Summary
ArfGAPs are known to be involved in cargo sorting in COPI transport. However, the role of ArfGAPs in post-Golgi membrane traffic has not been defined. To determine the function of ArfGAPs in post-Golgi traffic, we used siRNA to examine each of 25 ArfGAPs for effects on cation-independent mannose 6-phosphate receptor (CIMPR) localization. We found that down-regulation of ArfGAP3 resulted in the peripheral localization of CIMPR. The effect was specific for ArfGAP3 and dependent on its GAP activity, because the phenotype was rescued by ArfGAP3 but not by ArfGAP1, ArfGAP2 or the GAP domain mutants of ArfGAP3. ArfGAP3 localized to the trans-Golgi network and early endosomes. In cells with reduced expression of ArfGAP3, Cathepsin D maturation was slowed and its secretion was accelerated. Also retrograde transport from the endosomes to the trans-Golgi network of endogenous CIMPR, but not truncated CIMPR lacking the luminal domain, was perturbed in cells with reduced expression of ArfGAP3. Furthermore the exit of epidermal growth factor receptor (EGFR) from the early endosomes and degradation of EGFR after EGF stimulation was slowed in cells with reduced expression of ArfGAP3. ArfGAP3 associates with Golgi-localized, γ-ear-containing, ADP-ribosylation factor binding proteins (GGAs), and ArfGAP3 knockdown reduces membrane association of GGAs. A possible mechanism explaining our results is that ArfGAP3 regulates transport from early endosomes to late endosomes. We suggest a model in which ArfGAP3 regulates Golgi association of GGA clathrin adaptors.
Keywords: ADP-ribosylation factor, ArfGTPase-activating protein, membrane traffic, Golgi apparatus, Mannose 6-phosphate receptor
Results and Discussion
ArfGAP3 depletion changed the distribution of CIMPR
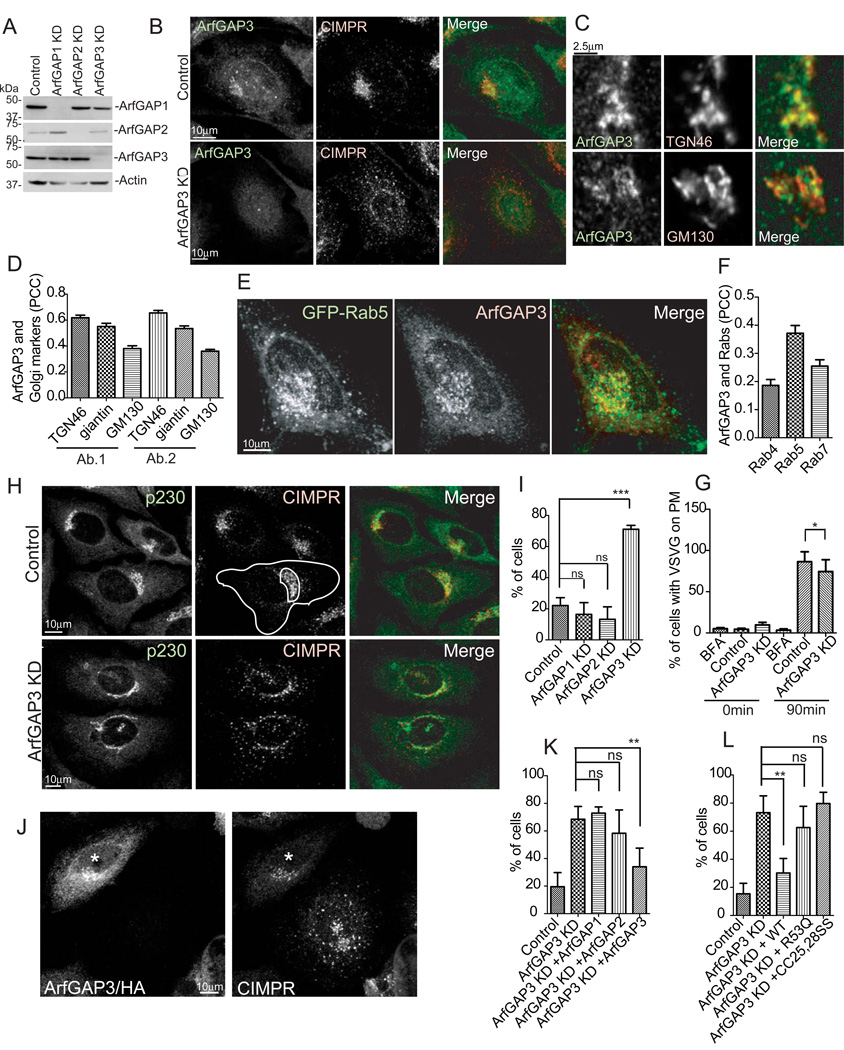
To screen for ArfGAPs that function in post-Golgi traffic we treated HeLa cells with siRNA targeting 25 ArfGAPs and examined the localization of CIMPR (see supplemental information). In cells transfected with ArfGAP3 siRNA, CIMPR was dispersed in the periphery (Figure 1B) as compared to control cells in which CIMPR concentrated in the juxtanuclear region. We confirmed that siRNA treated cells (henceforth called ArfGAP3 knockdown cells) had reduced levels of ArfGAP3 protein by western blot (Figure 1A) and immunofluorescence (Figure 1B).
Figure 1. CIMPR was redistributed to the periphery in ArfGAP3 knockdown (KD) cells.
