Abstract
Here we find, using functional Magnetic Resonance Imaging (fMRI), that object manipulation knowledge is accessed by way of the ventral object processing pathway. We exploit the fact that parvocellular channels project to the ventral but not the dorsal stream, and show that increased neural responses for tool stimuli are observed in the inferior parietal lobule when those stimuli are visible only to the ventral object processing stream. In a control condition, tool-preferences were observed in a superior and posterior parietal region for stimuli titrated so as to be visible by the dorsal visual pathway. Functional connectivity analyses confirm the dissociation between sub-regions of parietal cortex according to whether their principal afferent input is via the ventral or dorsal visual pathway. These results challenge the ‘Embodied Hypothesis of Tool Recognition’, according to which tool identification critically depends on simulation of object manipulation knowledge. Instead, these data indicate that retrieval of object-associated manipulation knowledge is contingent on accessing the identity of the object, a process that is subserved by the ventral visual pathway.
1. Introduction
Visual object processing has been argued to be organized at a macroscopic level into two functionally independent visual pathways (e.g., Goodale and Milner, 1992). The ventral visual pathway projects from primary visual cortex (V1) to ventral occipital-temporal cortex, and supports form-based object identification and analysis of surface properties such as color and texture (Cant and Goodale, 2007; Goodale and Milner, 1992; Grill-Spector et al., 2001). Lesions to ventral stream structures classically result in impaired visual object recognition and perceptual decisions (e.g., judging the orientation of a line) but spared reaching and grasping (e.g., Goodale and Milner, 1992). The dorsal visual pathway projects from V1 to dorsal occipital and posterior parietal cortex. It supports volumetric and spatial analysis of objects in the service of object-directed reaching and grasping. Patients with lesions to dorsal stream structures can have difficulty with reaching and/or grasping the same visual stimuli for which they can recognize and about which they can make normal perceptual judgments (e.g., Jeannerod et al., 1994; Perenin and Vighetto, 1988).
A number of fMRI studies have shown that viewing common tools leads to differential blood oxygen level-dependent (BOLD) responses in localized regions within the temporal and parietal lobes, compared to a range of baseline categories (e.g., animals, vehicles, places; e.g., Chao et al., 1999; Chao and Martin, 2000; Mahon et al., 2007; Noppeney et al., 2006). Viewing tools elicits differential BOLD contrast in the medial fusiform gyrus, a structure unequivocally within the ventral visual pathway. Tool stimuli also elicit differential BOLD responses in the left posterior middle temporal gyrus, on the lateral surface of the temporal lobe. Whether the left posterior middle temporal gyrus that is tool responsive should be considered a part of the dorsal stream or the ventral stream, or both, is an open issue: it is just anterior to visual motion area MT/V5 which is unequivocally a part of the dorsal stream (Ungerleider and Mishkin, 1982; see also Beauchamp et al., 2002) but lesions to the middle temporal gyrus are associated with lexical semantic and conceptual level impairments for tools (e.g., Damasio et al., 2004). Finally, tool stimuli elicit differential BOLD responses in the left parietal lobule, across a large swath of cortex extending from posterior parietal cortex anteriorly along the intraparietal sulcus (IPS), and inferiorly into the supramarginal gyrus of the inferior parietal lobule (e.g., Chao et al., 1999; Chao and Martin, 2000; Mahon et al., 2007; Noppeney et al., 2006).
Recent work has shown that there is significant interaction between the ventral and dorsal streams, and that a better characterization than the two streams as independent pathways is that they are two ways of distinguishing the afferent input to dissociable computations relevant to object directed action. For instance, the ventral stream has been shown to be able to support some visuomotor behavior, and visuomotor performance in the context of ventral stream lesions may not be completely spared, even in simple tasks, particularly when these visuomotor actions are not under online guidance (e.g., Goodale et al., 1994; Karnath et al., 2009; for a review see Himmelbach et al., 2012; Schenk and McIntosh, 2009). In addition, the dorsal stream is not a monolithic entity, and should certainly not be ‘equated’ with parietal cortex (Goodale and Milner, 1992). Rizzolatti and Matelli (2003) argued that the classic dorsal stream can be further subdivided into a dorso-dorsal pathway, comprising (among other areas) area V6 and the superior parietal lobule (SPL), and dedicated to the online control of visuomotor behavior, and a ventro-dorsal pathway, corresponding (among other areas) to the inferior parietal lobule (IPL), and concerned with object-directed actions (left hemisphere), action understanding, and spatial analysis (right hemisphere). Finally, it has been shown that there is strong interconnectivity between the dorsal and ventral visual streams (e.g., Binkofski et al., 2007; Nelissen and Vanduffell, 2011; Pisella et al., 2006; Rushworth et al., 2006; Zhong and Rockland, 2003). For instance, the IPL has connections with aspects of the ventral temporal cortex (Binkofski et al., 2007; Borra et al., 2008; Nelissen and Vanduffell, 2011), and the IPL is increasingly being thought of as the locus of integration of abstract (potentially ‘semantic’) information about object use that arrives from ventral and lateral temporal cortices, and visuomotor information coming from dorsal stream regions (V6, superior parietal lobule), into a coherent object-specific action plan (e.g., Binkofski and Buxbaum, 2012; Frey, 2007; Grafton, 2010; Randerath et al., 2010). Overall, these data and arguments suggest that while the general distinction between a dorsal and a ventral stream holds, there is some overlap in their functions and there is certainly ample interactivity between the two streams.
One way to address the distinction between ventral and dorsal visual streams, the cross-talk between them, and their relation to the organization of semantic memory is by studying how information about manipulable objects such as tools and utensils is represented and organized. Functionally appropriate tool use depends on specific motor information being brought into register with specific visual information. Broadly speaking, object-directed actions can be separated into a reach-to-grasp component, and complex object-associated manipulations. Reach-to-grasp actions are visuomotor acts that are largely constrained by the physical characteristics of the objects; by the current location of the hands, intervening obstacles, and target objects: but do not draw on stored ‘semantic’ knowledge1. Thus, all of the positional and volumetric information necessary to reach toward and grasp an object (albeit not necessarily in a functionally appropriate way) is provided by the visual input. By contrast, complex object-associated manipulations describe the way that objects are manipulated in order for the object to be used in a functionally appropriate way (e.g., the hammering action when using a hammer). However, it is important to note that object function and object manipulation knowledge doubly dissociate, and are known to be subserved by functionally and neuroanatomically separate systems. This double dissociation has been shown in neuropsychological patients (Buxbaum and Saffran, 2002; Negri et al., 2007; Garcea et al., in press; Sirigu et al., 1991), functional MRI (Boronat et al., 2005; Canessa et al.,2008; Kellenbach et al., 2003), behavioral responses in normal subjects (Tucker & Ellis, 1998; Garcea and Mahon, 2012), and with transcranial magnetic stimulation (Ishibashi et al., 2011; Pelgrims et al., 2011; Pobric et al., 2010).
