Abstract
In eukaryotes, cellular levels of adenosine monophosphate (AMP) signal the metabolic state of the cell. AMP concentrations increase significantly upon metabolic stress, such as glucose deprivation in yeast. Here we show that several DEAD-box RNA helicases are sensitive to AMP, which is not produced during ATP hydrolysis by these enzymes. We find that AMP potently inhibits RNA binding and unwinding by the yeast DEAD-box helicases Ded1p, Mss116p, and eIF4A. However, the yeast DEAD-box helicases Sub2p and Dbp5p are not inhibited by AMP. Our observations identify a subset of DEAD-box helicases as enzymes with the capacity to directly link changes in AMP concentrations to RNA metabolism.
In eukaryotes, cellular AMP levels correlate with the metabolic state of the cell 1,2,3,4.Metabolic stress, such as glucose deprivation in yeast 5,6, or exertion of muscle cells in metazoans lead to a temporary increase in AMP levels 7,8. It is well known that AMP modulates activities of several metabolic enzymes, including glycogen phosphorylase and phosphofructokinase 9,10. However, only a single eukaryotic enzyme, AMP-activated protein kinase, is known to directly affect gene expression in response to changes in AMP concentrations 11. No enzymes have been identified that can directly link gene expression at the RNA stage to changes in AMP concentrations.
Here we show that several DEAD-box RNA helicases are sensitive to AMP. Enzymes from the large and highly conserved DEAD-box helicase family are involved in virtually all aspects of eukaryotic RNA metabolism, from pre-mRNA splicing to RNA decay 12. DEAD-box RNA helicases bind and remodel RNA and RNA-protein complexes in an ATP-dependent fashion12. ATP drives and modulates RNA binding and duplex unwinding, while being hydrolyzed to ADP and inorganic phosphate 12. The reaction product ADP also binds to DEAD-box helicases and influences ATP-dependent RNA binding and remodeling activities 13.
Recent crystal structures of several DEAD-box helicases showed AMP in their ATP binding site 14,15,16. AMP is not a product of ATP hydrolysis by DEAD-box or other helicases 14, and AMP accommodation is thus not expected as a result of ATP turnover. Since DEAD-box helicases are involved in most cellular RNA transactions, AMP binding by these enzymes might therefore impact RNA metabolism at many points. For such effects, AMP would need to influence the interaction of the enzymes with RNA, or otherwise alter the biochemical behavior of the helicases. Whether AMP impacts enzymatic or other biochemical features of DEAD-box helicases is not known. Here, we have examined the effects of AMP on the biochemical activity of several yeast DEAD-box helicases.
AMP inhibits RNA binding by the DEAD-box helicase Ded1p
We first determined AMP affinity for the yeast protein Ded1p. This enzyme is involved in translation initiation and ribosome biogenesis 17,18. For comparison, we also measured ADP binding to Ded1p. Both, ADP and AMP competitively inhibited ATP binding by Ded1p (Supplementary Fig.S1), consistent with crystal structures of DEAD-box helicases showing accommodation of ADP and AMP in the ATP binding site 14,15,16.
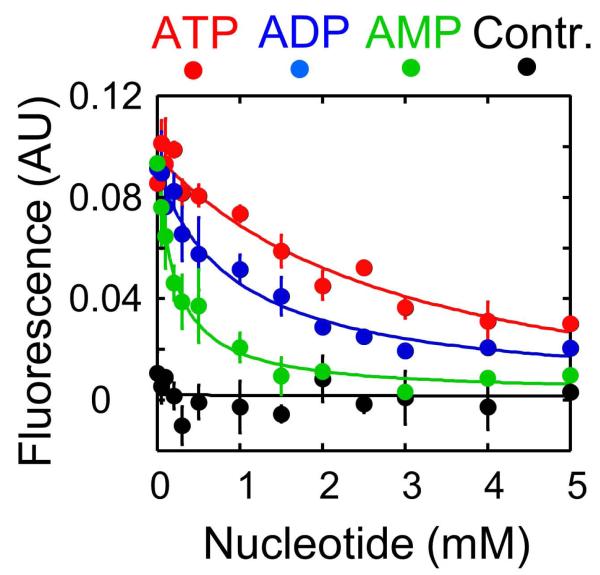
Nucleotide affinities to Ded1p without RNA were measured by competition between fluorescent mantADP and ATP, ADP, or AMP, respectively (Fig. 1, Supplementary Fig. 2). AMP bound to Ded1p with an affinity higher than that for both ADP and ATP (Fig. 1, Supplementary Table 1).These observations show that under the reaction conditions, AMP binds to Ded1p with an affinity within the range of physiological AMP concentrations, which vary in yeast from [AMP]0 ~ 20 - 200 μM in the absence of metabolic stress to [AMP]MS >1,000 μM under metabolic stress 5,6.
