Abstract
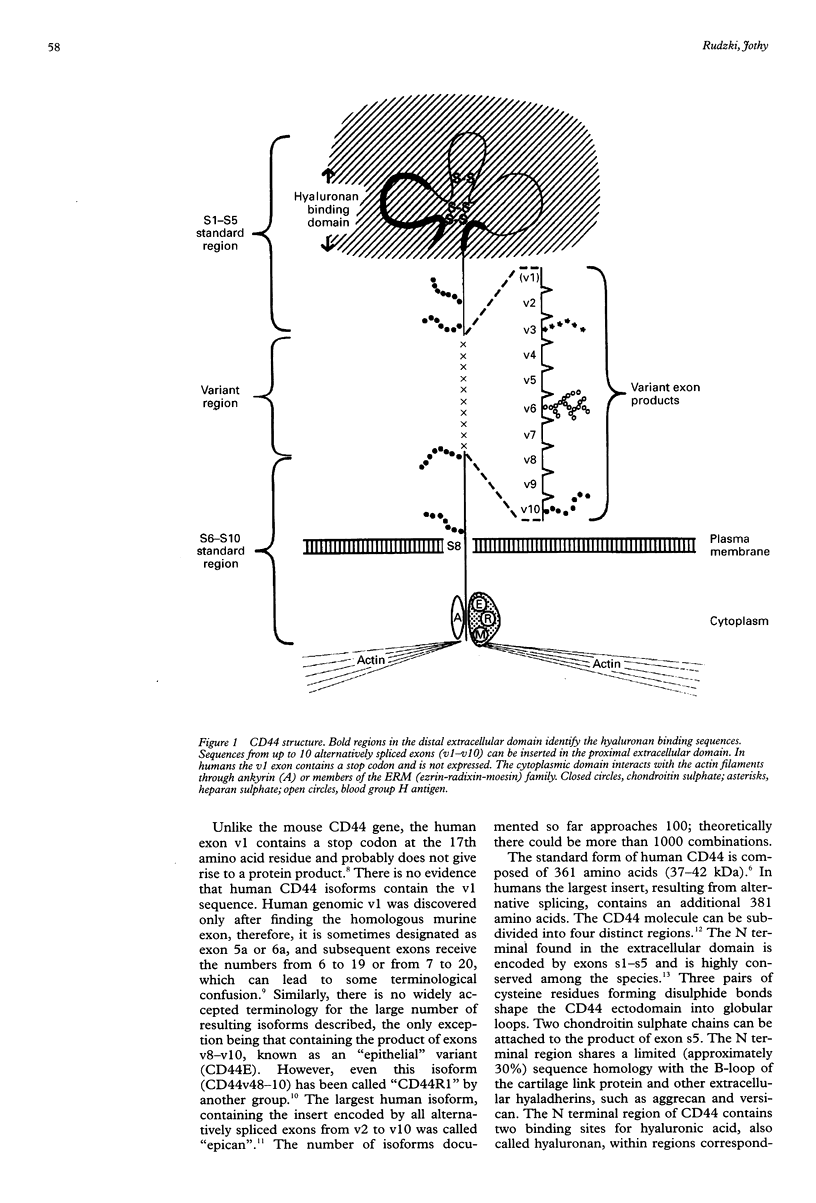
CD44 is a family of transmembrane glycoproteins that act mainly as a receptor for hyaluronan. It can also bind some other extracellular matrix ligands (chondroitin sulphate, heparan sulphate, fibronectin, serglycin, osteopontin) with lower affinity. CD44 is encoded by a single gene containing 20 exons, 10 of which (v1-v10) are variant exons inserted by alternative splicing. The standard, ubiquitously expressed isoform of CD44, does not contain sequences encoded by these variant exons. Numerous variant isoforms of CD44 containing different combinations of exons v1-v10 inserted into the extracellular domain can be expressed in proliferating epithelial cells and activated lymphocytes. CD44 plays a significant role in lymphocyte homing. Both alternative splicing and glycosylation influence receptor function of the molecule, usually reducing its affinity to hyaluronan. The cytoplasmic domain of CD44 communicates with the cytoskeleton via ankyrin and proteins belonging to the ezrin-moesin-radixin family. Relatively little is known about the intracellular events following interactions of CD44 with its ligands. Some variant isoforms, especially those containing sequences encoded by v6-v10, are overexpressed in both human and animal neoplasms. In a rat pancreatic adenocarcinoma model one of the variant CD44 isoforms was proved to be determinant in the metastatic process. For some human neoplasms (carcinomas of the digestive tract, non-Hodgkin's lymphomas, thyroid carcinomas, and others) correlations have been made between the particular pattern of CD44 variants produced by neoplastic cells and clinicopathological parameters of tumours, such as grade, stage, presence of metastases, and survival. In vitro studies indicate that modifications of CD44 expression result in different ligand recognition and influence cell motility, invasive properties, and metastatic potential of experimental tumours. Investigation of CD44 neoexpression can be useful both in early cancer diagnosis and in predicting tumour behaviour. It can also contribute to better understanding of molecular mechanisms leading to neoplastic transformation.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbasi A. M., Chester K. A., Talbot I. C., Macpherson A. S., Boxer G., Forbes A., Malcolm A. D., Begent R. H. CD44 is associated with proliferation in normal and neoplastic human colorectal epithelial cells. Eur J Cancer. 1993;29A(14):1995–2002. doi: 10.1016/0959-8049(93)90461-n. [DOI] [PubMed] [Google Scholar]
- Arch R., Wirth K., Hofmann M., Ponta H., Matzku S., Herrlich P., Zöller M. Participation in normal immune responses of a metastasis-inducing splice variant of CD44. Science. 1992 Jul 31;257(5070):682–685. doi: 10.1126/science.1496383. [DOI] [PubMed] [Google Scholar]
- Ariza A., López D., Mate J. L., Isamat M., Musulén E., Pujol M., Ley A., Navas-Palacios J. J. Role of CD44 in the invasiveness of glioblastoma multiforme and the noninvasiveness of meningioma: an immunohistochemistry study. Hum Pathol. 1995 Oct;26(10):1144–1147. doi: 10.1016/0046-8177(95)90278-3. [DOI] [PubMed] [Google Scholar]
- Bartolazzi A., Jackson D., Bennett K., Aruffo A., Dickinson R., Shields J., Whittle N., Stamenkovic I. Regulation of growth and dissemination of a human lymphoma by CD44 splice variants. J Cell Sci. 1995 Apr;108(Pt 4):1723–1733. doi: 10.1242/jcs.108.4.1723. [DOI] [PubMed] [Google Scholar]
- Bartolazzi A., Peach R., Aruffo A., Stamenkovic I. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med. 1994 Jul 1;180(1):53–66. doi: 10.1084/jem.180.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista D. S., Xuan J. W., Hota C., Chambers A. F., Harris J. F. Inhibition of Arg-Gly-Asp (RGD)-mediated cell adhesion to osteopontin by a monoclonal antibody against osteopontin. J Biol Chem. 1994 Sep 16;269(37):23280–23285. [PubMed] [Google Scholar]
- Bennett K. L., Jackson D. G., Simon J. C., Tanczos E., Peach R., Modrell B., Stamenkovic I., Plowman G., Aruffo A. CD44 isoforms containing exon V3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol. 1995 Feb;128(4):687–698. doi: 10.1083/jcb.128.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett K. L., Modrell B., Greenfield B., Bartolazzi A., Stamenkovic I., Peach R., Jackson D. G., Spring F., Aruffo A. Regulation of CD44 binding to hyaluronan by glycosylation of variably spliced exons. J Cell Biol. 1995 Dec;131(6 Pt 1):1623–1633. doi: 10.1083/jcb.131.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch M., Mitchell S., Hart I. R. Isolation and characterization of human melanoma cell variants expressing high and low levels of CD44. Cancer Res. 1991 Dec 15;51(24):6660–6667. [PubMed] [Google Scholar]
