Abstract
Insulin initiates diverse hepatic metabolic responses, including gluconeogenic suppression and induction of glycogen synthesis and lipogenesis1,2. The liver possesses a rich sinusoidal capillary network with increased hypoxia and decreased gluconeogenesis in the perivenous zone3. Here, diverse vascular endothelial growth factor (VEGF) inhibitors improved glucose tolerance in normal or diabetic db/db mice, potentiating hepatic insulin signaling, decreasing gluconeogenic gene expression, increasing glycogen storage and suppressing hepatic glucose production (HGP). VEGF inhibition induced hepatic hypoxia via sinusoidal vascular regression and sensitized liver insulin signaling through hypoxia inducible factor-2α (HIF-2α) stabilization. Notably, liver-specific constitutive activation of HIF-2α, but not HIF-1α, was sufficient to augment hepatic insulin signaling via direct and indirect induction of insulin receptor substrate 2 (IRS2), an essential insulin receptor adaptor protein4–6. Further, liver IRS2 was both necessary and sufficient to mediate HIF-2α and VEGF inhibition effects on glucose tolerance and hepatic insulin signaling. These results demonstrate an unsuspected intersection between HIF-2α–mediated hypoxic signaling and hepatic insulin action via IRS2 induction, which can be co-opted by VEGF inhibitors to modulate glucose metabolism. These studies also indicate distinct roles in hepatic metabolism for HIF-1α, which promotes glycolysis7–9, versus HIF-2α, which suppresses gluconeogenesis, and suggest novel treatment approaches for type 2 diabetes mellitus.
The liver regulates systemic energy reserves by controlling carbohydrate and lipid metabolism in response to dietary and systemic cues. Hepatic insulin stimulation recruits insulin receptor substrate (IRS) proteins to the insulin receptor, with activation of AKT, GSK3β and mTOR, coordinately suppressing hepatic gluconeogenesis and inducing glycogen synthesis and lipogenesis1,2. The liver perivenous zone experiences relative hypoxia accompanied by suppression of gluconeogenesis3. During normoxia, prolyl hydroxylase domain–containing enzymes (PHD1–3) and factor inhibiting HIF (FIH) hydroxylate members of the HIF transcription factor family (HIF1–3), resulting in von Hippel-Lindau (VHL)-dependent proteosomal degradation; hypoxic inhibition of this hydroxylation stabilizes HIFs and induces HIF transcriptional targets10.
The VEGF family contains VEGF-A-D and PlGF, each with distinct affinities for VEGF receptors 1–3 (VEGFR1–3) and neuropilins. VEGFR1/Flt1 is a high-affinity receptor for VEGF-A, -B and PlGF versus VEGFR2/Flk1, which is a low-affinity receptor for VEGF-A, -C and –D11,12. VEGF inhibitor treatment decreases fasting blood glucose levels and improves glucose tolerance in mice and humans through unclear mechanisms13,14, and specific VEGF-B inhibition improves glucose tolerance through enhanced peripheral glucose uptake15. Here, we utilized single intravenous injection of adenoviruses encoding the soluble extracellular ligand-binding domains of VEGFR1/Flt1 (Ad sFlt1) or VEGFR2/Flk1 fused to an antibody Fc fragment (Ad sFlk1) to achieve hepatic secretion of Flt1 or Flk1 ectodomains into the circulation; both ectodomains elicit potent and durable VEGF-A neutralization in vivo16,17. Ad sFlt1-mediated VEGF inhibition improved the response to i. p. glucose injection in both glucose tolerance tests (GTT) and insulin tolerance tests (ITT) in either C57Bl/6 (Fig. 1a) or diabetic db/db mice (Fig. 1b) compared to control treatment as confirmed by AUC analysis (Supplementary Fig. 1a–d). Similar results were obtained with Ad sFlk1 (Fig. 1b and Supplementary Fig. 1c,d). Recombinant aflibercept/VEGF Trap, encoding a VEGFR1/VEGFR2 ectodomain fusion that binds VEGF-A, -B and PlGF18,19, also improved glucose tolerance versus control treatment in C57Bl/6 or db/db mice (Fig. 1c, d and Supplementary Fig. 1e,f), as did both the anti-VEGF-A monoclonal antibody (mAb) B20.4.1.120, and the anti-VEGFR2 monoclonal antibody DC10121 (Supplementary Fig. 1g,h), neither of which interfere with VEGF-B signaling.
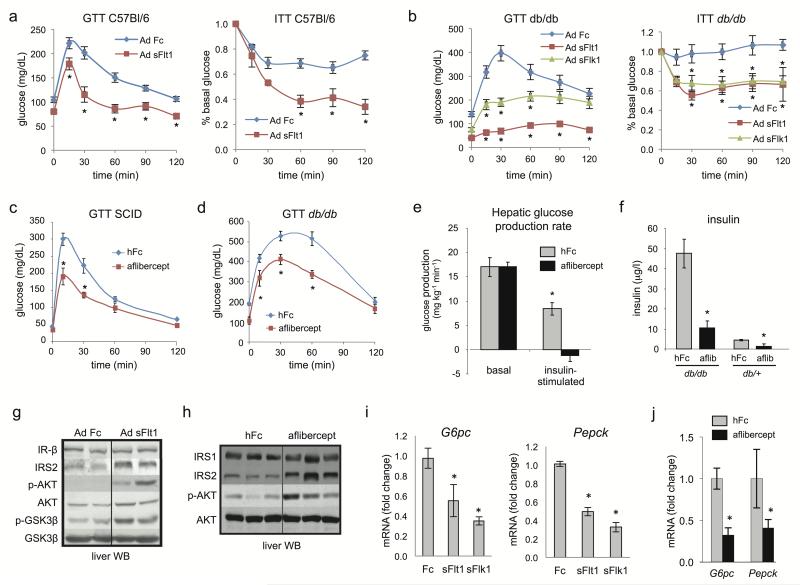
Figure 1. VEGF inhibition improves hepatic insulin action.