As noted above, viewing tool stimuli leads to fMRI activation in a large swath of left hemisphere parietal regions, from posterior parietal/dorsal occipital cortex (~V6), through IPS, including the superior parietal lobule, and the supramarginal gyrus of the inferior parietal lobule in the left hemisphere. Recent data suggests that the parietal regions that comprise this tool network may be assigned to different tool-related functions (e.g., Buxbaum et al., 2006, 2007; Vingerhoets, 2008; Vingerhoets et al., 2009). For instance, Vingerhoets et al. (2009) suggested that different parts of the inferior parietal cortex are responsible for different aspects of gesture planning and coordination necessary for tool use. This complex mosaic of functions and the associated integrative nature of tool-related parietal cortex fits well with the diverse profiles often found in limb apraxia after left parietal lobe damage (e.g., Goldenberg, 2009; Goldenberg and Hagmann, 1998; Goldenberg and Spatt, 2009; Sunderland et al., 2013). Apraxic patients may present with deficits in imitating meaningless and/or meaningful gestures, in pantomiming tool use, and actual tool use. Those impairments can be selective in some patients, and the hallmark of the impairment is that it cannot be reduced to motoric, perceptual, or general cognitive deficits. Decades of research in apraxia has led to the development of several models to explain the relevant phenomena. One model is based on the idea that what is impaired in some patients with apraxia is the ability to apprehend the spatial relations between the effectors and the objects, and between the interactive parts of objects (e.g., the relationship between the flat surfaces of hammers and nails; e.g., Goldenberg, 2008). Another proposal is that some patients with apraxia may have a deficiency of working memory/executive systems that are critical for integrating semantic and motoric information (e.g., Randerath et al., 2010).
In spite of the relatively developed nature of neurocognitive models of object-directed action, it remains an open issue whether object associated manipulation knowledge is accessed via the dorsal visual pathway. At a minimum, complex object associated actions draw on knowledge about object function in order to implement the correct object associated manipulation. It would also seem to be a reasonable hypothesis that knowledge of object function is contingent on knowledge of object identity—i.e., to know the function of an object you have to know its identity. That kind of purely ‘conceptual’ analysis would suggest that object manipulation knowledge is retrieved in a way that is contingent on accessing the identity of the object. Here we sought to test this hypothesis using fMRI in normal participants.
Is manipulation knowledge accessed via the ventral visual pathway?
It is widely accepted that activation of the left supramarginal gyrus when viewing tools indexes the retrieval of complex object-associated manipulation knowledge (e.g., Boronat et al., 2005; Mahon et al., 2007; Rumiati et al., 2004). A number of authors have argued that the mere presentation of a manipulable object automatically potentiates object use information (e.g., Creem-Regehr and Lee, 2005; Garcea and Mahon, 2012; Grèzes et al., 2003; Mahon and Caramazza, 2008; Tucker and Ellis, 1998). Furthermore, some models have emphasized that complex object associated manipulation knowledge is accessed independently of, and prior to, the computation of meaning from the visual input. This view, which can be referred to as the ‘Embodied Hypothesis of Tool Recognition’, assumes that the retrieval of motor knowledge about how to manipulate tools is a necessary and intermediary step in identifying a tool from visual input (e.g., Gallese and Lakoff, 2005; Martin et al., 2000; Noppeney et al., 2006). Specifically, tool concepts include, constitutively, manipulation knowledge, and thus, in order to retrieve a tool concept from visual input, manipulation knowledge would have to be retrieved (i.e., simulated). According to this view, manipulation knowledge would necessarily be accessed independently of the ventral visual pathway. Otherwise if the stimulus were first processed by the ventral pathway, then it would have already passed through the classic channels of object recognition and, presumably, the identity of the stimulus would have already been accessed.
As sketched above in at least one form, an alternative view to the Embodied Hypothesis of Tool Recognition is that motor information about object manipulation is accessed subsequent to processing of the visual stimulus by the ventral visual pathway, i.e., subsequent to object identification. On this alternative, motor information does not form a constitutive aspect of object recognition processes, as it is accessed contingent upon visual identification in the ventral stream. There are different forms that such a view could take. For instance, Arbib et al. (Arbib, 2008; Fagg and Arbib, 1998) proposed that the mere visual inspection of an object leads to the processing of the many possible motor interactions afforded by an object. This processing is carried out in a set of dorsal visual stream and frontal premotor regions, and is dependent on the physical properties of objects that are relevant for interacting with it. However, it is the recognition of an object, mediated by ventral temporal regions, that restricts the set of affordances to those which match the typical use of the object (i.e., the ‘target’ complex object manipulation knowlege). From a slightly different perspective, but not incompatible with that view, the grounding by interaction proposal of Mahon and Caramazza (2008) argues that motor information is not constitutive of the conceptual representation of an object. Motor information, on that view, may play an important role by grounding the ‘tokening’ of a concept in the current context and/or particular instantiation, but does not figure causally in the process of accessing object identity from visual input. Rather, and perhaps as suggested by the proposal of Arbib and colleagues, motor information is activated automatically and that automatic activation may serve other purposes that are not related with object identification per se, but to the preparation of the system to act, should action be called for. These views agree that object concepts are not distributed over motor information, and that object recognition is fundamentally a ventral stream process. They may differ in the emphasis placed on different ways in which object-directed action knowledge could be relevant to object processing, for instance, as a way to prepare for action, or as a way to contextualize a particular instantiation of an object.
Here we use fMRI and images of tools and animals that are titrated such that their visual processing is biased toward either the ventral or dorsal pathways to ajudicate between these two hypotheses of how manipulation knowledge is accessed, and ultimately, how tools are visually recognized. For images of tools and animals that ‘are visible’ only by the ventral, but not by the dorsal visual pathway, the Embodied Hypothesis of Tool Recognition predicts that there should be no tool-preferences observed in parietal cortex. This is because the Embodied Cognition Hypothesis posits that parietal activation for tools reflects the operation of the dorsal pathway; thus psychophysical manipulations that bias the processing of stimuli towards the ventral stream (and away from the dorsal stream) will prevent those stimuli from driving BOLD responses in parietal cortex. In contrast, according to an alternative theoretical view, such as the Grounding by Interaction Hypothesis (Mahon and Caramazza, 2008; see also Fagg and Arbib, 1998; Binkofski and Buxbaum, 2012), knowledge about how to manipulate an object requires information about the identity and associated function of an object, and hence is accessed via processing of the visual stimuli by the ventral visual pathway. The prediction of that alternative is that that there should be tool-preferences in parietal cortex, but restricted to the left inferior parietal lobule that represents complex object-associated manipulation knowledge, and which has strong connectivity with ventral stream regions.
To evaluate these issues, we exploit an asymetry in how parvocellular and koniocellular channels within the visual system project to the dorsal and ventral visual pathways. Midget ganglion cells are color sensitive and hence excited by chromatically-defined red/green isoluminant stimuli (e.g., Dacey 2000; Livingstone and Hubel, 1988; Kveraga et al., 2007), relay information through the parvocellular pathway and almost exclusively to ventral stream structures (e.g., Ferrera et al., 1992; Livingstone and Hubel, 1988; Merigan and Maunsell, 1993). In contrast, bistratified ganglion cells and koniocellular intralaminar cells from the Lateral Geniculate Nucleus (LGN) respond to blue-only stimuli (e.g., Casagrande, 1994; Dacey, 2000; Henry and Reid, 2000), and project directly to areas within the dorsal stream, in particular area V5/MT (Sincich et al., 2004). Furthermore, some of the phenomena of ‘blindsight’, when patients with primary visual cortex lesions can still perform visuomotor tasks, have been attributed to intact processing within the koniocellular pathway (e.g., Vakalopoulos, 2005), suggesting that koniocellular channels project to V5, and ultimately parietal structures, without passing through early visual cortex (see also Das and Huxlin, 2010).
