Abstract
1,5-disubstituted tetrazoles are an important drug-like scaffold known for their ability to mimic the cis-amide bond conformation. The scaffold is readily accessible via substitution of the carboxylic acid component of the Ugi multi-component reaction (MCR) with TMSN3 in what is herein denoted the Ugi-azide reaction. This full paper presents a concise, novel, general strategy to access a plethora of new heterocylic scaffolds utilizing tethered aldo/keto-acids/esters in the Ugi-azide reaction followed by a ring closing event that generates novel highly complex bis-heterocyclic lactam-tetrazoles.
Introduction
The design of peptidomimetics to circumvent small molecule biological stability issues, thus delivering improved pharmacokinetic profiles, has gained massive interest over the last twenty years.1 In particular, cis-amide bonds have been shown to play key roles in protein secondary structures involved in several important biological systems.2 In studies to determine effective mimics of the cis-amide bond, the tetrazole ring and more specifically the 1,5-disubstituted tetrazole, has proven to be a valuable bioisostere, extensively reported on by Marshall et al.3 The biological significance of related ring systems has grown in recent years with a number of tetrazole analogs reported to exhibit biological activity toward the cannabinoid-1 receptor (CB1),4 fatty acid amide hydrolase,5 melanin-concentrating hormone receptor 1,6 polo-like kinase 1,7 and to act as orally effective human growth hormone secretagogues.8 Clearly, development of concise routes to novel 1,5-disubstituted tetrazole chemical space has the potential to deliver small molecule partners or probes for new or established protein receptors, enabling studies on protein function or even initiation of translational campaigns.
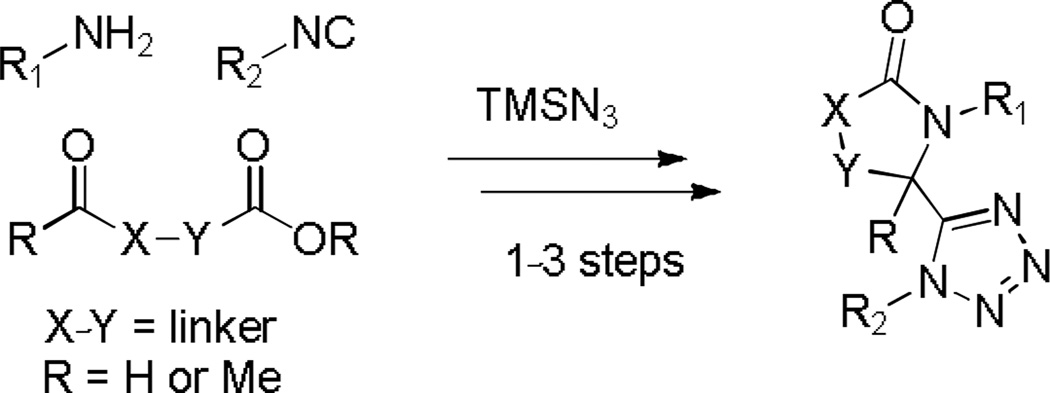
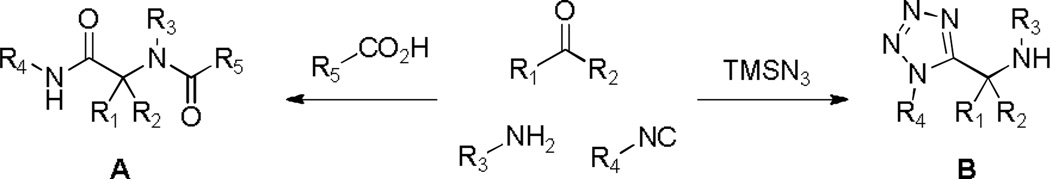
The classical Ugi MCR is comprised of four components, an aldehyde, amine, isocyanide and carboxylic acid, which on mixing generate the peptidic-like structure A containing 4 points of diversification (Scheme 1). As such, it is probably the premiere isocyanide based MCR, and subsequent chemical manipulation of the flexible product has received immense interest in the medicinal chemistry community providing access to arrays of highly diverse small molecules.9 Moreover, an offspring of the Ugi reaction, denoted the Ugi-azide reaction, offers a concise chemical route to 1,5-disubstituted tetrazoles which is initiated with simple replacement of the carboxylic acid with TMSN3, delivering 1,5-disubstituted tetrazoles B (Scheme 1).10 Through use of a variety of assorted reagents and systematically exploring different ring closing possibilities of the Ugi-azide product B, unique scaffolds such as ketopiperazine-tetrazoles,11 azepine-tetrazoles,12 benzodiazepine-tetrazoles13 and quinoxaline-tetrazoles14 have been successfully generated.
Scheme 1.
Ugi and Ugi-azide MCR
Recently, we reported on the use of methyl levulinate 1, a tethered keto-ester, in the Ugi-azide MCR followed by subsequent rigidification.15 This full paper details the versatility and generality of the methodology with greatly enhanced scope in tether diversity between the aldehyde and electrophilic acid or ester appendage (Scheme 2), enabling access to multiple collections of bis-heterocyclic lactam-tetrazoles recently submitted to the United States Molecular Libraries Small Molecule Repository (MLSMR).
Scheme 2.
General strategy to lactam-tetrazoles
Results and discussion
Syntheses of tetrazolyl-pyrrolidinones and -indolinones
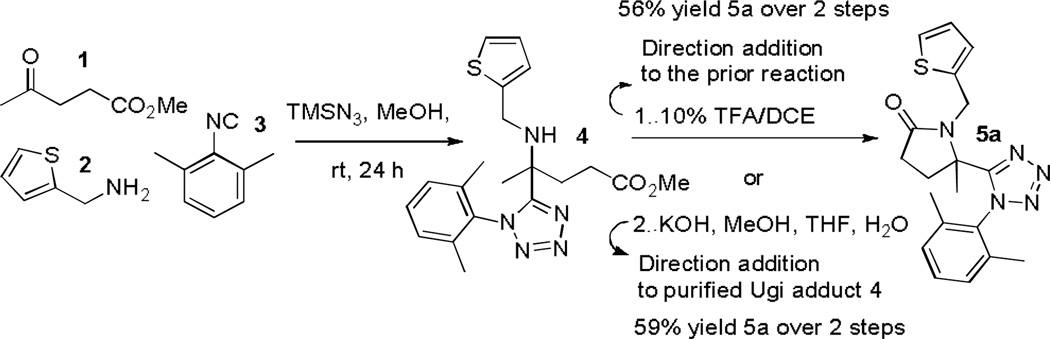
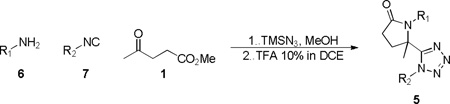
Initial studies concentrated on exploring the suitability of commercially available methyl levulinate 1 to provide the tetrazolyl-lactam 5a with supporting reagents thiophen-2-ylamine 2 and 2-isocyano-1,3-dimethylbenzene 3 (Scheme 3).15 After formation of the Ugi product 4, direct addition of a solution of 10% TFA in DCE [Note: without removal of MeOH] facilitated lactam formation to give 5a in 56% yield over two steps (Scheme 3). Interestingly, when methanol was removed prior to addition and dissolution of 4 in 10% TFA/DCE, 5a was only observed in negligible amounts. The Ugi product was thus isolated and subjected to basic conditions (Scheme 3, Scheme 2. KOH, MeOH, THF, H2O). Gratifyingly, 2a was attained in comparable yields (59% over 2 steps), presumably through cyclization of the secondary amine directly onto a newly formed carboxylic acid from the methyl ester moiety. With two complementary routes in hand the more operationally friendly acid mediated protocol was employed to establish the reactivity domain through preparation of a further eight tetrazolyl-pyrrolidinones 2 (Table 1). Furthermore, the methodology was importantly shown to be compatible with plate based production, delivering 96 congeners in rapid fashion.1
Scheme 3.
Access to tetrazolyl-pyrrolidinone 5a
Table 1.
Tetrazolyl-pyrrolidinones series
 | |||
|---|---|---|---|
| R1 | R2 | Product | Yield (%) |
| 5b | 78 | ||
| 5c | 54 | ||
| 5d | 69 | ||
| 5e | 59 | ||
| 5f | 52 | ||
| 5g | 64 | ||
| 5h | 40 | ||
| 5i | 48 | ||
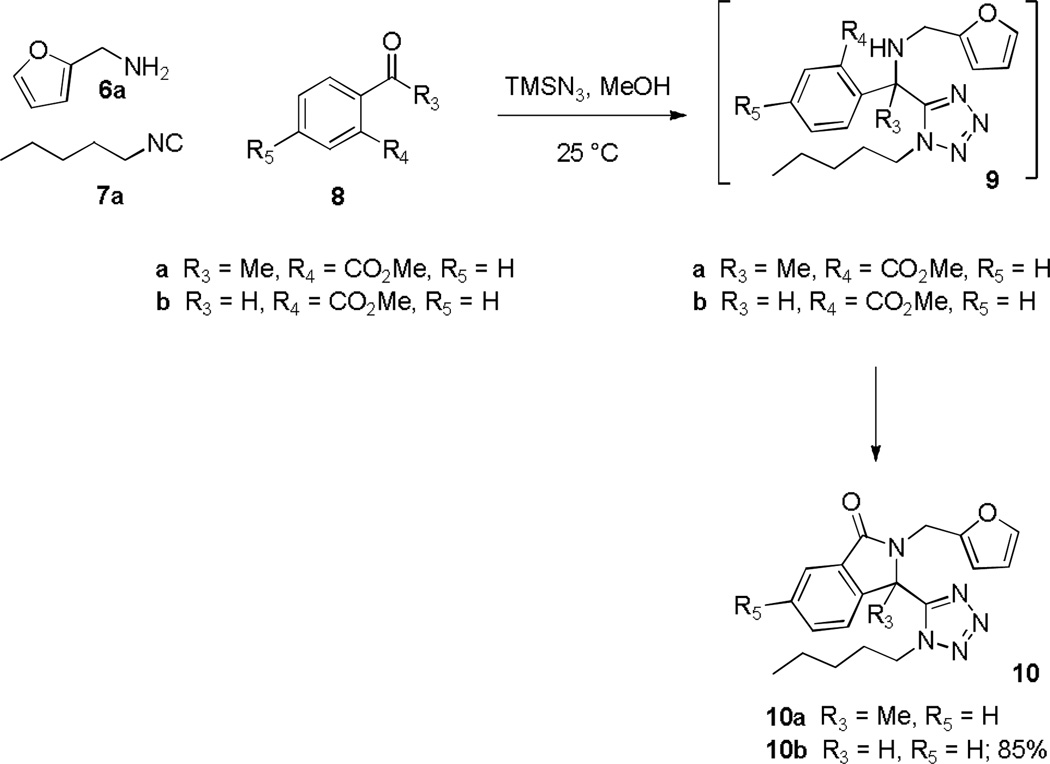
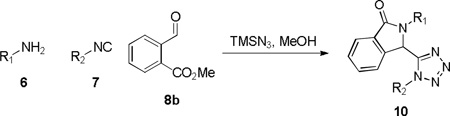
Encouraged by this operationally friendly protocol, we embarked upon additional studies of new tether diversity exploring the production of tetrazolyl-indolinones 10. Preliminary attempts focused on the use of methyl 2-acetylbenzoate 8a, n-pentyl isocyanide 7a and furfurylamine 6a in the Ugi-azide MCR (Scheme 4). Unexpectedly, the condensation performed poorly for the acetophenone 8a, whereas the aldehyde congener, methyl 2-formylbenzoate 8b, performed in exemplary fashion and 10b was formed directly without the need for addition of acid (85% isolated yield, 2 steps).
