Abstract
The use of lipoplexes for the intracellular delivery of nucleic acids typically involves the optimization of several parameters that are known to affect delivery. Researchers commonly vary charge ratio, and often incorporate different amounts of helper lipids (e.g., cholesterol) to optimize formulations for transfection in cell culture and in vivo. The results of such experiments are often interpreted in the context of nuclease resistance and cell association, but effects on the protein corona are usually not considered. While many studies have demonstrated that lipoplex structure and function can be dramatically compromised in the presence of serum, little attention has been paid to the adsorption of specific proteins and how this might be affected by formulation parameters. In this study, we characterize changes in the protein corona that occur as DOTAP-based lipoplexes are formulated with different amounts of cholesterol and prepared at different charge ratios. Our results demonstrate a significant effect of lipid composition on both total protein adsorption as well as the individual proteins from fetal calf serum that are associated with lipoplexes. In addition, we show that PEGylation increases protein adsorption with our formulations; effects that depend on the type of PEG conjugate employed in the lipoplex. Attempts to identify a specific protein responsible for enhancing transfection were unsuccessful.
Keywords: serum protein adsorption, lipoplex, cholesterol, MALDI/MS, protein corona, charge ratio, transfection
Introduction
The ability to deliver nucleic acids (genes, siRNA, miRNA, aptamers, antisense) to cells offers an opportunity to develop entirely new types of therapeutics that can replace or silence gene expression associated with disease [1-5]. Although the majority of clinical trials have utilized viral vectors, synthetic delivery systems offer a safer alternative that do not elicit a specific immune response to the vector, which ultimately allows for repeat administration. However, nonviral vectors do activate innate immunity, and opsonization has been shown to alter uptake by specific cells (e.g., liver macrophages) [6,7]. Many studies have documented the interactions between nonviral vectors and serum, and the adverse effects on delivery are well-established [8,9]. More specifically, experiments with lipoplexes have clearly shown that transfection is typically compromised after exposure to serum, and this effect is directly relevant to clearance in vivo [10-12]. Adsorption of serum proteins is not limited to lipoplexes, and similar effects of serum protein binding have been shown with polymer-based delivery systems [13,14]. More recently, the protein “corona” that accumulates upon transfer into physiological fluids has received considerable attention with respect to its influence on nanoparticles [15,16].
While the vast majority of research on the impacts of serum have not identified specific proteins responsible for observed effects, some studies have utilized western blots to identify individual proteins that are adsorbed to particulate delivery systems [13,17,18]. These studies have concluded that some proteins facilitate uptake/clearance by the reticuloendothelial system (RES), while other proteins have the ability to prevent RES uptake thereby prolonging circulation times in blood. More specifically, the binding of apolipoproteins (e.g., ApoE) are thought to enhance uptake by hepatocytes, whereas albumin adsorption is thought to allow delivery systems to avoid RES uptake, extend circulation times, and promote accumulation in tumors [19,20]. In fact, researchers have demonstrated that pre-coating delivery systems with albumin reduces hepatic uptake of polymeric nanospheres and liposomes in vivo [21]. Additional studies have proposed that albumin adsorption improves intracellular trafficking by facilitating endosomal release [22,23]. Although increases in the amount of protein adsorbed to particles have been correlated with increased clearance [17,24], the work described above clearly indicates that the adsorption of specific proteins (e.g., albumin, ApoE, fibrinogen) can also play a role in uptake/clearance. In addition, some reports have suggested that the proteins composing the “corona” vary with time, and therefore that the properties of the vector are dynamic and dependent on the proteins adsorbed at any specific time [15,16]. It follows that formulation variables commonly used in preparing delivery systems (e.g., lipid composition, size, surface charge, PEGylation) may also affect serum protein adsorption, which ultimately affects vector performance (e.g., transfection). While previous work has documented differences in protein adsorption to various formulations, there has yet to be a systematic study of the effect of formulation variables on specific protein adsorption.
We have previously documented the effect of lipoplex cholesterol content on serum protein interactions, and shown that high cholesterol contents greatly reduce the amount of protein adsorbed to lipoplexes [25,26]. In the current study, we utilize mass spectrometry to identify the individual proteins from fetal calf serum that are adsorbed to lipoplex formulations possessing different cholesterol contents. In addition, we identify changes in specific protein adsorption associated with varying charge ratios and PEGylation, and compare these changes to transfection efficiency in cell culture. Our data indicate that these formulation variables have profound effects on the individual proteins that adsorb to lipoplexes upon serum exposure, and this may contribute to the observed differences in transfection.
Materials and Methods
Lipoplex Preparation
N-(1-(2, 3-dioleoyloxy) propyl)-N, N, N-trimethylammonium chloride (DOTAP), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG), cholesterol, and egg phosphatidylcholine (PC) were purchased from Avanti Polar Lipids (Alabaster, AL). Cholesterol-PEG was synthesized using the modified procedure of Zhao et al [27] as previously described [28]. Lipid formulations were prepared by mixing stock lipids in chloroform and subsequently evaporating chloroform from the mixtures under a stream of nitrogen gas (15 min). The resulting lipid film was dried overnight under vacuum to remove residual chloroform. Films were re-suspended at 65 °C in double distilled water and sonicated for 1 minute. Lipoplexes were prepared at different +/− charge ratios by mixing equal volumes of plasmid encoding luciferase with the liposome suspension as previously described [25]. Complexation occurred instantly upon mixing, and the complexes were incubated at room temperature for 15 minutes prior to use in experiments.
Transfection Protocol
MCF-7 cells (American Type Culture Collection # HTB-22; human breast adenocarcinoma cells) were cultured at 37 °C, 5% carbon dioxide with 100% humidity in Minimum Essential Media (MEM), 10% fetal calf serum (FCS), 50 U/ml penicillin, 50 μg/ml streptomycin (Cellgro MediaTech Inc., Manassas, VA). Media was filtered through either a 0.22 μm syringe filter or a 0.22 μm stericup to ensure sterility prior to use (Thermo-Fisher Rockford, IL). For transfection experiments, cells were seeded at 20,000 cells/well in 96-well plates 24 hours prior to treatment. On the day of the experiment, cells were washed twice with phosphate buffered saline (PBS) before adding 100 μl of transfection media. Lipoplexes which had been pre-incubated 1:1 v/v in MEM or FCS for 30 minutes prior to dilution in 100% MEM, were administered to cells for transfection. Formulations were added to the center of each well (2 μl total volume containing 1 μg total DNA) and incubated for 4 hours. In some cases, lipoplexes were added directly to transfection media containing 50% MEM-50% FCS to mimic in vivo serum protein conditions, and transfection was conducted as described above. After 4 hours, the transfection media was removed, and cells were washed twice with PBS before being reintroduced to their normal 10% FCS growth media. After resting for 48 hours, the cells were again washed twice with PBS before being lysed with Promega lysis buffer (30 μl) in the −80 °C freezer according to manufacturer's instructions (Promega, Madison, WI). Lysate was assayed for protein content using a Bio-Rad protein assay (Bio-Rad, Hercules, CA) on a 96-well THERMOmax plate reader (Molecular Devices, Sunnyvale, CA). Luminescence was quantified with a Monolight Luminometer according to manufacturer's instructions (BD Biosciences, San Jose, CA).
