Abstract
Abnormal myelin gene expression in the central nervous system (CNS) is associated with many mental illnesses, including psychiatric disorders and drug addiction. We have previously shown that prenatal exposure to nicotine, the major psychoactive component in cigarette smoke, alters myelin gene expression in the CNS of adolescent rats. To examine whether this effect is specific for adolescents, we examined myelin gene expression in the CNS of juveniles and adults. Pregnant Sprague-Dawley rats were treated with nicotine (3 mg/kg/day; GN) or saline (GS) via osmotic mini pumps from gestational days 4 to 18. Both male and female offspring were sacrificed at postnatal day P20-21 (juveniles), P35-36 (adolescents), or P59-60 (adults). Three limbic brain regions, the prefrontal cortex (PFC), caudate putamen (CPu), and nucleus accumbens (NAc), were dissected. The expression of genes encoding major myelin components was evaluated using quantitative RT-PCR. We found that GN altered myelin gene expression in juveniles with brain region and sex differences. The pattern of alteration was different from that observed in adolescents. Although these genes were expressed normally in male adults, we observed decreased expression in GN-treated female adults, especially in the CPu. Thus, GN altered myelin gene expression throughout postnatal development and adulthood. The effect on adolescents was quite different from that at other ages, which correlated with the unique symptoms of many psychiatric disorders during adolescence.
Keywords: nicotine, gestational, myelin, smoking, sex, development
Introduction
Maternal smoking during pregnancy (MSDP) has been associated with many neurobehavioral problems in the offspring. Those whose mothers smoke during pregnancy are more likely to show reduced cognitive abilities [10] and to develop neuropsychiatric disorders such as attention deficit hyperactivity disorder, conduct disorder, depression, autism, and drug addiction [24].
To evaluate the underlying mechanisms, we established a rat model of gestational exposure to a moderate dose of nicotine (GN) [35], the major psychoactive component of tobacco. Our previous studies focusing on adolescents showed that GN altered behavioral responses to addictive substances [16, 17], cell death/survival pathways [45], and expression of cell adhesion molecules in the central nervous system (CNS) [7]. Recently, we found that central myelin gene expression was also changed during adolescence by GN treatment in a brain region- and sex-dependent manner [8]. These studies suggest that nicotine replacement therapy during pregnancy may carry many of the same risks to the offspring as maternal smoking.
Myelin is a membrane structure produced by oligodendrocytes (OLGs) in the CNS and consists of many specific proteins and large amounts of glycolipids and cholesterol [3, 12]. Myelin basic protein (MBP) and proteolipid protein (PLP) contribute approximately 85% of the protein content of myelin. The remaining 15% includes 2′, 3′-cyclic nucleotide 3′-phosphodiesterase (CNP), myelin oligodendrocyte glycoprotein (MOG), myelin-associated oligodendrocytic basic protein (Mobp), and T-cell differentiation protein (Mal) [3]. Deficits in these major myelin components lead not only to abnormal myelin structure but also to axonal degeneration [3].
The functional acknowledgment of the OLG–myelin complex has greatly advanced in the past decades. Myelin structure not only increases the conduction velocity of action potentials [22], but also interacts with axons to support neuronal survival and modulate neurotransmission [13, 34, 39]. OLGs also synthesize neurotrophic factors to promote neuronal survival and axonal growth [46]. Recently, OLG-myelin complex has attracted new interest because of its apparent involvement in drug addiction and various psychiatric disorders such as schizophrenia, bipolar disorder, and major depression [6, 15].
The process of myelination involves OLG-precursor migration, proliferation, and differentiation into OLGs followed by maturation and formation of a myelin sheath around axons [3]. Myelination initiates during embryonic development, continues into adolescence, and still exhibits great plasticity in the adult nervous system [2, 4, 31]. As the major resource for cholesterol production in the brain and high energy demands for producing and maintaining the massive membrane structure, the process of myelination is vulnerable to environmental challenges [2]. Our recent study suggested that prenatal nicotine exposure affects myelination in the adolescent brain. However, it is not clear whether this effect is specific to adolescents. Therefore, we examined major myelin gene expression in GN-treated rats at different ages, including juveniles and young adults.
