance of the RNU2 array [18] (GenBank accession numbers L37793 and U57614.1). In this study, the regions flanking the RNU2 locus were also cloned and sequenced, which subsequently allowed the establishment of the gene order on chromosome 17: BRCA1 – left junction – RNU2 locus – right junction – chromosome 17 telomere [19].
Field Inversion Gel Electrophoresis (FIGE) analysis of >80 chromosomes from diverse human populations showed that the length of individual RNU2 tandem arrays varied from ∼40 to ∼200 kb (∼6 to >30 repeats): 57% of them were between 100 and 200 kb (16–30 repeats), 32% were between 40 and 100 kb (6–16 repeats) and 11% were longer than the 200 kb limit of the FIGE conditions used (>30 repeats) [20]. More recently, the study of 210 HapMap individuals with Pulse Field Gel Electrophoresis (PFGE) technique revealed a wider range of allelic size (6 to more than 60 copies) [21].
The first attempts to characterise the RNU2 array made in the eighties and the nineties were halted before CNVs started to focus the attention of scientists, certainly because its absence from the human genome reference sequence made it disappear into the dustbin of obsolete and discredited sequences. Therefore, this macrosatellite did not benefit at all from the huge acceleration in human knowledge acquisition of the last 15 years resulting from the implementation of new technologies.
Here we present the precise localization of the RNU2 locus within the chromosome 17 reference assembly and the first direct visualisation of this highly polymorphic CNV by FISH on combed DNA.
Materials and Methods
Ethics Statement
The studied subjects belonged to BRCA1 families and either carried the BRCA1 mutation present in the family or were non-carriers [22]. Informed consent was not required as the data were analyzed anonymously. Nevertheless, the subjects belong to a study which has been reviewed and approved by the appropriate ethics committee (Comité de Protection des Personnes Ile de France III, 3 october 2006, agreement n°2373).
Public Access Database Interrogation and Analysis of the Human Chromosome 17 Reference Sequence and of Clones' Sequence
The human genome reference sequence, working draft assemblies, clone sequences and annotations were obtained from the “UCSC Genome Bioinformatics Site” (http://genome.ucsc.edu). Gene-specific information was obtained from the “Entrez Gene” NCBI's database (http://www.ncbi.nlm.nih.gov/gene). Clone alignments were performed using the BLAST2Seq at the NCBI website (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE_TYPE=BlastSearch&PROG_DEF=blastn&BLAST_PROG_DEF=megaBlast&SHOW_DEFAULTS=on&BLAST_SPEC=blast2seq&LINK_LOC=align2seq).
Cell Lines
Human lymphoblastoid cell lines (LCLs) established by Epstein-Barr virus immortalization of subject's blood lymphocytes were maintained in RPMI 1640 medium (Life Technologies, Saint Aubin, France) supplemented with 10% fetal calf serum (VWR, Fontenay sous Bois, France) and 1% penicillin– streptomycin (Life Technologies).
Plug Preparation and Molecular Combing of DNA
EBV-immortalized lymphoblastoid cells were embedded in agarose blocks (1.2% NuSieve GTG Agarose, Lonza, Levallois-Perret, France) as previously described [23]. DNA was purified in an ESP solution: EDTA 0.5 M pH 8.0, 1% Sarcosyl (Sigma-Aldrich, Saint Quentin Fallavier, France), 2 mg/mL Proteinase K (Eurobio, Courtaboeuf, France) overnight and then agarose was melted at 68°C for 20 min and digested by 1.5 U of β-agarase (New England Biolabs, Evry, France) overnight in a M.E.S solution (2-N-Morpholino-Ethane sulfonique 500 mM pH 5.5). The resulting DNA solution was incubated with a silanized coverslip (CombiCoverslips, Genomic Vision, Paris, France), which was then removed from the solution at a constant speed of 300 µm/sec with the molecular combing system (MCS, Genomic Vision). This protocol allows maintenance of a constant DNA stretching factor of 2 kb/ µm [24]. Combicoverslips with combed DNA were then baked for 4 hours at 60°C. The quality of combing (linearity and density of DNA molecules) was estimated under an epi-fluorescence microscope equipped with an FITC filter set and a 40× air objective on freshly combed coverslips mounted in 20 µL of a 1 ml ProLong-gold solution containing 1 µL of Yoyo-1 solution (both from Life Technologies).
Metaphase Chromosome Spreading
Metaphase spreads were prepared from patient derived lymphocytes using standard procedures.
