Abstract
Objective
To evaluate ankle joint abnormalities in a knee osteoarthritis (OA) cohort.
Methods
Participants (n=159) with symptomatic and radiographic OA in at least one knee underwent technetium-99m methylene diphosphonate bone scan (scored 0–3) of the ankles and forefeet. Knee radiographs were graded for OA features of joint space narrowing (JSN) and osteophyte (OST). Ankle symptoms and history of ankle injury were assessed by self-report. Knee alignment was measured from a long-limb radiograph. Ankle radiographs were obtained on those who returned for follow-up (n=138) and were graded for ankle tibiotalar JSN and OST.
Design
Ankle scintigraphic abnormalities were frequent (31% of individuals, one-third bilateral). Ankle symptoms were reported by 23% of individuals and history of ankle injury by 24%. Controlling for gender, age, BMI, and contralateral predictor, ankle scintigraphic abnormalities were associated with: ipsilateral ankle symptoms (P=0.005); contralateral knee JSN (P=0.001), knee OST (P=0.006) and knee malalignment (P=0.08); and history of ankle injury or surgery of either ankle (P<0.0001). At follow-up, scintigraphic abnormalities of the ankle were strongly associated with presence of tibiotalar radiographic OA (P<0.0001).
Conclusions
Although considered rare, we observed a high prevalence of radiographic features of ankle OA in this knee OA cohort. History of overt ankle injury did not appear to account for the majority of ankle abnormalities. These results are consistent with a probable kinematic association of knee OA pathology and contralateral ankle abnormalities and suggest that interventions targeting mechanical factors may be needed to prevent ankle OA in the setting of knee OA.
Keywords: osteoarthritis, ankle, knee, scintigraphy, alignment
Introduction
The ankle joint has been a particular focus of investigations for its apparent resistance to osteoarthritis (OA) compared with sites such as the knee [1]. The difference in OA susceptibility and prevalence between knee and ankle joints has been posited to be due to different repair responses of these two joints based on differences in their metabolic, biochemical and biomechanical properties [1–11]. The literature related to the prevalence of symptomatic ankle OA is limited, but available references are in general agreement that ankle OA is rare--occurring at a rate of only 1–4% [12]. Trauma is generally believed to account for as much as 80% of ankle OA [13–16]; whereas trauma is believed to account for only 12% of knee OA [15].
When ankle OA does occur, it has a major impact on quality of life. According to one study, the impairment associated with ankle OA, as measured with the SF-36 standardized health survey, is equivalent to end-stage renal disease or congestive heart failure [17]. Therefore, it would be advantageous to gain a greater understanding of the etiologies of ankle OA and develop strategies to prevent it. Although it has been established that OA in a lower extremity joint can affect other kinematically related joints of the lower extremities, the vast majority of studies have focused on the interaction of knees and hips [18–21]. In sum, these studies have revealed a marked increased risk of OA in a contralateral cognate joint (knee/knee, hip/hip), and a modest increased risk of OA in a contralateral non-cognate joint (knee/hip, hip/knee). Three previous studies evaluated the association of knee and ankle OA [22–24]. These revealed an increased risk of ankle OA ipsilateral to knee OA; possible contralateral associations controlling for ipsilateral disease were not explored.
The goal of our study was to gain insights into the high prevalence of ankle abnormalities observed in our Prediction of Osteoarthritis Progression (POP) knee OA cohort [25, 26]. We hypothesized that knee OA would influence the prevalence and severity of ankle abnormalities.
Methods
Participants
All 159 participants of the NIH sponsored POP study were included in this substudy, which was approved in accordance with the policies of the Duke Institutional Review Board. Recruitment was independent of foot or ankle status and based solely on knee related factors. Participants met American College of Rheumatology criteria for symptomatic OA of at least one knee; they also met radiographic criteria for OA with a Kellgren-Lawrence (KL) [27] score of 1–3 in at least one knee. Knee symptoms were ascertained by the National Health and Nutrition Examination Survey I criterion of pain, aching or stiffness on most days of any one month in the past year [28]. The overall distribution of knee KL grades was: KL0 2%; KL1 22%, KL2 20%, KL3 44%, and KL4 9% (at baseline 3% of knees were replaced). A total of 96% of participants had evidence of bilateral radiographic OA based on an OA definition of KL>1 or TKR for OA; a total of 87% had bilateral knee symptoms. KL grade was used as an inclusion criterion, however, this substudy utilized the more precise individual radiographic features of knee OA for all analyses. Exclusion criteria included the following: bilateral knee KL4 scores; exposure to a corticosteroid (either parenteral or oral) within 3 months prior to the study evaluation; knee arthroscopic surgery within 6 months prior to the study evaluation; history of avascular necrosis, inflammatory arthritis, Paget’s disease, joint infection, periarticular fracture, neuropathic arthropathy, reactive arthritis, or gout; and current anticoagulation. A total of 308 non-replaced knees and 318 ankles were available for analysis from the 159 study participants. At baseline, ankle symptoms (“joint that has bothered you in the last year”), and a history of ankle joint injury and ankle surgery were ascertained by self-report. A total of 138 participants returned for 3-year follow-up at which time it was possible to obtain more explicit information on ankle injury and surgery for both ankles as follows: “Has a doctor ever told you that you broke or fractured your ankle?”; “Do you have a history of ankle surgery?”; “Please list any injury to your ankles” (and whether the injury required the use of a cane, crutch, surgery, cast, taping or ace bandage).