a–d. Glucose tolerance tests (GTT) and insulin tolerance tests (ITT) in adult C57Bl/6J (a) or db/db mice (b) treated with a single i.v. injection of Ad sFlt1/sVEGFR1, Ad sFlk1/sVEGFR2 or Ad Fc (n=8 each, 109 pfu) after 15 days. The initial average blood glucose levels for ITT in a. were Fc=129, sFlt1=72.8 and in b. were Fc=162, sFlt1=55.4, sFlk1=109, all mg/dL. c,d. GTT of adult SCID mice (n=5) or db/db mice (n=5) treated with aflibercept or hFc after 15 days. e. Hyperinsulinemic euglycemic clamp analysis of aflibercept- and hFc-treated db/db mice after 2 weeks. f. ELISA determination of fasting plasma insulin concentration from mice as in d. g,h. Insulin signaling pathway determination in fasted liver extracts after 14 days. i,j. Analysis of G6pc and Pepck mRNA by qRT-PCR from liver from Ad Fc, Ad sFlt1 and Ad sFlk1-injected mice (n=5, ad lib, day 14) (i) or adult SCID mice treated with aflibercept, (n=5, fasted, day 14) (j). Values are expressed as mean ± s.e.m. * = P<0.05.
VEGF inhibitors decreased fasting or fed glucose levels (Supplementary Fig. 2a–e) and aflibercept did not increase plasma insulin or decrease glucagon (Supplementary Fig. 2f,g). In a hyperinsulinemic euglycemic clamp study, two-week aflibercept treated db/db mice exhibited higher insulin sensitivity, enhanced insulin-induced suppression of hepatic glucose production (HGP) (Fig. 1e and Supplementary Fig. 3) and substantially improved hyperinsulinemia (Fig. 1f) compared to control hFc-treated db/db mice. This occurred without altering insulin-stimulated whole-body glucose disposal, peripheral tissue-specific glucose uptake, or hepatic CREB or AMPK signaling (Supplementary Fig. 4a,b).
The insulin-potentiating effects of VEGF inhibition on HGP prompted evaluation of insulin receptor (IR) signaling in liver. Both aflibercept and Ad sFlt1 treatment increased phosphorylation of AKT (p-AKT) and GSK3β (p-GSK3β), augmented expression of IRS2, but not IRS1 or IR itself (Fig. 1g, h), and suppressed phosphoenolpyruvate kinase (Pepck) and glucose-6-phosphatase catalytic subunit (G6pc) versus controls (Fig. 1i,j). Similar effects were also observed with Ad sFlk1 treatment (Fig. 1i and Supplementary Fig. 4c,d). Both sFlt1 and sFlk1 repressed liver G6PC and elevated glycogen in fasted liver compared to Fc-treated mice (Supplementary Fig. 4e). Together, this data indicated that VEGF inhibition suppresses HGP through hepatic insulin signaling sensitization.
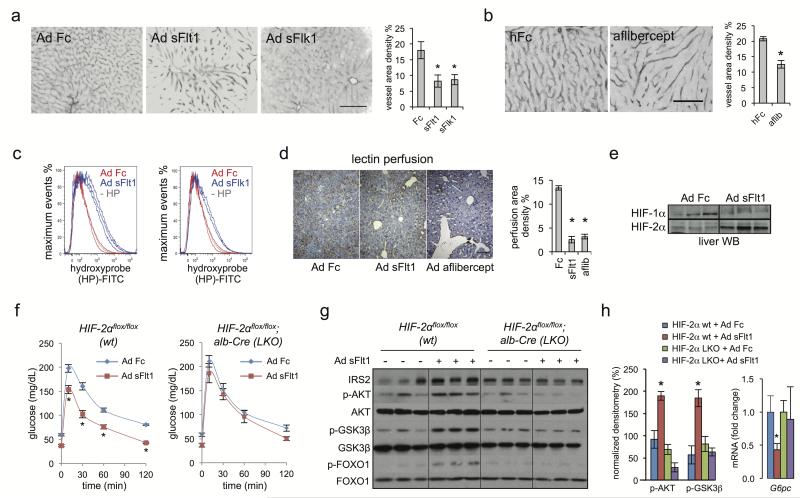
VEGF inhibition induces capillary regression in both normal adult organs and solid tumors, although effects in liver have not been described13,22,23. Ad sFlt1, Ad sFlk1 and recombinant aflibercept induced marked and reversible regression of CD31+ hepatic sinusoids compared to controls (Fig. 2a,b and Supplementary Fig. 5a,b), as did DC101 and B20.4.1.1 (Supplementary Fig. 5c,d). A sensitive FACS-based hypoxyprobe method detected a clear hypoxic shift in liver upon in vivo VEGF antagonism (Fig. 2c) versus Fc-treated animals. Ad sFlt1 and aflibercept also decreased functional perfusion in mouse liver upon intravascular biotin infusion (Fig. 2d). Further, microarray analysis of aflibercept-treated mouse liver revealed upregulation of several hypoxia-inducible genes, including Cited2, Loxl2, and Pfk1 (Supplementary Fig. 6a), while HIF-2α, but not HIF-1α protein was stabilized in Ad sFlt1-treated mouse liver (Fig. 2e). Notably, Ad sFlt1 improved glucose tolerance in unexcised Hif2αflox/flox mice, but this effect was significantly blunted upon liver-specific Hif-2α deletion (Hif-2α LKO) in Hif2αflox/flox;albumin-Cre mice9 (Fig. 2f and Supplementary Fig. 7a). Similarly, Ad sFlt1 induction of hepatic IRS2, p-AKT or p-GSK3β and G6pc suppression was blunted in Hif-2α LKO mice (Fig. 2g,h), indicating that VEGF inhibition requires liver Hif2α to sensitize liver insulin signaling. Additionally, adenoviral shRNA knockdown of liver Hif2α also reversed sFlt1 effects on glucose tolerance and insulin signaling (Supplementary Fig. 7b–e).