Particle Sizing by DLS
Sizes of the lipoplexes were measured by dynamic light scattering with a Nicomp 380 ZLS (PSS Nicomp, Santa Barbara, CA) as previously described [25]. The instrument was set on vesicle mode using the volume-weighted Gaussian distribution analysis. Samples were pre-incubated 1:1 v/v in either MEM or FCS for 30 minutes (before and after FCS exposure, respectively) prior to performing the size measurements. Incubated samples were then diluted 100-fold in PBS prior to sizing to reduce light scattering.
Serum Protein Pull-down Experiments
Lipoplex formulations were mixed 1:1 v/v with FCS, and incubated at room temperature for 30 minutes (incubation time was varied to obtain the data in Fig. 1). Samples were centrifuged at 13,000 × g for 60 min to pellet lipoplexes. The supernatant was removed, and the pellet was resuspended in 10% w/v sodium dodecyl sulfate (SDS). Surface proteins were removed by boiling (95 °C) re-suspended pellets in SDS for 5 minutes according to the methods of Tandia et al.[29]. Samples were then dialyzed into PBS using a low MW (2000 Da) dialysis cassette from Pierce Thermo-Fisher (Rockford, IL) for 24 h with three buffer changes. Total protein content of each pull-down was quantified with the Bio-Rad protein assay mentioned above. The remainder of the sample was analyzed by mass spectrometry as described below.
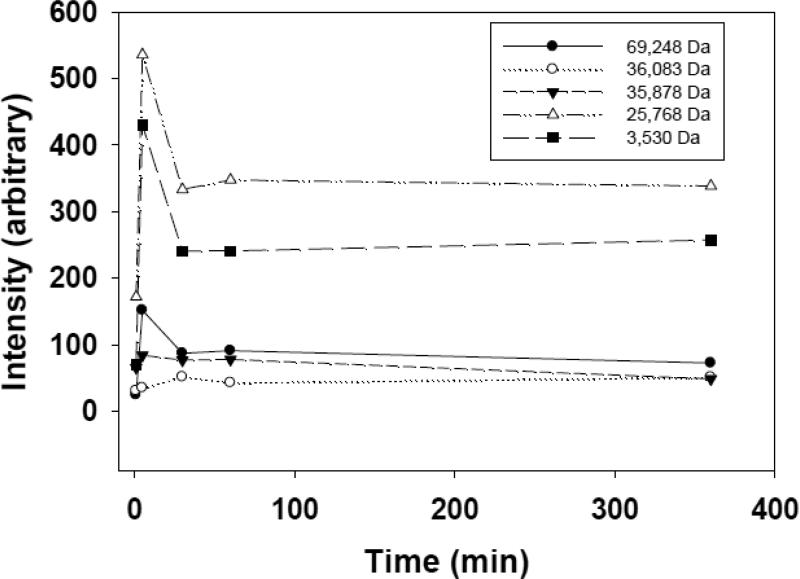
Figure 1.
Time course for the adsorption of individual serum proteins. Lipoplexes (20% DOTAP/80% Chol; +/− = 4) were incubated in fetal calf serum and isolated at different times. MALDI/MS analysis identified five different proteins in the corona, the levels of which varied after 5 min, but remained stable after 30 min.
Protein Analysis by MALDI/MS
Dialyzed proteins from the pull-down experiments were prepared for mass spectrometry using Millipore C4 ZipTips (Billerica, MA) and trifluoroacetic acid (TFA) as previously described [26]. Each sample was then mixed 1:1 v/v with saturated matrix solution (α-cyano-4-hydroxycinnamic [CHCA] for protein detection at ~10 mg/ml or saturated in 0.1%TFA/50% ACN/water). According to the manufacturer, this matrix is best for identifying proteins below 100 kD, so very high molecular weight species may not have been detected by our methods. The 1:1 matrix/protein solutions (1 μl) were then spotted on a clean plate. The plate was loaded in the Bruker Omniflex MALDI-TOF (Bruker Corp., Fremont, CA) and spectra analyzed by Flex Analysis software as previously described [26]. Due to the mass accuracy and possible modification/degradation of proteins extracted from the protein corona, it is difficult to definitively identify proteins associated with each approximate mass with this procedure. However similar to other profiling studies, MALDI provided insight into differences in protein coronas based on formulation that could be further investigated [30]. Additional experiments were performed utilizing protease digestion for identification of individual proteins present in the corona (see below).
Digestion Procedure for Mass Spec Analysis
Chemical denaturation for mass spectrometry analysis was performed according to the procedure described by Russell and Park [31]. Briefly, 1 M guanidine hydrochloride and 5 mM dithiothreitol were added to each pull-down after dialysis, and the mixtures were incubated at 70 °C for 20 minutes as previously described [26]. After denaturation was complete, 25 mM iodoacetamide was added, and this mixture was incubated at room temperature for 30 minutes. Porcine trypsin was added to this mixture at approximately a 1:40 weight ratio of trypsin to protein. The solution was allowed to digest for 5 hours at 37 °C as previously described [26].
Analysis of Digested Protein
Digested protein of most samples was analyzed in the Bruker Omniflex MALDI-TOF as described above. Samples containing large numbers of adsorbed proteins (e.g., 50%/50% formulation) required slightly different instrumentation to identify the proteins detected by the procedure described above. Briefly, 1 μl of digested protein was loaded onto a Magic AQ C18 reverse phase nano column (Bruker, Fremont, CA) using a nano-Advance autosampler and nano flow UPLC (Bruker). Alternatively 0.5 μl of the cleaned sample was spotted onto a stainless steel target plate with 0.5ul of CHCA matrix (10 mg/ml in 50%ACN, 0.5%TFA in water) and allowed to dry in a fume hood. The plate was loaded into a 4800 MALDI Tof-Tof (AB Sciex) and 500 laser shots were collected to generate the MS spectra. The 15 most intense ions were then selected for MS/MS fragmentation. Mobile phases (Thermo-Fisher Rockford, IL) consisted of H2O with 0.1% formic acid (A) and 89.95% acetonitrile, 9.95% H2O, 0.1% formic acid (B). Peptides were chromatographically separated at a flow rate of 800 nl/min using a gradient of 5-45% over 10 minutes followed by a column wash at 95% B mobile phase for 3 minutes. An Amazon Speed ion trap equipped with a Captive Spray source (Bruker, Fremont, CA) was used for MS/MS analysis of the eluting peptides, and the data was searched using Mascot Server version 2.2 (Matrix Science). Proteins not identified by this procedure are listed as “unidentified”, and may correspond to fragments released due to activation of specific proteases (e.g., in opsonization).
The 4800 MALDI Tof-Tof was calibrated using Proteo Mass calibration peptides from Sigma. The peptides range in mass from 750-3500 Daltons allowing the software to generate a 5 point curve suitable for calibrating the masses from 500-4000m/z to well under 100ppm (typically <50ppm). For additional precision 0.1ug Glu-Fibrinopeptide, 1570.688m/z, was added to 1ml of matrix as an internal standard allowing for mass accuracies of <10ppm.