A. HeLa cells were transfected with control, ArfGAP1, 2 or 3 siRNAs. 30 µg of the cell lysates were examined by immunoblotting. B. HeLa cells treated with either control or ArfGAP3 siRNA were double-stained with ArfGAP3 and CIMPR antibodies. C. HeLa cells were double-stained with anti-ArfGAP3 antibody and TGN46 or GM130. D. Z-stacks of images were analyzed for colocalization of ArfGAP3 with TGN46, giantin, and GM130. The average PCCs (n=30) are presented with S.E.M. Two different antibodies against ArfGAP3 were used (Ab.1 and Ab.2). E. Hela cells were transfected with GFP-Rab5 and stained with anti-ArfGAP3. F. Quantification of colocalization between ArfGAP3 and overexpressed Rabs (Rab4, Rab5 and Rab7). The average PCCs (n=30) are presented with S.E.M. G. VSVG ts045 transport to the cell surface was measured by flow cytometry. H. Control HeLa cells and ArfGAP3 KD cells were double-stained with CIMPR and p230. Total and Golgi area intensity of fluorescence were measured as shown by the outline. I. The percentage of cells with dispersed CIMPR was quantified for control and knockdown cells. J. Cells overexpressing HA-tagged ArfGAP3, indicated with the asterisk, retained a juxtanuclear localization of CIMPR. K. The effect of ArfGAP3 depletion on CIMPR distribution was reversed by ArfGAP3 but not ArfGAP1 or ArfGAP2 overexpression. L. GAP activity of ArfGAP3 was required for CIMPR localization. * indicates significantly different, p < 0.05; **, p < 0.01; ***, p < 0.001. ns, not significant.
ArfGAP3 is reported to be involved in COPI transport in the cis-Golgi [1–4] whereas our results indicated function of ArfGAP3 in post-Golgi trafficking. For a more detailed localization of ArfGAP3, we double-stained cells with antibodies against ArfGAP3 and the trans-Golgi network (TGN) marker TGN46, the medial-Golgi marker giantin, or the cis-Golgi marker GM130. ArfGAP3 colocalized with TGN46 to a greater extent than with GM130 (Figure 1 C, D). (Figure 1D). HA-tagged Rab4, GFP-tagged Rab5 and RFP-tagged Rab7 were overexpressed as markers for early/recycling, early and early/late endosomes. ArfGAP3 colocalized the most with Rab5, less with Rab7 and Rab4 (Figure 1E, F, Figure S1 A, B). Thus, ArfGAP3 predominantly localizes at the TGN and early endosomes, consistent with a function of ArfGAP3 in post-Golgi trafficking.
To rule out a general disruption of Golgi function in ArfGAP3 knockdown cells, we measured the transport of a temperature-sensitive mutant of Vesicular Stomatitis Virus G-protein (VSVG) to the cell surface (Figure 1G). We used cells treated with Brefeldin A (BFA), which disrupts the Golgi, as a positive control. In ArfGAP3 knockdown cells, transport was decreased only 12% compared to control cells. The distribution of Golgi marker proteins was not perturbed (Figure 1H, 2H; Figure S2 A and C; Figure 2J). Thus, the Golgi was largely intact and functional.
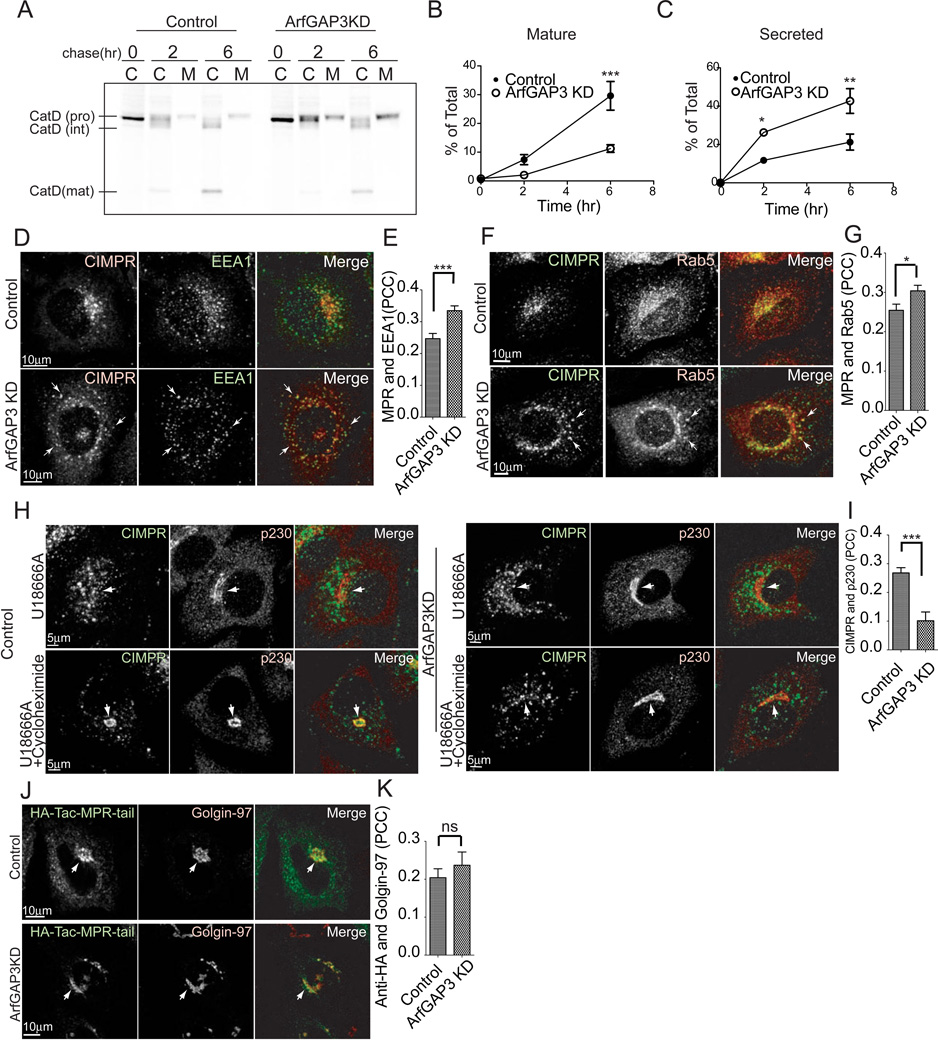
Figure 2. CIMPR transport is perturbed in ArfGAP3 KD cells.