Figure 2. VEGF inhibition induces hepatic vascular regression, liver hypoxia and HIF-2α stabilization to augment hepatic insulin signaling.
a, b. Liver CD31 immunofluorescence and vessel area density percentage following Ad Fc, Ad sFlt1, Ad sFlk1, or aflibercept treatment after 14 days. Scale bar = 100 μm. c. FACS determination of hypoxia in mouse hepatocytes 14 days after Ad sFlt1-, Ad Flk1- (blue curves) or control Ad Fc- (red curves) treatment. Hypoxyprobe injection in mice was followed by anti-hydroxyprobe FITC-conjugated secondary antibody in disaggregated hepatocytes. The gray curve indicates a control liver without hypoxyprobe injection. d. Streptavidin-HRP staining of biotinylated L. esculentum lectin liver perfusion 22 days after Ad Fc, Ad sFlt1 and aflibercept treatment. Quantification of perfusion area density percentage is shown (n=3). e. HIF-2α and HIF-1α liver nuclear protein levels after 5 days Ad Fc and Ad sFlt1 treatment. f. GTT in 8–10 week old WT (Hif2αflox/flox) or HIF2LKO (Hif2αflox/flox; albumin-Cre) mice (n=6) 14 days after i.v. injection with Ad sFlt1 or Ad Fc. g. Liver Western blot from mice treated as in f. after 16 h fast. h. Densitometric quantitation of g. and G6pc liver qRT-PCR from f. Values are mean ± s.e.m. * = P<0.05.
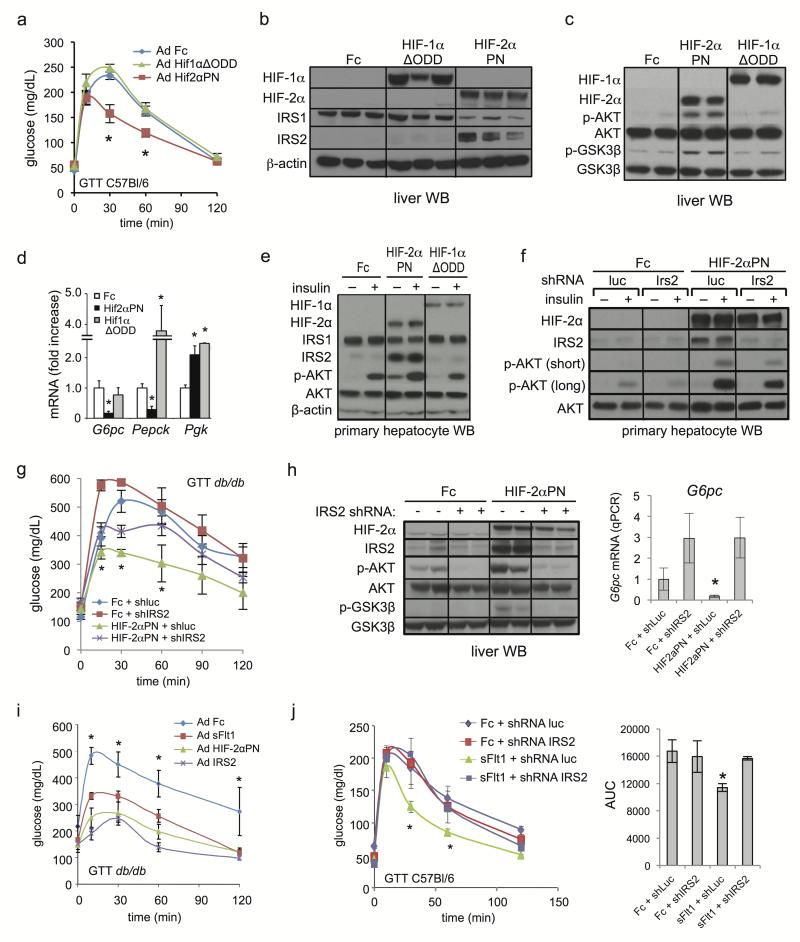
We next examined whether hepatic HIF-2α activation was sufficient to augment liver insulin receptor signaling. Adenoviral hepatic expression of a constitutively active allele of Hif2α mutated at inhibitory hydroxylation sites Pro531 and Asn847 (Ad Hif2αPN) or lacking the inhibitory oxygen-dependent degradation domain (ODD) (Ad Hif2αΔODD) significantly improved glucose tolerance, blood glucose and insulin tolerance under fed or fasted conditions compared to Ad Fc controls; a Hif1αΔODD mutant had comparatively less activity (Fig. 3a and Supplementary Fig. 8a–f). Similarly, Ad Hif2αPN or Ad Hif2αΔODD elevated hepatic IRS2 protein, p-AKT, p-GSK3β and p-FOXO1 under fasted or fed conditions while Hif1αΔODD was comparatively less active (Fig. 3b,c and Supplementary Fig. 8g). In parallel, HIF-2α constitutive activation repressed G6pc and Pepck more strongly than did HIF-1α (Fig. 3d and Supplementary Fig. 8h). In primary mouse hepatocytes, HIF-2α but not HIF-1α activation markedly elevated IRS2 levels and potently synergized with insulin to elevate AKT phosphorylation compared to control, indicating a cell-autonomous mechanism (Fig. 3e).
Figure 3. Activation of hepatic HIF-2α but not HIF-1α signaling is sufficient to improve liver insulin action.
a. GTT of C57Bl/6 mice 3d after single i.v. injection of Ad Fc, Ad Hif1αΔODD or Ad Hif2αPN. b, c. Liver extracts from animals in a. were analyzed by Western blot for IRS proteins (b) or for insulin signaling intermediates (c), 5d, ad lib. d. qPCR analysis of liver gluconeogenic gene expression, 5d, fasted. e. Western blot of hepatocytes 24 hours after Ad Fc, Ad Hif1αΔODD or Ad Hif2αPN infection, with or without 100 nM insulin stimulation. f. Western blot of primary mouse hepatocytes infected with Ad Fc or Ad Hif2αPN +/− Ad shRNA luc or Ad shRNA Irs2 for 24h, with and without 100 nM insulin stimulation. Short and long exposures for p-AKT are shown. g. GTT from db/db mice (n=8/group) 4 days after Ad Hif2αPN or Ad Fc treatment ± either Ad shRNA luciferase (shLuc) or Ad shRNA Irs2 (shIRS2). h. Western blot and qRT-PCR analysis of G6pc mRNA from liver of animals in g., ad lib. i. GTT from 8–10 week db/db mice treated with Ad Fc, Ad sFlt1, Ad Hif2αPN or Ad Irs2 after 5 days. j. GTT and AUC from C57Bl/6 mice at 5 days after injection with Ad Fc or Ad sFlt1 ± Ad shRNA luc or Ad shRNA Irs2 (n=6). Values are mean ± s.e.m. * = P<0.05.
