Abstract
In the majority of HIV-1 infected individuals, the adaptive immune response drives virus escape resulting in persistent viremia and a lack of immune-mediated control. The expression of negative regulatory molecules such as PD-1 during chronic HIV infection provides a useful marker to differentiate functional memory T cell subsets and the frequency of T cells with an exhausted phenotype. In addition, cell-based measurements of virus persistence equate with activation markers and the frequency of CD4 T cells expressing PD-1. High-level expression of PD-1 and its ligands PD-L1 and - L2 are found on hematopoietic and non-hematopoietic cells, which are regulated by chronic antigen stimulation, Type 1 and Type II interferons (IFNs), and homeostatic cytokines. In HIV infected subjects, PD-1 levels on CD4 and CD8 T cells continue to remain high following combination anti-retroviral therapy (cART). System biology approaches have begun to elucidate signal transduction pathways regulated by PD-1 expression in CD4 and CD8 T cell subsets that become dysfunctional through chronic TCR activation and PD-1 signaling. In this review, we summarize our current understanding of transcriptional signatures and signal transduction pathways associated with immune exhaustion with a focus on recent work in our laboratory characterizing the role of PD-1 in T cell dysfunction and HIV pathogenesis. We also highlight the therapeutic potential of blocking PD-1-PD-L1 and other immune checkpoints for activating potent cellular immune responses against chronic viral infections and cancer.
1. Introduction
In HIV-1 infection, viral replication causes profound CD4 T cell loss, compromises mucosal barrier function, and leads to chronic immune activation and dysfunction that is not fully restored following cART. The CD8 T cell repertoire in HIV-1 infected subjects is functionally heterogeneous with a high frequency of cells arrested in an intermediate T cell differentiation stage and fail to transit to functional memory during persistent infection. In chronic untreated infection, functionally exhausted T cells are unable to proliferate or produce IL-2 and inflammatory cytokines in response to antigen stimulation [1]. Anergy is likely the consequence of a program of coordinately regulated factors induced by NFAT and negative regulatory signals that block proximal TCR signaling and downstream RAS/MEK/ERK, JNK, and PI3K/AKT/mTOR pathways, and cell cycle progression [2–4]. Furthermore, dysfunctional cells display markers associated with replicative senescence: CD28− CD57+ CD95+, γ–H2AXfoci, MAPKK3/6, telomere erosion, and low autophagic flux [5]. Although we have acquired a significant understanding of T cell phenotypes in HIV infection, many questions remain regarding the molecular mechanisms involved in induction and maintenance of exhausted phenotypes and the ability to restore function.
CD8 T cells upregulate multiple inhibitory receptors, including PD-1, 2B4, CTLA-4, CD160, and LAG-3, in response to chronic antigen stimulation and express low and intermediate levels of CD127. Numerous studies have indicated that multiple inhibitory pathways work together to promote T cell exhaustion and tolerance in allogeneic tolerance models [6, 7]. Of note, co-inhibitory molecules (CTLA-4, PD-1, CD160) are also implicated in the normal course of immunity providing signals that reestablish homeostasis and counterbalance the deleterious effects of prolonged immune activation [8–10]. PD-1 plays an essential role in attenuating CD4-mediated immunopathology during Mycobacterium tuberculosis infection and in autoimmune Type 1 diabetes [11–18].
The role of PD-1 in suppressing the antiviral response was first demonstrated by the rapid clearance of adenoviral infections in Pdcd1−/− mice compared to wild type. [19]. The role of PD-1 in acute versus chronic viral infections was further delineated by Barber et al in their study of LCMV infection. In the acute LCMV Armstrong infection model, viral clearance occurred within a week, during which a transient spike in PD-1 levels was observed [11]. CD8 T cells subsequently differentiated into highly multifunctional effector cells with increased IFNγ, TNFα and IL-2 expression and secretion of effector molecules granzyme and perforin. The increase in functional CD8 T cells resulted in efficient viral clearance and establishment of robust CD8 memory cells. In contrast, in the model of chronic LCMV clone 13 infection, antigenic persistence resulted in high levels of PD-1 expression on CD8 T cells, loss of effector function, and an immune exhausted phenotype [11]. CD8 T cells that exhibited an exhausted phenotype showed a progressive loss in proliferation, IL-2 and TNF-α production, IFN-γ and cytotoxic ability [12, 16, 20–25] and the ability to become memory cells [26]. A similar role for PD-1 in skewing exhausted CD4 and CD8 T cell phenotypes has been reported in other chronic viral infections such as Hepatitis C [27, 28], Hepatitis B [29], in SIV [30] and HIV [31–33] as well as in cancer [34]. In the context of chronic virus infections and cancers, therapeutic interventions aimed at blocking PD-1 from interacting with its ligand offer a promising new strategy to restore virus and tumor-specific CD8 T cell proliferation and effector cytokine production that may lead to the control of virus replication and tumor growth [11, 16].
2. PD-1 Expression in Chronic HIV Infection
Pioneering work by Day et al. and Trautmann et al. correlated the levels of PD-1 expression on antigen-specific CD8 T cells with immune dysfunction and viral load during chronic HIV infection [21, 35]. Similar to results obtained by blocking PD-1 signaling in murine models of chronic viral infection, these studies also showed that blocking PD-1-PD-L1 interaction restored the proliferative and survival capability of HIV-specific CD8 T cells in vitro. Zhang et al. showed that PD-1 was expressed on T cells at lower levels in long-term non-progressors (LTNPs) compared to typical progressors. CD8 T cell differentiation was skewed within these two patient groups, with decreased proliferation of HIV specific effector memory cells in typical progressors but not in LTNPs. A recent study identified defects in pathways associated with metabolic and mitochondrial function in CD8 PD-1hi T cells during chronic HIV infection [36].
PD-1 expression is induced by the strength and duration of TCR-peptide/MHC interaction and CD28 costimulation and is sustained by chronic antigen stimulation [37, 38]. PD-1 expression is also induced by γ-chain cytokines IL-2, IL-4, IL-7, IL-15, IL-21, as well as TGF-β and is highly expressed on T cells during chronic HIV/SIV infection [39, 40]. Cells with high PD-1 surface expression correlate with a phenotype typically associated with apoptotic cell death [35, 41–44].
PD-1 is downmodulated after viral epitope escape during LCMV infection, indicating that continued antigen stimulation may be required to maintain PD-1 surface expression [1]. A study by Conrad et al. reported that in chronic HIV infection, PD-1 expression is highest on the dominant clonotype [45] within the antigen-specific response further supporting the idea that for CD8 T cells, PD-1 expression is primarily antigen driven. However, there was no correlation between the magnitude of the immune response and PD-1 expression, indicating that although PD-1 expression is a sensitive marker of antigen recognition, T cells that express higher levels of PD-1 also exhibit reduced expansion in response to antigen [1, 6, 37, 45, 46]. In addition, in vitro studies have demonstrated that PD-1 expression can be induced by cytokine stimulation alone, which suggests that antigen-independent pathways can regulate the expression of PD-1 in chronic infection and conditions of low-level systemic inflammation [47]. These findings are supported by the observation that high PD-1 and PD-L1 expression persists in HIV patients successfully treated with cART and undetectable viral loads [21, 48, 49]. It is important to note that increased PD-1 expression is not limited to HIV-specific CD8 T cells, but is observed in total CD8 and CD4 T cell populations in viremic and in virally suppressed patients [50] [51]. PD-1 expression plays an important role in CD4 T cell dysfunction during HIV infection [52]. Said et al. showed that the translocation of microbial products from the gut to the blood could induce PD-1 expression on monocytes. Upregulation of PD-1 resulted in enhanced IL-10 production and the inhibition, by IL-10 of CD4 T cell proliferation and effector functions. Therapeutic interventions that inhibit PD-1 and other coinhibitory signals with blocking antibodies may reduce the threshold of activation needed for TCR signaling and improve CD4 and CD8 T cell effector responses against chronic virus infections and cancer [53].
