Abstract
Glycation involves the non-enzymatic addition of reducing sugars and/or their reactive degradation products to amine groups on proteins. This process is promoted by the presence of elevated blood glucose concentrations in diabetes and occurs with various proteins that include human serum albumin (HSA). This review examines work that has been conducted in the study and analysis of glycated HSA. The general structure and properties of HSA are discussed, along with the reactions that can lead to modification of this protein during glycation. The use of glycated HSA as a short-to-intermediate term marker for glycemic control in diabetes is examined, and approaches that have been utilized for measuring glycated HSA are summarized. Structural studies of glycated HSA are reviewed, as acquired for both in vivo and in vitro glycated HSA, along with data that have been obtained on the rate and thermodynamics of HSA glycation. In addition, this review considers various studies that have investigated the effects of glycation on the binding of HSA with drugs, fatty acids and other solutes and the potential clinical significance of these effects.
Keywords: Human serum albumin, Glycation, Glycated albumin, Diabetes, Protein modification, Drug-protein binding
1. Introduction
Diabetes occurs in 366 million people worldwide and is projected to affect 552 million people by 2030 [1]. In the United States alone, an estimated 25.8 million people have this disease [2]. Diabetes can be described as a group of disorders that result from insulin deficiency and/or insulin resistance. Insulin is a hormone produced in the pancreas and is responsible for the regulation of glucose in the circulation [3]. The presence of either insulin deficiency or insulin resistance in diabetes produces an abnormal increase in blood glucose, resulting in a condition known as hyperglycemia. The high blood glucose concentrations seen in diabetes can lead to a number of detrimental health effects, ranging from an increased risk of heart disease and stroke to kidney disease, blindness, and lower limb amputations [4–8].
Two of the most prevalent forms of this disease are type 1 and 2 diabetes [1]. In type 1 diabetes, insufficient production of insulin is caused by an autoimmune response in which the body’s immune system attacks insulin-producing pancreatic beta cells [1,2]. Type 1 diabetes is also known as juvenile-onset, immune-mediated or insulin-dependent diabetes. Patients with this type of diabetes require daily insulin injections for survival. Type 1 diabetes accounts for approximately 5–10% of individuals who have diabetes. Type 2 diabetes is also known as non-insulin dependent or adult-onset diabetes; this is the most common form of diabetes and accounts for 90–95% of individuals with this disease. Type 2 diabetes is caused by an inadequate cellular response to insulin (i.e., by muscle, liver, or adipose cells), or “insulin resistance”, and is strongly associated with factors that include obesity, age, and family history [1,2].
Many of the long-term effects of diabetes can be related to the process of protein glycation. Glycation involves the non-enzymatic addition of reducing sugars and/or their reactive degradation products to primary or secondary amine groups on proteins [9,10]. Early stage glycation involves the nucleophilic attack of a reducing sugar with primary amine groups on proteins to form a Schiff base, as shown in Figure 1(a). This intermediate product can then undergo a slow rearrangement to create a more stable Amadori product, or ketoamine (i.e., fructosyl-lysine, or FL, when this reaction involves glucose) [11–15]. Further oxidation, dehydration, and cross-linking steps can then occur, such as through the formation of reactive dicarbonyl compounds. These latter compounds exhibit significantly enhanced reactivity for sites such as arginine and lysine residues on proteins, as illustrated in Figure 1(b), with the result being the creation of advanced glycation end-products (AGEs) [12].
Figure 1.
The process of glycation, which begins during early stage glycation (a) with the reaction of a reducing sugar such as D-glucose with a free amine group on a protein to form a reversible Schiff base, followed by the formation of a more stable Amadori product (i.e., fructosyl-lysine, or FL, in the case of glucose). The open chain glucose adducts can also convert to the closed forms that are shown above, which represent an N-substituted aldosylamine in the case of the Schiff base or an N-substituted 1-amino-1-deoxyketose in the case of the Amadori product [15]. These early stage glycation reactions can later be followed by additional events during advanced stage glycation (b) that lead to the formation of advanced glycation end-products (AGEs). More details on the structures of FL and common AGEs are provided in Figure 6.
The study of proteins containing either early stage glycation products or AGEs has become of great interest due to the suspected effects of glycation on protein function and tissue damage during diabetes. This effect has been well-studied for proteins with long life spans, such as collagen and lens crystallin [16,17], as well for some proteins with shorter life spans, such as hemoglobin [18]. However, there has also been an increasing interest in the glycation of human serum albumin (HSA) and closely-related proteins (e.g., bovine serum albumin, or BSA). This interest is illustrated in Figure 2 by the increasing number of publications that have appeared on this topic over the last few decades, and especially over the last ten years.
Figure 2.
Number of publications that have appeared over the last three decades that are related to the topic of glycation involving albumin or human serum albumin (HSA). These numbers were obtained on SciFinder in February 2013 using the key terms “glycated” or “glycosylated” and “albumin” or “HSA”.
This review will discuss past and recent reports that have examined the glycation of albumins, and especially HSA. This will include a discussion of the structure and function of HSA, the process of glycation, and the measurement of glycated HSA for clinical purposes. The types of structural modifications that can appear as a result of glycation on albumin and the analysis of these modifications will also be considered. The rate and thermodynamics of glycation for HSA will be examined. In addition, the binding of drugs and solutes to glycated HSA and the possible clinical significance of glycation-related modifications on the function and behavior of HSA will be discussed.
2. Structure and function of human serum albumin
HSA is the most abundant protein in human plasma or serum. This protein is normally present in serum at concentrations ranging from 30–50 g/L and accounts for approximately 60% of the total protein content in serum [19,20]. HSA has a molecular weight of 66.7 kDa and is composed of a single polypeptide chain with 585 amino acids and 17 disulfide chains. The crystal structure of HSA reveals a globular heart-shaped protein composed of approximately 67% α-helices, 23% extended chains, and 10% β-turns (see Figure 3) [19,21–25]. The N-terminus and 59 lysine residues can act as potential sites for the formation of early stage glycation products on this protein, as illustrated by the reaction in Figure 1, although not all of these groups are equally susceptible to this reaction [19,26]. HSA also has 24 arginines that, along with the lysines and N-terminus, could potentially be involved in the formation of AGEs [19].
Figure 3.
The crystal structure of HSA. The locations of the main drug binding sites in this protein (i.e., Sudlow sites I and II) are shown, as well as the locations for several lysines that have often been reported to take part in glycation. This structure was generated using Protein Data Bank (PDB) file ID: 1AO6.
HSA is involved in many physiological processes. For instance, this protein aids in the regulation of osmotic pressure and pH in blood. In addition, HSA mediates lipid metabolism, sequesters toxins, and works as an antioxidant (i.e., it binds free radicals) [19]. Another important function of HSA is its action as a transport protein for a wide range of solutes that include some low mass hormones, fatty acids, and drugs [19,24,27–32]. There are various binding sites located in the structure of this protein. For example, crystallographic studies have shown that HSA has various binding sites for fatty acids [27,30]. This protein also has two major binding sites for drugs (i.e., Sudlow sites I and II, as shown in Figure 3), in addition to several minor sites for small solutes [19,24,31–34]. Sudlow site I is located in subdomain IIA of HSA and is also known as the warfarin-azapropazone binding site. This region tends to bind to bulky heterocyclic compounds, such as warfarin, azapropazone, and phenylbutazone [19,24,31,35]. Sudlow site II is located in subdomain IIIA of HSA and is also referred to as the indole-benzodiazepine binding site. This site is the primary binding region on HSA for aromatic compounds such as ibuprofen and indole-containing compounds (e.g., L-tryptophan) [19,24,31,36].
3. Measurement of glycated albumin
The measurement of glycated HSA (or “glycated albumin”, GA, as it is often called in the clinical literature) has been of interest for a number of years as a complementary tool to standard assays using glucose or glycated hemoglobin (i.e., hemoglobin A1c, or HbA1c) to monitor glycemic control in diabetic patients. Blood glucose is a standard test for the diagnosis and monitoring of diabetes, but only provides information on the glycemic status of a patient at a particular moment in time [37]. HbA1c is an accepted biomarker for providing a long-term record of glycemic control over a period of 2–3 months, as made possible by the life span of approximately 90–120 days for hemoglobin in red blood cells [38,39]. However, it has been suggested that HbA1c may not be suitable for evaluating patients with unstable blood glucose concentrations, and the accuracy of this marker can be affected by the presence of hemoglobin variants [40], recent blood transfusions or diseases such as hemolytic anemia and renal failure, which can affect the production and effective life span of red blood cells in the circulation [41,42].
These limitations in current methods have generated interest in other markers and tests for the assessment of glucose control, especially for examining the control of blood glucose over short-to-intermediate lengths of time [38]. The utilization of glycated HSA for this purpose has been suggested because the half-life of HSA in the blood is only 12–21 days [37–39]. This means that assays for glycated HSA, or related markers, can provide information on glucose control over a period of roughly of 2–3 weeks. This feature, in turn, could help physicians to detect deteriorating control of blood glucose before any noticeable changes in HbA1c occur [37,43,44].
Fructosamine assays have been explored by clinical laboratories as means for measuring glycation that is mainly related to HSA [37,38,45]. The term “fructosamine” (often abbreviated as “FA”) typically refers to all ketoamine linkages that result from the glycation of serum proteins. Because HSA is the most abundant of the serum proteins, fructosamine is predominantly a measure of glycated HSA, contributing ~90% to the total [41,42]. As a result, fructosamine assays tend to be used when glycemic control needs to be examined over a period of 1–3 weeks [37,38,42]. Colorimetric methods such as the thiobarbituric acid (TBA), nitroblue tetrazolium (NBT) and hydrazine-based assays have been employed to measure glycated serum proteins [45–47]. For example, the NBT assay relies on the ability of N-substituted ketoamines to reduce the dye nitroblue tetrazolium in a basic solution, generating a colored product [45,48]. Although fructosamine assays are popular in some countries, they have not seen as much use in the U.S., partly due to difficulties encountered in the commercialization of such methods [38]. Further details on the performance characteristics of these techniques are provided in Refs. [38,42–47].
The concentration of glycated HSA can be measured directly by a number of techniques. These methods include boronate affinity chromatography [38,49–55], immunoassay-related techniques (e.g., enzyme-linked immunosorbent assays or radioimmunoassays) [38,56,57] and enzymatic methods [58–61]. Profiling of glycated proteins in human serum, including HSA, has also been explored using boronate affinity chromatography followed by tandem mass spectrometric detection [62]. The boronate affinity chromatographic methods make use of the interaction between sugar residues and a binding agent such as phenylboronic acid; this process allows for the retention and separation of glycated HSA from non-glycated HSA (see Figure 4), with the retained species later being detected and quantified by an on-line or off-line method (e.g., mass spectrometry or an immunoassay) [38,49–53,55]. Boronates have also been used in an enzyme-linked boronate immunoassay to measure glycated HSA [57]. One current clinical assay based on an enzymatic method utilizes an albumin-specific proteinase (i.e., ketoamine oxidase) to digest glycated HSA, followed by treatment with a fructosaminase to oxidize the glycated amino acids and produce hydrogen peroxide, which is then measured [59–61]. Raman spectroscopy [63], refractive index measurements [64], capillary electrophoresis [65] and other electrophoretic methods [66,67] have also been explored as alternative techniques for measuring glycated albumin.