The functional relevance of IRS2 to HIF-2α regulation of hepatic insulin signaling was explored both in vitro and in vivo. In primary hepatocytes, the synergistic activation of AKT phosphorylation by Ad Hif2αPN and insulin was attenuated by Irs2 knockdown (Ad shRNA IRS2) (Fig. 3f). Further, in db/db mice, the ability of Ad Hif2αPN to improve glucose tolerance, increase liver AKT phosphorylation and repress G6pc were attenuated by concomitant Irs2 knockdown (Fig. 3g,h and Supplementary Fig. 8i). Hepatic Irs2 overexpression via Ad IRS2 markedly improved glucose tolerance in db/db mice versus Ad Fc in agreement with previous results24 and phenocopied Ad Hif2αPN or VEGF inhibition (Fig. 3i and Supplementary Fig. 8j), consistent with liver IRS2 induction being sufficient to mediate Ad Hif2αPN or VEGF inhibition effects on glucose tolerance. Similarly, Ad sFlt1 improvement of glucose tolerance was also attenuated by liver Irs2 shRNA knockdown (Fig. 3j).
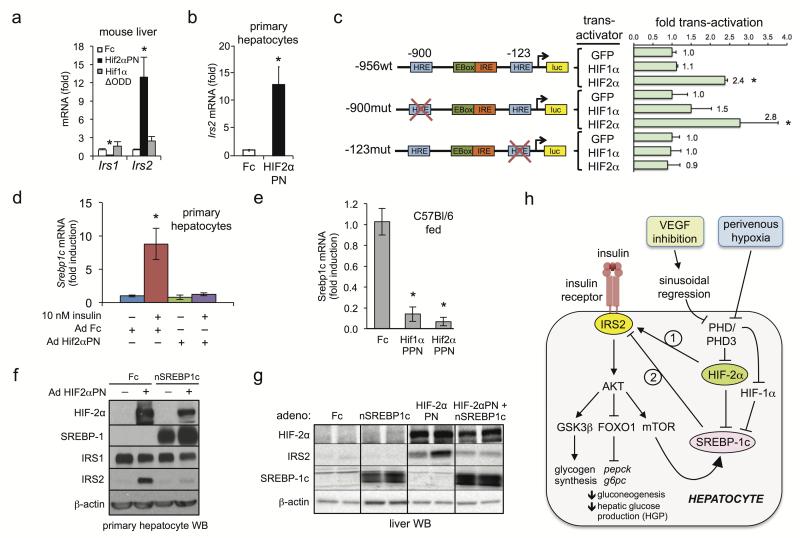
HIF-2α induced liver Irs2 mRNA both in vivo and in primary hepatocytes (Fig. 4a,b), suggesting direct transcriptional regulation. Canonical hypoxia-response elements (HREs) are present at −900 and −123 in the mouse and human IRS promoter regions (Fig. 4c). The proximal −123 but not the distal −900 HRE was required for HIF-2α transactivation of an IRS2-luciferase reporter construct in insulin-starved H2 hepatoma cells; notably, HIF-1α was ineffective (Fig. 4c) and both HIF-1α and HIF-2α potently activated 5xHRE luciferase constructs (data not shown). Additionally, microarray of aflibercept-treated mouse liver revealed down-regulation of numerous SREBP-1c target genes (Supplementary Fig. 6b). In addition to well-described lipogenic functions and induction by feeding or insulin1, SREBP-1c represses Irs2 in fed or fasted mice by competitively inhibiting FOXO binding to Irs2 promoter insulin responsive elements25. Further, liver Srepb1c and Irs2 expression are inversely correlated in multiple settings25,26, suggesting possible HIF-2α induction of IRS2 via Srebp1c repression. Constitutive HIF-2α activation potently abrogated both insulin-stimulated Srebp-1c expression in primary hepatocytes (Fig. 4d) and Srebp-1c expression in fed mice (Fig. 4e). However, HIF-1α activation also lowered Srebp-1c mRNA levels (Fig. 4e) without affecting glucose tolerance or inducing IRS2 (Fig. 3, Supplementary Fig. 8a–g), suggesting that Srebp1c repression was not itself sufficient for HIF-2α induction of IRS2. Forced adenoviral overexpression of a cleaved active form of SREBP-1c (Ad nSrebp1c)27 reversed Ad Hif2αPN stimulation of IRS2 both in insulin-starved primary hepatocytes (Fig. 4f) and in vivo under re-fed conditions (Fig. 4g). Thus, HIF-2α suppression of the Irs2 repressor Srebp1c, while not itself sufficient, facilitates HIF-2α–Irs2 direct trans-activation (Fig. 4h).