Figure 1. Nucleotide to Ded1p.
Binding of ATP (red), ADP (blue), and AMP (green) to Ded1p in the absence of RNA.Black circles show a control reaction (ADP titration) without Ded1p. Data points represent averages from at least four independent measurements, error bars mark one standard deviation. Solid lines show the best fit to the binding isotherm. Obtained data are compiled in Supplementary Table S1. Nucleotide binding was monitored by competition with mantADP (Supplementary Fig.S2). Steady-state fluorescence was measured in a buffer containing 40 mM Tris·HCl (pH 8.0), 50 mM NaCl, 0.5 mM MgCl2, 2 mM DTT, 0.01% (vol/vol) IGEPAL, and 1 μM Ded1p at 19°C,using a FluoroMax-3 spectrofluorimeter (JobinYvon, Spex Instruments S.A., Inc.) and a 3×3×35 mm quartz cuvette with 150 μl of reaction buffer. Purified Ded1p was expressed and purified as previously described 33. Tryptophan residues were excited at 295 nm (2 nm bandwidth) to minimize inner filter effects of the nucleotide in solution. Emission was monitored from 305-500 nm (2 nm bandwidth). MantADP (mADP) was excited at 360 nm (1 nm bandwidth) and emission was monitored from 400-500 nm (1nm bandwidth). All reactions contained equimolar concentrations of nucleotide and Mg2+ and 0.5 mM Mg2+ excess over the nucleotide. Affinities of unlabeled ATP, ADP and AMP were measured by competitive binding with 50 μM mADP.
We next examined the impact of AMP, ADP, and ATP on RNA binding, using a 10 nt single stranded RNA, which represents a minimal binding site for Ded1p. We determined the apparent equilibrium constants for a basic thermodynamic framework of nucleotide and RNA binding by Ded1p (Fig.2a). This approach was necessary because the low affinity of Ded1p for the 10 nt RNA (K1/2 = 36.9 ± 2.4 μM, Fig. 2a) precluded reliable measurements with RNA concentrations approaching saturation of Ded1p. To establish the framework, we measured the RNA-stimulated ATPase activity of Ded1p as a function of RNA and AMP or ADP concentration. Apparent equilibrium binding constants were determined by a global fit of all data to the thermodynamic scheme (Fig.2a,b, Supplementary Table 1, Supplementary Figs. S3, S4; for experimental details and data analysis see Supplementary Methods).
Figure 2. Thermodynamic framework for nucleotide and RNA binding by Ded1p.
