Abstract
Background
Circulating levels of pro-inflammatory advanced glycation end products (AGEs) are increased in diabetes and other conditions characterized by chronically elevated oxidant stress (OS). OS also increases after acute trauma and is implicated in the development of complications such as multiple organ failure. Herein, we assess the effect of acute OS on circulating levels of AGEs in a cohort of acute trauma victims.
Methods
An observational study was performed at a large Level 1 Trauma Center. Blood samples for measurement of two AGEs, carboxymethyllysine (CML) and methylglyoxal (MG), were obtained at admission, and serially afterwards in patients admitted to the ICU. Demographics, dietary history, markers of injury severity and ICU morbidity and mortality data were collected.
Results
One hundred and fifty-six trauma patients (TP) (age: 39±17 years, 83% males, injury severity score: 18±14) were included in the study. TP had significantly higher serum AGE levels than normal healthy controls (CML, TP 12.4±8.2 U/mL vs. controls 8.9±5.3 U/mL, p<0.001; MG, TP 2.1±1.4 nmol/mL vs. controls 0.79±0.3 nmol/mL, p< 0.001). Admission serum AGE levels in 49 severe TP admitted to the ICU were lower than those who were not. However, among the ICU patients, serum AGEs increased further for about 7 days in patients with an uncomplicated course, and remained markedly elevated in those with a complicated course.
Conclusions
Circulating AGEs are transiently increased after acute trauma and persistently elevated AGE levels are associated with greater severity of injury.
Keywords: dietary AGEs, glycation, oxidative stress, nutrition
Introduction
Trauma remains the most common cause of death for all individuals between the ages of 1 and 44 years and is the third most common cause of death regardless of age [1]. Following the initial injury, trauma causes a rapid activation of macrophages with an increased release of pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-6 [2–8], as well as increased activity of the multi-ligand receptor for advanced glycation endproducts (RAGE), activated by high mobility group box 1 (HMGB1), which is released early after trauma [9, 10]. A rapid and sustained inflammatory response is thought to lead to activation of mitogen-activated protein kinases (MAPK), NF-κB, and Rho GTPases [11]. Experimental and clinical evidence suggests that these mediators are involved in the pathogenesis of the multiple organ dysfunction syndrome, which leads to high morbidity and death in patients with severe injury [8]. While these studies have provided information on the molecular mechanisms involved in this early inflammatory response, the potential role of advanced glycation endproducts (AGEs) has not been well established.
AGEs are known pro-inflammatory and pro-oxidative compounds largely shown to be elevated in states of chronic inflammation and oxidative stress (OS), such as diabetes and chronic kidney disease (CKD) [11]. Traditionally, AGEs have been considered to be generated largely endogenously in response to hyperglycemia, but it is now clear they can also form under conditions of high OS, even in the absence of high blood glucose. However, it is now recognized that modern foods, often processed under dry heat conditions, contribute significantly to the body AGE pool [12]. Studies in healthy subjects as well as in patients with diabetes or CKD indicate a link between exogenous AGEs, circulating AGEs and markers of chronic inflammation and chronic OS [13–15]. Based on recent evidence, large amounts of AGEs, largely due to high dietary AGE intake, can accumulate in tissues and cells over time, a small fraction of which is reflected in the circulation [11]. Less is known about circulating AGE levels during conditions of acute trauma and the data have been contradictory [16, 17]. Here we hypothesized that pre-existing or newly formed AGEs might be released in the circulation from tissues after extensive trauma injury promoting acutely elevated OS possibly contributing to the high morbidity linked to severe trauma.
The aim of this study was therefore two-fold: 1) to measure levels of circulating AGEs in trauma patients on presentation to the trauma center; and 2) to determine whether there is a correlation between these levels with prior dietary AGE intake, with levels of other markers of OS, and with outcome in victims of acute multiple trauma.
Materials and methods
A prospective, observational study was performed at a large, urban, level 1 trauma center. All adult (≥ 18 years) patients presenting to the trauma center who had blood drawn for initial laboratory workup were considered eligible and informed consent was obtained from the patient or from the patient’s surrogate. The University of Miami Miller School of Medicine Institutional Review Board approved this study. The study was performed in compliance with the World Medical Association Declaration of Helsinki regarding ethical conduct of research involving human subjects. Patients were excluded if they were < 18 years old, or if they were not expected to survive past 24 h of hospitalization.