Figure 4. HIF-2α regulates IRS2 through Srebp1c-dependent and –independent mechanisms.
a. qRT-PCR analysis of Irs1 and Irs2 in C57lBl/6 mouse liver 5 days after Ad Hif2αPN or Hif1αΔODD treatment. b. qRT-PCR analysis of IRS2 in primary hepatocytes 24h post-infection with the indicated adenoviruses. c. Luciferase reporter assay in mouse H2 hepatocytes with human IRS2 promoter-luciferase constructs containing wild-type sequence (wt) or mutations of distal (−900mut) or proximal (−123mut) HREs with Ad GFP, Ad Hif2αΔODD or Ad Hif1αΔODD. d. Srebp1c qRT-PCR from primary hepatocytes infected with Ad Fc or Ad Hif2αPN ± insulin. e. Mouse liver Srebp1c qRT-PCR 5 days after Ad Fc, Ad Hif1αΔODD or Ad Hif2αPN treatment. f. Western blot of primary hepatocytes infected with either Ad Fc or Ad Hif2αPN ± Ad nSrebp-1c. g. IRS2 and nSREPB1c liver Western blot after Ad Fc or Ad Hif2αPN treatment ± Ad nSrebp1c. h. Model. HIF-2α signaling positively regulates the insulin receptor signaling pathway in hepatocytes through induction of IRS2 expression. HIF-2α, but not HIF-1α regulates IRS2 expression directly by promoter trans-activation; both HIF-2α and HIF-1α suppress Srebp1c which can facilitate HIF2α transactivation of IRS2. Physiologic liver hypoxia and VEGF inhibition via vascular regression represent two stimuli capable of activating the HIF2α→IRS2 pathway, possibly mediated by PHD3 (see accompanying manuscript by Taniguchi et al.). Values are expressed as mean ± s.e.m. * = P<0.05.
These studies reveal a previously unsuspected cross-talk between the liver hypoxia and insulin signaling pathways whereby HIF-2α strongly augments insulin-dependent AKT activation and gluconeogenic suppression (Fig. 4h). Here, complementary in vitro and in vivo studies demonstrate that HIF-2α positively regulates hepatic insulin signaling by up-regulating IRS2. This IRS2 induction is specific to HIF-2α and not HIF-1α, paralleling studies where the former and not the latter exerts dominant roles in regulation of liver lipogenesis32, of hepatic Epo9,28 and of other loci such as Oct4, cyclin D1 and DMT129–31. In knockdown and overexpression studies, liver IRS2 is both necessary and sufficient for HIF-2α-mediated improvement of glucose tolerance and hepatic insulin signaling. The improved glucose tolerance upon liver IRS2 overexpression agrees strongly with prior studies where increased hepatic IRS2 expression is fully sufficient to potently ameliorate diabetes in db/db mice and activate fasted liver insulin signaling24.
Although we have established a clear functional role for IRS2, we cannot fully exclude parallel IRS2-independent pathways. Possibilities for IRS2-independent HIF effects include insulin-independent, direct association of PGC-1α, HNF4 and/or C/EBPα with Pepck and G6pc promoter elements32,33. Alternatively, EPO has been reported to either improve peripheral glucose uptake34 or inhibit gluconeogenesis by modulation of inflammation35, although EPO does not induce liver IRS2 (S. P. and C. J. K., unpublished data). Our demonstration that the HIF-2α/ IRS2 axis couples hypoxia sensing to gluconeogenic repression via potentiation of hepatic insulin signaling complements observations of hypoxic regulation of IRS2 and invasion in breast cancer cells36. Notably, upon hepatic Vhl deletion stabilizing both HIF-2α and HIF-1α, a Srebp1c gene signature is induced by superimposed deletion of Hif2α but not Hif1α, suggesting the possibility of HIF-2α-specific Srebp1c repression32. HIF-2α induced IRS2 but decreased IRS1, suggestive of potential reciprocal regulation; however, liver IRS1 down-regulation typically impairs rather than improves glucose tolerance4,5.
The beneficial effects of HIF-2α on animal models of insulin resistance suggest the potential therapeutic utility of prolyl hydroxylase inhibitors that block VHL-dependent HIF degradation for type 2 diabetes mellitus therapy. This could occur via isoform-specific PHD3 pharmacologic inhibition (see accompanying paper by Taniguchi et al.) that could circumvent the hepatic lipid accumulation associated with conventional liver HIF-2α activation.32,37 A second strategy for pharmacologic manipulation of the hepatic HIF-2α/IRS2 axis is represented by VEGF inhibition, which decreases pancreatic islet microvessel density13 with specific VEGF-B antagonism enhancing peripheral glucose uptake15. However, we describe that VEGF inhibitors such as aflibercept, which binds VEGF-A, -B and PlGF18, can exert primary action on liver via insulin sensitization rather than on skeletal muscle. Such alternative mechanisms are further supported by the ability of non-VEGF-B VEGF inhibitors such as sFlk1, B20.4.1.1 and DC101 to modulate glucose tolerance and/or hepatic insulin signaling. Conceivably, VEGF-A and VEGF-B could coordinately regulate glucose metabolism via hepatic insulin signaling and peripheral glucose uptake, respectively15, with additional mechanisms certainly possible. Since HIF-2α and HIF-1α exhibit similar hypoxic stabilization profiles38, the specific VEGF inhibitor stabilization of HIF-2α versus HIF-1α could occur via selective PHD3 inhibition (see accompanying manuscript by Taniguchi et al.) or alternative non-hypoxic pathways17, and underlie observations of improved glucose control in diabetic VEGF inhibitor-treated patients14. It is further interesting to contemplate the potential contribution of decreased plasma glucose to anti-tumor effects of VEGF inhibition39.
Overall, our data indicate distinct roles in energy metabolism for hepatic HIF-2α, which sensitizes liver insulin signaling and suppresses gluconeogenesis, versus hepatic HIF-1α, which promotes glycolysis7–9. In vivo, physiologic perivenous liver hypoxia3 could trigger the HIF-2α/IRS2 axis, preferentially enhance insulin signaling and suppress perivenous Pepck and G6pc, explaining the enrichment of gluconeogenesis in the periportal zone3. Finally, the mechanistic interplay in liver between the hypoxia/HIF-2α and insulin receptor signaling pathways, as demonstrated here, has potentially broad implications for the study of metabolism and therapeutic approaches to type 2 diabetes mellitus.