In summary then, the logic of this investigation is that the existence of tool-preferences restricted to inferior regions of left parietal cortex for chromatically-defined red/green isoluminant stimuli (i.e., P-biased stimuli) would indicate that those inferior parietal regions receive input from the ventral stream. For comparison, and as an internal control, blue-only tool stimuli (i.e., K-biased stimuli) are expected to activate superior and posterior parietal regions that are known to receive their principal inputs from the dorsal visual pathway, presumably via a direct geniculate projection to MT/V5.
To anticipate our findings, we find that P-biased stimuli drive tool-preferences selectively in inferior parietal regions, while K-biased stimuli selectively drive tool-preferences in posterior/superior parietal regions. We then test the core assumption behind our manipulations by computing functional connectivity between the inferior and superior/posterior tool preferring regions and the ventral stream and MT/V5. As would be predicted, the inferior tool-preferring parietal region exhibits functional connectivity to the ventral stream but not to MT/V5, while the posterior/superior tool-preferring parietal region exhibits functional connectivity to MT/V5 but not to the ventral stream.
2. Materials and Methods
2.1 Participants
Twenty-one individuals participated in the study (mean age = 23.2 ± 3.3 years; 11 female participants). All participants had normal or corrected to normal vision, no history of neurological disorders, and participated in accordance with the guidelines of the University of Rochester’s Research Subjects Review Board. Data from two participants were not analyzed as it was discovered that they were not right handed; the rest of the participants were right handed.
2.2 Experimental Stimuli
Twenty grey-scale pictures and twenty line drawings of animals and tools (10 per category) were used. There were no statistically significant differences between the images of tools and animals in mean luminance; neither were there differences in lexical frequency, familiarity and imageability values for the words corresponding to the images used (Baayen et al., 1993; Coltheart, 1981). The stimuli were enclosed in a 245 by 240 rectangular pixel frame, and subtended ~5 degrees of visual angle (viewing angle ~ 47 pixels per degree). The grey-scale pictures were presented over a uniform grey background (see Figure 1). The chromatic profiles of the line drawings were modified with a series of in-house scripts (Matlab) on a participant-by-participant basis, in accordance with guidelines for creating P-biased and K-biased stimuli (Cavanagh et al., 1992; Kveraga et al, 2007). For thresholding purposes, line drawings of ellipsoids were used. These chromatic values were determined just prior to and during the acquisition of the T1 image, which was always the first scan acquired in the session (see below).
Figure 1. Examples of Stimuli Used in the Experiment.
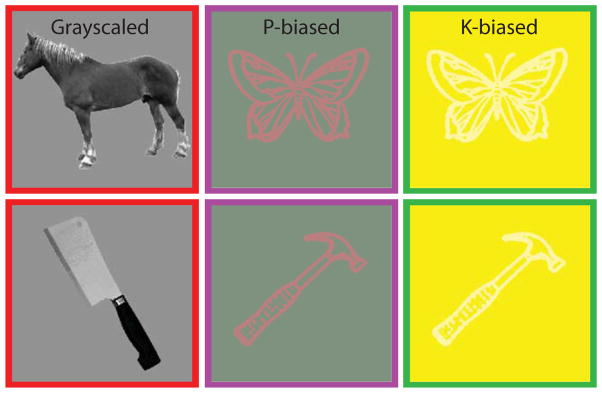
The items presented in grayscale and as line drawings were different. The images in the P-biased condition were isoluminant red/green, whereas the foreground and background (also isoluminant) of the K-biased stimuli were chromatically defined by blue only.
For P-biased stimuli, we defined the red/green isoluminant point with heterochromatic flicker photometry. Following Kveraga et al. (2007), the background and foreground of the images alternated between pure red and pure green at a frequency of about 14 Hz. Participants controlled the output of the red channel until the background and foreground images no longer appeared to flicker. Participants repeated this task 10 times, and the values were averaged to determine the chromatic values for the steady stimuli that were used in the experiment. Stimuli using these red and green values for the foreground and background, respectively, were isoluminant and hence defined only by chromatic differences within long and medium wavelength cones (see Figure 1). As noted above, this condition is known to selectively stimulate the parvocellular pathway; hence we referred to it as the P-biased condition.
For the K-biased condition, we used a procedure to create tritanopic stimuli (i.e., stimuli carried by signals arriving from the blue, short-wavelength cones; Cavanagh et al., 1992; Wald, 1964). Participants were exposed to an intense yellow field that effectively saturates the responses of the red and green cones (e.g., Wald, 1964). Variations in blue stimulation will then be carried only by the responses of blue short-wavelength cones (e.g., Cavanagh et al., 1987; Lee and Stromeyer, 1989; Wald, 1964). Thus, a blue-only colored rotating disk was superimposed on top of the yellow field. Because motion perception for equiluminous stimuli is disturbed (e.g., Moreland, 1982), and equiluminant motion appears slower, participants were asked to monitor the speed of the rotating disk while controlling the output of the blue channel. Participants would vary the intensity of the blue channel until they noticed a marked drop in the speed of the blue rotating disk (repeated 10 times, values averaged). The values obtained were used to color the foreground drawing against an intense yellow background. The foreground drawing would, therefore, be visible only through koniocellular channels, as it would be perceived as a homogeneous rectangular field by other pathways (see Figure 1).
2.3 Procedure
Stimulus presentation was controlled with ‘A Simple Framework’ (Schwarzbach, 2011), using Psychtoolbox in MATLAB (Brainard, 1997). Stimuli were back projected on a screen (temporal resolution = 120Hz) that participants viewed with a mirror attached to the head coil. Participants viewed the tool and animal stimuli passively (no response) in a miniblock design. Each miniblock lasted 8 seconds, and consisted of the presentation of 10 animals or 10 tools from a single condition (e.g., 10 tools from the P-biased condition, or 10 animals from the K-Biased Condition, etc.). The pictures were presented once in each miniblock (Duration = 800ms, ISI = 0), and miniblocks were separated by 8 seconds of fixation. There were two miniblocks of each condition per run. The same tool and animal stimuli were presented in two additional conditions that were not relevant to issues discussed in this manuscript. Each run contained a full balanced experimental design, and lasted approximately 5 minutes. Each run was then an independent modular ‘replication’ containing all the experimental manipulations. Participants completed between 3 and 8 runs (one participant completed 3 runs, one completed 4 runs, two completed 5 runs, four completed 6 runs, ten completed 7 runs, and one completed 8 runs); the reason for the unequal runs was that participants completed as many runs as they were comfortable remaining in the scanner.
2.4 MRI Parameters
Whole brain BOLD imaging was conducted on a 3-Tesla Siemens MAGNETOM Trio scanner with a 32-channel head coil at the Rochester Center for Brain Imaging. High-resolution structural T1 contrast images were acquired using a magnetization prepared rapid gradient echo (MPRAGE) pulse sequence at the start of each session (TR = 2530 ms, TE = 3.44 ms flip angle = 7 degrees, FOV = 256 mm, matrix = 256 × 256, 1×1×1mm sagittal left-to-right slices). An echo-planar imaging pulse sequence was used for T2* contrast (TR = 2000 ms, TE = 30 ms, flip angle = 90 degrees, FOV = 256 mm, matrix 64 × 64, 30 sagittal left-to-right slices, voxel size = 4×4×4mm). The first 6 volumes of each run were discarded to allow for signal equilibration (four volumes at the scanner, i.e., not saved, and 2 in preprocessing).