Probe Preparation
Probes were obtained by labelling PCR-amplified fragments using primers designed with the Primer3 v.0.4.0 software (http://frodo.wi.mit.edu/primer3/) and synthesized by Eurofins MWG Operon (Ebersberg, Germany). The entire RNU2 repeat unit was amplified with primers ReRNU2F/R (5′-GCCAAAAGGACGAGAAGAGA-3′ (59°C)/5′-GGAGCTTGCTCTGTCCACTC-3′ (60°C)) for metaphase chromosome FISH experiments. For combed DNA FISH experiments, 2 regions of the repeat unit were chosen and amplified with primers L4F/R and L5F/R in order to include no more than 300 bp of repeat sequences (such as Alu or LTR sequences) according to the Repeat Masker software (http://www.repeatmasker.org/cgi-bin/WEBRepeatMasker) and 4 regions flanking the RNU2 array with primers FP1F/R, FP2F/R, FP3F/R and FP4F/R. Long-range PCRs were performed in 20 µL reactions using Long PCR Enzyme Mix (Thermo Fisher Scientific, Illkirch, France), following these cycling conditions: 94°C for 2 min, 10 cycles of (96°C for 20 s, Tm°C for 30 s, 68°C for 45 s/kb), 25 cycles of (96°C for 20s, Tm°C for 30s, 68°C for 45 s/kb+10 s/cycle), 68°C for 10 min. Primer sequences and temperature of annealing (in brackets) were the following: L4F 5′-GCGGCCCACAAGATAAGATA-3′ (59°C); L4R 5′-ACGACGCAGTTAGGAGGCTA-3′ (59°C); L5F 5′-CTACACAGCCCAGGACACG-3′ (59°C); L5R 5′-GTTGGCCATGCCTTAAAGTG-3′ (59°C); FP1F 5′-CCAAATTTTCCAAGAGACTGACTT-3′ (59°C); FP1R 5′-GGAGTGAACAGGTGAGAGGATTAT-3′ (59°C); FP2F 5′-GAGCCAAAAATGGATACCTAGAGA-3′ (59°C); FP2R 5′-TGATCCCTGATATCCAATAACCTT-3′ (59°C); FP3F 5′-TACCCCCTTCCTAGCCCTTA-3′ (59°C); FP3R 5′-TCATGCAGCCTGGTACAGAG -3′ (58°C); FP4F 5′-ACCGGGCTGTGTAGAAATTG-3′ (58°C); FP4R 5′-ACCTCATCCTGGCTTACAGG-3′ (58°C). The sizes of the PCR fragments were 434 bp for L4, 1,959 bp for L5, 4,393 bp for FP1, 4,860 bp for FP2, 7,009 bp for FP3 and 5,340 bp for FP4. PCR products have been cloned within the pCR2.1-TOPO XL vector (Life Technologies) according to the manufacturer's instructions.
The probe used in metaphase chromosome FISH experiments was labelled with fluorescein using the nick translation method. Probes used in combed DNA FISH experiments were labelled by random-priming: 200 ng of each probe were incubated during 10 min at 100°C with 1× random primers (Life Technologies), and then cooled at 4°C during 5 min. Klenow enzyme (40U) and dNTP 1× were added. Depending on the emission color chosen, dNTPs 1mM coupled with biotin (for red emission), digoxygenin (for blue emission), or Alexa-488 (for green emission) were also added. These mixes were incubated overnight at 37°C, and the priming reaction were then stopped with EDTA 2.10−2 mM pH 8.
Fluorescent In Situ Hybridization
On metaphase chromosomes
Hybridization was performed as described previously [25] with the probe described above and a 17q subtelomeric probe labelled with rhodamine (Cytocell, Cambridge, UK). After denaturation, overnight hybridization and post-hybridization washes, slides were DAPI counterstained and were read using a fluorescent microscope equipped with a CCD camera.
