Abstract
Background
HIV is highly stigmatized, compromising both treatment and prevention in resource-limited settings.
Purpose
To study the relationship between internalized HIV-related stigma and serostatus disclosure and to determine the extent to which this association varies with the degree of social distance.
Methods
We fit multivariable Poisson regression models, with cluster-correlated robust estimates of variance, to data from 259 persons with HIV enrolled in an ongoing cohort study in rural Uganda.
Results
Persons with more internalized stigma were less likely to disclose their seropositivity. The magnitude of association increased with social distance such that the largest association was observed for public disclosures and the smallest association was observed for disclosures to sexual partners.
Conclusions
Among persons with HIV in rural Uganda, internalized stigma was negatively associated with serostatus disclosure. The inhibiting effect of stigma was greatest for the most socially distant ties.
Keywords: HIV, social stigma, disclosure, Uganda
Introduction
The process through which persons with HIV disclose their seropositivity to others is a complex psychosocial challenge with important personal and public health implications. From a public health perspective, disclosure to serodiscordant sexual partners has been described as a “prosocial” behavior that facilitates conversations about protected sexual intercourse to prevent secondary transmission of HIV (1, 2). Several observational studies conducted in sub-Saharan Africa have linked non-disclosure of seropositivity to HIV transmission risk behaviors, including unprotected sexual intercourse and multiple partnering (3–6). Consistent with these studies, one mathematical modeling exercise suggested that increasing serostatus disclosure at a population level could reduce HIV transmission risks by as much as 40 percent (7).
People with HIV may realize personal benefits when disclosing their serostatus to other social ties enhances their access to social support (8–10). The extent to which social support improves adaptive coping and psychosocial management of HIV has been well documented (11–18). These benefits are even more important in resource-limited settings where formal safety nets are limited or absent and social relationships serve as an important source of risk-sharing and informal insurance (19, 20). In one multi-country qualitative study, people with HIV in Nigeria, Tanzania, and Uganda were found to rely heavily on social ties to overcome conditions of resource scarcity and achieve sustained HIV treatment adherence (21).
Potentially undermining these substantive public health and personal benefits is the stigma attached to HIV in many countries worldwide (22–27). HIV-related stigma is a multifaceted construct, conceptualized as a devalued or discredited status that society attaches to specific conditions or attributes, such as HIV or mental illness (28). The primary focus of this study was internalized HIV-related stigma, or the extent to which persons with HIV accept their discredited status as valid and develop self-defacing internal representations of themselves (29).
Conceptual Model
The hypothesized negative effects of stigma may be understood through a model of psychological inhibition (30), in which people with HIV feel they cannot disclose their seropositivity to others for fear of the potentially unpleasant consequences of revealing their discredited status. As described conceptually by Alonzo and Reynolds (31), in the setting of social stigma, persons with HIV may delay disclosure until HIV disease progression renders further concealment impossible. These hypotheses are borne out in cross-sectional studies conducted in several sub-Saharan African countries, where the stigma of HIV is frequently cited as a barrier to disclosure (32) and where persons with HIV who perceive greater stigma are less likely to disclose their seropositivity to others (4, 33, 34). Although no studies of people with HIV in resource-limited settings have controlled for measures of health status, two U.S.-based studies showed that asymptomatic men were less likely to disclose their seropositivity to close social ties compared to symptomatic men who had experienced considerable disease progression (35, 36).
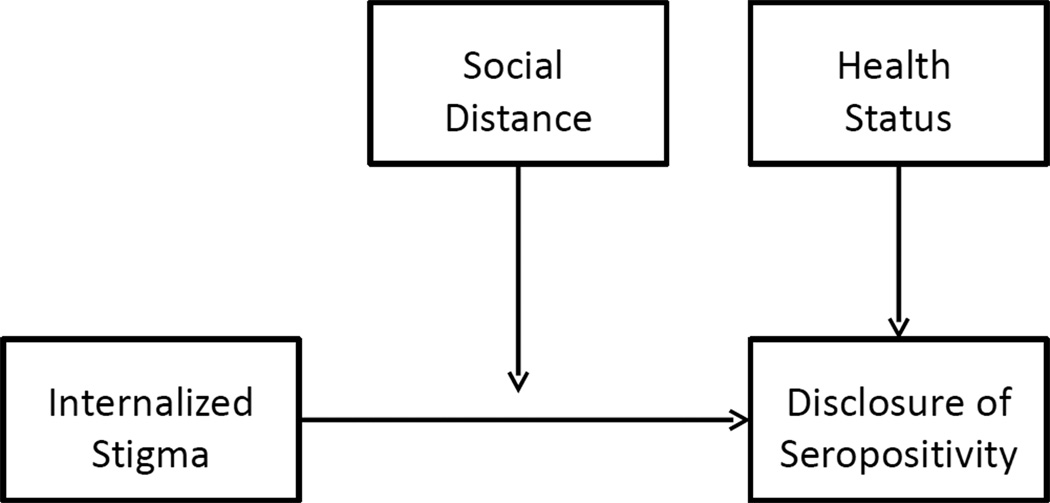
If psychological inhibition is a substantive explanation for why social stigma undermines disclosure of HIV seropositivity, then the inhibiting effects should be expected to attenuate with increasing social distance. Social distance is typically conceptualized along various dimensions, such as affectivity, or subjective feelings towards others (37); collectively recognized norms, such as bonds of kinship (38); or frequency of interaction (39). Thus, at one end of the continuum, complete strangers would be considered socially distant ties. At the other extreme, socially proximate ties might be exemplified by Goffman (28)’s “wise” persons, or those who have accepted the stigmatized person despite his or her devalued status: “the marginal men before whom the individual with a fault need feel no shame nor exert self-control, knowing that in spite of his failing he will be seen as an ordinary other” (p.42). Although previous work in this area has differentiated between disclosures to different types of social ties (8, 35, 36, 40), no studies have investigated the extent to which the inhibiting effects of stigma on disclosure vary across different types of social ties. Our conceptual discussion here is summarized in Figure 1.