Materials and Methods
Animals and tissue collection
Sprague-Dawley rats were maintained in a temperature (21°C)- and humidity (50%)-controlled room on a 12-h light–dark cycle (lights on 0700–1900) with unlimited access to food and water. Pregnant rats (Harlan, San Diego, CA) were treated with either nicotine at a concentration of 3 mg/kg/day (calculated free base) or saline via osmotic minipumps (Alzet Model 2002) as described previously [35]. The minipump has a 14-day delivery period and was implanted subcutaneously on the back of each dam on gestational day 4. Blood concentrations resulting from this dose of nicotine are equivalent to those found in humans who smoke about 1½ packs of cigarettes per day [29]. After birth, pups were cross-fostered on drug-naive dams to minimize the effects of abnormal maternal behaviors or milk output attributable to nicotine treatment. As previously reported [16], GN treatment did not influence dam weight gain, litter size, or pup weight gain during postnatal development. Pups were weaned on postnatal day (P) 21, and for each sex at each age, non-sibling animals were used. Brain tissues from the prefrontal cortex (PFC), dorsal caudate putamen (CPu), and nucleus accumbens (NAc) were collected from pups at P20-21 (juveniles), P35-36 (adolescents), or P59-60 (adults) and stored at −80°C until being used for quantitative real-time polymerase chain reaction (qRT-PCR) assay (N = 5 or 6). The tissues were excised using a brain punch tissue set (Stoelting, WI) and rat brain matrices (Kent Scientific, CT) according to coordinates from Paxinos and Watson [37]. All experiments were carried out in accordance with the Institutional Animal Care and Use Committees at the University of California, Irvine, and University of Virginia and were consistent with Federal guidelines.
Quantitative real-time PCR array
We examined eight genes encoding major myelin components, namely, myelin basic protein (Mbp), proteolipid protein 1 (Plp1), 2′,3′-cyclic nucleotide 3′ phosphodiesterase (Cnp), T-cell differentiation protein (Mal), gap junction protein, gamma 3 (Gjc3), myelin-associated oligodendrocytic basic protein (Mobp), myelin oligodendrocyte glycoprotein (Mog), and aspartoacylase (Aspa) using qRT-PCR. The primers were designed using Primer Express (v. 3.0) software (Applied Biosystems) and spanned introns to avoid amplifying genomic DNA. The amplicon sequences were subjected to a BLAST search to ensure the specificity of the primers for the target genes and synthesized by Fisher Scientific (Pittsburgh, PA). All the primers were tested for their specificity by checking cycle number and the dissociation curve prior to inclusion in the qRT-PCR array. The primer sequences are listed in Supplementary Table 1.
The RNA was isolated from each brain region using TRIZol reagent (Invitrogen) according to the manufacturer's instructions and amplified as described previously for adequate cDNA [7]. The qRT-PCR was conducted as described previously [21, 27]. Briefly, the RT product was amplified in a volume of 10 μl containing 5 μl of 2× Power SYBR® Green PCR Master Mix (Applied Biosystems) and combined sense and antisense primers (3 μl; final concentration 250 nM) in a 384-well plate using the 7900HT Sequence Detection System (Applied Biosystems). Expressions of all genes were normalized to the expression of actin and GAPDH and then analyzed using a comparative Ct method [47]. Because data normalized to GAPDH yielded results similar to those normalized by actin, only the results normalized by actin are provided in this report.
Data analysis
Instead of comparing gene expression directly across ages and sexes, we calculated the ratios of gene expression in the GN group versus the corresponding GS group at each age and for each sex. This eliminates the potential impact of age and sex differences on brain structure and tissue collection.
Data were analyzed by mixed-design ANOVA with between-subjects factors (Age, Sex, Brain Region, and Drug) and within-subject factor (Gene). Significant main effects and interactions were further analyzed by appropriate ANOVA and post-hoc analysis with Bonferroni correction for multiple comparisons. Significant alteration in mRNA expression was defined as a fold change > 20% with a p value < 0.05.
Results
ANOVA analysis revealed the expression levels of myelin genes were altered by GN treatment with a significant interaction of treatment with brain region, sex, and age (F4, 122 = 6.665, p < 0.0001).