(a) Apparent equilibrium dissociation constants (K1/2) for the minimal thermodynamic scheme of nucleotide and RNA binding to Ded1p(E: Enzyme, R: RNA). Constants were determined by global fitting the fraction of inorganic phosphate generated during ATP hydrolysis assays (for experimental details and data analysis see Supplementary Methods). (b) Quality assessment of the global data fit. To visualize the goodness of fit to the data set, experimental data (y-axis) were plotted versus the calculated f[Pi] for the entire model (x-axis). Data points for ADP are blue, those for AMP green. Black lines represent a linear fit through the data. The correlation coefficient is given as R2. For quality assessment of data subsets (e.g., ATP alone, high and low RNA or ADP/AMP) see Supplementary Fig.S4b-f. (c) Binding of a 10 nt single stranded RNA to 1 μM Ded1p without nucleotide (black), and with saturating ATP (red), ADP (blue), or with AMP (green). All reactions contained equimolar concentrations of nucleotide and Mg2+ and 0.5 mM Mg2+ excess over the nucleotide. For RNA sequences see Supplementary Materials. Fractions of bound RNA with ADP, AMP and without nucleotide were determined from the equilibrium constants shown in panel (a). Nucleotide concentrations were extrapolated to infinity. Error bars represent the 95% confidence interval obtained from the global fit of all data to the complete thermodynamic model (Supplementary Methods, Supplementary Table S1). (d) Cooperativity between RNA and nucleotide binding, as calculated from the difference in free energies for nucleotide binding with and without RNA. (ΔΔG° = ΔG° (nt)-RNA − ΔG° (nt)+RNA, with ΔG° = −RTln(1/K1/2)). Positive ΔΔG° values indicate cooperativity between nucleotide and RNA binding, negative values indicate anti-cooperative binding.
Compared to the reaction without nucleotide, AMP weakened RNA binding by Ded1p, whereas ADP and ATP promoted RNA binding (Fig.2c). In addition, the data revealed positive cooperativity between RNA and ATP or ADP binding (Fig.2d). That is, RNA binding also promotes ATP and ADP association. However, AMP and RNA binding display negative cooperativity (Fig.2d), indicating that AMP and RNA binding antagonize each other.
These observations correlate with an AMP-induced change in the structure of the ATP binding site in DEAD-box helicases, compared to structures with ATP or ADP 14,15,16,19,20,21,22 (Supplementary Fig.S5). Structures as well as our RNA and nucleotide binding data are consistent with a scenario where AMP prevents the DEAD-box helicase to close the two helicase core domains, thereby preventing formation of the complete RNA binding site, which spans both RecA-like domains 23,19. Hence, negative cooperativity between AMP and RNA binding is seen. In contrast, ATP promotes closure of the two domains, which is reflected in the positive cooperativity between ATP and RNA binding. The positive cooperativity between ADP and RNA binding suggests that ADP also promotes the closed conformation of Ded1p with RNA bound, similar to ATP and RNA binding.
AMP inhibits RNA unwinding by Ded1p
We next examined whether AMP also impacted ATP-driven RNA duplex unwinding by Ded1p (Fig. 3). AMP strongly reduced unwinding activity (Fig.3a). Notably, AMP inhibited unwinding significantly stronger than ADP at identical concentrations, consistent with the differences in the affinities for both nucleotides seen above (Figs.1, 2). We detected virtually no unwinding inhibition with cyclic AMP (cAMP) or adenosine (Fig.3a, b, first column), indicating that the phosphate configuration of AMP is critical for nucleotide binding.
Figure 3. Impact of AMP on RNA helicase activity.