- Bourguignon L. Y., Lokeshwar V. B., Chen X., Kerrick W. G. Hyaluronic acid-induced lymphocyte signal transduction and HA receptor (GP85/CD44)-cytoskeleton interaction. J Immunol. 1993 Dec 15;151(12):6634–6644. [PubMed] [Google Scholar]
- Bourguignon L. Y., Walker G., Suchard S. J., Balazovich K. A lymphoma plasma membrane-associated protein with ankyrin-like properties. J Cell Biol. 1986 Jun;102(6):2115–2124. doi: 10.1083/jcb.102.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks L., Niedobitek G., Agathanggelou A., Farrell P. J. The expression of variant CD44 in nasopharyngeal carcinoma is unrelated to expression of LMP-1. Am J Pathol. 1995 May;146(5):1102–1112. [PMC free article] [PubMed] [Google Scholar]
- Cannistra S. A., Kansas G. S., Niloff J., DeFranzo B., Kim Y., Ottensmeier C. Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res. 1993 Aug 15;53(16):3830–3838. [PubMed] [Google Scholar]
- Chaudhry A., Gobl A., Eriksson B., Skogseid B., Oberg K. Different splice variants of CD44 are expressed in gastrinomas but not in other subtypes of endocrine pancreatic tumors. Cancer Res. 1994 Feb 15;54(4):981–986. [PubMed] [Google Scholar]
- Clarke M. R., Landreneau R. J., Resnick N. M., Crowley R., Dougherty G. J., Cooper D. L., Yousem S. A. Prognostic significance of CD44 expression in adenocarcinoma of the lung. Clin Mol Pathol. 1995 Aug;48(4):M200–M204. doi: 10.1136/mp.48.4.m200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. Human leukocyte differentiation antigens: monoclonal antibody computer databases as a tool for the future. Mol Cell Probes. 1987 Mar;1(1):61–72. doi: 10.1016/0890-8508(87)90007-7. [DOI] [PubMed] [Google Scholar]
- Combaret V., Coll J. L., Favrot M. C. Expression of integrin and CD44 adhesion molecules on neuroblastoma: the relation to tumor aggressiveness and embryonic neural-crest differentiation. Invasion Metastasis. 1994;14(1-6):156–163. [PubMed] [Google Scholar]
- Combaret V., Lasset C., Frappaz D., Bouvier R., Thiesse P., Rebillard A. C., Philip T., Favrot M. C. Evaluation of CD44 prognostic value in neuroblastoma: comparison with the other prognostic factors. Eur J Cancer. 1995;31A(4):545–549. doi: 10.1016/0959-8049(95)00027-g. [DOI] [PubMed] [Google Scholar]
- Cooper D. L., Dougherty G. J. To metastasize or not? Selection of CD44 splice sites. Nat Med. 1995 Jul;1(7):635–637. doi: 10.1038/nm0795-635. [DOI] [PubMed] [Google Scholar]
- Culty M., Shizari M., Thompson E. W., Underhill C. B. Binding and degradation of hyaluronan by human breast cancer cell lines expressing different forms of CD44: correlation with invasive potential. J Cell Physiol. 1994 Aug;160(2):275–286. doi: 10.1002/jcp.1041600209. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human brain-granulocyte-T lymphocyte antigen probably homologous to the W 3/13 antigen of the rat. Eur J Immunol. 1980 Oct;10(10):745–749. doi: 10.1002/eji.1830101004. [DOI] [PubMed] [Google Scholar]
- Dall P., Heider K. H., Hekele A., von Minckwitz G., Kaufmann M., Ponta H., Herrlich P. Surface protein expression and messenger RNA-splicing analysis of CD44 in uterine cervical cancer and normal cervical epithelium. Cancer Res. 1994 Jul 1;54(13):3337–3341. [PubMed] [Google Scholar]
- Denhardt D. T., Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993 Dec;7(15):1475–1482. [PubMed] [Google Scholar]
- Dougherty G. J., Cooper D. L., Memory J. F., Chiu R. K. Ligand binding specificity of alternatively spliced CD44 isoforms. Recognition and binding of hyaluronan by CD44R1. J Biol Chem. 1994 Mar 25;269(12):9074–9078. [PubMed] [Google Scholar]
- Driessens M. H., Stroeken P. J., Rodriguez Erena N. F., van der Valk M. A., van Rijthoven E. A., Roos E. Targeted disruption of CD44 in MDAY-D2 lymphosarcoma cells has no effect on subcutaneous growth or metastatic capacity. J Cell Biol. 1995 Dec;131(6 Pt 2):1849–1855. doi: 10.1083/jcb.131.6.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dämmrich J., Vollmers H. P., Heider K. H., Müller-Hermelink H. K. Importance of different CD44v6 expression in human gastric intestinal and diffuse type cancers for metastatic lymphogenic spreading. J Mol Med (Berl) 1995 Aug;73(8):395–401. doi: 10.1007/BF00240138. [DOI] [PubMed] [Google Scholar]