Prefrontal cortex
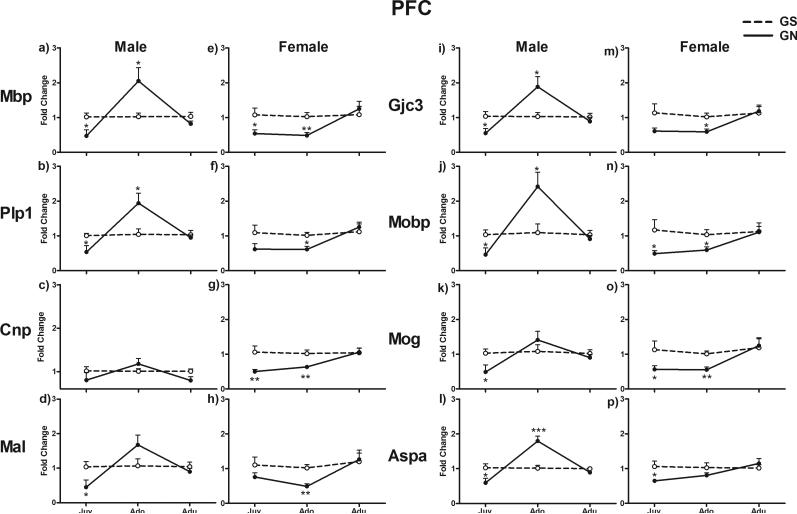
In the PFC, myelin gene expression was altered by GN treatment with a significant interaction of treatment with Sex and Age (F2, 37 = 7.487; p = 0.002). All examined genes showed similar patterns of alteration within each age and sex, as indicated by an insignificant Gene effect and insignificant interactions of Gene with other factors (Fig.1). In juveniles, these genes generally showed decreased expression in GN-treated animals compared with GS controls in both males (p < 0.05 for Mbp, Plp1, Mal, Gjc3, Mobp, Mog, and Aspa) and females (p < 0.05 for Mbp, Mobp, Mog, and Aspa; p < 0.01 for Cnp). In adolescents, these genes were upregulated in GN-treated males (p < 0.05 for Mbp, Plp1, Gjc3, Mobp; p < 0.001 for Aspa), whereas they were downregulated in GN-treated females (p < 0.05 for Plp1, Gjc3, and Mobp; p < 0.01 for Mbp, Cnp, Mal, and Mog). These genes were expressed normally in both GN-treated male and female adults.
Figure 1.
Fold change in mRNA expression of eight myelin genes in the PFC in juvenile, adolescent, and adult rats exposed to gestational nicotine (GN; solid lines) or saline (GS; dotted lines). Data from males and females are presented separately. Data are expressed as means ± SEM (N = 4 or 5). *p <0.05; **p <0.01; ***p <0.001 significantly different from GS controls. Abbreviations for Figures 1–3: Aspa = aspartoacylase; Cnp = 2′,3′-cyclic nucleotide 3′ phosphodiesterase; Gjc3 = gap junction protein, gamma 3; Mal = T-cell differentiation protein; Mbp = myelin basic protein; Mobp = myelin-associated oligodendrocytic basic protein; Mog = myelin oligodendrocyte glycoprotein; and Plp1 = proteolipid protein 1.
Caudate putamen
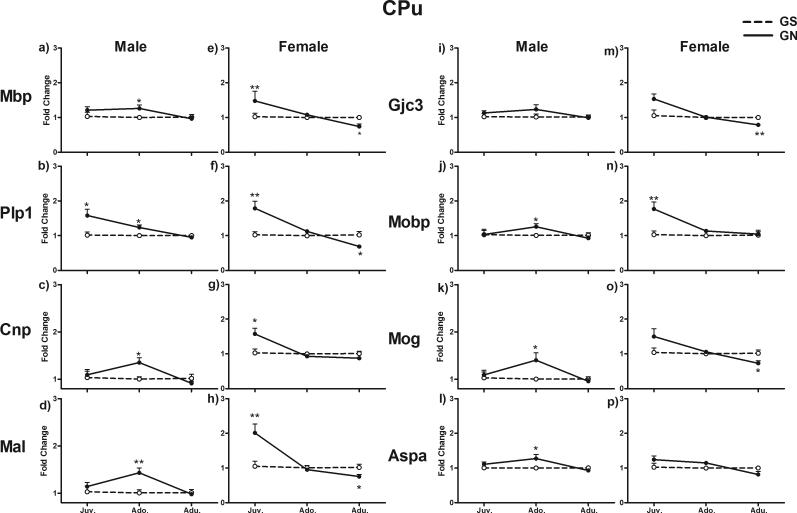
In the CPu, GN altered myelin gene expression with a significant interaction of treatment with Sex and Age (F2, 40 = 6.007, p = 0.005) (Fig. 2). In male juveniles, the expression of Plp1 was significantly increased in the GN group compared with GS controls (p < 0.05), whereas all other genes were expressed normally. In contrast, these genes were generally upregulated in GN females (p < 0.05 for Cnp; p < 0.01 for Mbp, Plp1, Mal, and Mobp). In adolescents, the expression of these genes was generally increased in GN males (p < 0.05 for Mbp, Plp1, Cnp, Mobp, Mog, and Aspa; p < 0.01 for Mal), whereas they were expressed normally in GN females. In adults, these genes were expressed normally in GN males while generally having decreased expression in GN females compared with GS controls (p < 0.05 for Mbp, Plp1, Mal, and Mog; p < 0.01 for Gjc3).
