Abstract
Understanding the contribution of cerebellar dysfunction to complex neurological diseases such as autism spectrum disorders (ASD) is an ongoing topic of investigation. In a recent paper, Tsai et al. (Nature 488(7413) 647–51, 2012) used a powerful combination of conditional mouse genetics, electrophysiology, behavioral tests and pharmacological manipulations to address the role of Tuberous sclerosis complex 1 (Tsc1) in Purkinje cells and cerebellar function. The authors make the staggering discovery that morphological and electrophysiological defects in Purkinje cells are linked to systems wide ASD-like behavioral deficits. In this journal club, I discuss the major findings of this paper and critically assess the implications of this seminal work.
Keywords: ASD, Tsc1, Purkinje cells, behavior, synapses
Introduction
Autism Spectrum Disorder (ASD) is arguably one of the most puzzling, yet intriguing neurodevelopmental disorders. The first description of this disease 60 years ago suggested a significant malfunction in cognition and behavior in affected children (1). For a period of time it was believed that parenting and environmental factors were responsible for the absence of social interactions, repetitive behavior, impaired language and an obsessive need for “sameness”. More detailed and careful analysis of postmortem and imaging studies instead implicated fundamental defects in brain pathology, in regions including the limbic system, hippocampus, amygdala, cerebellum, corpus callosum, basal ganglia and brainstem. Furthermore, it has become well accepted that ASD is a neurological genetic disorder with prevalence for mutations in genes associated with synapses, and is therefore often referred to as “the disease of synapses”. Numerous reports suggest that majority of ASD patients exhibit widespread motor impairments (2). Since the cerebellum is one of the primary centers for motor coordination, its potential involvement in ASD has long been proposed.
The cerebellum is composed of only ten major neuronal types and three glial populations (3). Purkinje cells, the only output of the cerebellar cortex, are the fundamental element around which the cerebellum circuit is organized, both in its structure and its function (3). They receive all the synaptic information that comes from outside of the cerebellum, process it, and relay the information to the cerebellar and vestibular nuclei in the brain stem (4). The cerebellar nuclei then connect the cerebellum to multiple regions in the brain and spinal cord.
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder caused by mutations in the two genes; TSC1, which is located on chromosome 9, and TSC2, which is found on chromosome 16. Upon dimerization, TSC1 and TSC2 together are able to negatively regulate mammalian target of Rapamycin (mTOR) signaling. TSC primarily affects the skin, brain, eyes, heart, lungs and kidneys. It results in development of non-cancerous tumors called tubers. The severity of TSC symptoms ranges from mild to severe, with a select population of TSC patients exhibiting cognitive deficits. The cognitive deficits range from developmental delay to mental retardation and autism. Surprisingly, the cerebellum has emerged as an important brain structure in TSC disorder, as the number of tubers present in the affected cerebellum is thought to be associated with severity of autistic behaviors (5–7). Curiously, in milder cases neuroimaging techniques are not able to detect clear-cut cerebellar derangements, but this does not exclude the possibility of cell-specific malfunctions due to structure and/or functional deficits that are beyond the resolution of current imaging techniques.
It is therefore of utmost significance to define and understand the role of the cerebellum and its cell types in children affected with TSC, and the connection to ASD. Towards that goal, a recent outstanding paper by Tsai et al. (8) combined complex mouse genetics, behavior and electrophysiology to uncover the importance of the cerebellum in TSC-associated ASD behaviors. They investigated the role of Tsc1 in controlling neuronal network function and the behavioral consequences of altering its function in the cerebellum. To that end, Tsai et al. (8) used a Purkinje cell specific Cre driver, L7 (L7-Cre), and a Tsc1 floxed allele (Tsc1flox) (9, 10) to selectively manipulate Tsc1 function in Purkinje cells. They demonstrated that both L7-Cre;Tsc1flox/+ (L7;Tsc1flox/+, heterozygous mutants) and L7-Cre;Tsc1flox/flox (L7;Tsc1flox/flox, homozygous mutants) mice had a decrease in Purkinje cell excitability and displayed ASD-like behaviors.