Figure 1.
Conceptual model of internalized stigma, social distance, and disclosure of HIV seropositivity
Hypotheses
The present study departs from previous work in its use of longitudinal data from sub-Saharan Africa to estimate the association between HIV-related stigma and disclosure of seropositivity to sexual partners and other social ties, and in its assessment of how the strength of the association varies according to social distance. Consistent with the empirical evidence reviewed above, we hypothesized that persons with a greater degree of internalized stigma would be less likely to disclose their seropositivity. Following the previous conceptual work on social distance, we hypothesized that the magnitude of the stigma-disclosure relationship would be greater with increasing social distance, i.e., internalized stigma would have the least inhibiting effects on disclosures to sexual partners and friends, and the most inhibiting effects on more socially distant ties.
Methods
Study population, design, and data collection
Mbarara District is located in a rural area of Uganda southwest of Kampala and is reachable by a five-hour automobile drive. Mbarara town (population 82,000) is the primary commercial hub, but the majority of district residents live in outlying rural areas. The local economy is largely based on subsistence agriculture, and food insecurity is common, especially among people with HIV (20, 41, 42). In this setting, considerable stigma is attached to HIV/AIDS (43–45). Data for this study were drawn from the Uganda AIDS Rural Treatment Outcomes (UARTO) study, a cohort of treatment-naïve persons with HIV initiating antiretroviral therapy who were recruited from the Mbarara Immune Suppression Syndrome Clinic. All participants provided written informed consent, either with a signature or, if there were cultural literacy reasons why a signature was not appropriate, a thumbprint. Upon enrolling in the study, participants were seen every 3–4 months for blood draws and structured interviews to assess changes in health status and psychosocial wellbeing. Interviews took place in a private office near the clinic. Study interviews were conducted in a private research office located near the clinic. Questionnaires were administered by research assistants with Bachelor’s degrees who spoke the local language (Runyankore). Midway through the study, while recruitment of new treatment-naïve participants into UARTO was still ongoing, assessments for internalized HIV-related stigma were added to the survey instrument. Because HIV treatment itself may be associated with declines in stigma (46, 47), the analytic sample was limited to only those participants enrolled after the stigma assessments were added, i.e., so that the first stigma assessment for each included participant would represent a treatment-naïve baseline. Consistent with local etiquette and custom, at the conclusion of each interview participants were offered a nominal incentive for their time, such as 1 kg of sugar or a bar of soap. Ethical approval for all study procedures was obtained from the Committee on Human Research, University of California at San Francisco; the Partners Human Research Committee, Massachusetts General Hospital; and the Institutional Review Committee, Mbarara University of Science and Technology.
Primary outcomes
The primary outcomes were disclosure to social ties of varying degrees of social distance. Persons who reported being sexually active with a primary sexual partner in the previous three months were asked if they had verbally disclosed their seropositivity to that partner. All persons were asked if in the previous three months they had verbally disclosed their seropositivity to a family member, friend, or neighbor, or if they had made a public disclosure. All persons were also asked if in the previous three months they had disclosed their seropositivity to a religious leader. Finally, at each assessment participants were asked to estimate, on a five-point Likert scale (ranging from “no one” to “everyone”), the number of people in their households whom they believed knew about their seropositivity.
Explanatory variables
Internalized HIV-related stigma was measured with the Internalized AIDS-Related Stigma Scale, a six-item scale originally developed for use among persons with HIV in the U.S., South Africa, and Swaziland (48) and subsequently shown to have a coherent internal structure and good reliability and construct validity among persons with HIV in rural Uganda (49). Higher scores on this scale indicate a greater degree of internalized stigma.