A. Cells were pulse-labeled with [35S] methionine/cysteine for 1 hr, chased for 2 or 6 hr, and then cell-associated and medium-associated proteins were immunoprecipitated with anti-Cathepsin D antibody. A representative experiment out of three is shown. The lanes indicated C are the cell-associated and lanes labeled M are Medium-associated radiolabeled Cathepsin D. B. Mature Cathepsin D was decreased in ArfGAP3 KD cells. The amount of mature Cathepsin D detected in the autoradiograms presented in panel (A) was quantified. C. Quantification of pro-Cathepsin D detected in the cell culture media. More Cathepsin D was secreted in ArfGAP3 KD cells. D. Control and ArfGAP3 KD cells were double-stained with CIMPR and EEA1 antibodies. Arrows indicate colocalization of CIMPR with EEA1. E. Quantification of colocalization between CIMPR and EEA1. The average PCCs (n=30) are presented with S.E.M. F. Control and ArfGAP3 KD cells were double-stained with CIMPR and Rab5 antibodies. Arrows indicate colocalization of CIMPR with Rab5. G. Quantification of colocalization between CIMPR and Rab5. The average PCCs (n=30) are presented with S.E.M. H. Endosome to TGN transport of endogenous CIMPR is slowed in ArfGAP3 knockdown cells. Cells were treated with 3 µg/ml U18666A for 36 hr, thereby trapping CIMPR in endosomes. Cells were subsequently treated with 40 µg/ml cycloheximide for 3 hr, which results in the transport of CIMPR to the TGN in control cells but not in ArfGAP3 KD cells. The cells were stained with CIMPR and p230. Arrows indicate the Golgi area stained with p230. I. Quantification of colocalization between endogenous CIMPR and p230 in cells treated with U18666A and cycloheximide. The average PCCs (n=30) are presented with S.E.M. J. ArfGAP3 does not affect CIMPR lacking luminal domain. HeLa cells overexpressing HA-Tac-MPR-tail were incubated with anti-HA antibody for 3 min, washed and chased for 30 min. The cells were stained with Golgin-97. Arrows indicate HA-Tac-MPR-Tail localized at the Golgi. K. Quantification of colocalization between internalized anti-HA antibody and Golgin-97. The average PCCs (n=30) are presented with S.E.M. * indicates significant difference, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant.
We quantified CIMPR distribution (Figure 1H, see supplemental information). CIMPR localized to the periphery in 71% of ArfGAP3 knockdown cells in contrast to 22% of control cells (Figure 1I). Overexpression of ArfGAP3 rescued CIMPR dispersion due to ArfGAP3 knockdown (Figure 1J, K), excluding an off-target effect of the siRNA. ArfGAP3 is reported to function in ER-Golgi transport redundantly with ArfGAP1 and 2 [3]. However, CIMPR distribution in ArfGAP1 and ArfGAP2 knockdown cells was not different than in control cells (Figure 1I), and dispersion was not rescued by myc-tagged ArfGAP1 or HA-tagged ArfGAP2 (Figure 1K). These results indicate that the effect on CIMPR distribution was specific for ArfGAP3.
To determine whether ArfGAP3 GAP activity is required for CIMPR localization, we used two GAP-dead mutants, [R53Q]ArfGAP3 and [CC25,28SS]ArfGAP3. [R53Q]ArfGAP3 has a mutation in the catalytic arginine [5]. [CC25, 28SS]ArfGAP3 has double mutations in the GAP domain and is unable to bind to Arf•GTP and has no GAP activity. As shown in Figure 1L, neither mutant rescued the effect of ArGAP3 knockdown. The result indicates that GAP activity is required for ArfGAP3 function.
Reduced expression of ArfGAP3 perturbs the transport of CIMPR
To determine whether function of CIMPR is affected by ArfGAP3 knockdown, we examined processing and secretion of a ligand of CIMPR, the lysosomal enzyme Cathepsin D [6]. In pulse-chase experiments, we observed an increase of secreted Cathepsin D in the medium and a decrease of the mature form in ArfGAP3 knockdown cell lysates (Figure 2A-C).
To determine the compartment to which CIMPR was relocalized in ArfGAP3 knockdown cells we analyzed its colocalization with the early endosomal markers, EEA1 and Rab5. More CIMPR colocalized with EEA1 and Rab5 in ArfGAP3 knockdown cells than in controls (Figure 2D-G). Colocalization with the recycling endosome marker, transferrin (Figure S1 C, D) and the late endosome markers CD63 and Rab9 was unchanged (Figure S3A-D). These results indicate that CIMPR relocalized in part to early endosomes in ArfGAP3 knockdown cells.
Endocytosis and recycling of transferrin were unchanged (Figure S1E, F), suggesting constitutive endocytosis and recycling pathways were not perturbed in ArfGAP3 knockdown cells.