2.5 fMRI Data analysis
fMRI data were analyzed with the Brain Voyager software package (Version 2.1) and in-house scripts drawing on the BVQX toolbox for MATLAB. Preprocessing of the functional data included, in the following order, slice time correction (sinc interpolation), motion correction with respect to the first volume of the first functional run, and linear trend removal in the temporal domain (cutoff: 2 cycles within the run). Functional data were registered (after contrast inversion of the first volume) to high-resolution de-skulled anatomy on a participant-by-participant basis in native space. For each participant, echo-planar and anatomical volumes were transformed into standardized (Talairach and Tournoux, 1988) space. Functional data were smoothed at 6mm (1.5 voxels) at FWHM, and interpolated to 3×3×3mm voxels.
We used the general linear model to fit beta estimates to the events of interest. Experimental events were convolved with a standard 2 gamma hemodynamic response function. In addition, the first derivatives of the six motion parameters describing volume-to-volume motion were added (not convolved) as predictors of no interest to attract variance associated with motion. There were 6 motion regressors (not convolved), and 10 regressors: the two-by-three design of the category of the stimulus (tools and animals), and chromatic condition (P-Biased, K-Biased, Grayscale Images, and two additional manipulations of the stimuli not analyzed herein). All analyses treated subjects as a random factor, and there were thus 18 degrees of freedom in the group-level analyses.
Functional connectivity analyses were time course based and were run using the inferior and posterior/superior regions identified as exhibiting tool-preferences for P- and K-biased stimuli, respectively (see below for findings), and two theoretically defined target ROIs – the left medial fusiform and bilateral MT/V5 ROIs. ROIs for the left medial fusiform gyrus and MT/V5 (bilaterally) were defined based on the peak Talairach coordinates from previously published work (Mahon et al., 2007; Tootell et al., 1995).
Mahon et al. (2007) had participants silently name pictures of tools, manipulable objects that did not have a systematic relationship between structure and manner of manipulation, large non-manipulable objects, and animals. The medial fusiform gyrus (bilaterally) was defined by contrasting all nonliving stimuli against animals. Here, we created a 10 mm sphere around the peak Talairach coordinates obtained by Mahon et al. (2007) for the left medial fusiform gyrus (x = −24, y = −48, z = 8).
Tootell et al. (1995) presented participants with high-contrast moving stimuli and compared the activation elicited by those stimuli against that obtained for stationary stimuli. This analysis led to the definition of an area that was almost exclusively driven by moving stimuli - the bilateral MT/V5 complex. In our study, we created two 7.5 mm spheres (that match, in total volume, the left medial fusiform gyrus sphere) around Tootell et al.’s (1995) Talairach coordinates for the bilateral MT/V5 complex (x = +/− 45, y = −76, z = 3).
Functional connectivity was computed among ROIs over the averaged time course of all voxels in the ROI. The resulting r values were then Fisher transformed, and entered into the ROI-based group-level functional connectivity analysis.
3. Results
We first computed the contrast map showing parietal regions exhibiting differential BOLD contrast when viewing grayscale tools compared to viewing grayscale animals (p < 0.01, corrected). As can be seen in Figure 2A, a large region of parietal cortex in the left hemisphere, encompassing superior and posterior parietal regions, the IPS, as well as inferior and lateral parietal cortex exhibited differential BOLD responses for tools compared to animals. Moreover, other regions that have typically emerged when contrasting tools against other categories of objects were also observed to be more activated for tools in our study. In particular both the left middle temporal gyrus (LMTG, p < 0.01, corrected; peak coordinates x = −39, y = −52, z = −8) and the medial aspect of the left fusiform gyrus (p < 0.01, uncorrected; peak coordinates x = −21, y = −49, z = −14), exhibited stronger responses to grey-scale pictures of tools than of animals. This replicates a number of previous studies showing that simply viewing tools leads to the automatic engagement of a set of temporal and parietal regions that collectively represent visual and praxis information necessary for object-directed action (e.g., Chao and Martin, 2000; Creem-Regehr and Lee, 2005; Noppeney et al., 2006; Mahon et al., 2007).
Figure 2. Tool Preferences in Parietal Cortex.
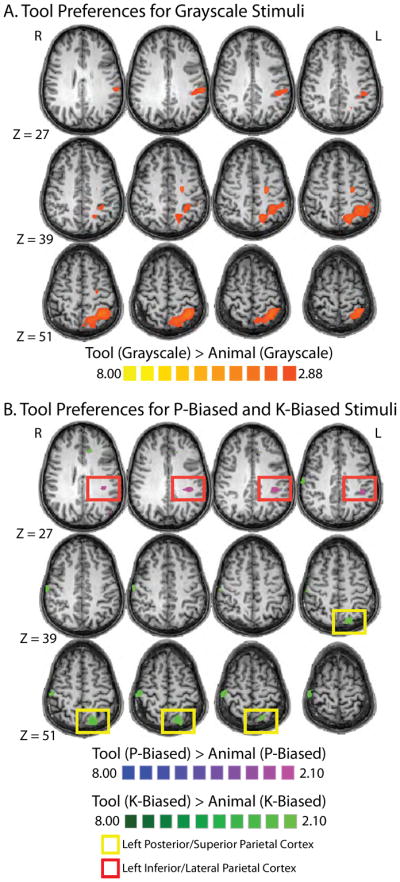
Tool-preferring regions within parietal cortex for A) grayscale images (p < 0.01, corrected); and B) chromatically defined line-drawings (P- and K-biased; p < 0.05, corrected).
We then tested whether left parietal tool preferring regions could be dissociated according to the way in which the line drawing stimuli were chromatically defined. Specifically, as discussed in the introduction, we predicted that i) left inferior parietal tool responsive cortex, because of its interconnectivity with P-dominated ventral stream regions, would be selectively activated for the contrast of P-biased tool stimuli against P-biased animal stimuli, while ii) left superior and posterior parietal tool-preferring cortex, would be selectively activated for the contrast of K-biased tool stimuli compared to K-biased animal stimuli, because of the direct connections between LGN and the dorsal stream area MT for the koniocellular pathway.
As can be seen in Figure 2B, P-biased tool-preferences were restricted to inferior aspects of left parietal tool-responsive cortex (p < 0.05, corrected; peak coordinates x = −33, y = −34, z = 28). In contrast, tool-preferences for K-biased stimuli were restricted to posterior/superior aspects of tool-responsive parietal cortex (in or around area V6/V6a; e.g., Fang and He, 2005; Pitzalis et al., 2006; Simon et al., 2002; p < .05, corrected; peak coordinates x = −18, y = −70, z = 52; all analyses are whole-brain analyses).