There were several different measures of health status. The structured interview questionnaire inquired about whether or not the participant had experienced in the previous month any of 29 potentially HIV-related symptoms, such as “enlarged bumps in your neck, armpits, or groin,” “problems with weight loss or wasting,” or “change in the way your body looks such as fat deposits or weight gain.” The extent to which the participant found each symptom bothersome was scored on a four-point Likert-type scale ranging from 1 (“not at all”) to 4 (“a lot”). Responses to the 29 questions were used to create an equally weighted average of the z-scores (50), with the sign of the measure oriented so that greater values of the HIV-related symptom index are associated with a greater symptom burden. Health-related quality of life was measured with the Medical Outcomes Study-HIV Health Survey (MOS-HIV) mental health summary and physical health summary scores. The MOS-HIV consists of 35 items grouped into 11 domains, with higher mental and physical health summary scores reflecting a better health-related quality of life (51). The individual domains are scored as summated rating scales from 0–100, and the overall mental and physical health summary scores are transformed to t-scores with a mean of 50 and a standard deviation of 10.
Statistical analysis
All statistical analyses were conducted using the Stata/MP software package (version 12.0, StataCorp LP, College Station, Tex.). Poisson regression models were fit to the pooled data, with internalized stigma as the exposure of interest and each of the binary disclosure outcomes analyzed in a separate regression model. Pooling the data allowed for multiple disclosures over time. For example, a participant could have reported disclosing to a friend and a neighbor in the three months prior to baseline, reported not making any disclosures at the next quarterly interview, then reported disclosing to another friend at the third quarterly interview, etc. This is consistent with prior work describing HIV serostatus disclosure as a lifelong process that may involve selective disclosure and repeated disclosure decisions to be made over time (40, 52). Because nearly all participants (433 [95.0%]) had disclosed to a family member at baseline, predictors of subsequent disclosure to family members were not analyzed. Given the binary nature of the dependent variable, the exponentiated regression coefficients were interpreted as risk ratios rather than as incidence rate ratios (53, 54).
Because participants’ estimates of the number of people in their households whom they believed knew about their seropositivity was scored on a five-point Likert scale, this outcome was modeled as an ordinal dependent variable. Because the Brant (55) test revealed violations of the proportional odds assumption (χ2=67.3, P<0.001), an unconstrained partial proportional odds regression model was employed to relax the proportional odds constraint (56). As with a conventional ordered logistic regression model, the exponentiated coefficients associated with the exposure variables are interpreted as the odds that the respondent is in a higher category of the dependent variable (i.e., more household members knowing their serostatus).
In addition to the health status variables described above, the estimates were also adjusted for socio-demographic variables that have previously been shown to be associated with HIV serostatus disclosure, including age (33), sex (26, 27), educational attainment (26), marital status (57), alcohol use (58), and economic well-being (59). Hazardous drinking was defined as a positive screen on the three-item consumption subset of the Alcohol Use Disorders Identification Test (60). Economic well-being was derived from principal components analysis applied to a series of 25 binary variables for household-owned assets and housing characteristics, with higher values of the unitless index indicating greater asset wealth (61). All socio-demographic variables were considered time-varying with the exception of sex, educational attainment, and economicwell being, which were measured at baseline only.
Cluster-correlated robust estimates of variance were employed throughout, in order to correct standard errors for clustering of observations within study participants over time (62–64) and, for the Poisson regression models, to relax the assumption that the outcome data were derived from a Poisson distribution (54, 65). Covariates were lagged by three months in order to ensure a temporal lag between the exposures and the outcomes. Average marginal effects were calculated in order to aid exposition with regards to the magnitudes of the estimated associations (66). We also calculated predictive margins (67) to estimate the probability of disclosure at different levels of internalized stigma, with the other explanatory variables evaluated at their mean values.
Additional regression models were used to explore the robustness of the initial findings. First, because prior disclosures may affect the likelihood of subsequent disclosures (57), a binary variable indicating whether the participant had disclosed their seropositivity to anyone in the previous three months was added to the regression models. Second, to assess for potential variation in the primary exposure over time, duration of treatment was added to the regression models, and the statistical significance of the interaction terms between internalized stigma and duration of treatment was tested. Third, because the stigma of HIV is often layered over other inequities in resource-limited settings and because the effects of internalized stigma may vary by gender depending on the setting (26, 27), the statistical significance of the interaction terms between internalized stigma and gender were tested.