Demographic information and a detailed dietary history were collected at the time of enrollment. Dietary information was used to calculate AGE intake as previously described [12]. For patients admitted to the ICU, daily organ dysfunction score, infections, and other complications were recorded.
The patients’ age ranged from 18 to 93 years with 83% males. A population of healthy subjects with the same age range with 55% males was used as a control.
Blood AGE levels were obtained at initial presentation to the trauma center and throughout the course of ICU hospitalization, namely on day 7, every 7 days thereafter and at discharge. Blood samples were centrifuged and aliquots of plasma or serum were used for routine measurements by the hospital clinical laboratory or frozen (at −80° C) until assessed by the division of Experimental Diabetes and Aging, The Mount Sinai School of Medicine, New York, NY, USA. Serum samples were tested for εN-carboxymethyllysine (CML) (4G9 mab, Alteon, Inc., Northvale, NJ, USA) and for methylglyoxal (MG) derivatives, such as MG-H1 (MG3D11 mab) by ELISAs, based on standards characterized by HPLC and GC-MS, as previously described [18]. The test sensitivity for CML and MG is 0.1 U/mL and 0.004 nmol/mL, respectively; the intra-assay variation is±2.6% (CML) and±2.8% (MG) and the inter-assay variation is±4.1% (CML) and±5.2% (MG). Plasma 8-isoprostane levels were measured by a commercially available ELISA kit (Cayman Chemical Company, Ann Arbor, MI, USA).
Statistical analysis
Data are presented as mean±SD, unless otherwise stated. Differences of means between groups were analyzed by the Student‘s t-test or ANOVA (followed by Bonferroni correction for multiple comparisons) depending on the number of groups. Correlation analyses were also examined by Pearson ’ s correlation coefficient. Significant differences were defined as a p-value < 0.05 and are based on two-sided tests. Data analysis used the SPSS statistical program (SPSS 17.0 for Windows, Chicago, IL, USA).
Results
Serum AGEs at presentation
Baseline characteristics of the trauma patients are presented in Table 1. One hundred and fifty-six subjects were enrolled with 49 of them admitted to the ICU. The remaining 107 were either transferred to the ward or discharged home (Table 1). As would be expected, the injury severity score (ISS) and the length of hospital stay (LOS) were significantly higher and the Glascow Coma Score (GCS) significantly lower in those admitted to the ICU.
Table 1.
Demographics and serum AGEs on admission.
| Total | Non-ICU | ICU | pa | Healthy | pb | |
|---|---|---|---|---|---|---|
| n | 156 | 107 | 49 | |||
| Age, years | 39±17 | 38±15 | 43±21 | 0.168 | 41±10 | 0.307 |
| Gender, %Male/Female | 83/17 | 83/17 | 81/19 | 0.878 | 55/45 | 0.006 |
| BMI | 27.9±7 | 28.2±7 | 27.5±5 | 0.509 | 25.6±5 | 0.006 |
| ISS | 18±14 | 11±8 | 31±16 | 0.001 | NA | NA |
| GCS | 13.5±3 | 14±2 | 12±4 | 0.008 | NA | NA |
| LOS, days | 6(0–0139) | 4(0–135) | 22(139) | 0.001 | NA | NA |
| Serum Creatinine, mg/dL | NA | NA | 0.89±0.23 | NA | 0.84±0.17 | NA |
| Serum CML, U/mL | 12±5 | 13±5 | 9.8±5 | 0.001 | 7.4±5 | 0.001 |
| Serum MG, nmol/mL | 2.17±1.40 | 2.40±0.15 | 1.62±0.89 | 0.001 | 0.74±0.37 | 0.001 |
| 8-iso, pg/mL | 167±142 | 280±225 | 120±70 | 0.105 | 178±98 | 0.466 |
All data are expressed as mean±SD. 8-iso, plasma 8-isoprostanes; ISS, Injury Severity Score; GCS, Glascow Coma Score; LOS, length of hospital stay in days expressed as median and range.
p, statistical difference between ICU and non-ICU groups;
p, statistical difference between total trauma and healthy controls.