(a) Effect of adenosine nucleotides on duplex unwinding by Ded1p. Representative non-denaturing PAGE of unwinding time courses (30 min) with Ded1p.Cartoons show the mobility of unwound and duplex substrates, the asterisk marks the radiolabel. Unwinding reactions for Ded1p were performed as previously described 24 (19 °C, 20 μl reaction buffer containing 40 mMTris·HCl (pH 8.0), 50 mMNaCl, 0.5 mM MgCl2, 2 mM DTT, 0.6 unit•μl−1RNasin, 0.01% (vol/vol) IGEPAL, 0.1 μM Ded1p, 0.5 nM 13 bp RNA duplex). Ded1p was pre-incubated with the 13 bp duplex substrate with a 25 nt 3’ss overhang (for sequences see Supplementary Materials) and the reactions were initiated with 0.5 mM ATP in the presence or absence of 0.5 mMindicated nucleotide (Total [Mg2+]: 1mM with ATP alone, 1.5 mM in all other reactions). At each time-point, a 3 μl portion was removed, and the reaction was stopped with 3 μl of 1% SDS, 50 mM EDTA, 20% (v/v) Glycerol, and 0.1% (w/v) Bromphenol Blue/Xylene Cylanol. Samples were applied to a 15% native polyacrylamide gel, and electrophoresed at 20 V/cm at 4°C. Gels were dried and radioactivity was quantified with a PhosphorImager (GE) and the ImageQuant software (Mol. Dynamics).First order unwinding rate constants were calculated as previously described 13.(b) Observed first order unwinding rate constants (kunw) in the presence of indicated nucleotides, calculated for Ded1p from panel (a) and for several DEAD-box helicases (Mss116p, eIF4A, Sub2p, Dbp5p) and the non-DEAD-box RNA helicase Mtr4p. Error bars mark one standard deviation of at least three independent experiments. Unwinding reactions with the other RNA helicases were measured as described above, with the following modifications to obtain optimal unwinding rate constants: Mss116p: 10 mM Tris·HCl (pH 7.5), 100 mM KCl, Sub2p: 10 mM Tris·HCl (pH 7.0), 50 mM KCl at 30°C, Dbp5p: 40 mM Hepes (pH 7.5) at 30°C, eIF4A: 40 mM Hepes (pH 7.5), 8% (vol/vol) PEG6000 at 25°C and a 10bp duplex, and Mtr4p: 40 mM MOPS (pH 6.5), 100 mM NaCl, (16 bp duplex) at 30°C. Mtr4p was expressed and purified as described 33,34. eIF4A was expressed and purified according to a protocol identical to that described for Ded1p 33. Mss116p, Dbp5p, and Sub2p were generous gifts from Dr. Alan Lambowitz (University of Texas, Austin, TX), Dr. Karsten Weis (University of California, Berkeley, CA), and Dr. DitlevBroderson (University of Aarhus, Aarhus, Denmark). The proteins were expressed and purified as described 26,35. Radiolabeled substrates were prepared as previously described 36.
To account for the differences in Mg2+ coordination between ATP and ADP, and the other nucleotides, we measured effects of free Mg2+. No significant inhibition was seen (Fig. 3b, first column). Identical inhibition patterns were observed with different reaction buffers, and under different reaction conditions (Supplementary Fig.S6). Collectively, these results indicate that the strong inhibition of duplex unwinding by AMP is specific to AMP, and not due to free Mg2+ or accommodation of any adenine-based nucleotide by Ded1p.
AMP inhibits RNA unwinding by other, but not by all DEAD-box helicases
Having shown that Ded1p binds AMP and that AMP inhibits both RNA binding and unwinding, we next tested whether other DEAD-box helicases reacted to AMP in a similar fashion. We probed the effect of AMP on the ATP-driven RNA unwinding activity of four additional DEAD-box helicases from yeast: Mss116p, involved in mitochondrial RNA metabolism, eIF4A (Tif1p), involved in translation initiation, Sub2p, involved in mRNA export and pre-mRNA splicing, and Dbp5p, involved in RNA export 24,25,26,27.
Like Ded1p, Mss116p and eIF4A were inhibited by AMP, less by ADP, and not significantly by cAMP or adenosine (Fig.3b). In contrast, Sub2p and Dbp5p showed only significant inhibition by ADP, while inhibition by AMP was moderate and did not exceed the degree of inhibition seen with cAMP, adenosine or free Mg2+ (Fig.3b). We also examined the Ski2-like RNA helicase Mtr4p, a non-DEAD-box helicase from S. cerevisiae (Fig.3b). Mtr4p showed only inhibition by ADP, neither AMP, cAMP or adenosine had strong effects (Fig.3b). The observations with this series of yeast RNA helicases thus indicate that some, but not all DEAD-box helicases are sensitive to AMP.
It is not obvious how the highly conserved helicase core shared by all DEAD-box helicases allows some of the enzymes, but not others, to sense AMP. Examination of the correlation between ATP, ADP, and RNA binding for the DEAD-box helicases for which this information is available 28,29,30,31, does not reveal uniformly negative or positive cooperativity between nucleotide and RNA binding (Supplementary Fig.S7), suggesting that RNA binding is also modulated differently by ADP and ATP in DEAD-box helicases.