Results
The sample consisted of 259 treatment-naïve persons with HIV initiating ART. They were followed from August 2007 to July 2010, for a median duration of follow-up of 1.8 years (mean, 1.6 years; standard deviation, 0.6 years; interquartile range, 1.4–2.1 years). Nineteen participants were lost to follow up (7.4%), and 14 participants died (5.5%). Baseline summary characteristics of the sample are displayed in Table 1. At baseline, among 125 participants who reported being sexually active with a primary sexual partner, 83 (66%) had disclosed their seropositivity to that partner. Among all participants, the proportion of participants who had disclosed their seropositivity at baseline varied by type of social tie: family member (245 [95%]), friend (191 [74%]), neighbor (101 [39%]), and religious leader (42 [16%]). Only 11 participants (4%) reported having made a public disclosure. Approximately one-half of participants (144 [55%]) stated that they believed “most people” or “everyone” in their household knew about their seropositivity. At baseline, the median value of the internalized stigma scale was 1 (mean, 1.2; standard deviation, 1.7; interquartile range, 0–2). More than one-half of participants (163 [63%]) endorsed at least one item of the internalized stigma scale.
Table 1.
Baseline characteristics of the sample (N=259)
| Median (interquartile range) or No. (%) | |
|---|---|
| Age, years | 34 (28–40) |
| Time since diagnosis (years) | 0.7 (0.3–1.8) |
| Women | 173 (67%) |
| Achieved secondary education or more | 56 (22%) |
| Married | 112 (43%) |
| Household asset wealth * | −0.6 (−1.5 to 1.1) |
| Positive screen for hazardous drinking † | 32 (12%) |
| Physical health summary score ‡ | 45 (35–53) |
| Mental health summary score ¶ | 43 (35–51) |
| HIV-related symptom index § | 0.3 (−0.1 to 0.8) |
Derived from principal components analysis applied to a series of 25 binary variables for household-owned assets and housing characteristics (61)
Positive screen on the three-item consumption subset of the Alcohol Use Disorders Identification Test (60)
Physical health summary score of the Medical Outcomes Study-HIV Health Survey (MOS-HIV) (51)
Mental health summary score of the MOS-HIV
Derived from equally weighted responses to questions about 29 potentially HIV-related symptoms (50)
In univariable regression models, internalized stigma had a negative and statistically significant association with serostatus disclosure. The relative risks varied with social distance, ranging from 0.92 (95% confidence interval [CI], 0.87–0.98) for disclosures to primary sexual partners to 0.69 (95% CI, 0.60–0.81) for public disclosures. With regards to the health status variables, greater HIV-related symptom burden was negatively associated with disclosures to primary sexual partners (relative risk [RR], 0.78; 95% CI, 0.62–0.98) but not with other disclosures, and neither of the MOS-HIV summary scores had statistically significant univariable associations with any of the outcomes.
After multivariable adjustment, the primary associations of interest changed little. As shown in Table 2, the estimated adjusted relative risks [ARR] ranged from 0.94 (95% CI, 0.90–0.99) for disclosures to primary sexual partners and 0.70 (95% CI, 0.61–0.80) for public disclosures. Contrary to what was hypothesized on the basis of previous findings, neither HIV-related symptoms nor the MOS-HIV summary scores had statistically significant multivariable-adjusted associations with any of the outcomes.
Table 2.
Correlates of serostatus disclosure, across different types of social ties varying in social proximity
| Adjusted relative risk (95% confidence interval) |
|||||
|---|---|---|---|---|---|
| To sexual partner | To friend | To neighbor | To religious leader | Public | |
| Internalized stigma scale, per point | 0.94 (0.90–0.99) | 0.96 (0.94–0.98) | 0.89 (0.86–0.93) | 0.79 (0.73–0.86) | 0.70 (0.61–0.80) |
| Age, per 5 years | 0.99 (0.93–1.05) | 1.02 (1.00–1.04) | 1.05 (1.02–1.09) | 1.16 (1.09–1.24) | 1.26 (1.13–1.42) |
| Female | 0.89 (0.73–.109) | 1.03 (0.96–1.10) | 1.11 (0.98–1.26) | 1.37 (0.98–1.91) | 1.26 (0.80–2.00) |
| Achieved secondary education | 1.06 (0.86–1.31) | 0.95 (0.88–1.03) | 0.77 (0.64–0.93) | 0.88 (0.60–1.28) | 0.87 (0.52–1.47) |
| Married | 2.93 (1.99–4.32) | 1.00 (0.95–1.06) | 0.91 (0.81–1.02) | 0.82 (0.63–1.08) | 0.83 (0.56–1.24) |
| Household asset wealth | 0.98 (0.93–1.03) | 0.99 (0.98–1.00) | 0.97 (0.94–1.00) | 0.93 (0.85–1.01) | 0.89 (0.80–0.99) |
| Positive screen for hazardous drinking | 1.10 (0.85–1.42) | 1.04 (0.96–1.13) | 0.98 (0.85–1.14) | 0.78 (0.52–1.17) | 0.97 (0.56–1.65) |
| MOS-HIV physical health summary score | 1.00 (0.99–1.01) | 1.00 (0.99–1.00) | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 1.01 (0.99–1.03) |
| MOS-HIV mental health summary score | 1.00 (0.99–1.01) | 1.00 (0.99–1.00) | 1.00 (0.99–1.00) | 0.99 (0.98–1.01) | 0.99 (0.97–1.02) |
| Symptom burden | 0.83 (0.64–1.08) | 0.99 (0.94–1.05) | 1.05 (0.93–1.18) | 1.13 (0.88–1.45) | 1.21 (0.75–1.96) |
Expressed in terms of average marginal effects, each additional point on the internalized stigma scale was associated with a 3.5 (95% CI, 1.5–5.5) to 8.4 (95% CI, 5.4–11.4) percentage point lower probability of disclosure, depending on the type of social tie (Table 3). These effects were large in magnitude relative to the baseline probabilities of disclosure. As can be observed from the difference in average predicted probabilities of disclosure evaluated at the lowest vs. highest stigma scores, the inhibiting effect of internalized stigma was smallest for disclosures to sexual partners and friends and largest for disclosures to neighbors and religious leaders, and for public disclosures.