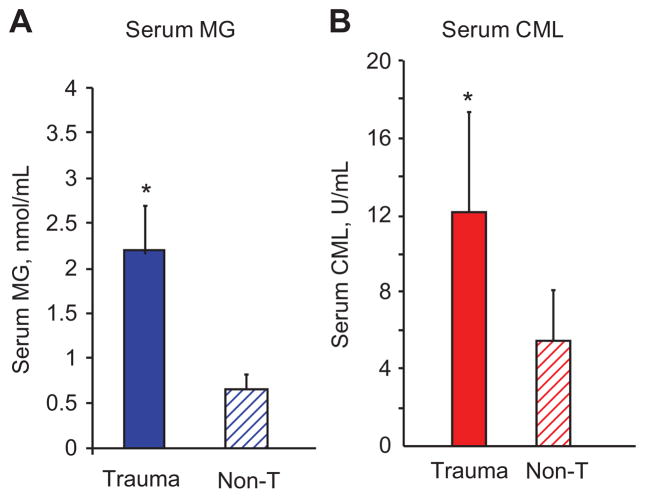
At presentation to the trauma center, serum levels of both CML (sCML) and MG (sMG) were significantly higher in trauma patients, compared to age-matched healthy controls (Table 1; Figure 1A, B). Blood AGE levels in diabetic trauma patients (n=8; only 1 admitted to the ICU) were higher than in non-diabetic trauma patients (n=148) and in healthy controls (n=91). At presentation, no correlation was observed between GCS, levels of blood glucose or serum creatinine and circulating levels of either CML or MG. Of note, levels of circulating AGEs were initially lower in those requiring ICU admission compared to those who did not, but still higher than healthy controls (Table 1). Peak serum AGE values after admission to the ICU, however, showed a significant increase for both CML (17.4±7 U/mL) and MG (2.47±1.12 nmol/mL). LOS was inversely, albeit modestly, correlated with admission levels of sCML (r=−0.218, p=0.008) and sMG (r=−0.250, p=0.002).
Figure 1. Circulating AGE levels on admission to Trauma Center.
Levels of serum CML and MG in trauma patients (n=156) based on ELISAs (see Materials and methods) are compared to values obtained in a group of age-matched healthy controls (n=91) under fasting conditions. Data are expressed as mean±SEM, * statistically significant difference between trauma patients and healthy controls (p<0.005).
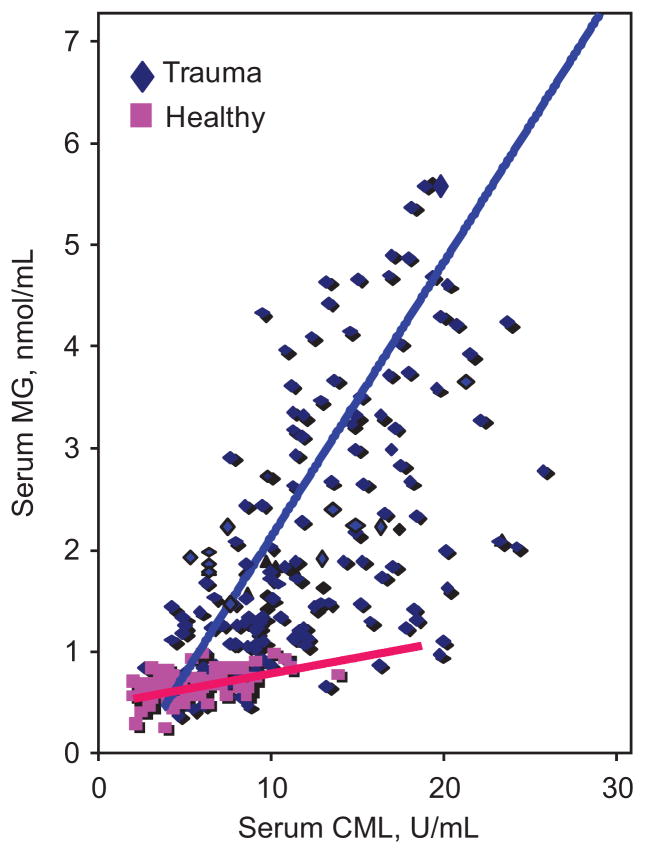
Serum levels of CML correlated highly with levels of MG (r=0.77, p=0.001) (Figure 2). The line of association between both serum AGEs in trauma patients had a very different slope than for healthy subjects largely reflecting a steep elevation of the highly reactive MG derivatives (trauma: y=0.145x+0.408; healthy: y=0.026x+0.523) (Figure 2).