To determine whether retrograde transport from the endosome to the Golgi was affected in ArfGAP3 knockdown cells, we examined the transport of a construct that has the cytoplasmic tail of CIMPR conjugated with HA-tagged interleukin 2 receptor luminal and transmembrane regions (HA-Tac-MPR-tail). We internalized anti-HA antibody to follow the transport of HA-Tac-MPR-tail from the plasma membrane (PM) to the Golgi. In 30 min, anti-HA antibody reached the Golgi and colocalized with Golgin-97, a TGN marker similarly in control and ArfGAP3 knockdown cells (Figure 2J, K). We also examined the retrograde transport of Shiga toxin B subunit, the cytoplasmic tail of Furin conjugated with CD8, and the cytoplasmic tail of sortilin conjugated with HA-Tac, and found no differences between control and ArfGAP3 knockdown cells (Figure S2A-C). These results suggest that retrograde transport is not generally affected by ArfGAP3 depletion. We found that trafficking of the truncated MPR-tail differs from that of the full-length CIMPR (Figure S2D,E) consistent with previous studies [7]. We analyzed the retrograde transport of GFP-MPR-full by internalizing anti-GFP antibody for 30 min (Figure S2F). Internalized anti-GFP colocalized with TGN46 less in ArfGAP3 knockdown cells than in control cells (Figure S2F-H). Due to the low efficiency of transport of internalized GFP-MPR-full to the Golgi in control cells (Figure S2G), we used U18666A, which causes the accumulation of cholesterol in late endosomes and redistributes CIMPR to endosomal structures [7, 8, 9]. This peripherally localized CIMPR is shifted to the TGN upon cycloheximide treatment [7]. CIMPR was redistributed to the periphery in control and ArfGAP3 knockdown cells in the presence of U18666A (Figure 2H). Upon treatment with cycloheximide, CIMPR was transported back to the TGN in control cells but remained in the periphery in ArfGAP3 knockdown cells (Figure 2H, I). These results indicate that the transport from the endosome to the TGN of full length CIMPR is specifically perturbed in ArfGAP3 knockdown cells.
The transport of epidermal growth factor receptor was also perturbed in ArfGAP3 knockdown cells
We next determined if the degradation pathway was affected by ArfGAP3 knockdown. After confirming equivalent levels of steady-state CIMPR expression between control and ArfGAP3 knockdown cells (Figure S3E), we monitored CIMPR degradation after treatment with cyclohexamide. (Figure S3F). No difference was detected between control and ArfGAP3 knockdown cells (Figure S3F). However, CIMPR was only slowly degraded, with significant CIMPR remaining after 7.5hr of cycloheximide treatment in control cells, making detection of a small effect on the degradation pathway difficult.
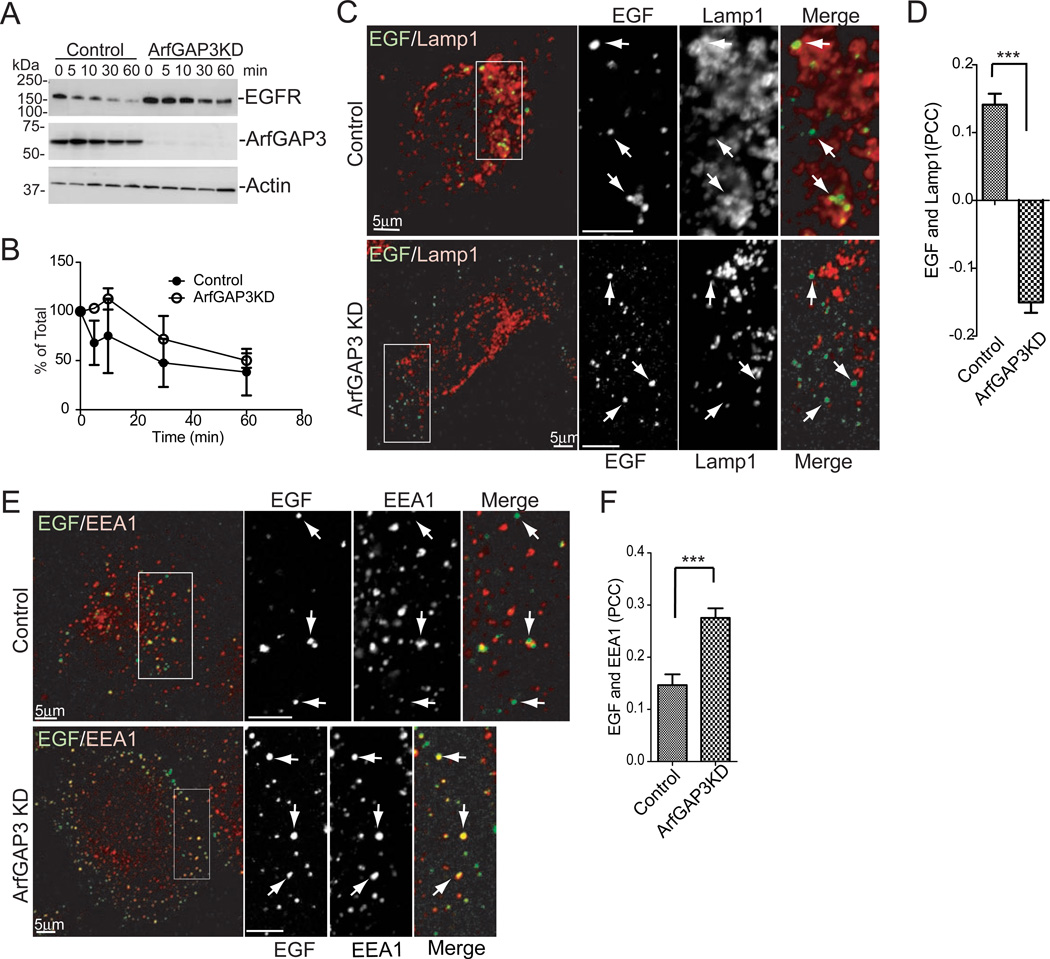