Radiographic Imaging
Posteroanterior fixed-flexion weight-bearing knee radiographs were obtained with the SynaFlexer™ lower limb positioning frame (Synarc, San Francisco) and scored for joint space narrowing (JSN) and osteophyte (OST) on a 0–3 scale using a standardized atlas [29]. Knee alignment was measured to within 0.5 degrees on a weight-bearing long-limb anteroposterior radiograph as previously reported [30]; alignment of 180° was taken as the reference value and considered neutral, angles <180° were defined as va rus alignment, and angles >180° were defined as valgus alignment. Knee malalignment was defined as the degrees from neutral. There was one unreadable long-limb film, therefore, data for this variable were available on 307 knees. At follow-up, weight-bearing ankle radiographs (mortise and lateral views) were obtained as previously described [31]. There are no radiographic atlases for standardized grading of ankle OA so ankle radiographs were scored by analogy to knee OA scoring based on the Altman atlas [29]. Ankle radiographs were scored (0–3) for tibiotalar JSN (medial and lateral), and OST (medial, lateral, anterior and posterior), and KL grade (0–4) by a reader (JR) with high intra-rater reliability for all three measures (Intraclass Correlation Coefficients (ICCs) >0.90) based on blinded rescoring of 30 ankle radiographs several weeks after the initial scoring.
Scintigraphic Imaging
Bone scintigraphic images were obtained of each ankle (anteroposterior, mediolateral, lateromedial, and posteroanterior views) at 2.5 hours after injection of technetium-99m methylene diphosphonate; the tibiotalar joint (for four quadrants: medial, lateral, tibial, talar) and forefoot were scored semi-quantitatively by an experienced reader (RC) on a 0–3 scale (0=normal, 1=mild, 2=moderate, 3=intense). For the purposes of this study, the forefoot was defined as the region distal to the ankle (involving regions distal to the tibiotalar and subtalar joint). As reported previously, the reliability of scoring scintigrams was high for the medial and lateral tibiotalar joint (ICCs 0.86–0.97) and good for the forefoot (ICC 0.677) [26]. Bone scintigraphy was performed at baseline (n=159) and at the time of follow-up 3 years later (n=138).
Statistical Analyses
Analysis of outcomes measured at the individual level was performed as follows: bivariate analyses for bone scan abnormality of any ankle or forefoot were analyzed by Student’s t-test for age and body mass index (BMI), and percentages and logistic regression for gender. Corrected McNemar’s Chi-square statistic was used to compare the prevalence of scintigraphic abnormalities in the medial versus lateral ankle and tibial versus talar ankle. Bivariate analyses of bone scan abnormality and history of joint injury were analyzed by Student’s t-test.
Final prediction of ankle or forefoot abnormalities was analyzed at the level of joint by poisson regression, controlling for age, gender and BMI. The analysis of ankle and forefoot outcomes utilized methods that incorporated into the analytic structure the inherent correlation due multiple observations for each individual using repeated measures generalized estimating equations (GEE) [32] for the poisson distributed outcomes. Significance levels were determined by the Wald Chi-Square, controlling for the demographics, age, gender and BMI. Modeling the correlation between limbs and assessing the impact of values from the index limb on the opposing limb provided an analytic challenge; to do this, the contralateral limb was incorporated into the analysis. For each outcome and predictor, the following analyses were performed: (1) ‘Ipsilateral’--the impact of the predictor on the outcome from the index leg using both limbs as replicates, (e.g. predicting ankle/forefoot values given the knee values on both the right and left side); (2) ‘Contralateral’--prediction of the contralateral outcome given the index predictor (e.g. predicting the impact on the opposing ankle/forefoot given values from the index knee combining the separate values for both the right and left sides); and (3) ‘Ipsilateral controlled for contralateral predictor’ and ‘Contralateral controlling for ipsilateral predictor’--prediction of the index outcome given values from both the index and opposing predictors (e.g. predicting values for each ankle/forefoot given predictors from both knees). This latter analysis was of greatest interest, allowing us to assess the impact of the contralateral predictor controlling for the equivalent value on the index limb. We hypothesized that affected knees would impact the contralateral ankles, even controlling for the on-side or ipsilateral values. The parameter estimates allowed for the assessment of whether there was a greater impact for the index predictor or the contralateral predictor. Results were qualitatively and quantitatively similar for analyses performed unadjusted and adjusted for age, gender and BMI; therefore results of adjusted analyses (hazard ratios, 95% confidence intervals and p values) are provided in all the tables. For any particular model, statistical significance was set at a Type I error level alpha of 0.05. Since the analyses are exploratory, no adjustment for multiple tests was applied.