Figure 2. Relationship between serum CML and MG in trauma patients compared to controls.
Levels of sCML were plotted against levels of sMG for trauma patients on initial presentation to the trauma center and compared with similar association in healthy controls under fasting condition. There is a close correlation between sCML and sMG levels in trauma patients (r=0.45, p=0.001) as is in healthy controls (r=0.78, p=0.001). The slopes of the curves, however, were significantly different (p=0.002) (trauma: y=0.145x+0.408; healthy: y=0.026x+0.523).
Upon admission, namely within hours from trauma, circulating levels of 8-isoprostanes were not increased in trauma patients, compared to healthy controls (Table 1), and were not as elevated as previously seen in patients with conditions characterized by chronic high OS such as CKD [19]. However, repeated measurements in a subgroup of patients admitted to the ICU (n=14) showed a significant increase in plasma 8-isoprostanes over time (days to weeks) from 124±66 pg/mL to 219±101 pg/mL (p=0.008). As previously shown, there was a positive correlation of levels of 8-isoprostanes with CML (r=0.469, p=0.012) but not with MG derivatives.
Dietary AGEs in trauma patients
Since serum AGE levels are influenced by diet, a dietary assessment was obtained in study participants. The estimated total daily dietary caloric and AGE intake (~15 AGE Eq/day) at presentation was similar to that reported for non-trauma normal cohorts [12]. No correlation, however, was found between dietary AGE intake and serum AGE levels on presentation to the trauma center.
ICU course, trauma patients – longitudinal studies
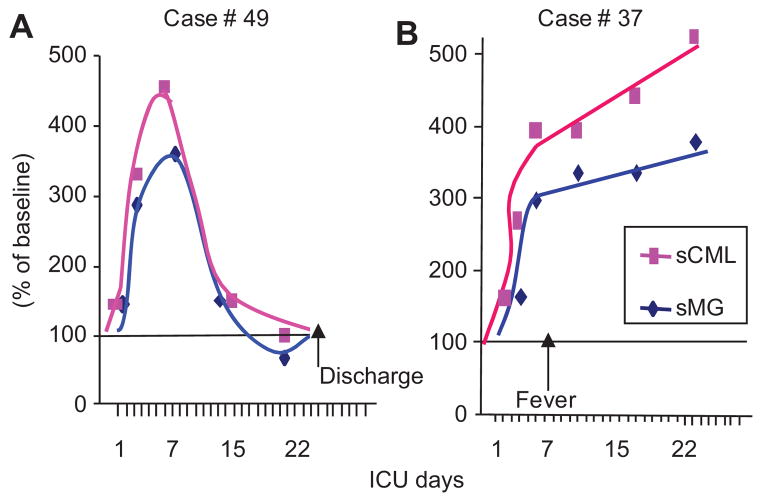
The levels of sCML and sMG were monitored at 7-day intervals in those patients admitted to the ICU. Both serum AGEs rose in parallel in the ICU by 2–4-fold above the already elevated admission levels (Figure 3A, B). However, at least two distinct patterns emerged with respect to sCML and sMG levels, which appeared to be related to the clinical course. In subjects with an uncomplicated clinical ICU course, sCML and sMG levels rose by 2–3-fold in the first week, but returned to the ICU baseline within 2 weeks (Figure 3A). By comparison, in subjects who suffered one or more episodes of sepsis, fever, respiratory or other distress, sAGE levels remained high or continued to rise for >3–4 weeks (Figure 3B).
Figure 3. The kinetics of serum AGE levels in severe acute trauma requiring ICU vary with the clinical course.
Representative post-trauma ICU cases showing the course of sAGEs, CML and MG derivatives while in ICU. (A) Course of an uncomplicated case depicting an initial elevation and a subsequent rapid return to baseline, (B) course of a case complicated by secondary infection detected at day 7. Data are presented as percent of baseline serum CML and MG (based on ELISAs), obtained on admission to the center.