We next examined the degradation of Epidermal Growth Factor Receptor (EGFR). Upon EGF stimulation, EGFR is efficiently transported to the lysosomes via early and late endosomes [10]. In ArfGAP3 knockdown cells, the degradation of EGFR was slower than in control cells (Figure 3A, B). This effect could be due to a decrease in lysosomal enzyme activity, or a block in transport of EGFR to late endosomes/lysosomes. EGF partially colocalized with the lysosomal marker Lamp1 after 30 min of internalization in control cells treated with the lysosomal inhibitor leupeptin whereas colocalization was significantly reduced in leupeptin-treated ArfGAP3 knockdown cells (Figure 3C,D), indicating the transport of EGF to the late endosomes/lysosomes is perturbed in ArfGAP3 knockdown cells. Colocalization of EGF with the early endosome marker EEA1 was increased in ArfGAP3 knockdown cells (Figure 3E, F), indicating the transport of EGF out of early endosomes is slowed. We found no significant difference in the colocalization of the fluid-phase marker BSA with Lamp1 in control and ArfGAP3 knockdown cells (Figure S3G and H). We also assayed endosomal acidification with transferrin conjugated with fluorescein isothiocyanate and found via the null point method [11] that endosomal acidification was not perturbed in ArfGAP3 knockdown cells (data not shown). These results indicate that the effect of ArfGAP3 knockdown is specific for EGFR and lysosomal biogenesis is not significantly perturbed.
Figure 3. EGFR transport is perturbed in ArfGAP3 KD cells.
A. Effect of ArfGAP3 depletion on EGFR degradation. The cells were serum starved overnight then treated with EGF for the indicated times. EGFR levels were measured by immunoblotting. A representative experiment (out of three) is shown. B. Quantification of EGFR degradation in ArfGAP3 depleted cells. Immunoblots from three experiments were quantified by densitometry using ImageJ. The values at each time point were normalized to time 0. Average values ± S.E.M. are plotted. C. ArfGAP3 KD decreased targeting of EGF to the LAMP1 compartment. The cells were treated with 1 mg/ml Leupeptin for 4 hr, then serum starved for 1hr. Alexa Fluor 488 conjugated EGF was internalized for 3 min, washed and chased for 30 min. The cells were fixed and stained with Lamp1. Arrows indicate structures containing EGF. D. Quantification of colocalization between EGF and Lamp1 for experiments described in (C). The average PCCs (n=30) are presented with S.E.M. E. The cells were treated as in B and C and stained with EEA1. Arrows indicate structures containing EGF. F. Quantification of colocalization between EGF and EEA1. The average PCCs (n=30) are presented with S.E.M. *** indicates significant difference, p < 0.001
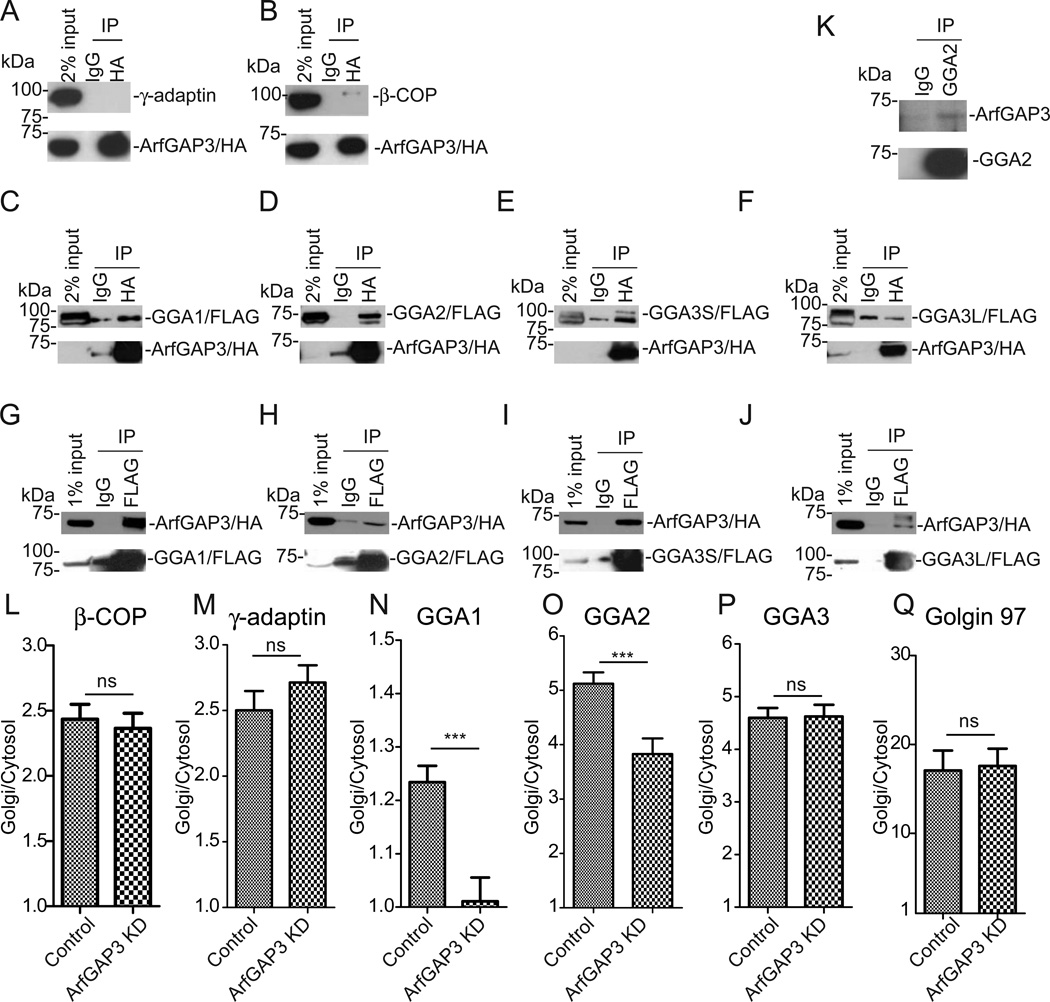
Our results indicate that ArfGAP3 functions at the TGN-endosome interface. We examined if ArfGAP3 associates with TGN/endosomal coat proteins. Clathrin adaptor AP-1 and Golgi-localized, γ-ear-containing, ADP-ribosylation factor binding proteins (GGAs) are known to regulate MPR transport [12–15]. Also, ArfGAP3 is thought to regulate COPI in the early secretory pathway [1–4] and possibly endosomal COPI [16–21]. First, to examine if AP-1 or COPI associates with ArfGAP3, we overexpressed HA-tagged ArfGAP3 in HEK 293 cells, immunoprecipitated with anti-HA antibody, and blotted for an AP-1 or COPI subunit (Figure 4A and 4B). We could not detect the association