Figure 4.

Use of a column and support containing a boronate ligand (e.g., phenylboronic acid) for the retention of glycated HSA. The active binding agent in this process is the tetrahedral boronate anion, which forms under alkaline conditions.
The clinical assays for glycated HSA typically express their results in terms of the ratio of the amount of glycated HSA versus the total amount of HSA that is present. This feature means that these methods are not generally affected by changes in the overall concentration of HSA [38,41]. Prior work from the 1980s estimated that 6–13% of HSA was glycated in healthy individuals, with this amount increasing by up to 20–30% in diabetic patients [26,38,68–70]. A recent review noted that a normal reference range of 11–16% is used with a commercial enzymatic-based method, and current assays based on affinity chromatography often have lower reference values in the range of 0.6–3% [38]. However, all current methods tend to give typical glycated HSA values for diabetic patients that are 2- to 5-fold higher than normal levels [38]. Altered albumin metabolism can affect glycated HSA measurements in patients that suffer from proteinuria, hepatic cirrhosis or hypothyroidism [41]. In addition, elevated amounts of glycated HSA have been noted to occur with several vascular complications and to be linked to immune function, oxidative stress, cholesterol homeostasis, and atherosclerosis [39].
4. Structural analysis of glycated albumin
The process of glycation has been found to have possible effects on both the structure and function of HSA [26,71]. For instance, several reports have found altered drug binding with this protein as a result of glycation [71–75], as will be discussed in Section 6. The study of alterations in the structure of HSA following glycation has involved the use of radiolabeling [76–78], colorimetric assays [48], fluorescence spectroscopy [26,79–82], infrared spectroscopy [79], circular dichroism [71,82,83], and NMR spectroscopy [84]. In addition, work based on immunoassays [79–85], electrophoresis [81,86] and HPLC [78,81,87–89] has provided information on the total glycation levels or on the amount of specific AGEs that are present within albumin. Some of these approaches again include the prior isolation of modified albumin by methods such as boronate affinity chromatography [38,49–55,76,78,79,90–92].
More detailed information on glycated-related modifications has been obtained by using mass spectrometry. For instance, liquid chromatography-mass spectrometry (LC-MS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) have been used to locate and identify glycation sites in albumin [92–97], to identify glycated peptides obtained from serum proteins [91], and to look at the sites involved in the related process of galactation (i.e., the addition of galactose to an amine group) [98] and in the formation of some AGEs [99,100]. High resolution mass spectrometry (HRMS) [101] and tandem mass spectrometry (MS/MS) [101,102] have also been used to detect glycated peptides or to identify glycation sites. Fourier transform mass spectrometry (FTMS) has been utilized to detect and identify glycated peptides from HSA [103]. Gas chromatography-mass spectrometry (GC-MS) has been employed to investigate glycation at the N-terminus of HSA [104]. In addition, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) has been used to estimate the overall extent of modification for glycated HSA, to study glycated BSA, to locate sites for early stage glycation products in HSA, to identify various AGEs in HSA, and to compare the extent of modification at various regions of glycated HSA [80,92,102,105–111].
The process of glycation can be divided into several stages [10–12]. The first stage of this process, as shown earlier in Figure 1(a), involves the combination of a reducing sugar such as glucose with primary amine groups (e.g., lysine residues or the N-terminus of a protein) to form a reversible Schiff base; this product can then slowly rearrange to form an Amadori product [10–14]. Fructosyl-lysine (FL, see Figure 1) is the major Amadori product produced in vivo and has been found to occur at various locations in glycated HSA. Table 1 summarizes the results of numerous studies that have examined the glycation process and the creation of Amadori products for both in vitro and in vivo glycated HSA [76,78,80,93–95,97,101,105–108,110].
Table 1.
Modification sites and adducts that have been reported for glycated human serum albumin (HSA)a
| Modification process [Ref.] | Sample (Glycation Conditions)b & Analysis Methods | Modification Sites Identified | Major Adducts |
|---|---|---|---|
| Glycation [76] | In vivo sample; tritium labeling, boronate affinity chromatography, cation exchange chromatography & amino acid analysis | Major site: K525 | FL/Amadori products |
| Glycation [78] | In vivo sample; tritium labeling, boronate affinity chromatography, HPLC & amino acid analysis | Major site: K525; other significant sites: K199, K233, K281, K439; additional sites identified: K12, K317, K351, K534 | FL/Amadori products |
| Glycation [94] | In vitro sample(330-fold mol excess glucose vs. 1.5 mM HSA, 37°C, 28 days); LC-MS/MS | K12, K20, K41, K51, K73, K136, K137, K159, K162, K199, K205, K233, K262, K276, K281, K313, K317, K323, K351, K378, K389, K402, K432, K436, K439, K475, K500, K519, K525, K541, K545, K557, K574 | FL/Amadori products |
| Glycation [95] | In vivo sample; LC-MS | K525 > K233 > K136/K137 > K199, K64/K73, K439, K317, K274/K276 > K534 > K389c | FL/Amadori products |
| Glycation [93] | In vivo samples; boronate affinity chromatography & LC-MS/MS | K524/K525 > K162 > K276 > K359 > K351 > K233; additional sites identified: K12, K51, K64, K174, K181, K262, K378, K414, K475, K541, K545d | FL/Amadori products |
| Glycation [101] | In vitro sample (370-fold mol excess glucose vs. lyophilized HSA, 80°C, 25 min); 13C- labeling, HPLC, HRMS & MS/MS | K4, K41, K73, K137, K181, K190/K195, K199/K205, K225/K233, K323, e K351, K378, K413/K414, K432, K436/K439, K466, K519, K524/K525, K536, K545, K564/K573 | FL/Amadori products |
| Glycation [97] | Plasma sample incubated with 30 mM glucose (37 °C, 24 h); 12C/13C- labeling, boronate affinity chromatography, LC-MS/MS | Preferential sites: K525, K240, K233, K51, K136, K137, K73; other sites: K41, K64, K93, K106, K159, K174, K181, K195, R218, K262, K274, K323, K359, K372, K378, K389, K402, K413, K432, K436, K439, K444, K466, R472, K475, K500, K519, K573d | FL/Amadori products and AGEs |
| Glycation & AGE formation [96] | In vitro sample (330-fold mol excess glucose vs. 1.5 mM HSA, 37°C, 28 days); LC- MS/MS, MALDI- TOF MS | K51, K137, K162, K225, K233, K276, K313, K323, K351, K378, K413, K444, K525, K536, K545, K573 | FL/Amadori products and AGEs |
| Glycation & AGE formation [80] | In vitro sample (3125-fold mol excess glucose vs. 16 μM HSA, 40 °C, up to 115 days); MALDI-TOF MS | Potential sites: K41, K64, K93, K106, K317, K323, K359, K372, K402, K439, K444, K525, K541, K545, K557, K560, K564, K573, K574, R10, R98; Other potential sites: K4, K51, K281, K286, K313, R160, R209 | FL/Amadori products and AGEs |
| Glycation & AGE formation [107] | In vitro sample (commercial preparation of minimally-glycated HSA); peptide fractionation, MALDI-TOF MS | K12, K51, K159, K199, K205, K286, K378, K439, K525, K538; R160, R222, R472 | FL/Amadori products and AGEs (e.g., AFGP, ArgP CEL, CML, 3DH-H1, G- H1, Pyr, THP) |
| Glycation & AGE formation [106] | In vitro samples (24- to 48-fold mol excess glucose vs. 0.63 mM HSA, 37°C, 2-5 weeks); peptide fractionation, MALDI-TOF MS | Significant sites: K525/K524/R521 > N- Terminus, K93/R98, K276/K286, K414/K439 > R197 > K199, K281, R428; Other sites: K4, K12, K174, K190, K205, K212, K262, K313, K317, K413, K545, K557, K560, K564, K573, K574; R10, R186, R209, R410 | FL/Amadori products and AGEs (e.g., AFGP, ArgP, CEL, CML, 3- DG-H1, G-H1, IB, MG-H1, Pyr, THP) |
| Glycation & AGE formation [105] | In vitro sample (commercial preparation of minimally-glycated HSA); 16O/18O- labeling, peptide fractionation, MALDI-TOF MS | K159, K212, K323, K413, K432, K439, K525; R81, R114, R218 | FL/Amadori products and AGEs (e.g., AFGP, CEL, CML, Pyr) |
| Glycation & AGE formation [108] | In vitro samples (24- fold mol excess glucose vs. 0.63 mM HSA, 37 °C, 2–5 weeks); 16O/18O- labeling, peptide fractionation, MALDI-TOF MS | Regions with highest levels of modification (likely sites): 1–10 (N-terminus*/K4/R10) or 42–51 (K51), 521–531 (K525*/K524), 275–286 (K281*/K276/K286), 21–41 (K41), 82–93 (K93), 87–100 (K93/R98), 101–119 (K106/R114/R117), 146–160 (K159/R160), 360–372 (K372); other regions: 426–442 (K439*/R428), 61–82 (K64/K73/R81), 142–153 (R144/R145), 154–167 (K159), 189–208 (K199), 209–227 (K212/R218), 241–257 (R257), 324–336 (R336), 373–389 (K378/K389), 414–428 (K414/R428), 502–518 (K519)f | FL/Amadori products and AGEs (e.g., AFGP, CEL, 3-DG-H1, G- H1, Pyr, MG- H1) |
| Glycation & AGE formation [110] | In vivo sample; 16O/18O-labeling, peptide fractionation, MALDI-TOF MS | K12, K162, K174, K190, K199, K205, K276, K281, K286, K313, K317, K372, K432, K525, K545, K557, K560, K564, K573/K574; R10, R98, R160, R197, R209, R428, R484, R485 | FL/Amadori products and AGEs (e.g., AFGP, CEL, CML, 3-DG- H1, G-H1, MG-H1, Pyr, THP) |
| AGE formation with methylglyoxal [99,100] | In vitro samples (5- fold mol excess of glucose vs. 0.1 mM HSA at 37 °C, 24 h); LC-MS/MS | R410 > R114, R186, R218, R428 | MG-H1, ArgP, CEL, methylglyoxal- derived lysine dimer |
| Galactation [98] | In vitro samples (8- to 1500-fold mol excess galactose vs. 1.2 μM HSA, 37–56°C, 0.01–8 days); boronate affinity chromatography, LC-MS/MS | K12, K233, K276/K281, K414, K525 | Galactosyl- lysine |
A ranking of the modified residues is provided if such information appeared in the given references. If no ranking was provided, then the modification sites are listed in their order of appearance in HSA. List of abbreviations: AFGP, 1-alkyl-2-formyl-3,4-glycosyl-pyrrole; AGE, advanced glycation end-product; ArgP, argpyrimidine; CEL, Nε-carboxyethyl-lysine; CML, Nε-carboxmethyl-lysine; 3-DG-H1, 3-deoxyglucosone-derived hydroimidazolone, isomer 1; FL, fructosyl-lysine; G-H1, glyoxal-derived hydroimidazolone, isomer 1; IB, imidazolone B; MG-H1, methylglyoxal-derived hydroimidazolone, isomer 1; Pyr, pyrraline; THP, tetrahydropyrimidine.