Figure 2.
Fold change in mRNA expression of eight myelin genes in the CPu in juvenile, adolescent and adult rats exposed to gestational nicotine (GN; solid lines) or saline (GS; dotted lines). Data for males and females are presented separately. Data are expressed as means ± SEM (N = 4 or 5). *p <0.05; **p <0.01; ***p <0.001 significantly different from GS controls.
Nucleus accumbens
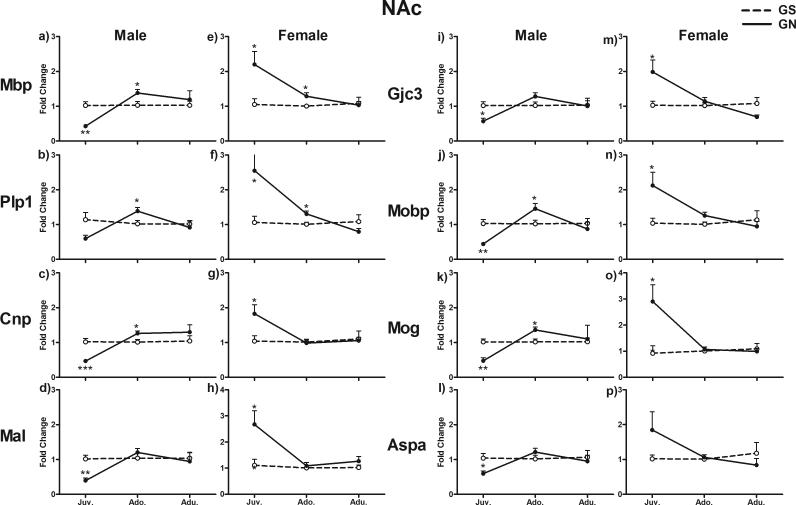
In the NAc, GN altered myelin gene expression with a significant interaction of treatment with Sex and Age (F2, 45 = 15.275, p = 0.001). All examined genes showed similar patterns of alterations within each age and sex, as indicated by an insignificant Gene effect and insignificant interactions of Gene with other factors (Fig. 3). In juveniles, these genes showed decreased expression in GN-treated males (p < 0.05 for Gjc3 and Aspa; p < 0.01 for Mbp, Mal, Mobp, and Mog; p < 0.001 for Cnp) and increased expression in GN-treated females compared with GS controls (p < 0.05 for Mbp, Plp1, Cnp, Mal, Gjc3, Mobp, and Mog). These genes generally showed upregulation in adolescent males (p < 0.05 for Mbp, Plp1, Cnp, Mobp, and Mog), whereas only Mbp (p < 0.05) and Plp1 (p < 0.05) were upregulated in GN-treated adolescent females. These genes were expressed normally in both adult males and females.
Figure 3.
Fold change in mRNA expression of eight myelin genes in the NAc in juvenile, adolescent, and adult rats exposed to gestational nicotine (GN; solid lines) or saline (GS; dotted lines). Data from males and females are presented separately. Data are expressed as means ± SEM (N = 4 or 5). *p <0.05; **p <0.01; ***p <0.001 significantly different from GS controls.
Discussion
Myelination in the CNS continues into adolescence and exhibits large plasticity in adulthood [2, 4, 31]. By examining major myelin gene expression, our study suggested that central myelination is disturbed by GN treatment in both developing and mature brains. This effect was complicated and depended on sex and the brain region examined.