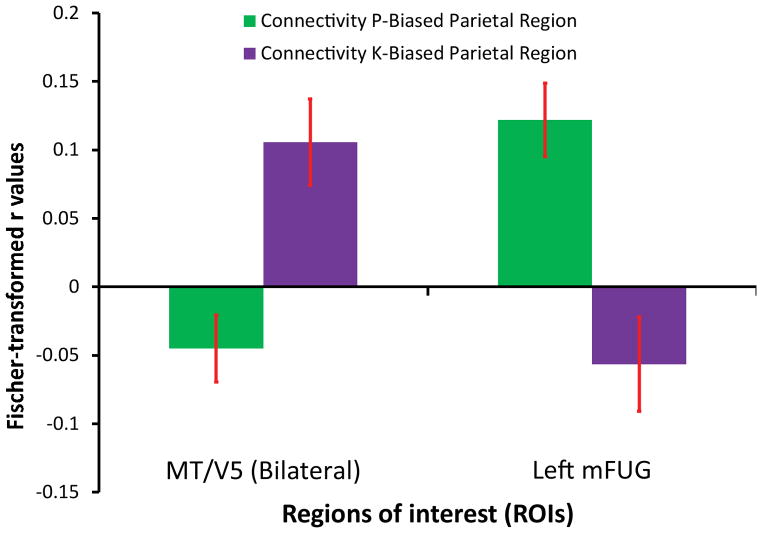
In order to independently test our assumption that the P- and K-biased stimuli were driving activation within the hypothesized networks, two functional connectivity analyses were conducted. The average time courses from the ROIs defined as showing tool-preferences for P- and K-biased stimuli (Figure 2A) were used as seeds in the functional connectivity analyses, and the group-level average connectivity to predefined target ROIs was computed. The target ROIs were the left medial fusiform gyrus (a ventral stream region), which should process, inter alia, parvocellular visual information, and MT/V5 (bilaterally; a dorsal stream region) which should process koniocellular information. Thus, the expectation would be that the left inferior parietal seed, where P-biased tool-preferences were observed, would exhibit privileged connectivity to the left medial fusiform gyrus, whereas the left superior/posterior parietal ROI, where K-biased tool-preferences were observed, would express privileged connectivity to MT/V5. That is, ventral stream P-dominated regions (e.g., the left medial fusiform gyrus) should be functionally connected with the region that demonstrated a bias for tools under parvocellular stimulation, whereas dorsal stream K-biased regions (i.e., area MT; Sincich et al., 2004) should be functionally connected with the region that demonstrated a bias for tools under koniocellular stimulation. As shown in Figure 3 this is exactly the pattern that was obtained. The cross over interaction (F (1,18) = 20.92, p < 0.001, MSE = 0.25, η2 = 0.991) as well as the simple main effects within each target ROI (left medial fusiform gyrus: t (18) = 3.59, p < 0.003; MT/V5: t (18) = 3.26, p < 0.005) were all significant.
Figure 3. Functional Connectivity Analysis.
Group-level statistics were computed over the averaged Fisher transformed r values for the connectivity values between each parietal seed, defined by tool-preferences for P-biased and K-biased stimuli, and each target ROI (MT/V5 and Left Medial Fusiform Gyrus). The error bars represent the standard errors of the mean across subjects.
4. General Discussion
The data that we have reported show that tool-preferences within different sub-regions of left parietal cortex can be dissociated according to whether they are principally driven by analysis of the visual input by the ventral or the dorsal visual pathways. We manipulated the chromatic profiles of line-drawings of animal and tool stimuli such that they were biased toward being processed by the parvocellular or koniocellular pathways. The parvocellular pathway projects almost exclusively to the ventral visual stream (e.g., Ferrera et al., 1992; Livingstone and Hubel, 1988; Merigan and Maunsell, 1993). The koniocellular pathway presents a somewhat less restrictive route to anterior visual areas, but is known to project directly to area MT/V5 (Sincich et al., 2004) – a region within the dorsal visual pathway – and may support intact visuomotor performance in blindsight patients (e.g., Vakalopoulos, 2005). Tool-preferences for P-biased stimuli in parietal cortex were restricted to the inferior parietal lobule, while tool-preferences for K-biased stimuli were restricted to superior and posterior aspects of left parietal cortex. In addition, we showed that these two tool-preferring parietal regions, doubly dissociated by P- and K-biased stimuli, could also be doubly dissociated by their functional connectivity. While the K-biased left parietal region showed greater connectivity with MT/V5 than with the left medial fusiform gyrus, the P-biased left parietal region showed the opposite effect: greater functional connectivity with the left medial fusiform guys than with MT/V5.
These data demonstrate that tool-preferences in the left inferior parietal lobule are dependent on input from the ventral visual pathway. We know, on the basis of long standing lesion work, that damage to the left inferior parietal lobule is associated with impairments for manipulating objects correctly according to their function (Buxbaum and Saffran, 2002; Moreaud et al., 1998; for review, see Johnson-Frey, 2004; Mahon and Caramazza, 2005). The available imaging work converges with the view that complex object-associated manipulation knowledge is represented in the left inferior parietal lobule, and that activation of that structure when viewing tools reflects the automatic retrieval of such manipulation knowledge (e.g., Boronat et al., 2005; Mahon et al., 2007; Rumiati et al., 2004). Our data further show that this region receives a principal input from ventral stream structures that are known to support visual identification. This is in line with recent data that suggest that the inferior parietal lobule (where our P-biased tool-specific activation was found) may integrate relatively abstract (e.g., ‘semantic’) information about the target object with potential available motor plans (e.g., Arbib, 2008). That integrative function of the left inferior parietal lobule, and specifically the left supramarginal gyrus, may be a key step in the selection of the appropriate manipulation for a given object, and for reaching toward objects in a way that anticipates the eventual manipulation that will be applied to them. For instance, if you are reaching to a hammer in order to pound a nail, it will be grasped in a specific way even if that is not the simplest or most comfortable grasp point; however, if the hammer is being grasped simply to move it, then it may be grasped in a more efficient and biomechanically ‘comfortable’ fashion. This type of view of the function of the left inferior parietal lobule, that it integrates multiple sources of information in the service of planning actions, reinforces the emerging notion that parietal cortex does not monolithically reflect dorsal stream activity. Rather, our findings, and other findings reviewed above, fit more naturally with an understanding of the parietal action system as having significant internal organization that can be distinguished (at least in part) according to its afferent inputs (Rizzolatti & Matelli, 2003; Vingerhoets et al., 2009).
Tool preferences for K-biased tool stimuli were observed in posterior/superior parietal regions, likely in the vicinity of visual area V6/V6a (for comparison, see e.g., Pitzalis et al., 2006). This region is known to be involved in volumetric analysis and to project to the superior parietal lobule (see area cIPS, or caudal IPS, as described in Culham et al., 2003). Fang and He (2005) found a potentially similar region of the dorsal stream to be activated when participants were shown images of tools that were rendered invisible using Continuous Flash Suppression, an interocular suppression technique. Using the same psychophysical technique as Fang and He (2005), we have previously shown selective modulation of behavioral responses for tool stimuli compared to a range of other categories (Almeida et al., 2008, 2010), consistent with the view that this region is involved in the extraction of information about for instance, the principal axis of elongation of an object as it is relevant for grasping (e.g., Almeida et al., under review; Sakuraba et al., 2012).
Importantly, K-biased stimuli were, in all important respects, similar to the P-biased stimuli; they were equiluminant and chromatically defined, and they were line drawings. In fact, the only difference was that the K-biased stimuli exploited the responses of the blue cones and of the koniocellular pathway. To our knowledge, this is the first demonstration of modulation of high-level visual processing by koniocellular signals, and as such provides some suggestion for a functional role of the direct anatomical projection between LGN and MT/V5 (Sincich et al., 2004). Our functional connectivity analyses further show that the regions that exhibit tool-preferences for K-biased stimuli are functionally connected with area MT/V5.