Results
It is well-recognized that the adsorption of serum proteins to delivery vehicles can have dramatic, negative effects on particle characteristics and delivery [7-12]. Previous work with both liposomes and lipoplexes has demonstrated the ability of cholesterol to enhance resistance to serum-induced aggregation and dissociation [17,24,25,32-34]. Moreover, lipoplexes formulated with progressively higher cholesterol contents are better able to withstand serum-induced aggregation and maintain transfection rates in full-strength serum [8]. In contrast to the detrimental effects that are commonly observed with lipoplexes after serum exposure [10-12], we have recently reported that serum significantly enhances transfection by lipoplexes possessing very high cholesterol contents (80 mol%) [26]. It follows that proteins recruited to the surface of these lipoplexes may contribute to transfection, and that the cholesterol content may affect the type of proteins that are recruited to the surface as well as the quantity of proteins adsorbed on the particle surface.
Previous studies with other particulate delivery systems have reported dynamic changes in the protein corona, and thus our initial experiment was designed to monitor the levels of individual proteins adsorbed over time. Figure 1 depicts the levels of individual proteins associated with lipoplexes (20% DOTAP/80% cholesterol; +/− = 4) at different times ranging from 5 min to 6 hr. A total of five proteins were adsorbed after a 5-minute incubation, and these same five proteins were observed throughout the six hour incubation (Fig. 1). Furthermore, we did not observe any other proteins adsorbing to this lipoplex over the duration of the experiment. Three of the five proteins initially adsorbed at higher levels than that observed at later timepoints, and levels of all adsorbed proteins were virtually constant from 30 min to 6 hr. Although detailed quantification of each protein was not performed, all samples were collected on the same day, and subsequent extractions and analyses were performed at the same time under identical conditions. Therefore, although the different signal intensities do not accurately reflect the relative levels of different proteins, the approximately constant signal intensity for each individual protein indicates that the level of each specific protein comprising the corona is stable after 30 min. This experiment was repeated and virtually identical results were obtained.
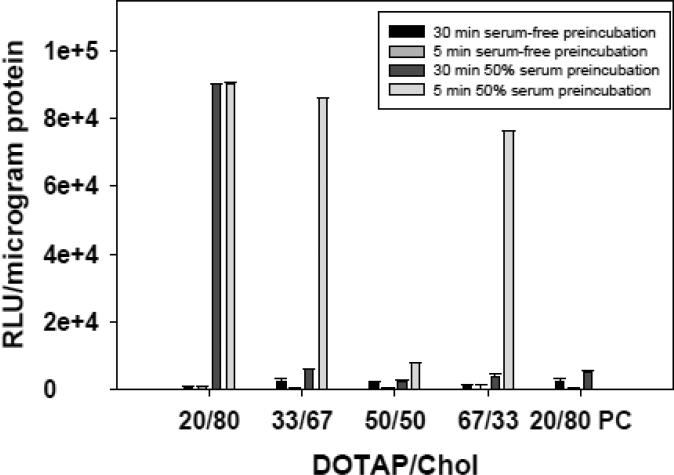
Considering the different levels of proteins adsorbed at 5 min as compared to 30 min, we performed an experiment to determine if these changes in the protein corona affect transfection. Because transfection experiments involve incubating lipoplexes with cells for hours, we designed our experiments such that lipoplexes exposed to serum for different times (5 vs. 30 min) were used to transfect cells in serum-free media. Equivalent incubations were also performed in serum-free media for comparison. The results in Figure 2 show that the time of serum exposure had no effect on transfection by the 20% DOTAP/80% Chol formulation, suggesting that the increased adsorption observed initially (depicted in Fig. 1) had minimal effects on transfection with this formulation. Furthermore, the effects of serum pre-incubation are dramatic and result in significant enhancements in transfection rates (Fig. 2). In contrast, serum pre-incubation time had striking effects on some lipoplexes (+/− = 4) formulated at lower cholesterol contents which exhibited high transfection rates after a 5-min serum incubation, but displayed low rates (comparable to no serum exposure) after a 30-min pre-incubation. Surprisingly, serum had relatively small effects on the widely-used 50% DOTAP/50% Chol formulation as well as the 20% DOTAP/80% PC formulation, both of which exhibited very low transfection rates under all conditions (Fig. 2). A control experiment demonstrated that addition of serum to the cell culture media at levels comparable to that adsorbed onto the surface of serum-incubated lipoplexes (simulating release of the corona in serum free media after a 30-min pre-incubation) had no effect on transfection (data not shown).
Figure 2.
The effect of serum exposure on lipoplexes possessing different cholesterol contents. Lipoplexes (+/− = 4) were incubated in either serum-free media or 50% serum for 5 min and 30 min prior to incubating with cells in serum-free media. After a 4-hour incubation, the ability of lipoplexes to transfect MCF-7 cells in culture was assessed at 48 h. A formulation substituting phosphatidylcholine for cholesterol (20/80 PC) is shown for comparison. Each bar represents the mean transfection of 8 individual wells, and the error bars represent the standard errors.
In an attempt to gain insight into the effects of cholesterol content on serum protein adsorption, the proteins associated with the lipoplex formulations depicted in Fig. 2 were characterized by MALDI/MS (Table I). The findings reveal an astonishing effect of cholesterol content on both the total amount of protein and the number of specific proteins adsorbed to lipoplexes. More specifically, a wide variety of serum proteins were associated with lipoplexes possessing 33% and 50% cholesterol, whereas protein adsorption to lipoplexes formulated with higher cholesterol contents (67%, 80%) was limited to only a small number of proteins. This “selectivity” of protein binding is reflected in the much larger amounts of total protein bound to lipoplexes possessing 33% and 50% cholesterol (Table I). However, the formulation lacking cholesterol (20% DOTAP/80% PC) adsorbed much larger amounts of protein, but displayed relatively selective binding of individual proteins similar to that observed with lipoplexes formulated at high cholesterol contents. Despite profound differences among the formulations with regard to the quantities of protein bound and the recruitment of specific proteins, particle sizes were comparable for all formulations at this charge ratio (170-220 nm) both before and after serum exposure (Table I).
Table I.
The Effect of Cholesterol Content on the Adsorption of Individual Serum Proteins. Lipoplexes were formulated at +/− = 4, and proteins associated with each formulation were identified by MALDI/MS. The total quantity of adsorbed protein on each formulation is also shown, as is the diameter (nm) of each lipoplex before and after incubation in fetal calf serum (FCS).