Scheme 4.
Synthesis of 2-(furan-2-ylmethyl)-isoindolin-1-one 10b
Eight analogs were thus prepared using an assortment of primary amines and isocyanides (Table 2) and the chemistry was progressed to plate based production delivering 96 additional analogs of indolinone-tetrazoles 8.18
Table 2.
Indolinone tetrazole series
 | |||
|---|---|---|---|
| R1 | R2 | Product | Yield (%) |
 |
10c | 51 | |
| 10d | 58 | ||
 |
10e | 66 | |
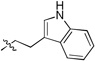 |
10f | 43 | |
| 10g | 51 | ||
| 10h | 58 | ||
| 10i | 29 | ||
| 10j | 36 | ||
Syntheses of tetrazolyl-piperidinones, -ketopiperazines, and-thiomorpholinones
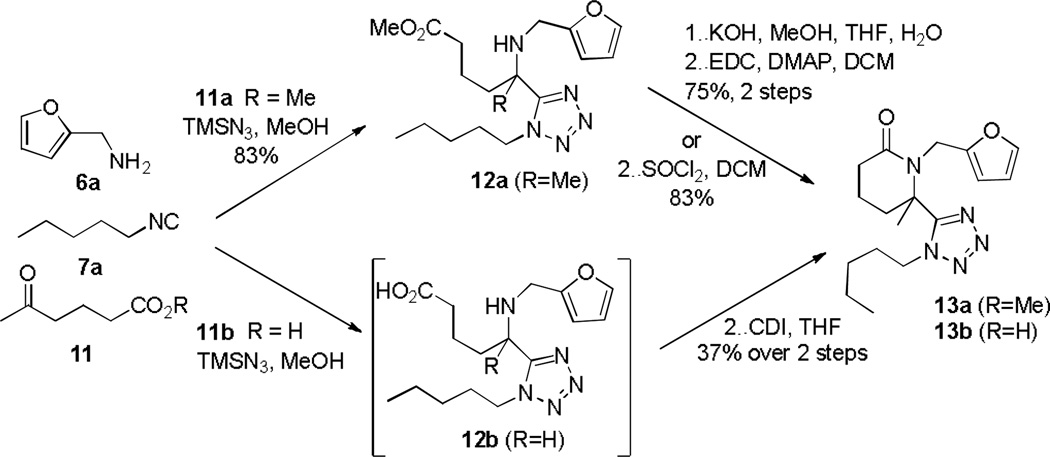
Using the same diversity reagents 6a and 7a, the feasibility of expanding the generality of this methodology to afford 6 membered rings was subsequently investigated with bifunctional reagents methyl ester 11a and free carboxylic acid 11b. Thus, after Ugi-azide reaction of 6a, 7a and 11a, TFA was added to the methanolic reaction medium of 12a and contrary to observations with its 5-membered ring congener no cyclization to desired product 13a was observed.
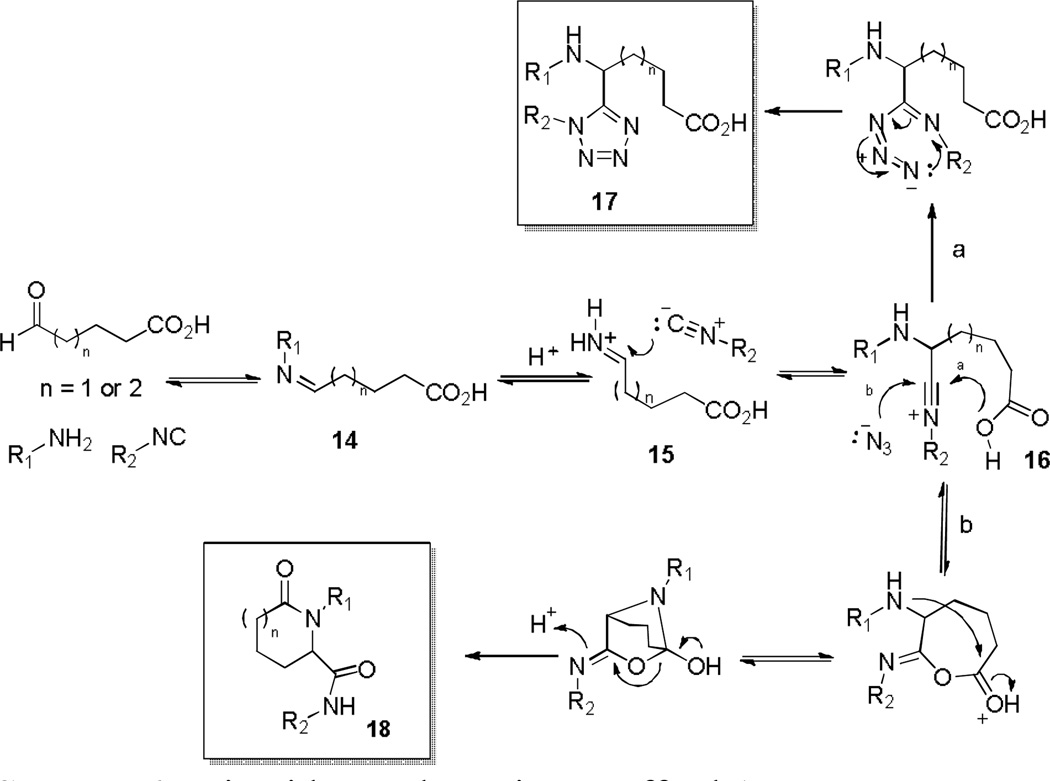
From prior experience with 4 (Scheme 3) possessing both ester and amine functionalities akin to 12a, lactamization was attempted via two additional steps i.e. ester cleavage to the free acid and amide bond formation to afford the lactam. Thus, reaction of methyl 5-oxohexanoate 11a in the azide modified Ugi reaction with 6a and 7a (Scheme 5) gave the Ugi amino-ester 12a (83% yield). Basic hydrolysis of 12a and subsequent 1-ethyl-3-(3-dimethyllaminopropyl)-carbodiimide (EDC) or SOCl2 mediated intramolecular amide coupling provided 13 in 75% and 83% yield respectively over the final two steps. Looking to improve the route to 13, we investigated utilizing the tethered keto-acid 11b in the Ugi-Azide MCR and, to our delight, the Ugi product 12b as opposed to 18 was formed, Scheme 6. Without purification of 12b, coupling agent 1,1’-carbonyldiimidazole (CDI) was able to catalyze lactam formation to provide tetrazolyl-piperidinone 13b in 37% overall yield for the combined two steps (Scheme 5). With a general one-pot, two-step optimized procedure in hand, five other examples were prepared using an assortment of primary amines and isocyanides reported previously.16 Mechanistically, this was intriguing and a postulated sequence of events is depicted in Scheme 6.19 Thus, condensation of an aldo-acid and a primary amine forms imine 15. Upon imine protonation, isocyanide addition forms the intermediate nitrilium ion 16. The classical intramolecular Ugi MCR (Path a) would typically afford lactam 18 after Mumm rearrangement. However, the small and highly nucleophilic azide ion intercepts 16 (Path b) in preference to intramolecular ring closure with the free carboxylic acid, affording 17 which is ready for CDI mediated ring closure. Further efforts were devoted to enrich molecular diversity through the assembly of more elaborated heterocyclic cores and three additional libraries incorporating unique molecular features and six membered rings found in medicinally valuable compounds were thus evaluated for route feasibility. Thus, integration of a sulphur atom into the 6-membered ring to generate thiomorpholinone derivatives 20 was attempted. This peculiar moiety has been the subject of intense study due to its pharmacological properties depicted as a 1,4-benzothiazine framework in the calcium antagonist Semotiadil.20 When keto-acid 19a (R = H) was mixed with 2,5-dimethoxybenzylamine and cyclopentyl isocyanide, competition between path a and b, illustrated in Scheme 6 gave two products, i.e. 17 and 18 derivatives. Subsequently, the crude mixture was treated with CDI to give 20a in only 21% yield. Due to this low yield, we turned our attention to methyl 2-((2-oxopropyl)thio)acetate 19b (R = Me), prepared from methyl thioglycolate and chloroacetone, which delivered 20a in 69% yield in two steps. Accordingly, a variety of primary amines, isocyanides and 19b were evaluated to establish a preliminary reactivity domain and furnish an array of five unique thiomorpholinones 20a-e (Table 3).
Scheme 5.
Optimization of the preparation of piperidine-tetrazole 13
Scheme 6.
Ugi-azide condensation to afford 17
Table 3.
Array of thiomorpholinone-tetrazole derivatives 20
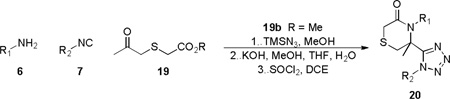 | ||||
|---|---|---|---|---|
| R1 | R2 | Product | Ugi (%) |
Final Steps* (%) |
 |
20a | 78 | 88 | |
| 20b | 61 | 66 | ||
| 20c | 42 | 22 | ||
| 20d | 86 | 73 | ||
| 20e | 61 | 96 | ||
Basic hydrolysis and SOCl2 activation
Another enticing moiety that drew our attention was the 4-sulfonyl-2-piperazinone skeleton embedded in 22. This conformationally restricted motif represents an essential structural feature of human factor Xa and gene transcription inhibitors.21,22 Indeed, sulfonamide keto-acids have previously been reported as highly compatible bifunctional reagents in intramolecular Ugi three component condensations affording 5-carbamoyl-5-methyl-4-sulfonyl-2-piperazinones in a single step.23 The sulfonamide keto-ester 21 was thus synthesized from glycine methyl ester in two steps consisting of sequential sulfonylation and alkylation using 18-crown-6 as a phase-transfer catalyst and a relatively mild base (K2CO3). A series of six 4-sulfonyl-2-piperazinones 22 were generated to confirm the utility of 21 in producing novel tetrazolo-fused analogs, thereby further expanding the generality of the lactam-tetrazole forming methodology depicted in Scheme 2 (Table 4).
Table 4.
Array of 4-sulfonyl-2-piperazinone-tetrrazole derivatives
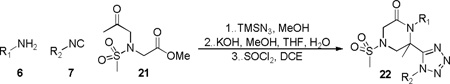 | ||||
|---|---|---|---|---|
| R1 | R2 | Product | Ugi (%) |
Final Steps* (%) |
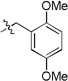 |
22a | 16 | 78 | |
| 22b | 27 | 93 | ||
| 22c | 59 | 82 | ||
| 22d | 55 | 86 | ||
| 22e | 74 | 58 | ||
| 22f | 64 | 64 | ||
Basic hydrolysis and SOCl2 activation
Additional attempts to further diversify the portfolio of scaffolds derived from this methodology were carried out by fusing heteroaromatic rings onto the bifunctional input. In particular, derivatives of 4,5,6,7-tetrahydropyrazolo[1,5-a]-pyrazine-4-one represent an uncultivated set of pharmaceutically relevant compounds where the scaffold is currently found in both fibrinogen and vitronectin receptor antagonists.24,25 Intrigued by the potential new biological applications of this rare bifunctional substituted-pyrazole methyl ester26, it was thus employed with supporting reagents 6 and 7 in the development of new synthetic route to scaffold 24. Alkylation of methyl 1H-pyrazole-3-carboxylate with chloroace- tone under phase transfer conditions in the presence of K2CO3 and 18-crown-6 provided 23 in a single step. In analogous fashion to prior methods reported herein, 23 was mixed with a variety of primary amines and isocyanides in the Ugi-Azide MCR. Subsequent basic hydrolysis and SOCl2-mediated ring closure furnished a compilation of five 4,5,6,7-tetrahydropyrazolo[1,5-a]-pyrazine-4-one derivatives 24a-e depicted in Table 5 with moderate to good isolated yields.