3. PD-1 in Aviremic HIV Infected Subjects
Several studies have examined PD-1 expression on peripheral blood T cells in HIV elite controller (EC) and aviremic cART treated cohorts since higher frequencies of both CD4 and CD8 cells expressing PD-1 are associated with viremia and disease progression. These studies showed that HIV antigen-induced CD8 proliferation, production of IFNγ, macrophage inflammatory protein (MIP)-1β, and TNF-α was higher in ECs and aviremic cART treated subjects than in typical or fast progressors [22, 54, 55]. Whittall et al. observed a significant reduction in PD-1 expression in the EC and LTNP cohorts compared to fast progressors [38]. The protective phenotype in EC and LTNP correlated with CD40L expression and a significant increase in the production of chemokines and cytokines CCL-3 (MIP-1α), CCL-4 (MIP-1β), and IL-6 [38].
Whereas PD-1 expression on CD8 cells appears to correlate with ongoing viral replication, high levels of PD-1 expression on CD4 cells appears to predict suboptimal CD4 T cell recovery after long term cART. GALT CD4 and CD8 T cells generally have higher expression of PD-1 and CTLA-4 than peripheral T cells [56]. Interestingly, the level of expression of PD-1 and CTLA-4 on CD4 T cells was consistently higher than levels observed in CD8 T cells in GALT, which further reinforces previous findings that inflammatory cytokines differentially regulate PD-1 expression on CD4 and CD8 T cells [56]. Despite higher levels of activation markers in the GALT, detection of viral DNA is considerably lower than in peripheral blood and isolation of cells with multi-spliced viral RNA is rare [57, 58].
5. Increasing CD8 T cell dysfunction is associated with the expression of multiple negative regulators
Since PD-1 expression is transiently upregulated on activated CD8 T cells, it is often difficult to distinguish at the population level between recently activated antigen-specific cells and cells with high levels of PD-1 characteristic of an exhausted phenotype. CD4 T cells activated by HIV exposed DCs upregulate the expression of multiple inhibitory molecules, including CTLA-4, CD160, 2B4, and Tim-3 that might additively contribute to the level of T cell dysfunction [59–61]. Recent studies of anti-tumor T cell responses support the concept that co-expression of several negative regulators on CD8 T cells confer increased levels of cellular dysfunction [62–64]. A study by Peretz et al. examined the co-expression of PD-1 with CD160, another negative regulator of T cell function, during different stages of HIV infection [60]. Their study identified a PD-1/CD160 double positive subset specifically present in chronic infection that not only had the highest level of PD-1 expression, but also demonstrated the least functional phenotype [60]. Transcriptional analysis revealed down-regulation of several transcriptional nodes involved in the regulation of T cell survival and effector function, details of which are discussed further in section 11 [60, 65, 66]. Further studies are needed to determine the long-term outcome of cells that fail to downregulate the PD-1 receptor and whether immune cells expressing multiple coinhibitory molecules can be rescued from senescence in vivo or are more susceptible to undergo apoptosis upon antigen activation.
A study by Lichterfeld et al. demonstrated that HIV-specific CTLs have shortened telomeric DNA and significant reduction in telomerase activity [67]. The telomere length in HIV-specific CD8 T cells approaches the Hayflick limit for terminal senescence during progressive HIV infection [22, 55, 68]. The shortened telomeres observed in HIV-specific CD8 T cells correlated with reduced expression of proteins of the shelterin complex and disruption of shelterin complex formation [69]. The shelterin complex is a group of nucleoproteins found at the terminal ends of chromosomal DNA whose function is to protect telomeric DNA and regulate telomerase activity [70, 71]. When the shelterin complex is dissociated, telomeric DNA is exposed. This is recognized as a double strand break and initiates the DNA damage response for repair to limit structural DNA damage, such as chromosomal recombination or non-homologous end joining [72, 73]. Telomere length and telomerase activity could be rescued in HIV specific CD8 T cells treated with PD-1 blocking antibodies [67]. This lead to improved clinical outcomes in SIV infected rhesus macaques treated with PD-1 blocking antibody [74]. These data suggest that HIV-specific CD8 T cells with shortened telomeres are not in a state of irreversible DNA repair and that recovery of telomere length may contribute to the rescue of cytotoxic effector functions [69]. However, Lichterfeld et al. also found that despite an observed increase in telomere length, PD-1 blocking antibody treatment did not result in stabilization to normal levels in the protein expression of the components of the shelterin complex that protect exposed telomere ends and which could influence long term survival [69].
6. PD-1 Signaling
PD-1 consists of a single CD28-like extracellular Ig domain and a 20 amino acid stalk. Its cytoplasmic tail contains two conserved tyrosine-based signaling motifs, an immunoreceptor tyrosine-based inhibition motif (ITIM), followed by an immunoreceptor tyrosine-based switch motif (ITSM), both of which are phosphorylated upon PD-1 engagement [21, 75]. Current models for the inhibitory action of PD-1 on proximal T cell signaling suggest that these motifs recruit negative regulators to the immunological synapse and block Lck kinase activity and phosphorylation of the TCR. The identity of PD-1-binding partners and proximal protein tyrosine kinase (PTK) signaling and protein tyrosine phosphatases (PTPs) involved in PD-1 mediated inhibition of TCR signaling is an ongoing area of investigation [76, 77]. As the effect of PD-1 triggering by PD-L1 or PD-L2 leads to blocking antigen-specific proliferation, cytokine production, cytolytic function, and pro-survival pathways this suggests that PD-1 could interfere with a very proximal step in T cell activation [78].