Online Methods
Animals
All mice were males and on a 12-h light/dark cycle in a pathogen-free animal facility and unless otherwise specified were C57Bl/6J (JAX). The generation of Hif2α conditional alleles and albumin-Cre transgenic mice have been previously described40,41. Hepatocyte-specific deletion of Hif2α was achieved through generating mice that were homozygous for the Hif2α 2-lox alleles and expressed the albumin-Cre transgene. Littermates not carrying the albumin-Cre transgene were used as control animals. Mutant mice were generated in a mixed genetic background (BALB/c, 129Sv/J, and C57BL/6). 8–10 week-old Lepr db/db and Lepr db/+ mice were purchased from Jackson Laboratory. 8–10 week-old C57BL/6 mice were purchased from Taconic. Mice were injected with adenoviruses through the tail vein at between 1×108 to 5×108 plaque-forming units in 0.1 mL PBS. (Ad sFlt1, Ad sFlk1, Ad Fc 1×109 pfu; Ad HIF2αPN, Ad HIF2ΔODD, Ad HIF1ΔODD, Ad IRS2, Ad Fc 3×108 and 1.5×108; Ad shRNA IRS2, Ad shRNA luciferase 5×108 and 6×108) Mice received VEGF Trap protein (aflibercept) subcutaneously two times per week at 25 mg/kg. For control groups, mice were treated with humanized Fc (Regeneron). DC101 (a rat IgG1 monoclonal antibody targeting mouse VEGFR2)21 was produced by Antibody Solution from conditioned media of the cognate hybridoma ATCC HB-11534. SCID mice received thrice-weekly subcutaneous injections of DC101 or control rat IgG (Sigma) at 40 mg/kg for 2 weeks. The anti-VEGF-A monoclonal antibody (mAb) B20.4.1.120, or control anti-ragweed mAb (Genentech) were administered to C57Bl/6 mice at 5 mg/kg, 3x/week i. p. All animals studies were performed in accordance with the National Institutes of Health guidelines for use and care of live animals and were approved by the Stanford University Institutional Animal Care and Use Committee A3213-01.
Adenoviruses
The construction of Ad-sFlt1 (encoding N-terminal hemagglutinin-tagged murine soluble VEGFR1 containing the first three Ig domains), Ad Flk1-Fc (encoding a full-length murine soluble VEGFR2 ectodomain fused to mouse IgG2α Fc) and Ad Fc (encoding IgG2α Fc alone) have been previously described16,42. Human Hif2αPN was generated by site-directed mutagenesis replacing Pro531 and Asn847 by alanines. The human Hif1α allele lacking the inhibitory oxygen-dependent degradation domain (Hif1αΔODD) was a kind gift of Cecile Chartier; similar methods were used to generate human Hif2αΔODD. To generate adenoviruses expressing various shRNAs, an adenoviral shRNA shuttle vector was generated in which a CMV promoter drives a GFP/3' UTR miR-30 expression cassette in an Ad strain 5 backbone. miR-30-flanked shRNA sequences against mouse Hif2α, Irs2, Dec1 and Dec2 were obtained from Open Biosystems and cloned into the miR-30 expression cassette via XhoI/EcoRI restriction sites. Sequence information available upon request. The human nSrebp-1c adenovirus for in vitro primary hepatocyte infection was from Eton Bioscience, Inc. The human nSrebp-1c adenovirus used for in vivo mouse experiments has been described27. All adenoviral inserts were cloned into the E1 region of E1−E3− Ad strain 5 by homologous recombination. Adenoviruses were produced in 293 cells and purified by double CsCl gradient purification as described16.
Glucose Tolerance Test
Glucose tolerance tests were performed by intraperitoneal injection of glucose (2 g kg−1 for non-diabetic mice and 0.5 g kg−1 for diabetic db/db mice) after a 16 h overnight fast. Tail whole blood samples were taken before injection (time 0) and at 15, 30, 60, and 120 min post-injection.
Insulin Tolerance Test
Insulin tolerance tests were performed by intraperitoneal injection of insulin (Eli Lilly) (0.75 U/kg for non-diabetic mice and 1.5 U/kg for diabetic db/db mice) after a 5 hour fast. Whole blood samples were collected from the tail before insulin injection (time 0) and at 15, 30, 60, and 120 minutes post-injection. Glucose levels were normalized to fasted glucose levels at time 0, and AUC was calculated in GraphPad Prism using a baseline of y=1.
Hyperinsulinemic euglycemia clamp study
Studies were performed by the Mouse Metabolic Pathophysiology Core (MMPC) at Vanderbilt University as previously described43 Insulin clamps were performed on 5-h fasted mice. [3-3H]glucose was primed (0.8 μCi) and continuously infused for a 120-min equilibration period (0.04 μCi/min) and a 2-h clamp period (0.08 μCi/min). Baseline blood or plasma parameters were determined in blood samples collected at −10 and −5 min. At t = 0, insulin infusion (2.0 mU·kg−1·min−1) was started and continued for 155 min. Blood glucose was clamped using a variable rate of glucose infusion (GIR). Mice received heparinized saline-washed erythrocytes from donors at 4.5 μL/min to prevent a fall of hematocrit. Insulin clamps were validated by assessment of blood glucose over time. Blood glucose was monitored every 10 min, and the GIR was adjusted as needed. Blood was taken at 80–120 min for the determination of [3-3H]glucose. Clamp insulin was determined at t = 120 and 155 min. At 120 min, 13 μCi of 2[14C]deoxyglucose ([14C]2DG) was administered as an intravenous bolus. Blood was taken at 2–35 min for the determination of [14C]2DG. After the last sample, mice were anesthetized and tissues were collected. Plasma insulin was determined by the Vanderbilt Hormone Assay and Analytical Services Core. Radioactivity of [3-3H]glucose, [14C]2DG, and [14C]2DG-6-phosphate were determined by liquid scintillation counting. Glucose appearance (Ra) and disappearance (Rd) rates were determined using non-steady-state equations44. Endogenous glucose production (endoRa) was determined by subtracting the GIR from total Ra. The glucose metabolic index (Rg) was calculated as previously described45.
FACS-based hypoxia detection
C57BL/6 mice were injected intravenously with Fc, Flt1, and Flk1 adenoviruses as described above. At day 14, mice were injected intraperitoneal with pimonidazole hydrochloride 100 mg/kg (Hypoxyprobe, Inc., Burlington MA), and liver was harvested after 2 hours and disassociated into a single cell suspension using Collagenase IV (Sigma), permeabilized with TritonX, and incubated for one hour with FITC-conjugated anti-hypoxyprobe antibody (Hypoxyprobe, Inc., Burlington MA). Cells were analyzed on a BD LSR II Flow Cytometer (Becton Dickinson) using FlowJo software.