Results
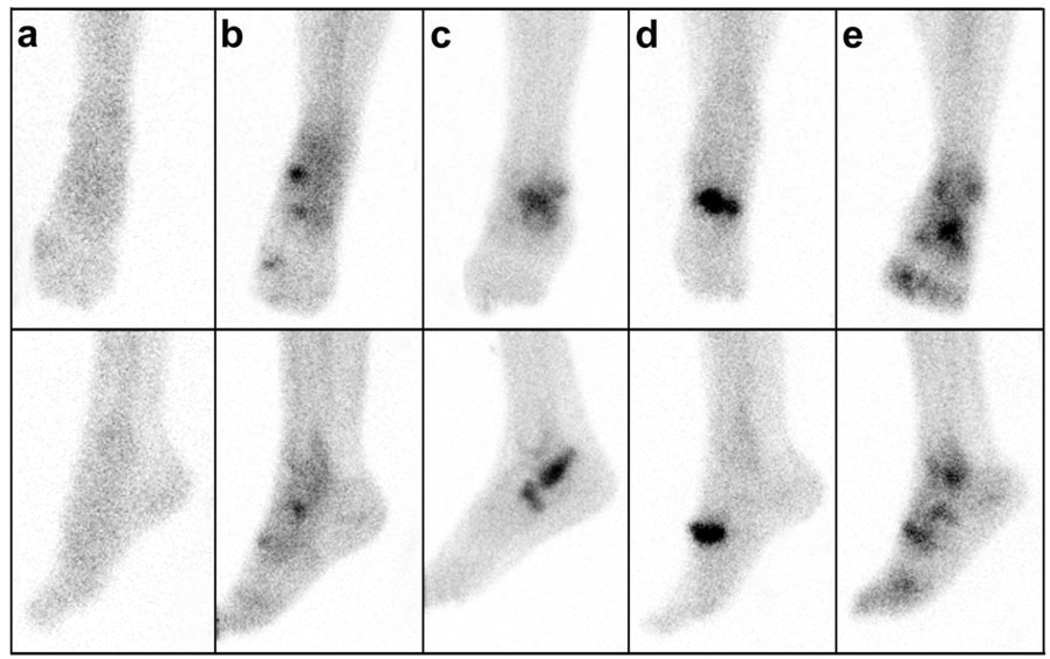
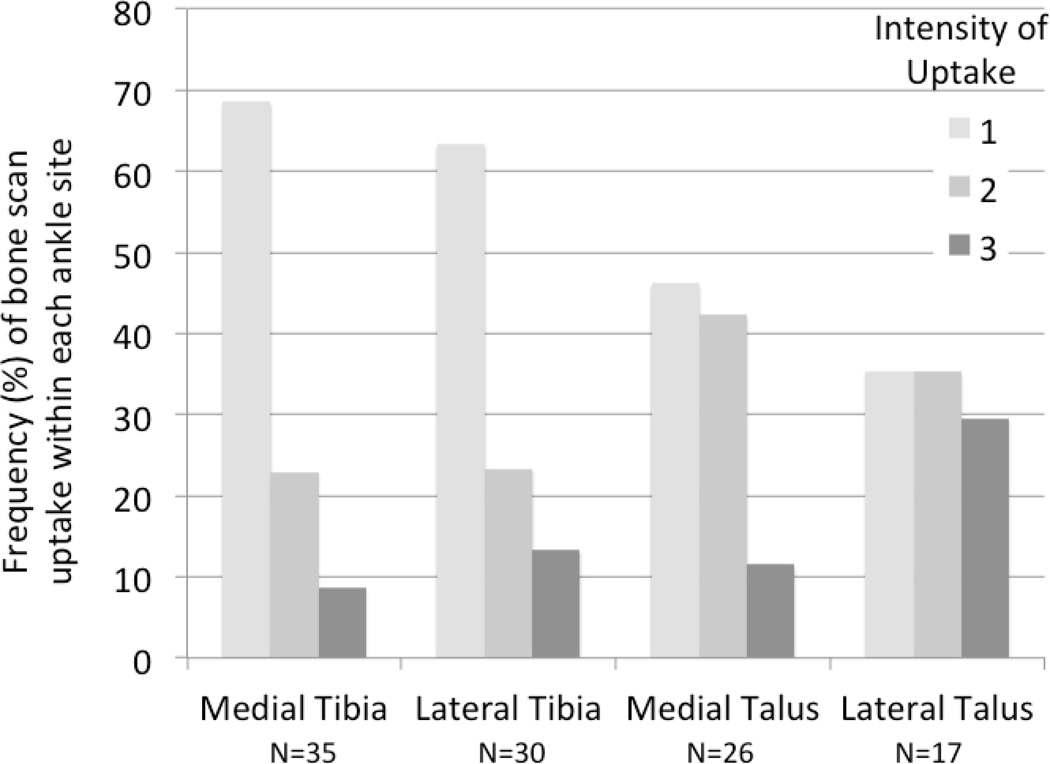
The cohort was 74% female, mean+SD age 63.7±11.8 years, and BMI 31.3±6.7 kg/m2. Additional characteristics of this cohort have been described previously [25]. Representative ankle scintigrams are provided in Figure 1 demonstrating the ability to localize uptake in the medial and lateral compartments of the tibiotalar joint and the forefoot. Scintigraphic abnormalities of the ankle and forefoot were frequent, 31% and 50% respectively. The frequency and localization of uptake among the 70 ankles with any scintigraphic abnormalities was 74% medial and 53% lateral (of these 27% had uptake in both compartments), 76% tibial and 46% talar (of these 21% had uptake in both compartments). Overall, medial ankle scintigraphic abnormalities were significantly more prevalent than lateral (P=0.037 corrected McNemar Chi-square statistic); and tibial abnormalities were significantly more prevalent than talar (P=0.0065 corrected McNemar Chi-square statistic) (Figure 2). BMI was greater in the group with any ankle scintigraphic abnormalities; age and gender did not differ in the two scintigraphic groups (NO compared with ANY ankle abnormality) (Table 1). Age and BMI were greater in the group with any forefoot scintigraphic abnormalities; gender did not differ in the two scintigraphic groups (NO compared with ANY forefoot abnormality) (Table 1).
Figure 1. Ankle scintigraphic images.
Representative late phase technetium-99m–disphosphonate bone scintigraphic images of ankle joints depicting the various and distinct patterns of uptake. Shown are anteroposterior views (top row) and mediolateral views (bottom row). These images illustrate examples of uptake in a) normal joint, b) medial ankle and forefoot, c) lateral ankle and forefoot, d) forefoot only, and e) generalized medial and lateral ankle and forefoot.
Figure 2. Frequency and localization of scintigraphic abnormalities in the ankle.