7. PD-1 Interference with the most proximal TCR signaling event
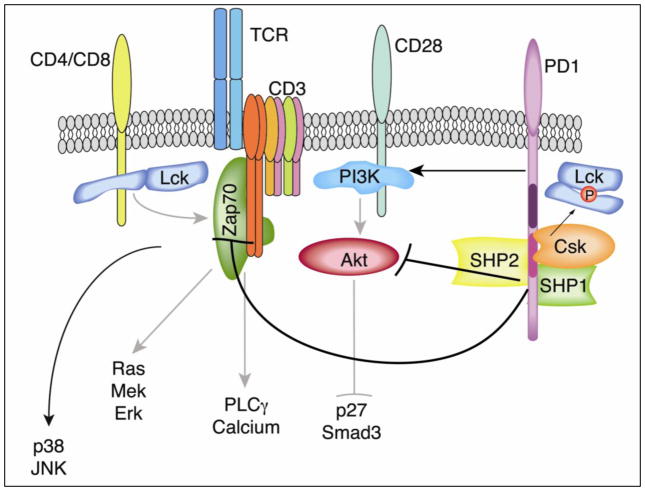
Sheppard et al. demonstrated that PD-1 signaling could interfere with the earliest tyrosine phosphorylation events in TCR signaling [75]. Specifically, they showed a decrease in phosphorylation of ZAP-70 and the CD3ζ chain, similar to the defect in phosphorylation of Ig, Syk, PLCγ and ERK observed in B cells as a result of PD-1 triggering [79]. In addition, immunoprecipitation of biotinylated, phosphorylated peptides of the ITIM and ITSM domains of the PD-1 cytoplasmic tail identified 4 proteins that bind to specific residues of PD-1. The tyrosine phosphatases SHP-1 and SHP-2 were found to bind to the phosphotyrosines in the ITSM motif. Surprisingly, two Src family kinases csk and lck were identified as binding to ITSM and ITIM, respectively. Thus, these four signaling proteins represent the most likely mediators of PD-1 signaling function [Fig. 1]. The observation that Lck and Csk can both be recruited to PD-1 provides for an interesting potential mechanism of TCR attenuation. Activation of lck represents a critical step in the initiation of TCR signaling [80]. Csk-mediated phosphorylation of Lck Tyr505 results in an inactive lck conformation through cis binding of the lck SH2 domain. This is functionally opposed by the receptor-like tyrosine phosphatase CD45, which dephosphorylates Tyr505, and phosphorylation of the conserved Tyr394 in the activation loop of the catalytic domain of Lck conferring increased kinase activity and enabling Lck to phosphorylate TCR/CD3 complex ITAMs. Recruitment of both of these kinases to the cytoplasmic tail of PD-1 during TCR activation and formation of the immunological synapse represents a potential mechanism through which PD-1 can inhibit the earliest events in proximal TCR signaling by inactivating lck [81, 82].
Figure 1. PD-1 ligation inhibits proximal TCR signaling and CD28-mediated costimulation required for cell cycle progression.
Colocalization of PD-1 to TCR microclusters following engagement with its ligand PDL-1/PDL-2 mediates a phosphorylation dependent inhibition of TCR stimulation [99]. Inhibition of membrane proximal phosphorylation of CD3ζ chain and ZAP-70 [87] and the downstream Ras/Mek/Erk, and AKT signaling pathways promotes accumulation of CDK inhibitory protein p27kip1 and cell cycle arrest [3]. Inhibitory effects of PD-1 ligation have been attributed to the recruitment of the tyrosine phosphatase SHP-2, by the ITSM of PD-1 to the immunological synapse resulting in dephosphorylation of TCR signaling molecules [99]. Immunopreciptation studies demonstrate that the tyrosine kinases Csk and Lck are also recruited to PD-1 [87]. Since the target of Csk is the inhibitory Y505 of Lck, this suggests a potential mechanism for PD-1 in inactivating Lck and shutting down the induction of the TCR-mediated tyrosine phosphorylation cascade.
8. SHP-2 as a mediator of PD-1 signaling
Several studies have demonstrated an association of the tyrosine phosphatases SHP-1 and SHP-2 with PD-1 [79, 83–86]. These molecules make appealing candidates for mediators of PD-1 signaling as they provide a simple potential mechanism of signal attenuation by tyrosine dephosphorylation. Although either SHP-1 or SHP-2 can mediate PD-1 inhibition of TCR signaling when artificially co-localized to TCR microclusters, only SHP-2 is recruited physiologically [87]. SHP-2 appears to be the more important player inPD -1 signaling in T cells. Recent work by Yokosuka et al. underscores the importance of SHP-2 in PD-1 mediated inhibition of TCR signaling [87]. Using a planar bilayer system, they were able to determine that following stimulation of both TCR and PD-1, 98% of activated TCRs localized to microclusters containing PD-1. In the absence of PD-L1 ligation, PD-1 localized away from TCR microclusters. Co-localization of PD-1 to TCR microclusters was essential for destabilization of the immune synapse and efficient at inhibiting TCR signaling independent of PD-1 phosphorylation. The latter was supported by mutation of the ITIM and ITSM tyrosines to phenylalanine, which resulted in recruitment of PD-1 to microclusters but an inability to inhibit TCR signals.
Phosphorylation of SHP-2 occurs upon stimulation of both PD-1 and TCR [84]; the critical phosphotyrosine residue within the PD-1 cytoplasmic tail is also required for recruitment of SHP-2 [83]. Additionally, SHP-2 can inhibit integrin signaling, which may partially account for the destabilization of the immune synapse caused by PD-1 engagement [88]. The current model to explain how SHP-2 could mediate PD-1 inhibition of TCR signaling is that PD-1 recruits SHP-2 to TCR microclusters where it dephosphorylates tyrosines within the proximal TCR signaling complex. While this may be correct, evidence supporting this model is sparse. Thus, it appears that the effect of SHP-2 on TCR signaling seems to be highly dependent on the context with which SHP-2 is recruited to the TCR signaling complex.
9. PD-1 Inhibition of Cell Cycle
Recent studies have identified differences in PD-1 and CTLA-4 signaling on inhibition of PI3K-Akt and the downstream effects on IL-2 production [89, 90]. The observation that CD3ζ chain and ZAP-70 phosphorylation is abrogated in response to PD-1 signaling suggests that PD-1 is acting on the earliest TCR signaling events [75]. Recent work by Patsoukis et al. has elegantly defined the mechanism by which PD-1 engagement mediates cell cycle arrest [3]. This group demonstrated that PD-1 downregulates transcription of the gene that encodes for Skp2, a recognition component of the ubiquitin E3-ligase complex Skp, Cullin, F-boxcontaining complex (SCF). Down-regulation of Skp2 prevents the ubiquitination and degradation of p27 during the induction of cell cycle. PD-1 mediated stabilization of p27 and increased transcriptional activity of Smad3 to initiate the cascade of events that ultimately result in G1 cell cycle arrest. SMAD3 increases expression of CDK inhibitor p15INK4 and inhibits the CDK activator CDC25A resulting in inhibition of cyclin dependent kinases CDK2, CDK4, and CDK6 essential for progression through G1 and S phase of the cell cycle. These downstream effects were found to be the result of both defective activation of the Ras-MEK-ERK pathway and inhibition of Akt activation. Interestingly, p38 and JNK activation remained unaffected, indicating that PD-1 signaling selectively targets TCR signaling pathways rather than representing a global abrogation of TCR signaling.