Loss of Tsc1 in Purkinje cells causes deleterious affects on morphology and circuitry
One of the most useful advents in mouse genetics was the development of the cre/lox recombination system. In this approach, when floxed alleles are combined with the right Cre drivers they can provide spatial and temporal control over gene function in an exclusive part of the body or even cell type. Tsai et al. (8) used the cre/lox system elegantly to address the role of Tsc1 in Purkinje cells and more broadly to uncover the role of the cerebellum in TSC. To target recombination exclusively in Purkinje cells, the authors used an L7-Cre transgenic line (10) that expresses Cre starting in the first postnatal week in a pattern that is mainly restricted to Purkinje cells, with only scattered and infrequent recombination elsewhere in the brain. To ensure that the L7-Cre allele results in efficient removal of Tsc1 function, the authors first analyzed expression of the downstream effector of mTOR signaling, phosphorylated S6 (pS6), using immunohistochemistry and found the expected increase in this protein’s expression in affected Purkinje cells of L7;Tsc1flox/+ (heterozygous) and L7;Tsc1flox/flox (homozygous mutant) animals in comparison to controls. Previous studies in dissociated hippocampal cells suggested that loss of Tsc1 leads to an increase in soma size (11). In accordance, Tsai et al. (2012) found an increase in the size of Purkinje cell soma in the L7;Tsc1flox/flox mutants, but not in the L7;Tsc1flox/+ heterozygous mice.
One of the most consistent and apparent abnormalities reported in the vast majority of ASD cases are significant deficits in the number of the Purkinje cells (12, 13). This anomaly remains one of the most reliable and reproducible observations in ASD autopsied brains. Because the Purkinje cells are a central and integral part of the cerebellar cytoarchitecture and network, it is essential that their numbers are developmentally controlled and maintained throughout life. L7;Tsc1flox/flox mutants exhibited loss of Purkinje cells starting at 2 months of age, a phenotype that became progressively worse by 4 months of age. In contrast, the authors reported similar Purkinje cell numbers between L7;Tsc1flox/+ heterozygous and control animals at 4 months of age. The authors confirmed that the loss of the Purkinje cells in L7;Tsc1flox/flox mutants was due to their gradual cell death as visualized by TUNEL and caspase 3 immunostaining. One of the possible causes of cell death is oxidative stress. Exposure to maternal infection and environmental toxins such as heavy metals, mercury, and pesticides are known sources of oxidative stress and additionally have been implicated in ASD (14). All these afflictions on the cell cause oxidative stress, which in turn results in damage to proteins, DNA and lipids. Interestingly, Tsai et al. (8) found increased levels of markers for the endoplasmic reticulum and for oxidative stress (GRP78 and HO1) in L7;Tsc1flox/flox mutant animals.
Since dendritic spines represent major sites of excitatory synaptic contacts, their morphology and density have been used as an indicator of assessing the quantity, quality and organization of connections in the brain. Increased spine densities in cortical pyramidal neurons have been reported in various animal models of ASD (15). Tsai et al. (8) reported that both L7;Tsc1flox/flox homozygous and L7;Tsc1flox/+ heterozygous mutants showed an increase in spine density on the Purkinje cell dendrites, suggesting an additional role of Tsc1 in Purkinje cells. Interestingly, Tsc loss in cortical and hippocampal neurons results in a decrease in the density of spines (11, 16, 17). The opposite effects on the two different brain regions could suggest an additional level of complexity of TSC1 and TSC2 signaling within distinct brain regions. Future studies should be dedicated to investigating and understanding the molecular mechanisms that govern regional differences in Tsc function.