of AP-1 with ArfGAP3 (Figure 4A) whereas we detected the association of COPI with ArfGAP3 (Figure 4B), consistent with a previous report [22]. Mammalian GGAs have three isoforms, GGA1, GGA2, and GGA3 [23, 24]. GGA3 has two splicing variants, a ubiquitously expressed short form (GGA3S) and a brain specific long form (GGA3L) [25, 26]. The short form lacks the binding site for CIMPR [25]. To examine if GGAs associate with ArfGAP3, we double transfected cells with HA-tagged ArfGAP3 and FLAG-tagged GGA1, GGA2, GGA3S or GGA3L, and performed immunoprecipitation with anti-HA. We found that FLAG-tagged GGA1, GGA2, and GGA3S were co-precipitated with HA-tagged ArfGAP3 (Figure 4C-E), whereas FLAG-tagged GGA3L was not (Figure 4F). We also performed immunoprecipitation with anti-FLAG and then blotted with anti-HA (Figure 4GJ). In these experiments, HA-tagged ArfGAP3 was co-precipitated with all GGAs (Figure 4G-J). We performed immunoprecipitation from untransfected cells with anti-GGA2 antibody (Figure 4K). ArfGAP3 precipitated with GGA2 (Figure 4K), indicating endogenous ArfGAP3 and GGA2 associate.
Figure 4. ArfGAP3 associates with GGAs.
A,B. 293T cells were transfected with HA-tagged ArfGAP3 and immunoprecipitated with anti-HA, blotted with anti-γ-adaptin (AP-1 subunit) (A) or anti-β-COP (COPI subunit) (B). C-F. 293T cells were double-transfected with HA-tagged ArfGAP3 and FLAG-tagged GGA1 (C), GGA2 (D), GGA3S (E), GGA3L (F), immunoprecipitated with anti-HA and blotted with anti-FLAG antibody. G-H. 293T cells were double-transfected with HA-tagged ArfGAP3 and FLAG-tagged GGA1 (G), GGA2 (H), GGA3S (I), GGA3L (J), immunoprecipitated with anti-FLAG and blotted with anti-HA antibody. K. Coimmunoprecipitation was performed with anti-GGA2 antibody and blotted with anti-ArfGAP3. A representative experiment (out of three) is shown. L-QHeLa cells were stained with β-COP (L), γ-adaptin (M), GGA1 (N), GGA2 (O), GGA3 (P) and Golgin 97 (Q). Quantification of the ratio of Golgi/Cytosol was presented with S.E.M in control and ArfGAP3 KD cells. * indicates significant difference, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant.
We next examined whether ArfGAP3 affected the association of coat proteins with the TGN/endosomes. ArfGAP3 is an ArfGAP that catalyzes GTP hydrolysis on Arf. We reasoned that if ArfGAP3 is down-regulated in cells, Arf•GTP would accumulate, and this accumulation would increase the efficiency of recruitment of coat proteins to membrane. We examined membrane association of coat proteins using image-based analysis (see supplementary information, Figure S4 A and B). We measured the ratio of Golgi/Cytosol for ; AP-1, COPI, GGA1,GGA2, GGA3, and Golgin-97 (Golgin-97 is reported to be recruited to the Golgi membrane by Arf-like protein, Arl1 [27]) (Figure 4L-Q), in control and ArfGAP3 knockdown cells. We found no difference in Golgi association of COPI, AP-1, GGA3 and Golgin 97 (Figure 4L, M, P and Q). In contrast, we found a small but significant decrease in Golgi/endosomal association of GGA1 and GGA2 (Figure 4N and 4O). Our results indicate that ArfGAP3 regulates GGA1/2.