Adoptive cell transfer studies of tumor infiltrating lymphocytes for the immunotherapy of cancer have demonstrated a role for PD-1 signaling not only in the inhibition of Akt and Erk pathways but also in TCR induced calcium flux [85]. These studies showed a partial loss in PD-1 mediated inhibition upon treatment with sodium stibogluconate, a chemical inhibitor of the tyrosine phosphatases SHP-1 and SHP-2. These observations bring into question whether PD-1 is mediating direct dephosphorylation of Akt and PI3K or if it is working further upstream. The observation that IL-2 can restore Ras-MEK-ERK signaling but not the AKT/PI3K pathway suggests that PD-1 inhibition is more complex than inhibition of global tyrosine phosphorylation during the proximal TCR signaling events. However, additional studies are necessary to understand the exact mechanisms. Multiple overlapping mechanisms are likely involved as PD-1 has been also shown to upregulate Cbl activity that might also inhibit Akt activity through stabilization of PTEN [76, 91] The fact that p38 and JNK signaling pathways are still intact after PD-1 stimulation even though these pathways are downstream of TCR suggests that PD-1 does not simply facilitate a global inhibition of TCR. Instead, this seems to suggest that PD-1 inhibition targets specific pathways that may help to define a unique PD-1 signature associated with TCR inhibition and that is non-overlapping with other negative regulatory molecules. New insights gained from systems biology approaches may help to further elucidate critical pathways of chronic TCR activation and PD-1 signaling and provide insight into the role of PD-1 in immune dysfunction and cell survival. Indeed, system biology approaches have proven successful for identifying gene expression signatures and biomarkers that predict disease pathogenesis and responses to vaccination and are currently being used to help identify individuals where blocking PD-1 and other coinhibitory molecules could restore and augment antiviral and anti-tumor immune responses.
11. Transcriptional Profiles and Pathways associated with Progressive Immune Dysfunction
Although PD-1 signaling is known to inhibit proximal TCR signaling, proliferation, and T cell function, a recent study showed differential expression of only three transcription factors upregulated by PD-1 ligation in HIV progressors compared to elite controllers: i) Basic leucine zipper transcription factor ATF-like (BATF), ii) signal transducer and activator of transcription-1 (STAT1), and iii) interferon regulatory factor-9 (IRF9) [92]. BATF is a negative regulator of AP-1 activity and is sufficient to inhibit T cell proliferation and cytokine production, whereas silencing BATF expression reduces PD-1 mediated inhibition. BATF plays an essential role in the upregulation of BCL-6 and cMAF for CD4 T follicular helper and Th17 differentiation and B cell class-switch recombination [93] and reciprocally regulates BLIMP expression during CD8 memory differentiation in humans [92, 94]. Furthermore, BCL-6 represses the expression of miRNAs that inhibit PD-1 expression indicating a positive feedback loop for PD-1 expression through the BATF/BCL-6 axis [95, 96]. Other PD-1 signature genes enriched in exhausted CD8 T cells showed significant correlation with genes with known inhibitory function including CD244, KLRG1, KLRK1, and KLRD1. In healthy adults, the Killer cell Ig-like receptor family (KIRL) genes are among the most significantly downregulated genes in CD8 CCR7lo PD-1hi cells compared to CD8 CCR7lo PD-1lo cells [97]. In contrast, the KIRL family of genes is significantly upregulated in CD8 PD-1hi CD160hi double positive cells in chronic HIV infection. These DP cells are less responsive to PD-1 blocking when compared to single positive CD8 PD-1hi CD160lo cells from the same individual. Similarly, it has been shown that HCV-specific CD8 T cells that are highly PD-1 positive respond poorly to PD-1-PD-L1 blockade [98]. In chronic infection, exhausted CD8 T cells lack repressive histone marks (H3K9me3 and H3K27me3) and fail to remethylate DNA at the PDCD1 promoter [60]. This is due to the downregulation of the Dnmt3a isoform2 (Dnmt3a2) in exhausted CD8 cells (18- and 7- fold relative to day 4 effector and functional memory CD8 T cells). Since expression of the KIRL family is also regulated by DNA methylation, global hypomethylation of target genes might represent a key mechanism for maintaining nonfunctional T cells.
A recent study by Peretz et al. found that co-expression of PD-1 and CD160 is associated with greater T cell dysfunction in chronic HIV-infection [60]. Sorted CD8 T cell subsets from HIV-infected patients expressing PD-1 and/or CD160 were analyzed for their transcriptional profile with the objective of defining a signature that could differentiate between activation and exhaustion. Unsupervised cluster analysis showed that both double positive and single positive PD-1 subsets clustered apart to create two statistically significant populations with unique transcriptional programs. Several genes involved in the inhibition of survival were activated (SUMO2, KIF7) in the CD8 PD-1+CD160+ double positive (DP) subset while target genes of Wnt/Notch signaling were induced (Wnt7a, AXIN2) in the PD-1+CD160− single positive (SP) subset. A similar observation was made in a recent study that compared the differential utilization of transcription factor hub genes and pathways from longitudinal samples of memory and exhausted T cell subsets [99]. Although TCF1 (Tcf7), a key transcription factor in the WNT/βcatenin pathway for self renewal is downregulated in exhausted CD8 PD-1hi T cells, other TCF4 or non-canonical Wnt signaling pathways might be operative in SP CD8 cells, whereas CD8 PD-1hi CD160hi double positive cells downregulated the expression of Wnt target genes.
Comprehensive gene array profiling on sorted HIV-specific CD8 T cells at different stages of disease as well as in subjects who naturally control viral replication revealed that genes involved in response to cellular and oxidative stress are strongly expressed among patients who naturally control viral load and express low levels of PD-1 and CD160 on responding T cells [26, 59, 100]. Gene set enrichment analysis confirmed this observation and identified pathways downregulated in protein folding as well as several targets of the KEAP/NRF2 regulatory pathway. NRF2 is a potent transcriptional activator involved in the inducible expression of many cytoprotective genes in response to oxidative stresses target and inflammation and acts on major cellular functions such as repair and removal of damaged proteins (STIP1, HSP) and cell survival. Nrf2 (NF-erythroid derived 2-like) is upregulated through the AKT and ERK1/2 signal transduction pathways and regulated post-translationally by KEAP mediated proteasomal degradation. Therefore, KEAP upregulation and NRF2 downregulation seen in the double positive CD8 PD1hi CD160hi subset could be an important determinant in predicting the function and survival of exhausted and effector CD8 T cells populations in settings of chronic inflammation.
Pathway analysis has become the first choice for extracting information and explaining the biological relevance of high-throughput molecular data [101]. The ability to infer negative regulatory networks from expression data and how transcription factors selectively and dynamically activate different transcriptional targets is a major challenge. Currently, we infer regulatory networks from expression data using GSEA knowledge base driven pathway analysis methods. Functional class sorting aggregates gene level statistics for all differentially expressed genes in a pathway to generate a single pathway level statistic that is tested for significance by the null hypothesis. The combination of transcriptional profiling, cellular phenotype and functional analyses have allowed us to derive correlations between transcriptional pathways and innate and adaptive immune mechanisms leading to immune exhaustion and disease progression. New bioinformatics tools and methods such as GENIE3 which uses random forests to construct trees to identify transcription factors whose expression levels at a given time can predict the expression levels of each target gene are needed to help overcome current limitations in identifying interdependence between pathways in a dynamic system [102]. Simultaneous integration of gene expression data, proteomic, and metabolomic data with physiological endpoints using computational techniques such as the Sparce Partial Least Squared (sPLS) method, that combines both integration and simultaneous variable selection on two datasets in a one-step strategy, has allowed us to identify biomarkers of immune failure not detected by independent analysis of single datasets alone. The sPLS method, an adaptation of PLS, addresses our need to perform integrative analyses with features like transcriptional and metabolic profiles, cytometric profiles, CD4 counts, plasma biomarkers and proinflammatory cytokines, and clinical indices [103]. The method is implemented in the R package mixOmics, and has been validated empirically on simulated and real biological data in our preliminary studies [104].