On combed DNA
One tenth of each random priming mix was precipitated during 1 hour at −80°C with 10 µg of Human Cot1 DNA, 2 µg herring sperm DNA, one tenth of volume of NaAc 3 M pH 5.2 and 2.5 volumes of Ethanol 100%. After centrifugation during 30 min at 4°C and at 13,500 rpm, the supernatant was discarded and the pellet dried at 37°C and dissolved with hybridization buffer (deionized formamide, SSC (salt sodium citrate) 2X, Sarcosyl 0.5%, NaCl 10 mM, SDS 0.5%, Blocking Aid). 20 µL of the mixes were laid on a coverslip with combed DNA, denatured at 95°C during 5 min, and incubation was then performed overnight at 37°C in a hybridizer (Dako, Les Ulis, France). For probe detection, hybridized coverslips were washed three times (3 min each) with formamide-SSC 2X, and three times with SSC 2X. Coverslips were then incubated 20 min at 37°C in a wet room with the first reagents: Streptavidine-A594 for Biotin-dNTP (1), Rabbit anti-A488 antibody for Alexa-A488-dNTP (2), and Mouse anti-Dig AMCA antibody for Digoxygenin-dNTP (3). Coverslips were washed with three successive baths of SSC 2X-Tween20 1%. Similarly, coverslips were incubated with the second reagents: Goat anti-streptavidine biotinylated antibody (1), Goat anti-rabbit A488 antibody (2) and Rat anti-mouse AMCA antibody (3). Coverslips were washed and incubated with the third reagents: Streptavidine A594 (1), and goat anti-rat A350 antibody (3). Coverslips were dehydrated with three successive baths of ethanol (70-90-100%). Image acquisition was performed with a customized automated fluorescence microscope (Image Xpress Micro, Molecular Devices, Sunnyvale, CA, USA) at 40× magnification, and image analysis and signal measurement were performed with ImageJ (available from NIH) and GVlab (Genomic Vision) softwares. Allelic number of copies was determined by counting the number of signals corresponding to a repeat unit only on fibres for which intact flanking probes could be observed. In all cases, the number of copies has been determined by at least two individuals, resulting in differences of one copy at the most. For the nicest 72 fibres obtained from 21 individuals, we determined the individual exact stretching factor by measuring the length of a motif covering 128 kb within the BRCA1 bar code, which in turn allowed us to determine the physical distance separating BRCA1 and the RNU2 locus.
Results
Precise Localisation of the RNU2 Array
The organization of the RNU2-BRCA1 region as published in the literature is presented in Figure 1A: the genes described within this interval are NBR1, BRCA1P1 (a BRCA1 pseudogene) and NBR2 [16], [19]. The distance between the RNU2 locus and D17S1322, a microsatellite located within BRCA1 intron 19, is reported to be ∼175 kb based on physical maps. This would locate BRCA1 ∼113 kb away from the RNU2 locus. In contradiction with the literature, a single RNU2 gene described as a pseudogene, RNU2-4P (289 bp long), also known as RNU2P2, is found on the chromosome 17 reference assembly Build 37 in the first intron of an uncharacterised gene named LOC100130581, ∼187 kb away from BRCA1 (Figure 1B and Table S1 in Additional file). Along with NBR1, BRCA1P1 and NBR2, one more gene, TMEM106A, has been identified by sequence analysis within this region.
Figure 1. Schematic representation of the chromosome 17q21 region around the BRCA1 gene.

(A) Gene locations and physical map distances as reported in the literature [16], [19]. (B) Gene locations within a 300 Kb window as shown in the UCSC Genome Browser. Arrows indicate transcription direction. BRCA1P1: BRCA1 pseudogene.
As shown previously [11], [26]–[27], FISH on mitotic metaphase chromosomes using a probe obtained by labelling a 6.1 kb PCR fragment amplified with primers flanking the RNU2 repeat unit gave a unique signal over band 17q21 (Figure 2), which indicated that the repeat unit is located at the same cytogenetic band as the BRCA1 gene. Furthermore, the high intensity of the signal was consistent with the repeat unit being present in multiple copies.
Figure 2. Visualization by FISH on mitotic metaphase chromosome of the RNU2 locus.

Two probes were used, one consisting of the 6.1
Seven RNU2 genes could be found in Entrez Gene (NCBI's repository for gene-specific information), among which five are considered to be pseudogenes. RNU2-1 (GenBank accession number NR_002716.3), assigned to chromosome band 17q12-q21, is identical to the gene found in the RNU2 repeat unit, but this locus, which in Build 36 was annotated on an unplaced contig based on a single unfinished BAC (Bacterial Artificial Chromosome) sequence, is no longer present in Build 37 as the BAC was removed from the assembly. A portion of the RNU2 repeat unit (corresponding to positions 1440-3036 of U57614.1) is nevertheless present at position 41,399,577-41,401,198 (Figure 3A). The right junction of the RNU2 array sequenced in 1995 [18] (416 bp: 36 bp of the repeat unit+380 bp of flanking sequence) and located telomeric to the RNU2 locus [19] could be found as well at position 41,401,163-41,401,579, while the left junction (92 bp: 47 bp of the repeat unit+45 bp of flanking sequence) is missing from the human genome assembly, probably due to sequence assembly errors. In light of these data, we hypothesised that the RNU2 array was located between positions 41,399,577, and 41,401,198, where the right junction and part of the RNU2 array repeat unit can be found.