It is also important to note that the critical contrast maps that were computed were all independent: in principle, tool preferences for P-biased and K-biased stimuli could have overlapped, which would suggest concurrent dorsal and ventral stream input. Interestingly, however, the inferior parietal lobule, known to be involved in representing manipulation knowledge, emerged only for the P-biased contrast. At the very least this indicates independent input from ventral temporal regions to the computation of object manipulation information, and suggests that object identity information accessed via the ventral stream is retrieved prior to the activation of complex object manipulation in the left inferior parietal lobule.
An important objection that may be raised is whether our findings in fact provide any direct causal information on the direction of influence between ventral temporal cortex and the inferior parietal lobule. In other words, our analyses of connectivity do not contain any causal information in and of themselves, and thus our conclusion rests on the supposition that red/green isoluminant stimuli are selectively visible by parvocellular pathways, and hence by the ventral but not the dorsal visual pathways. It may well be that in certain contexts, particularly those involving overt actions, there could be a bidirectional exchange of information between parietal and temporal cortex—we would not want to deny that, as it seems to be a very reasonable and perhaps likely possibility. Such motor-to-visual interactions may in fact be suggested by theoretical alternatives to the embodied cognition tool recognition hypothesis, such as the FARS model of Arbib and colleagues (Fagg and Arbib, 1998) and the Grounding by Interaction Hypothesis (Mahon and Caramazza, 2008; 2009). Moreover, although it has been shown that potentiation-for-action (i.e., activation of motor information) can happen automatically, it may be the case that priming action contexts enhances potentiation-for-action (e.g. Jax and Buxbaum, 2010; Helbig et al., 2006, 2010; Randerath et al., 2012; Tucker & Ellis, 1998), potentially shifting the principal directionality of influence between motor-relevant knowledge and high level visual object recognition processes.
In summary, our findings indicate that motor-relevant information indexed by left parietal activation when viewing tools can be dissociated according to whether that information is extracted by the dorsal or ventral pathways. The fact that tool preferences are observed in the left inferior parietal lobule for stimuli that are visible only by the ventral visual pathway undermines the Embodied Cognition Hypothesis of tool recognition, because it shows that access to object manipulation knowledge (parietal activation) is contingent on identification of the stimuli (ventral stream processing). The fact that tool preferences for K-biased stimuli are restricted to posterior/superior parietal regions suggests a rich functional role for koniocellular channels in object processing.
More generally, our findings dovetail with the idea that low-level subcortical constraints within the visual system may constrain the way object knowledge is organized in the brain (e.g., Mahon et al., 2013). Particular aspects of the networks dedicated to high-level and complex computations may be more dependent on particular types of input. In the case we have reported, the tool network may be fractionated according to the type of input that preferentially drives different processes, thus providing new leverage on understanding the factors that shape the organization of conceptual knowledge.
Acknowledgments
J.A. was supported by funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement n° PCOFUND-GA-2009-246542 and from the Foundation for Science and Technology of Portugal Project Grants PTDC/PSI-PCO/114822/2009 and PTDC/MHC-PCN/3575/2012. This research was supported by NIH grant R21 NS076176 to BZM.
Footnotes
This is not to say that such actions do not draw on any stored information; they draw on a repertoire of skills that have been practiced (i.e., reaching and grasping); rather, they do not seem to require information that is elaborated and generalized to the ‘type’ of object that is being grasped (for discussion, see Wu, 2008).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida J, Mahon BZ, Caramazza A. The role of the dorsal visual processing stream in tool identification. Psychological Science. 2010;21:772–778. doi: 10.1177/0956797610371343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J, Mahon BZ, Nakayama K, Caramazza A. Unconscious processing dissociates along categorical lines. Proceedings of the National Academy of Sciences, USA. 2008;105:5214–15218. doi: 10.1073/pnas.0805867105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J, Mahon BZ, Zapater-Raberov V, Dziuba A, Cabaço T, Marques JF, Nakayama K, Caramazza A. Grasping with the eyes: the role of elongation in visual manipulable object recognition. Cognitive Affective and Behavioral Neuroscience. doi: 10.3758/s13415-013-0208-0. under review. [DOI] [PubMed] [Google Scholar]
- Arbib M. From grasp to language: embodied concepts and the challenge of abstraction. Journal of Physiology Paris. 2008;102(1–3):4–20. doi: 10.1016/j.jphysparis.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, van Rijn H. The CELEX Lexical Database [CD-ROM] Philadelphia: University of Pennsylvania, Linguistic Data Consortium; 1993. [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel Visual Motion Processing Streams for Manipulable Objects and Human Movements. Neuron. 2002;34:149–159. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buxbaum LJ. Two action systems in the human brain. Brain and Language. 2012 doi: 10.1016/j.bandl.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Reetz K, Blangero A. Tactile agnosia and tactile apraxia: crosstalk between the action and perception streams in AIP. Behavioural and Brain Sciences. 2007;30:2001–2002. [Google Scholar]
- Boronat CB, Buxbaum LJ, Coslett HB, Tang K, Saffran EM, Kimberg DY, et al. Distinctions between manipulation and function knowledge of objects: Evidence from functional magnetic resonance imaging. Cognitive Brain Research. 2005;23(2–3):361–373. doi: 10.1016/j.cogbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Borra E, Belmalih A, Calzavara R, Gerbella M, Murata A, Rozzi S, Luppino G. Cortical connections of the macaque anterior intraparietal (AIP) area. Cerebral Cortex. 2008;18:1094–1111. doi: 10.1093/cercor/bhm146. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Grossman M, Coslett HB. Left inferior parietal representations for skilled hand-object interactions: Evidence from stroke and corticobasal degeneration. Cortex. 2007;43(3):411–23. doi: 10.1016/s0010-9452(08)70466-0. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Tang K, Detre JM. Neural substrates of knowledge of hand postures for object grasping and functional object use: Evidence from fMRI. Brain Research. 2006;1117(1):175–185. doi: 10.1016/j.brainres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Saffran EM. Knowledge of object manipulation and object function: Dissociations in apraxic and nonapraxic subjects. Brain and Language. 2002;82(2):179–199. doi: 10.1016/s0093-934x(02)00014-7. [DOI] [PubMed] [Google Scholar]
- Canessa N, Borgo F, Cappa SF, Perani D, Falini A, Buccino G, Tettamanti M, Shallice T. The different neural correlates of action and functional knowledge in semantic memory: An fMRI study. Cerebral Cortex. 2008;18:740–751. doi: 10.1093/cercor/bhm110. [DOI] [PubMed] [Google Scholar]
- Cant JS, Goodale MA. Attention to form or surface properties modulates different regions of human occipitotemporal cortex. Cerebral Cortex. 2007;17:713–731. doi: 10.1093/cercor/bhk022. [DOI] [PubMed] [Google Scholar]