ASD has been defined as a “disease of the synapse” and previous studies suggested synaptic deficits associated with loss of Tsc1 (11). The microcircuitry of the cerebellum is well-understood, and the Purkinje cells play a central role in processing incoming signals (3). The two main afferent systems conducting excitatory inputs into the cerebellum are the climbing and mossy fibers. Climbing fibers originate from the inferior olivary nucleus and each one establishes multiple synaptic contacts with the proximal dendrites of a single Purkinje cell. Because Purkinje cells in both L7;Tsc1flox/flox homozygous and L7;Tsc1flox/+ heterozygous mutant animals exhibit morphological defects, the authors investigated whether there was a defect in synaptic inputs. Interestingly, Tsai et al. (8) found no difference in the amplitude of individual climbing fiber inputs between L7;Tsc1flox/flox homozygous mutant and control animals. Furthermore, the authors found no differences in synaptic plasticity or amplitude ratios of excitatory postsynaptic currents (EPSCs) and inhibitory postsynaptic currents (IPSCs) between L7;Tsc1flox/flox homozygous mutant and control animals. However, the intrinsic excitability of Purkinje cells in L7;Tsc1flox/+ heterozygous and L7;Tsc1flox/flox homozygous mutant mice was significantly lower, and fewer evoked potentials were induced by current injection. Altogether, these data suggest that while the Purkinje cells receive normal synaptic input, their spontaneous and current- evoked output is deficient. It will be interesting to further investigate how deficient output of Purkinje cells affects the cerebellar nuclei and their connections outside the cerebellum.
Purkinje cell specific Tsc1 conditional mutants exhibit ASD-like behavior
ASD diagnosis is based entirely upon behavioral criteria, as no consistent diagnostic molecular markers are available. While the symptoms of the disorder may vary, it is generally accepted that an individual will be diagnosed as ASD if he/she lacks social reciprocity, exhibits deficits in nonverbal and verbal communication, language delays, repetitive and/or compulsive behaviors, insistence on sameness and upset to change.
The similarities between mice and humans in their brain anatomy and genetics, and in their molecular signaling and response to pharmacological treatments strongly support the further development and use of mouse models as a tool for understanding the mechanisms underlying ASD behaviors. Mice are highly social creatures that engage in reciprocal social interactions, as well as variety of sexual, aggressive and parenting behaviors. Tsai et al. (8) tested Tsc1 cerebellum specific mutants (both heterozygous and homozygous animals) in an impressive battery of tests targeted to assess ASD-like behaviors.
The three chamber task
The three chamber social behavioral task established by the Crawley laboratory (18) provides a powerful automated approach for testing social behaviors in mice. In the social approach aspect of the test, a subject mouse is scored for the time spent in a side chamber with a novel mouse, which is contained within a wire cup, versus time spent in a side chamber with a non-social novel object (identical wire cup). The longer time spent in a chamber with a novel mouse as opposed to non-animate object is termed “sociability”. The second part of the three chamber social behavioral task tests for “social novelty”. A novel stranger mouse is placed in one side of the chamber, while the familiar mouse is in the opposite chamber. The time spent in the chamber with the familiar mouse versus the novel stranger mouse is recorded. The authors examined 7–9 weeks old control, L7;Tsc1flox/+ heterozygous and L7;Tsc1flox/flox homozygous mutant mice and found no significant differences in the time spent between the novel mouse versus the novel object in both L7;Tsc1flox/+ heterozygous and L7;Tsc1flox/flox homozygous mutant mice in comparison to controls which spent significantly more time interacting with the novel mouse. Additionally, both L7;Tsc1flox/+ heterozygous and L7;Tsc1flox/flox homozygous mutant mice exhibited no preference for the social novelty, whereas control animals spent significantly more time interacting with the novel stranger mouse. The lack of social interactions was not due to the motor deficits in the heterozygous and homozygous mutant mice as the initial signs of ataxia were not observed until 7–8 weeks of age in L7;Tsc1flox/flox homozygous mutant mice. Purkinje cell phenotype of 2 month old L7;Tsc1flox/+ heterozygous mutant mice suggests that deficits in social interactions are not caused by the Purkinje cell loss but rather their increased spine density and reduced excitability. Next, the authors tested whether the mutant mice exhibited defects in recognizing olfaction cues, as it is crucial for social interactions. Interestingly, both 8–12 weeks old L7;Tsc1flox/+ heterozygous and L7;Tsc1flox/flox homozygous mutant mice displayed no defects in recognizing olfaction cues that are non-social in nature (water, almond, banana), but had significant defects in social cues, which likely contributed to social defects.