| Charge ratio = 4 | |||||
|---|---|---|---|---|---|
| DOTAP/Chol | 20:80 | 33:67 | 50:50 | 67:33 | 20:80 PC |
| Unidentified (3,530 Da) | x | x | x | x | - |
| Unidentified (8,000 Da) | - | - | x | x | - |
| Unidentified (13,300 Da) | - | - | - | x | - |
| Unidentified (16,500 Da) | - | - | x | - | - |
| Cationic trypsin (25,768 Da) | x | - | x | x | - |
| Immunoglobin gamma-2 C region (35,878 Da) | x | - | x | - | x |
| Immunoglobin gamma-1 C region (36,083 Da) | x | - | x | x | - |
| Alpha-2-HS-glycoprotein (38,394 Da) | - | - | x | - | - |
| Albumin (69,248 Da) | x | - | x | x | x |
| Krev interaction trapped protein 1 (84,237 Da) | - | - | x | x | - |
| Collagen Alpha-1 Chain (93,594 Da) | - | - | - | x | - |
| mg protein/g lipid | 76.4 | 101.5 | 181.1 | 172.7 | 280.9 |
| diameter before FCS | 209.1 | 190.2 | 204.6 | 185.3 | 179.5 |
| diameter after FCS | 217.3 | 181.4 | 219.6 | 173.1 | 184.2 |
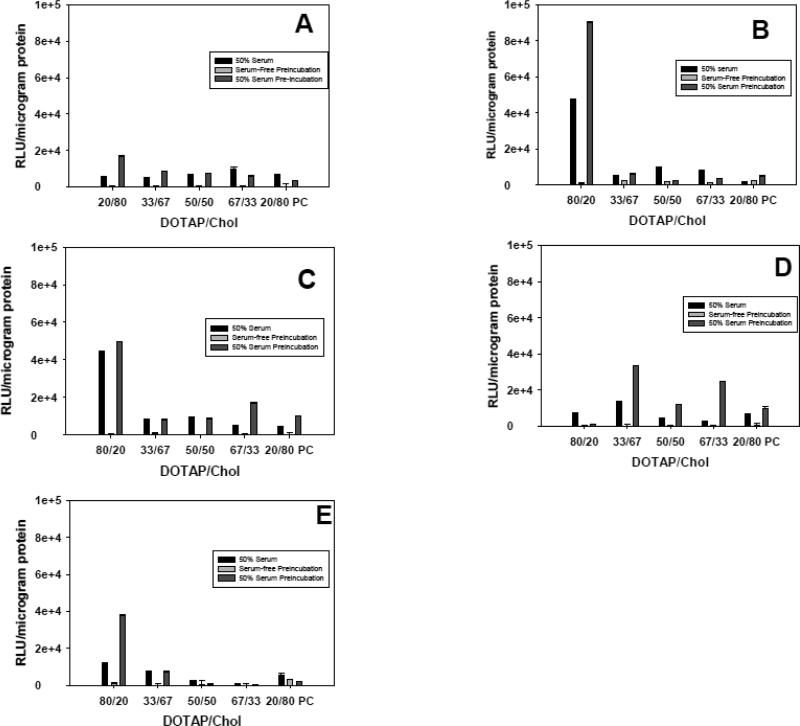
In addition to assessing the effects of cholesterol content, we also monitored the effects of charge ratio ranging from 0.25 to 8 on transfection. For these experiments, we assessed transfection in 50% serum, and in serum-free media after a 30-min pre-incubation in either 50% serum or serum-free media. The results indicate that charge ratio has a marked effect on lipoplexes possessing the highest cholesterol content (80 mol%), but a minimal effect on transfection with the other formulations (Fig. 3). Surprisingly, transfection rates of the 20% DOTAP/80% cholesterol formulation were progressively enhanced as charge ratio increased from 1 to 2 to 4, but was sharply reduced at the highest charge ratio (+/− = 8). Therefore, the enhanced transfection observed as charge ratio is increased from 1 to 4 cannot be explained by greater interactions with the cell surface due to increased cationic charge. Similarly, it is reasonable to assume that increasing charge ratio might enhance protein adsorption due to greater electrostatic interactions with serum components, however this was not observed (Table II). In fact, increasing the charge ratio from 4 to 8 caused a sharp reduction in both the amount of protein adsorbed as well as the numbers of individual proteins associated with every lipoplex (compare Tables I + II).
Figure 3.
The effect of lipoplex cholesterol content and charge ratio on transfection. Lipoplexes were prepared at different cholesterol contents, and used to transfect MCF-7 cells in 50% serum or in serum-free media after pre-incubation in 50% serum or serum-free media. Experiments were performed with lipoplexes formulated at different +/− charge ratios: 8 (A), 4 (B), 2 (C), 1 (D), 0.25 (E). A formulation substituting phosphatidylcholine for cholesterol (20/80 PC) is shown for comparison. Each bar represents the mean transfection of 8 individual wells, and the error bars represent the standard errors.
Table II.
The Effect of Cholesterol Content on the Adsorption of Individual Serum Proteins. Lipoplexes were formulated at +/− = 8, and proteins associated with each formulation were identified by MALDI/MS. The total quantity of adsorbed protein on each formulation is also shown, as is the diameter (nm) of each lipoplexe before and after incubation in fetal calf serum (FCS).
| charge ratio = 8 | |||||
|---|---|---|---|---|---|
| DOTAP/Chol | 20:80 | 33:67 | 50:50 | 67:33 | 20:80 PC |
| Unidentified (3,530 Da) | - | - | - | x | - |
| Unidentified (4,100 Da) | - | - | - | x | - |
| Unidentified (8,000 Da) | - | - | x | - | - |
| Unidentified (13,300 Da) | - | - | x | x | - |
| Unidentified (16,500 Da) | - | x | x | x | - |
| Cationic trypsin (25,768 Da) | - | - | - | - | x |
| Immunoglobin gamma-2 C region (35,878 Da) | - | - | - | x | - |
| Immunoglobin gamma-1 C region (36,083 Da) | - | - | x | x | - |
| Alpha-2-HS-glycoprotein (38,394 Da) | - | - | x | x | - |
| Albumin (69,248 Da) | x | x | x | x | x |
| mg protein/g lipid | 69.1 | 75.3 | 164.0 | 116.4 | 262.2 |
| diameter before FCS | 171.9 | 111.6 | 161.7 | 179.8 | 116.4 |
| diameter after FCS | 140.9 | 127.1 | 175.3 | 184 | 109.4 |
Consistent with the reduction in protein binding at higher charge ratios, an increase in protein binding was generally observed when lipoplexes were formulated at charge ratios of 1 and 2 regardless of cholesterol content (Tables III and IV, respectively), and dramatic increases in size observed with neutral lipoplexes (Table IV). Negatively-charged lipoplexes adsorbed relatively low levels of protein regardless of cholesterol content, similar to that observed at high charge ratios (Table V). Taken together, the results in Tables I-V demonstrate that cholesterol content and charge ratio have predominant effects on both the amount of protein bound and the number of proteins associated with each lipoplex. Surprisingly, the addition of serum caused relatively minor degrees of aggregation, and diameters of all lipoplexes remained below 300 nm at non-neutral charge ratios (with the exception of the 50% DOTAP/50% cholesterol formulation at +/− = 2).
Table III.
The Effect of Cholesterol Content on the Adsorption of Individual Serum Proteins. Lipoplexes were formulated at +/− = 2, and proteins associated with each formulation were identified by MALDI/MS. The total quantity of adsorbed protein on each formulation is also shown, as is the diameter (nm) of each lipoplex before and after incubation in fetal calf serum (FCS).
| charge ratio = 2 | |||||
|---|---|---|---|---|---|
| DOTAP/Chol | 20:80 | 33:67 | 50:50 | 67:33 | 20:80 PC |
| Unidentified (2,210 Da) | - | - | x | x | - |
| Unidentified (3,530 Da) | - | - | - | x | - |
| Unidentified (4,100 Da) | - | - | x | - | - |
| Unidentified (8,000 Da) | x | x | - | - | - |
| Unidentified (13,300 Da) | x | x | - | - | x |
| Unidentified (16,500 Da) | x | x | x | x | x |
| Cationic trypsin (25,768 Da) | x | x | x | x | - |
| Immunoglobin gamma-2 C region (35,878 Da) | x | x | x | x | x |
| Immunoglobin gamma-1 C region (36,083 Da) | - | - | x | x | - |
| Albumin (69,248 Da) | x | x | - | - | - |
| mg protein/g lipid | 127.3 | 166.2 | 215.6 | 187.6 | 288.7 |
| diameter before FCS | 287.5 | 256.9 | 354.8 | 259.2 | 197.4 |
| diameter after FCS | 296.1 | 238.4 | 301.7 | 275 | 203.6 |
Table IV.