- Eibl R. H., Pietsch T., Moll J., Skroch-Angel P., Heider K. H., von Ammon K., Wiestler O. D., Ponta H., Kleihues P., Herrlich P. Expression of variant CD44 epitopes in human astrocytic brain tumors. J Neurooncol. 1995 Dec;26(3):165–170. doi: 10.1007/BF01052619. [DOI] [PubMed] [Google Scholar]
- Figge J., del Rosario A. D., Gerasimov G., Dedov I., Bronstein M., Troshina K., Alexandrova G., Kallakury B. V., Bui H. X., Bratslavsky G. Preferential expression of the cell adhesion molecule CD44 in papillary thyroid carcinoma. Exp Mol Pathol. 1994 Dec;61(3):203–211. doi: 10.1006/exmp.1994.1037. [DOI] [PubMed] [Google Scholar]
- Finke L. H., Terpe H. J., Zörb C., Haensch W., Schlag P. M. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet. 1995 Mar 4;345(8949):583–583. [PubMed] [Google Scholar]
- Finn L., Dougherty G., Finley G., Meisler A., Becich M., Cooper D. L. Alternative splicing of CD44 pre-mRNA in human colorectal tumors. Biochem Biophys Res Commun. 1994 Apr 29;200(2):1015–1022. doi: 10.1006/bbrc.1994.1551. [DOI] [PubMed] [Google Scholar]
- Friedrichs K., Franke F., Lisboa B. W., Kügler G., Gille I., Terpe H. J., Hölzel F., Maass H., Günthert U. CD44 isoforms correlate with cellular differentiation but not with prognosis in human breast cancer. Cancer Res. 1995 Nov 15;55(22):5424–5433. [PubMed] [Google Scholar]
- Fujita N., Yaegashi N., Ide Y., Sato S., Nakamura M., Ishiwata I., Yajima A. Expression of CD44 in normal human versus tumor endometrial tissues: possible implication of reduced expression of CD44 in lymph-vascular space involvement of cancer cells. Cancer Res. 1994 Jul 15;54(14):3922–3928. [PubMed] [Google Scholar]
- Gansauge F., Gansauge S., Zobywalski A., Scharnweber C., Link K. H., Nussler A. K., Beger H. G. Differential expression of CD44 splice variants in human pancreatic adenocarcinoma and in normal pancreas. Cancer Res. 1995 Dec 1;55(23):5499–5503. [PubMed] [Google Scholar]
- Goldstein L. A., Butcher E. C. Identification of mRNA that encodes an alternative form of H-CAM(CD44) in lymphoid and nonlymphoid tissues. Immunogenetics. 1990;32(6):389–397. doi: 10.1007/BF00241632. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Banting G., Wiles M. V., Tunnacliffe A., Parkar M., Solomon E., Dalchau R., Fabre J. W. The gene, MIC4, which controls expression of the antigen defined by monoclonal antibody F10.44.2, is on human chromosome 11. Eur J Immunol. 1982 Aug;12(8):659–663. doi: 10.1002/eji.1830120807. [DOI] [PubMed] [Google Scholar]
- Gorham H., Sugino T., Bolodeoku J., Yoshida K., Goodison S., Tarin D. Distribution of CD44 messenger RNA in archival paraffin wax embedded tumours and normal tissues viewed by in situ hybridisation. Clin Mol Pathol. 1996 Jun;49(3):M147–M150. doi: 10.1136/mp.49.3.m147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorham H., Sugino T., Woodman A. C., Tarin D. Cellular distribution of CD44 gene transcripts in colorectal carcinomas and in normal colonic mucosa. J Clin Pathol. 1996 Jun;49(6):482–488. doi: 10.1136/jcp.49.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross N., Beretta C., Peruisseau G., Jackson D., Simmons D., Beck D. CD44H expression by human neuroblastoma cells: relation to MYCN amplification and lineage differentiation. Cancer Res. 1994 Aug 1;54(15):4238–4242. [PubMed] [Google Scholar]
- Guo Y. J., Liu G., Wang X., Jin D., Wu M., Ma J., Sy M. S. Potential use of soluble CD44 in serum as indicator of tumor burden and metastasis in patients with gastric or colon cancer. Cancer Res. 1994 Jan 15;54(2):422–426. [PubMed] [Google Scholar]
- Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Fosang A. J. Proteoglycans: many forms and many functions. FASEB J. 1992 Feb 1;6(3):861–870. [PubMed] [Google Scholar]
- Haynes B. F., Liao H. X., Patton K. L. The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cells. 1991 Sep;3(9):347–350. [PubMed] [Google Scholar]
- He Q., Lesley J., Hyman R., Ishihara K., Kincade P. W. Molecular isoforms of murine CD44 and evidence that the membrane proximal domain is not critical for hyaluronate recognition. J Cell Biol. 1992 Dec;119(6):1711–1719. doi: 10.1083/jcb.119.6.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider K. H., Hofmann M., Hors E., van den Berg F., Ponta H., Herrlich P., Pals S. T. A human homologue of the rat metastasis-associated variant of CD44 is expressed in colorectal carcinomas and adenomatous polyps. J Cell Biol. 1993 Jan;120(1):227–233. doi: 10.1083/jcb.120.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlich P., Zöller M., Pals S. T., Ponta H. CD44 splice variants: metastases meet lymphocytes. Immunol Today. 1993 Aug;14(8):395–399. doi: 10.1016/0167-5699(93)90141-7. [DOI] [PubMed] [Google Scholar]