Figure 3. Localisation of the RNU2 macrosatellite within the chromosome 17 sequence assemblies from NCBI Build 37.p10.

(A) Schema of the region surrounding the RNU2 array. The location of the portion of the RNU2 repeat unit (not comprising the RNU2 gene) and of the right junction found in the assemblies are depicted, as well as the probes used in molecular combing experiments that flank the RNU2 array (FP1-4), and the NBR1 and TMEM106A genes. (B) Clones covering the region. The reference sequence assemblies is based upon the complete sequence of 3 overlapping BACs, RP11-242D8, CTD-3014M21 and RP11-100E5 (AC060780.18, AC109326.11 and AC087650.12 respectively), represented by brown arrows. The complete sequence of the WI2-3095P13 fosmid (AC160862.2, green arrow) matches the reference sequence. The sequence of the ABC10-44487500M2 fosmid (AC231386.2, green arrow) matches the reference sequence up to its centromeric extremity where it contains several RNU2 repeat units (depicted in a dotted curl). The five unassembled contigs of the working draft sequence of the RP11-570A16 BAC clone (AC087365.3) showing homology with the reference sequence are represented by a purple arrow. Contig 15 has been mis-assembled, as it contains several RNU2 repeat units (depicted in dotted curls) at both its extremities.
In order to sustain this hypothesis, we extracted from the databases the sequences covering this region and analyzed them. The complete sequence of a 41 kb fosmid (ABC10-44487500M2) reported in AC231386.2 confirmed the localisation of the RNU2 macrosatellite, as it displayed 5 complete repeat units followed by sequences matching Build 37 from position 41,399,577 to 41,413,658 (Figure 3B). We also analysed the unfinished sequence of the RP11-570A16 BAC clone (AC087365.3), namely 16 unordered contigs covering 104,495 bp. Part or the entire sequence of the RNU2 array repeat unit is found in all but contigs 1, 5 and 6. Contig 1, which contains TMEM106A and the end of NBR1, and contigs 6 and 5 match adjacent sequences on chromosome 17 (Figure 3). The main parts of contigs 15 and 16 also match adjacent sequences, with an overlap of 1.3 kb between contigs 15 and 16 corresponding to a portion of the RNU2 array repeat unit, and of ∼500 bp between contigs 5 and 15. This ∼500 bp overlap precedes a portion of the RNU2 array repeat unit sequence in contig 15, while it is at the end of contig 5, which suggests that contig 15 has been incorrectly assembled and that all the sequences matching the RNU2 array repeat unit should be placed at the other end of the contig. Indeed, the assembly of these contigs is comforted not only by the chromosome 17 reference sequence assemblies but also by the complete sequence of a fosmid (AC160862.2) that covers this region. In conclusion, these data are all in agreement with a localisation of the RNU2 macrosatellite between positions chr17∶41,399,577, and chr17∶41,401,198, which puts it ∼124 kb telomeric to the BRCA1 gene and ∼63 kb centromeric to the RNU2-4P gene.