We suggest that ArfGAP3 affects transport from early endosomes to late endosomes. First, CIMPR is partly relocalized to early endosomes in ArfGAP3 knockdown cells (Figure 2D-G). Second, ArfGAP3 affected EGFR transport from early to late endosomes (Figure 3E, F). Third, a significant pool of ArfGAP3 localized at early endosomes (Figure 1E, F). Fourth, ArfGAP3 affected transport of full-length CIMPR but not truncated CIMPR lacking the luminal region. Waguri et al [7] proposed that the luminal region of CIMPR has a role in endosomal retention of the receptor. Full-length CIMPR is thought to be transported from early to late endosomes and then returns to the TGN [30], whereas truncated CIMPR is possibly transported from early endosomes to the TGN directly [31]. An effect of ArfGAP3 knockdown on full-length CIMPR and EGFR but not on truncated CIMPR is most easily explained if ArfGAP3 affects transport from early to late endosomes. We also considered the possibility that ArfGAP3 regulates CIMPR transport from late endosomes to the TGN, the step affected by knockdown of GCC188 and by RhoBTB3 [32–34]. However, in the case of knockdown of GCC188 or RhoBTB3, CIMPR colocalized with Rab9 but not EEA1. Our working hypothesis is summarized in Figure S4C.
We found that ArfGAP3 associates with GGAs and Golgi/endosomal association of GGA1 and GGA2 is decreased in ArfGAP3 knockdown cells. These findings are not predicted by the standard model in which Arf•GTP recruits coat and the GAP functions as a negative regulator of coat binding to membrane. However, evidence is accumulating that the ArfGAPs can have a positive function related to coat assembly [28, 29, 35–37]. It is plausible that the decreased association of GGA1 and GGA2 with the Golgi/endosomal compartment on ArfGAP3 knockdown reflects ArfGAP3 functions as an Arf effector, interacting with GGA1/2 and driving the assembly of GGA1/2 to promote formation of vesicles, rather than functioning solely as a negative regulator of Arf.
In summary, we report the unexpected finding that ArfGAP3 functions non-redundantly in the post-Golgi compartment where it regulates transport of CIMPR and EGFR. Ongoing studies are focused on identifying binding partners of ArfGAP3 as well as other approaches for elucidating its role in cargo sorting.
Supplementary Material
Highlights.
Transport of the cation independent mannose-6-phosphate receptor requires ArfGAP3.
Lysosomal degradation of the epidermal growth factor receptor requires ArfGAP3.
ArfGAP3 binds to GGAs.
ArfGAP3 depletion reduces membrane association of GGA1/2.
Acknowledgments
We thank Elizabeth Sztul for helpful discussions. The work was supported by NIH Intramural Program (Project BC 007365).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Frigerio G, Grimsey N, Dale M, Majoul I, Duden R. Two human ARFGAPs associated with COP-I-coated vesicles. Traffic. 2007;8:1644–1655. doi: 10.1111/j.1600-0854.2007.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weimer C, Beck R, Eckert P, Reckmann I, Moelleken J, Brugger B, Wieland F. Differential roles of ArfGAP1, ArfGAP2, and ArfGAP3 in COPI trafficking. J Cell Biol. 2008;183:725–735. doi: 10.1083/jcb.200806140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saitoh A, Shin HW, Yamada A, Waguri S, Nakayama K. Three homologous ArfGAPs participate in coat protein I-mediated transport. J Biol Chem. 2009;284:13948–13957. doi: 10.1074/jbc.M900749200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kartberg F, Asp L, Dejgaard SY, Smedh M, Fernandez-Rodriguez J, Nilsson T, Presley JF. ARFGAP2 and ARFGAP3 are essential for COPI coat assembly on the Golgi membrane of living cells. J Biol Chem. 2010;285:36709–36720. doi: 10.1074/jbc.M110.180380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo R, Ahvazi B, Amariei D, Shroder D, Burrola B, Losert W, Randazzo PA. Kinetic analysis of GTP hydrolysis catalysed by the Arf1-GTP-ASAP1 complex. Biochem J. 2007;402:439–447. doi: 10.1042/BJ20061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Figura K, Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]
- 7.Waguri S, Tomiyama Y, Ikeda H, Hida T, Sakai N, Taniike M, Ebisu S, Uchiyama Y. The luminal domain participates in the endosomal trafficking of the cation-independent mannose 6-phosphate receptor. Exp Cell Res. 2006;312:4090–4107. doi: 10.1016/j.yexcr.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T, Beuchat MH, Lindsay M, Frias S, Palmiter RD, Sakuraba H, Parton RG, Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- 9.Tomiyama Y, Waguri S, Kanamori S, Kametaka S, Wakasugi M, Shibata M, Ebisu S, Uchiyama Y. Early-phase redistribution of the cation-independent mannose 6-phosphate receptor by U18666A treatment in HeLa cells. Cell Tissue Res. 2004;317:253–264. doi: 10.1007/s00441-004-0941-3. [DOI] [PubMed] [Google Scholar]
- 10.Haglund K, Dikic I. The role of ubiquitylation in receptor endocytosis and endosomal sorting. J Cell Sci. 2012;125:265–275. doi: 10.1242/jcs.091280. [DOI] [PubMed] [Google Scholar]
- 11.Yamashiro DJ, Maxfield FR. Acidification of morphologically distinct endosomes in mutant and wild-type Chinese hamster ovary cells. J Cell Biol. 1987;105:2723–2733. doi: 10.1083/jcb.105.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer C, Zizioli D, Lausmann S, Eskelinen EL, Hamann J, Saftig P, von Figura K, Schu P. mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000;19:2193–2203. doi: 10.1093/emboj/19.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh P, Griffith J, Geuze HJ, Kornfeld S. Mammalian GGAs act together to sort mannose 6-phosphate receptors. J Cell Biol. 2003;163:755–766. doi: 10.1083/jcb.200308038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirst J, Sahlender DA, Choma M, Sinka R, Harbour ME, Parkinson M, Robinson MS. Spatial and functional relationship of GGAs and AP-1 in Drosophila and HeLa cells. Traffic. 2009;10:1696–1710. doi: 10.1111/j.1600-0854.2009.00983.x. [DOI] [PubMed] [Google Scholar]