The Effect of Cholesterol Content on the Adsorption of Individual Serum Proteins. Lipoplexes were formulated at +/− = 1, and proteins associated with each formulation were identified by MALDI/MS. The total quantity of adsorbed protein on each formulation is also shown, as is the diameter (nm) of each lipoplexe before and after incubation in fetal calf serum (FCS).
| charge ratio = 1 | |||||
|---|---|---|---|---|---|
| DOTAP/Chol | 20:80 | 33:67 | 50:50 | 67:33 | 20:80 PC |
| Unidentified (2,210 Da) | - | - | x | x | - |
| Unidentified (3,530 Da) | - | - | - | x | - |
| Unidentified (4,100 Da) | x | - | x | - | - |
| Unidentified (8,000 Da) | - | - | x | x | - |
| Unidentified (12,600 Da) | x | - | x | x | - |
| Unidentified (13,300 Da) | - | x | - | - | x |
| Unidentified (16,500 Da) | x | x | x | x | x |
| Cationic trypsin (25,768 Da) | - | x | x | x | - |
| Immunoglobin gamma-2 C region (35,878 Da) | x | x | x | x | x |
| Immunoglobin gamma-1 C region (36,083 Da) | x | - | x | x | - |
| Albumin (69,248 Da) | - | x | - | - | - |
| Krev interaction trapped protein 1 (84,237 Da) | - | - | x | x | - |
| Collagen Alpha-1 Chain (93,594 Da) | - | - | x | x | - |
| mg protein/g lipid | 65.5 | 212.7 | 294.5 | 253.1 | 358.2 |
| diameter before FCS | 814.1 | 837.9 | 371.4 | 457.1 | 272.7 |
| diameter after FCS | 827.5 | 871.5 | 380.2 | 474.2 | 283.9 |
Table V.
The Effect of Cholesterol Content on the Adsorption of Individual Serum Proteins. Lipoplexes were formulated at +/− = 0.25, and proteins associated with each formulation were identified by MALDI/MS. The total quantity of adsorbed protein on each formulation is also shown, as is the diameter (nm) of each lipoplexe before and after incubation in fetal calf serum (FCS).
| charge ratio = 0.25 | |||||
|---|---|---|---|---|---|
| DOTAP/Chol | 20:80 | 33:67 | 50:50 | 67:33 | 20:80 PC |
| Unidentified (8,000 Da) | - | - | x | x | - |
| Unidentified (12,600 Da) | x | - | x | x | - |
| Unidentified (13,300 Da) | - | - | - | - | x |
| Unidentified (16,500 Da) | - | x | x | x | x |
| Cationic trypsin (25,768 Da) | - | - | x | x | - |
| Immunoglobin gamma-2 C region (35,878 Da) | - | - | x | x | x |
| Immunoglobin gamma-1 C region (36,083 Da) | x | - | - | x | - |
| Albumin (69,248 Da) | - | x | x | x | x |
| mg protein/g lipid | 72.7 | 80.4 | 142.9 | 154.9 | 200.0 |
| diameter before FCS | 272.7 | 283.8 | 266 | 249.7 | 243.1 |
| diameter after FCS | 217.4 | 261.4 | 268.7 | 283.8 | 267.5 |
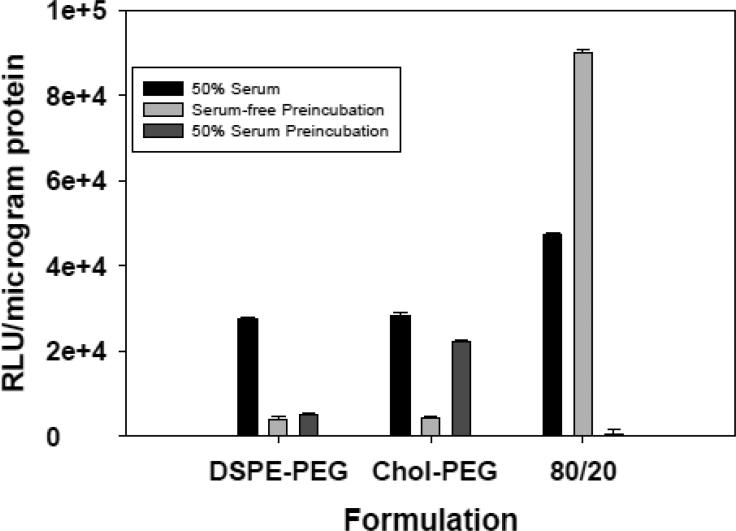
In addition to the changes in associated proteins and particle size noted above, it is clear that the 20% DOTAP/80% cholesterol formulation at a charge ratio of 4 exhibits significantly higher transfection rates than the other lipoplexes (Fig. 3). Therefore, we chose this formulation to assess the effects of PEGylation on both transfection and protein binding. As shown in Figure 4, PEGylation of this formulation with either 2% DSPE-PEG or Chol-PEG diminished transfection as compared to the non-PEGylated formulation. Characterization of protein adsorption revealed that the total amount of protein bound was increased by PEGylation, and a greater number of proteins were associated with lipoplexes incorporating DSPE-PEG (Table VI). Taken together, the overall effect of PEGylation was to decrease transfection and enhance protein binding, but differences were observed between DSPE-PEG and Chol-PEG. While protein binding and transfection will likely vary with PEG content, only 2% PEG was investigated; a concentration that is commonly employed in both cell culture and in vivo.
Figure 4.
The effect of PEGylation on transfection. PEG-lipid conjugates were incorporated into lipoplexes (20% DOTAP/80% Chol; +/− = 4) to achieve 20% DOTAP/78% Chol/2% PEG conjugate. These preparations were used to transfect MCF-7 cells in 50% serum or in serum-free media after pre-incubation in 50% serum or serum-free media. Transfection with the non-PEGylated lipoplex is shown for comparison. Each bar represents the mean transfection of 8 individual wells, and the error bars represent the standard errors.
Table VI.