A total of 318 ankle joints were scored for intensity of scintigraphic uptake based on a four-point scale (0=none, 1=mild, 2=moderate, 3=intense uptake) by compartment (medial or lateral) and bone site (talar and/or tibial). A total of 108 sites in 70 ankles had abnormal scintigraphic uptake (score >1). The frequency of bone scan uptake for each level of intensity (0–3) is depicted as a % for each site.
Table 1.
Demographic characteristics of the cohort by scintigraphic abnormality.
| Joint Site | Scintigraphic Finding |
N | Age Mean (SD) |
BMI Mean (SD) |
Gender (% male) |
|---|---|---|---|---|---|
| Ankle | None Abnormal | 109 | 62.9 (11.9) | 30.2 (5.8) | 24.7% |
| Any Abnormal | 49 | 65.4 (11.6) | 33.7 (7.9) | 26.5% | |
| Group Difference | −2.59 | −3.4 | 0.91 | ||
| (95% CI) | (−6.6, 1.4) | (−5.7, −1.2) | (0.42, 1.97) | ||
| p value | 0.20 | 0.0025 | 0.81 | ||
| Forefoot | None Abnormal | 79 | 61.7 (11.8) | 30.0 (5.3) | 31.6% |
| Any Abnormal | 79 | 65.6 (11.7) | 32.6 (7.6) | 19.0% | |
| Group Difference | −3.94 | −2.62 | 1.98 | ||
| (95% CI) | (−7.6, −0.2) | (−4.7, −0.5) | (0.94, 4.12) | ||
| p value | 0.036 | 0.014 | 0.070 | ||
Group difference by Student’s t-test for age and BMI, and by odds ratio for gender.
One or both ankles bothered 23% of participants; among these individuals, 53% had bilateral ankle symptoms (Table 2). Although ankle symptoms were associated with ankle scintigraphic abnormalities they were not associated with forefoot scintigraphic abnormalities (P=0.72). Ankle scintigraphic abnormalities were associated with ipsilateral but not contralateral ankle symptoms; this association persisted after controlling for contralateral symptoms (Table 3). Overall the risk of an abnormal ankle scintigram in the setting of ankle symptoms was increased 2.3 fold.
Table 2.
Baseline prevalence of ankle and forefoot abnormalities in the POP knee OA cohort.
| Clinical characteristic | Unilateral | Bilateral | Any Side |
|---|---|---|---|
| Abnormal Ankle Bone Scans N (% of individuals) |
28 (17.6%) |
21 (13.2%) |
49 (30.8%) |
| Abnormal Forefoot Bone Scans N (% of individuals) |
29 (18.2%) |
50 (31.5%) |
79 (49.7%) |
| Ankle Symptoms N (% of individuals) |
17 (10.7%) |
19 (12.0%) |
36 (22.6%) |
| Self-reported ankle Injury N (% of individuals) |
7 (2.2%) |
0 (0%) |
7 (4.4%) |
| Self-reported ankle surgery N (% of individuals) |
5 (1.6%) |
2 (0.6%) |
7 (4.4%) |
| Self-reported ankle injury or surgery N (% of individuals) |
12 (3.8%) |
2 (0.6%) |
14 (8.8%) |
Table 3.
Ipsilateral and contralateral associations with ankle scintigraphic abnormalities.
| Risk of Ankle Bone Scan Abnormalities | |||
|---|---|---|---|
| Predictor | Side of Association | Hazard Ratio (95% CI) |
P value |
| Ankle Symptoms |
Ipsilateral | 2.13 (1.10, 4.12) |
0.03 |
| Ipsilateral (controlled for contralateral predictor) |
2.30 (1.28, 4.13) |
0.005 | |
| Contralateral (controlled for ipsilateral predictor) |
0.89 (0.48, 1.63) |
0.70 | |
| Contralateral | 1.60 (0.83, 3.09) |
0.16 | |
| Knee JSN | Ipsilateral | 1.14 (0.89, 1.46) |
0.30 |
| Ipsilateral (controlled for contralateral predictor) |
1.08 (0.88, 1.34) |
0.45 | |
| Contralateral (controlled for ipsilateral predictor) |
1.41 (1.14, 1.73) |
0.001 | |
| Contralateral | 1.40 (1.12, 1.76) |
0.003 | |
| Knee OST | Ipsilateral | 1.09 (0.99, 1.20) |
0.07 |
| Ipsilateral (controlled for contralateral predictor) |
1.04 (0.95, 1.14) |
0.42 | |
| Contralateral (controlled for ipsilateral predictor) |
1.12 (1.03, 1.22) |
0.006 | |
| Contralateral | 1.14 (1.05, 1.24) |
0.002 | |
| Knee Malalignment |
Ipsilateral | 1.09 (1.02, 1.17) |
0.01 |
| Ipsilateral (controlled for contralateral predictor) |
1.02 (0.91, 1.15) |
0.68 | |
| Contralateral (controlled for ipsilateral predictor) |
1.09 (0.99, 1.21) |
0.08 | |
| Contralateral | 1.11 (1.05, 1.18) |
0.0005 | |
| Ankle Injury/Surgery |
Ipsilateral | 6.31 (4.16, 9.57) |
<0.0001 |
| Ipsilateral (controlled for contralateral predictor) |
3.81 (2.49, 5.84) |
<0.0001 | |
| Contralateral (controlled for ipsilateral predictor) |
2.96 (1.87, 4.70) |
<0.0001 | |
| Contralateral | 5.68 (3.60, 8.95) |
<0.0001 | |
Results adjusted for age, gender and BMI with Generalized Estimating Equations to control for ankle correlation within an individual; CI=Confidence Interval; JSN=joint space narrowing; OST=osteophyte; Knee malalignment defined as degrees deviation from 180° or neutral alignment
Knee OA, defined as JSN≥1 or knee OST>1, was significantly associated (P=0.001– 0.006) with scintigraphic abnormalities of the contralateral ankle (Table 3). Knee OA was not associated with ipsilateral ankle scintigraphic abnormalities after adjusting for presence of contralateral knee OA. Knee malalignment was associated with scintigraphic abnormalities of the contralateral but not ipsilateral ankle (P=0.02 adjusted for age, gender and contralateral knee malalignment); however, the strength of this association was somewhat diminished with additional adjustment for BMI (P=0.08). We did not find any association of knee symptoms alone with ankle or forefoot bone scan abnormality (data not shown). These findings demonstrate a probable kinematic association of knee OA pathology and contralateral ankle abnormalities.