Repetitive behavior
To model the repetitive behaviors that are common to ASD, Tsai et al. (8) exploited a reversal learning paradigm using the water T maze and found significantly impaired reversal learning in the 8–12 weeks old L7;Tsc1flox/flox homozygous mutant mice when compared to controls. Repetitive grooming is another type of repetitive behavior. Interestingly, both 8–12 weeks old L7;Tsc1flox/+ heterozygous and L7;Tsc1flox/flox homozygous mutant mice exhibited excessive repetitive grooming behavior in comparison to control animals.
Vocalization
Testing communication in mice can present a challenge, but complex vocalizations between separated pups and the dam have been shown to be a useful measure of social communication in rodents (18). The use of sensitive ultrasonic microphones and advanced software has revealed multiple and distinct calls in mice. Tsai et al. (8) recorded higher rates of ultrasonic vocalizations in both L7;Tsc1flox/+ heterozygous and L7;Tsc1flox/flox homozygous mutant pups in comparison to control pups at postnatal day 7 and 10, developmental time prior to the reported Purkinje cell death. It will be interesting, and perhaps even necessary, to resolve how cerebellar function and olfaction intersect during social behaviors. Moreover, future experiments could exploit L7;Tsc1flox/+ heterozygous and L7;Tsc1flox/flox mutant animals in testing the myriad of pharmaceutical drugs used for ameliorating and treating different aspects of abnormal behavior.
All together behavioral and histological results suggest that the changes, caused by the loss of Tsc1, in the Purkinje cell morphology and function, and not cell death, are sufficient to result in abnormal behavior, including social interactions, learning and memory. Pharmaceutical drug screens that exclusively target alteration in dendritic spine density or enhancing functional output would be of major clinical importance to our understanding of ASD and the future design of medications for the disorder. While it is clear from both L7;Tsc1flox/+ and L7;Tsc1flox/flox mutant animals that the Purkinje cells participate in both sociability and social memory, it is not clear whether the same neuronal networks govern these distinct social behaviors. In this regard, it will be interesting to investigate the relationship between the hippocampus, amygdala and cerebellum in controlling social memory, and screen for drugs that might enhance memory by targeting one or more of these structures.
Previous studies in both human and murine models showed that inhibition of mTOR signaling in TSC patients or Tsc1 mutant mice resulted in improvement of anatomical and behavioral phenotypes (19). Treatment with Rapamycin or RAD001 has been shown to improve survival and weight gain, to reduce seizures, and to improve some cognitive function and anatomic abnormalities (16, 20). Tsai et al. (8) investigated whether treatment with mTOR inhibitors could ameliorate the deficits observed in the Purkinje cell specific Tsc1 mutant mice. The authors treated control and mutant mice with Rapamycin starting at postnatal day 7 to coincide with the onset of Cre expression (8). Consistently with previous reports, Rapamycin treatment in L7;Tsc1flox/flox mutant animals improved anatomical integrity, specifically preventing Purkinje cell loss and decreasing soma size. Furthermore, when the L7;Tsc1flox.flox mutant mice were treated with the mTOR inhibitor Rapamycin, their social behavior was improved. Additionally, Rapamycin-treated L7;Tsc1flox/flox mutant mice displayed no differences in acquisition and reversal learning in the water T-maze in comparison to control animals.