12. Therapeutic Potential of blocking PD-1 and its Ligands
Combining immunological, biochemical and systems biology data provides significant support for PD-1 as an important target for therapeutic interventions. Indeed, studies in HIV, Hepatitis B, and Hepatitis C have demonstrated an effect of blocking PD-1-PD-L1/2 interaction on the rescue of T cell effector functions [21, 27, 35, 74, 98, 105–108]. IFNs are strong inducers of PD-L1 (B7-H1) and PD-L2 (B7-H2) expression on hematopoietic and non-hematopoietic cells via STAT1 activation and IFN regulatory factor 1 (IRF1). PD-L1 has two binding partners, PD-1 and B7-1, and the significance of the PD-L1-B7-1 interaction in regulating self-reactive T cell responses has been shown in models of autoimmunity. Several groups have reported that blocking PD-L1 may have a higher efficacy in rescuing T cell function than targeting PD-1 itself [21, 48]. A recent study by Rosignoli et al. comparing PD-1 with PD-L1 blocking antibody found that blocking PD-L1 resulted in a five-fold increase in HIV Gag-specific proliferation in PBMCs from cART treated patients [50], whereas studies in cancer have shown that blocking PD-1 might be more effective than antibodies targeting its ligand, PD-L1 [126,127].
We, as well as other groups, are currently conducting safety and efficacy studies with PD-1 blocking antibodies in the rhesus macaque SIV infection model [74, 109]. Preliminary results show that PD-1 blockade leads to the expansion of SIV specific CD8 T cells, rescue of cytotoxic functions, and a significant reduction in viral load [74]. Interestingly, PD-1 blocking treatment also resulted in expansion of memory B cells and an increase in antibodies to SIV envelope [74]. This same group has recently shown that activated memory B cells expressing PD-1 are rapidly lost in SIV-infected macaques who experience rapid progressive disease [109]. PD-1 blockade in these animals allowed for greater antibody responses to both SIV and non-SIV antigens [109]. Another study using PD-1 blocking antibody in SIV infected macaques showed increased survival and evidence for restoration of mucosal barrier integrity [110]. These results were associated with the induction of a Type I IFN gene signature in blood and in tissue isolated from GALT of treated animals. In summary, these preclinical studies provide a strong rationale for initiating human clinical trials targeting PD-1 with blocking antibodies in HIV infected patients in combination with anti-retroviral therapy.
Although cART has been shown to partially restore the immune function of exhausted antigen-specific CD8 T cells of HIV infected subjects [111], blocking PD-1 or combinations of co-inhibitory molecules, might serve a dual role by reactivating latent virus in CD4 cells and by relieving a functional block on virus-specific CD8 memory T cells to enhance antigen-specific proliferation and effector cell differentiation. A recent study showed that in HIV infected patients successfully treated with cART, pre-stimulation of autologous CD8 CTL ex vivo with Gag peptides and IL-2 was required to activate killing of virus-infected CD4 cells reactivated in vitro with SAHA [112]. The implications of this study for eradicating latent virus reservoirs is that Gag-specific CD8 CTLs preexist in cART treated HIV infected subjects (in this small study) in an arrested state of differentiation and that antigen stimulation can rapidly induce anti-viral effector functions. Thus, for the majority of successfully treated HIV patients, a push-pull approach blocking negative regulatory signals with PD-1 blocking antibody while activating antigen-specific CD8 TTM and TEM effector cells by vaccination and cytokines such as IL-15 or IL-7 might prove most effective for CD8 CTL killing of virus infected cells and prevent reactivated virus infection from replenishing latent reservoirs (Fig. 2). Targeting immune checkpoints carries a general risk for autoimmune reactions and it will be important to determine the risk/benefit ratio of anti-PD-1 therapy in HIV infected patients receiving cART. We are currently performing these safety and proof of concept studies using PD-1 blocking antibody therapy in SIV infected, cART treated macaques.
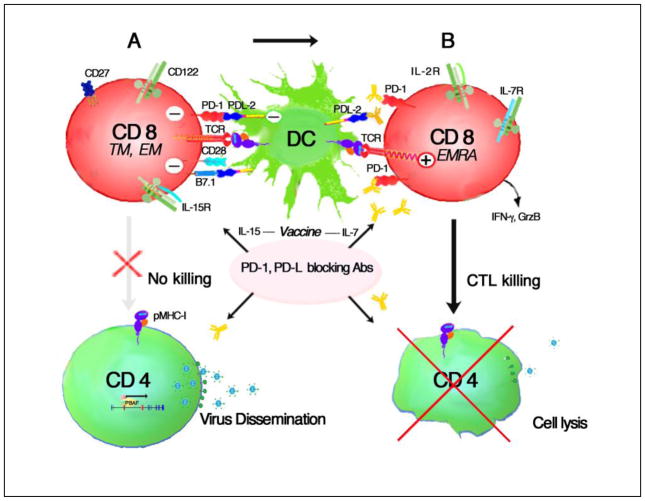
Figure 2. Functional activation of exhausted antigen-specific CD8 CTL by PD-1/PD-L1/L2 blocking antibodies.
High levels of PD-1 expression are found on antigen-specific CD8 TTM and TEM cells in HIV infection even in patients successfully treated with HAART. CD8 cells also express B7.1 that can bind PD-L2 expressed on DCs further suppressing CD8 differentiation to effectors (A). Blocking PD-1 or its ligand with monoclonal antibodies can prevent negative signals delivered through PD-L1 and PD-L2 ligand binding and increase the sensitivity of the TCR to antigen. In the absence of PD-1 coinhibitory signals, CD8 cells can proliferate, differentiate into highly effective cytotoxic T effector cells and produce effector molecules such as IFN-γ and Granzyme B. IL-15 can drive TEMRA and CTL differentiation to kill CD4 T cells that are replicating virus and administering IL-7 concurrently with vaccination strategies might further enhance CD8 killing.
13. Blocking Immune Checkpoints in Cancer
The safety, antitumor activity, and pharmacokinetics of blocking negative regulatory receptors to enhance anti-tumor immunity lead to approval by the FDA in 2011 of Ipilimumab, a CTLA-4 blocking antibody, for the treatment of advanced melanoma. Two large Phase I trials blocking PD-1-PD-L1 interactions in patients with advanced cancers including melanoma and non-small cell lung cancer (NSCLC) have recently reported highly promising results for targeting PD-1 in cancer. Topalian et al. used an IgG4 monoclonal antibody (BMS-936558) that targets PD-1 while Brahmer; et al. used a monoclonal antibody that targeted PD-L1. Tumor response rates were between 18–28% for patients treated with anti-PD-1 antibody and 6–17% for the anti-PD-L1 antibody [113, 114]. Of the patients that tested positive for PD-L1 expression there was a response rate of 36% suggesting that PD-L1 might be a useful biomarker predictive of efficacy. Although these results are encouraging for the use of blocking PD-1 monoclonal antibodies for activating anti- tumor responses, preclinical models suggest combinatorial strategies that block multiple immune checkpoints combined with vaccination, molecular adjuvants and/or cytokines, might improve T cell effector function and antitumor responses [115, 116].