Despite the overall prevalence of individuals with ankle abnormalities by bone scintigraphy (31%), surprisingly few individuals in this cohort reported a history of ankle injury (overall 4.4%) or surgery (4.4%) at the time of their first evaluation. Nevertheless, even controlling for report of injury or surgery of the other limb, any history of ankle injury or surgery was strongly associated with both ipsilateral and contralateral ankle scintigraphic abnormalities (Table 3).
A total of 138 participants returned three years after the baseline evaluation. This afforded the opportunity to obtain ankle radiographs and repeat ankle scintigrams, The prevalence of radiographic tibiotalar joint OA was high as defined by KL grade≥1 (79%) or KL grade≥2 (15%), JSN≥1 (15%), or OST≥1 (74%). The distribution of ankle KL grades was KL0 21%, KL1 64%, KL2 12%, KL3 2%, KL4 1%. On both sides, ankle scintigraphic uptake correlated strongly with severity of radiographic ankle OA based on KL grade (R=0.49 average of both sides, P<0.0001). At follow up, ankle scintigraphic abnormality was independently associated with tibiotalar JSN (R=0.35 average of both sides, p<0.0001) and tibiotalar OST score (R=0.35 average of both sides, P=0.0008). These data demonstrate that the severity of ankle scintigraphic abnormalities correlated with the severity of ankle radiographic OA in this cohort.
The 3-year follow-up also afforded an opportunity to obtain more extensive data on the occurrence, severity, type, and date of ankle injury in order to further explore the possible association of ankle abnormalities and ankle injury in this cohort. Significant ankle injury was defined as one requiring a cast, cane, crutch or surgery. At the time of follow-up, a total of 36 incidents of ankle injury (in 33 individuals, 20 on right side, 16 on left side) were reported to predate the first study visit; 2 additional incidents of ankle injury were reported to have occurred between the baseline and follow-up visit. Among the participants who returned for follow-up, all but 1 of the original reports of ankle injury were reported again. A large proportion of the individuals with ankle scintigraphic abnormalities at baseline returned for follow-up (N=41, 84%). In this group, ankle scintigraphic uptake at baseline correlated with the more complete accounting of history of prior ankle injury (P=0.007 Chi Square adjusted for age, gender and BMI). However, even with a more complete accounting of prior ankle injuries, still the majority (76%) of ankles with baseline scintigraphic abnormalities occurred in individuals without a history of prior significant injury. These data suggest that ankle injury alone cannot account for the ankle scintigraphic abnormalities and features of radiographic ankle OA observed in this knee OA cohort.
The interrelationship of knee and forefoot was similar to that observed for the knee and ankle; namely, knee OA and knee malalignment were significantly associated with contralateral forefoot scintigraphic abnormalities but not ipsilateral abnormalities (Table 4).
Table 4.
Association of knee pathology with forefoot scintigraphic abnormalities.
| Risk of Forefoot Bone Scan Abnormalities | |||
|---|---|---|---|
| Predictor | Side of Association | Hazard Ratio (95% CI) |
P value |
| Knee JSN | Ipsilateral | 1.07 (0.94, 1.23) |
0.30 |
| Ipsilateral (controlled for contralateral predictor) |
1.02 (0.91, 1.15) |
0.73 | |
| Contralateral (controlled for ipsilateral predictor) |
1.25 (1.10, 1.42) |
0.0005 | |
| Contralateral | 1.24 (1.08, 1.42) |
0.0018 | |
| Knee OST | Ipsilateral | 1.05 (0.99, 1.11) |
0.09 |
| Ipsilateral (controlled for contralateral predictor) |
1.01 (0.96, 1.07) |
0.65 | |
| Contralateral (controlled for ipsilateral predictor) |
1.10 (1.05, 1.15) |
<0.0001 | |
| Contralateral | 1.10 (1.04, 1.16) |
0.0004 | |
| Knee Malalignment |
Ipsilateral | 1.05 (0.00, 1.11) |
0.11 |
| Ipsilateral (controlled for contralateral predictor) |
1.01 (0.95, 1.07) |
0.76 | |
| Contralateral (controlled for ipsilateral predictor) |
1.06 (1.00, 1.11) |
0.03 | |
| Contralateral | 1.07 (1.01, 1.12) |
0.015 | |
Results adjusted for age, gender and BMI with Generalized Estimating Equations to control for ankle correlation within an individual; CI=Confidence Interval; JSN=joint space narrowing; OST=osteophyte; Knee malalignment defined as degrees deviation from 180° or neutral a lignment
Discussion
The main purpose of this study was to evaluate the interrelationship of knee pathology and ankle/forefoot abnormalities. Past studies identified an association of knee OA and ipsilateral ankle OA [23], and noted that severe ankle degeneration did not exist in the absence of severe knee degeneration [24]; in addition, several studies noted the occurrence of bilateral ankle OA [22–24]. To our knowledge however, this is the first study to demonstrate a clear association of knee OA with contralateral ankle pathology. In contrast to the former studies, upon accounting for contralateral knee pathology, we have discerned a relationship of knee radiographic OA and severity of knee malalignment with contralateral ankle pathology as demonstrated by bone scintigraphy. We observed a similar contralateral interrelationship of knee pathology with forefoot scintigraphic abnormalities. At baseline only 9% of participants in this study reported previous ankle injury or surgery; at follow-up with more extensive inquiry, this increased to 22%. While a history of ankle injury was clearly associated with the presence of ankle pathology, less than one-quarter of ankles with scintigraphic abnormalities were associated with prior injury. These data suggest that ankle injury alone cannot fully account for the ankle scintigraphic abnormalities and features of radiographic ankle OA in this knee OA cohort. The association of knee pathology with contralateral ankle pathology is the first to our knowledge to suggest that knee OA is a risk factor for contralateral ankle pathology.