Discussion
This study demonstrates that serum levels of AGEs are markedly elevated shortly after acute trauma. This finding cannot be explained by acute elevations of blood glucose, acute renal failure or on the basis of dietary AGE consumption. It occurred in subjects without prior medical history, although the pre-existence of diabetes made the phenomenon more pronounced. Serum AGEs in a subgroup of patients who ended up being admitted to the ICU continued to rise by 6–8-fold above normal if in critical condition, complicated by secondary events.
Elevations in circulating AGEs of this magnitude have been well documented previously in CKD and in diabetic patients, but the rise in both conditions is chronic and progressive [13–15]. Rapid increases in serum AGEs have been previously documented in acute clinical conditions such as sepsis [16, 17] and these may reflect new AGE formation linked to OS, a result likely to apply to the traumatic stress response [2–10]. In this respect, CML is a stable terminal oxidative end product and MG is an earlier intermediate, a less stable but potent glycating agent [11]. These two compounds are produced in excess in diabetes, but also in conditions unrelated to diabetes, i.e., CKD, aging and increased dietary AGE intake, promoting a high tissue OS state, inflammation and injury [11]. Release of large amounts of intracellular AGEs to the circulation could promote an immediate systemic rise in ROS resulting in high OS and inflammatory events [11]. Unexpected, however, was the initial absence of elevated plasma 8-iso-prostane levels, previously used as markers of systemic OS in conditions such as diabetes and CKD [13–15]. One potential explanation for the absence of acutely elevated levels of these native lipid peroxidants, other than methodological, including the time elapsed until sample procurement, here being in the order of hours after trauma, might be a longer time period required for their induction and release in the circulation. In this context, levels of 8-isoprostane were noted to increase significantly above the admission level in patients who had follow-up measurements at later times while in the ICU. High serum AGEs may therefore precede and/or promote changes in other markers of systemic OS, however, further studies are required to establish this view.
Although in this study levels of AGEs were assessed in the intravascular space, it is reasonable to assume they largely reflected intracellular AGEs released in the circulation after severe tissue injury secondary to trauma. These AGEs would include glycating intermediates, such as MG-H1, which are known as OS promoting agents [11]. As such, they are known to increase ROS generation and to increase major signaling and transcriptional factors that control inflammatory and apoptotic responses [20]. Together with the depleted anti-oxidant defenses in trauma patients this increase in pro-oxidant AGEs could further compromise their ability to balance a subsequently fully developed high OS and inflammatory state. Based on the above, however, we could not explain the observed inverse correlation between serum AGEs and ISS on admission, except to speculate that the cohort available for the study was relatively small. Our findings of acutely increased circulating AGE levels after trauma are indeed consistent with findings in patients in the ICU setting [16, 17], in one of which circulating AGEs inversely correlated with sepsis severity as seen in our study [17]. Larger trials are required to illuminate these interesting questions.
Although there was no correlation between levels of blood glucose and serum AGEs, we cannot rule out a contribution of transient hyperglycemia to the increase in post-trauma serum AGEs [21]. Hyperglycemia in critically ill patients occurs as part of the response to injury and is independent of pre-existing diabetes [22, 23]. Intracellular hyperglycemia can lead to the generation of both ROS as well as AGEs, but the exact mechanisms by which it leads to poor post-trauma outcomes remain less well defined. Since in this study glycemia was regulated by insulin administration based on standard ICU protocol, it is unlikely that it was a major underlying contributor to the rapid AGE surge. Of interest, a high AGE state, as with an increased OS state, is associated with insulin resistance in diabetic patients, attributable to the direct pro-inflammatory effects of AGEs, independently of blood glucose levels [15]. Studies in both humans and animals have shown that AGEs regulate SIRT1, a major deacetylase, which controls insulin action as well as inflammatory response, i.e., via hyper-acetylation of NF-κB p65 in tissues [15, 24].