Available crystal structures do not reveal features in the ATP binding sites that could explain differing effects of AMP on the DEAD-box helicases tested. All residues implicated in AMP binding are highly conserved across all DEAD-box proteins 12,14,15,16. However, susceptibility to AMP may correlate with subtle differences in Mg2+ binding. Ded1p, Mss116p, and eIF4A, which are strongly inhibited by AMP show much less inhibition by free Mg2+than Dbp5p and Sub2p. These two proteins are not inhibited by AMP, but show stronger inhibition by free Mg2+ (Fig.3b). Crystal structures suggest that AMP binding to DEAD-box proteins does not involve Mg2+, while ADP and ATP binding does (Supplementary Fig.S4). It is not apparent which structural characteristics confer these potential differences in Mg2+ binding.
AMP sensing by DEAD-box proteins as possible direct link between energy and RNA metabolism
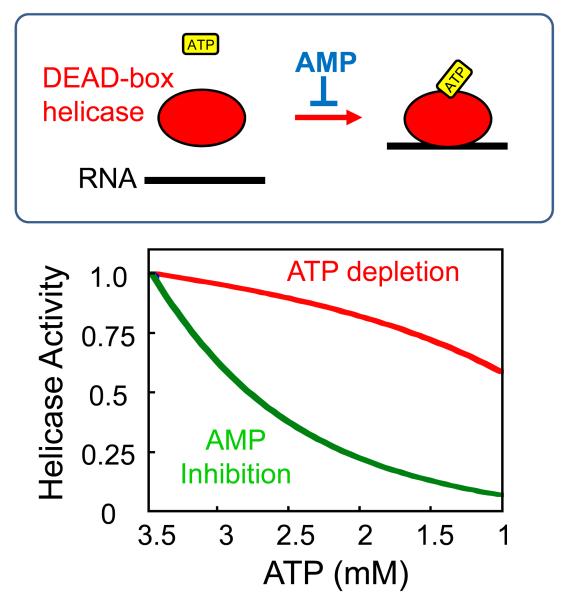
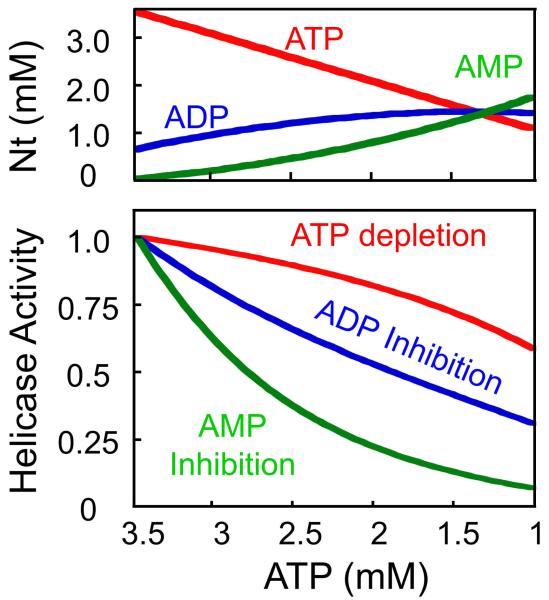
Notwithstanding its exact structural basis, AMP sensing is a novel function for the subset of DEAD-box RNA helicases that accommodate this nucleotide. These DEAD-box RNA helicases are thus the first enzymes with the potential to link RNA metabolism to changes in AMP levels, and thereby to the metabolic state of the cell. Such a connection would be direct, without detours through signaling cascades. The measured nucleotide affinities for Ded1p are particularly well suited for a powerful response to the rise in AMP levels seen during metabolic stress such as glucose deprivation in yeast 5,11 (Fig.4). Simulations show that comparably little inhibition of the helicase occurs as a result of stress-associated ATP depletion or increase in ADP levels (Fig. 4, lower panel). Both, the decrease in ATP concentration and the increase in ADP concentration are moderate, compared to the greater increase in AMP levels upon stress induction (Fig.4, upper panel). The helicase strongly responds to the rising AMP concentration, making AMP a highly efficient modulator of the enzyme (Fig.4).