- Hijiya N., Setoguchi M., Matsuura K., Higuchi Y., Akizuki S., Yamamoto S. Cloning and characterization of the human osteopontin gene and its promoter. Biochem J. 1994 Oct 1;303(Pt 1):255–262. doi: 10.1042/bj3030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst E., Meijer C. J., Radaskiewicz T., van Dongen J. J., Pieters R., Figdor C. G., Hooftman A., Pals S. T. Expression of a human homing receptor (CD44) in lymphoid malignancies and related stages of lymphoid development. Leukemia. 1990 May;4(5):383–389. [PubMed] [Google Scholar]
- Ichikawa W. Positive relationship between expression of CD44 and hepatic metastases in colorectal cancer. Pathobiology. 1994;62(4):172–179. doi: 10.1159/000163907. [DOI] [PubMed] [Google Scholar]
- Iida N., Bourguignon L. Y. New CD44 splice variants associated with human breast cancers. J Cell Physiol. 1995 Jan;162(1):127–133. doi: 10.1002/jcp.1041620115. [DOI] [PubMed] [Google Scholar]
- Isacke C. M. The role of the cytoplasmic domain in regulating CD44 function. J Cell Sci. 1994 Sep;107(Pt 9):2353–2359. doi: 10.1242/jcs.107.9.2353. [DOI] [PubMed] [Google Scholar]
- Ishii S., Ford R., Thomas P., Nachman A., Steele G., Jr, Jessup J. M. CD44 participates in the adhesion of human colorectal carcinoma cells to laminin and type IV collagen. Surg Oncol. 1993 Aug;2(4):255–264. doi: 10.1016/0960-7404(93)90015-q. [DOI] [PubMed] [Google Scholar]
- Jackson D. G., Bell J. I., Dickinson R., Timans J., Shields J., Whittle N. Proteoglycan forms of the lymphocyte homing receptor CD44 are alternatively spliced variants containing the v3 exon. J Cell Biol. 1995 Feb;128(4):673–685. doi: 10.1083/jcb.128.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S. T., Bargatze R. F., Herron L. R., Butcher E. C. A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol. 1986 Oct;16(10):1195–1202. doi: 10.1002/eji.1830161003. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol. 1992 Feb;116(3):817–825. doi: 10.1083/jcb.116.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensuu H., Ristamäki R., Klemi P. J., Jalkanen S. Lymphocyte homing receptor (CD44) expression is associated with poor prognosis in gastrointestinal lymphoma. Br J Cancer. 1993 Aug;68(2):428–432. doi: 10.1038/bjc.1993.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaijk P., Troost D., Morsink F., Keehnen R. M., Leenstra S., Bosch D. A., Pals S. T. Expression of CD44 splice variants in human primary brain tumors. J Neurooncol. 1995 Dec;26(3):185–190. doi: 10.1007/BF01052621. [DOI] [PubMed] [Google Scholar]
- Kainz C., Kohlberger P., Tempfer C., Sliutz G., Gitsch G., Reinthaller A., Breitenecker G. Prognostic value of CD44 splice variants in human stage III cervical cancer. Eur J Cancer. 1995 Sep;31A(10):1706–1709. doi: 10.1016/0959-8049(95)00353-k. [DOI] [PubMed] [Google Scholar]
- Kalomiris E. L., Bourguignon L. Y. Mouse T lymphoma cells contain a transmembrane glycoprotein (GP85) that binds ankyrin. J Cell Biol. 1988 Feb;106(2):319–327. doi: 10.1083/jcb.106.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann M., Heider K. H., Sinn H. P., von Minckwitz G., Ponta H., Herrlich P. CD44 variant exon epitopes in primary breast cancer and length of survival. Lancet. 1995 Mar 11;345(8950):615–619. doi: 10.1016/s0140-6736(95)90521-9. [DOI] [PubMed] [Google Scholar]
- Kim H., Yang X. L., Rosada C., Hamilton S. R., August J. T. CD44 expression in colorectal adenomas is an early event occurring prior to K-ras and p53 gene mutation. Arch Biochem Biophys. 1994 May 1;310(2):504–507. doi: 10.1006/abbi.1994.1199. [DOI] [PubMed] [Google Scholar]
- Knudson C. B., Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993 Oct;7(13):1233–1241. [PubMed] [Google Scholar]
- Koopman G., Heider K. H., Horst E., Adolf G. R., van den Berg F., Ponta H., Herrlich P., Pals S. T. Activated human lymphocytes and aggressive non-Hodgkin's lymphomas express a homologue of the rat metastasis-associated variant of CD44. J Exp Med. 1993 Apr 1;177(4):897–904. doi: 10.1084/jem.177.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiishi I., Shizari M., Underhill C. B. CD44 can mediate the adhesion of platelets to hyaluronan. Blood. 1994 Jul 15;84(2):390–396. [PubMed] [Google Scholar]
- Kugelman L. C., Ganguly S., Haggerty J. G., Weissman S. M., Milstone L. M. The core protein of epican, a heparan sulfate proteoglycan on keratinocytes, is an alternative form of CD44. J Invest Dermatol. 1992 Dec;99(6):886–891. doi: 10.1111/1523-1747.ep12614896. [DOI] [PubMed] [Google Scholar]