- Casagrande VA. A third parallel visual pathway to primate area V1. Trends in Neuroscience. 1994;17:305–310. doi: 10.1016/0166-2236(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Adelson EH, Heard P. Vision with equiluminant colour contrast: 2. A large scale technique and observations. Perception. 1992;21:219–226. doi: 10.1068/p210219. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Anstis SM, MacLeod DIA. Equiluminance: Spatial and temporal factors and the contribution of blue-sensitive cones. Journal of the Optical Society of America A. 1987;4:1428–1438. doi: 10.1364/josaa.4.001428. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. NeuroImage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about object. Nature Neuroscience. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC Psycholinguistic Database. Quarterly Journal of Experimental Psychology. 1981;33A:497–505. [Google Scholar]
- Creem-Regehr SH, Lee JN. Neural representations of graspable objects: are tools special? Cognitive Brain Research. 2005;22 (3):457–469. doi: 10.1016/j.cogbrainres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Culham JC, Danckert SL, DeSouza JF, Gati JS, Menon RS, Goodale MA. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Experimental Brain Research. 2003;153:180–189. doi: 10.1007/s00221-003-1591-5. [DOI] [PubMed] [Google Scholar]
- Dacey DM. Parallel pathways for spectral coding in primate retina. Annual Review of Neuroscence. 2000;23:743–775. doi: 10.1146/annurev.neuro.23.1.743. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92(1–2):179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Das A, Huxlin KR. New approaches to visual rehabilitation for cortical blindness: outcomes and putative mechanisms. Neuroscientist. 2010;16(4):374–87. doi: 10.1177/1073858409356112. [DOI] [PubMed] [Google Scholar]
- Fagg AH, Arbib MA. Modeling Parietal-Premotor Interactions in Primate Control of Grasping. Neural Networks. 1998;11:1277–1303. doi: 10.1016/s0893-6080(98)00047-1. [DOI] [PubMed] [Google Scholar]
- Fang F, He S. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nature Neuroscience. 2005;8(10):1380–1385. doi: 10.1038/nn1537. [DOI] [PubMed] [Google Scholar]
- Ferrera VP, Nealey TA, Maunsell JH. Mixed parvocellular and magnocellular geniculate signals in visual area V4. Nature. 1992;27:756–761. doi: 10.1038/358756a0. [DOI] [PubMed] [Google Scholar]
- Frey SH. What puts the how in where? Tool use and the divided visual streams hypothesis. Cortex. 2007;43(3):368–375. doi: 10.1016/s0010-9452(08)70462-3. [DOI] [PubMed] [Google Scholar]
- Gallese V, Lakoff G. The brain’s concepts: The role of the sensory-motor system in conceptual knowledge. Cognitive Neuropsychology. 2005;22:455–479. doi: 10.1080/02643290442000310. [DOI] [PubMed] [Google Scholar]
- Garcea FE, Dombovy M, Mahon BZ. Preserved tool knowledge in the context of impaired action knowledge: Implications for models of semantic memory. Frontiers in Human Neuroscience. 7:120. doi: 10.3389/fnhum.2013.00120. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea FE, Mahon BZ. What is in a tool concept? Dissociating manipulation knowledge from function knowledge. Memory and Cognition. 2012;40:1303–1313. doi: 10.3758/s13421-012-0236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G. Apraxia. In: Goldenberg G, Miller B, editors. Handbook of clinical neurology. 3. Edinburgh: Elsevier; 2008. pp. 323–338. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Apraxia and the parietal lobes. Neuropsychologia. 2009;47(6):1449–1459. doi: 10.1016/j.neuropsychologia.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Hagmann S. Tool use and mechanical problem solving in apraxia. Neuropsychologia. 1998;36(7):581–589. doi: 10.1016/s0028-3932(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Spatt J. The neural basis of tool use. Brain. 2009;132(6):1645–1655. doi: 10.1093/brain/awp080. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Jakobson LS, Keillor JM. Differences in the visual control of pantomimed and natural grasping movements. Neuropsychologia. 1994;32(10):1159–78. doi: 10.1016/0028-3932(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in Neurosciences. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grafton ST. The cognitive neuroscience of prehension: recent developments. Experimental Brain Research. 2010;204(4):475–491. doi: 10.1007/s00221-010-2315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Tucker M, Armony JL, Ellis R, Passingham RE. Objects automatically potentiate action: an fMRI study of implicit processing. European Journal of Neuroscience. 2003;17:2735–2740. doi: 10.1046/j.1460-9568.2003.02695.x. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Research. 2001;41(10–11):1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Helbig HB, Graf M, Kiefer M. The role of action representations in visual object recognition. Experimental Brain Research. 2006;174(2):221–228. doi: 10.1007/s00221-006-0443-5. [DOI] [PubMed] [Google Scholar]
- Helbig HB, Steinwender J, Graf M, Kiefer M. Action observation can prime visual object recognition. Experimental Brain Research. 2010;200(3–4):251–258. doi: 10.1007/s00221-009-1953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SHC, Reid RC. The koniocellular pathway in primate vision. Annual Review of Neuroscience. 2000;23:127–153. doi: 10.1146/annurev.neuro.23.1.127. [DOI] [PubMed] [Google Scholar]
- Himmelbach M, Boehme R, Karnath HO. 20 years later: A second look on DF’s motor behaviour. Neuropsychologia. 2012;50(1):139–44. doi: 10.1016/j.neuropsychologia.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Ishibashi R, Lambon Ralph MA, Saito S, Pobric G. Different roles of lateral anterior temporal and inferior parietal lobule in coding function and manipulation tool knowledge: Evidence from an rTMS study. Neuropsychologia. 2011;49:1128–1135. doi: 10.1016/j.neuropsychologia.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Jax S, Buxbaum LJ. Response interference between functional and structural actions linked to the same familiar object. Cognition. 2010;115(2):350–355. doi: 10.1016/j.cognition.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M, Decety J, Michel F. Impairment of grasping movements following a bilateral posterior parietal lesion. Neuropsychologia. 1994;32(4):369–380. doi: 10.1016/0028-3932(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey S. The neural bases of complex tool use in humans. TRENDS in Cognitive Sciences. 2004;8:71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Rüter J, Mandler A, Himmelbach M. The Anatomy of Object Recognition—Visual Form Agnosia Caused by Medial Occipitotemporal Stroke. Journal of Neuroscience. 2009;29:5854–5862. doi: 10.1523/JNEUROSCI.5192-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenbach ML, Brett M, Patterson K. Actions speak louder than functions: The importance of manipulability and action in tool representation. Journal of Cognitive Neuroscience. 2003;15:20–46. doi: 10.1162/089892903321107800. [DOI] [PubMed] [Google Scholar]