Table 5.
Array of 4,5,6,7-tetrahydropyrazolo[1,5-a]-pyrazine-4-one tetrazole derivatives 24
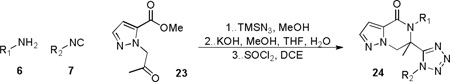 | ||||
|---|---|---|---|---|
| R1 | R2 | Product | Ugi (%) |
Final Steps* (%) |
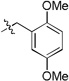 |
24a | 74 | 51 | |
| 24b | 73 | 57 | ||
| 24c | 67 | 78 | ||
| 24d | 51 | 72 | ||
| 24e | 42 | 55 | ||
Basic hydrolysis and SOCl2 activation
Syntheses of tetrazolyl-azepanones, -thiazepanones, and -benzoxazepinones
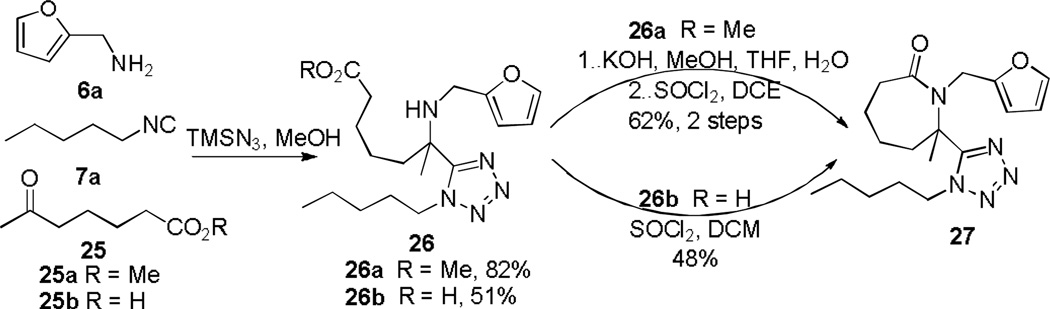
With five diverse scaffolds in hand, we subsequently expanded the generality to variations of seven-membered ring lactams. Initial efforts were devoted to preparations of 1-(furan-2-ylmethyl)-7-methyl-7-(1-pentyl-1H-tetrazol-5-yl)azepan-2-one 27 analogs (Scheme 7). In similar fashion with attempts to prepare tetrazolyl-piperidines 13, TFA failed to deliver 27 after direct addition of TFA/DCE to a methanolic solution of the on-going Ugi-azide reaction with 25a (R=Me) and supporting reagents 6a and 7a. However, unlike 13 which was accessible via CDI or EDC-mediated amide coupling, 27 was not obtained after similar coupling methods were employed with crude 26b (R=H). Azepine-tetrazole 27 was ultimately generated in 48% yield through SOCl2 triggered in situ acyl chloride formation on partially purified 26b (Scheme 7). Due to its high polarity, complete isolation of 26b did prove difficult and hence attention was turned to the Ugi amino-ester 26a, synthesized from methyl 6-oxoheptanoate 25a (82% yield). Yield improvement to 62% was observed when 27 was produced from 26a (R=H) in two steps comprising consecutive basic hydrolysis and in situ acyl chloride formation (Scheme 7). This method was exemplified by the assembly of four analogs and has been previously reported.16
Scheme 7.
Optimization of azepinone-tetrazoles 27
Thiazepanone derivatives 29 were successfully prepared in similar fashion to the thiomorpholinones 20. The key reagent in production of this derivative, namely methyl 2-((3-oxobutyl)thio)acetate 28, was prepared from methyl thioglycolate and 4-chlorobutan-2-one in one step. Upon completion of the Ugi-azide condensation, MCR intermediates were subjected to the optimized two-step protocol to ultimately afford five examples 29a-e in good overall yields for the 3 step process (Table 6).
Table 6.
Array of [1,4]thiazepanone derivatives 29
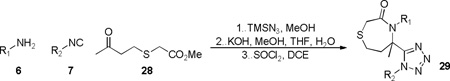 | ||||
|---|---|---|---|---|
| R1 | R2 | Product | Ugi (%) |
Final Steps* (%) |
 |
29a | 68 | 57 | |
| 29b | 70 | 66 | ||
| 29c | 70 | 54 | ||
| 29d | 61 | 45 | ||
| 29e | 75 | 51 | ||
Basic hydrolysis and SOCl2 activation
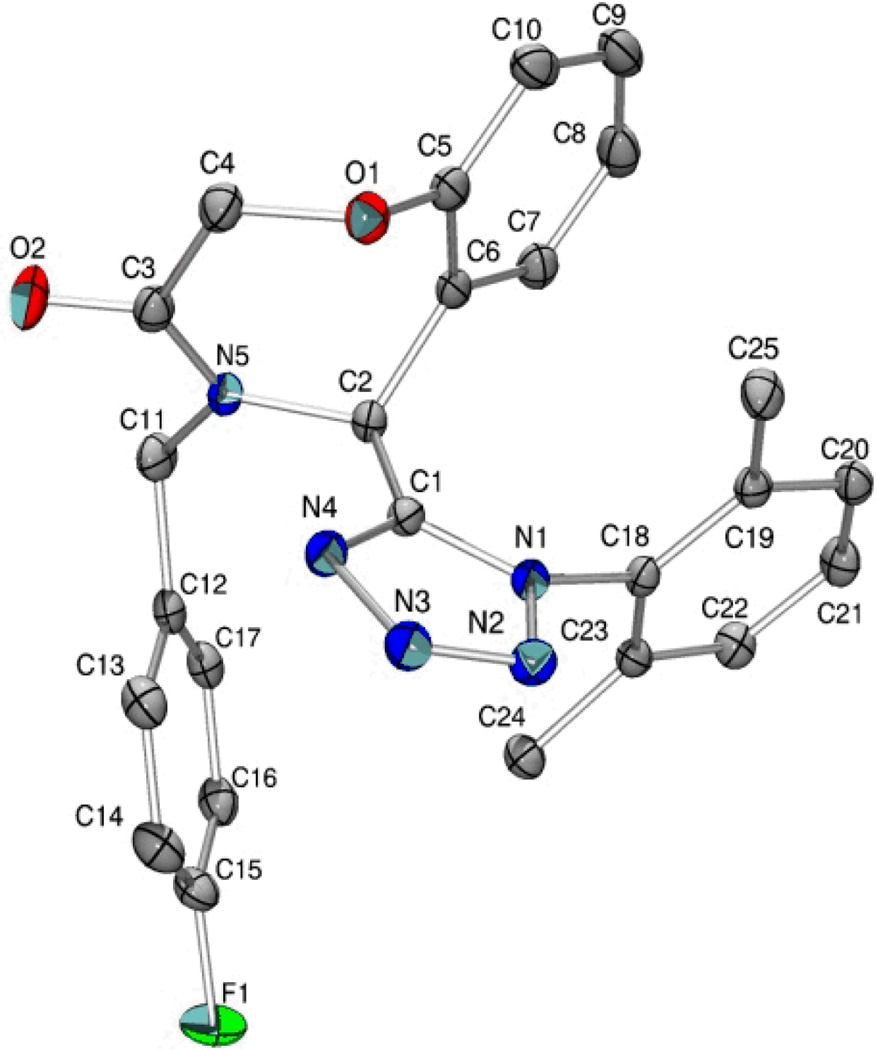
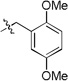
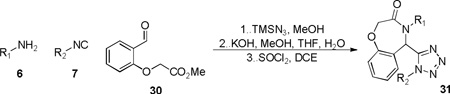
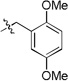
A final example highlighting the generality of this methodology enabled access to the tetrazolo-benzoxazepinones 31. Akin to the [1,4]thiazepanone 29, the benzo[1,4]oxazepinone fragment may also be viewed as a relatively under-developed scaffold in the pharmaceutical sector, although, a handful of articles do describe some utility with the chemotype observed embedded in ACE/neutral endopeptidase (NEP)27 and HIV-128 inhibitors. Methyl esterification of 2-(2-formylphenoxy)acetic acid generated methyl 2-(2-formylphenoxy)acetate 30, that was used as the tethered bifunctional component (Table 7). Six analogs 31a-31f were synthesized and the structure of 31c was confirmed by X-ray crystallography (Figure 1).29
Table 7.
Array of benzo[1,4]oxazepinone derivatives 31
 | ||||
|---|---|---|---|---|
| R1 | R2 | Product | Ugi e(%) |
Final Steps* (%) |
 |
31a | 80 | 29 | |
| 31b | 63 | 31 | ||
| 31c | 74 | 84 | ||
| 31d | 78 | 70 | ||
| 31e | 77 | 35 | ||
| 31f | 66 | 62 | ||
Basic hydrolysis and SOCl2 activation
Figure 1.
X-Ray crystal structure of 31c
Conclusions
A straightforward, robust and extremely versatile strategy coupling the Ugi-Azide MCR with amidative post-condensation modifications enabling ring-closure to a variety of pharmacologically relevant scaffolds has been established. In this context, tethered aldo-esters/keto-acids/keto-esters were key bifunctional precursors that in combination with supporting isonitrile and amine reagents typically afforded the Ugi-azide adduct in good yield. Not surprisingly during the optimization of the post-condensation ring closing methodology, it was apparent that stronger activating reagents were required as the desired lactam ring size increased, yet all of the 5 to 7-exo-trig processes remained, as expected, ultimately feasible.30 By means of this general route, nine bis-heterocyclic tetrazolo-scaffolds and related congener sets were prepared incorporating a wide array of bifunctional input linker diversity (Scheme 2, x = linker) and additional diversity elements from supporting reagents 6 and 7. Coupled with the cis-amide bond surrogacy possessed by the 1,5-disubstituted tetrazole nucleus, these new bis-heterocylic scaffolds represent potential innovative new molecular probes to interrogate peptidergic biological systems.
Experimental
General remarks
All reagents were purchased from Acros Organics, Alfa Aesar, Sigma Aldrich and TCI America. Microwave assisted reactions were conducted in a 10-ml vial on a CEM microwave initiator. The flash column chromatography was carried out on Teledyne Isco CombiFlash Rf 200. 1H and 13C NMR spectra were recorded in CDCl3 or DMSOd6 on a Varian 400 MHz spectrometer. Chemical shifts in 1H NMR spectra are reported in parts per million (ppm, d) downfield from the internal standard Me4Si (TMS). Chemical shifts in 13C NMR spectra are reported relative to the central line of the chloroform signal (d = 77.70 ppm) or the DMSO signal (d = 40.0 ppm). Low-resolution mass spectra were obtained with a Shimadzu Prominence UFLCXR/LCMS-2020/ELSD-LTII instrument. High-resolution mass spectra were obtained with 9.4 Tesla Bruker FT/ICR-MS instrument.
General procedure for aldo/keto-esters (8b, 19b, 28 and 30)
All can be found in the electronic supplementary information.
General experimental procedure for synthesis of indolinone tetrazoles (10b-10j)
Methyl 2-formylbenzoate 8b (0.250 mmol), R1NH2 6 (0.250 mmol), TMSN3 (0.250 mmol) and R2NC 7 (0.250 mmol) were dissolved in MeOH (1.0 ml) in a 10 ml vial. The reaction was allowed to run at room temperature for 24 h. The crude mixture was concentrated in vacuo and purified by flash chromatography (Hexane/EtOAc) to afford the indolinone tetrazoles.