A typical ratio of glucose to HSA that is found in blood during diabetes is 24:1 (mol/mol) and the normal concentration of HSA in serum is generally 0.53–0.75 mM (i.e., 35–50 g/L).
The order of these results are those for the “Diabetic patient” in Table 1 of Ref. [95]. A similar order was obtained for other samples that were examined.
The values differ from the published residues by 24 amino acids to exclude the signaling peptide that was included in the sequence in this study and to make the listed sequence assignments consistent with the rest of the table.
This paper included an entry for K322 which should have instead been K323, according to a personal communication with the authors.
This study focused on quantitative studies based on the examination of a decrease in the abundance of non-modified peptides from the indicated regions. The suspected modification sites leading to each decrease are those contained in the given regions, as based on the sequence of HSA, identified modifications, and prior studies of glycated HSA; in cases where a particular residue is a well-known modification site from prior work, this residue is indicated by an asterisk. This list combines the modified regions that were seen for two in vitro samples that were incubated either two or five weeks with the stated amount of glucose. For 502–518, which contains no lysine or arginine, K519 is given as a possible site of modification because it is immediately next to the enzymatic digestion site for this peptide.
The combined list of modification sites that have been reported for FL and Amadori products includes all 59 lysines in HSA (see Figure 5). However, only a relatively small number of these residues appear to account for most of the Amadori products and modifications that are found in glycated HSA. As Figure 5 illustrates, lysine 525 has consistently been found to be a major site for the formation of such products within both in vivo and in vitro glycated HSA [76,78,108]. Other glycation sites that have been observed in at least half of the reported studies in Table 1 include lysines 439, 199, 51, 378, 545, 12, 233, 276, 281, 317 and 323. It is interesting to note that lysine 525 and many of these same lysines have been reported to be important sites for the addition of galactose to HSA in vitro [98]. An additional 23 lysine residues have been found to be modified for glycated HSA in at least four of the reports listed in Table 1. Besides these lysine residues, the N-terminus has been noted in some reports to be a site for glycation in HSA [80,104,106,108]. Factors involved in determining the relative susceptibility of these lysines and the N-terminus to glycation include local acid-base catalysis effects, the accessibility of the site to the solvent or environment, and the local pKa for the amine group at each site [78,96,106,108,110].
Figure 5.
Number of reports from the literature (see Table 1) that have identified glycation-related modifications at the various lysine or arginine residues in HSA. The three regions of the bar graphs show the number of reports that have involved in vitro samples (bottom section, dark gray; up to 8 total papers), in vivo samples (middle section, light gray; up to 5 total papers) or plasma that was spiked with glucose and incubated under in vitro conditions (top section, intermediate gray; up to 1 paper).
The next stage of the glycation process involves the oxidation of adducts on glycated proteins or free sugars to form intermediate reactive dicarbonyl compounds, or α-oxaloaldehydes [28,88,112–118]. The α-oxaloaldehydes that have been most commonly correlated with diabetes, kidney disease, and cardiovascular disease in vivo are glyoxal, methylglyoxal, and 3-deoxyglycosone [89,115]. The concentrations of these α-oxaloaldehydes typically increase from sub-nanomolar concentrations in normal individuals to micromolar concentrations in a number of diseases [116]. α-Oxaloaldehydes can react with both lysines and arginines to form AGEs, as illustrated in Figure 1(b), and can also lead to the modification of cysteine residues [81]. AGE formation on plasma proteins is mediated by a number of competing processes that include the oxidation of sugars, lipids, or protein-bound sugar adducts and the counteracting removal of reactive dicarbonyl compounds by the glyoxylase pathway or through natural protein degradation [116].
Table 1 includes a list of AGEs that have been observed in studies of both in vitro and in vivo glycated HSA [80,96,97,105–108,110]. Examples of these AGEs are shown in Figure 6. As indicated in Table 1, methylglyoxal-derived hydroimidazolone isomer 1 (MG-H1) and glyoxal-derived hydroimidazolone isomer 1 (G-H1) have been seen in several studies for in vitro or in vivo glycated HSA. The corresponding hydroimidazolone derived from 3-deoxyglucosone (3-DG-H1, isomer 1) has also been observed in these samples. Other possible AGEs that have been reported for both in vivo and in vitro samples of glycated HSA include other products related to arginine, such as tetrahydropyrimidine (THP); products related to lysines, such as Nε-carboxyethyl-lysine (CEL), Nε-carboxymethyl-lysine (CML) and pyrraline (Pyr); and products related to either lysines or arginines, such as 1-alkyl-2-formyl-3,4-glycosyl-pyrrole (AFGP). In addition, the arginine-related products argpyrimidine (ArgP) and imidazolone B (IB) have been observed for glycated HSA in vitro [99,100,106,107].
Figure 6.
Structures of fructosyl-lysine (FL) and examples of advanced glycation end-products (AGEs) that have been reported for glycated HSA (see summary in Table 1).
Some studies have compared the relative amounts of these AGEs that can be found in HSA and the residues that may take part in these modifications. For instance, minimally glycated HSA that was prepared in vitro was found to contain mainly FL and CML plus minor amounts of others AGEs (e.g., ArgP, 3DG–H1, G-H1, MG-H1, THP and some 3-deoxyglucosone-derived lysine dimer); in vitro highly glycated HSA that was examined by the same methods contained the same AGEs plus pyrraline and high amounts of FL [88,89]. Quantitative screening for several types of AGEs in plasma proteins, including HSA, indicated that hydroimidazolones were a major type of modification that occurred in this group. Of these, MG-H1 was estimated to be present in about 2% of HSA from normal healthy individuals, followed by 1% for 3DG–H (e.g., 3DG–H1) and 0.1% for G-H1 [117]. AGEs such as CML, CEL were found in the same types of samples at amounts just below those of G-H1, while only small amounts of cross-linked species like pentosidine and methylglyoxal-derived lysine dimer were detected. The levels of these modifications were found, in general, to increase in patients with renal failure [117].
It has been noted that the reaction of methylglyoxal with 0.11 mM HSA at pH 7.4 and 37° C tends to be selective for arginine residues at a methylglyoxal concentration of 1 mM, with a much higher methyglyoxal concentration (100 mM) leading to increased modification for arginines as well as the modification of some lysines [81]. Of the 24 arginines that are present in HSA, 21 of these have already been proposed to take part in some type of AGE formation, as is illustrated in Figure 5. This observation agrees qualitatively with in vitro studies of HSA from Ref. [81], in which the incubation of this protein with over a 900-fold mol excess of methylglyoxal resulted in the modification of an average of 19 out of 24 arginines per HSA. The arginines which have been found to be modified in four or more studies of AGEs for in vitro or in vivo glycated HSA are R128, R428, R10, R98, R114, and R160; another four arginines have been noted to be modified in at least three studies of glycated HSA. Studies that have examined the in vitro reaction of methylglyoxal with HSA have found that R410 is a particularly active site for this pair of reactants, followed by R114, R186, R218 and R428 [99,100].
The use of glycated HSA as a biomarker for glycemic control, as discussed in Section 3, is based on the fact that the extent of glycation for this protein will depend on the time allowed for glycation and the amount of glucose that is available for reaction with HSA. As will be shown in Section 5, the types of modifications that occur on glycated HSA will also depend on these factors. Furthermore, this means some variations in glycation-related modifications may occur between samples of in vitro glycated HSA that are prepared under different conditions [106,108], between in vivo glycated HSA and in vitro glycated HSA that is prepared under non-physiological conditions [119], or even between in vivo samples that are acquired from different patients [93,111]. Despite this variability, there are strong similarities between the results of structural studies that have been obtained for in vivo and in vitro glycated HSA. For instance, lysine 525 has consistently been found to be a major glycation site in both types of samples [76,78,108]. In addition, all lysines that have been found to be modified in five or more studies of glycated HSA have been detected both in vivo and in vitro (see Figure 5); glycation at many of the remaining lysines in HSA has been observed in these two types of samples as well. There is also good agreement between the types of AGEs that have been found for in vivo and in vitro glycated HSA (see Table 1) and in the arginines that have been most often observed to take part in glycation-related modifications in these samples (see Figure 5).
Several recent studies have further noted close similarities between in vivo results and those for in vitro glycated HSA that has been prepared using glucose/HSA concentrations and incubation conditions that mimic those found in serum during diabetes. These similarities include the specific residues that are being modified [76,78,105–108,110], the types of modifications that occur [105–107,110], the relative order of modified lysines versus lysine 525 in terms of their formation of fructosyl-lysine [93,108], and the observed modification patterns versus in vivo glycated HSA that has a similar extent of overall glycation [106,108,110]. Together, these data indicate that in vitro glycated HSA can be a useful model for in vivo glycated HSA in many situations, especially if the two types of samples have been created or generated under similar reaction conditions.
5. Kinetic and thermodynamic studies of albumin glycation
The rate of modification and thermodynamics of the glycation process for HSA have been examined in a few studies. For instance, early work in this area confirmed the reaction model that is shown in Figure 1(a) for the early stage glycation of HSA [15]. This process involves biphasic kinetics, in which the reversible combination of glucose with a free amine group to create a Schiff base quickly reaches an equilibrium (i.e., in a matter of hours under the conditions used in Ref. [15]), followed by a much slower conversion of the Schiff base to an Amadori product. The average rate constants for the initial association or dissociation of glucose with amine groups on HSA were determined to be 1.5 M−1 h−1 (or 4.2 × 10−4 M−1 s−1) and 0.39 h−1 (or 1.1 × 10−4 s−1), respectively (Note: unless otherwise indicated, the rate constants and equilibrium constants listed in this review were obtained at pH 7.4 and 37_6 C). The average association equilibrium constant for this process was 3.9 (± 0.3) M−1. The rate constant for the formation of Amadori products was estimated to be 0.026 h−1 (7.2 × 10−6 s−1), with these products being found to be quite stable and having a half-life of at least 3 weeks [15].