Variation of the Number of RNU2 Array Repeat Unit in the Human Population
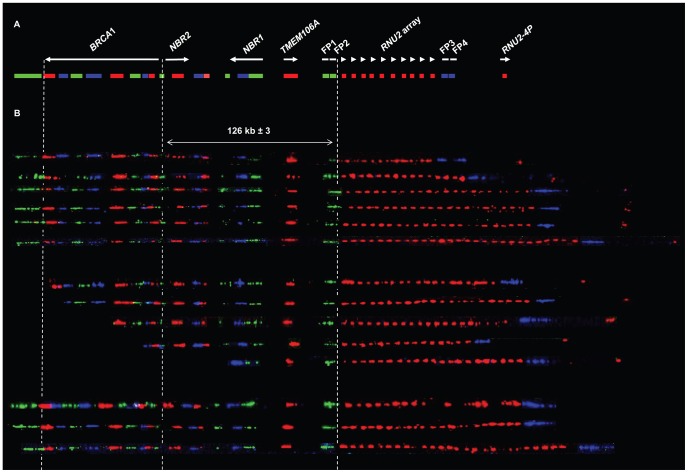
We next undertook to directly visualize the proximity of the RNU2 array with the BRCA1 gene by using the molecular combing technology. We completed the existing bar code that allows to get a panoramic view of BRCA1 and its flanking genes, namely TMEM106A, NBR1, BRCA1P1, and NBR2 [23], with a probe obtained by labeling two PCR fragments amplified with primers flanking close regions devoid of repeat sequences within the RNU2 array repeat unit (1.96 and 0.46 kb). We also generated four probes expected to hybridize regions flanking the RNU2 macrosatellite based on our assumption of its location, respectively 7.3 kb downstream in the case of probes FP1 and FP2, on the centromeric side, and 2 kb upstream in the case of probes FP3 and FP4, on the telomeric side (Figure 4). Hybridisation of these probes with combed DNA of very good quality generated a consistent pattern of signals covering a genomic region >350 kb. This pattern shows the juxtaposition, from chromosome 17 centromere to telomere, of the BRCA1 bar code, FP1-2 probes, RNU2, FP3-4 probes and RNU2-4P (the RNU2 probes cross-react with the RNU2 pseudogene), thus validating our tentative map (Figure 4). The average size of the interval between the end of the BRCA1 gene and the RNU2 array boundary was 126 kb±3 when measuring 72 fibres from 21 individuals (expected size based on the chromosome 17 reference assembly: 123.7 kb). The distance between the array boundary and RNU2-4P seemed consistent with that expected from our tentative map (63.4 kb), but the paucity of the number of fibres displaying both BRCA1 and RNU2-4P precluded us from doing precise measures. Measurement of the RNU2 signals gave an average size of 2.15 kb±0.63, while the average size for the gap between two RNU2 signals was 4.30 kb±2.21, as expected on the basis of the sequence of the RNU2 array repeat unit.
Figure 4. Visualization by molecular combing of the 17q21 region around BRCA1.
(A) Schematization of the genomic morse code used. The BRCA1 Genomic Morse Code (GMC) depicted (v4.0) is an improvement of the published code (v1.0) [23]. It covers a genomic region of 200 kb and consist in 17 signals of a distinct color (green, red or blue), each composed of 1 to 3 small horizontal bars corresponding to a single DNA probe. The signals for the flanking probes FP1-4 are each composed of 2 green or blue horizontal bars, while the signal for the RNU2 array repeat unit is composed of 1 red horizontal bar. Of note, the probe for the RNU2 array cross-reacts with RNU2-4P. (B) Fourteen fibres displaying different numbers of RNU2 signals are shown. The first six fibres display the entire bar code from the BRCA1 GMC to RNU2-4P, while the followings miss either the beginning of the BRCA1 GMC or RNU2-4P.
In total, we analysed 41 individuals with this technique. All but one of them displayed two populations of fibres containing different numbers of RNU2 signals, confirming the high level of heterozygosity of the RNU2 macrosatellite, which reached 0.98 in our small sample. Examples of fibres displaying different numbers of repeats are shown in Figure 4. The 28 different alleles that we identified among the 46 unrelated chromosomes analysed (five of which carrying a BRCA1 mutation) are presented in Table 1: 14 of them (50%) were found only once while twelve were found twice (43%), one three times (3.5%) and one six times (3.5%). The number of RNU2 array repeat units was found to range from 6 to 82 copies, and most of the alleles differed from their closest allele by one copy.
Table 1. Description of the RNU2 array alleles identified in 46 unrelated chromosomes.