Figure 4. Modulation of the helicase activity of Ded1p to stress-induced changes in nucleotide concentrations.
Upper panel: Simulated changes in nucleotide levels in response to metabolic stress, such as glucose depletion. Curves were calculated as described by Hardie et al., based on published parameters for the adenylate kinase reaction 11. For the calculation, the reaction is assumed at equilibrium with a constant total concentration of adenylate nucleotides. Experimental measurements of AMP concentrations suggest that this simulation might underestimate the AMP increase associated with metabolic stress5. Lower panel: Response of Ded1p (represented as ATP bound state of the enzyme) to ATP depletion (red), inhibition by ADP (blue), or inhibition by AMP (green), using affinities given in Fig. 2a.
To which extent changes in AMP levels affect Ded1p, Mss116p or eIF4A in the cell remains to be defined. However, it is well established that Ded1p functions in the cellular response to metabolic stress 32. Ded1p is present in stress granules that form upon glucose deprivation in yeast 32. AMP-mediated inhibition of Ded1p, a protein involved in ribosome biogenesis and translation initiation could contribute to slowing and the ultimate stop of these energy-intensive processes under metabolic stress.
Supplementary Material
Highlights.
Several DEAD-box RNA helicases are sensitive to AMP, which is not produced during ATP hydrolysis by these enzymes.
AMP potently inhibits RNA binding and unwinding by the yeast DEAD-box helicases Ded1p, Mss116p, and eIF4A, but not the yeast DEAD-box helicases Sub2p and Dbp5.
Observations identify a subset of DEAD-box helicases as enzymes with the capacity to directly link changes in AMP concentrations to RNA metabolism.
Acknowledgements
We thank Dr. Richard Hanson (Case Western Reserve University), members of our laboratory, and members of the Center for RNA Molecular Biology at Case Western Reserve University for comments on the manuscript. We thank Zhaofeng Gao (Case Western Reserve University) for preparation of eIF4A, and Sukanya Srinivasan (Case Western Reserve University) for preparation of Mtr4p. We are grateful to Dr. Piet deBoer for access to a FluoroMax-3 spectrofluorimeter, Drs. Anna Mallam, Mark Del Campo and Alan Lambowitz (University of Texas, Austin, TX) for purified Mss116, Drs. Ben Montpetit and Karsten Weis (University of California, Berkeley, CA) for purified Dbp5p, and Andreas Bøggild and Dr. Ditlev Broderson (University of Aarhus, Aarhus, Denmark) for purified Sub2p. This work was supported by the NIH (GM067700 to E.J.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Corton JM, Gillespie JG, Hardie DG. Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol. 1994;4:315–24. doi: 10.1016/s0960-9822(00)00070-1. [DOI] [PubMed] [Google Scholar]
- 2.Sakamoto K, Zarrinpashneh E, Budas GR, Pouleur AC, Dutta A, Prescott AR, et al. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKalpha2 but not AMPKalpha1. Am J Physiol Endocrinol Metab. 2006;290:E780–8. doi: 10.1152/ajpendo.00443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W, Yang X, Lopez de Silanes I, Carling D, Gorospe M. Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J Biol Chem. 2003;278:27016–23. doi: 10.1074/jbc.M300318200. [DOI] [PubMed] [Google Scholar]
- 4.Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18:3004–9. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson WA, Hawley SA, Hardie DG. Glucose repression/derepression in budding yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP:ATP ratio. Curr Biol. 1996;6:1426–34. doi: 10.1016/s0960-9822(96)00747-6. [DOI] [PubMed] [Google Scholar]