Table 3.
Magnitudes and moderators of the association between internalized stigma and disclosure
| To sexual partner | To friend | To neighbor | To religious leader | Public | |
|---|---|---|---|---|---|
| Average marginal effect† (95% confidence interval [CI]) | −0.04 (−0.01 to −0.07) | −0.04 (−0.01 to −0.05) | −0.08 (−0.05 to −0.10) | −0.08 (−0.05 to −0.11) | −0.05 (−0.03 to −0.07) |
| Predicted probability of disclosure‡ (95% CI) | |||||
| No stigma | 0.65 (0.58–0.71) | 0.95 (0.92–0.97) | 0.77 (0.73–0.81) | 0.45 (0.39–0.51) | 0.20 (0.16–0.24) |
| Highest stigma score | 0.46 (0.34–0.58) | 0.75 (0.66–0.85) | 0.39 (0.30–0.48) | 0.11 (0.06–0.16) | 0.02 (0.01–0.04) |
| Stigma × treatment duration, z-score | 1.02 | 0.63 | 1.48 | 0.59 | 1.41 |
| Stigma × gender, z-score | 2.57 | 0.25 | 0.82 | 0.92 | 0.36 |
The average marginal effect represents the change in the probability of disclosure per one-point change in the internalized stigma scale.
The predicted probability of disclosure represents the probability of disclosure at different levels of internalized stigma, with the other explanatory variables evaluated at their mean values.
Other important patterns were identified in the data. The association between marital status and disclosure was statistically significant only for disclosures to sexual partners (ARR, 2.93; 95% CI, 1.99–4.32). Household asset wealth was negatively associated with disclosure, but the association was statistically significant only for public disclosures (ARR, 0.89; 95% CI, 0.80–0.99). Inclusion of prior disclosure in the regression models did not change the statistical significance of the primary estimates. The interaction terms between stigma and treatment duration were not statistically significant. The interaction term for gender was statistically significant only for disclosures to sexual partners, but the differences in magnitude were small: each additional point on the internalized stigma scale was associated with a 3.8 (95% CI, 0.6–7.0) percentage point lower probability of disclosure by men and 3.4 (95% CI, 0.5–6.2) percentage point lower probability of disclosure by women.
Participants with a greater degree of internalized stigma were also more likely to have disclosed to a lower proportion of household members (odds ratio [OR], 0.72; 95% CI, 0.66–0.78). Among persons with the lowest possible level of internalized stigma, the average predicted probability of being in the lowest category of household disclosure (“no one knows”) was 1.1 percent (95% CI, 0.6–1.7) and the predicted probability of being in the highest category (“everyone knows”) was 72.4 percent (95% CI, 68.2–76.5). In comparison, among persons with the maximum internalized stigma score, the predicted probability of being in the lowest category was 7.7 percent (95% CI, 3.0–12.4), and the predicted probability of being in the highest category was 26.7 percent (95% CI, 17.8–35.6). With multivariable adjustment, the estimated effect of internalized stigma remained statistically significant (adjusted odds ratio [AOR], 0.65; 95% CI, 0.53–0.78).
Discussion
In this analysis of data from a cohort of persons with HIV initiating treatment in rural Uganda, persons with a greater degree of internalized stigma were less likely to disclose their seropositivity to social ties of all types. The public health implications of our findings about nondisclosure to sexual partners are especially relevant to sub-Saharan African settings where nondisclosure has been linked to HIV transmission risk behaviors (3–6) and where a large proportion of new heterosexually transmitted HIV infections among adults occur in the context of serodiscordant marriages or cohabiting relationships (68, 69). The discussion about how best to support persons with HIV in disclosing their seropositivity to serodiscordant sexual partners has assumed even greater public health significance in light of recently published data showing efficacy of early treatment initiation to reduce sexual transmission of HIV between men and women in stable, serodiscordant partnerships who were willing to disclose their serostatus to each other (70).