2-(furan-2-ylmethyl)-3-(1-pentyl-1H-tetrazol-5-yl)isoindo-lin-1-one (10b)
white solid (m.p. 95–97 °C); 85% yield (one step); 1H NMR (400 MHz, CDCl3) δ ppm 8.02 – 7.97 (m, 1H), 7.65 – 7.56 (m, 2H), 7.28 – 7.22 (m, 2H), 6.26 – 6.21 (m, 2H), 6.19 – 6.15 (m, 1H), 4.78 (d, J = 15.6 Hz, 1H), 4.57 (d, J = 15.6 Hz, 1H), 3.57 (ddd, J = 14.8, 8.8, 6.3 Hz, 1H), 3.43 (ddd, J = 14.8, 8.8, 6.3 Hz, 1H), 1.38 – 1.24 (m, 1H), 1.19 – 1.08 (m, 1H), 1.08 – 0.95 (m, 2H), 0.89 – 0.78 (m, 2H), 0.71 (t, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 167.7, 150.2, 148.6, 142.9, 140.3, 133.0, 131.2, 130.1, 124.7, 123.1, 110.7, 109.6, 55.1, 55.0, 47.6, 38.1, 28.4, 28.2, 21.7, 13.6; [M+H]+ = 352.4; HRMS (ESI): m/z calcd for (C19H22N5O2): 352.1768; found: 352.1772.
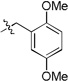
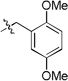
3-(1-cyclopentyl-1H-tetrazol-5-yl)-2-(2,5-dimethoxybenzyl) isoindolin-1-one (10c)
yellow solid (m.p. 118–119 °C); 51% yield (one step); 1H NMR (400 MHz, CDCl3) δ ppm 8.01 – 7.94 (m, 1H), 7.62 – 7.50 (m, 2H), 7.21 – 7.15 (m, 1H), 6.96 – 6.91 (m, 1H), 6.77 (ddd, J = 8.9, 3.0, 1.6 Hz, 2H), 6.71 (dd, J = 8.9, 1.3 Hz, 2H), 6.20 (s, 1H), 4.96 (d, J = 14.5 Hz, 1H), 4.41 (d, J = 14.4 Hz, 1H), 3.99 – 3.88 (m, 1H), 3.74 (s, 3H), 3.68 (s, 3H), 2.01 – 1.75 (m, 3H), 1.74 – 1.59 (m, 1H), 1.59 – 1.47 (m, 1H), 1.41 – 1.28 (m, 1H), 1.19 – 1.07 (m, 1H), 1.06 – 0.92 (m, 1H); 13C NMR (100 MHz, CDCl3) δ ppm 168.4, 153.5, 151.4, 150.3, 140.8, 132.8, 131.3, 129.8, 124.4, 124.3, 123.1, 116.7, 114.4, 111.3, 59.3, 55.74, 55.68, 55.4, 39.9, 33.4, 32.6, 24.7, 24.6; [M+H]+ = 420.3; HRMS (ESI): m/z calcd for (C23H26N5O3): 420.20302; found: 420.20308.
3-(1-benzyl-1H-tetrazol-5-yl)-2-(thiophen-2-ylmethyl)iso-indolin-1-one (10d)
yellow solid (m.p. 134–136 °C); 58% yield (one step); 1H NMR (400 MHz, CDCl3) δ ppm 7.95 (d, J = 7.6 Hz, 1H), 7.53 (t, J = 7.6 Hz, 1H), 7.38 (td, J = 7.6, 1.3 Hz, 1H), 7.24 – 7.18 (m, 2H), 7.16 (td, J = 7.3, 1.4 Hz, 2H), 6.97 – 6.93 (m, 1H), 6.90 (ddd, J = 5.1, 3.5, 1.7 Hz, 1H), 6.83 – 6.79 (m, 1H), 6.68 (d, J = 7.9 Hz, 2H), 6.15 (s, 1H), 4.89 (dd, J = 15.4, 4.5 Hz, 2H), 4.62 (d, J = 15.3 Hz, 1H), 4.12 (d, J = 15.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ ppm 167.5, 150.4, 139.7, 137.3, 132.9, 132.3, 131.2, 130.0, 128.8, 128.7, 127.8, 127.2, 127.1, 126.5, 124.6, 123.1, 54.0, 51.2, 39.3; [M+H]+ = 388.3; HRMS (ESI): m/z calcd for (C21H18N5OS): 388.12266; found: 388.12239.
2-(2,5-dimethoxybenzyl)-3-(1-(2,6-dimethylphenyl)-1H-tetrazol-5-yl)isoindolin-1-one (10e)
white solid (m.p. 167–169 °C); 66% yield (one step); 1H NMR (400 MHz, CDCl3) δ ppm 7.61 – 7.57 (m, 1H), 7.46 – 7.41 (m, 1H), 7.41 – 7.36 (m, 1H), 7.30 – 7.26 (m, 2H), 7.19 – 7.12 (m, 2H), 6.80 – 6.73 (m, 4H), 6.09 (s, 1H), 4.95 (d, J = 15.0 Hz, 1H), 4.10 (d, J = 15.0 Hz, 1H), 3.78 (s, 3H), 3.69 (s, 3H), 2.04 (s, 3H), 1.22 (s, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 167.6, 153.6, 152.4, 151.5, 139.7, 135.3, 135.0, 131.7, 131.5, 131.2, 130.7, 129.5, 128.7, 128.3, 124.8, 123.9, 123.2, 116.1, 113.8, 111.4, 55.8, 55.7, 54.4, 39.8, 17.6, 17.0; [M+H]+ = 456.3; HRMS (ESI): m/z calcd for (C26H26N5O3): 456.20302; found: 456.20242.
2-(2-(1H-indol-3-yl)ethyl)-3-(1-(tert-butyl)-1H-tetrazol-5-yl)isoindolin-1-one (10f)
white solid (m.p. 108–110 °C); 43% yield (one step); 1H NMR (400 MHz, DMSOd6) δ ppm 10.83 (s, 1H), 7.88 – 7.80 (m, 1H), 7.63 – 7.56 (m, 2H), 7.37 – 7.27 (m, 3H), 7.11 (s, 1H), 7.04 (t, J = 7.5 Hz, 1H), 6.92 (t, J = 7.2 Hz, 1H), 6.68 (s, 1H), 3.94 – 3.83 (m, 1H), 3.15 – 2.93 (m, 2H), 2.82 – 2.71 (m, 1H), 1.81 (s, 9H); 13C NMR (100 MHz, DMSOd6) δ ppm 167.71, 153.00, 143.32, 136.63, 132.83, 131.69, 129.76, 127.31, 123.80, 123.29, 123.25, 121.51, 118.80, 118.22, 111.94, 111.26, 62.67, 55.24, 42.53, 40.60, 40.39, 40.18, 39.97, 39.76, 39.56, 39.35, 30.27, 24.28; [M+H]+ = 401.4; HRMS (ESI): m/z calcd for (C23H25N6O): 401.20844; found: 401.20824.
3-(1-cyclohexyl-1H-tetrazol-5-yl)-2-(4-hydroxyphenethyl) isoindolin-1-one (10g)
white solid (m.p. 162–164 °C); 51% yield (one step); 1H NMR (400 MHz, CDCl3) δ ppm 8.04 – 7.94 (m, 1H), 7.66 – 7.55 (m, 2H), 7.26 – 7.22 (m, 1H), 7.06 – 7.00 (m, 2H), 6.84 – 6.75 (m, 2H), 6.52 – 6.40 (m, 1H), 6.09 (s, 1H), 4.22 – 4.11 (m, 1H), 3.13 (tt, J = 11.6, 3.7 Hz, 1H), 3.05 – 2.90 (m, 2H), 2.80 – 2.69 (m, 1H), 2.00 – 1.49 (m, 8H), 1.15 – 1.03 (m, 2H), 0.92 (dd, J = 16.4, 11.1 Hz, 1H), 0.68 – 0.54 (m, 1H); 13C NMR (100 MHz, CDCl3) δ ppm 168.0, 155.1, 149.5, 140.2, 132.9, 131.6, 130.2, 129.7, 128.9, 124.4, 123.0, 115.7, 58.9, 54.9, 42.7, 33.3, 32.7, 32.6, 25.2, 25.1, 24.4; [M+H]+ = 404.4; HRMS (ESI): m/z calcd for (C23H26N5O2): 404.20810; found: 404.20828.
3-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-1H-tetrazol-5-yl)-2-isobutylisoindolin-1-one (10h)
yellow solid (m.p. 66–67 °C); 58% yield (one step); 1H NMR (400 MHz, CDCl3) δ ppm 7.70 – 7.64 (m, 1H), 7.58 – 7.45 (m, 2H), 7.29 (d, J = 7.4 Hz, 1H), 6.68 – 6.64 (m, 1H), 6.23 (s, 1H), 6.17 – 6.14 (m, 1H), 4.32 – 4.11 (m, 5H), 3.66 (dd, J = 13.9, 9.5 Hz, 1H), 2.48 (dd, J = 13.9, 5.7 Hz, 1H), 2.09 – 1.96 (m, 1H), 0.94 (d, J = 6.6 Hz, 3H), 0.86 (d, J = 6.7 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 167.6, 151.7, 145.5, 143.5, 140.2, 132.2, 132.0, 129.6, 125.1, 124.0, 122.8, 118.3, 117.6, 114.6, 64.3, 64.1, 54.6, 48.0, 27.5, 20.3, 19.7; [M+H]+ = 392.3; HRMS (ESI): m/z calcd for (C21H22N5O3): 392.17172; found: 392.17184.
2-(1-benzylpiperidin-4-yl)-3-(1-(4-methoxyphenyl)-1H-tetrazol-5-yl)isoindolin-1-one (10i)
light tangerine solid (m.p. 83–84 °C); 29% yield (one step); 1H NMR (400 MHz, CDCl3) δ ppm 7.62 (d, J = 7.4 Hz, 1H), 7.52 (td, J = 7.5, 1.3 Hz, 1H), 7.45 (t, J = 7.4 Hz, 1H), 7.35 – 7.22 (m, 7H), 6.70 – 6.64 (m, 2H), 6.63 – 6.57 (m, 2H), 6.29 (s, 1H), 4.15 – 4.03 (m, 1H), 3.77 (s, 3H), 3.47 (s, 2H), 2.97 – 2.89 (m, 1H), 2.88 – 2.80 (m, 1H), 2.08 – 1.99 (m, 3H), 1.69 (d, J = 9.4 Hz, 1H), 1.72 – 1.64 (m, 1H), 1.48 – 1.39 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 168.2, 160.9, 153.2, 141.1, 138.3, 132.4, 132.1, 129.7, 128.9, 128.2, 127.0, 126.7, 125.3, 124.0, 122.7, 114.3, 62.6, 55.6, 52.8, 52.7, 51.6, 30.1, 29.9; [M+H]+ = 481.3; HRMS (ESI): m/z calcd for (C28H29N6O2): 481.23465; found: 481.23441.