14. Conclusion
Recent studies have lead to significant advances in our understanding of the central role PD-1 and other negative regulatory molecules play in regulating immune function, as well as the potential therapeutic benefit of blocking PD-1-PD-L1 interactions in augmenting CD8 and CD4 T cell effector functions. These studies have provided the basis for the development of humanized monoclonal antibodies that block PD-1 inhibitory signaling to enhance immune effector responses for the eradication of tumors and chronic infectious diseases, with the potential for development of PD-1 agonists that can suppress the immune mediated pathology in autoimmune diseases. Clinical trials in cancer treatment and macaque SIV studies using PD-1 blocking antibodies have produced promising results in vivo that merit continued investigation into identifying patient populations that might benefit from these interventions. Recent clinical trials in cancer highlight the fact that response to treatment with PD-1 or PD-L1 blocking antibodies is variable and clinical responses are not seen in a large proportion of treated patients. Systems level investigations in the era of personalized medicine is needed to identify mechanisms and molecular pathways that can predict the ability to restore immune function and identify the patient populations most likely to respond to PD-1 blockade. Additionally, future studies focused on elucidating additive effects of blocking PD-1 and other negative regulatory molecules and immunosuppressive cytokines will help to identify combinatorial approaches that can enhance T cell effector responses to vaccination and therapeutic interventions.
Highlights.
System biology approaches toward understanding immune dysfunction in HIV infection
Transcriptional programs regulating anergy and cell survival in chronic HIV infection
PD-1 expression correlates with both functional and dysfunctional immune responses
Blocking PD-1 and other immune checkpoints in HIV potentiates CD8 effector function.
New computational methods are needed to model regulation of transcriptional networks
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. Journal of Virology. 2009 May;83:4386–94. doi: 10.1128/JVI.02524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fehr T, Lucas CL, Kurtz J, Onoe T, Zhao G, Hogan T, et al. A CD8 T cell-intrinsic role for the calcineurin-NFAT pathway for tolerance induction in vivo. Blood. 2010 Feb 11;115:1280–7. doi: 10.1182/blood-2009-07-230680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012 Jun 26;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells AD. New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J Immunol. 2009 Jun 15;182:7331–41. doi: 10.4049/jimmunol.0803917. [DOI] [PubMed] [Google Scholar]
- 5.Phadwal K, Alegre-Abarrategui J, Watson AS, Pike L, Anbalagan S, Hammond EM, et al. A novel method for autophagy detection in primary cells: impaired levels of macroautophagy in immunosenescent T cells. Autophagy. 2012 Apr;8:677–89. doi: 10.4161/auto.18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, et al. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathogens. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas CL, Workman CJ, Beyaz S, LoCascio S, Zhao G, Vignali DA, et al. LAG-3, TGF-beta, and cell-intrinsic PD-1 inhibitory pathways contribute to CD8 but not CD4 T-cell tolerance induced by allogeneic BMT with anti-CD40L. Blood. 2011 May 19;117:5532–40. doi: 10.1182/blood-2010-11-318675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterson AM, Brown KE, Keir ME, Vanguri VK, Riella LV, Chandraker A, et al. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol. 2011 Aug 1;187:1097–105. doi: 10.4049/jimmunol.1003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Riella LV, Chock S, Liu T, Zhao X, Yuan X, et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol. 2011 Aug 1;187:1113–9. doi: 10.4049/jimmunol.1100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science. 2012 Apr 27;336:485–9. doi: 10.1126/science.1217718. [DOI] [PubMed] [Google Scholar]
- 11.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006 Feb 9;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nature Immunology. 2007 Nov;8:1246–54. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 13.Lazar-Molnar E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proceedings of the National Academy of Sciences of the United States of America. 2010 Jul 27;107:13402–7. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee LF, Logronio K, Tu GH, Zhai W, Ni I, Mei L, et al. Anti-IL-7 receptor-alpha reverses established type 1 diabetes in nonobese diabetic mice by modulating effector T-cell function. Proceedings of the National Academy of Sciences of the United States of America. 2012 Jul 31;109:12674–9. doi: 10.1073/pnas.1203795109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008 May;28:710–22. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 16.Okazaki T, Honjo T. Rejuvenating exhausted T cells during chronic viral infection. Cell. 2006 Feb 10;124:459–61. doi: 10.1016/j.cell.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Penaranda C, Kuswanto W, Hofmann J, Kenefeck R, Narendran P, Walker LS, et al. IL-7 receptor blockade reverses autoimmune diabetes by promoting inhibition of effector/memory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2012 Jul 31;109:12668–73. doi: 10.1073/pnas.1203692109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Periasamy S, Dhiman R, Barnes PF, Paidipally P, Tvinnereim A, Bandaru A, et al. Programmed death 1 and cytokine inducible SH2-containing protein dependent expansion of regulatory T cells upon stimulation With Mycobacterium tuberculosis. The Journal of Infectious Diseases. 2011 May 1;203:1256–63. doi: 10.1093/infdis/jir011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. The Journal of experimental medicine. 2003 Jul 7;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. Journal of Immunology. 2009 May 15;182:5891–7. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nature Medicine. 2006 Oct 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 22.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006 Jun 15;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. Journal of Immunology. 2003 Jan 1;170:477–86. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 24.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Current Opinion in Immunology. 2007 Aug;19:408–15. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. Journal of Virology. 2003 Apr;77:4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angelosanto JM, Blackburn SD, Crawford A, Wherry EJ. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J Virol. 2012 Aug;86:8161–70. doi: 10.1128/JVI.00889-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. Journal of Virology. 2007 Mar;81:2545–53. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. Journal of Immunology. 2002 Sep 15;169:3447–58. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 29.Rehermann B, Chang KM, McHutchinson J, Kokka R, Houghton M, Rice CM, et al. Differential cytotoxic T-lymphocyte responsiveness to the hepatitis B and C viruses in chronically infected patients. Journal of Virology. 1996 Oct;70:7092–102. doi: 10.1128/jvi.70.10.7092-7102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007 Aug 1;110:928–36. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. The Journal of experimental medicine. 2000 Jul 3;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001 Mar 1;410:106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 33.Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, et al. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. The Journal of experimental medicine. 2003 Dec 15;198:1909–22. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nature Medicine. 1999 Jun;5:677–85. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 35.