This study additionally demonstrated that internalized stigma inhibited disclosure of seropositivity to intimate social ties other than sexual partners. Moreover, the magnitudes of the estimated associations between internalized stigma and serostatus disclosure generally declined with social distance: namely, the inhibiting effect of internalized stigma was greatest for public disclosures vs. disclosures to sexual partners or friends. Improved policy and programming in the area of stigma reduction is also likely to improve overall well being for persons with HIV given that disclosure of seropositivity is a pivotal step in accessing social support (8–10), which is itself linked to improvements in HIV treatment adherence (11, 13–15, 17, 21). Relationships with more distal social ties may serve qualitatively different functions compared to intimate ties, such as providing different opportunities for social engagement (71) or access to material resources (39, 72). These benefits underscore their significance as well as the importance of better understanding the public health implications of encouraging disclosure to more distal social ties.
Given the paucity of longitudinal studies in this area, our findings provide strong support for the imperative of developing effective anti-stigma interventions in resource-limited settings. Most anti-stigma intervention studies have generally emphasized information delivery or counseling-based approaches targeted towards the general public, i.e., people presumed not to be infected with HIV. A primary implication of our study, however, is that interventions to encourage disclosures of seropositivity based solely on informational approaches alone are unlikely to be effective (73). These education-based behavioral interventions should be packaged with interventions to specifically address the stigma of HIV. Given that the stigma of HIV is a multifaceted construct and enabled by differential power relations between stigmatized and nonstigmatized groups (74), new multipronged approaches to stigma reduction in resource-limited settings are clearly needed.
Castro and Farmer (75) have advanced the argument that “structural violence determines, in large part, who suffers from AIDS-related stigma and discrimination” (p.55). This argument is consistent with the experiences of people with HIV in resource-limited settings such as Uganda, where norms of economic reciprocity serve as a form of informal insurance (76–78): the economic impacts of HIV/AIDS have exacerbated both instrumental and symbolic stigma (79, 80). The stigma of HIV is both symbolic (81), in that it derives from the widely assumed association between HIV and economic incapacity/physical disability, and instrumental (82–84), in that it serves to exclude economically inadequate persons from participation in an interdependent economy of mutual benefit. Some observers have speculated that economic strengthening or livelihoods interventions may play a role in reducing HIV-related stigma (79, 85). These hypotheses have not been formally tested but are consistent with qualitative research conducted in Nigeria, Tanzania, and Uganda by Ware, Idoko, Kaaya et al. (21), who conclude that the prospect of being excluded from solidarity networks is a primary reason why the stigma of HIV is so feared.
Relatively uncommon in the literature are intervention studies where disclosure of HIV serostatus has been emphasized as a primary outcome (73). It should be acknowledged, however, that the outcomes of HIV serostatus disclosure are not unambiguously positive. Disclosure may result in positive health or social outcomes, as in the literature reviewed previously, but due to the stigma of HIV often the reactions of significant others can be mixed or even negative. People with HIV in a wide range of countries have reported negative or even violent reactions after disclosing their serostatus to sexual partners and other intimate ties (25, 35, 86). In order to avoid undesirable outcomes, interventions targeting disclosure behaviors should be sensitive to these potential negative consequences.
Interpretation of the study findings is subject to several limitations. First, the structured questionnaire did not inquire as to the identity of the specific persons to whom the participant had made a disclosure at each point in time. It could therefore not be determined whether participants who made disclosures subsequent to treatment initiation were describing the same or different persons. In general, random measurement error in the dependent variable would tend to bias the estimates towards the null. However, if more stigmatized participants were more likely to respond affirmatively while also interpreting the question as referring to disclosures to the same person over time, this would bias the estimates away from the null. On its face, the joint probability of this differential response and differential interpretation was unlikely to have occurred. Furthermore, the unconstrained partial proportional odds regression model employed an ordinal dependent variable that was not subject to the same type of potential measurement error. The consistency between these two analyses thus increases our confidence in the validity of our primary analysis. Second, differential loss to follow-up could have affected the estimates. If participants who were lost to follow-up were both more stigmatized and less likely to disclose their seropositivity to others, this could have biased the estimates away from the null. However, relatively few participants in the study were lost to follow up. Those who were ultimately lost to follow-up had a similar baseline degree of internalized stigma and pattern of disclosures compared to those not lost to follow up (with P-values for equality-of-medians and chi-squared tests ranging from 0.24–0.82), allaying concerns about this potential source of bias. Third, we imposed on the data our conceptualization of social distance. Surveying participants about the emotional intensity of their bonds to different types of social ties would have yielded a more direct measure of tie strength (87, 88). Fourth, reduced self-efficacy for disclosing seropositivity may simply be a part of the construct of internalized stigma. If so, the outcomes would be, by definition, incorporated into the exposures, again biasing the estimates away from the null. However, this is unlikely to be the case given prior work on the factor structure of HIV-related stigma, which has demonstrated negative self-image and disclosure concerns to be two separate factors (89). Furthermore, the finding that the intensity of the stigma/non-disclosure relationship was smallest for more proximal social ties suggests that the primary exposure variable measured a construct that is conceptually distinct from the outcome. Fifth, we fit separate regression models for disclosures to each type of social tie. Doing so amounts to assuming that the error terms are uncorrelated across equations, or that a person who is inclined to disclose his or her seropositivity to one type of social tie is no more likely to disclose to another type of social tie. Combining information across equations would likely have resulted in a gain in efficiency, suggesting that the estimates presented here are biased towards the null.