The Adsorption of Individual Serum Proteins to PEGylated Lipoplexes. Lipoplexes were formulated at +/− = 4 and 20% DOTAP/80% Chol with either 2% DSPE-PEG or 2% Chol-PEG, and proteins associated with each formulation were identified by MALDI/MS. The data for the unPEGylated formulation (20:80) from Table I are included for comparison. The total quantity of adsorbed protein on each formulation is also shown, as is the diameter (nm) of each lipoplex before and after incubation in fetal calf serum (FCS).
| DSPE-PEG (2%) | Chol-PEG (2%) | 20:80 | |
|---|---|---|---|
| Unidentified (3,530 Da) | x | - | x |
| Unidentified (4,100 Da) | - | x | - |
| Unidentified (14,300 Da) | x | x | - |
| Unidentified (16,500 Da) | x | - | - |
| Cationic Trypsin (25,768 Da) | - | - | x |
| Immunoglobin gamma-2 C region (35,878 Da) | x | - | x |
| Alpha-2-HS-glycoprotein (38,394 Da) | x | - | x |
| Albumin (69,248 Da) | x | x | x |
| mg protein/g lipid | 175.8 | 109.8 | 76.4 |
| diameter before FCS | 216 | 212.3 | 209.1 |
| diameter after FCS | 249.3 | 227.4 | 217.3 |
Discussion
The ability to resist the detrimental effects of serum (e.g., aggregation, dissociation) is clearly advantageous for delivery vehicles designed for intravenous administration. Numerous studies have demonstrated that the concentration of serum used for in vitro experiments has a dramatic effect on vector integrity and performance in cell culture, i.e., the media conditions utilized for maintaining cells in culture (10% serum) do not provide adequate serum levels to mimic vector response to intravenous administration [8,11,35]. Accordingly, reports have advocated for the use of high serum levels (≥ 50%) in experiments designed to assess the serum stability of synthetic delivery systems [5,8,35]. Recent work from our laboratory has demonstrated very similar outcomes between cell culture and animal experiments in terms of optimal cholesterol content, ligand concentration, and ligand-mediated transfection enhancement when high serum levels are utilized to characterize vector performance in cell culture [36,37]. In contrast to the detrimental effects of serum that are commonly observed, some studies have suggested that the mechanism of cell uptake can be altered by serum proteins, and that adsorbed proteins can enhance transfection under certain circumstances [22,26,38,39]. However, it is generally thought that adsorption of serum proteins in vivo (opsonization) hastens clearance and reduces therapeutic efficacy [6,7,17,18].
The goal of this study was to characterize the effects of formulation variables on serum protein binding and transfection. Our previous work has documented the beneficial effects of incorporating cholesterol into lipoplexes, and thus we endeavored to characterize the effect of cholesterol content on serum protein adsorption and transfection. Although the ability of cholesterol to improve the serum stability of liposomes has been known for decades [32,33], recent studies with lipoplexes have reported that cholesterol can also affect intracellular trafficking behavior that results in improved transfection [37,40]. In investigating the effect of cholesterol content on protein binding, it was important to first characterize the dynamic changes in the protein corona that occur upon serum exposure. Our results indicate that the proteins that associate with the 20% DOTAP/80% cholesterol formulation are consistent over a six hour period, although the relative levels of the different proteins fluctuate over the first 30 min (Fig. 1). This finding is consistent with our earlier studies utilizing FRET to monitor the complexation of DNA with this same lipoplex formulation which concluded that interactions stabilized after 30 min [8]. Our results with cultured cells indicate that these transient changes in the protein corona do not affect transfection with this formulation, although some lipoplexes possessing reduced cholesterol contents had variable transfection rates over the first 30 min (Fig. 2). However, with these formulations, transfection after a 30 min pre-incubation in serum was comparable to that observed when lipoplexes remained in 50% serum for 4 hours, suggesting that subsequent alterations in the protein corona have minimal effects on transfection (Figs. 2,3B). These findings are consistent with studies showing rapid and non-exchangeable binding to liposomes [32], but differ from some studies that have described an “evolution” of the protein corona wherein adsorption is dynamic over four to six hours [13,15]. While it has long been recognized that lipid composition can affect protein adsorption, further studies would be needed to determine the degree to which the kinetics of protein adsorption are also affected by lipid composition and serum source (e.g., mouse, human). It should also be noted that experimental conditions (e.g., pH, ionic strength) can also affect protein binding.
Characterization of the proteins associated with lipoplexes reveals an astonishing effect of cholesterol content on both the total amount of protein adsorbed as well as the individual proteins in the corona (Table I). Perhaps the most striking result is the dramatic reduction in protein binding as cholesterol content is increased from 50 to 67 mol%. In addition to the dramatic reduction in the amount of protein bound to lipoplexes possessing high cholesterol contents, there is a reduction in the number of proteins associated with these lipoplexes. The ability of high cholesterol contents to reduce protein binding is consistent with earlier studies concluding that the presence of up to 80 mol% cholesterol in membranes from lens cells serves to prevent protein-membrane interactions involved in cataract formation [41,42]. The precise mechanism by which cholesterol exerts this effect is unclear, but the increased membrane rigidity imparted by cholesterol would be expected to hinder protein insertion into the bilayer.
Similar to the effect of cholesterol content, our results show that charge ratio has a dramatic effect on both the amount of protein adsorbed as well as the individual proteins associated with each formulation (Tables I-V). Although previous studies have concluded that the amount of protein adsorbed determines transfection efficiency [17,24], we do not observe a consistent correlation between protein bound and transfection (compare Fig. 4 and Tables I-V). In addition, we do not observe any individual proteins that are only present under conditions that result in high transfection, consistent with our recent studies [26]. Previous reports have linked albumin adsorption with high transfection [20-23], and some studies have suggested the presence of “dysopsonins” that would prevent interactions with detrimental proteins [43]. An alternative explanation might be that it is the precise lipid composition which affects interactions with cell membranes that facilitate transfection, but the dramatic drop in transfection at different charge ratios despite identical lipid composition (charge ratio is altered by DNA content) argues against this potential explanation (Fig. 4).
One strategy that is commonly used to combat the detrimental effects of serum protein binding is to incorporate PEGylated components into delivery vehicles [20,27,28,43]. Many studies have documented the ability of PEG to enhance circulation times, and it is typically argued that PEG decreases clearance by providing a steric barrier that resists protein binding [44,45]. Indeed, the use of PEGylation to stabilize particulate systems has become so ubiquitous that little attention is paid to studies documenting the negative effects of PEGylation on protein binding, delivery, and circulation times [5,36,46-48]. In our hands, the commonly-used DSPE-PEG dramatically reduces transfection rates and increases both the amount of protein bound as well as the number of individual proteins associated with 20%/80% lipoplexes (Fig. 4, Table VI). The fact that less detrimental effects were observed with the cholesterol-PEG conjugate indicates that the creation of an anionic lipid via conjugation of PEG to DSPE may be problematic, consistent with previous studies implicating the anionic oxygen moiety in complement activation [49]. Recent studies have concluded that the negative effects of PEG on intracellular delivery are due to PEG hindering fusion with internal membranes, thereby reducing endosomal escape [50]. It follows that the Chol-PEG conjugate may be more amenable to dissociation/degradation within the endosome.