Given the reports that ankle OA is rare [33, 34], we were surprised to observe a high prevalence of abnormal ankle scintigrams (31% of individuals) indicating an abnormally high level of ankle joint bone remodeling in this knee OA cohort. A similar high prevalence (29% of individuals) of ankle abnormalities (radiographic OA) was observed by Tallroth et. al. in another knee OA cohort with participants of similar age to ours [23]. The prevalence of ankle joint pathology in these knee OA cohorts exceeded even the highest prevalence rates of ankle OA derived from cadaveric studies on non-selected populations (5–18%) [2, 35, 36]. The prevalence of ankle symptoms in our knee OA cohort (23% of individuals) also far exceeded the prevalence of self-reported ankle symptoms (4%) and physician observed ankle abnormalities (0.8%) in the general population in the US Health and Nutrition Examination Survey (NHANES I - 1971 to 1975) [37]. The strong relationship of ankle abnormalities with knee OA in our cohort suggests that knee pathology may contribute to the excess prevalence of ankle abnormalities observed in our study.
Biomechanical factors have been posited to be involved in the non-random evolution of OA in “kinematically related joints” of the lower extremities [19, 22, 24]. An early gait study showed that knee OA was associated with redistribution of load from the impaired to less or non-impaired joints through a multijoint change in dynamics [38]. A decade later, knee OA was shown to increase loading at the ankle; in the setting of knee OA, more rapidly increasing joint forces were posited to lead to initiation and more rapid progression of OA at joints adjacent to the knee [39]. Another gait study showed that individuals with severe knee OA had greater ankle dorsiflexion moments and higher ankle internal rotation moment [40, 41]. Other studies have shown reduced ankle power [42] including reduced ankle plantar-flexion power in individuals with knee OA [43]. Thus, as aptly stated by Muehleman [24]--a malaligned knee affects the alignment of the entire kinetic chain. Based on these studies we conclude that altered mechanics, as a result of knee OA and knee malalignment, are likely contributing to the associated contralateral ankle abnormalities.
Based on the sequence of joint replacements within a patient with knee or hip OA, a nonrandom evolution of OA in these joints has been established [18]. With regard to hip OA, the reason for the contralateral hip/knee OA risk relationship was demonstrated to be based on mechanical alteration; end-stage hip OA was associated with significantly higher peak external knee adduction moment and peak medial compartment load [19, 20]. Individuals with hip OA also have altered gait mechanics consisting of a shorter stride length, less contralateral and especially ipsilateral hip motion, modified ankle motion bilaterally, and a different intra-limb coordination pattern compared to control subjects [44]. Since individuals with hip OA have altered gait mechanics that might impact the incidence of ankle pathology, it would be of great interest in future to explore the association of hip and ankle OA and their laterality.
Degenerative changes in the ankle are reported to increase with age, to be more severe in men than in women, to be predominantly bilateral, and to correlate with weight [7]. With respect to ankle abnormalities, we did not observe an age or gender bias in this knee OA cohort. Unilateral abnormalities were more common than bilateral and ankle scintigraphic abnormalities were independently associated with ankle symptoms but not BMI (data not shown). Our finding that medial ankle scintigraphic abnormalities were more prevalent than lateral agrees with a prior conclusion from a study of cadaveric ankle joint degeneration [22].
Although our study was limited by the lack of baseline ankle radiographs, bone scintigraphic abnormalities of the ankle at follow-up were strongly correlated with radiographic abnormalities of the ankle at follow-up suggesting that ankle scintigraphic abnormalities reflect underlying features of radiographic ankle OA. Additional limitations include the fact that injury data were self-reported and subject to recall bias; however, with the exception of 1 event, all the initially reported events were reported at three-year follow-up along with additional events that were captured with inclusion of a variety of more explicit questions about ankle events. Third, although these data suggest that knee OA leads to contralateral features of radiographic ankle OA, causality cannot be definitively established without serial knee and ankle radiographs.
In summary, our data provide evidence for a much higher prevalence of ankle joint abnormalities than expected based on much of the existing literature. We demonstrate a high prevalence of ankle scintigraphic abnormalities and radiographic features of ankle OA in the setting of radiographic knee OA and an association of knee OA and knee malalignment with contralateral ankle pathology. These data suggest that knee OA is likely a significant risk factor for ankle abnormalities, particularly ankle abnormalities contralateral to an osteoarthritic knee. The known mechanical interaction of lower extremity joints in a kinetic chain suggests that therapeutic interventions targeting mechanical factors may be needed to prevent ankle OA in the setting of knee OA.