In this study, we selectively examined a few major myelin genes that play important roles in the CNS. MBP is the major protein maintaining myelin structure [28], and ASPA is an important enzyme for myelin lipid synthesis [44]. Although we only reported mRNA expression in this study, our previous study has shown that the alteration in protein expression of MBP is consistent with that of mRNA in adolescent brains [8]. The membrane-associated proteins such as MAL and GJE3 interact with neighbor cells to support myelin structure and function [11, 40]. Other proteins, such as PLP1 and CNP, are less involved in myelination but help shape the underlying axon and also support axon function [19, 26]. Both MOBP and MOG are located selectively on the outside surface of myelin sheaths and have been related to the autoimmune response [30, 33]. Abnormal expression of these genes therefore can lead to abnormal myelin structure and deficits in neuronal function.
We have previously shown opposite sex differences in GN-induced alterations in myelin gene expression, which is specifically observed in the PFC of adolescents, with increased expression in GN males but decreased expression in GN females [8]. In comparing gene expression in juveniles, our data further suggest that the opposite sex differences also exist in the striatum during adolescent development, as these genes in GN-treated males were changed from downregulation in juveniles to upregulation in adolescents, whereas they were altered from upregulation in juvenile females to normal expression in adolescent females. Together, our data suggest that GN increases central myelination in adolescent males but decreases it in the females in all the brain regions examined. The opposite sex differences may result from the surge of gonadal hormones during puberty, because gonadal hormones stimulate OLG development and myelination [23, 43]. Gestational nicotine exposure delays the onset of puberty in female animals [32], whereas early onset of puberty in males has been linked to MSDP [18].
In contrast to those seen in the adolescents, the sex differences in juveniles were observed mainly in the striatum, with opposite sex differences especially in the NAc. The sex differences in the juvenile striatum were opposite to those in adolescents, which were characterized by either downregulation or normal myelin gene expression in GN-treated males but upregulation in GN females. The observation of sex differences in juveniles suggests a mechanism independent of pubertal gonadal hormone surges. Many studies have suggest that sexual dimorphism in the brain results not only from gonadal hormones but also from sex chromosomes (see [1] for a review). Plp1, one of the major myelin protein genes, is an X chromosome-linked gene [20], although it is still not clear whether it is related to observed sex differences. Heterogeneity in brain development may contribute to the brain region differences, as PFC has prolonged postnatal development and matures relatively later than other brain regions [25, 42].
By examining young adults, our data showed that GN's effect continued into adulthood, especially in adult females, suggesting that females are more vulnerable to GN's effects than are males. A decrease in myelin gene expression in the cortex and striatum has been observed in drug abusers and patients with schizophrenia, bipolar disorder, and major depressive disorder [41]. The downregulation of myelin genes in GN-treated female adults implies that females are susceptible to these disorders as a result of a long-term effect of prenatal exposure to smoking. Given the role of gonadal hormones in myelination [9], a close monitor on the estrous cycle and a further study on later ages will help to understand whether the GN's effects on adults are related to hormone changes.
Together, our data show that GN induced abnormal myelin gene expression throughout postnatal development and adulthood. Myelin deficits are observed in many psychiatric disorders and drug addiction [14]. These disorders are evidenced by unique symptoms during adolescence. For example, the onset of major depression, bipolar illness, and schizophrenia often is observed in adolescents [36]. Other syndromes with a childhood onset, such as attention deficit hyperactivity disorder and Tourette's syndrome, frequently either remit or change symptomatology during the adolescent period [5, 38]. Although abnormal myelin gene expression was observed in juveniles and adults, the effects on adolescents were quite different, which correlates with the unique symptoms of psychiatric disorders in adolescents.
Supplementary Material
Highlights.
Postnatal myelin gene expression was examined in rats exposed to gestational nicotine (GN).
Myelin gene expression in the brain was altered in GN-treated juveniles, adolescents, and adults.
Age, brain region, and sex differences were observed in GN's effect on myelin gene expression.
The myelin gene expression response to GN in adolescence is unique.
Long-term effects on myelin gene expression were observed in female but not male adults.