In conclusion, our results indicate that increased cholesterol contents serve to attenuate the amount of protein that binds to lipoplexes, and the number of proteins that adsorb was also reduced in formulations containing ≥ 67 mol% cholesterol. The effect of charge ratio was surprising in that higher cationic charge ratios reduced protein adsorption to lipoplexes regardless of cholesterol content, and negatively charged lipoplexes also exhibited reduced protein adsorption. In addition to the quantity of protein bound, cholesterol content and charge ratio had significant effects on the adsorption of individual proteins, demonstrating that the effect of these common formulation variables is not straightforward. Therefore, common optimization experiments (i.e., altering charge ratio and/or helper lipid content) should be interpreted with caution in terms of understanding their effects on delivery mechanisms. Indeed, the effect of higher charge ratios is often interpreted simply as enhancing cationic interactions with anionic cell membranes, with little consideration of how such changes might impact the protein corona. Although we did not observe a specific protein that adsorbed only to formulations exhibiting high transfection, protein adsorption has been shown to affect uptake and clearance, and thus the ability of lipoplexes to preferentially associate with specific proteins deserves further attention. The effects of serum protein binding on lipid organization must also be considered separately from the specific proteins adsorbed. In this context, it is important to note that our recent study has documented that serum protein binding can cause the formation of cholesterol domains that are known to enhance transfection [26].
Supplementary Material
Acknowledgements
This work was supported by NIH/NIGMS grant #1 RO1GM093287-01A1. This study also utilized the University of Colorado School of Pharmacy Mass Spectrometry Core Facility and the Medicinal Chemistry Core facility via Colorado CTSA grant 5UL1RR025780 from NCRR/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, Bousso P, Deist FL, Fischer A. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 2.Hyde SC, Southern KW, Gileadi U, Fitzjohn EM, Mofford KA, Waddell BE, Gooi HC, Goddard CA, Hannavy K, Smyth SE, Egan JJ, Sorgi FL, Huang L, Cuthbert AW, Evans MJ, Colledge WH, Higgins CF, Webb AK, Gill DR. Repeat administration of DNA/liposomes to the nasal epithelium of patients with cystic fibrosis. Gene Ther. 2000;7:1156–1165. doi: 10.1038/sj.gt.3301212. [DOI] [PubMed] [Google Scholar]
- 3.Nabel GJ, Nabel EG, Yang ZY, Fox BA, Plautz GE, Gao X, Huang L, Shu S, Gordon D, Chang AE. Direct gene transfer with DNA-liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans. Proc Natl Acad Sci U S A. 1993;90:11307–11311. doi: 10.1073/pnas.90.23.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Restifo NP, Ying H, Hwang L, Leitner WW. The promise of nucleic acid vaccines. Gene Ther. 2000;7:89–92. doi: 10.1038/sj.gt.3301117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu L, Anchordoquy TJ. Drug Delivery Trends in Clinical Trials and Translational Medicine: challenges and opportunities in the delivery of nucleic acid-based therapeutics. J Pharm Sci. 2011;100:38–52. doi: 10.1002/jps.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moghimi SM, Patel HM. Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system – The concept of tissue specificity. Adv Drug Del Rev. 1998;32:45–60. doi: 10.1016/s0169-409x(97)00131-2. [DOI] [PubMed] [Google Scholar]
- 7.Moghimi SM, Patel HM. Differential properties of organ-specific serum opsonins for liver and spleen macrophages. Biochim. Biophys. Acta. 1989;984:379–383. doi: 10.1016/0005-2736(89)90306-4. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Anchordoquy TJ. The role of lipid charge density in the serum stability of cationic lipid/DNA complexes. Biochim Biophys Acta Biomembranes. 2004;1663:143–157. doi: 10.1016/j.bbamem.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Zelphati O, Uyechi LS, Barron LG, Szoka FC., Jr. Effect of serum components on the physico-chemical properties of cationic lipid/oligonucleotide complexes and on their interactions with cells. Biochim Biophys Acta. 1998;1390:119–133. doi: 10.1016/s0005-2760(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 10.Yang JP, Huang L. Overcoming the inhibitory effect of serum on lipofection by increasing the charge ratio of cationic liposome to DNA. Gene Ther. 1997;4:950–960. doi: 10.1038/sj.gt.3300485. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Tseng WC, Stolz DB, Wu SP, Watkins SC, Huang L. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection. Gene Ther. 1999;6:585–594. doi: 10.1038/sj.gt.3300865. [DOI] [PubMed] [Google Scholar]
- 12.Tandia B-M, Lonez C, Vandenbranden M, Ruysschaert J-M, Elouahabi A. Lipid mixing between lipoplexes and plasma lipoproteins is a major barrier for intravenous transfection mediated by cationic lipids. J Biol Chem. 2005;280:12255–12261. doi: 10.1074/jbc.M414517200. [DOI] [PubMed] [Google Scholar]
- 13.Nagayama S, Ogawara K-I, Fukuoka Y, Higaki K, Kimura T. Time-dependent changes in opsonin amount associated on nanoparticles alter their hepatic uptake characteristics. Int J Pharm. 2007;342:215–221. doi: 10.1016/j.ijpharm.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Alexis F, Pridgen E, Molnar LK, Farokhazad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dell'Orco D, Lundqvist M, Oslakovic C, Cedervall T, Linse S. Modeling the time evolution of the nanoparticle-protein corona in a body fluid. PLoS One. 2010;5:e10949. doi: 10.1371/journal.pone.0010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundqvist M, Stigler J, Cedervall T, Berggard T, Flanagan MB, Lynch I, Elia G, Dawson K. The evolution of the protein corona around nanoparticles: a test study. ACS NANO. 2011;5:7503–7509. doi: 10.1021/nn202458g. [DOI] [PubMed] [Google Scholar]
- 17.Semple SC, Chonn A, Cullis PR. Interactions of liposomes and lipid-based carrier systems with blood proteins: relation to clearance behavior in vivo. Adv Drug Del Rev. 1998;32:3–17. doi: 10.1016/s0169-409x(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 18.Scherphof GL, Kamps JAAM. Receptor versus non-receptor mediated clearance of liposomes. Adv Drug Del Rev. 1998;32:81–97. doi: 10.1016/s0169-409x(97)00133-6. [DOI] [PubMed] [Google Scholar]
- 19.Furumoto K, Ogawara K, Yoshida M, Takakura Y, Hashida M, Higaki K, Kimura T. Biliary excretion of polystyrene microspheres depends on the type of receptor-mediated uptake in rat liver. Biochim Biophys Acta. 2001;1526:221–226. doi: 10.1016/s0304-4165(01)00132-5. [DOI] [PubMed] [Google Scholar]
- 20.Furumoto K, Yokoe J-I, Ogawara K, Amano S, Takaguchi M, Higaki K, Kai T, Kimura T. Effect of coupling albumin onto surface of PEG liposome on its in vivo disposition. Int J Pharm. 2007;329:110–116. doi: 10.1016/j.ijpharm.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Ogawara K, Furumoto K, Nagayama S, Minato K, Higaki K, Kai T, Kimura T. Pre-coating with serum albumin reduces receptor-mediated hepatic disposition of polystyrene nanospheres: implications for rational design of nanoparticles. J Cont Rel. 2004;100:451–455. doi: 10.1016/j.jconrel.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Simoes S, Slepushkin V, Pires P, Gaspar R, Pedroso de Lima MC, Duzgunes N. Human serum albumin enhaqnces DNA transfection by lipoplexes and confers resistance to inhibition by serum. Biochim Biophys Acta. 2000;1463:459–469. doi: 10.1016/s0005-2736(99)00238-2. [DOI] [PubMed] [Google Scholar]