- Kveraga K, Boshyan J, Bar M. Magnocellular projections as the trigger of top-down facilitation in recognition. The Journal of Neuroscience. 2007;27:13232–13240. doi: 10.1523/JNEUROSCI.3481-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Stromeyer CF., III Contribution of human short-wave cones to luminance and motion detection. Journal of Physiology. 1989;413:563–593. doi: 10.1113/jphysiol.1989.sp017669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: Anatomy, Physiology, and Perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. The orchestration of the sensory-motor systems: Clues from neuropsychology. Cognitive Neuropsychology. 2005;22:480–494. doi: 10.1080/02643290442000446. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. A Critical Look at the Embodied Cognition Hypothesis & a New Proposal for Grounding Conceptual Content. Journal of Physiology - Paris. 2008;102:59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. Concepts & Categories: A Cognitive Neuropsychological Perspective. Annual Review of Psychology. 2009;60:27–51. doi: 10.1146/annurev.psych.60.110707.163532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon B, Kumar N, Almeida J. Spatial frequency tuning reveals visuomotor interactions between the dorsal and ventral visual systems. Journal of Cognitive Neuroscience. 2013 doi: 10.1162/jocn_a_00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Milleville S, Negri GAL, Rumiati RI, Caramazza A, Martin A. Action-related properties of objects shape object representations in the ventral stream. Neuron. 2007;55:507–520. doi: 10.1016/j.neuron.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Ungerleider LG, Haxby JV. Category specificity and the brain: The sensory/motor model of semantic representations of objects. In: Gazzaniga MS, editor. The New Cognitive Neurosciences. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- Merigan WH, Maunsell JHR. How parallel are the primate visual pathways? Annual Review of Neuroscience. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Moreaud O, Charnallet A, Pellat J. Identification without manipulation: A study of the relations between object use and semantic memory. Neuropsychologia. 1998;36(12):1295–1301. doi: 10.1016/s0028-3932(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Moreland JD. Spectral sensitivity measured by motion photometry. In: Verriest G, editor. Colour Deficiencies. VI. The Hague: Junk; 1982. pp. 61–66. [Google Scholar]
- Nelissen K, Vanduffell W. Grasping-Related Functional Magnetic Resonance Imaging Brain Responses in the Macaque Monkey. The Journal of Neuroscience. 2011;31(22):8220–8229. doi: 10.1523/JNEUROSCI.0623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri GAL, Rumiati RI, Zadini A, Ukmar M, Mahon BZ, Caramazza A. What is the role of motor simulation in action and object recognition? Evidence from apraxia. Cognitive Neuropsychology. 2007;24:795–816. doi: 10.1080/02643290701707412. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ, Penny WD, Friston KJ. Two distinct neural mechanisms for category-selective responses. Cerebral Cortex. 2006;16:437–445. doi: 10.1093/cercor/bhi123. [DOI] [PubMed] [Google Scholar]
- Pelgrims B, Olivier E, Andres M. Dissociation between manipulation and conceptual knowledge of object use in supramarginalis gyrus. Human Brain Mapping. 2011;32:1802–1810. doi: 10.1002/hbm.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perenin M, Vighetto A. Optic ataxia: a specific disruption in visuomotor mechanisms. I. Different aspects of the deficit in reaching for objects. Brain. 1988;111(3):643–674. doi: 10.1093/brain/111.3.643. [DOI] [PubMed] [Google Scholar]
- Pisella L, Binkofski F, Lasek K, Rossetti Y. No double-dissociation between optic ataxia and visual agnosia: Multiple sub-streams for multiple visuo-manual integrations. Neuropsychologia. 2006;44(13):2734–2748. doi: 10.1016/j.neuropsychologia.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Pitzalis S, Galletti C, Huang RS, Patria F, Committeri G, Galati G, Fattori P, Sereno MI. Wide-field retinotopy defines human cortical visual area v6. Journal of Neuroscience. 2006;26(30):7962–73. doi: 10.1523/JNEUROSCI.0178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Jeffries E, Lambon Ralph A. Amodal semantic representations depend on both anterior temporal lobes: Evidence from repetitive transcranial magnetic stimulation. Neuropsychologia. 2010;48:1336–1342. doi: 10.1016/j.neuropsychologia.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Randerath J, Goldenberg G, Spijkers W, Li Y, Hermsdörfer J. Different left brain regions are essential for grasping a tool compared with its subsequent use. Neuroimage. 2010;53(1):171–180. doi: 10.1016/j.neuroimage.2010.06.038. [DOI] [PubMed] [Google Scholar]
- Randerath J, Martin KR, Frey SH. Are tool properties always processed automatically? The role of tool use context and task complexity. Cortex. 2012 doi: 10.1016/j.cortex.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M. Two different streams form the dorsal visual system: anatomy and functions. Experimental Brain Research. 2003;153(2):146–157. doi: 10.1007/s00221-003-1588-0. [DOI] [PubMed] [Google Scholar]
- Rumiati RI, Weiss PH, Shallice T, Ottoboni G, Noth J, Zilles K, Fink GR. The neural basis of pantomiming the use of visually presented objects. NeuroImage. 2004;21:1224–31. doi: 10.1016/j.neuroimage.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Rushworth M, Behrens T, Johansen-Berg H. Connection patterns distinguish 3 regions of human parietal cortex. Cerebral Cortex. 2006;16(10):1418–1430. doi: 10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- Sakuraba S, Sakai S, Yamanaka M, Yokosawa K, Hirayama K. Does the human dorsal stream really process a category for tools? Journal of Neuroscience. 2012;32(11):3949–53. doi: 10.1523/JNEUROSCI.3973-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk T, McIntosh RD. Do we have independent visual streams for perception and action? Cognitive Neuroscience. 2009;1(1):52–62. doi: 10.1080/17588920903388950. [DOI] [PubMed] [Google Scholar]
- Schwarzbach J. A simple framework (ASF) for behavioral and neuroimaging experiments based on the psychophysics toolbox for MATLAB. Behavior Research Methods. 2011;43(4):1194–201. doi: 10.3758/s13428-011-0106-8. [DOI] [PubMed] [Google Scholar]
- Simon O, Mangin JF, Cohen L, Le Bihan D, Deahene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 2002;33(3):475–87. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Park KF, Wohlgemuth MJ, Horton JC. Bypassing V1: a direct geniculate input to area MT. Nature Neuroscience. 2004;7(10):1123–1128. doi: 10.1038/nn1318. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR, Poncet M. The role of sensorimotor experience in object recognition. Brain. 1991;114:2555–2573. doi: 10.1093/brain/114.6.2555. [DOI] [PubMed] [Google Scholar]
- Sunderland A, Wilkins L, Dineen R, Dawson SE. Tool-use and the left hemisphere: What is lost in ideomotor apraxia? Brain and Cognition. 2013;81(2):183–192. doi: 10.1016/j.bandc.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Tootell RBH, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW. Functional analysis of human MT and related visual cortical areas using functional magnetic resonance imaging. Journal of Neuroscience. 1995;15:3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M, Ellis R. On the relations between seen objects and components of potential actions. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:830–846. doi: 10.1037//0096-1523.24.3.830. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. The MIT Press; Cambridge MA: 1982. [Google Scholar]
- Vakalopoulos C. A theory of blindsight—the anatomy of the unconscious: a proposal for the koniocellular projections and intralaminar thalamus. Medical Hypotheses. 2005;65(6):1183–90. doi: 10.1016/j.mehy.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G. Knowing about tools: Neural correlates of tool familiarity and experience. Neuroimage. 2008;40:1380–1391. doi: 10.1016/j.neuroimage.2007.12.058. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, Acke F, Vandemaele P, Achten E. Tool responsive regions in the posterior parietal cortex: Effect of differences in motor goal and target object during imagined transitive movements. Neuroimage. 2009;47:1832–1843. doi: 10.1016/j.neuroimage.2009.05.100. [DOI] [PubMed] [Google Scholar]
- Wald G. The receptors of human color vision. Science. 1964;145(3636):1007–16. doi: 10.1126/science.145.3636.1007. [DOI] [PubMed] [Google Scholar]
- Wu WC. Visual attention, conceptual content, and doing it right. Mind. 2008;117:1003–1033. [Google Scholar]
- Zhong Y-M, Rockland KS. Inferior parietal lobule projections to anterior inferiortemporal cortex (area TE) in Macaque monkey. Cerebral Cortex. 2003;13:527–540. doi: 10.1093/cercor/13.5.527. [DOI] [PubMed] [Google Scholar]