Acknowledgments
This project was in part supported by National Institutes of Health grants DA-013783 and DA-026356 to Ming D. Li.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arnold AP. Sex chromosomes and brain gender, Nature reviews. Neuroscience. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- 2.Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiology of aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49-62. [DOI] [PubMed] [Google Scholar]
- 3.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 4.Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of general psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- 5.Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157:816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- 6.Bora E, Yucel M, Fornito A, Pantelis C, Harrison BJ, Cocchi L, Pell G, Lubman DI. White matter microstructure in opiate addiction. Addiction biology. 2012;17:141–148. doi: 10.1111/j.1369-1600.2010.00266.x. [DOI] [PubMed] [Google Scholar]
- 7.Cao J, Dwyer JB, Mangold JE, Wang J, Wei J, Leslie FM, Li MD. Modulation of cell adhesion systems by prenatal nicotine exposure in limbic brain regions of adolescent female rats. Int J Neuropsychopharmacol. 2011;14:157–174. doi: 10.1017/S1461145710000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao J, Wang J, Dwyer JB, Gautier NM, Wang S, Leslie FM, Li MD. Gestational nicotine exposure modifies myelin gene expression in the brains of adolescent rats with sex differences. Translational psychiatry. 2013;3:e247. doi: 10.1038/tp.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerghet M, Skoff RP, Swamydas M, Bessert D. Sexual dimorphism in the white matter of rodents. Journal of the neurological sciences. 2009;286:76–80. doi: 10.1016/j.jns.2009.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clifford A, Lang L, Chen R. Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: a literature review. Neurotoxicology and teratology. 2012;34:560–570. doi: 10.1016/j.ntt.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Cotrina ML, Nedergaard M. Brain connexins in demyelinating diseases: therapeutic potential of glial targets. Brain research. 2012;1487:61–68. doi: 10.1016/j.brainres.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Monasterio-Schrader P, Jahn O, Tenzer S, Wichert SP, Patzig J, Werner HB. Systematic approaches to central nervous system myelin. Cellular and molecular life sciences : CMLS. 2012;69:2879–2894. doi: 10.1007/s00018-012-0958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y, Dreyfus CF. Oligodendrocytes as providers of growth factors. J Neurosci Res. 2002;68:647–654. doi: 10.1002/jnr.10245. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y. Convergence and divergence in the etiology of myelin impairment in psychiatric disorders and drug addiction. Neurochemical research. 2008;33:1940–1949. doi: 10.1007/s11064-008-9693-x. [DOI] [PubMed] [Google Scholar]
- 15.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends in neurosciences. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franke RM, Belluzzi JD, Leslie FM. Gestational exposure to nicotine and monoamine oxidase inhibitors influences cocaine-induced locomotion in adolescent rats. Psychopharmacology. 2007;195:117–124. doi: 10.1007/s00213-007-0876-y. [DOI] [PubMed] [Google Scholar]
- 17.Franke RM, Park M, Belluzzi JD, Leslie FM. Prenatal nicotine exposure changes natural and drug-induced reinforcement in adolescent male rats. The European journal of neuroscience. 2008;27:2952–2961. doi: 10.1111/j.1460-9568.2008.06253.x. [DOI] [PubMed] [Google Scholar]
- 18.Fried PA, James DS, Watkinson B. Growth and pubertal milestones during adolescence in offspring prenatally exposed to cigarettes and marihuana. Neurotoxicology and teratology. 2001;23:431–436. doi: 10.1016/s0892-0362(01)00161-1. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, Schneider A, Zimmermann F, McCulloch M, Nadon N, Nave KA. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths IR, Schneider A, Anderson J, Nave KA. Transgenic and natural mouse models of proteolipid protein (PLP)-related dysmyelination and demyelination. Brain Pathol. 1995;5:275–281. doi: 10.1111/j.1750-3639.1995.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 21.Gutala R, Wang J, Kadapakkam S, Hwang Y, Ticku M, Li MD. Microarray analysis of ethanol-treated cortical neurons reveals disruption of genes related to the ubiquitin proteasome pathway and protein synthesis. Alcohol Clin Exp Res. 2004;28:1779–1788. doi: 10.1097/01.alc.0000148117.17707.b4. [DOI] [PubMed] [Google Scholar]
- 22.Hursh JB. Conduction velocity and diameter of nerve fiber. AM physiological Soc. 1939 [Google Scholar]