- Labarrière N., Piau J. P., Otry C., Denis M., Lustenberger P., Meflah K., Le Pendu J. H blood group antigen carried by CD44V modulates tumorigenicity of rat colon carcinoma cells. Cancer Res. 1994 Dec 1;54(23):6275–6281. [PubMed] [Google Scholar]
- Laurent T. C., Fraser J. R. Hyaluronan. FASEB J. 1992 Apr;6(7):2397–2404. [PubMed] [Google Scholar]
- Lesley J., He Q., Miyake K., Hamann A., Hyman R., Kincade P. W. Requirements for hyaluronic acid binding by CD44: a role for the cytoplasmic domain and activation by antibody. J Exp Med. 1992 Jan 1;175(1):257–266. doi: 10.1084/jem.175.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J., Hyman R., Kincade P. W. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- Lesley J., Schulte R., Hyman R. Binding of hyaluronic acid to lymphoid cell lines is inhibited by monoclonal antibodies against Pgp-1. Exp Cell Res. 1990 Apr;187(2):224–233. doi: 10.1016/0014-4827(90)90085-o. [DOI] [PubMed] [Google Scholar]
- Liao H. X., Lee D. M., Levesque M. C., Haynes B. F. N-terminal and central regions of the human CD44 extracellular domain participate in cell surface hyaluronan binding. J Immunol. 1995 Oct 15;155(8):3938–3945. [PubMed] [Google Scholar]
- Lokeshwar V. B., Bourguignon L. Y. Post-translational protein modification and expression of ankyrin-binding site(s) in GP85 (Pgp-1/CD44) and its biosynthetic precursors during T-lymphoma membrane biosynthesis. J Biol Chem. 1991 Sep 25;266(27):17983–17989. [PubMed] [Google Scholar]
- Lokeshwar V. B., Bourguignon L. Y. The lymphoma transmembrane glycoprotein GP85 (CD44) is a novel guanine nucleotide-binding protein which regulates GP85 (CD44)-ankyrin interaction. J Biol Chem. 1992 Nov 5;267(31):22073–22078. [PubMed] [Google Scholar]
- Lokeshwar V. B., Fregien N., Bourguignon L. Y. Ankyrin-binding domain of CD44(GP85) is required for the expression of hyaluronic acid-mediated adhesion function. J Cell Biol. 1994 Aug;126(4):1099–1109. doi: 10.1083/jcb.126.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C. R., Terpe H. J., Stauder R., Marston W. L., Stark H., Günthert U. Expression and modulation of CD44 variant isoforms in humans. J Cell Biol. 1994 Jan;124(1-2):71–82. doi: 10.1083/jcb.124.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manten-Horst E., Danen E. H., Smit L., Snoek M., Le Poole I. C., Van Muijen G. N., Pals S. T., Ruiter D. J. Expression of CD44 splice variants in human cutaneous melanoma and melanoma cell lines is related to tumor progression and metastatic potential. Int J Cancer. 1995 Jun 22;64(3):182–188. doi: 10.1002/ijc.2910640307. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Sugiyama M., Matsumura S., Hayle A. J., Robinson P., Smith J. C., Tarin D. Unusual retention of introns in CD44 gene transcripts in bladder cancer provides new diagnostic and clinical oncological opportunities. J Pathol. 1995 Sep;177(1):11–20. doi: 10.1002/path.1711770104. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Tarin D. Significance of CD44 gene products for cancer diagnosis and disease evaluation. Lancet. 1992 Oct 31;340(8827):1053–1058. doi: 10.1016/0140-6736(92)93077-z. [DOI] [PubMed] [Google Scholar]
- Milstone L. M., Hough-Monroe L., Kugelman L. C., Bender J. R., Haggerty J. G. Epican, a heparan/chondroitin sulfate proteoglycan form of CD44, mediates cell-cell adhesion. J Cell Sci. 1994 Nov;107(Pt 11):3183–3190. doi: 10.1242/jcs.107.11.3183. [DOI] [PubMed] [Google Scholar]
- Miyake K., Medina K. L., Hayashi S., Ono S., Hamaoka T., Kincade P. W. Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow cultures. J Exp Med. 1990 Feb 1;171(2):477–488. doi: 10.1084/jem.171.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Underhill C. B., Lesley J., Kincade P. W. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med. 1990 Jul 1;172(1):69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J. W., Kruyt P. M., Sewnath M., Oosting J., Seldenrijk C. A., Weidema W. F., Offerhaus G. J., Pals S. T. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet. 1994 Nov 26;344(8935):1470–1472. doi: 10.1016/s0140-6736(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Mulder J. W., Wielenga V. J., Polak M. M., van den Berg F. M., Adolf G. R., Herrlich P., Pals S. T., Offerhaus G. J. Expression of mutant p53 protein and CD44 variant proteins in colorectal tumorigenesis. Gut. 1995 Jan;36(1):76–80. doi: 10.1136/gut.36.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S., Shimabukuro Y., Miki Y., Saho T., Hino E., Kasai D., Nozaki T., Kusumoto Y., Okada H. Inducible binding of human lymphocytes to hyaluronate via CD44 does not require cytoskeleton association but does require new protein synthesis. J Immunol. 1994 Jan 15;152(2):467–477. [PubMed] [Google Scholar]