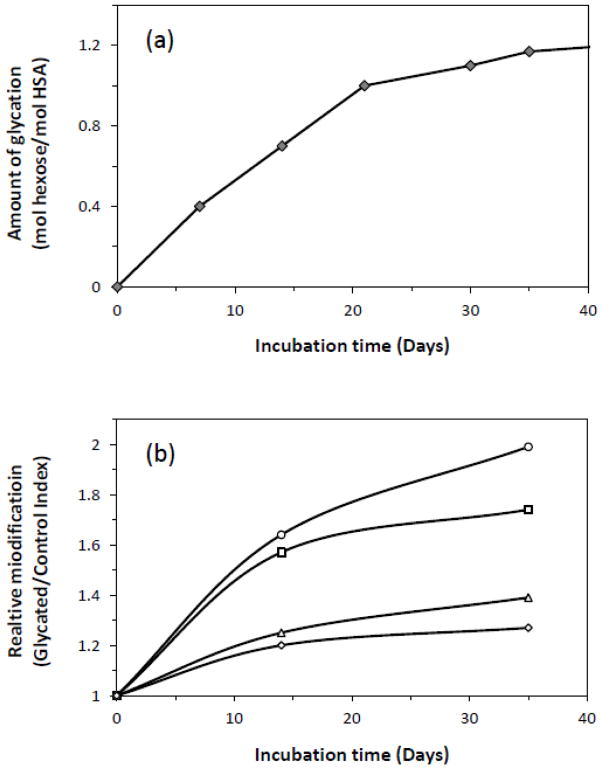
The net rate of glycation and modification for HSA has been investigated in a number of studies under in vitro conditions. The results of one such study are shown in Figure 7(a), as based on the measurement of fructosamine and the overall degree of glycation [106]. Similar trends have been noted when using the change in mass that is observed over time for in vitro glycated HSA [111]. The results in both Figures 7(a) and (b) were carried out under incubation conditions and using concentrations of glucose and HSA that are representative of physiological conditions. The data show that most modifications occur within the first three weeks of incubation, followed by a more gradual change over longer incubation times [106,111]. Comparable trends have been noted in other reports examining the reaction of HSA or BSA with glucose or similar reagents [77,80,120–122]. Besides incubation time, the effects of glucose concentration and pH on glycation have also been considered [122].
Figure 7.
Change in (a) the total amount of glycation or (b) the amount of modification at specific residues as a function of time for in vitro glycated HSA. The data in both plots were acquired at pH 7.4 and 37° C for 0.63 mM HSA incubated with 15 mM glucose. In (b) the relative extent of modification is given by the value of the measured glycated/control index, as obtained for residues 1–10 (○), 521–531 (□), 189–208 (△), and 426–442 (◇) and containing potential modification sites such as the N-terminus, R521/K525, K199/K205 and R428/K439. Adapted with permission for Refs. [106,108].
The formation over time of early stage glycation products versus AGEs has been investigated as well. As an example, both semi-quantitative and quantitative studies of in vitro glycated HSA that was prepared under physiological or diabetic-type conditions (i.e., a 24- to 48-fold mol excess of glucose versus 0.63 mM HSA) have found that early stage glycation products such as FL dominate after incubation times of two weeks, with AGEs becoming more apparent after longer reaction times of up to five weeks [106,108]. A similar trend has been noted in vitro when using a larger excess of glucose versus HSA during glycation (i.e., 3125-fold mol excess of glucose versus 16 μM HSA) [80].
In some cases, the rate of formation of glycation products at specific regions of HSA has been determined, as shown in Figure 7(b). It has been found through this research that different regions of this protein do vary in their rates of glycation product formation [108]. For instance, the pseudo-first order rate constants for the formation of glycation products at regions containing the N-terminus, K525, K439/R428 and K199 (or K205) have been estimated to be 2.5 × 10−5, 2.2 × 10−5, 1.1 × 10−5 and 1.1 × 10−5 min−1, respectively, over the first two weeks of incubation, with values of 1.3 × 10−5 min−1 to 0.6 × 10−5 min−1 being obtained over five weeks of incubation. Similar modifications at other regions of glycated HSA have been found to have pseudo-first order rate constants in the range of 10−6 to 10−5 min−1 [108].
6. Binding of glycated albumin to drugs, fatty acids and other solutes
Because glycation can modify the structure of HSA, there has been interest in how these changes may affect the ability of HSA to act as a binding agent for drugs and other solutes [70–75,82,110,119,123–131]. This topic has been of particular interest in recent years as experiments examining the structure of glycated HSA have found that modifications in this protein can occur on several residues that are at or near Sudlow sites I and II, as illustrated in Figure 3 [99,100,105–108]. Various methods have been employed to examine the binding of solutes with glycated HSA to see if any changes occur versus the interactions that are seen for normal HSA. These techniques have included fluorescence spectroscopy [71,82,83,119], circular dichroism [83], ultrafiltration [119,123,130], equilibrium dialysis or rate of dialysis [70,82,124,131,132], and chromatographic techniques such as the Hummel-Dreyer method [130] or various forms of high performance affinity chromatography [72–75,126–129].
Several reports have studied the effects that glycation may have on drug and solute binding at the major binding regions for these agents on HSA. For instance, warfarin and its enantiomers have been used as site-selective probes for Sudlow site I of HSA [71–75,83,110,126,128,133]. The changes that have been observed for binding by warfarin to HSA have been found to vary with the binding conditions and the extent of glycation for HSA. For instance, early reports using fluorescence spectroscopy or equilibrium dialysis at 20–25°C noted a 6.9-fold decrease or 1.8-fold increase in the affinity for warfarin with in vitro glycated HSA that had high amounts of glycation (i.e., as prepared using up to a 165- to 3300-fold mol excess of glucose versus HSA) [70,71,74]. An affinity decrease of ~20% for warfarin was observed using a pooled sample of in vivo glycated HSA isolated from diabetic patients; in the same study, a decrease in affinity of 30–60% was seen for in vitro glycated HSA that had been prepared by incubating a pooled sample of normal HSA with a 67- to 270-fold mol excess of glucose [119]. Other experiments have examined the binding of warfarin with in vitro glycated HSA that had been prepared by using glycation levels similar to those present in patients with diabetes, with no significant change in overall binding or interactions at Sudlow site I observed when compared to normal HSA [74]. Little or no change in binding was also observed for warfarin at pH 7.0 and room temperature with in vitro glycated HSA that had moderate amounts of modification [83], and when using chromatographic-based zonal elution studies to examine the overall binding of warfarin with entrapped samples of normal HSA or in vitro glycated HSA with modification levels comparable to those seen in diabetic patients [126]. One practical consequence of these latter observations is that no appreciable effect of glycation would be expected in clinical and pharmaceutical studies that use warfarin as a site-selective probe with HSA that has levels of modification similar to those seen in diabetes [74,126].
Similar experiments have examined the binding of L-tryptophan as a site-selective probe for Sudlow site II of glycated HSA and normal HSA [72–75,110,126,128,134]. Early studies using HSA that was glycated in vitro in the presence of bound palmitic acid did not show any apparent change in the affinity of L-tryptophan versus normal HSA at 25°C [132]. However, a later study found a decrease in binding at 4° C and when using a low amount of in vitro glycation (i.e., 9-fold mol excess of glucose versus 0.6 mM HSA), while an increase in binding was seen at a higher glycation level (i.e., a 46-fold mol excess of glucose versus 0.6 mM HSA) [82]. This result is consistent with recent competition experiments carried out at 37°C and using in vitro glycated HSA that contained an extent of modification similar to those found in diabetes; in this latter work, a 4.7- to 5.8-fold increase in the association equilibrium constant for L-tryptophan at Sudlow site II was measured for glycated HSA [74]. A similar, large increase in affinity was seen at 37°C with comparable HSA samples but using a high-throughput chromatographic method to examine the overall binding of L-tryptophan with normal HSA and in vitro glycated HSA [126]. Thus, in the case of L-tryptophan, glycation would be expected to have an effect in clinical and pharmaceutical research that uses this solute as a site-selective probe with HSA [74,126].
Another group of drugs that may have their binding to HSA affected by glycation are sulfonylureas [72,73,75,110,126–129]. Sulfonylureas are a form of oral medication that is used in the treatment of type 2 diabetes. These drugs are highly bound to HSA and have been found to have multi-site interactions at both Sudlow sites I and II [72,73,75,110,127,128,135]. Table 2 summarizes the results of studies have examined the binding of several sulfonylureas with glycated HSA that has been prepared in vitro and with various degrees of glycation similar to those found in diabetic patients [72,73,75,128]. As this table indicates, the amount of HSA glycation has been found to affect the affinity of this protein for these sulfonylurea drugs. The size of this effect can vary from one drug to the next in the same class or between different binding sites for the same drug, and can lead to either an increase or decrease in affinity at a given site. For instance, the changes in affinity seen at Sudlow sites I and II in Table 2 range from 0.6-fold to 6.0-fold versus normal HSA for the given set of sulfonylurea drugs. Similar effects and variations in affinity at comparable levels of glycation have been noted in recent studies examining the binding by many of the same sulfonylurea drugs with in vivo samples of glycated HSA [110].
Table 2.
Changes in the binding of various sulfonylurea drugs at Sudlow sites I and II when comparing in vitro glycated HSA with normal HSA
| Relative change in association equilibrium constant (↑ or ↓)a | ||||||
|---|---|---|---|---|---|---|
| Drug & binding site | gHSA1 | gHSA2 | gHSA3 | |||
| Sudlow site I | ||||||
| Acetohexamide | ↑ 1.4-fold | N.S. (↓ 10%)b | N.S. (< 5%) | |||
| Tolbutamide | ↑ 1.3-fold | ↑ 1.2-fold | ↑ 1.2-fold | |||
| Gliclazide | N.S. (< 10%) | ↑ 1.9-fold | N.S. (↑ 11%)b | |||
| Glibenclamidec | N.S. (< 5%) | ↑ 1.7-fold | ↑ 1.9-fold | |||
| Sudlow site II | ||||||
| Acetohex amide | ↓ 0.6-fold | ↓ 0.8-fold | N.S. (<10%) | |||
| Tolbutamide | ↑ 1.1-fold | ↑ 1.4-fold | ↑ 1.2-fold | |||
| Gliclazide | ↓ 0.8-fold | ↑ 1.3-fold | ↓ 0.6-fold | |||
| Glibenclamidec | ↑ 4.3-fold | ↑ 6.0-fold | ↑ 4.6-fold |
This table is based on data obtained from Refs. 72, 73, 75 and 128. The results for each drug were obtained using the same preparations of in vitro glycated HSA and were measured at 37°C and in the presence of pH 7.4, 0.067 M potassium phosphate buffer. The levels of glycation for the protein samples were as follows: gHSA1, 1.31 (±0.05); gHSA2, 2.34 (±0.13); and gHSA3, 3.35 (±0.14) mol hexose/mol HSA. The relative changes listed are all versus the following association equilibrium constants that have been measured for normal HSA: Sudlow site I acetohexamide = 4.2 × 104 M−1, tolbutamide = 5.5 × 104 M−1, gliclazide = 1.9 × 104 M−1, glibenclamide = 2.4 × 104 M−1; Sudlow site II - acetohexamide = 13.0 × 104 M−1, tolbutamide = 5.3 × 104 M−1, gliclazide = 6.0 × 104 M−1, glibenclamide = 3.9 × 104 M−1. The term “N.S.” stands for “not significant” and indicates an association equilibrium constant that was not significantly different from that for normal HSA at the 95% confidence level.
