Abstract
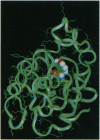
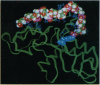
The full sequence of the genome-linked viral protein (VPg) cistron located in the central part of potato virus Y (common strain) genome has been identified. The VPg gene codes for a protein of 188 amino acids, with significant homology to other known potyviral VPg polypeptides. A three-dimensional model structure of VPg is proposed on the basis of similarity of hydrophobic-hydrophilic residue distribution to the sequence of malate dehydrogenase of known crystal structure. The 5' end of the viral RNA can be fitted to interact with the protein through the exposed hydroxyl group of Tyr-64, in agreement with experimental data. The complex favors stereochemically the formation of a phosphodiester bond [5'-(O4-tyrosylphospho)adenylate] typical for representatives of picornavirus-like viruses. The chemical mechanisms of viral RNA binding to VPg are discussed on the basis of the model structure of protein-RNA complex.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambros V., Baltimore D. Protein is linked to the 5' end of poliovirus RNA by a phosphodiester linkage to tyrosine. J Biol Chem. 1978 Aug 10;253(15):5263–5266. [PubMed] [Google Scholar]
- Bairoch A., Boeckmann B. The SWISS-PROT protein sequence data bank, recent developments. Nucleic Acids Res. 1993 Jul 1;21(13):3093–3096. doi: 10.1093/nar/21.13.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Birktoft J. J., Rhodes G., Banaszak L. J. Refined crystal structure of cytoplasmic malate dehydrogenase at 2.5-A resolution. Biochemistry. 1989 Jul 11;28(14):6065–6081. doi: 10.1021/bi00440a051. [DOI] [PubMed] [Google Scholar]
- Bowie J. U., Reidhaar-Olson J. F., Lim W. A., Sauer R. T. Deciphering the message in protein sequences: tolerance to amino acid substitutions. Science. 1990 Mar 16;247(4948):1306–1310. doi: 10.1126/science.2315699. [DOI] [PubMed] [Google Scholar]
- Chelvanayagam G., Roy G., Argos P. Easy adaptation of protein structure to sequence. Protein Eng. 1994 Feb;7(2):173–184. doi: 10.1093/protein/7.2.173. [DOI] [PubMed] [Google Scholar]
- Dandekar T., Argos P. Folding the main chain of small proteins with the genetic algorithm. J Mol Biol. 1994 Feb 25;236(3):844–861. doi: 10.1006/jmbi.1994.1193. [DOI] [PubMed] [Google Scholar]
- Godzik A., Skolnick J. Sequence-structure matching in globular proteins: application to supersecondary and tertiary structure determination. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12098–12102. doi: 10.1073/pnas.89.24.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka M., Yoshida Y., Masaki H., Namba S., Yamashita S., Tsuchizaki T., Uozumi T. Cloning and sequencing of the 3' half of a potato virus Y (O strain) genome encoding the 5k protein, protease, polymerase and coat protein. Nucleic Acids Res. 1992 Jul 11;20(13):3515–3515. doi: 10.1093/nar/20.13.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert M., Böhm G., Jaenicke R. Structural relationships of homologous proteins as a fundamental principle in homology modeling. Proteins. 1993 Oct;17(2):138–151. doi: 10.1002/prot.340170204. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamtekar S., Schiffer J. M., Xiong H., Babik J. M., Hecht M. H. Protein design by binary patterning of polar and nonpolar amino acids. Science. 1993 Dec 10;262(5140):1680–1685. doi: 10.1126/science.8259512. [DOI] [PubMed] [Google Scholar]
- Lim W. A., Sauer R. T. The role of internal packing interactions in determining the structure and stability of a protein. J Mol Biol. 1991 May 20;219(2):359–376. doi: 10.1016/0022-2836(91)90570-v. [DOI] [PubMed] [Google Scholar]
- Lüthy R., Bowie J. U., Eisenberg D. Assessment of protein models with three-dimensional profiles. Nature. 1992 Mar 5;356(6364):83–85. doi: 10.1038/356083a0. [DOI] [PubMed] [Google Scholar]
- Murphy J. F., Rhoads R. E., Hunt A. G., Shaw J. G. The VPg of tobacco etch virus RNA is the 49-kDa proteinase or the N-terminal 24-kDa part of the proteinase. Virology. 1990 Sep;178(1):285–288. doi: 10.1016/0042-6822(90)90405-g. [DOI] [PubMed] [Google Scholar]