- 23.Kafitz KW, Herth G, Bartsch U, Guttinger HR, Schachner M. Application of testosterone accelerates oligodendrocyte maturation in brains of zebra finches. Neuroreport. 1992;3:315–318. doi: 10.1097/00001756-199204000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev Neuropsychol. 2009;34:1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R. Experience and the developing prefrontal cortex. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17186–17193. doi: 10.1073/pnas.1121251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 27.Li MD, Kane JK, Wang J, Ma JZ. Time-dependent changes in transcriptional profiles within five rat brain regions in response to nicotine treatment, Brain research. Molecular brain research. 2004;132:168–180. doi: 10.1016/j.molbrainres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Martini R, Mohajeri MH, Kasper S, Giese KP, Schachner M. Mice doubly deficient in the genes for P0 and myelin basic protein show that both proteins contribute to the formation of the major dense line in peripheral nerve myelin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:4488–4495. doi: 10.1523/JNEUROSCI.15-06-04488.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matta SG, Elberger AJ. Combined exposure to nicotine and ethanol throughout full gestation results in enhanced acquisition of nicotine self-administration in young adult rat offspring. Psychopharmacology. 2007;193:199–213. doi: 10.1007/s00213-007-0767-2. [DOI] [PubMed] [Google Scholar]
- 30.Mayer MC, Meinl E. Glycoproteins as targets of autoantibodies in CNS inflammation: MOG and more. Therapeutic advances in neurological disorders. 2012;5:147–159. doi: 10.1177/1756285611433772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. Journal of neurochemistry. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- 32.Meyer DC, Carr LA. The effects of perinatal exposure to nicotine on plasma LH levels in prepubertal rats. Neurotoxicology and teratology. 1987;9:95–98. doi: 10.1016/0892-0362(87)90084-5. [DOI] [PubMed] [Google Scholar]
- 33.Montague P, McCallion AS, Davies RW, Griffiths IR. Myelin-associated oligodendrocytic basic protein: a family of abundant CNS myelin proteins in search of a function. Developmental neuroscience. 2006;28:479–487. doi: 10.1159/000095110. [DOI] [PubMed] [Google Scholar]
- 34.Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- 35.Park MK, Loughlin SE, Leslie FM. Gestational nicotine-induced changes in adolescent neuronal activity. Brain research. 2006;1094:119–126. doi: 10.1016/j.brainres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature reviews. Neuroscience. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C. The rat brain in stereotxic coordinates. Academic Press Inc.; San Diego: 2005. [Google Scholar]
- 38.Peterson BS. Considerations of natural history and pathophysiology in the psychopharmacology of Tourette's syndrome. J Clin Psychiatry. 1996;57(Suppl 9):24–34. [PubMed] [Google Scholar]
- 39.Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, Benoit-Marand M, Chen C, Moore H, O'Donnell P, Brunner D, Corfas G. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci U S A. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaeren-Wiemers N, Bonnet A, Erb M, Erne B, Bartsch U, Kern F, Mantei N, Sherman D, Suter U. The raft-associated protein MAL is required for maintenance of proper axon--glia interactions in the central nervous system. J Cell Biol. 2004;166:731–742. doi: 10.1083/jcb.200406092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sokolov BP. Oligodendroglial abnormalities in schizophrenia, mood disorders and substance abuse. Comorbidity, shared traits, or molecular phenocopies? Int J Neuropsychopharmacol. 2007;10:547–555. doi: 10.1017/S1461145706007322. [DOI] [PubMed] [Google Scholar]
- 42.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 43.Stocker S, Guttinger HR, Herth G. Exogenous testosterone differentially affects myelination and neurone soma sizes in the brain of canaries. Neuroreport. 1994;5:1449–1452. doi: 10.1097/00001756-199407000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Leone P, Wu G, Francis JS, Li H, Jain MR, Serikawa T, Ledeen RW. Myelin lipid abnormalities in the aspartoacylase-deficient tremor rat. Neurochem Res. 2009;34:138–148. doi: 10.1007/s11064-008-9726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei J, Wang J, Dwyer JB, Mangold J, Cao J, Leslie FM, Li MD. Gestational nicotine treatment modulates cell death/survival-related pathways in the brains of adolescent female rats. Int J Neuropsychopharmacol. 2011;14:91–106. doi: 10.1017/S1461145710000416. [DOI] [PubMed] [Google Scholar]
- 46.Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:4967–4974. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.