Dietary AGE intake has been found to be an excellent determinant of circulating AGEs under stable or chronic conditions [13–15]. The dietary history obtained revealed a relatively high level of AGE consumption similar to that previously reported for a normal population [12 – 15]. While the levels of serum AGE found in these subjects either upon admission or throughout their hospital stay could not be explained based solely on a high dietary AGE intake, the pre-trauma dietary history supports the conclusion that sufficiently large tissue or intracellular deposits of AGEs would be available for release into the vascular space upon an abrupt disruption of tissue integrity. The increased associative slope of circulating CML and MG in acute trauma patients compared to that in healthy controls supports this view and points to the potential for highly reactive MG-intermediates to impart broad systemic toxicity under these conditions.
Among the limitations of the present pilot study are a relatively small sample size, using a single center and a predominantly Hispanic population, which may not reflect the general population. Limited follow-up measurements of baseline parameters after admission restricted the assessment of their temporal course. Also, the study was not powered for the purpose of drawing conclusions about potential outcomes. It should also be pointed out that the measurements of serum CML and MG were based on ELISA methods that, while well validated, are semi-quantitative, and focus on a few out of a large and heterogeneous group of AGEs [11, 23]. Further studies utilizing analytical approaches, such as LC MS/MS, will be needed to identify and characterize additional AGEs formed under these acute conditions.
In conclusion, this pilot observational study demonstrates a clear association between acute trauma and markedly elevated levels of highly reactive and thus potentially toxic AGEs in serum. Persistent AGE elevation in the circulation appears to parallel trauma severity and complications in the ICU. The findings, while requiring further investigation, may highlight the potential use of rapidly elevated serum AGE levels as a new marker of severe systemic stress in acute trauma victims, and of a potentially adverse impact on outcome.
Acknowledgments
Research funding: None declared.
Support: NIH AG-23188 and AG-09453 (H. Vlassara).
Footnotes
Authors’ conflict of interest disclosure: The authors stated that there are no conflicts of interest regarding the publication of this article.
Employment or leadership: None declared.
Honorarium: None declared.
Contributor Information
Carl I. Schulman, Division of Trauma and Surgical Critical Care, DeWitt Daughtry Family Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, USA
Jaime Uribarri, Mount Sinai School of Medicine, One Gustave Levy Place, New York, NY 10029 USA.
Weijing Cai, Mount Sinai School of Medicine, New York, NY, USA.
Ron Manning, Division of Trauma and Surgical Critical Care, DeWitt Daughtry Family Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, USA.
David C. Landy, Division of Trauma and Surgical Critical Care, DeWitt Daughtry Family Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, USA
Margaret Gallardo, Division of Trauma and Surgical Critical Care, DeWitt Daughtry Family Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, USA.
Angela Castillo, Division of Trauma and Surgical Critical Care, DeWitt Daughtry Family Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, USA.
Nicholas Namias, Division of Trauma and Surgical Critical Care, DeWitt Daughtry Family Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, USA.
Gary E. Striker, Mount Sinai School of Medicine, New York, NY, USA
Alan Livingstone, Division of Trauma and Surgical Critical Care, DeWitt Daughtry Family Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, USA.
Helen Vlassara, Mount Sinai School of Medicine, New York, NY, USA.