Quantitation of microvessel density
Vessel area density percentage of CD31 immunhistochemical and immunofluorescence sections (n≥5 fields) were analyzed by pixel intensity measurements in ImageJ.
RNA isolation and real-time PCR
Total RNA was isolated using Trizol (Invitrogen). RNA samples were reversed transcribed into cDNA using a reverse transcription kit (Invitrogen). Individual gene expression was quantified by SYBR green real-time PCR (Biorad) and normalized to β-actin. Primer sequences for real-time PCRs are available upon request.
ELISA
Serum insulin was measured by enzyme-linked immunosorbent assay (ALPCO). Serum glucagon was measured by mouse glucagon ELISA (ALPCO).
Protein Immunoblot
The antibodies used were β-actin and GAPDH (Abcam); IRS1 and IRS2 (Millipore); AKT, p-AKT, GSK3β, p-GSK3β, FOXO1, and p-FOXO1 (Cell Signaling); anti-HIF2α (Santa-Cruz); anti-HIF1α (Bethyl). The following antibodies were from Cell Signaling: p-ACC1, ACC1, AMPKα, p-AMPKα, AMPKβ, p-AMPKβ. Liver lysates were prepared in RIPA buffer. Liver nuclear extracts were prepared using previously described nuclear extraction protocols9. Quantifications were performed by chemoluminescence and densitometric scanning of the films under linear exposure conditions. Phosphorylated-AKT was normalized to total AKT, phosphorylated-GSK3β was normalized to total GSK3β and IRS2 was normalized to actin loading control.
Isolation and culture of primary mouse hepatocytes
Primary hepatocytes were isolated from 10–12 week-old C57Bl/6 mice using a collagenase perfusion method from UCSF Liver Center (SFGH, San Francisco, CA). Isolated hepatocytes were resuspended in William's E media and plated on collagen I-coated plates in serum-free media. For hypoxia chamber experiments, cells were incubated at the specific O2 concentration for 24 h followed by RNA extraction and gene expression analysis. For in vitro insulin stimulation assay, freshly harvested mouse primary hepatocytes were cultured in serum overnight. Adenoviruses were added to serum-free media 12 hours after plating for 24 hours at a multiplicity of infection (M.O.I) of 10 (Ad Fc, Ad HIF2αPN and Ad HIF1ΔODD) or MOI of 20 (Ad shRNA IRS2 and Ad shRNA luciferase). Following adenoviral infection, cells were stimulated with 100 nM bovine pancreas insulin (Sigma) for 10 minutes followed by protein extraction and western blot analysis. For in vitro Irs2 and Srebp-1c expression analysis, primary hepatocytes were infected with adenovirus 12 hours after plating for 24 hours at a M.O.I of 10 in serum-free media. After 24 hours, cells were stimulated with 10 nM insulin for 6 hours. RNA was extracted and qRT-PCR was performed as described above.
Lectin perfusion
Adult C57Bl/6 mice received single i.v. injection of Ad Fc, Ad sFlt1 or Ad VEGF Trap/aflibercept17 (encoding the aflibercept coding sequence) at 109 pfu each. Ad day 22, mice were anesthetized with Avertin and 100 μl of biotinylated L. esculentum lectin (1 mg/ml in 0.9% NaCl; Vector Laboratories) was injected by tail vein, allowed to circulate for 2 minutes and then the mouse was perfused with 1% PFA via the left ventricle. Paraffin sections of liver were developed with streptavidin-HRP and photographed at 20x magnification.
Transfections
Human IRS2 promoter-luciferase constructs containing wild-type sequence (wt) or mutations of the distal (−900mut) or proximal (−123mut) HREs were generated by site-directed mutagenesis and transfected into mouse H2 hepatocytes with internal Renilla luciferase control in the presence of Ad GFP, Ad Hif2αΔODD or Ad Hif1αΔODD.
Microarray analysis
For comparison of gene expression, 8–10 week-old female SCID C17 mice were injected with VEGF Trap (n = 5) or hFc (n = 4) at 25 mg/kg twice weekly for 9 weeks. RNA from liver was extracted as described above and 30 μg of total RNA was used to generate cDNA. Hybridization of sample cDNA to Mouse Exonic Evidence Based Oligonucleotide (MEEBO) arrays was performed by the Stanford Functional Genomics Facility (SFGF). Statistical analysis of gene expression was performed using Significance Analysis of Microarrays software (SAM) and heat-map representation was generated through TreeView.
Statistical analysis
All bars show mean ± s.e.m. Significance was calculated using a student's t-test or one-way ANOVA with Newman-Keuls post-test for comparison of groups greater than 2. * = P < 0.05.
Supplementary Material
Acknowledgements
We are grateful to Ron DePinho, Lauren Harshman, Betty Tam, Cecile Chartier, and members of the Kuo laboratory for insightful comments, to Chris Her, Joanna Kovalski, Karen Thabet and Dhruv Nandamundi for technical assistance and to Germaine Fuh and Genentech for B20.4.1.1 antibody. Fellowship support was from the Stanford MSTP (K. W. and L. M. M.), NIH/NIGMS GM-07365 Training Grant (K. W.), Stanford Cardiovascular Institute T32 Training Grant 1K12HL087746 (S. P.), Molecular and Cellular Immunobiology Program Training Grant 5T32AI07290 (L. M. M.), NIH ARRA Supplement 1R01HL074267 (L. M. M), RSNA Resident Research Grants 1018 and 1111 (C. M. T.), and Molecular Endocrinology Training Program Grant T32DK007563 (K. X. M.). This work was supported by P30 DK026743 (Cell Biology Core Facility, UCSF Liver Center) to J. J. M.; NIH RO1DK043748, P60 DK020593 and U24DK059637 to O. P. G.; NIH CA67166 and the Sydney Frank Foundation to A. J. G.; and a Stanford Developmental Cancer Research Award and NIH R01HL074267, R01NS064517 and R01CA158528 to C. J. K.