2-cyclopropyl-3-(1-(naphthalen-2-yl)-1H-tetrazol-5-yl)iso-indolin-1-one (10j)
light tangerine solid (m.p. 88–89 °C); 36% yield (one step); 1H NMR (400 MHz, CDCl3) δ ppm 7.85 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 8.7 Hz, 1H), 7.59 – 7.51 (m, 4H), 7.46 (d, J = 7.5 Hz, 1H), 7.40 (d, J = 7.7 Hz, 1H), 7.33 (d, J = 7.6, H), 7.10 (d, J = 2.0 Hz, 1H), 6.96 (dd, J = 8.7, 2.2 Hz, 1H), 6.17 (s, 1H), 2.47 – 2.39 (m, 1H), 0.95 – 0.88 (m, 1H), 0.81 – 0.72 (m, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 168.4, 152.3, 140.4, 133.5, 132.5, 132.2, 129.8, 129.8, 128.3, 128.2, 128.0, 127.7, 124.7, 124.0, 122.8, 121.7, 55.8, 24.2, 6.6, 5.1; [M+H]+ = 368.3; HRMS (ESI): m/z calcd for (C22H18N5O): 368.1506; found: 368.1513.
General experimental procedure for synthesis of tetrazolyl-thiomorpholinones (20a–e), tetrazolyl ketopiperazines (22a–f and 24a–e), tetrazolyl-thiazepanones (29a–e), and tetrazolyl-benzoxazepinones (31a–f)
Bifunctional reagents (20, 22, 24, 29 or 31, 0.250 mmol), R1NH2 (0.250 mmol), TMSN3 (0.250 mmol) and R2NC (0.250 mmol) were dissolved in MeOH (1.0 ml) in a 10 ml vial. The reaction was allowed to run at room temperature for 24 h. The crude mixture was concentrated in vacuo and purified by flash chromatography (Hexane/EtOAc) to afford the Ugi-tetrazoles. Subsequently, MeOH (1.5 ml), THF (0.75 ml), and H2O (0.5 ml) were added, followed by 0.03 ml of a 1g/1ml solution of KOH in H2O and the reaction mixture was irradiated in a microwave initiator at 100 °C for 5 min. Upon acidification with 1 M HCl solution to pH 2, the hydrolyzed product was then extracted with EtOAc (3 × 2 ml) and the organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was dissolved in DCE (2 ml) followed by addition of SOCl2 (1.5 eq) and the reaction was refluxed at 85 °C. When completed, TEA (0.5 ml) was added and the mixture was stirred for 2 h before being purified by flash chromatography (hexane/EtOAc) on silica gel to afford final products.
5-(1-cyclopentyl-1H-tetrazol-5-yl)-4-(2,5-dimethoxybenzyl)-5-methylthiomorpholin-3-one (20a)
viscous liquid; 69% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 6.76 – 6.66 (m, 3H), 4.98 (d, J = 16.5 Hz, 1H), 4.88 – 4.77 (m, 1H), 3.80 – 3.70 (m, 4H), 3.67 (s, 3H), 3.63 (d, J = 17.2 Hz, 1H), 3.53 (d, J = 17.2 Hz, 1H), 3.20 (d, J = 14.4 Hz, 1H), 3.08 (d, J = 14.3 Hz, 1H), 2.31 – 2.20 (m, 1H), 2.20 – 2.02 (m, 5H), 1.98 (s, 3H), 1.83 – 1.66 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 166.5, 155.8, 153.5, 150.2, 125.9, 114.4, 111.5, 110.6, 63.1, 60.6, 55.6, 55.5, 43.6, 40.0, 35.2, 34.4, 32.7, 27.1, 25.2, 25.1; [M+H]+ = 418.2; HRMS (ESI): m/z calcd for (C20H28N5O3S): 418.19074; found: 418.19068.
5-(1-cyclohexyl-1H-tetrazol-5-yl)-4-(4-hydroxyphenethyl)-5-methylthiomorpholin-3-one (20b)
viscous liquid; 40% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.00 (dd, J = 8.3, 1.6 Hz, 2H), 6.78 (dd, J = 8.3, 1.6 Hz, 2H), 4.51 (tt, J = 11.7, 3.8 Hz, 1H), 4.19 – 4.06 (m, 1H), 3.81 (td, J = 11.9, 4.3 Hz, 1H), 3.55 (d, J = 17.3 Hz, 1H), 3.45 (d, J = 17.3 Hz, 1H), 3.18 – 2.98 (m, 2H), 2.65 (td, J = 12.0, 5.2 Hz, 1H), 2.42 (td, J = 11.8, 5.0 Hz, 1H), 2.27 (d, J = 11.4 Hz, 1H), 2.19 (s, 3H), 2.14 – 1.67 (m, 4H), 1.57 – 1.42 (m, 1H), 1.40 – 1.21 (m, 4H); 13C NMR (100 MHz, CDCl3) δ ppm 165.9, 155.2, 155.0, 140.5, 129.8, 115.6, 62.4, 60.2, 58.9, 49.0, 39.7, 34.0, 33.8, 33.0, 27.3, 25.7, 25.4, 24.8, 24.6; [M+H]+ = 402.2; HRMS (ESI): m/z calcd for (C20H28N5O2S): 402.1958; found: 402.1957.
4-(3-(1H-imidazol-1-yl)propyl)-5-(1-(2,6-dimethylphenyl)-1H-tetrazol-5-yl)-5-methylthiomorpholin-3-one (20c)
viscous liquid; 9% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.46 – 7.38 (m, 2H), 7.24 (d, J = 7.6 Hz, 2H), 7.03 (s, 1H), 6.87 (s, 1H), 4.08 – 3.96 (m, 1H), 3.96 – 3.83 (m, 1H), 3.55 – 3.44 (m, 1H), 3.37 (d, J = 17.0 Hz, 1H), 3.12 (d, J = 15.7 Hz, 1H), 3.05 (d, J = 15.7 Hz, 1H), 2.85 (d, J = 14.1 Hz, 1H), 2.73 – 2.58 (m, 1H), 2.39 – 2.23 (m, 1H), 1.87 (s, 3H), 1.86 (s, 3H), 1.56 (s, 3H), 1.26 (s, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 165.7, 157.4, 137.0, 136.1, 135.8, 132.8, 131.3, 129.7, 129.24, 129.21, 118.6, 62.5, 45.0, 44.0, 38.4, 32.0, 30.4, 24.9, 17.8, 17.5; [M+H]+ = 412.1; HRMS (ESI): m/z calcd for (C20H26N7OS): 412.1914; found: 412.1916.
4-(furan-2-ylmethyl)-5-methyl-5-(1-pentyl-1H-tetrazol-5-yl)thiomorpholin-3-one (20d)
white solid (m.p. 134–135 °C); 63% yield (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.25 – 7.21 (m, 1H), 6.32 – 6.22 (m, 1H), 6.16 – 6.11 (m, 1H), 4.39 (d, J = 15.4 Hz, 1H), 4.28 (d, J = 15.4 Hz, 1H), 4.18 – 4.04 (m, 1H), 3.86 – 3.73 (m, 1H), 3.50 (q, J = 17.2 Hz, 2H), 3.22 (d, J = 14.3 Hz, 1H), 2.95 (d, J = 14.3 Hz, 1H), 2.20 (s, 3H), 2.06 – 1.91 (m, 2H), 1.44 – 1.28 (m, 4H), 0.93 (t, J = 6.7 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 165.5, 155.4, 149.7, 141.7, 110.8, 109.7, 61.8, 48.8, 41.3, 39.0, 32.5, 29.2, 28.9, 2637, 22.1, 13.8; [M+H]+ = 350.2; HRMS (ESI): m/z calcd for (C16H24N5O2S): 350.16452; found: 350.1645.
4-cyclopropyl-5-methyl-5-(1-(naphthalen-2-yl)-1H-tetrazol-5-yl)thiomorpholin-3-one (20e)
light tangerine solid (m.p. 164–165 °C); 59% (three steps);1H NMR (400 MHz, CDCl3) δ ppm 8.06 (d, J = 8.7 Hz, 1H), 7.99 (d, J = 7.5 Hz, 1H), 7.93 (d, J = 9.6 Hz, 2H), 7.76 – 7.61 (m, 2H), 7.44 (dd, J = 8.7, 1.6 Hz, 1H), 3.22 (d, J = 16.2 Hz, 1H), 2.94 (dd, J = 15.0, 5.9 Hz, 2H), 2.84 (d, J = 14.0 Hz, 1H), 2.44 – 2.32 (m, 1H), 2.07 (s, 3H), 1.03 – 0.87 (m, 2H), 0.80 – 0.68 (m, 1H), 0.67 – 0.54 (m, 1H); 13C NMR (100 MHz, CDCl3) δ ppm 167.5, 158.0, 133.8, 132.5, 131.6, 130.2, 128.6, 128.5, 128.2, 128.1, 126.4, 123.3, 63.0, 38.4, 31.4, 28.5, 26.2, 9.0, 7.7; [M+H]+ = 366.1; HRMS (ESI): m/z calcd for (C19H20N5OS): 366.1383; found: 366.1383.
6-(1-cyclopentyl-1H-tetrazol-5-yl)-1-(2,5-dimethoxybenzyl)-6-methyl-4-(methylsulfonyl)piperazin-2-one (22a)
viscous liquid; 12% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 6.83 (s, 1H), 6.80 – 6.66 (m, 2H), 4.98 (d, J = 16.3 Hz, 1H), 4.77 – 4.67 (m, 1H), 4.26 (d, J = 16.8 Hz, 1H), 4.10 – 3.95 (m, 2H), 3.89 (d, J = 12.9 Hz, 1H), 3.75 (s, 3H), 3.65 (s, 3H), 3.47 (d, J = 12.9 Hz, 1H), 2.77 (s, 3H), 2.31 – 2.17 (m, 1H), 2.14 – 1.96 (m, 5H), 1.85 (s, 3H), 1.78 – 1.65 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 165.2, 154.4, 153.6, 150.3, 126.0, 115.4, 112.1, 110.8, 60.8, 60.4, 55.7, 55.6, 54.0, 49.0, 42.0, 35.9, 35.2, 34.2, 25.2, 25.1, 24.4; [M+H]+ = 479.2; HRMS (ESI): m/z calcd for (C21H31N6O5S): 479.2071; found: 479.2066.
6-(1-benzyl-1H-tetrazol-5-yl)-6-methyl-4-(methylsulfonyl)-1-(thiophen-2-ylmethyl)piperazin-2-one (22b)
viscous liquid; 25% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.42 – 7.32 (m, 3H), 7.28 – 7.22 (m, 1H), 7.07 (d, J = 7.4 Hz, 2H), 6.88 – 6.80 (m, 1H), 6.43 – 6.36 (m, 1H), 5.30 (d, J = 16.1 Hz, 1H), 4.96 (d, J = 16.2 Hz, 1H), 4.51 (d, J = 15.3 Hz, 1H), 4.41 (d, J = 15.3 Hz, 1H), 4.18 (d, J = 16.7 Hz, 1H), 3.75 (d, J = 16.7 Hz, 1H), 3.34 (d, J = 12.7 Hz, 1H), 3.11 (d, J = 12.7 Hz, 1H), 2.52 (s, 3H), 2.04 (s, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 164.5, 154.0, 138.4, 133.5, 129.5, 129.2, 127.6, 127.5, 127.2, 127.1, 126.4, 59.5, 52.0, 51.7, 49.0, 43.1, 35.1, 24.0; [M+H]+ = 447.0; HRMS (ESI): m/z calcd for (C19H23N6O3S2): 447.1268; found: 447.1265.