However, it remains unclear whether the electrophysiological deficits in L7;Tsc1flox/flox mutant animals can be rescued by Rapamycin treatment. Since the behavioral aspects of the mutant mice are rescued upon drug treatment, it will be important to examine what effect Rapamycin has on the overall neuronal network structure and connectivity, and elucidate the mechanisms that mediate any potential recovery of function. Moreover, one wonders how Purkinje cell function is affected in awake mice. It would be interesting to examine Purkinje cell complex and simple spike behavior using in vivo recordings in alert L7;Tsc1flox/flox mutant mice. Finally, it is important to mention that a recent study examined the role of Tsc2 in the Purkinje cells and similar to Tsai et al. (8), revealed ASD-like deficits in Tsc2 conditional knock out mice (21). Reith et al. (21) found that loss of Tsc2 causes the Purkinje cell degeneration, as well as abnormal repetitive and social behavior, which could be rescued by Rapamycin treatment.
Summary
The role of the cerebellum in ASD remains a controversial topic, mainly because it is unclear how it might contribute to abnormalities in social interactions and repetitive behaviors. The advances in mouse genetics, which allow for fine-tuned genetic temporal and spatial manipulations, have provided the necessary tools with which to unravel the molecular and cellular mechanisms underlying complex neurological disorders. Using such techniques, Tsai et al. (8) established an intimate link between abnormal cerebellar Purkinje cell function and behavioral deficits that are relevant to ASD. Furthermore, they shed light on the requirement of the TSC-mTOR pathway in regulating cerebellar physiology and remarkably, its potential as a therapeutic target in rescuing complex phenotypes with drugs such as Rapamycin. This study not only implicates the cerebellum as a key player of ASD-like behavior, but specifically demonstrates that abnormal cerebellar Purkinje cells function can be a major contributor to these behaviors. Purkinje cell axons represent the sole output of the cerebellar cortex and all synapse on cerebellar or vestibular nuclei. Cerebellar nuclei in turn connect broadly to various regions of the brain including association and paralimbic cortex in addition to motor cortex (22). In the future, the study design used by Tsai et al. (2012) could be adapted to other sophisticated approaches such as diffusion tensor imaging, which could reveal potential defects in cerebral cortical and subcortical structures that communicate with cerebellum. It will be important to identify other cell types and circuits that contribute to the development of complex neurological and psychiatric disorders. Moreover, it is possible that other ASD-linked disease loci cause similar or complementary defects in the brains of ASD patients; using the conceptual technical repertoire implemented by Tsai et al. (2012), examination of other mouse ASD models may unveil core defects shared by multiple disorders on the spectrum. Such knowledge will allow for more potent and direct pharmaceutical drug development, with less side effects and off-target complications.
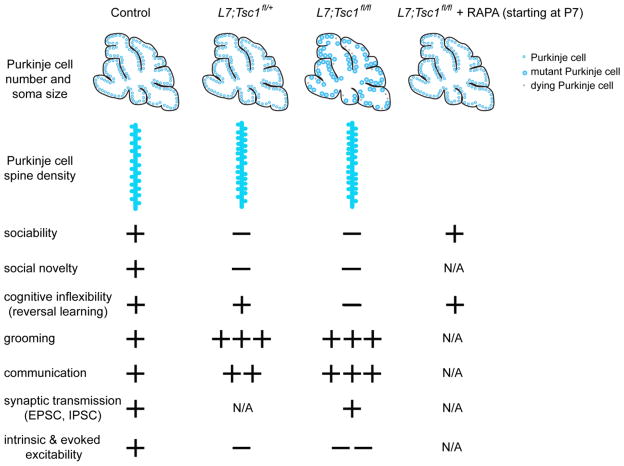
Fig. 1.