- Naitoh H., Yazawa S., Asao T., Nakajima T., Nakamura J., Takenoshita S., Nagamachi Y. The recognition of cancer-associated fucosylated antigens in colorectal cancer by a novel monoclonal antibody, YB-2. Surg Today. 1994;24(4):382–384. doi: 10.1007/BF02348574. [DOI] [PubMed] [Google Scholar]
- Neame S. J., Isacke C. M. Phosphorylation of CD44 in vivo requires both Ser323 and Ser325, but does not regulate membrane localization or cytoskeletal interaction in epithelial cells. EMBO J. 1992 Dec;11(13):4733–4738. doi: 10.1002/j.1460-2075.1992.tb05578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottenburg C., Rees G., St John T. Isolation of mouse CD44 cDNA: structural features are distinct from the primate cDNA. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8521–8525. doi: 10.1073/pnas.86.21.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill H. C. Antibody which defines a subset of bone marrow cells that can migrate to thymus. Immunology. 1989 Sep;68(1):59–65. [PMC free article] [PubMed] [Google Scholar]
- Peach R. J., Hollenbaugh D., Stamenkovic I., Aruffo A. Identification of hyaluronic acid binding sites in the extracellular domain of CD44. J Cell Biol. 1993 Jul;122(1):257–264. doi: 10.1083/jcb.122.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penno M. B., August J. T., Baylin S. B., Mabry M., Linnoila R. I., Lee V. S., Croteau D., Yang X. L., Rosada C. Expression of CD44 in human lung tumors. Cancer Res. 1994 Mar 1;54(5):1381–1387. [PubMed] [Google Scholar]
- Perschl A., Lesley J., English N., Trowbridge I., Hyman R. Role of CD44 cytoplasmic domain in hyaluronan binding. Eur J Immunol. 1995 Feb;25(2):495–501. doi: 10.1002/eji.1830250228. [DOI] [PubMed] [Google Scholar]
- Rall C. J., Rustgi A. K. CD44 isoform expression in primary and metastatic pancreatic adenocarcinoma. Cancer Res. 1995 May 1;55(9):1831–1835. [PubMed] [Google Scholar]
- Ristamäki R., Joensuu H., Salmi M., Jalkanen S. Serum CD44 in malignant lymphoma: an association with treatment response. Blood. 1994 Jul 1;84(1):238–243. [PubMed] [Google Scholar]
- Rodriguez C., Monges G., Rouanet P., Dutrillaux B., Lefrançois D., Theillet C. CD44 expression patterns in breast and colon tumors: a PCR-based study of splice variants. Int J Cancer. 1995 Oct 20;64(5):347–354. doi: 10.1002/ijc.2910640512. [DOI] [PubMed] [Google Scholar]
- Ruiz P., Schwärzler C., Günthert U. CD44 isoforms during differentiation and development. Bioessays. 1995 Jan;17(1):17–24. doi: 10.1002/bies.950170106. [DOI] [PubMed] [Google Scholar]
- Salles G., Zain M., Jiang W. M., Boussiotis V. A., Shipp M. A. Alternatively spliced CD44 transcripts in diffuse large-cell lymphomas: characterization and comparison with normal activated B cells and epithelial malignancies. Blood. 1993 Dec 15;82(12):3539–3547. [PubMed] [Google Scholar]
- Sato N., Funayama N., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992 Sep;103(Pt 1):131–143. doi: 10.1242/jcs.103.1.131. [DOI] [PubMed] [Google Scholar]
- Screaton G. R., Bell M. V., Bell J. I., Jackson D. G. The identification of a new alternative exon with highly restricted tissue expression in transcripts encoding the mouse Pgp-1 (CD44) homing receptor. Comparison of all 10 variable exons between mouse, human, and rat. J Biol Chem. 1993 Jun 15;268(17):12235–12238. [PubMed] [Google Scholar]
- Screaton G. R., Bell M. V., Jackson D. G., Cornelis F. B., Gerth U., Bell J. I. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiter S., Arch R., Reber S., Komitowski D., Hofmann M., Ponta H., Herrlich P., Matzku S., Zöller M. Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med. 1993 Feb 1;177(2):443–455. doi: 10.1084/jem.177.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiter S., Schadendorf D., Herrmann K., Schneider M., Rösel M., Arch R., Tilgen W., Zöller M. Expression of CD44 variant isoforms in malignant melanoma. Clin Cancer Res. 1996 Mar;2(3):447–456. [PubMed] [Google Scholar]
- Senger D. R., Brown L. F., Perruzzi C. A., Papadopoulos-Sergiou A., Van de Water L. Osteopontin at the tumor/host interface. Functional regulation by thrombin-cleavage and consequences for cell adhesion. Ann N Y Acad Sci. 1995 Apr 21;760:83–100. doi: 10.1111/j.1749-6632.1995.tb44622.x. [DOI] [PubMed] [Google Scholar]
- Sherman L., Skroch-Angel P., Moll J., Schwechheimer K., Ponta H., Herrlich P., Hofmann M. Schwann cell tumors express characteristic patterns of CD44 splice variants. J Neurooncol. 1995 Dec;26(3):171–184. doi: 10.1007/BF01052620. [DOI] [PubMed] [Google Scholar]