Acknowledgments
Role of the funding source
This study was supported, in whole or in part, by the National Institute on Aging at the National Institutes of Health (Claude D. Pepper Older Americans Independence Centers 5P30 AG028716); National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health (Grants RO1AR48769 and P01 AR050245), and the National Center for Research Resources at the National Institutes of Health (MO1-RR-30), supporting the Duke General Clinical Research Unit where this study was conducted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
VBK conceived and supervised all aspects of the study and wrote the manuscript; TWW assisted with the study design, statistical analysis, revision of the manuscript and final approval of the submitted version; JBR graded the ankle radiographs, reviewed the data, participated in manuscript revisions and final approval of the submitted version; REC assisted with study design, performed and graded the bone scans, reviewed the results, assisted with interpretation of the results, and participated in manuscript revisions; CFP performed the bulk of the statistical analyses, helped draft and revise the manuscript, and approved the submitted version.
Competing interest statement
None
References
- 1.Aurich M, Squires GR, Reiner A, Mollenhauer JA, Kuettner KE, Poole AR, et al. Differential matrix degradation and turnover in early cartilage lesions of human knee and ankle joints. Arthritis Rheum. 2005;52:112–119. doi: 10.1002/art.20740. [DOI] [PubMed] [Google Scholar]
- 2.Huch K, Kuettner KE, Dieppe P. Osteoarthritis in ankle and knee joints. Semin Arthritis Rheum. 1997;26:667–674. doi: 10.1016/s0049-0172(97)80002-9. [DOI] [PubMed] [Google Scholar]
- 3.Kang Y, Koepp H, Cole AA, Kuettner KE, Homandberg GA. Cultured human ankle and knee cartilage differ in susceptibility to damage mediated by fibronectin fragments. J Orthop Res. 1998;16:551–556. doi: 10.1002/jor.1100160505. [DOI] [PubMed] [Google Scholar]
- 4.Huch K. Knee and ankle: human joints with different susceptibility to osteoarthritis reveal different cartilage cellularity and matrix synthesis in vitro. Arch Orthop Trauma Surg. 2001;121:301–306. doi: 10.1007/s004020000225. [DOI] [PubMed] [Google Scholar]
- 5.Eger W, Schumacher BL, Mollenhauer J, Kuettner KE, Cole AA. Human knee and ankle cartilage explants: catabolic differences. J Orthop Res. 2002;20:526–534. doi: 10.1016/S0736-0266(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 6.Cole AA, Kuettner KE. Molecular basis for differences between human joints. Cellular & Molecular Life Sciences. 2002;59:19–26. doi: 10.1007/s00018-002-8401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole AA, Margulis A, Kuettner KE. Distinguishing ankle and knee articular cartilage. Foot Ankle Clin. 2003;8:305–316. doi: 10.1016/s1083-7515(03)00012-3. x. [DOI] [PubMed] [Google Scholar]
- 8.Kuettner KE, Cole AA. Cartilage degeneration in different human joints. Osteoarthritis Cartilage. 2005;13:93–103. doi: 10.1016/j.joca.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Fetter NL, Leddy HA, Guilak F, Nunley JA. Composition and transport properties of human ankle and knee cartilage. J Orthop Res. 2006;24:211–219. doi: 10.1002/jor.20029. [DOI] [PubMed] [Google Scholar]
- 10.Patwari P, Cheng DM, Cole AA, Kuettner KE, Grodzinsky AJ. Analysis of the relationship between peak stress and proteoglycan loss following injurious compression of human postmortem knee and ankle cartilage. Biomech Model Mechanobiol. 2007;6:83–89. doi: 10.1007/s10237-006-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aurich M, Mwale F, Reiner A, Mollenhauer JA, Anders JO, Fuhrmann RA, et al. Collagen and proteoglycan turnover in focally damaged human ankle cartilage: evidence for a generalized response and active matrix remodeling across the entire joint surface. Arthritis Rheum. 2006;54:244–252. doi: 10.1002/art.21535. [DOI] [PubMed] [Google Scholar]
- 12.Valderrabano V, Frigg A, Leumann A, Horisberger M. [Total ankle arthroplasty in valgus ankle osteoarthritis] Orthopade. 2011;40:971–974. doi: 10.1007/s00132-011-1825-3. 976–977. [DOI] [PubMed] [Google Scholar]
- 13.Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004:S6–S15. doi: 10.1097/01.blo.0000143938.30681.9d. [DOI] [PubMed] [Google Scholar]
- 14.Saltzman CL, Salamon ML, Blanchard GM, Huff T, Hayes A, Buckwalter JA, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44–46. [PMC free article] [PubMed] [Google Scholar]
- 15.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 16.Valderrabano V, Horisberger M. Ankle osteoarthritis – A review of the current state of knowledge. European Musculoskeletal Review. 2011:6. [Google Scholar]
- 17.Saltzman CL, Zimmerman MB, O’Rourke M, Brown TD, Buckwalter JA, Johnston R. Impact of comorbidities on the measurement of health in patients with ankle osteoarthritis. J Bone Joint Surg Am. 2006;88:2366–2372. doi: 10.2106/JBJS.F.00295. [DOI] [PubMed] [Google Scholar]
- 18.Shakoor N, Block JA, Shott S, Case JP. Nonrandom evolution of end-stage osteoarthritis of the lower limbs. Arthritis Rheum. 2002;46:3185–3189. doi: 10.1002/art.10649. [DOI] [PubMed] [Google Scholar]
- 19.Shakoor N, Hurwitz DE, Block JA, Shott S, Case JP. Asymmetric knee loading in advanced unilateral hip osteoarthritis. Arthritis Rheum. 2003;48:1556–1561. doi: 10.1002/art.11034. [DOI] [PubMed] [Google Scholar]