- 23.Kummitha CM, Malamas AS, Lu Z-R. Albumin pre-coating enhances intracellular siRNA delivery of multifunctional amphiphile/siRNA nanoparticles. Int J. Nanomed. 2012;7:5205–5214. doi: 10.2147/IJN.S34288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semple SC, Chonn A, Cullis PR. Influence of cholesterol on the association of plasma proteins with liposomes. Biochem. 1996;35:2521–2525. doi: 10.1021/bi950414i. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Anchordoquy TJ. Cholesterol domains in cationic lipid/DNA complexes improve transfection. Biochim Biophys Acta Biomembranes. 2008;1778:2177–81. doi: 10.1016/j.bbamem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Betker JL, Kullberg M, Gomez J, Anchordoquy TJ. Cholesterol domains enhance transfection. Ther Del. 2013;4:453–462. doi: 10.4155/tde.13.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao XB, Muthusamy N, Byrd JC, Lee RJ. Cholesterol as a bilayer anchor for PEGylation and targeting ligand in folate-receptor-targeted liposomes. J Pharm Sci. 2007;96:2424–2435. doi: 10.1002/jps.20885. [DOI] [PubMed] [Google Scholar]
- 28.Xu L, Wempe MF, Anchordoquy TJ. The effect of cholesterol domains on PEGylated liposomal gene delivery in vitro. Ther Del. 2011;2:451–60. doi: 10.4155/tde.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tandia B-M, Vandenbranden M, Wattiez R, et al. Identification of human plasma proteins that bind to cationic lipid/DNA complex and analysis of their effects on transfection efficiency: implications for intravenous gene transfer. Mol Ther. 2003;8:264–273. doi: 10.1016/s1525-0016(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 30.Sidransky D, Irizarry R, Califano JA, et al. Serum protein MALDI profiling to distinguish upper aerodigestive tract cancer patients from control subjects. J Natl Cancer Inst. 2003;95:1711–1717. doi: 10.1093/jnci/djg099. [DOI] [PubMed] [Google Scholar]
- 31.Park Z-Y, Russell DH. Identification of individual proteins in complex protein mixtures by high-resolution, high-mass accuracy MALDI TOF-mass spectrometry analysis of in-solution thermal denaturation/enzymatic digestion. Anal Chemistry. 2001;73:2558–2564. doi: 10.1021/ac001488p. [DOI] [PubMed] [Google Scholar]
- 32.Juliano RL, Lin G. The interaction of plasma proteins with liposomes: protein binding and effects on the clotting and complement systems. In: Tom BH, Six HR, editors. Liposomes and Immunobiology. Elsevier North Holland, Inc.; 1980. [Google Scholar]
- 33.Senior J, Gregoriadis G. Stability of small unilamellar liposomes in serum and clearance from the circulation: the effect of the phospholipid and cholesterol components. Life Sci. 1982;30:2123–2136. doi: 10.1016/0024-3205(82)90455-6. [DOI] [PubMed] [Google Scholar]
- 34.Crook K, Stevenson BJ, Dubouchet M, Porteous DJ. Inclusion of cholesterol in DOTAP transfection complexes increases the delivery of DNA to cells in vitro in the presence of serum. Gene Ther. 1998;5:137–143. doi: 10.1038/sj.gt.3300554. [DOI] [PubMed] [Google Scholar]
- 35.Templeton NS. Cationic liposomes as in vivo delivery vehicles. Curr Med Chem. 2003;10:1279–1287. doi: 10.2174/0929867033457421. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Betker JL, Yin H, Anchordoquy TJ. Ligands located within a cholesterol domain enhance gene delivery to the target tissue. J Cont Release. 2012;160:57–63. doi: 10.1016/j.jconrel.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu L L, Anchordoquy TJ. Effect of cholesterol nanodomains on the targeting of lipid-based gene delivery in cultured cells. Mol Pharm. 2010;7:1311–17. doi: 10.1021/mp100097b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caracciolo G, Callipo L, De Sanctis SC, Cavaliere C, Pozzi D, Lagana A. Surface adsorption of protein corona controls the cell internalization mechanism of DC-Chol-DOPE/DNA lipoplexes in serum. Biochim Biophys Acta. 2009;1798:536–543. doi: 10.1016/j.bbamem.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Akinc A, Querbes W, De S, Qin J, et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18:1357–64. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pozzi D, Marchini C, Cardarelli F, et al. Transfection efficiency boost of cholesterol-containing lipoplexes. Biochim. Biophys. Acta. 2012;1818:2335–2343. doi: 10.1016/j.bbamem.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Tang D, Borchman D, Yappert MC, Cenedella RJ. Influence of cholesterol on the interaction of α-crystallin with phospholipids. Exp Eye Res. 1998;66:559–567. doi: 10.1006/exer.1997.0467. [DOI] [PubMed] [Google Scholar]
- 42.Jacob RF, Cenedella RJ, Mason RP. Direct evidence for immiscible cholesterol domains in human ocular lens fiber cell plasma membranes. J Biol Chem. 1999;274:31613–18. doi: 10.1074/jbc.274.44.31613. [DOI] [PubMed] [Google Scholar]
- 43.Johnstone SA, Masin D, Mayer L, Bally MB. Surface-associated serum proteins inhibit the uptake of phophatidylserine and poly(ethylene glycol) liposomes by mouse macrophages. Biochim Biophys Acta. 2001;1513:25–37. doi: 10.1016/s0005-2736(01)00292-9. [DOI] [PubMed] [Google Scholar]
- 44.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA. 1991;88:11460–64. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dos Santos N, Allen C, Doppen A-M, Anantha M, et al. Influence of poly(ethylene glycol) grafting density and polymer length on liposomes: relating plasma circulation lifetimes to protein binding. Biochim Biophys Acta. 2007;1768:1367–77. doi: 10.1016/j.bbamem.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Bradshaw-Pierce EL, DeLille A, Gustafson DL, Anchordoquy TJ. In vivo comparative study of Lipid/DNA complexes with different in vitro serum stability: effects on biodistribution and tumor accumulation. J Pharm Sci. 2008;97:237–50. doi: 10.1002/jps.21076. [DOI] [PubMed] [Google Scholar]
- 47.Harvie P, Wong FMP, Bally MB. Use of poly(ethylene glycol)-lipid conjugates to regulate the surface attributes and transfection activity of lipid-DNA particles. J Pharm Sci. 2000;89:652–663. doi: 10.1002/(SICI)1520-6017(200005)89:5<652::AID-JPS11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 48.Ishida T, Ichihara M, Wang XY, Yamamoto K, Kimura J, Majima E, Kiwada H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J Cont Rel. 2006;112:15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Moghimi SM, Hamad I, Andresen TL, Jorgensen K, Szebeni J. Methylation of the phosphate oxygen moiety of phospholipid-methoxy(polyethylene glycol) conjugate prevents PEGylated liposome-mediated complement activation and anaphylatoxin production. FASEB J. 2006;20:E2057–67. doi: 10.1096/fj.06-6186fje. [DOI] [PubMed] [Google Scholar]
- 50.Bao Y, Jin Y, Chivukula P, Zhang J, et al. Effect of PEGylation on biodistribution and gene silencing of siRNA/lipid nanoparticle complexes. Pharm Res. 2013;30:342–351. doi: 10.1007/s11095-012-0874-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.