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006 Sep 21;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 36.Trautmann L, Mbitikon-Kobo FM, Goulet JP, Peretz Y, Shi Y, Van Grevenynghe J, et al. Profound metabolic, functional and cytolytic differences characterize HIV-specific CD8 T cells in primary and chronic HIV infection. Blood. 2012 Sep 6; doi: 10.1182/blood-2012-04-422550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottschalk RA, Hathorn MM, Beuneu H, Corse E, Dustin ML, Altan-Bonnet G, et al. Distinct influences of peptide-MHC quality and quantity on in vivo T-cell responses. Proc Natl Acad Sci U S A. 2012 Jan 17;109:881–6. doi: 10.1073/pnas.1119763109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whittall T, Peters B, Rahman D, Kingsley CI, Vaughan R, Lehner T. Immunogenic and tolerogenic signatures in human immunodeficiency virus (HIV)-infected controllers compared with progressors and a conversion strategy of virus control. Clinical and experimental immunology. 2011 Nov;166:208–17. doi: 10.1111/j.1365-2249.2011.04463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cumont MC, Monceaux V, Viollet L, Lay S, Parker R, Hurtrel B, et al. TGF-beta in intestinal lymphoid organs contributes to the death of armed effector CD8 T cells and is associated with the absence of virus containment in rhesus macaques infected with the simian immunodeficiency virus. Cell death and differentiation. 2007 Oct;14:1747–58. doi: 10.1038/sj.cdd.4402192. [DOI] [PubMed] [Google Scholar]
- 40.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunological Reviews. 2010 Jul;236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Far M, Halwani R, Said E, Trautmann L, Doroudchi M, Janbazian L, et al. T-cell exhaustion in HIV infection. Current HIV/AIDS reports. 2008 Feb;5:13–9. doi: 10.1007/s11904-008-0003-7. [DOI] [PubMed] [Google Scholar]
- 42.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. Journal of Virology. 2007 Sep;81:9249–58. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annual Review of Immunology. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nilsson J, Boasso A, Velilla PA, Zhang R, Vaccari M, Franchini G, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006 Dec 1;108:3808–17. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conrad JA, Ramalingam RK, Smith RM, Barnett L, Lorey SL, Wei J, et al. Dominant clonotypes within HIV-specific T cell responses are programmed death-1high and CD127low and display reduced variant cross-reactivity. Journal of Immunology. 2011 Jun 15;186:6871–85. doi: 10.4049/jimmunol.1004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salisch NC, Kaufmann DE, Awad AS, Reeves RK, Tighe DP, Li Y, et al. Inhibitory TCR coreceptor PD-1 is a sensitive indicator of low-level replication of SIV and HIV-1. Journal of Immunology. 2010 Jan 1;184:476–87. doi: 10.4049/jimmunol.0902781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. Journal of Immunology. 2008 Nov 15;181:6738–46. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 48.Forler D, Kocher T, Rode M, Gentzel M, Izaurralde E, Wilm M. An efficient protein complex purification method for functional proteomics in higher eukaryotes. Nature Biotechnology. 2003;21:89–92. doi: 10.1038/nbt773. [DOI] [PubMed] [Google Scholar]
- 49.Rosignoli G, Cranage A, Burton C, Nelson M, Steel A, Gazzard B, et al. Expression of PD-L1, a marker of disease status, is not reduced by HAART in aviraemic patients. AIDS. 2007 Jun 19;21:1379–81. doi: 10.1097/QAD.0b013e3281de7296. [DOI] [PubMed] [Google Scholar]
- 50.Rosignoli G, Lim CH, Bower M, Gotch F, Imami N. Programmed death (PD)-1 molecule and its ligand PD-L1 distribution among memory CD4 and CD8 T cell subsets in human immunodeficiency virus-1-infected individuals. Clinical and experimental immunology. 2009 Jul;157:90–7. doi: 10.1111/j.1365-2249.2009.03960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koizumi H, Hashimoto M, Fujiwara M, Murakoshi H, Chikata T, Borghan MA, et al. Different in vivo effects of HIV-1 immunodominant epitope-specific cytotoxic T lymphocytes on selection of escape mutant viruses. Journal of Virology. 2010 Jun;84:5508–19. doi: 10.1128/JVI.02483-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nature Medicine. 2010 Apr;16:452–9. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ha SJ, West EE, Araki K, Smith KA, Ahmed R. Manipulating both the inhibitory and stimulatory immune system towards the success of therapeutic vaccination against chronic viral infections. Immunological Reviews. 2008 Jun;223:317–33. doi: 10.1111/j.1600-065X.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 54.Zimmerli SC, Harari A, Cellerai C, Vallelian F, Bart PA, Pantaleo G. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proceedings of the National Academy of Sciences of the United States of America. 2005 May 17;102:7239–44. doi: 10.1073/pnas.0502393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nature Immunology. 2002 Nov;3:1061–8. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 56.Rueda CM, Velilla PA, Chougnet CA, Montoya CJ, Rugeles MT. HIV-Induced T-Cell Activation/Exhaustion in Rectal Mucosa Is Controlled Only Partially by Antiretroviral Treatment. PLoS ONE. 2012;7:e30307. doi: 10.1371/journal.pone.0030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marchetti G, Bellistri GM, Borghi E, Tincati C, Ferramosca S, La Francesca M, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008 Oct 1;22:2035–8. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 58.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. The Journal of Infectious Diseases. 2009 Apr 15;199:1177–85. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Che KF, Shankar EM, Muthu S, Zandi S, Sigvardsson M, Hinkula J, et al. P38MAPK/STAT3 Pathway Signaling Regulates Expression of Inhibitory Molecules in T-cells Activated by HIV-1 Exposed Dendritic Cells. Molecular medicine. 2012 Jul 3; doi: 10.2119/molmed.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peretz Y, He Z, Shi Y, Yassine-Diab B, Goulet JP, Bordi R, et al. CD160 and PD-1 Co-Expression on HIV-Specific CD8 T Cells Defines a Subset with Advanced Dysfunction. PLoS Pathogens. 2012 Aug;8:e1002840. doi: 10.1371/journal.ppat.1002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shankar EM, Che KF, Messmer D, Lifson JD, Larsson M. Expression of a broad array of negative costimulatory molecules and Blimp-1 in T cells following priming by HIV-1 pulsed dendritic cells. Molecular medicine. 2011 Mar-Apr;17:229–40. doi: 10.2119/molmed.2010.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Porichis F, Kwon DS, Zupkosky J, Tighe DP, McMullen A, Brockman MA, et al. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood. 2011 Jul 28;118:965–74. doi: 10.1182/blood-2010-12-328070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamamoto T, Price DA, Casazza JP, Ferrari G, Nason M, Chattopadhyay PK, et al. Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood. 2011 May 5;117:4805–15. doi: 10.1182/blood-2010-11-317297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. The Journal of experimental medicine. 2010 Sep 27;207:2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature Immunology. 2009 Jan;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011 Jul;12:663–71. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lichterfeld M, Mou D, Cung TD, Williams KL, Waring MT, Huang J, et al. Telomerase activity of HIV-1-specific CD8+ T cells: constitutive up-regulation in controllers and selective increase by blockade of PD ligand 1 in progressors. Blood. 2008 Nov 1;112:3679–87. doi: 10.1182/blood-2008-01-135442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lichterfeld M, Kaufmann DE, Yu XG, Mui SK, Addo MM, Johnston MN, et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. The Journal of experimental medicine. 2004 Sep 20;200:701–12. doi: 10.1084/jem.20041270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lichterfeld M, Cung T, Seiss K, Rosenberg ES, Pereyra F, Yu XG. Shelterin dysfunction and p16(INK4a)-mediated growth inhibition in HIV-1-specific CD8 T cells. Journal of Virology. 2012 May;86:5533–40. doi: 10.1128/JVI.00196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annual review of genetics. 