- 6.Walther T, Novo M, Rossger K, Letisse F, Loret MO, Portais JC, et al. Control of ATP homeostasis during the respiro-fermentative transition in yeast. Mol Syst Biol. 2010;6:344. doi: 10.1038/msb.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudley GA, Tullson PC, Terjung RL. Influence of mitochondrial content on the sensitivity of respiratory control. J Biol Chem. 1987;262:9109–14. [PubMed] [Google Scholar]
- 8.Dudley GA, Terjung RL. Influence of acidosis on AMP deaminase activity in contracting fast-twitch muscle. Am J Physiol. 1985;248:C43–50. doi: 10.1152/ajpcell.1985.248.1.C43. [DOI] [PubMed] [Google Scholar]
- 9.Helmreich E, Cori CF. The Role of Adenylic Acid in the Activation of Phosphorylase. Proc Natl Acad Sci U S A. 1964;51:131–8. doi: 10.1073/pnas.51.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansour TE. Studies on heart phosphofructokinase: purification, inhibition and activation. J Biol Chem. 1963;238:2285–2292. [Google Scholar]
- 11.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linder P, Jankowsky E. From unwinding to clamping - the DEAD box RNA helicase family. Nat Rev Mol Cell Biol. 2011;12:505–16. doi: 10.1038/nrm3154. [DOI] [PubMed] [Google Scholar]
- 13.Yang Q, Jankowsky E. ATP- and ADP-dependent modulation of RNA unwinding and strand annealing activities by the DEAD-box protein DED1. Biochemistry. 2005;44:13591–601. doi: 10.1021/bi0508946. [DOI] [PubMed] [Google Scholar]
- 14.Hogbom M, Collins R, van den Berg S, Jenvert RM, Karlberg T, Kotenyova T, et al. Crystal structure of conserved domains 1 and 2 of the human DEAD-box helicase DDX3X in complex with the mononucleotide AMP. J Mol Biol. 2007;372:150–9. doi: 10.1016/j.jmb.2007.06.050. [DOI] [PubMed] [Google Scholar]
- 15.Schutz P, Karlberg T, van den Berg S, Collins R, Lehtio L, Hogbom M, et al. Comparative structural analysis of human DEAD-box RNA helicases. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudolph MG, Heissmann R, Wittmann JG, Klostermeier D. Crystal structure and nucleotide binding of the Thermus thermophilus RNA helicase Hera N-terminal domain. J. Mol. Biol. 2006;361:731–743. doi: 10.1016/j.jmb.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 17.Hilliker A, Gao Z, Jankowsky E, Parker R. The DEAD-box protein Ded1 modulates translation by the formation and resolution of an eIF4F-mRNA complex. Mol. Cell. 2011;43:962–972. doi: 10.1016/j.molcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schäfer T, Strauss D, Petfalski E, Tollervey D, Hurt E. The path from nucleolar 90S to cytoplasmic 40S pre-ribosomes. EMBO J. 2003;22:1370–80. doi: 10.1093/emboj/cdg121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi H, Cordin O, Minder CM, Linder P, Xu RM. Crystal structure of the human ATP-dependent splicing and export factor UAP56. Proc Natl Acad Sci U S A. 2004;101:17628–33. doi: 10.1073/pnas.0408172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Campo M, Lambowitz AM. Structure of the Yeast DEAD box protein Mss116p reveals two wedges that crimp RNA. Mol Cell. 2009;35:598–609. doi: 10.1016/j.molcel.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montpetit B, Thomsen ND, Helmke KJ, Seeliger MA, Berger JM, Weis K. A conserved mechanism of DEAD-box ATPase activation by nucleoporins and InsP6 in mRNA export. Nature. 2011;472:238–42. doi: 10.1038/nature09862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sengoku T, Nureki O, Nakamura A, Kobayashi S, Yokoyama S. Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell. 2006;125:287–300. doi: 10.1016/j.cell.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 23.Mallam AL, Jarmoskaite I, Tijerina P, Del Campo M, Seifert S, Guo L, et al. Solution structures of DEAD-box RNA chaperones reveal conformational changes and nucleic acid tethering by a basic tail. Proc. Natl. Acad. Sci. USA. 2011;108:12254–12259. doi: 10.1073/pnas.1109566108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strasser K, Hurt E. Splicing factor Sub2p is required for nuclear mRNA export through its interaction with Yra1p. Nature. 2001;413:648–52. doi: 10.1038/35098113. [DOI] [PubMed] [Google Scholar]