- Murphy J. F., Rychlik W., Rhoads R. E., Hunt A. G., Shaw J. G. A tyrosine residue in the small nuclear inclusion protein of tobacco vein mottling virus links the VPg to the viral RNA. J Virol. 1991 Jan;65(1):511–513. doi: 10.1128/jvi.65.1.511-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas O., Laliberté J. F. The complete nucleotide sequence of turnip mosaic potyvirus RNA. J Gen Virol. 1992 Nov;73(Pt 11):2785–2793. doi: 10.1099/0022-1317-73-11-2785. [DOI] [PubMed] [Google Scholar]
- Rennell D., Bouvier S. E., Hardy L. W., Poteete A. R. Systematic mutation of bacteriophage T4 lysozyme. J Mol Biol. 1991 Nov 5;222(1):67–88. doi: 10.1016/0022-2836(91)90738-r. [DOI] [PubMed] [Google Scholar]
- Riechmann J. L., Laín S., García J. A. Highlights and prospects of potyvirus molecular biology. J Gen Virol. 1992 Jan;73(Pt 1):1–16. doi: 10.1099/0022-1317-73-1-1. [DOI] [PubMed] [Google Scholar]
- Riechmann J. L., Laín S., García J. A. The genome-linked protein and 5' end RNA sequence of plum pox potyvirus. J Gen Virol. 1989 Oct;70(Pt 10):2785–2789. doi: 10.1099/0022-1317-70-10-2785. [DOI] [PubMed] [Google Scholar]
- Robaglia C., Durand-Tardif M., Tronchet M., Boudazin G., Astier-Manifacier S., Casse-Delbart F. Nucleotide sequence of potato virus Y (N Strain) genomic RNA. J Gen Virol. 1989 Apr;70(Pt 4):935–947. doi: 10.1099/0022-1317-70-4-935. [DOI] [PubMed] [Google Scholar]
- Rothberg P. G., Harris T. J., Nomoto A., Wimmer E. O4-(5'-uridylyl)tyrosine is the bond between the genome-linked protein and the RNA of poliovirus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4868–4872. doi: 10.1073/pnas.75.10.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A., Blundell T. L. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993 Dec 5;234(3):779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Strauss E. G. Evolution of RNA viruses. Annu Rev Microbiol. 1988;42:657–683. doi: 10.1146/annurev.mi.42.100188.003301. [DOI] [PubMed] [Google Scholar]
- Sudarsanam S., March C. J., Srinivasan S. Homology modeling of divergent proteins. J Mol Biol. 1994 Aug 12;241(2):143–149. doi: 10.1006/jmbi.1994.1484. [DOI] [PubMed] [Google Scholar]
- Sweet R. M., Eisenberg D. Correlation of sequence hydrophobicities measures similarity in three-dimensional protein structure. J Mol Biol. 1983 Dec 25;171(4):479–488. doi: 10.1016/0022-2836(83)90041-4. [DOI] [PubMed] [Google Scholar]
- Takeda N., Kuhn R. J., Yang C. F., Takegami T., Wimmer E. Initiation of poliovirus plus-strand RNA synthesis in a membrane complex of infected HeLa cells. J Virol. 1986 Oct;60(1):43–53. doi: 10.1128/jvi.60.1.43-53.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole V., Dalmay T., Burgyán J., Balázs E. Cloning and sequencing of potato virus Y (Hungarian isolate) genomic RNA. Gene. 1993 Jan 30;123(2):149–156. doi: 10.1016/0378-1119(93)90118-m. [DOI] [PubMed] [Google Scholar]
- Tobin G. J., Young D. C., Flanegan J. B. Self-catalyzed linkage of poliovirus terminal protein VPg to poliovirus RNA. Cell. 1989 Nov 3;59(3):511–519. doi: 10.1016/0092-8674(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Tordo V. M., Chachulska A. M., Fakhfakh H., Le Romancer M., Robaglia C., Astier-Manifacier S. Sequence polymorphism in the 5'NTR and in the P1 coding region of potato virus Y genomic RNA. J Gen Virol. 1995 Apr;76(Pt 4):939–949. doi: 10.1099/0022-1317-76-4-939. [DOI] [PubMed] [Google Scholar]
- Vos P., Verver J., Jaegle M., Wellink J., van Kammen A., Goldbach R. Two viral proteins involved in the proteolytic processing of the cowpea mosaic virus polyproteins. Nucleic Acids Res. 1988 Mar 25;16(5):1967–1985. doi: 10.1093/nar/16.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C., Gregoret L. M., Agard D. A. Modeling side-chain conformation for homologous proteins using an energy-based rotamer search. J Mol Biol. 1993 Feb 20;229(4):996–1006. doi: 10.1006/jmbi.1993.1100. [DOI] [PubMed] [Google Scholar]
- Wimmer E. Genome-linked proteins of viruses. Cell. 1982 Feb;28(2):199–201. doi: 10.1016/0092-8674(82)90335-x. [DOI] [PubMed] [Google Scholar]
- Yue K., Dill K. A. Forces of tertiary structural organization in globular proteins. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):146–150. doi: 10.1073/pnas.92.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]