- 15.Puertollano R, Aguilar RC, Gorshkova I, Crouch RJ, Bonifacino JS. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science. 2001;292:1712–1716. doi: 10.1126/science.1060750. [DOI] [PubMed] [Google Scholar]
- 16.Aniento F, Gu F, Parton RG, Gruenberg J. An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu F, Aniento F, Parton RG, Gruenberg J. Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J Cell Biol. 1997;139:1183–1195. doi: 10.1083/jcb.139.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daro E, Sheff D, Gomez M, Kreis T, Mellman I. Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP. J Cell Biol. 1997;139:1747–1759. doi: 10.1083/jcb.139.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu F, Gruenberg J. ARF1 regulates pH-dependent COP functions in the early endocytic pathway. J Biol Chem. 2000;275:8154–8160. doi: 10.1074/jbc.275.11.8154. [DOI] [PubMed] [Google Scholar]
- 20.Gabriely G, Kama R, Gerst JE. Involvement of specific COPI subunits in protein sorting from the late endosome to the vacuole in yeast. Mol Cell Biol. 2007;27:526–540. doi: 10.1128/MCB.00577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razi M, Chan EY, Tooze SA. Early endosomes and endosomal coatomer are required for autophagy. J Cell Biol. 2009;185:305–321. doi: 10.1083/jcb.200810098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson PJ, Frigerio G, Collins BM, Duden R, Owen DJ. Gamma-COP appendage domain - structure and function. Traffic. 2004;5:79–88. doi: 10.1111/j.1600-0854.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 23.Boman AL, Zhang C, Zhu X, Kahn RA. A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol Biol Cell. 2000;11:1241–1255. doi: 10.1091/mbc.11.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dell'Angelica EC, Puertollano R, Mullins C, Aguilar RC, Vargas JD, Hartnell LM, Bonifacino JS. GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J Cell Biol. 2000;149:81–94. doi: 10.1083/jcb.149.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takatsu H, Katoh Y, Shiba Y, Nakayama K. Golgi-localizing, gamma-adaptin ear homology domain, ADP-ribosylation factor-binding (GGA) proteins interact with acidic dileucine sequences within the cytoplasmic domains of sorting receptors through their Vps27p/Hrs/STAM (VHS) domains. J Biol Chem. 2001;276:28541–28545. doi: 10.1074/jbc.C100218200. [DOI] [PubMed] [Google Scholar]
- 26.Wakasugi M, Waguri S, Kametaka S, Tomiyama Y, Kanamori S, Shiba Y, Nakayama K, Uchiyama Y. Predominant expression of the short form of GGA3 in human cell lines and tissues. Biochem Biophys Res Commun. 2003;306:687–692. doi: 10.1016/s0006-291x(03)01032-5. [DOI] [PubMed] [Google Scholar]
- 27.Lu L, Hong W. Interaction of Arl1-GTP with GRIP domains recruits autoantigens Golgin-97 and Golgin-245/p230 onto the Golgi. Mol Biol Cell. 2003;14:3767–3781. doi: 10.1091/mbc.E03-01-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiba Y, Luo R, Hinshaw JE, Szul T, Hayashi R, Sztul E, Nagashima K, Baxa U, Randazzo PA. ArfGAP1 promotes COPI vesicle formation by facilitating coatomer polymerization. Cell Logist. 2011;1:139–154. doi: 10.4161/cl.1.4.18896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiba Y, Randazzo PA. ArfGAP1 function in COPI mediated membrane traffic: currently debated models and comparison to other coat-binding ArfGAPs. Histol Histopathol. 2012;27:1143–1153. doi: 10.14670/HH-27.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeffer SR. Multiple routes of protein transport from endosomes to the trans Golgi network. FEBS Lett. 2009;583:3811–3816. doi: 10.1016/j.febslet.2009.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165:111–122. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy JV, Burguete AS, Sridevi K, Ganley IG, Nottingham RM, Pfeffer SR. A functional role for the GCC185 golgin in mannose 6-phosphate receptor recycling. Mol Biol Cell. 2006;17:4353–4363. doi: 10.1091/mbc.E06-02-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Espinosa EJ, Calero M, Sridevi K, Pfeffer SR. RhoBTB3: a Rho GTPase-family ATPase required for endosome to Golgi transport. Cell. 2009;137:938–948. doi: 10.1016/j.cell.2009.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown FC, Schindelhaim CH, Pfeffer SR. GCC185 plays independent roles in Golgi structure maintenance and AP-1-mediated vesicle tethering. J Cell Biol. 2011;194:779–787. doi: 10.1083/jcb.201104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.East MP, Kahn RA. Models for the functions of Arf GAPs. Semin Cell Dev Biol. 2011;22:3–9. doi: 10.1016/j.semcdb.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SY, Yang JS, Hong W, Premont RT, Hsu VW. ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J Cell Biol. 2005;168:281–290. doi: 10.1083/jcb.200404008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang JS, Lee SY, Gao M, Bourgoin S, Randazzo PA, Premont RT, Hsu VW. ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J Cell Biol. 2002;159:69–78. doi: 10.1083/jcb.200206015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.