1-(furan-2-ylmethyl)-6-methyl-4-(methylsulfonyl)-6-(1-pentyl-1H-tetrazol-5-yl)piperazin-2-one (22c)
viscous liquid; 48% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.25 – 7.21 (m, 1H), 6.30 – 6.25 (m, 1H), 6.19 (d, J = 3.2 Hz, 1H), 4.60 (d, J = 15.7 Hz, 1H), 4.36 (d, J = 15.7 Hz, 1H), 4.17 (d, J = 16.7 Hz, 1H), 4.13 – 4.07 (m, 1H), 4.01 (d, J = 16.7 Hz, 1H), 3.96 – 3.85 (m, 1H), 3.81 (d, J = 12.9 Hz, 1H), 3.50 (d, J = 13.0 Hz, 1H), 2.80 (s, 3H), 2.07 (s, 3H), 2.04 – 1.92 (m, 2H), 1.45 – 1.29 (m, 4H), 0.92 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 164.3, 154.3, 149.1, 142.1, 110.8, 110.0, 59.4, 53.5, 49.2, 49.0, 39.8, 36.1, 29.2, 28.8, 24.4, 22.1, 13.8; [M+H]+ = 410.9; HRMS (ESI): m/z calcd for (C17H27N6O4S): 411.1809; found: 411.1803.
6-(1-(2,6-dimethylphenyl)-1H-tetrazol-5-yl)-1-(4-fluoro-benzyl)-6-methyl-4-(methylsulfonyl)piperazin-2-one (22d)
viscous liquid; 47% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.43 (td, J = 7.7, 2.5 Hz, 1H), 7.34 – 7.20 (m, 2H), 7.12 – 7.05 (m, 2H), 6.96 (td, J = 8.7, 2.7 Hz, 2H), 4.99 (d, J = 16.1 Hz, 1H), 4.23 – 4.08 (m, 2H), 4.05 (d, J = 13.0 Hz, 1H), 3.58 (d, J = 16.2 Hz, 1H), 3.37 (d, J = 13.0 Hz, 1H), 2.81 (s, 3H), 2.04 (s, 3H), 1.96 (s, 3H), 1.31 (s, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 165.6, 160.7, 156.4, 135.6, 135.5, 133.1, 132.9, 131.4, 129.3, 129.1, 128.5, 128.4, 115.7, 115.5, 60.2, 53.9, 48.4, 47.3, 37.9, 22.8, 17.7, 17.6; [M+H]+ = 472.8; HRMS (ESI): m/z calcd for (C22H26FN6O3S): 473.1766; found: 473.1761.
6-(1-isopropyl-1H-tetrazol-5-yl)-6-methyl-4-(methylsulfo-nyl)-1-(3-(trifluoromethyl)benzyl)piperazin-2-one (22e)
viscous liquid; 43% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.53 (d, J = 7.6 Hz, 1H), 7.48 – 7.41 (m, 2H), 7.38 (d, J = 7.7 Hz, 1H), 5.40 (d, J = 16.1 Hz, 1H), 4.69 – 4.58 (m, 1H), 4.30 (d, J = 16.9 Hz, 1H), 4.12 – 3.94 (m, 2H), 3.82 (d, J = 16.1 Hz, 1H), 3.48 (d, J = 13.1 Hz, 1H), 2.77 (s, 3H), 1.78 (s, 3H), 1.68 (d, J = 6.4 Hz, 3H), 1.62 (d, J = 6.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 165.4, 153.8, 138.5, 130.5, 129.3, 124.5, 123.7, 60.6, 53.5, 53.3, 48.8, 47.9, 35.9, 24.8, 23.8, 23.0; [M+H]+ = 461.2; HRMS (ESI): m/z calcd for (C18H24F3N6O3S): 461.1577; found: 461.1582.
6-(1-cyclohexyl-1H-tetrazol-5-yl)-1-(4-methoxybenzyl)-6-methyl-4-(methylsulfonyl)piperazin-2-one (22f)
viscous liquid; 41% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.11 (d, J = 8.1 Hz, 2H), 6.81 (d, J = 8.4 Hz, 2H), 5.26 (d, J = 15.4 Hz, 1H), 4.28 (d, J = 16.7 Hz, 1H), 4.22 – 4.11 (m, 1H), 4.01 (d, J = 16.7 Hz, 1H), 3.89 (d, J = 12.9 Hz, 1H), 3.78 (s, 3H), 3.59 (d, J = 15.4 Hz, 1H), 3.41 (d, J = 12.9 Hz, 1H), 2.78 (s, 3H), 2.17 – 1.88 (m, 4H), 1.81 (s, 3H), 1.48 – 1.20 (m, 4H), 0.95 – 0.78 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 164.9, 159.0, 154.0, 129.4, 128.6, 114.1, 60.6, 60.5, 55.3, 54.0, 49.3, 47.6, 35.4, 34.0, 33.5, 29.7, 25.7, 25.5, 24.6; [M+H]+ = 463.2; HRMS (ESI): m/z calcd for (C21H31N6O4S): 463.2122; found: 463.2118.
6-(1-cyclopentyl-1H-tetrazol-5-yl)-5-(2,5-dimethoxybenzyl)-6-methyl-6,7-dihydropyrazolo[1,5-a]pyrazin-4(5H)-one (24a)
light brown solid (m.p. 171–172 °C); 38% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.61 – 7.58 (m, 1H), 7.03 (d, J = 2.6 Hz, 1H), 7.01 – 6.98 (m, 1H), 6.75 (dd, J = 9.0, 2.9 Hz, 1H), 6.69 (d, J = 8.9 Hz, 1H), 5.04 (d, J = 15.8 Hz, 1H), 4.76 (d, J = 13.3 Hz, 1H), 4.76 – 4.67 (m, 1H), 4.43 (d, J = 13.3 Hz, 1H), 4.38 (d, J = 15.8 Hz, 1H), 3.73 (s, 3H), 3.58 (s, 3H), 2.09 – 1.92 (m, 4H), 1.89 (s, 3H), 1.86 – 1.76 (m, 2H), 1.72 – 1.58 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 158.9, 153.7, 153.5, 150.6, 140.5, 133.0, 126.0, 116.0, 113.2, 111.0, 109.2, 60.8, 59.9, 55.8, 55.7, 55.6, 55.5, 40.2, 35.1, 33.5, 24.9, 24.1; [M+H]+ =438.3; HRMS (ESI): m/z calcd for (C22H28N7O3): 438.2248; found: 438.2242.
6-(1-(2,6-dimethylphenyl)-1H-tetrazol-5-yl)-5-(4-fluoro-benzyl)-6-methyl-6,7-dihydropyrazolo[1,5-a]pyrazin-4(5H)-one (24b)
white solid (m.p. 163–164 °C); 42% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.51 – 7.48 (m, 1H), 7.45 (t, J = 7.6 Hz, 1H), 7.31 (t, J = 7.2 Hz, 1H), 7.26 (d, J = 8.2 Hz, 1H), 7.19 (dd, J = 7.4, 5.5 Hz, 2H), 7.05 – 6.93 (m, 2H), 6.86 (d, J = 1.4 Hz, 1H), 5.41 (d, J = 16.4 Hz, 1H), 4.76 (d, J = 13.7 Hz, 1H), 4.29 (d, J = 13.7 Hz, 1H), 3.87 (d, J = 16.4 Hz, 1H), 1.89 (s, 5H), 1.63 (s, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 163.2, 160.7, 158.7, 156.5, 140.4, 136.1, 136.0, 134.1, 133.2, 132.3, 131.5, 129.3, 128.4, 115.7, 115.5, 109.0, 105.5, 59.8, 55.7, 45.6, 23.7, 17.7, 17.5; [M+H]+ = 432.2; HRMS (ESI): m/z calcd for (C23H23FN7O): 432.19426; found: 432.19474.
5-(furan-2-ylmethyl)-6-methyl-6-(1-pentyl-1H-tetrazol-5-yl)-6,7-dihydropyrazolo[1,5-a]pyrazin-4(5H)-one (24c)
viscous liquid; 38% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.62 – 7.57 (m, 1H), 7.28 – 7.25 (m, 1H), 7.00 – 6.97 (m, 1H), 6.34 – 6.24 (m, 1H), 6.15 – 6.10 (m, 1H), 4.76 – 4.63 (m, 2H), 4.57 – 4.46 (m, 2H), 4.04 – 3.90 (m, 1H), 3.88 – 3.73 (m, 1H), 2.08 (s, 3H), 1.92 – 1.79 (m, 1H), 1.77 – 1.63 (m, 1H), 1.40 – 1.17 (m, 4H), 0.88 (t, J = 6.9 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 157.8, 153.8, 149.4, 142.2, 140.6, 132.8, 110.9, 109.7, 109.4, 59.1, 55.3, 48.8, 39.1, 29.4, 28.6, 23.9, 22.0, 13.7; [M+H]+ = 370.1; HRMS (ESI): m/z calcd for (C19H22N5O2): 352.1768; found: 352.1772.
6-(1-isopropyl-1H-tetrazol-5-yl)-6-methyl-5-(3-(trifluoro-methyl)benzyl)-6,7-dihydropyrazolo[1,5-a]pyrazin-4(5H)-one (24d)
white solid (m.p. 187–188 °C); 37% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.57 (t, J = 1.9 Hz, 1H), 7.56 – 7.49 (m, 2H), 7.44 (t, J = 7.6 Hz, 1H), 7.39 (d, J = 7.7 Hz, 1H), 6.98 (t, J = 2.0 Hz, 1H), 5.38 (d, J = 16.1 Hz, 1H), 4.83 (d, J = 13.6 Hz, 1H), 4.61 – 4.52 (m, 2H), 4.25 (d, J = 16.1 Hz, 1H), 1.90 (s, 3H), 1.52 (d, J = 6.5 Hz, 3H), 1.42 (d, J = 6.5 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ ppm 159.0, 153.4, 140.7, 140.6, 138.9, 132.8, 130.7, 129.4, 124.6, 124.1, 124.1, 109.5, 60.1, 55.6, 53.1, 46.7, 24.4, 23.6, 22.8; [M+H]+ = 420.2; HRMS (ESI): m/z calcd for (C19H21F3N7O): 420.1754; found: 420.1751.
6-(1-(2,6-dimethylphenyl)-1H-tetrazol-5-yl)-5-(4-methoxy-benzyl)-6-methyl-6,7-dihydropyrazolo[1,5-a]pyrazin-4(5H)-one (24e)
viscous liquid; 23% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.49 (d, J = 2.0 Hz, 1H), 7.44 (t, J = 7.6 Hz, 1H), 7.30 (d, J = 7.6 Hz, 1H), 7.25 (d, J = 8.2 Hz, 1H), 7.12 (d, J = 8.6 Hz, 2H), 6.85 (d, J = 2.0 Hz, 1H), 6.81 (d, J = 8.7 Hz, 2H), 5.35 (d, J = 16.2 Hz, 1H), 4.80 (d, J = 13.6 Hz, 1H), 4.28 (d, J = 13.6 Hz, 1H), 3.76 (s, 3H), 3.75 (d, J = 16.2 Hz, 1H), 1.89 (d, J = 12.0 Hz, 6H), 1.64 (s, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 158.8, 158.7, 156.7, 140.3, 136.2, 135.9, 133.3, 132.3, 131.5, 130.4, 129.3, 128.1, 114.1, 108.9, 59.7, 55.9, 55.3, 45.6, 23.8, 17.7, 17.5; [M+H]+ = 443.8; HRMS (ESI): m/z calcd for (C24H26N7O2): 444.21425; found: 444.215.