- 25.Halls C, Mohr S, Del Campo M, Yang Q, Jankowsky E, Lambowitz AM. Involvement of DEAD-box Proteins in Group I and Group II Intron Splicing. Biochemical Characterization of Mss116p, ATP Hydrolysis-dependent and -independent Mechanisms, and General RNA Chaperone Activity. J. Mol. Biol. 2007;365:835–855. doi: 10.1016/j.jmb.2006.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weirich CS, Erzberger JP, Flick JS, Berger JM, Thorner J, Weis K. Activation of the DExD/H-box protein Dbp5 by the nuclear-pore protein Gle1 and its coactivator InsP6 is required for mRNA export. Nat Cell Biol. 2006;8:668–76. doi: 10.1038/ncb1424. [DOI] [PubMed] [Google Scholar]
- 27.Linder P. Yeast RNA helicases of the DEAD-box family involved in translation initiation. Biol Cell. 2003;95:157–67. doi: 10.1016/s0248-4900(03)00032-7. [DOI] [PubMed] [Google Scholar]
- 28.Lorsch JR, Herschlag D. The DEAD box protein eIF4A. 1. A minimal kinetic and thermodynamic framework reveals coupled binding of RNA and nucleotide. Biochemistry. 1998;37:2180–93. doi: 10.1021/bi972430g. [DOI] [PubMed] [Google Scholar]
- 29.Henn A, Cao W, Hackney DD, De La Cruz EM. The ATPase cycle mechanism of the DEAD-box rRNA helicase, DbpA. J Mol Biol. 2008;377:193–205. doi: 10.1016/j.jmb.2007.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henn A, Cao W, Licciardello N, Heitkamp SE, Hackney DD, De La Cruz EM. Pathway of ATP utilization and duplex rRNA unwinding by the DEAD-box helicase, DbpA. Proc Natl Acad Sci U S A. 2010;107:4046–50. doi: 10.1073/pnas.0913081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao W, Coman MM, Ding S, Henn A, Middleton ER, Bradley MJ, et al. Mechanism of Mss116 ATPase reveals functional diversity of DEAD-Box proteins. J Mol Biol. 2011;409:399–414. doi: 10.1016/j.jmb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beckham C, Hilliker A, Cziko A-M, Noueiry A, Ramaswami M, Parker R. The DEAD-box RNA helicase Ded1p affects and accumulates in Saccharomyces cerevisiae P-bodies. Mol Biol Cell. 2008;19:984–93. doi: 10.1091/mbc.E07-09-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fairman ME, Maroney PA, Wang W, Bowers HA, Gollnick P, Nilsen TW, et al. Protein displacement by DExH/D “RNA helicases” without duplex unwinding. Science. 2004;304:730–4. doi: 10.1126/science.1095596. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Jia H, Jankowsky E, Anderson JT. Degradation of hypomodified tRNA(iMet) in vivo involves RNA-dependent ATPase activity of the DExH helicase Mtr4p. RNA. 2008;14:107–16. doi: 10.1261/rna.808608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halls C, Mohr S, Del Campo M, Yang Q, Jankowsky E, Lambowitz AM. Involvement of DEAD-box proteins in group I and group II intron splicing. Biochemical characterization of Mss116p, ATP hydrolysis-dependent and -independent mechanisms, and general RNA chaperone activity. J Mol Biol. 2007;365:835–55. doi: 10.1016/j.jmb.2006.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jankowsky E, Gross CH, Shuman S, Pyle AM. The DExH protein NPH-II is a processive and directional motor for unwinding RNA. Nature. 2000;403:447–51. doi: 10.1038/35000239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.