In summary, this study found that internalized HIV-related stigma among persons with HIV in rural Uganda may hinder HIV prevention efforts by inhibiting disclosure of seropositivity to sexual partners, and that it may compromise overall health and well-being by inhibiting disclosure to significant social ties. Furthermore, the magnitude of the stigma-disclosure relationship intensified with social distance, as the inhibiting effect of stigma was greatest for the most socially distant ties. Interventions that successfully reduce internalized stigma among persons with HIV may have a significant public health impact in rural Uganda and other similar resource-limited settings.
ACKNOWLEDGMENTS
We thank the Uganda AIDS Rural Treatment Outcomes (UARTO) study participants who made this study possible by sharing their experiences; Nozmo F.B. Mukiibi for his contributions to study design and implementation; and Annet Kembabazi and Annet Kawuma for providing study coordination and administrative support. While these individuals are acknowledged for their assistance, no endorsement of manuscript contents or conclusions should be inferred. A preliminary version of this analysis was presented in part at the 18th Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, USA, March 2, 2011.
SOURCE OF FUNDING: This study was funded by U.S. National Institutes of Health R01 MH-054907, K23 MH-079713 and MH-079713-03S1, and P30 AI-027763. Additionally, the authors acknowledge the following sources of salary support: K23 MH-096620 (Tsai); K24 MH-087227 (Bangsberg); a Scholar Award through the Harvard University Center for AIDS Research (P30 AI-060354), the Eleanor and Miles Shore 50th Anniversary Fellowship Program for Scholars in Medicine, the Harvard Global Health Institute, and K23 MH-097667 (Katz); K23 MH-087228 (Haberer); and the Burke Family Foundation (Weiser).
Footnotes
Conflict of Interest Statement
The authors have no conflict of interest to disclose
REFERENCES
- 1.Duru OK, Collins RL, Ciccarone DH, et al. Correlates of sex without serostatus disclosure among a national probability sample of HIV patients. AIDS Behav. 2006;10:495–507. doi: 10.1007/s10461-006-9089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klitzman R, Exner T, Correale J, et al. It's not just what you say: relationships of HIV dislosure and risk reduction among MSM in the post-HAART era. AIDS Care. 2007;19:749–756. doi: 10.1080/09540120600983971. [DOI] [PubMed] [Google Scholar]
- 3.Loubiere S, Peretti-Watel P, Boyer S, Blanche J, Abega SC, Spire B. HIV disclosure and unsafe sex among HIV-infected women in Cameroon: results from the ANRS-EVAL study. Soc Sci Med. 2009;69:885–891. doi: 10.1016/j.socscimed.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 4.Simbayi LC, Kalichman SC, Strebel A, Cloete A, Henda N, Mqeketo A. Disclosure of HIV status to sex partners and sexual risk behaviours among HIV-positive men and women, Cape Town, South Africa. Sex Transm Infect. 2007;83:29–34. doi: 10.1136/sti.2006.019893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisele TP, Mathews C, Chopra M, et al. High levels of risk behavior among people living with HIV Initiating and waiting to start antiretroviral therapy in Cape Town South Africa. AIDS Behav. 2008;12:570–577. doi: 10.1007/s10461-007-9279-7. [DOI] [PubMed] [Google Scholar]
- 6.Kalichman SC, Ntseane D, Nthomang K, Segwabe M, Phorano O, Simbayi LC. Recent multiple sexual partners and HIV transmission risks among people living with HIV/AIDS in Botswana. Sex Transm Infect. 2007;83:371–375. doi: 10.1136/sti.2006.023630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinkerton SD, Galletly CL. Reducing HIV transmission risk by increasing serostatus disclosure: a mathematical modeling analysis. AIDS Behav. 2007;11:698–705. doi: 10.1007/s10461-006-9187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalichman SC, DiMarco M, Austin J, Luke W, DiFonzo K. Stress, social support, and HIV-status disclosure to family and friends among HIV-positive men and women. J Behav Med. 2003;26:315–332. doi: 10.1023/a:1024252926930. [DOI] [PubMed] [Google Scholar]