5-(1-cyclopentyl-1H-tetrazol-5-yl)-4-(2,5-dimethoxybenzyl)-5-methyl-1,4-thiazepan-3-one (29a)
viscous liquid; 39% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.23 (d, J = 2.6 Hz, 1H), 6.86 – 6.76 (m, 2H), 5.39 (d, J = 16.4 Hz, 1H), 4.84 – 4.72 (m, 1H), 4.54 (d, J = 16.3 Hz, 1H), 4.06 – 3.93 (m, 1H), 3.81 (s, 3H), 3.80 (s, 3H), 3.15 (d, J = 15.0 Hz, 1H), 2.82 – 2.61 (m, 3H), 2.32 – 1.89 (m, 7H), 1.83 – 1.66 (m, 2H), 1.70 (s, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 174.3, 157.7, 153.8, 145.0, 126.5, 114.0, 111.8, 60.1, 58.9, 56.0, 55.9, 41.4, 39.6, 34.6, 34.3, 30.1, 27.3, 25.1, 25.1; [M+H]+ = 432.2; HRMS (ESI): m/z calcd for (C21H30N5O3S): 432.20639; found: 432.2064.
5-(1-benzyl-1H-tetrazol-5-yl)-5-methyl-4-(thiophen-2-ylmethyl)-1,4-thiazepan-3-one (29b)
white solid (m.p. 192–193 °C); 46% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.43 – 7.31 (m, 3H), 7.26 – 7.22 (m, 1H), 7.13 – 7.01 (m, 2H), 6.93 – 6.87 (m, 1H), 6.80 – 6.74 (m, 1H), 5.42 (d, J = 15.8 Hz, 1H), 5.32 – 5.12 (m, 2H), 4.39 (d, J = 15.6 Hz, 1H), 3.59 – 3.44 (m, 1H), 3.12 (d, J = 15.3 Hz, 1H), 2.76 (d, J = 15.3 Hz, 1H), 2.67 – 2.52 (m, 2H), 2.00 – 1.87 (m, 1H), 1.81 (s, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 172.9, 157.9, 139.5, 133.2, 129.2, 129.1, 127.1, 127.0, 126.4, 126.3, 59.3, 51.8, 44.0, 39.0, 33.9, 28.2, 26.1; [M+H]+ = 400.2; HRMS (ESI): m/z calcd for (C19H22N5OS2): 400.12603; found: 400.12546.
4-(furan-2-ylmethyl)-5-methyl-5-(1-pentyl-1H-tetrazol-5-yl)-1,4-thiazepan-3-one (29c)
viscous liquid; 38% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.39 – 7.34 (m, 1H), 6.50 – 6.43 (m, 1H), 6.40 – 6.37 (m, 1H), 5.08 (d, J = 15.8 Hz, 1H), 4.55 (d, J = 15.8 Hz, 1H), 4.02 (t, J = 7.7 Hz, 2H), 3.97 – 3.83 (m, 1H), 3.04 (d, J = 14.8 Hz, 1H), 2.87 (dt, J = 14.8, 3.9 Hz, 1H), 2.64 (dt, J = 14.6, 4.4 Hz, 1H), 2.56 (d, J = 14.8 Hz, 1H), 2.14 (ddd, J = 15.1, 11.2, 4.0 Hz, 1H), 1.93 (s, 3H), 1.98 – 1.77 (m, 2H), 1.41 – 1.18 (m, 4H), 0.90 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 173.2, 157.7, 150.5, 141.6, 112.0, 110.0, 58.4, 48.6, 40.8, 39.5, 34.4, 30.5, 29.1, 28.7, 27.0, 22.1, 13.8; [M+H]+ = 364.2; HRMS (ESI): m/z calcd for (C17H26N5O2S): 364.18017; found: 364.18077.
5-(1-(2,6-dimethylphenyl)-1H-tetrazol-5-yl)-4-(4-methoxy-benzyl)-5-methyl-1,4-thiazepan-3-one (29d)
white solid (m.p. 182–184 °C); 27% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.45 (t, J = 7.6 Hz, 1H), 7.33 – 7.27 (m, 2H), 7.05 (d, J = 8.3 Hz, 2H), 6.80 (d, J = 8.6 Hz, 2H), 5.09 (d, J = 16.3 Hz, 1H), 3.89 – 3.79 (m, 1H), 3.76 (s, 3H), 3.16 – 2.97 (m, 3H), 2.88 (dd, J = 15.0, 6.9 Hz, 1H), 2.76 (dd, J = 12.5, 5.7 Hz, 1H), 2.20 – 2.08 (m, 1H), 1.94 (s, 3H), 1.93 (s, 3H), 1.61 (s, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 173.2, 159.3, 158.5, 136.6, 135.9, 132.5, 131.2, 130.6, 129.3, 129.1, 127.7, 114.0, 58.7, 55.2, 47.1, 41.3, 36.6, 33.8, 28.6, 18.0, 17.9; [M+H]+ = 438.0; HRMS (ESI): m/z calcd for (C23H28N5O2S): 438.19582; found: 438.19671.
5-(1-isopropyl-1H-tetrazol-5-yl)-5-methyl-4-(3-(trifluoro-methyl)benzyl)-1,4-thiazepan-3-one (29e)
viscous liquid; 38% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.74 (s, 1H), 7.67 (d, J = 7.6 Hz, 1H), 7.58 (d, J = 7.8 Hz, 1H), 7.52 (t, J = 7.7 Hz, 1H), 5.58 (d, J = 16.0 Hz, 1H), 4.63 – 4.49 (m, 1H), 4.39 (d, J = 15.9 Hz, 1H), 4.00 (ddd, J = 14.5, 11.2, 2.9 Hz, 1H), 3.17 (d, J = 15.0 Hz, 1H), 2.86 – 2.68 (m, 2H), 2.69 (d, J = 15.0 Hz, 1H), 2.16 – 2.05 (m, 1H), 1.76 (s, 3H), 1.62 – 1.48 (m, 6H); 13C NMR (100 MHz, CDCl3) δ ppm 174.2, 156.8, 138.9, 131.1, 131.0, 129.4, 125.3, 124.5, 122.6, 59.1, 52.2, 47.6, 39.5, 34.5, 30.1, 27.2, 23.2, 23.1; [M+H]+ = 302.1; HRMS (ESI): m/z calcd for (C18H23N5OS): 414.15699; found: 414.15684.
5-(1-cyclopentyl-1H-tetrazol-5-yl)-4-(2,5-dimethoxybenzyl)-4,5-dihydrobenzo[f][1,4]oxazepin-3(2H)-one (31a)
viscous liquid; 23% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.40 (tt, J = 8.0, 1.9 Hz, 1H), 7.21 – 7.05 (m, 3H), 6.87 – 6.77 (m, 3H), 5.62 (s, 1H), 5.39 (d, J = 15.1 Hz, 1H), 4.98 (d, J = 16.7 Hz, 1H), 4.43 (d, J = 16.7 Hz, 2H), 4.15 – 4.03 (m, 1H), 3.74 (s, 3H), 3.67 (s, 3H), 2.15 – 1.85 (m, 3H), 1.85 – 1.72 (m, 1H), 1.66 – 1.63 (m, 1H), 1.50 – 1.32 (m, 1H), 1.30 – 1.22 (m, 1H), 1.18 – 1.05 (m, 1H);13C NMR (100 MHz, CDCl3) δ ppm 169.8, 157.6, 153.8, 153.5, 151.8, 132.0, 130.6, 128.7, 125.1, 122.1, 115.9, 114.3, 111.8, 110.0, 73.4, 59.6, 56.1, 55.7, 55.6, 47.1, 32.9, 32.8, 24.5, 24.3; [M+H]+ = 450.3; HRMS (ESI): m/z calcd for (C24H28N5O4): 450.2136; found: 450.213.
4-cyclopropyl-5-(1-(naphthalen-2-yl)-1H-tetrazol-5-yl)-4,5-dihydrobenzo[f][1,4]oxazepin-3(2H)-one (31b)
viscous liquid; 20% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.88 (d, J = 7.7 Hz, 1H), 7.78 (d, J = 8.7 Hz, 1H), 7.72 (d, J = 7.6 Hz, 1H), 7.66 – 7.56 (m, 1H), 7.40 (d, J = 2.0 Hz, 1H), 7.10 (td, J = 7.6, 2.0 Hz, 1H), 7.00 (dd, J = 8.6, 2.1 Hz, 2H), 6.97 (d, J = 8.0 Hz, 2H), 6.60 – 6.45 (m, 2H), 5.63 (s, 1H), 4.92 (d, J = 16.5 Hz, 1H), 4.33 (d, J = 16.5 Hz, 1H), 3.08 – 2.97 (m, 1H), 1.05 – 0.76 (m, 5H); 13C NMR (100 MHz, CDCl3) δ ppm 171.25, 156.90, 156.02, 133.45, 132.38, 131.43, 130.51, 130.04, 129.75, 128.83, 128.18, 128.13, 127.89, 127.67, 125.42, 124.35, 122.42, 121.38, 77.31, 76.99, 76.68, 73.58, 58.71, 33.47, 8.77, 7.83; [M+H]+ = 398.2; HRMS (ESI): m/z calcd for (C23H20N5O2): 398.16115; found: 398.16105.
5-(1-(2,6-dimethylphenyl)-1H-tetrazol-5-yl)-4-(4-fluoro-benzyl)-4,5-dihydrobenzo[f][1,4]oxazepin-3(2H)-one (31c)
white solid (m.p. 178–179 °C); 62% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.26 – 7.14 (m, 4H), 7.08 (d, J = 7.6 Hz, 1H), 7.04 – 6.96 (m, 3H), 6.76 (d, J = 7.6 Hz, 1H), 6.70 – 6.62 (m, 1H), 5.96 (d, J = 7.4 Hz, 1H), 5.62 (d, J = 15.3 Hz, 1H), 5.11 (dd, J = 16.8, 2.2 Hz, 1H), 5.03 (d, J = 2.2 Hz, 1H), 4.50 (dd, J = 16.8, 2.5 Hz, 1H), 4.25 (d, J = 15.3 Hz, 1H), 1.77 (s, 3H), 1.22 (s, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 170.1, 163.7, 161.3, 157.1, 154.7, 136.9, 133.7, 131.7, 131.4, 131.3, 131.1, 130.7, 130.1, 130.0, 129.3, 128.8, 128.3, 124.8, 121.4, 115.9, 115.7, 73.3, 55.1, 50.9, 16.8, 16.4; [M+H]+ = 444.1; HRMS (ESI): m/z calcd for (C25H23FN5O2): 444.18303; found: 444.18303.
5-(1-isopropyl-1H-tetrazol-5-yl)-4-(3-(trifluoromethyl)benz-yl)-4,5-dihydrobenzo[f][1,4]oxazepin-3(2H)-one (31d)
viscous liquid; 55% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.63 – 7.56 (m, 1H), 7.52 – 7.42 (m, 4H), 7.24 – 7.17 (m, 1H), 7.15 (d, J = 7.7 Hz, 1H), 7.07 (d, J = 7.5 Hz, 1H), 5.83 (d, J = 16.0 Hz, 1H), 5.32 (s, 1H), 4.99 (d, J = 16.9 Hz, 1H), 4.52 (d, J = 16.9 Hz, 1H), 4.32 (d, J = 15.7 Hz, 1H), 3.97 – 3.82 (m, 1H), 1.43 (d, J = 5.6 Hz, 3H), 0.86 (d, J = 5.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 170.1, 157.6, 152.5, 137.1, 132.5, 131.6, 130.2, 129.4, 128.5, 125.6, 124.8, 124.5, 122.3, 73.5, 55.5, 51.5, 51.4, 22.3, 21.7; [M+H]+ = 432.2; HRMS (ESI): m/z calcd for (C21H21N5O2): 432.16419; found: 432.16378.