- Sherman L., Sleeman J., Herrlich P., Ponta H. Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol. 1994 Oct;6(5):726–733. doi: 10.1016/0955-0674(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Bishop J. M. Expression of CD44 is repressed in neuroblastoma cells. Mol Cell Biol. 1991 Nov;11(11):5446–5453. doi: 10.1128/mcb.11.11.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn H. P., Heider K. H., Skroch-Angel P., von Minckwitz G., Kaufmann M., Herrlich P., Ponta H. Human mammary carcinomas express homologues of rat metastasis-associated variants of CD44. Breast Cancer Res Treat. 1995;36(3):307–313. doi: 10.1007/BF00713402. [DOI] [PubMed] [Google Scholar]
- Sliutz G., Tempfer C., Winkler S., Kohlberger P., Reinthaller A., Kainz C. Immunohistochemical and serological evaluation of CD44 splice variants in human ovarian cancer. Br J Cancer. 1995 Dec;72(6):1494–1497. doi: 10.1038/bjc.1995.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate J., Trejdosiewicz L. K., Smith B., Selby P. J. Patterns of splice variant CD44 expression by normal human urothelium in situ and in vitro and by bladder-carcinoma cell lines. Int J Cancer. 1995 Aug 9;62(4):449–456. doi: 10.1002/ijc.2910620415. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Aruffo A., Amiot M., Seed B. The hematopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyaluronate-bearing cells. EMBO J. 1991 Feb;10(2):343–348. doi: 10.1002/j.1460-2075.1991.tb07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauder R., Eisterer W., Thaler J., Günthert U. CD44 variant isoforms in non-Hodgkin's lymphoma: a new independent prognostic factor. Blood. 1995 May 15;85(10):2885–2899. [PubMed] [Google Scholar]
- Sugino T., Gorham H., Yoshida K., Bolodeoku J., Nargund V., Cranston D., Goodison S., Tarin D. Progressive loss of CD44 gene expression in invasive bladder cancer. Am J Pathol. 1996 Sep;149(3):873–882. [PMC free article] [PubMed] [Google Scholar]
- Takada M., Yamamoto M., Saitoh Y. The significance of CD44 in human pancreatic cancer: I. High expression of CD44 in human pancreatic adenocarcinoma. Pancreas. 1994 Nov;9(6):748–752. doi: 10.1097/00006676-199411000-00013. [DOI] [PubMed] [Google Scholar]
- Takada M., Yamamoto M., Saitoh Y. The significance of CD44 in human pancreatic cancer: II. The role of CD44 in human pancreatic adenocarcinoma invasion. Pancreas. 1994 Nov;9(6):753–757. doi: 10.1097/00006676-199411000-00014. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Stamenkovic I., Cutler M., Saya H., Tanabe K. K. CD44 hyaluronate binding influences growth kinetics and tumorigenicity of human colon carcinomas. Oncogene. 1995 Dec 7;11(11):2223–2232. [PubMed] [Google Scholar]
- Takeuchi K., Yamaguchi A., Urano T., Goi T., Nakagawara G., Shiku H. Expression of CD44 variant exons 8-10 in colorectal cancer and its relationship to metastasis. Jpn J Cancer Res. 1995 Mar;86(3):292–297. doi: 10.1111/j.1349-7006.1995.tb03053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K. K., Ellis L. M., Saya H. Expression of CD44R1 adhesion molecule in colon carcinomas and metastases. Lancet. 1993 Mar 20;341(8847):725–726. doi: 10.1016/0140-6736(93)90490-8. [DOI] [PubMed] [Google Scholar]
- Tanabe K. K., Stamenkovic I., Cutler M., Takahashi K. Restoration of CD44H expression in colon carcinomas reduces tumorigenicity. Ann Surg. 1995 Oct;222(4):493–503. doi: 10.1097/00000658-199510000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpe H. J., Koopmann R., Imhof B. A., Günthert U. Expression of integrins and CD44 isoforms in non-Hodgkin's lymphomas: CD44 variant isoforms are preferentially expressed in high-grade malignant lymphomas. J Pathol. 1994 Oct;174(2):89–100. doi: 10.1002/path.1711740205. [DOI] [PubMed] [Google Scholar]
- Terpe H. J., Stark H., Prehm P., Günthert U. CD44 variant isoforms are preferentially expressed in basal epithelial of non-malignant human fetal and adult tissues. Histochemistry. 1994 Feb;101(2):79–89. doi: 10.1007/BF00269353. [DOI] [PubMed] [Google Scholar]
- Terpe H. J., Störkel S., Zimmer U., Anquez V., Fischer C., Pantel K., Günthert U. Expression of CD44 isoforms in renal cell tumors. Positive correlation to tumor differentiation. Am J Pathol. 1996 Feb;148(2):453–463. [PMC free article] [PubMed] [Google Scholar]