The association equilibrium constant for this drug and sample of glycated HSA was significantly different at the 90% confidence level from the reference value for the same drug with normal HSA but was not significantly different at the 95% confidence level.
Glibenclamide also binds to the digitoxin site of HSA, which may have displayed a slight decrease in binding in going from normal HSA to the samples of in vitro glycated HSA that are represented in this table [128].
As Table 2 indicates, there is growing evidence that the amount of glycation-related modifications can have a large effect on the binding of a drug or solute with glycated HSA. This has been noted not only for sulfonylurea drugs [72,73,75,110,128] but is also suggested by the apparent changes in affinity with glycation conditions that have been noted in the binding studies that were discussed earlier for warfarin [70,71,74,119,126] and L-tryptophan [74,82,126,132]. In some cases, such as for warfarin, a large change in the extent of glycation may be needed to see a change in binding, while for other solutes (e.g., L-tryptophan) these changes may begin at small or moderate amounts of modification. It has been further proposed through structural studies (see Section 4) that the variations in binding that have been noted at particular sites on HSA (e.g., Sudlow sites I and II) are probably related to that changes in the amount and types of modifications that occur in HSA with different degrees of glycation [72–75,82,83,106,108,110].
The binding of other solutes with glycated HSA has also been examined [26,70,83,119,122–125,130,131]. For instance, it has been reported that the dye Bromocresol Purple has a decrease in binding with glycated HSA, especially when this protein is reacted with glyceraldehyde, and that this change in binding is linked to the degree of modification [70]. A decrease in the binding capacity of salicylate has been observed for in vitro glycated HSA [122]. It was found for in vitro glycated HSA with moderate amounts of glycation that there is a decrease in the binding of dansylproline, which binds to Sudlow site II, but no measurable change for dansylamide, which binds to Sudlow site I; decreased binding was further seen for phenybutazone, ibuprofen and flufenamic acid [83]. The binding of in vivo glycated HSA with hemin did not change, but a 50% decrease in affinity for bilirubin was observed [26]. A decrease in affinity of 30–50% was seen for ketoprofen in its binding to a pooled sample of in vivo glycated HSA from diabetic patients, while either no change or up to a 50% decrease was observed for in vitro glycated HSA that had been prepared by using a 67- to 270-fold mol excess of glucose [119]. In some studies, no change was found in the free fraction of phenytoin in spiked serum samples obtained from diabetic patients or normal individuals [123,124], while another report found a significant decrease in the binding of phenytoin and valproate in such samples but no change for digitoxin [131]. Other studies have observed slightly decreased binding by diazepam to serum proteins in such samples [124,125].
In addition to elevated amounts of protein glycation, diabetic patients have been found to have higher concentrations of fatty acids in serum when compared to healthy individuals [136–138]. As an example, in one early study it was proposed that an increase in free fatty acid concentrations was correlated with an increase in the free fraction of valproic acid in spiked serum samples from diabetic patients versus normal individuals [123]. In another study, a 20-fold decrease in binding strength was reported for the fatty acid cis-parinaric acid when comparing in vivo and in vitro glycated HSA with normal HSA [26]. Fatty acids can have many binding regions on HSA and often interact strongly with these sites [27,30,139–143]. In addition, some fatty acids can have direct competition with drugs on HSA or lead to allosteric effects during the binding of drugs and other solutes with this protein [140,141]. One recent study examined the combined effect of glycation and several long chain fatty acids on the overall binding of HSA to a number of sulfonylurea drugs [144]. The results showed an increase in the global affinity of glycated HSA for the drugs that were examined, and a similar decrease in affinity to both normal HSA and glycated HSA for these drugs in the presence of many common long chain fatty acids [144].
A question that has often been raised in the investigation of drug and solute binding to glycated HSA is the possible clinical relevance of these interactions. The answer will depend on the drug or solute that is being considered, the typical therapeutic or physiological range of the drug/solute, the regions on HSA that are involved in the interaction, and the degree of glycation for HSA. In some cases this effect can be minimal, as has been observed for phenytoin or diazepam in spiked serum [123,124] and warfarin in the presence of in vitro glycated HSA that has amounts of modification similar to those seen in diabetes [73,83,126]. For other solutes and drugs, there may be a large change in affinity or binding, as has been observed in serum, for in vivo glycated HSA, or for in vitro glycated HSA with glycation levels similar to those seen in diabetes. Examples of this latter case include L-tryptophan [74,126], cis-parinaric acid [26], bilirubin [26], and ketoprofen [119]. Although further data is needed on these effects for a broader range of drugs, the results that have already been obtained suggest that Sudlow site II may be more prone to Sudlow site I to such changes. This generalization is illustrated by studies that have compared the binding of glycated HSA to drugs and probes that are known to bind to these two sites [72–75,83,110,126,127].
Even drugs with moderate changes in affinity might be affected to a significant degree if they have a relatively narrow therapeutic range and/or undesirable effects at concentrations flanking this range. For instance, the sulfonylurea results in Table 2 for in vitro glycated HSA and similar data for in vivo glycated HSA [110] predict that the free, biologically-active fractions of these drugs could differ by 0.6- to 1.7-fold from what would be expected in serum at typical therapeutic concentrations and in the presence of only normal HSA. This difference is of concern for these drugs because a lower-than-expected free fraction would lead to improper control of blood glucose, while a higher-than-expected free fraction might lead to hypoglycemia [72,75,128,129,135,145]. In addition to these direct changes in the free drug fractions, another way glycation may affect the behavior of such solutes is through the changes in drug-drug interactions that might occur as the affinities of one or both drugs are altered at Sudlow sites I and II or other regions of HSA [74,128].
7. Conclusions
The glycation of HSA has been a topic of interest for several decades and especially over the last ten years. Part of this interest is related to the clinical use of glycated HSA as a biomarker for the control of blood glucose over short-to-intermediate periods of time. To meet this need, a number of methods are now available to clinical laboratories for either the direct or indirect measurement of glycated HSA. With such methods, glycated HSA can be used as a complementary biomarker to blood glucose and Hb1Ac for monitoring glycemic control in diabetic patients.
There has also been increasing interest in the effects glycation may have on the structure of HSA. A variety of methods have been employed for examining the number, location and types of modifications that can occur in glycated HSA. Many possible modification sites have been detected on this protein for both early stage glycation products and AGEs, but a smaller subset of these sites appear to take part in most of these reactions. Some information on the similarities, or differences, between in vivo and in vitro glycated HSA has been acquired through such work. The overall rate of glycation, the thermodynamics of this process, and the rate of modification at specific regions have been considered in such studies. The role of glycation conditions on the types of modifications that are formed has also been examined.
Related work has appeared on the possible effects glycation may have on the properties of HSA, such as its ability to bind to drugs and other small solutes. This research has again employed various methods and has examined a growing list of solutes. This work has included compounds that are known to bind to HSA at specific regions (e.g., Sudlow sites I and II) and solutes with more complex binding, such as drugs that have multi-site binding to HSA (e.g., sulfonylurea drugs and fatty acids). The results have shown that the effects of glycation on binding by HSA can vary from one solute to the next, even within the same group of compounds, and can vary for the same solute at different sites on HSA. These effects can be clinically significant and appear to vary with the extent of glycation and the glycation conditions.
Given the importance of HSA and the relatively complex nature of glycation, it is anticipated that more research will be conducted in the future to obtain further information on this process and on how it affects HSA. This information could lead to the development of more effective assays for measuring glycated HSA as a biomarker for diabetes control. Such data would also be expected to create a better understanding of how diabetes and glycation can alter the structure and function of proteins like HSA. This improved understanding could, in turn, result in new tools for studying the biochemical effects of diabetes and in the development of better treatment regimes for diabetic patients.
Highlights.
Glycation of albumin involves the non-enzymatic addition of glucose to this protein.
Glycated albumin is a short-to-intermediate term biomarker for glucose control.
The glycation of albumin can affects the structure and function of this protein.
The process of glycation can alter the ability of albumin to bind drugs and solutes.