| Alleles (N = 28) | Number of repeat units | Number of occurrence | Frequency of each allele |
| 1 | 6 | 1 | 0.02 |
| 2 | 8 | 1 | 0.02 |
| 3 | 9 | 1 | 0.02 |
| 4 | 11 | 2 | 0.04 |
| 5 | 12 | 1 | 0.02 |
| 6 | 13 | 1 | 0.02 |
| 7 | 14 | 2 | 0.04 |
| 8 | 15 | 1 | 0.02 |
| 9 | 16 | 1 | 0.02 |
| 10 | 17 | 1 | 0.02 |
| 11 | 18 | 3 | 0.07 |
| 12 | 19 | 5 | 0.11 |
| 13 | 20 | 1 | 0.02 |
| 14 | 21 | 2 | 0.04 |
| 15 | 22 | 2 | 0.04 |
| 16 | 23 | 1 | 0.02 |
| 17 | 25 | 1 | 0.02 |
| 18 | 27 | 2 | 0.04 |
| 19 | 28 | 2 | 0.04 |
| 20 | 29 | 2 | 0.04 |
| 21 | 30 | 1 | 0.02 |
| 22 | 32 | 2 | 0.04 |
| 23 | 34 | 2 | 0.04 |
| 24 | 35 | 2 | 0.04 |
| 25 | 36 | 1 | 0.02 |
| 26 | 37 | 2 | 0.04 |
| 27 | 47 | 2 | 0.04 |
| 28 | 82 | 1 | 0.02 |
Discussion
The gaps in the finished human genome-assemblies are likely to host undiscovered CNVs. Some long-published and well documented structural variations are also missing from human genome-assemblies due to the difficulty to assemble repeated regions [28]–[29]. Indeed repeats confuse the assembly process, often resulting in contig mis-assembly [30]. The determination of which segments of the genome are affected by CNVs and the mapping of each CNV to a human genomic region is, however, an important step to assess the phenotypic and pathologic potency of these structural variations. To date, less than a dozen macrosatellites have been characterized although this type of CNVs consisting typically of dozens of repetitive units of several kilobases are among the most polymorphic structural variations and the most likely to impact chromatin organisation and human health [31].
Here, we have determined the exact localisation of the human RNU2 macrosatellite within chromosome 17 genome-assembly (Build 37), between positions chr17∶41,399,577, and chr17∶41,401,198, ∼124 kb telomeric to the BRCA1 gene and ∼63 kb centromeric to one of the numerous RNU2 pseudogenes, RNU2-4P, the only one present on chromosome 17. We validated this location by a FISH analysis of combed DNA (“molecular combing”) using a BRCA1 Genomic Morse Code [23] completed by probes complementary to the RNU2 array repeat unit and to flanking regions. This approach allowed us to determine the exact number of repeats carried by 46 independent chromosomes (41 individuals analysed in total), revealing 28 different alleles that display from 6 to 82 monomers. Up to now, two studies on the RNU2 macrosatellite alleles have been published in which the RNU2 array sizes were estimated from FIGE- or PFGE-separated EcoRI (a null cutter) genomic fragments visualised with a RNU2-specific probe [20]–[21]. Interestingly, the minimal number of repeats is the same in the three studies (i.e. 6). Liao et al. (1999) identified 15 different alleles in 28 chromosomes, but FIGE could not resolve alleles with array length >200 kb (33 copies) [20]. PFGE resolution appeared better as Schaap et al. (2013) were able to identify 58 different alleles (6–63 copies) differing from their closest allele by one copy by analysing 210 human DNA samples from four populations [21]. However, the electrophoresis-based methods may lack precision in determining the exact number of repeats for large arrays, especially for those exceeding 500 kb, while repeat number counting following molecular combing is not sensitive to array length as long as probes complementary to the RNU2 locus flanking regions are used to assess fibre integrity. Moreover, the molecular combing technique allows the identification of possible complex repeat patterns resulting from large insertions of foreign DNA into the array and/or repeat inversions. However, electrophoresis-based methods are better suited to detect mosaicism, which is quite common in the case of macrosatellites [31]. These two techniques are therefore complementary for the study of macrosatellite repeats.
U2 snRNAs play an essential role in formation of the catalytically active spliceosome by base pairing with both the intron branch point and the U6 snRNA [32]. A five nucleotide deletion in one of the five murine U2 snRNA genes causes ataxia and neurodegeneration, neuron loss being strongly dependent on the dosage of wild-type and mutant U2 snRNAs [33]. This finding suggests that RNU2 might be associated with disease in humans as well. Growing evidence links splicing factor dysfunction with disease, particularly cancer [34]. Furthermore, the proximity of this macrosatellite to the BRCA1 gene combined with its high degree of polymorphism raise the interesting possibility that it could be involved in breast cancer susceptibility. Indeed, investigations in mice have suggested that the effect of CNVs on the expression of flanking genes could extend up to 450 kb away from their location, all the more in the case of long CNVs (> 50kb) [35]. In humans, the stronger evidence of such an effect so far came from the study of the Williams-Beuren syndrome, where not only hemizygous genes that map within the microdeletion responsible for the disease but also normal copy neighboring genes show decreased relative levels of expression [36]. Our study, which gives a precise localization and better characterizes the RNU2 locus, provides the foundation for testing the association between copy number at this locus and breast cancer or other diseases risk.
Supporting Information
Genomic coordinates of 17q21 genes and sequences (Build 37.p10).