- Thomas L., Byers H. R., Vink J., Stamenkovic I. CD44H regulates tumor cell migration on hyaluronate-coated substrate. J Cell Biol. 1992 Aug;118(4):971–977. doi: 10.1083/jcb.118.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama-Sorimachi N., Sorimachi H., Tobita Y., Kitamura F., Yagita H., Suzuki K., Miyasaka M. A novel ligand for CD44 is serglycin, a hematopoietic cell lineage-specific proteoglycan. Possible involvement in lymphoid cell adherence and activation. J Biol Chem. 1995 Mar 31;270(13):7437–7444. doi: 10.1074/jbc.270.13.7437. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Oishi K., Sato N., Sagara J., Kawai A., Tsukita S. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994 Jul;126(2):391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tölg C., Hofmann M., Herrlich P., Ponta H. Splicing choice from ten variant exons establishes CD44 variability. Nucleic Acids Res. 1993 Mar 11;21(5):1225–1229. doi: 10.1093/nar/21.5.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl-Steidl M., Müller-Holzner E., Zeimet A. G., Adolf G. R., Daxenbichler G., Marth C., Dapunt O. Prognostic value of CD44 splice variant expression in ovarian cancer. Oncology. 1995 Sep-Oct;52(5):400–406. doi: 10.1159/000227497. [DOI] [PubMed] [Google Scholar]
- Underhill C. B., Chi-Rosso G., Toole B. P. Effects of detergent solubilization on the hyaluronate-binding protein from membranes of simian virus 40-transformed 3T3 cells. J Biol Chem. 1983 Jul 10;258(13):8086–8091. [PubMed] [Google Scholar]
- Underhill C. B., Green S. J., Comoglio P. M., Tarone G. The hyaluronate receptor is identical to a glycoprotein of Mr 85,000 (gp85) as shown by a monoclonal antibody that interferes with binding activity. J Biol Chem. 1987 Sep 25;262(27):13142–13146. [PubMed] [Google Scholar]
- Underhill C. B., Thurn A. L., Lacy B. E. Characterization and identification of the hyaluronate binding site from membranes of SV-3T3 cells. J Biol Chem. 1985 Jul 5;260(13):8128–8133. [PubMed] [Google Scholar]
- Underhill C. CD44: the hyaluronan receptor. J Cell Sci. 1992 Oct;103(Pt 2):293–298. doi: 10.1242/jcs.103.2.293. [DOI] [PubMed] [Google Scholar]
- Weber G. F., Ashkar S., Glimcher M. J., Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science. 1996 Jan 26;271(5248):509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- Wielenga V. J., Heider K. H., Offerhaus G. J., Adolf G. R., van den Berg F. M., Ponta H., Herrlich P., Pals S. T. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res. 1993 Oct 15;53(20):4754–4756. [PubMed] [Google Scholar]
- Xuan J. W., Hota C., Chambers A. F. Recombinant GST-human osteopontin fusion protein is functional in RGD-dependent cell adhesion. J Cell Biochem. 1994 Feb;54(2):247–255. doi: 10.1002/jcb.240540213. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A., Saito M., Gio T., Iida A., Takeuchi K., Hirose K., Nakagawara G., Urano T., Furukawa K., Shiku H. Expression of CD44 variant exons 8-10 in gastric cancer. Jpn J Cancer Res. 1995 Dec;86(12):1166–1171. doi: 10.1111/j.1349-7006.1995.tb03310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Yang B. L., Savani R. C., Turley E. A. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994 Jan 15;13(2):286–296. doi: 10.1002/j.1460-2075.1994.tb06261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Bolodeoku J., Sugino T., Goodison S., Matsumura Y., Warren B. F., Toge T., Tahara E., Tarin D. Abnormal retention of intron 9 in CD44 gene transcripts in human gastrointestinal tumors. Cancer Res. 1995 Oct 1;55(19):4273–4277. [PubMed] [Google Scholar]
- Yoshida K., Sugino T., Bolodeoku J., Warren B. F., Goodison S., Woodman A., Toge T., Tahara E., Tarin D. Detection of exfoliated carcinoma cells in colonic luminal washings by identification of deranged patterns of expression of the CD44 gene. J Clin Pathol. 1996 Apr;49(4):300–305. doi: 10.1136/jcp.49.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Katoh S., He Q., Oritani K., Miyake K., Lesley J., Hyman R., Hamik A., Parkhouse R. M., Farr A. G. Monoclonal antibodies to CD44 and their influence on hyaluronan recognition. J Cell Biol. 1995 Jul;130(2):485–495. doi: 10.1083/jcb.130.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Toyos J., Jalkanen S., Butcher E. C. Flow cytometric analysis of the Hermes homing-associated antigen on human lymphocyte subsets. Blood. 1989 Aug 1;74(2):751–760. [PubMed] [Google Scholar]
- van Muijen G. N., Danen E. H., Veerkamp J. H., Ruiter D. J., Lesley J., van den Heuvel L. P. Glycoconjugate profile and CD44 expression in human melanoma cell lines with different metastatic capacity. Int J Cancer. 1995 Apr 10;61(2):241–248. doi: 10.1002/ijc.2910610217. [DOI] [PubMed] [Google Scholar]