Acknowledgments
This work was supported, in part, by the National Institutes of Health under grants R01 DK069629 and R01 GM044931.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.International Diabetes Federation diabetes atlas. 5. Brussels, Belgium: International Diabetes Federation; 2011. [Google Scholar]
- 2.National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2011. U.S. Centers for Disease Control; Atlanta, GA: 2011. [Google Scholar]
- 3.Nelson DL, Cox MM, editors. Lehninger principles of biochemistry. New York: W. H. Freeman and Company; 2005. [Google Scholar]
- 4.Hartog JWL, Voors AA, Bakker SJL, Smit AJ, Veldhuisen DJV. Advanced glycation end-products (AGEs) and heart failure: pathophysiology and clinical implications. Eur J Heart Fail. 2007;9:1146–55. doi: 10.1016/j.ejheart.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Olijhoeka JK, Graafb YVD, Bangaa JD, Algrab A, Rabelinka TJ, Visserena FLJ. The metabolic syndrome is associated with advanced vascular damage in patients with coronary heart disease, stroke, peripheral arterial disease or abdominal aortic aneurysm. Eur Heart J. 2004;25:342–48. doi: 10.1016/j.ehj.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Perneger TV, Brancati FL, Whelton PK, Klag MJ. End-stage renal disease attributable to diabetes mellitus. Ann Intern Med. 1994;121:912–18. doi: 10.7326/0003-4819-121-12-199412150-00002. [DOI] [PubMed] [Google Scholar]
- 7.Turk Z, Misur I, Turk N. Temporal association between lens protein glycation and cataract development in diabetic rats. Acta Diabetol. 1997;34:49–54. doi: 10.1007/s005920050066. [DOI] [PubMed] [Google Scholar]
- 8.Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention Diabetes Care. 1989;12:24–31. doi: 10.2337/diacare.12.1.24. [DOI] [PubMed] [Google Scholar]
- 9.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344:109–16. [PMC free article] [PubMed] [Google Scholar]
- 10.Maillard LC. Action des acides amines sur les sucres: formation des melanoidines par voie methodique. C R Acad Sci. 1912;154:66–8. [Google Scholar]
- 11.Nursten H. The Maillard reaction. Royal Society of Chemistry; Cambridge, UK: 2005. [Google Scholar]
- 12.Lapolla A, Fedele D, Reitano R, Bonfante L, Guizzo M, Seraglia R, et al. Mass spectrometric study of in vivo production of advanced glycation end-products/peptides. J Mass Spectrom. 2005;40:969–72. doi: 10.1002/jms.842. [DOI] [PubMed] [Google Scholar]
- 13.Lapolla A, Fedele D, Seraglia R, Traldi P. The role of mass spectrometry in the study of non-enzymatic protein glycation in diabetes: an update. Mass Spectrom Rev. 2006;25:775–97. doi: 10.1002/mas.20090. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Ames JM, Smith RD, Baynes JW, Metz TO. A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: probing the pathogenesis of chronic disease. J Proteome Res. 2009;8:754–69. doi: 10.1021/pr800858h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baynes JW, Thorpe SR, Murtiashaw MH. Nonenzymatic glucosylation of lysine residues in albumin. Method Enzymol. 1984;106:88–98. doi: 10.1016/0076-6879(84)06010-9. [DOI] [PubMed] [Google Scholar]
- 16.Perejda A, Uitto J. Nonenzymatic glycosylation of collagen and other proteins: relationship to development of diabetic complications. Collagen Rel Res. 1982;2:81–8. [PubMed] [Google Scholar]
- 17.Rogozinski S, Blumenfeld O, Seifter S. The nonenzymatic glycosylation of collagen. Arch Biochem Biophys. 1983;221:428–37. doi: 10.1016/0003-9861(83)90161-3. [DOI] [PubMed] [Google Scholar]
- 18.Jones RL, Cerami A. Nonenzymatic glycosylation of proteins in diabetes mellitus. Recent Adv Diabetes. 1984;1:173–180. [Google Scholar]
- 19.Peters T., Jr . All about albumin: biochemistry, genetics, and medical applications. Academic Press; San Diego: 1996. [Google Scholar]
- 20.Tietz NW, editor. Textbook of clinical chemistry. Saunders; Philadelphia: 1986. [Google Scholar]
- 21.Colmenarejo G. In silico prediction of drug-binding strengths to human serum albumin. Med Res Rev. 2003;23:275–301. doi: 10.1002/med.10039. [DOI] [PubMed] [Google Scholar]
- 22.Dockal M, Carter DC, Ruker F. The three recombinant domains of human serum albumin. J Biol Chem. 1999;274:29303–10. doi: 10.1074/jbc.274.41.29303. [DOI] [PubMed] [Google Scholar]
- 23.He XM, Carter DC. Atomic structure and chemistry of human serum albumin. Nature. 1992;358:209–15. doi: 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
- 24.Otagiri M. A molecular functional study on the interactions of drugs with plasma proteins. Drug Metab Pharmacokinet. 2005;20:309–23. doi: 10.2133/dmpk.20.309. [DOI] [PubMed] [Google Scholar]
- 25.Choi EJ, Foster MD. The role of specific binding in human serum albumin adsorption to self-assembled monolayers. Langmuir. 2002;18:557–61. [Google Scholar]
- 26.Shaklai N, Garlick RL, Bunn HF. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem. 1984;259:3812–7. [PubMed] [Google Scholar]
- 27.Curry S, Mandelkow H, Brick P, Franks N. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nature Struct Biol. 1998;5:827–35. doi: 10.1038/1869. [DOI] [PubMed] [Google Scholar]
- 28.Koyama H, Sugioka N, Uno A, Mori S, Nakajima K. Effects of glycosylation of hypoglycemic drug binding to serum albumin. Biopharm Drug Dispos. 1997;18:791–801. doi: 10.1002/(sici)1099-081x(199712)18:9<791::aid-bdd66>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Ascoli GA, Domenici E, Bertucci C. Drug binding to human serum albumin: abridged review of results obtained with high-performance liquid chromatography and circular dichroism. Chirality. 2006;18:667–79. doi: 10.1002/chir.20301. [DOI] [PubMed] [Google Scholar]
- 30.Simard JR, Zunszain PA, Ha CE, Yang JS, Bhagavan NV, Petitpas I, et al. Locating high-affinity fatty acid-binding sites on albumin by X-ray crystallography and NMR spectroscopy. Proc Natl Acad Sci USA. 2005;102:17958–63. doi: 10.1073/pnas.0506440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sudlow G, Birkett DJ, Wade DN. Characterization of two specific drug binding sites on human serum albumin. Mol Pharmacol. 1975;11:824–32. [PubMed] [Google Scholar]
- 32.Sudlow G, Birkett DJ, Wade DN. Further characterization of specific drug binding sites on human serum albumin. Mol Pharmacol. 1976;12:1052–61. [PubMed] [Google Scholar]
- 33.Sengupta A, Hage DS. Characterization of the binding of digitoxin and acetyldigitoxin to human serum albumin by high-performance affinity chromatography. J Chromatogr B. 1999;725:91–100. doi: 10.1016/s0378-4347(98)00589-1. [DOI] [PubMed] [Google Scholar]
- 34.Hage DS, Sengupta A. Studies of protein binding to non-polar solutes by using zonal elution and high-performance affinity chromatography: interactions of cis- and trans-clomiphene with human serum albumin in the presence of β-cyclodextrin. Anal Chem. 1998;70:4602–9. doi: 10.1021/ac980734i. [DOI] [PubMed] [Google Scholar]
- 35.Loun B, Hage DS. Chiral separation mechanisms in protein-based HPLC columns. I. Thermodynamic studies of R- and S-warfarin binding to immobilized human serum albumin. Anal Chem. 1994;66:3814–22. doi: 10.1021/ac00093a043. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Hage DS. Characterization of the binding and chiral separation of D- and L-tryptophan on a high-performance immobilized human serum albumin column. J Chromatogr. 1993;645:241–50. doi: 10.1016/0021-9673(93)83383-4. [DOI] [PubMed] [Google Scholar]
- 37.Cohen M. Measurement of circulating glycated proteins to monitor intermediate-term changes in glycaemic control. Eur J Clin Chem Clin Biochem. 1992;30:851–9. [PubMed] [Google Scholar]
- 38.Roohk HV, Zaidi AR. A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol. 2008;2:1114–21. doi: 10.1177/193229680800200620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guerin-Dubourg A, Catan A, Bourdon E, Rondeau P. Structural modifications of human albumin in diabetes. Diabetes Metab. 2012;38:171–8. doi: 10.1016/j.diabet.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem. 2001;47:153–63. [PubMed] [Google Scholar]
- 41.Zheng CM, Ma WY, Wu CC, Lu KC. Glycated albumin in diabetic patients with chronic kidney disease. Clin Chim Acta. 2012;413:1555–61. doi: 10.1016/j.cca.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 42.Cohen RM, Sacks DB. Comparing multiple measures of glycemia: how to transition from biomarker to diagnostic test? Clin Chem. 2012;58:1615–7. doi: 10.1373/clinchem.2012.196139. [DOI] [PubMed] [Google Scholar]
- 43.Lee EY, Lee BW, Kim D, Lee Y, Kim KJ, Kang EY, et al. Glycated albumin is a useful glycation index for monitoring fluctuating and poorly controlled type 2 diabetes patients. Acta Diabetol. 2011;48:167–72. doi: 10.1007/s00592-010-0242-0. [DOI] [PubMed] [Google Scholar]
- 44.Mehrotra R, Kalantar-Zadeh K, Adler S. Assessment of glycemic control in dialysis patients: glycosylated hemoglobin or glycated albumin? Clin J Am Soc Nephrol. 2011;6:1520–2. doi: 10.2215/CJN.04210511. [DOI] [PubMed] [Google Scholar]
- 45.Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33:2153–63. [PubMed] [Google Scholar]
- 46.Mashiba S, Uchida K, Okuda S, Tomita S. Measurement of glycated albumin by the nitroblue tetrazolium colorimetric method. Clin Chim Acta. 1992;212:3–15. doi: 10.1016/0009-8981(92)90133-b. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi K, Yoshimoto K, Hirauchi K, Uchida K. A novel colorimetric method for determination of glycated protein based on 2-keto-glucose release with hydrazine. Biol Pharm Bull. 1993;16:195–8. doi: 10.1248/bpb.16.195. [DOI] [PubMed] [Google Scholar]
- 48.Baker JR, Zyzak DV, Thorpe SR, Baynes JW. Chemistry of the fructosamine assay: D-glucosone is the product of oxidation of Amadori compounds. Clin Chem. 1994;40:1950–5. [PubMed] [Google Scholar]
- 49.Yatscoff RW, Tevaarwerk GJ, MacDonald JC. Quantification of nonenzymically glycated albumin and total serum protein by affinity chromatography. Clin Chem. 1984;30:446–9. [PubMed] [Google Scholar]
- 50.Uchida T, Kozuma T, Yasukawa K, Shima K. Glycated albumin analyzer. Methods Chromatogr. 1996;1:33–41. [Google Scholar]
- 51.Poduslo JF, Curran GL. Increased permeability across the blood-nerve barrier of albumin glycated in vitro and in vivo from patients with diabetic polyneuropathy. Proc Natl Acad Sci USA. 1992;89:2218–22. doi: 10.1073/pnas.89.6.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yasukawa K, Abe F, Shida N, Koizumi Y, Uchida T, Noguchi K, et al. High-performance affinity chromatography system for the rapid, efficient assay of glycated albumin. J Chromatogr. 1992;597:271–5. doi: 10.1016/0021-9673(92)80120-j. [DOI] [PubMed] [Google Scholar]
- 53.Hage DS, Anguizola JA, Bi C, Li R, Matsuda R, Papastavros E, et al. Pharmaceutical and biomedical applications of affinity chromatography: recent trends and developments. J Pharm Biomed Anal. 2012;69:93–105. doi: 10.1016/j.jpba.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hage DS. Affinity chromatography: a review of clinical applications. Clin Chem. 1999;45:593–615. [PubMed] [Google Scholar]