Schematic overview of the cerebellar and behavioral phenotypes in control, L7;Tsc1fl/+, L7;Tsc1flox/flox and L7;Tsc1flox/flox treated with rapamycin. In control, adult Purkinje cells are organized in a tightly packed monolayer. Heterozygous loss of Tsc1 results in increased Purkinje cell spine density, defects in excitability, abnormal social and repetitive behavior, and altered communication in the pups. Complete loss of Tsc1 causes an increase in the size of Purkinje cell soma and cell death (gray cells). These morphological defects are associated with reduced Purkinje cell excitability, social deficits, impaired cognitive behavior and abnormal vocalizations. However, when L7;Tsc1flox/flox mutants are treated with rapamycin (RAPA) starting at P7, the Purkinje cell deficits are rescued (soma size and cell number), as well as social and cognitive behavior.
Footnotes
The author declares that there is no conflict of interest in the work presented in this manuscript.
References
- 1.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–50. [PubMed] [Google Scholar]
- 2.Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. J Autism Dev Disord. 2010 Oct;40(10):1227–40. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Bayer SA. Development of the Cerebellar System: In Relation to Its Evolution, Structure, and Functions. New York: CRC Press; 1996. [Google Scholar]
- 4.Sillitoe RVFY, Watson C. Cerebellum. In: Watson CPG, Puelles L, editors. The Mouse Nervous System. Elsevier Inc; 2012. pp. 360–97. [Google Scholar]
- 5.Eluvathingal TJ, Behen ME, Chugani HT, Janisse J, Bernardi B, Chakraborty P, et al. Cerebellar lesions in tuberous sclerosis complex: neurobehavioral and neuroimaging correlates. J Child Neurol. 2006 Oct;21(10):846–51. doi: 10.1177/08830738060210100301. [DOI] [PubMed] [Google Scholar]
- 6.Ertan G, Arulrajah S, Tekes A, Jordan L, Huisman TA. Cerebellar abnormality in children and young adults with tuberous sclerosis complex: MR and diffusion weighted imaging findings. J Neuroradiol. 2010 Oct;37(4):231–8. doi: 10.1016/j.neurad.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Weber AM, Egelhoff JC, McKellop JM, Franz DN. Autism and the cerebellum: evidence from tuberous sclerosis. J Autism Dev Disord. 2000 Dec;30(6):511–7. doi: 10.1023/a:1005679108529. [DOI] [PubMed] [Google Scholar]
- 8.Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012 Aug 30;488(7413):647–51. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, et al. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002 Mar 1;11(5):525–34. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 10.Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000 Nov-Dec;28(3–4):93–8. [PubMed] [Google Scholar]
- 11.Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci. 2005 Dec;8(12):1727–34. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- 12.Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005 Apr-May;23(2–3):183–7. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, et al. Consensus paper: pathological role of the cerebellum in autism. Cerebellum. 2012 Sep;11(3):777–807. doi: 10.1007/s12311-012-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sajdel-Sulkowska EM, Xu M, Koibuchi N. Increase in cerebellar neurotrophin-3 and oxidative stress markers in autism. Cerebellum. 2009 Sep;8(3):366–72. doi: 10.1007/s12311-009-0105-9. [DOI] [PubMed] [Google Scholar]
- 15.Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010 Jan 14;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- 16.Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, et al. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008 May 21;28(21):5422–32. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, et al. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007 May 23;27(21):5546–58. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010 Jul;11(7):490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai P, Sahin M. Mechanisms of neurocognitive dysfunction and therapeutic considerations in tuberous sclerosis complex. Curr Opin Neurol. 2011 Apr;24(2):106–13. doi: 10.1097/WCO.0b013e32834451c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, et al. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008 Aug;14(8):843–8. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reith RM, McKenna J, Wu H, Hashmi SS, Cho SH, Dash PK, et al. Loss of Tsc2 in Purkinje cells is associated with autistic-like behavior in a mouse model of tuberous sclerosis complex. Neurobiol Dis. 2012 Nov 1; doi: 10.1016/j.nbd.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Musolino PL, Stoodley CJ, Schmahmann JD. Essential anatomy of the cerebellum and related structures. In: Boltshauser EaSJ., editor. Cerebellar Disorders in Children. London: McKeith Press; 2012. p. 456. [Google Scholar]