(DOCX)
Acknowledgments
We thank Richard Redon for helpful discussions, for reviewing the manuscript and offering helpful comments and suggestions, as well as Francesca Damiola for her critical reading of the manuscript. We thank Gaël Yvert, Rémy Bonnavion and Ivan Mikaélian for helpful discussions, Anne Vannier for expert advice on molecular combing and Jennifer Abscheidt, Mélanie Léoné and José Garcia for skilled technical assistance.
Funding Statement
This work was supported by a grant from the “Fondation ARC pour la Recherche sur le Cancer” The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Eichler EE, Nickerson DA, Altshuler D, Bowcock AM, Brooks LD, et al. (2007) Completing the map of human genetic variation. Nature 447: 161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feuk L, Carson AR, Scherer SW (2006) Structural variation in the human genome. Nat Rev Genet 7: 85–97. [DOI] [PubMed] [Google Scholar]
- 3. Freeman JL, Perry GH, Feuk L, Redon R, McCarroll SA, et al. (2006) Copy number variation: new insights in genome diversity. Genome Res 16: 949–961. [DOI] [PubMed] [Google Scholar]
- 4. Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, et al. (2006) Global variation in copy number in the human genome. Nature 444: 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCarroll SA, Kuruvilla FG, Korn JM, Cawley S, Nemesh J, et al. (2008) Integrated detection and population-genetic analysis of SNPs and copy number variation. Nat Genet 40: 1166–1174. [DOI] [PubMed] [Google Scholar]
- 6. Warburton PE, Hasson D, Guillem F, Lescale C, Jin X, et al. (2008) Analysis of the largest tandemly repeated DNA families in the human genome. BMC Genomics 9: 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abyzov A, Urban AE, Snyder M, Gerstein M (2011) CNVnator: an approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Genome Res 21: 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Arsdell SW, Weiner AM (1984) Human genes for U2 small nuclear RNA are tandemly repeated. Mol Cell Biol 4: 492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Westin G, Zabielski J, Hammarstrom K, Monstein HJ, Bark C, et al. (1984) Clustered genes for human U2 RNA. Proc Natl Acad Sci U S A 81: 3811–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hammarstrom K, Santesson B, Westin G, Pettersson U (1985) The gene cluster for human U2 RNA is located on chromosome 17q21. Exp Cell Res 159: 473–478. [DOI] [PubMed] [Google Scholar]
- 11. Lindgren V, Ares M Jr, Weiner AM, Francke U (1985) Human genes for U2 small nuclear RNA map to a major adenovirus 12 modification site on chromosome 17. Nature 314: 115–116. [DOI] [PubMed] [Google Scholar]
- 12. Abel KJ, Boehnke M, Prahalad M, Ho P, Flejter WL, et al. (1993) A radiation hybrid map of the BRCA1 region of chromosome 17q12-q21. Genomics 17: 632–641. [DOI] [PubMed] [Google Scholar]
- 13. Albertsen HM, Smith SA, Mazoyer S, Fujimoto E, Stevens J, et al. (1994) A physical map and candidate genes in the BRCA1 region on chromosome 17q12-21. Nat Genet 7: 472–479. [DOI] [PubMed] [Google Scholar]
- 14. Black DM, Nicolai H, Borrow J, Solomon E (1993) A somatic cell hybrid map of the long arm of human chromosome 17, containing the familial breast cancer locus (BRCA1). Am J Hum Genet 52: 702–710. [PMC free article] [PubMed] [Google Scholar]
- 15. Flejter WL, Barcroft CL, Guo SW, Lynch ED, Boehnke M, et al. (1993) Multicolor FISH mapping with Alu-PCR-amplified YAC clone DNA determines the order of markers in the BRCA1 region on chromosome 17q12-q21. Genomics 17: 624–631. [DOI] [PubMed] [Google Scholar]
- 16. Liu X, Barker DF (1999) Evidence for effective suppression of recombination in the chromosome 17q21 segment spanning RNU2-BRCA1. Am J Hum Genet 64: 1427–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neuhausen SL, Swensen J, Miki Y, Liu Q, Tavtigian S, et al. (1994) A P1-based physical map of the region from D17S776 to D17S78 containing the breast cancer susceptibility gene BRCA1. Hum Mol Genet 3: 1919–1926. [DOI] [PubMed] [Google Scholar]