5-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-1H-tetrazol-5-yl)-4-isopentyl-4,5-dihydrobenzo[f][1,4]oxazepin-3(2H)-one (31e)
white solid (m.p. 209–210 °C); 27% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.31 – 7.22 (m, 1H), 7.00 (d, J = 8.0 Hz, 1H), 6.89 (td, J = 7.5, 1.1 Hz, 1H), 6.82 – 6.75 (m, 2H), 6.50 – 6.35 (m, 2H), 5.37 (s, 1H), 4.86 (dd, J = 16.6, 1.0 Hz, 1H), 4.38 – 4.18 (m, 5H), 4.16 – 4.04 (m, 1H), 3.34 – 3.21 (m, 1H), 1.65 – 1.53 (m, 1H), 1.53 – 1.43 (m, 2H), 0.92 (s, 3H), 0.91 (s, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 169.2, 156.9, 155.5, 145.5, 143.7, 131.5, 130.4, 128.9, 126.2, 124.5, 121.5, 118.9, 117.7, 115.3, 73.3, 64.4, 64.2, 56.7, 48.3, 36.7, 25.9, 22.6, 22.5; [M+H]+ = 436.3; HRMS (ESI): m/z calcd for (C23H26N5O4): 436.19793; found: 436.19759.
4-(benzo[d][1,3]dioxol-5-ylmethyl)-5-(1-butyl-1H-tetrazol-5-yl)-4,5-dihydrobenzo[f][1,4]oxazepin-3(2H)-one (31f)
viscous liquid; 41% (three steps); 1H NMR (400 MHz, CDCl3) δ ppm 7.48 – 7.40 (m, 1H), 7.22 – 7.09 (m, 2H), 7.02 (d, J = 7.5 Hz, 1H), 6.77 (dd, J = 7.7, 2.0 Hz, 1H), 6.73 – 6.67 (m, 2H), 6.02 – 5.92 (m, 2H), 5.70 (d, J = 15.0 Hz, 1H), 5.37 (s, 1H), 4.97 (dd, J = 16.8, 1.9 Hz, 1H), 4.46 (dd, J = 16.8, 2.2 Hz, 1H), 4.06 (d, J = 15.1 Hz, 1H), 3.82 – 3.59 (m, 2H), 1.52 – 1.36 (m, 1H), 1.19 – 0.94 (m, 3H), 0.74 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 169.7, 157.7, 153.5, 148.2, 147.5, 132.3, 130.3, 129.5, 128.6, 125.4, 122.2, 121.9, 108.9, 108.3, 101.2, 73.5, 54.3, 51.2, 47.6, 30.9, 19.4, 13.3; [M+H]+ = 422.1; HRMS (ESI): m/z calcd for (C22H24N5O4): 422.18228; found: 422.8213.
Supplementary Material
Acknowledgment
The authors thanked the National Institutes of Health (P41GM086190) for funding, Dr. Sue Roberts for the X-Ray crystallography work, Kristen Keck for compound purification, Alex Laetsch for compound management, and Dr. Fabio De Moliner for proof-reading.
Footnotes
Electronic Supplementary Information (ESI) available: General procedure, 1H and 13C NMR spectra for aldo/keto-esters (8b, 19b, 19 and 30). 1H and 13C NMR spectra for all new synthesized compounds (10, 10b–j, 20a–e, 22a–f, 24a–e, 29a–e and 31a–f).
Notes and references
- 1.Giannis A, Kolter T. Angew. Chem. Int. Ed. 1993;32:1244–1267. [Google Scholar]
- 2.Zabrocki J, Smith GD, Dunbar JB, Jr, Iijima H, Marshall GR. J. Am. Chem. Soc. 1988;110:5875–5880. [Google Scholar]
- 3.Creighton CJ, Leo GC, Du W, Reitz AB. Bioorg. Med. Chem. 2004;12:4375–4385. doi: 10.1016/j.bmc.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Kang SY, Lee SH, Seo HJ, Jung ME, Ahn K, Kim J, Lee J. Bioorg. Med. Chem. Lett. 2008;18:2385–2389. doi: 10.1016/j.bmcl.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 5.Alexander JP, Cravatt BF. J. Am. Chem. Soc. 2006;128:9699–9704. doi: 10.1021/ja062999h. [DOI] [PubMed] [Google Scholar]
- 6.(a) Hulme C, Tempest P, Ma V, Nixey T, Balow G. PCT Int. Appl. 2005;260 pp. WO 2005019167. [Google Scholar]; (b) Tempest P, Nixey T, Ma V, Balow G, van Staden C, Salon J, Rorer K, Baumgartner J, Hale C, Bannon T, Hungate R, Hulme C. Abstract of Papers. Anaheim CA: 227th National Meeting of the American Chemical Society; 2004. Mar-Apr. MEDI 298. [Google Scholar]
- 7.Nixey T, Boylan J, Hulme C, Powers D, Smith A, Wong A. Abstract of Papers. Atlanta, GA: 231st National Meeting of the American Chemical Society; 2006. Mar 26–30, MEDI 277. [Google Scholar]
- 8.Li J, Chen SY, Li JJ, Wang H, Hernandez AS, Tao S, Musial CM, Qu F, Swartz S, Chao ST, Flynn N, Murphy BJ, Slusarchyk DA, Seethala R, Yan M, Sleph P, Grover G, Smith MA, Beehler B, Giupponi L, Dickinson KE, Zhang H, Humphreys WG, Patel BP, Schwinden M, Stouch T, Cheng PTW, Biller SA, Ewing WR, Gordon D, Robl JA, Tino JA. J. Med. Chem. 2007;50:5890–5893. doi: 10.1021/jm7010595. [DOI] [PubMed] [Google Scholar]
- 9.(a) Weber L. Curr. Opin. Chem. Biol. 2000;4:295–302. doi: 10.1016/s1367-5931(00)00092-2. [DOI] [PubMed] [Google Scholar]; (b) Hulme C, Gore V. Curr. Med. Chem. 2003;10:51–80. doi: 10.2174/0929867033368600. [DOI] [PubMed] [Google Scholar]; (c) Hulme C, Dietrich J. Mol. Divers. 2009;13:195–207. doi: 10.1007/s11030-009-9111-6. [DOI] [PubMed] [Google Scholar]
- 10.Ugi I. Angew. Chem. Int. Ed. 1962;1:8–21. [Google Scholar]
- 11.Nixey T, Kelly M, Hulme C. Tetrahedron Lett. 2000;41:8729–8733. [Google Scholar]
- 12.(a) Nixey T, Kelly M, Semin D, Hulme C. Tetrahedron Lett. 2002;43:3681–3684. [Google Scholar]; (b) Nayak M, Batra S. Tetrahedron Lett. 2010;51:510–516. [Google Scholar]
- 13.Borisov RS, Polyakov AI, Medvedeva LA, Khrustalev VN, Guranova NI, Voskressensky LG. Org. Lett. 2010;12:3894–3897. doi: 10.1021/ol101590w. [DOI] [PubMed] [Google Scholar]
- 14.Kalinski C, Umkehrer M, Gonnard S, Jager N, Ross G, Hiller W. Tetrahedron Lett. 2006;47:2041–2044. [Google Scholar]
- 15.Gunawan S, Petit J, Hulme C. ACS Comb. Sci. 2012;14:160–163. doi: 10.1021/co200209a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunawan S, Keck K, Laetsch A, Hulme C. Mol. Divers. 2012;16:601–606. doi: 10.1007/s11030-012-9373-2. [DOI] [PubMed] [Google Scholar]
- 17.Details of the nine compounds with all characterization data and reagents diversity for the four 24-well plates production can be found in ref 15
- 18.(a) While the manuscript was in preparation, it was realized a very close MCR based methodology to prepare compounds 10 had already been reported in Marcos CF, Marcaccini S, Menchi G, Pepino R, Torroba T. Tetrahedron Lett. 2008;49:149–152. However, unprecedented significant scope expansion and combinatorial applications are herein described for this series. (b) Details of the reagents diversity for the four 24-well plates production along with purity and yield can be found in the supplementary information.
- 19.Zhang J, Jacobson A, Rusche JR, Herlihy W. J. Org. Chem. 1999;64:1074–1076. doi: 10.1021/jo982192a. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe Y, Osanai K, Nishi T, Miyawaki N, Shii D, Honda T, Shibano T. Bioorg. Med. Chem. Lett. 1996;6:1923–1926. [Google Scholar]
- 21.(a) Choi-Sledeski YM, Kearner R, Poli G, Pauls H, Gardner C, Gong Y, Becker M, Davis R, Spada A, Liang G, Chu V, Brown K, Collussi D, Leadley R, Jr, Rebello S, Moxey P, Morgan S, Bentley R, Kasiewski C, Mignan S, Guilloteau J-P, Mikol V. J. Med. Chem. 2003;46:681–684. doi: 10.1021/jm020384z. [DOI] [PubMed] [Google Scholar]; (b) Nishida H, Miyazaki Y, Mukaihira T, Saitoh F, Fukui M, Harada K, Itoh M, Muraoka A, Matsusue T, Okamoto A, Hasaka Y, Matsumoto M, Ohnishi S, Mochizuki H. Chem. Pharm. Bull. 2002;50:1187–1194. doi: 10.1248/cpb.50.1187. [DOI] [PubMed] [Google Scholar]
- 22.Boger D, Goldberg J, Shigeki A, Yves C, Vogt P. Helv. Chim. Acta. 2000;83:1825–1845. [Google Scholar]
- 23.Ilyin AP, Trifilenkov AS, Kurashvili ID, Krasavin M, Ivachtchenko AV. J. Comb. Chem. 2005;7:360–363. doi: 10.1021/cc0500147. [DOI] [PubMed] [Google Scholar]
- 24.Askew BC, McIntyre CJ, Hunt CA, Claremon DA, Baldwin JJ, Anderson PS, Gould RJ, Lynch RJ, Chang CCT, Cook JJ, Lynch JJ, Holahan MA, Sitko GR, Stranieri MT. Bioorg. Med. Chem. Lett. 1997;7:1531–1536. [Google Scholar]
- 25.Wehner V, Stilz HU, Peyman A, Knolle J, Ruxer JM, Carniato D, Lefrancois JM, Gadek TR, McDowell R. Chem. Abstr. 1998;129:81970. DE Patent 19653647, 1998. [Google Scholar]
- 26.Ilyn AP, Trifilenkov AS, Tsirulnikov SA, Kurashvily ID, Ivachtchenko AV. J. Comb. Chem. 2005;7:806–808. doi: 10.1021/cc0500250. [DOI] [PubMed] [Google Scholar]
- 27.Robl JA, Simpkins LM, Asaad MM. Bioorg. Med. Chem. Lett. 2000;10:257–260. doi: 10.1016/s0960-894x(99)00671-x. [DOI] [PubMed] [Google Scholar]
- 28.(a) Klunder JM, Hargrave KD, West M, Cullen E, Pal K, Behnke ML, Kapadia SR, McNeil DW, Wu JC, Chow GC. J. Med. Chem. 1992;35:1887–1897. doi: 10.1021/jm00088a027. [DOI] [PubMed] [Google Scholar]; (b) Aiello F, Brizzi A, Garofalo A, Grande F, Ragno G, Dayam R, Neamati N. Bioorg. Med. Chem. 2004;15:4459–4466. doi: 10.1016/j.bmc.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 29.CCDC 936637 (31c) contains the supplementary crystallographic data for this paper. This data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 30.Baldwin JE. J. Chem. Soc., Chem. Commun. 1976:734–736. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.