- 55.Cohen MP, Clements RS. Measuring glycated proteins: clinical and methodological aspects. Diabetes Technol Therapeut. 1999;1:57–69. doi: 10.1089/152091599317585. [DOI] [PubMed] [Google Scholar]
- 56.Cohen MP, Hud E. Measurement of plasma glycoalbumin levels with a monoclonal antibody based ELISA. J Immunol Methods. 1989;122:279–83. doi: 10.1016/0022-1759(89)90275-5. [DOI] [PubMed] [Google Scholar]
- 57.Ikeda K, Sakamoto Y, Kawasaki Y, Miyake T, Tanaka K, Urata T, et al. Determination of glycated albumin by enzyme-linked boronate immunoassay (ELBIA) Clin Chem. 1998;44:256–63. [PubMed] [Google Scholar]
- 58.Paroni R, Ceriotti F, Galanello R, Battista LG, Panico A, Scurati E, et al. Performance characteristics and clinical utility of an enzymatic method for the measurement of glycated albumin in plasma. Clin Biochem. 2007;40:1398–405. doi: 10.1016/j.clinbiochem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Kohzuma T, Koga M. Lucica GA-L glycated albumin assay kit. A new diagnostic test for diabetes mellitus. Mol Diagnost Therap. 2010;14:49–51. doi: 10.1007/BF03256353. [DOI] [PubMed] [Google Scholar]
- 60.Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta. 2002;324:61–71. doi: 10.1016/s0009-8981(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Dou C, Yuan C, Datta A. Development of an automated enzymatic assay for the determination of glycated serum protein in human serum. Clin Chem. 2005;51:1991–2. doi: 10.1373/clinchem.2005.053447. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Q, Tang N, Schepmoes AA, Phillips LS, Smith RD, Metz TO. Proteomic profiling of nonenzymatically glycated proteins in human plasma and erythrocyte membranes. J Proteome Res. 2008;7:2025–32. doi: 10.1021/pr700763r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dingari NC, Horowitz GL, Kang JW, Dasari RR, Barman I. Raman spectroscopy provides a powerful diagnostic tool for accurate determination of albumin glycation. PLoS One. 2012;7:e32406. doi: 10.1371/journal.pone.0032406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhernovaya OS, Tuchin VV, Meglinski IV. Monitoring of blood proteins glycation by refractive index and spectral measurements. Laser Phys Lett. 2008;5:460–4. [Google Scholar]
- 65.Hinton DJS, Ames JM. Analysis of glycated protein by capillary electrophoresis. Int Congress Ser. 2002;1245:471–4. [Google Scholar]
- 66.Pereira Morais MP, Mackay JD, Bhamra SK, Buchanan JG, James TD, Fossey JS, van den Elsen JMH. Analysis of protein glycation using phenylboronate acrylamide gel electrophoresis. Proteomics. 2010;10:48–58. doi: 10.1002/pmic.200900269. [DOI] [PubMed] [Google Scholar]
- 67.Martin GJ, Rand JS, Hickey SA. Separation of serum glycated proteins by agarose gel electrophoresis and nitroblue tetrazolium staining in diabetic and normal cats. Vet Clin Pathol. 2006;35:307–10. doi: 10.1111/j.1939-165x.2006.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 68.Thornalley PJ. Dicarbonyl intermediates in the Maillard reaction. Ann NY Acad Sci. 2005;1043:111–7. doi: 10.1196/annals.1333.014. [DOI] [PubMed] [Google Scholar]
- 69.Mendez DL, Jensen RA, McElroy LA, Pena JM, Esquerra RM. The effect of non-enzymatic glycation on the unfolding of human serum albumin. Arch Biochem Biophys. 2005;444:92–9. doi: 10.1016/j.abb.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 70.Fitzpatrick G, Duggan PF. The effect of non-enzymatic glycation on ligand binding to human serum albumin. Biochem Soc Trans. 1987;15:267–8. [Google Scholar]
- 71.Nakajou K, Watanabe H, Kragh-Hansen U, Maruyama T, Otagiri M. The effect of glycation on the structure, function and biological fate of human serum albumin as revealed by recombinant mutants. Biochim Biophys Acta. 2003;1623:88–97. doi: 10.1016/j.bbagen.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Joseph KS, Anguizola J, Jackson AJ, Hage DS. Chromatographic analysis of acetohexamide binding to glycated human serum albumin. J Chromatogr B. 2010;878:2775–81. doi: 10.1016/j.jchromb.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joseph KS, Anguizola J, Hage DS. Binding of tolbutamide to glycated human serum albumin. J Pharm Biomed Anal. 2011;54:426–32. doi: 10.1016/j.jpba.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joseph KS, Hage DS. The effects of glycation on the binding of human serum albumin to warfarin and L-tryptophan. J Pharm Biomed Anal. 2010;53:811–8. doi: 10.1016/j.jpba.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsuda R, Anguizola J, Joseph KS, Hage DS. High-performance affinity chromatography and the analysis of drug interactions with modified proteins: binding of gliclazide with glycated human serum albumin. Anal Bioanal Chem. 2011;401:2811–9. doi: 10.1007/s00216-011-5382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garlick RL, Mazer JS. The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J Biol Chem. 1983;258:6142–6. [PubMed] [Google Scholar]
- 77.Day JF, Thorpe SF, Baynes JW. Nonenzymatically glycosylated albumin. J Biol Chem. 1979;254:595–7. [PubMed] [Google Scholar]
- 78.Iberg N, Fluckiger R. Nonenzymatic glycosylation of albumin in vivo: identification of multiple glycosylated sites. J Biol Chem. 1986;261:13542–5. [PubMed] [Google Scholar]
- 79.Arif B, Jalaluddin M, Moinuddin A, Ahmad J, Arif Z, Alam K. Structural and immunological characterization of Amadori-rich human serum albumin: role in diabetes mellitus. Arch Biochem Biophys. 2012;522:17–25. doi: 10.1016/j.abb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 80.Zoellner H, Hou JY, Hochgrebe T, Poljak A, Duncan MW, Golding J, et al. Fluorometric and mass spectrometric analysis of nonenzymatic glycosylated albumin. Biochem Biophys Res Comm. 2001;284:83–9. doi: 10.1006/bbrc.2001.4924. [DOI] [PubMed] [Google Scholar]
- 81.Westwood ME, Thornalley PJ. Molecular characteristics of methylglyoxal-modified bovine and human serum albumins. Comparison with glucose-derived advanced glycation endproduct-modified serum albumins. J Prot Chem. 1995;14:359–72. doi: 10.1007/BF01886793. [DOI] [PubMed] [Google Scholar]
- 82.Barzegar A, Moosavi-Movahedi AA, Sattarahmady N, Hosseinpour-Faizi MA, Aminbakhsh M, Ahmad F, et al. Spectroscopic studies of the effects of glycation of human serum albumin on L-trp binding. Prot Pep Lett. 2007;14:13–8. doi: 10.2174/092986607779117191. [DOI] [PubMed] [Google Scholar]
- 83.Okabe N, Hashizume N. Drug binding properties of glycosylated human serum albumin as measured by fluorescence and circular dichroism. Biol Pharm Bull. 1994;17:16–21. doi: 10.1248/bpb.17.16. [DOI] [PubMed] [Google Scholar]
- 84.Howard MJ, Smales CM. NMR analysis of synthetic human serum albumin α-helix 28 identifies structural distortion upon Amadori modification. J Biol Chem. 2005;280:22582–9. doi: 10.1074/jbc.M501480200. [DOI] [PubMed] [Google Scholar]
- 85.Taneda S, Monnier VM. ELISA of pentosidine, an advanced glycation endproduct, in biological specimens. Clin Chem. 1994;40:1766–73. [PubMed] [Google Scholar]
- 86.Lee C, Yim MB, Chock PB, Yim YS, Kang SO. Oxidation-reduction properties of methylglyoxal-modified protein in relation to free radical generation. J Biol Chem. 1998;273:25272–8. doi: 10.1074/jbc.273.39.25272. [DOI] [PubMed] [Google Scholar]
- 87.Schleicher E, Wieland OH. Specific quantitation by HPLC of protein (lysine) bound glucose in human serum albumin and other glycosylated proteins. J Clin Chem Clin Biochem. 1981;19:81–7. doi: 10.1515/cclm.1981.19.2.81. [DOI] [PubMed] [Google Scholar]
- 88.Ahmed N, Argirov OK, Minhas HS, Cordeiro CAA, Thornalley PJ. Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate and application to Nε-carboxymethyl-lysine- and Nε-(1-carboxyethyl)lysine-modified albumin. Biochem J. 2002;364:1–14. doi: 10.1042/bj3640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahmed N, Thornalley PJ. Chromatographic assay of glycation adducts in human serum albumin glycated in vitro by derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and intrinsic fluorescence. Biochem J. 2002;364:15–24. doi: 10.1042/bj3640015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lui X, Scouten WH. In: Handbook of affinity chromatography. Hage DS, editor. Chap 8 CRC Press; Boca Raton, FL: 2006. [Google Scholar]
- 91.Zhang Q, Monroe ME, Schepmoes AA, Clauss RW, Gritsenko MA, Meng D, et al. Comprehensive identification of glycated peptides and their glycation motifs in plasma and erythrocytes of control and diabetic subjects. J Proteome Res. 2011;10:3076–88. doi: 10.1021/pr200040j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frolov A, Hoffman R. Analysis of Amadori peptides enriched by boronic acid affinity chromatography. Ann NY Acad Sci. 2008;1126:253–6. doi: 10.1196/annals.1433.060. [DOI] [PubMed] [Google Scholar]
- 93.Frolov A, Hoffmann R. Identification and relative quantification of specific glycation sites in human serum albumin. Anal Bioanal Chem. 2010;397:2349–56. doi: 10.1007/s00216-010-3810-9. [DOI] [PubMed] [Google Scholar]
- 94.Gadgil HS, Bondarenko PV, Treuheit MJ, Ren D. Screening and sequencing of glycated proteins by neutral loss scan LC/MS/MS method. Anal Chem. 2007;79:5991–9. doi: 10.1021/ac070619k. [DOI] [PubMed] [Google Scholar]
- 95.Kisugi R, Kouzuma T, Yamamoto T, Akizuki S, Miyamoto H, Someya Y, et al. Structural and glycation site changes of albumin in diabetic patient with very high glycated albumin. Clin Chim Acta. 2007;382:59–64. doi: 10.1016/j.cca.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 96.Lapolla A, Fedele D, Reitano R, Arico NC. Enzymatic digestion and mass spectrometry in the studies of advanced glycation end products/peptides. J Am Soc Mass Spectrom. 2004;15:496–509. doi: 10.1016/j.jasms.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 97.Priego-Capote F, Scherl A, Muller M, Waridel P, Lisacek F, Sanchez JC. Glycation isotopic labeling with 13C-reducing sugars for quantitative analysis of glycated proteins in human plasma. Mol Cell Proteomics. 2010;9:579–97. doi: 10.1074/mcp.M900439-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frost L, Chaudhry M, Bell T, Cohenford M. In vitro galactation of human serum albumin: analysis of the protein’s galactation sites by mass spectrometry. Anal Biochem. 2011;410:248–56. doi: 10.1016/j.ab.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahmed N, Thornalley PJ. Peptide mapping of human serum albumin modified by methylglyoxal in vitro and in vivo. Ann NY Acad Sci. 2005;1043:260–6. doi: 10.1196/annals.1333.031. [DOI] [PubMed] [Google Scholar]
- 100.Ahmed N, Dobler D, Dean M, Thornalley PJ. Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J Biol Chem. 2005;280:5724–32. doi: 10.1074/jbc.M410973200. [DOI] [PubMed] [Google Scholar]