- 20.Shakoor N, Dua A, Thorp LE, Mikolaitis RA, Wimmer MA, Foucher KC, et al. Asymmetric loading and bone mineral density at the asymptomatic knees of patients with unilateral hip osteoarthritis. Arthritis Rheum. 2011;63:3853–3858. doi: 10.1002/art.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayre EC, Jordan JM, Cibere J, Murphy L, Schwartz TA, Helmick CG, et al. Quantifying the association of radiographic osteoarthritis in knee or hip joints with other knees or hips: the Johnston County Osteoarthritis Project. J Rheumatol. 2010;37:1260–1265. doi: 10.3899/jrheum.091154. [DOI] [PubMed] [Google Scholar]
- 22.Koepp H, Eger W, Muehleman C, Valdellon A, Buckwalter JA, Kuettner KE, et al. Prevalence of articular cartilage degeneration in the ankle and knee joints of human organ donors. J Orthop Sci. 1999;4:407–412. doi: 10.1007/s007760050123. [DOI] [PubMed] [Google Scholar]
- 23.Tallroth K, Harilainen A, Kerttula L, Sayed R. Ankle osteoarthritis is associated with knee osteoarthritis Conclusions based on mechanical axis radiographs. Arch Orthop Trauma Surg. 2008;128:555–560. doi: 10.1007/s00402-007-0502-9. [DOI] [PubMed] [Google Scholar]
- 24.Muehleman C, Margulis A, Bae WC, Masuda K. Relationship between knee and ankle degeneration in a population of organ donors. BMC Med. 2010;8:48. doi: 10.1186/1741-7015-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraus VB, McDaniel G, Worrell TW, Feng S, Vail TP, Varju G, et al. Association of bone scintigraphic abnormalities with knee malalignment and pain. Ann Rheum Dis. 2009;68:1673–1679. doi: 10.1136/ard.2008.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Addison S, Coleman RE, Feng S, McDaniel G, Kraus VB. Whole-body bone scintigraphy provides a measure of the total-body burden of osteoarthritis for the purpose of systemic biomarker validation. Arthritis Rheum. 2009;60:3366–3373. doi: 10.1002/art.24856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis MA, Ettinger WH, Neuhaus JM. Obesity and osteoarthritis of the knee: evidence from the National Health and Nutrition Examination Survey (NHANES I) Seminars in Arthritis & Rheumatism. 1990;20:34–41. doi: 10.1016/0049-0172(90)90045-h. [DOI] [PubMed] [Google Scholar]
- 29.Altman RD, Gold GE. AtAtlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):A1–A56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Kraus VB, Vail TP, Worrell T, McDaniel G. A comparative assessment of alignment angle of the knee by radiographic and physical examination methods. Arthritis Rheum. 2005;52:1730–1735. doi: 10.1002/art.21100. [DOI] [PubMed] [Google Scholar]
- 31.McDaniel G, Renner JB, Sloane R, Kraus VB. Association of knee and ankle osteoarthritis with physical performance. Osteoarthritis Cartilage. 2011 doi: 10.1016/j.joca.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeger S, Liang K. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 33.Chandnani V, Resnick D. Roentgenologic diagnosis. In: R Moskowitz, D Howell, V Goldberg, H Mankin., editors. Osteoarthritis Diagnosis and Medical/Surgical Management. Philadelphia: W.B: Saunders Co; 1992. pp. 263–311. [Google Scholar]
- 34.Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800–1806. doi: 10.1007/s11999-008-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meachim G. Cartilage fibrillation at the ankle joint in Liverpool necropsies. J Anat. 1975;119:601–610. [PMC free article] [PubMed] [Google Scholar]
- 36.Muehleman C, Bareither D, Huch K, Cole AA, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5:23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham LS, Kelsey JL. Epidemiology of musculoskeletal impairments and associated disability. Am J Public Health. 1984;74:574–579. doi: 10.2105/ajph.74.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pai YC, Chang HJ, Chang RW, Sinacore JM, Lewis JL. Alteration in multijoint dynamics in patients with bilateral knee osteoarthritis. Arthritis Rheum. 1994;37:1297–1304. doi: 10.1002/art.1780370905. [DOI] [PubMed] [Google Scholar]
- 39.Mundermann A, Dyrby CO, Andriacchi TP. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis Rheum. 2005;52:2835–2844. doi: 10.1002/art.21262. [DOI] [PubMed] [Google Scholar]
- 40.Astephen JL, Deluzio KJ, Caldwell GE, Dunbar MJ, Hubley-Kozey CL. Gait and neuromuscular pattern changes are associated with differences in knee osteoarthritis severity levels. J Biomech. 2008;41:868–876. doi: 10.1016/j.jbiomech.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Astephen JL, Deluzio KJ, Caldwell GE, Dunbar MJ. Biomechanical changes at the hip, knee, and ankle joints during gait are associated with knee osteoarthritis severity. J Orthop Res. 2008;26:332–341. doi: 10.1002/jor.20496. [DOI] [PubMed] [Google Scholar]
- 42.Al-Zahrani KS, Bakheit AM. A study of the gait characteristics of patients with chronic osteoarthritis of the knee. Disabil Rehabil. 2002;24:275–280. doi: 10.1080/09638280110087098. [DOI] [PubMed] [Google Scholar]
- 43.McGibbon CA, Krebs DE. Compensatory gait mechanics in patients with unilateral knee arthritis. J Rheumatol. 2002;29:2410–2419. [PubMed] [Google Scholar]
- 44.Ornetti P, Laroche D, Morisset C, Beis JN, Tavernier C, Maillefert JF. Three-dimensional kinematics of the lower limbs in hip osteoarthritis during walking. J Back Musculoskelet Rehabil. 2011;24:201–208. doi: 10.3233/BMR-2011-0295. [DOI] [PubMed] [Google Scholar]