Footnotes
Author contributions K. W., S. P., L. M. M., C. T., S. W., C. W-M. C. and K. X. M. designed and performed experiments; D. K. and J. Y. provided technical assistance; L. M., E. L., J. J. M. and M. C. S. provided reagents, O. P. M., G. T. and A. J. G. and C. J. K. designed experiments, K. W., S. P., L. M. M., C. T., O. P. M. and C. J. K. wrote the manuscript.
Competing Financial Interests S. W., K. A., L. M. and G. T. are employees of Regeneron Pharmaceuticals, which manufactures aflibercept.
References
- 1.Leavens KF, Birnbaum MJ. Insulin signaling to hepatic lipid metabolism in health and disease. Crit Rev Biochem Mol Biol. 2011;46:200–215. doi: 10.3109/10409238.2011.562481. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 3.Jungermann K. Zonation of metabolism and gene expression in liver. Histochem Cell Biol. 1995;103:81–91. doi: 10.1007/BF01454004. [DOI] [PubMed] [Google Scholar]
- 4.Kubota N, et al. Dynamic functional relay between insulin receptor substrate 1 and 2 in hepatic insulin signaling during fasting and feeding. Cell Metab. 2008;8:49–64. doi: 10.1016/j.cmet.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Dong X, et al. Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. The Journal of clinical investigation. 2006;116:101–114. doi: 10.1172/JCI25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong XC, et al. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8:65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong L, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140:280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu CJ, et al. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol Cell Biol. 2006;26:3514–3526. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rankin EB, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaelin WG, Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 12.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 13.Kamba T, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 14.Billemont B, et al. Blood glucose levels in patients with metastatic renal cell carcinoma treated with sunitinib. British journal of cancer. 2008;99:1380–1382. doi: 10.1038/sj.bjc.6604709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagberg CE, et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490:426–430. doi: 10.1038/nature11464. [DOI] [PubMed] [Google Scholar]
- 16.Kuo CJ, et al. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci USA. 2001;98:4605–4610. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tam BY, et al. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nature medicine. 2006;12:793–800. doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulos N, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holash J, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang WC, et al. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem. 2006;281:951–961. doi: 10.1074/jbc.M508199200. [DOI] [PubMed] [Google Scholar]
- 21.Prewett M, et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- 22.Fan X, et al. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. Faseb J. 2008;22:3571–3580. doi: 10.1096/fj.08-111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mancuso MR, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canettieri G, et al. Dual role of the coactivator TORC2 in modulating hepatic glucose output and insulin signaling. Cell metabolism. 2005;2:331–338. doi: 10.1016/j.cmet.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Ide T, et al. SREBPs suppress IRS-2-mediated insulin signalling in the liver. Nature cell biology. 2004;6:351–357. doi: 10.1038/ncb1111. [DOI] [PubMed] [Google Scholar]
- 26.Shimomura I, et al. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. [PubMed] [Google Scholar]
- 27.Lecomte V, et al. A new role for sterol regulatory element binding protein 1 transcription factors in the regulation of muscle mass and muscle cell differentiation. Mol Cell Biol. 2010;30:1182–1198. doi: 10.1128/MCB.00690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeo EJ, Cho YS, Kim MS, Park JW. Contribution of HIF-1alpha or HIF-2alpha to erythropoietin expression: in vivo evidence based on chromatin immunoprecipitation. Ann Hematol. 2008;87:11–17. doi: 10.1007/s00277-007-0359-6. [DOI] [PubMed] [Google Scholar]
- 29.Covello KL, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mastrogiannaki M, et al. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119:1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9:152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rankin EB, et al. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol. 2009;29:4527–4538. doi: 10.1128/MCB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang XL, et al. Ablation of ARNT/HIF1beta in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metab. 2009;9:428–439. doi: 10.1016/j.cmet.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scully MS, et al. A novel EPO receptor agonist improves glucose tolerance via glucose uptake in skeletal muscle in a mouse model of diabetes. Exp Diabetes Res. 2011;2011:910159. doi: 10.1155/2011/910159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng R, Zhu D, Bi Y, Yang D, Wang Y. Erythropoietin inhibits gluconeogenesis and inflammation in the liver and improves glucose intolerance in high-fat diet-fed mice. PLoS One. 2013;8:e53557. doi: 10.1371/journal.pone.0053557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mardilovich K, Shaw LM. Hypoxia regulates insulin receptor substrate-2 expression to promote breast carcinoma cell survival and invasion. Cancer Res. 2009;69:8894–8901. doi: 10.1158/0008-5472.CAN-09-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qu A, et al. Hypoxia-inducible transcription factor 2alpha promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology. 2011;54:472–483. doi: 10.1002/hep.24400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bracken CP, et al. Cell-specific regulation of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha stabilization and transactivation in a graded oxygen environment. The Journal of biological chemistry. 2006;281:22575–22585. doi: 10.1074/jbc.M600288200. [DOI] [PubMed] [Google Scholar]
- 39.Ho VW, et al. A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res. 2011;71:4484–4493. doi: 10.1158/0008-5472.CAN-10-3973. [DOI] [PubMed] [Google Scholar]
- 40.Gruber M, et al. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci USA. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Postic C, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 42.Jacobi J, et al. Discordant effects of a soluble VEGF receptor on wound healing and angiogenesis. Gene Ther. 2004;11:302–309. doi: 10.1038/sj.gt.3302162. [DOI] [PubMed] [Google Scholar]
- 43.Ayala JE, et al. Hyperinsulinemic-euglycemic clamps in conscious, unrestrained mice. J Vis Exp. 2011 doi: 10.3791/3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956;187:15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- 45.Mulligan KX, Morris RT, Otero YF, Wasserman DH, McGuinness OP. Disassociation of muscle insulin signaling and insulin-stimulated glucose uptake during endotoxemia. PLoS One. 2012;7:e30160. doi: 10.1371/journal.pone.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.