- 101.Stefanowicz P, Kijewska M, Kluczyk A, Szewczuk Z. Detection of glycation sites in proteins by high-resolution mass spectrometry combined with isotopic labeling. Anal Biochem. 2010;400:237–43. doi: 10.1016/j.ab.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 102.Brancia FL, Bereszczak JZ, Lapolla A, Fedele D, Baccarin L, Seraglia R, Traldi P. Comprehensive analysis of glycated human serum albumin tryptic peptides by off-line liquid chromatography followed by MALDI analysis on a time-of-flight/curved field reflectron tandem mass spectrometer. J Mass Spectrom. 2006;41:1179–85. doi: 10.1002/jms.1083. [DOI] [PubMed] [Google Scholar]
- 103.Marotta E, Lapolla A, Fedelle D, Senesi A, Reitano R, Witt M, et al. Accurate mass measurements by Fourier transform mass spectrometry in the study of advanced glycation end products/peptides. J Mass Spectrom. 2003;38:196–205. doi: 10.1002/jms.431. [DOI] [PubMed] [Google Scholar]
- 104.Robb DA, Olufemi OS, Williams DA, Midgley JM. Identification of glycation at the N-terminus of albumin by gas chromatography-mass spectrometry. Biochem J. 1986;261:871–8. doi: 10.1042/bj2610871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barnaby OS, Wa C, Cerny RL, Clarke W, Hage DS. Quantitative analysis of glycation sites on human serum labeling using 16O/18O labeling and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Clin Chim Acta. 2010;411:1102–10. doi: 10.1016/j.cca.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barnaby OS, Cerny RL, Clarke W, Hage DS. Comparison of modification sites formed on human serum albumin at various stages of glycation. Clin Chim Acta. 2011;412:277–85. doi: 10.1016/j.cca.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wa C, Cerny RL, Clarke WA, Hage DS. Characterization of glycation adducts on human serum albumin by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Clin Chim Acta. 2007;385:48–60. doi: 10.1016/j.cca.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barnaby OS, Cerny RL, Clarke W, Hage DS. Quantitative analysis of glycation patterns in human serum albumin using 16O/18O-labeling and MALDI-TOF MS. Clin Chim Acta. 2011;412:1606–15. doi: 10.1016/j.cca.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee BS, Krishnanchettiar S, Lateef SS, Gupta S. Analyses of the in vitro non-enzymatic glycation of peptides/proteins by matrix-assisted laser desorption/ionization mass spectrometry. Int J Mass Spectrom. 2007;260:67–74. [Google Scholar]
- 110.Anguizola J, Joseph KS, Barnaby OS, Matsuda R, Alvarado G, Clarke W, et al. Development of affinity microcolumns for drug-protein binding studies in personalized medicine: interactions of sulfonylurea drugs with in vivo glycated human serum albumin. Anal Chem. 2013;85:4453–60. doi: 10.1021/ac303734c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thornalley PJ, Argirova M, Ahmed N, Mann VM, Argirov O, Dawnay A. Mass spectrometric monitoring of albumin in uremia. Kidney Int. 2000;58:2228–34. doi: 10.1111/j.1523-1755.2000.00398.x. [DOI] [PubMed] [Google Scholar]
- 112.Kato H, Hayase F, Shin DB, Oimomi M, Baba S. 3-Deoxyglucosone, an intermediate product of the Maillard reaction. Prog Clin Biol Res. 1989;304:69–84. [PubMed] [Google Scholar]
- 113.Stitt AW, Curtis TM. Advanced glycation and retinal pathology during diabetes. Pharmacol Rep. 2005;57:156–68. [PubMed] [Google Scholar]
- 114.Takeuchi M, Kikuchi S, Sasaki N, Suzuki T, Watai T, Iwaki M, et al. Involvement of advanced glycation end-products (AGEs) in Alzheimer’s disease. Curr Alzheimer Res. 2004;1:39–46. doi: 10.2174/1567205043480582. [DOI] [PubMed] [Google Scholar]
- 115.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 116.Thornalley PJ. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems-role in ageing and disease. Drug Metabol Drug Interact. 2008;23:125–150. doi: 10.1515/dmdi.2008.23.1-2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thornalley PJ. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem J. 2003;375:581–92. doi: 10.1042/BJ20030763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end products: a review. Diabetologia. 2001;44:129–46. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 119.Baraka-Vidot J, Guerin-Dubourg A, Bourdon E, Rondeau P. Impaired drug-binding capacities of in vitro and in vivo glycated albumin. Biochimie. 2012;94:1960–7. doi: 10.1016/j.biochi.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 120.Voziyan PA, Khalifah RG, Thibaudeau C, Yildiz A, Jacob J, Serianni AS, et al. Modification of proteins in vitro by physiological levels of glucose. J Biol Chem. 2003;278:46616–24. doi: 10.1074/jbc.M307155200. [DOI] [PubMed] [Google Scholar]
- 121.Syrovy I. Glycation of albumin: reaction with glucose, fructose, galactose, ribose or glyceraldehydes measured using four methods. J Biochem Biophys Meth. 1994;28:115–21. doi: 10.1016/0165-022x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 122.Mereish KA, Rosenberg H, Cobby J. Glucosylated albumin and its influence on salicylate binding. J Pharmaceut Sci. 1982;1:235–8. doi: 10.1002/jps.2600710223. [DOI] [PubMed] [Google Scholar]
- 123.Gatti G, Crema F, Attardo-Parrinello G, Fratino P, Aguzzi F, Perucca E. Serum protein binding of phenytoin and valproic acid in insulin-dependent diabetes mellitus. Therapeut Drug Monitor. 1987;9:389–91. doi: 10.1097/00007691-198712000-00005. [DOI] [PubMed] [Google Scholar]
- 124.McNamara PJ, Blouin RA, Brazzell RK. The protein binding of phenytoin, propranolol, diazepam and AL01576 (an aldose reductase inhibitor) in human and rat diabetic serum. Pharmaceut Res. 1988;5:261–5. doi: 10.1023/a:1015966402084. [DOI] [PubMed] [Google Scholar]
- 125.Ruiz-Cabello F, Erill S. Abnormal serum protein binding of acidic drugs in diabetes mellitus. Clin Pharmacol Ther. 1984;36:691–5. doi: 10.1038/clpt.1984.241. [DOI] [PubMed] [Google Scholar]
- 126.Jackson AJ, Anguizola J, Pfaunmiller EL, Hage DS. Use of entrapment and high-performance affinity chromatography to compare the binding of drugs and site-specific probes with normal and glycated human serum albumin. Anal Bioanal Chem. 2013;405:5833–841. doi: 10.1007/s00216-013-6981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Joseph KS, Hage DS. Characterization of the binding of sulfonylurea drugs to HSA by high-performance affinity chromatography. J Chromatogr B. 2010;878:1590–8. doi: 10.1016/j.jchromb.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Matsuda R, Anguizola J, Joseph KS, Hage DS. Analysis of drug interactions with modified proteins by high-performance affinity chromatography: binding of glibenclamide to normal and glycated human serum albumin. J Chromatogr A. 2012;1265:114–122. doi: 10.1016/j.chroma.2012.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tsuchiya S, Tamiko S, Sekiguchi S. Nonenzymatic glucosylation of human serum albumin and its influence on binding capacity of sulfonylurea drugs. Biochem Pharmacol. 1984;33:2967–71. doi: 10.1016/0006-2952(84)90595-1. [DOI] [PubMed] [Google Scholar]
- 130.Koizumi K, Ikeda C, Ito M, Suzuki J, Kinoshita T, Yasukawa K, et al. Influence of glycosylation on the drug binding of human serum albumin. Biomed Chromatogr. 1998;12:203–10. doi: 10.1002/(SICI)1099-0801(199807/08)12:4<203::AID-BMC736>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 131.Doucet J, Fresel J, Hue G, Moore N. Protein binding of digitoxin, valproate and phenytoin in sera from diabetics. Eur J Clin Pharmacol. 1993;45:577–9. doi: 10.1007/BF00315318. [DOI] [PubMed] [Google Scholar]
- 132.Bohney JP, Feldhoff RC. Effects of nonenzymatic glycosylation and fatty acids on tryptophan binding to human serum albumin. 1992;43:1829–34. doi: 10.1016/0006-2952(92)90717-w. [DOI] [PubMed] [Google Scholar]
- 133.Joseph KS, Moser AC, Basiaga S, Schiel JE, Hage DS. Evaluation of alternatives to warfarin as probes for Sudlow site I of human serum albumin: characterization by high-performance affinity chromatography. J Chromatogr A. 2009;1216:3492–500. doi: 10.1016/j.chroma.2008.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Conrad ML, Moser AC, Hage DS. Evaluation of indole-based probes for high-throughput screening of drug binding to human serum albumin: analysis by high-performance affinity chromatography. J Sep Sci. 2009;32:1145–55. doi: 10.1002/jssc.200800567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Davis SN. In: Goodman and Gilman’s the pharmacological basis of therapeutics. 11. Brunton LL, Lazo JS, Parker KL, editors. Ch 60 New York: McGraw Hill; 2006. [Google Scholar]
- 136.Hagstrom-Toft E, Hellstrom L, Moberg E. Lipolytic response during spontaneous hypoglycaemia in insulin-dependent diabetic subjects. Horm Metab Res. 1998;30:586–93. doi: 10.1055/s-2007-978938. [DOI] [PubMed] [Google Scholar]
- 137.Chase PH, Glasgow AM. Juvenile diabetes mellitus and serum lipids and lipoprotein levels. Am J Dis Children. 1976;130:1113–7. doi: 10.1001/archpedi.1976.02120110075010. [DOI] [PubMed] [Google Scholar]
- 138.Bomba-Opon D, Wielgos M, Szymanska M, Bablok L. Effects of free fatty acids on the course of gestational diabetes mellitus. Neuroendocrinol Lett. 2006;27:277–80. [PubMed] [Google Scholar]
- 139.Spector AA. Fatty acid binding to plasma albumin. J Lipid Res. 1975;16:165–179. [PubMed] [Google Scholar]
- 140.Noctor TAG, Wainer IW, Hage DS. Allosteric and competitive displacement of drugs from human serum albumin by octanoic acid, as revealed by high-performance liquid affinity chromatography, on a human serum albumin-based stationary phase. J Chromatogr. 1992;577:305–15. doi: 10.1016/0378-4347(92)80252-l. [DOI] [PubMed] [Google Scholar]
- 141.Bhattacharya AA, Grüne T, Curry S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J Mol Biol. 2000;303:721–32. doi: 10.1006/jmbi.2000.4158. [DOI] [PubMed] [Google Scholar]
- 142.Petitpas I, Grüne T, Bhattacharya AA, Curry S. Crystal structures of human serum albumin complexed with monounsaturated and polyunsaturated fatty acids. J Mol Biol. 2000;314:955–60. doi: 10.1006/jmbi.2000.5208. [DOI] [PubMed] [Google Scholar]
- 143.Richieri GVS, Anel A, Kleinfeld AM. Interactions of long-chain fatty acids and albumin: determination of free fatty acid levels using the fluorescent probe ADIFAB. Biochemistry. 1993;32:7574–80. doi: 10.1021/bi00080a032. [DOI] [PubMed] [Google Scholar]
- 144.Basiaga SB, Hage DS. Chromatographic studies of changes in binding of sulfonylurea drugs to human serum albumin due to glycation and fatty acids. J Chromatogr B. 2010;878:3193–7. doi: 10.1016/j.jchromb.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Harrower AD. Comparative tolerability of sulphonylureas in diabetes mellitus. Drug Saf. 2000;22:313–20. doi: 10.2165/00002018-200022040-00004. [DOI] [PubMed] [Google Scholar]