References
- 1.Miniño AM, Heron MP, Murphy SL, Kochanek KD. Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics System. Deaths: final data for 2004. Natl Vital Stat Rep. 2007;55:1–119. [PubMed] [Google Scholar]
- 2.Roumen RM, Hendriks T, van der Ven-Jongekrijg J, Nieuwenhuijzen GA, Sauerwein RW, van der Meer JW, et al. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma: relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993;218:769–76. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala A, Wang P, Ba ZF, Perrin MM, Ertel W, Chaudry IH. Differential alterations in plasma IL-6 and TNF levels following and hemorrhage. Am J Physiol. 1991;260:R167–R171. doi: 10.1152/ajpregu.1991.260.1.R167. [DOI] [PubMed] [Google Scholar]
- 4.Ertel W, Keel M, Bonaccio M, Steckholzer U, Gallati H, Kenney JS, et al. Release of anti-inflammatory mediators after mechanical trauma correlates with severity of injury and clinical outcome. J Trauma. 1995;39:879–85. doi: 10.1097/00005373-199511000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997;25:1813–9. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Waydhas C, Nast-Kolb D, Jochum M, Trupka A, Lenk S, Fritz H, et al. Inflammatory mediators, infection, sepsis, and multiple organ failure after severe trauma. Arch Surg. 1992;127:460–7. doi: 10.1001/archsurg.1992.01420040106019. [DOI] [PubMed] [Google Scholar]
- 7.Roumen RM, Redl H, Schlag G, Zilow G, Sandtner W, Koller W, et al. Inflammatory mediators in relation to the development of multiple organ failure in patients after severe blunt trauma. Crit Care Med. 1995;23:474–80. doi: 10.1097/00003246-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Partrick DA, Moore EE, Moore FA, Biffl WL, Barnett CC., Jr Release of anti-inflammatory mediators after major torso trauma correlates with the development of postinjury multiple organ failure. Am J Surg. 1999;178:564–9. doi: 10.1016/s0002-9610(99)00240-8. [DOI] [PubMed] [Google Scholar]
- 9.Bopp C, Hofer S, Weitz J, Bierhaus A, Nawroth PP, Martin E, et al. sRAGE is elevated in septic patients and associated with patients outcome. J Surg Res. 2008;147:79–83. doi: 10.1016/j.jss.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Abraham E. Unraveling the role of high mobility group box protein 1 in severe trauma. Crit Care. 2009;13:1004–1005. doi: 10.1186/cc8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlassara H, Striker G. AGE restriction in diabetes mellitus: a paradigm shift. Nat Rev Encrinolo. 2011;7:1610–6. doi: 10.1038/nrendo.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911–6. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA. 2002;99:15596–601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Zhu L, et al. Protection against loss of innate defenses in adulthood by low AGE intake: role of a new anti-inflammatory AGE-receptor-1. J Clin Endocrinol Metab. 2009;94:4483–91. doi: 10.1210/jc.2009-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uribarri J, Cai W, Ramdas M, Goodman S, Pyzick R, Chen X, et al. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1. Diabetes Care. 2011;34:1610–6. doi: 10.2337/dc11-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Suzuki T, Ueda Y, et al. Circulating levels of advanced glycation end products (AGE) and interleukin-6 (IL-6) are independent determinants of serum asymmetric dimethylarginine (ADMA) levels in patients with septic shock. Pharmacol Res. 2009;60:515–8. doi: 10.1016/j.phrs.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Andrades ME, Lorenzi R, Nagai R, Fonseca Moreira JC, Ritter C, Dal-Pizzol F. Plasma glycation levels are associated with the severity of sepsis. Eur J Clin Invest. 2012;42:1055–60. doi: 10.1111/j.1365-2362.2012.02694.x. [DOI] [PubMed] [Google Scholar]
- 18.Cai W, Gao QD, Zhu L, Peppa M, He C, Vlassara H. Oxidative stress-inducing carbonyl compounds from common foods: novel mediators of cellular dysfunction. Mol Med. 2002;8:337–46. [PMC free article] [PubMed] [Google Scholar]
- 19.Linden E, Cai W, He JC, Xue C, Li Z, Winston J, et al. Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin J Am Soc Nephrol. 2008;3:691–8. doi: 10.2215/CJN.04291007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabbani N, Thornalley PJ. Glyoxalase in diabetes, obesity and related disorders. Semin Cell Dev Biol. 2011;22:309–17. doi: 10.1016/j.semcdb.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Williams MA, Enquobahrie DA, Zimmer J, Qiu CF, Hevner K, Abetew D, et al. Maternal plasma advanced glycation end products concentrations in response to oral 50-gram glucose load in mid-pregnancy: a pilot study. Clin Lab. 2012;58:1045–50. [PubMed] [Google Scholar]
- 22.Vlasselaers D. Blood glucose control in the intensive care unit: discrepancy between belief and practice. Crit Care. 2010;14:145– 146. doi: 10.1186/cc8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellanos MR, Rothman J, Kleiner M. Tight glycemic control in critically ill patients. J Am Med Assoc. 2010;303:16. doi: 10.1001/jama.2010.515. [DOI] [PubMed] [Google Scholar]
- 24.Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci USA. 2012;109:15888–93. doi: 10.1073/pnas.1205847109. [DOI] [PMC free article] [PubMed] [Google Scholar]