2008;42:301–34. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 71.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes & development. 2005 Sep 15;19:2100–10. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 72.Dimitrova N, de Lange T. MDC1 accelerates nonhomologous end-joining of dysfunctional telomeres. Genes & development. 2006 Dec 1;20:3238–43. doi: 10.1101/gad.1496606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998 Feb 6;92:401–13. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 74.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009 Mar 12;458:206–10. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004 Sep 10;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 76.Karwacz K, Bricogne C, MacDonald D, Arce F, Bennett CL, Collins M, et al. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO molecular medicine. 2011 Oct;3:581–92. doi: 10.1002/emmm.201100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vang T, Liu WH, Delacroix L, Wu S, Vasile S, Dahl R, et al. LYP inhibits T-cell activation when dissociated from CSK. Nature Chemical Biology. 2012 May;8:437–46. doi: 10.1038/nchembio.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riley JL. PD-1 signaling in primary T cells. Immunological Reviews. 2009 May;229:114–25. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001 Nov 20;98:13866–71. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992 Aug 21;70:585–93. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 81.Davis SJ, van der Merwe PA. Lck and the nature of the T cell receptor trigger. TRENDS in Immunology. 2011 Jan;32:1–5. doi: 10.1016/j.it.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Schoenborn JR, Tan YX, Zhang C, Shokat KM, Weiss A. Feedback circuits monitor and adjust basal Lck-dependent events in T cell receptor signaling. Sci Signal. 2011 Sep 13;4:ra59. doi: 10.1126/scisignal.2001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. Journal of Immunology. 2004 Jul 15;173:945–54. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 84.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001 Mar;2:261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 85.Wang SF, Fouquet S, Chapon M, Salmon H, Regnier F, Labroquere K, et al. Early T cell signalling is reversibly altered in PD-1+ T lymphocytes infiltrating human tumors. PLoS One. 2011;6:e17621. doi: 10.1371/journal.pone.0017621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brockdorff J, Williams S, Couture C, Mustelin T. Dephosphorylation of ZAP-70 and inhibition of T cell activation by activated SHP1. Eur J Immunol. 1999 Aug;29:2539–50. doi: 10.1002/(SICI)1521-4141(199908)29:08<2539::AID-IMMU2539>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 87.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012 Jun 4;209:1201–17. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kwon J, Qu CK, Maeng JS, Falahati R, Lee C, Williams MS. Receptor-stimulated oxidation of SHP-2 promotes T-cell adhesion through SLP-76-ADAP. EMBO J. 2005 Jul 6;24:2331–41. doi: 10.1038/sj.emboj.7600706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Molecular and Cellular Biology. 2005 Nov;25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henson SM, Macaulay R, Franzese O, Akbar AN. Reversal of functional defects in highly differentiated young and old CD8 T cells by PDL blockade. Immunology. 2012 Apr;135:355–63. doi: 10.1111/j.1365-2567.2011.03550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo H, Qiao G, Ying H, Li Z, Zhao Y, Liang Y, et al. E3 ubiquitin ligase Cbl-b regulates Pten via Nedd4 in T cells independently of its ubiquitin ligase activity. Cell reports. 2012 May 31;1:472–82. doi: 10.1016/j.celrep.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nature Medicine. 2010 Oct;16:1147–51. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nature Immunology. 2011 Jun;12:536–43. doi: 10.1038/ni.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011 Oct 28;35:583–95. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009 Aug 21;325:1001–5. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. 2012 Apr 15;188:3734–44. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duraiswamy J, Ibegbu CC, Masopust D, Miller JD, Araki K, Doho GH, et al. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. Journal of Immunology. 2011 Apr 1;186:4200–12. doi: 10.4049/jimmunol.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008 Jun;134:1927–37. 1937 e1–2. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network Analysis Reveals Centrally Connected Genes and Pathways Involved in CD8(+) T Cell Exhaustion versus Memory. Immunity. 2012 Nov 12; doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peretz Y, Cameron C, Sekaly RP. Dissecting the HIV-specific immune response: a systems biology approach. Curr Opin HIV AIDS. 2012 Jan;7:17–23. doi: 10.1097/COH.0b013e32834ddb0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khatri P, Sirota M, Butte AJ. Ten years of pathway analysis: current approaches and outstanding challenges. PLoS Comput Biol. 2012;8:e1002375. doi: 10.1371/journal.pcbi.1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huynh-Thu VA, Irrthum A, Wehenkel L, Geurts P. Inferring regulatory networks from expression data using tree-based methods. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Le Cao KA, Martin PG, Robert-Granie C, Besse P. Sparse canonical methods for biological data integration: application to a cross-platform study. BMC Bioinformatics. 2009;10:34. doi: 10.1186/1471-2105-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Le Cao KA, Gonzalez I, Dejean S. integrOmics: an R package to unravel relationships between two omics datasets. Bioinformatics. 2009 Nov 1;25:2855–6. doi: 10.1093/bioinformatics/btp515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. Journal of Virology. 2007 Apr;81:4215–25. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Golden-Mason L, Klarquist J, Wahed AS, Rosen HR. Cutting edge: programmed death-1 expression is increased on immunocytes in chronic hepatitis C virus and predicts failure of response to antiviral therapy: race-dependent differences. Journal of Immunology. 2008 Mar 15;180:3637–41. doi: 10.4049/jimmunol.180.6.3637. [DOI] [PubMed] [Google Scholar]
- 107.Boettler T, Panther E, Bengsch B, Nazarova N, Spangenberg HC, Blum HE, et al. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. Journal of Virology. 2006 Apr;80:3532–40. doi: 10.1128/JVI.80.7.3532-3540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. Journal of Experimental Medicine. 2006 Oct 2;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Titanji K, Velu V, Chennareddi L, Vijay-Kumar M, Gewirtz AT, Freeman GJ, et al. Acute depletion of activated memory B cells involves the PD-1 pathway in rapidly progressing SIV-infected macaques. The Journal of Clinical Investigation. 2010 Nov;120:3878–90. doi: 10.1172/JCI43271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dyavar Shetty R, Velu V, Titanji K, Bosinger SE, Freeman GJ, Silvestri G, et al. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. The Journal of Clinical Investigation. 2012 May 1;122:1712–6. doi: 10.1172/JCI60612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Janbazian L, Price DA, Canderan G, Filali-Mouhim A, Asher TE, Ambrozak DR, et al. Clonotype and repertoire changes drive the functional improvement of HIV-specific CD8 T cell populations under conditions of limited antigenic stimulation. J Immunol. 2012 Feb 1;188:1156–67. doi: 10.4049/jimmunol.1102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012 Mar 23;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012 Jun 28;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012 Jun 28;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pilon-Thomas S, Mackay A, Vohra N, Mule JJ. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. Journal of Immunology. 2010 Apr 1;184:3442–9. doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010 Dec 15;16:6019–28. doi: 10.1158/1078-0432.CCR-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]