- 18. Pavelitz T, Rusche L, Matera AG, Scharf JM, Weiner AM (1995) Concerted evolution of the tandem array encoding primate U2 snRNA occurs in situ, without changing the cytological context of the RNU2 locus. EMBO J 14: 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pavelitz T, Liao D, Weiner AM (1999) Concerted evolution of the tandem array encoding primate U2 snRNA (the RNU2 locus) is accompanied by dramatic remodeling of the junctions with flanking chromosomal sequences. EMBO J 18: 3783–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liao D, Pavelitz T, Kidd JR, Kidd KK, Weiner AM (1997) Concerted evolution of the tandemly repeated genes encoding human U2 snRNA (the RNU2 locus) involves rapid intrachromosomal homogenization and rare interchromosomal gene conversion. EMBO J 16: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schaap M, Lemmers RJ, Maassen R, van der Vliet PJ, Hoogerheide LF, et al. (2013) Genome-wide analysis of macrosatellite repeat copy number variation in worldwide populations: evidence for differences and commonalities in size distributions and size restrictions. BMC Genomics 14: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sinilnikova OM, Mazoyer S, Bonnardel C, Lynch HT, Narod SA, et al. (2006) BRCA1 and BRCA2 mutations in breast and ovarian cancer syndrome: reflection on the Creighton University historical series of high risk families. Fam Cancer 5: 15–20. [DOI] [PubMed] [Google Scholar]
- 23. Cheeseman K, Rouleau E, Vannier A, Thomas A, Briaux A, et al. (2012) A diagnostic genetic test for the physical mapping of germline rearrangements in the susceptibility breast cancer genes BRCA1 and BRCA2. Hum Mutat 33: 998–1009. [DOI] [PubMed] [Google Scholar]
- 24. Michalet X, Ekong R, Fougerousse F, Rousseaux S, Schurra C, et al. (1997) Dynamic molecular combing: stretching the whole human genome for high-resolution studies. Science 277: 1518–1523. [DOI] [PubMed] [Google Scholar]
- 25. Schluth-Bolard C, Delobel B, Sanlaville D, Boute O, Cuisset JM, et al. (2009) Cryptic genomic imbalances in de novo and inherited apparently balanced chromosomal rearrangements: array CGH study of 47 unrelated cases. Eur J Med Genet 52: 291–296. [DOI] [PubMed] [Google Scholar]
- 26. Bailey AD, Li Z, Pavelitz T, Weiner AM (1995) Adenovirus type 12-induced fragility of the human RNU2 locus requires U2 small nuclear RNA transcriptional regulatory elements. Mol Cell Biol 15: 6246–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu A, Fan HY, Liao D, Bailey AD, Weiner AM (2000) Activation of p53 or loss of the Cockayne syndrome group B repair protein causes metaphase fragility of human U1, U2, and 5S genes. Mol Cell 5: 801–810. [DOI] [PubMed] [Google Scholar]
- 28. International Human Genome Sequencing Consortium (2004) Finishing the euchromatic sequence of the human genome. Nature 431: 931–945. [DOI] [PubMed] [Google Scholar]
- 29. Eichler EE, Clark RA, She X (2004) An assessment of the sequence gaps: unfinished business in a finished human genome. Nat Rev Genet 5: 345–354. [DOI] [PubMed] [Google Scholar]
- 30. Phillippy AM, Schatz MC, Pop M (2008) Genome assembly forensics: finding the elusive mis-assembly. Genome Biol 9: R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tremblay DC, Alexander G Jr, Moseley S, Chadwick BP (2010) Expression, tandem repeat copy number variation and stability of four macrosatellite arrays in the human genome. BMC Genomics 11: 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wahl MC, Will CL, Luhrmann R (2009) The spliceosome: design principles of a dynamic RNP machine. Cell 136: 701–718. [DOI] [PubMed] [Google Scholar]
- 33. Jia Y, Mu JC, Ackerman SL (2012) Mutation of a U2 snRNA gene causes global disruption of alternative splicing and neurodegeneration. Cell 148: 296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Padgett RA (2012) New connections between splicing and human disease. Trends Genet 28: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henrichsen CN, Vinckenbosch N, Zollner S, Chaignat E, Pradervand S, et al. (2009) Segmental copy number variation shapes tissue transcriptomes. Nat Genet 41: 424–429. [DOI] [PubMed] [Google Scholar]
- 36. Merla G, Howald C, Henrichsen CN, Lyle R, Wyss C, et al. (2006) Submicroscopic deletion in patients with Williams-Beuren syndrome influences expression levels of the nonhemizygous flanking genes. Am J Hum Genet 79: 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genomic coordinates of 17q21 genes and sequences (Build 37.p10).
(DOCX)