- 9.Holt R, Court P, Vedhara K, Nott KH, Holmes J, Snow MH. The role of disclosure in coping with HIV infection. AIDS Care. 1998;10:49–60. doi: 10.1080/09540129850124578. [DOI] [PubMed] [Google Scholar]
- 10.Paxton S. The paradox of public HIV disclosure. AIDS Care. 2002;14:559–567. doi: 10.1080/09540120208629674. [DOI] [PubMed] [Google Scholar]
- 11.DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23:207–218. doi: 10.1037/0278-6133.23.2.207. [DOI] [PubMed] [Google Scholar]
- 12.Hansen NB, Vaughan EL, Cavanaugh CE, Connell CM, Sikkema KJ. Health-related quality of life in bereaved HIV-positive adults: relationships between HIV symptoms, grief, social support, and Axis II indication. Health Psychol. 2009;28:249–257. doi: 10.1037/a0013168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simoni JM, Frick PA, Huang B. A longitudinal evaluation of a social support model of medication adherence among HIV-positive men and women on antiretroviral therapy. Health Psychol. 2006;25:74–81. doi: 10.1037/0278-6133.25.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychol. 2000;19:124–133. [PubMed] [Google Scholar]
- 15.Gonzalez JS, Penedo FJ, Antoni MH, et al. Social support, positive states of mind, and HIV treatment adherence in men and women living with HIV/AIDS. Health Psychol. 2004;23:413–418. doi: 10.1037/0278-6133.23.4.413. [DOI] [PubMed] [Google Scholar]
- 16.Weaver KE, Llabre MM, Duran RE, et al. A stress and coping model of medication adherence and viral load in HIV-positive men and women on highly active antiretroviral therapy (HAART) Health Psychol. 2005;24:385–392. doi: 10.1037/0278-6133.24.4.385. [DOI] [PubMed] [Google Scholar]
- 17.Koenig LJ, Pals SL, Bush T, Pratt Palmore M, Stratford D, Ellerbrock TV. Randomized controlled trial of an intervention to prevent adherence failure among HIV-infected patients initiating antiretroviral therapy. Health Psychol. 2008;27:159–169. doi: 10.1037/0278-6133.27.2.159. [DOI] [PubMed] [Google Scholar]
- 18.Kalichman SC, Benotsch EG, Weinhardt L, Austin J, Luke W, Cherry C. Health-related Internet use, coping, social support, and health indicators in people living with HIV/AIDS: preliminary results from a community survey. Health Psychol. 2003;22:111–116. doi: 10.1037//0278-6133.22.1.111. [DOI] [PubMed] [Google Scholar]
- 19.Dercon S. Income risk, coping strategies, and safety nets. World Bank Research Observer. 2002;17:141–166. [Google Scholar]
- 20.Tsai AC, Bangsberg DR, Emenyonu N, Senkungu JK, Martin JN, Weiser SD. The social context of food insecurity among persons living with HIV/AIDS in rural Uganda. Soc Sci Med. 2011;73:1717–1724. doi: 10.1016/j.socscimed.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ware NC, Idoko J, Kaaya S, et al. Explaining adherence success in sub-Saharan Africa: an ethnographic study. PLoS Med. 2009;6:e11. doi: 10.1371/journal.pmed.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalichman SC, Simbayi LC, Jooste S, et al. Development of a brief scale to measure AIDS-related stigma in South Africa. AIDS Behav. 2005;9:135–143. doi: 10.1007/s10461-005-3895-x. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe WR, Weiser SD, Bangsberg DR, et al. Effects of HIV-related stigma among an early sample of patients receiving antiretroviral therapy in Botswana. AIDS Care. 2006;18:931–933. doi: 10.1080/09540120500333558. [DOI] [PubMed] [Google Scholar]
- 24.Roura M, Urassa M, Busza J, Mbata D, Wringe A, Zaba B. Scaling up stigma? The effects of antiretroviral roll-out on stigma and HIV testing. Early evidence from rural Tanzania. Sex Transm Infect. 2009;85:308–312. doi: 10.1136/sti.2008.033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medley A, Garcia-Moreno C, McGill S, Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother-to-child transmission programmes. Bull World Health Organ. 2004;82:299–307. [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MB, Wu Z, Rotheram-Borus MJ, Detels R, Guan J, Li L. HIV-related stigma among market workers in China. Health Psychol. 2005;24:435–438. doi: 10.1037/0278-6133.24.4.435. [DOI] [PubMed] [Google Scholar]
- 27.Swendeman D, Rotheram-Borus MJ, Comulada S, Weiss R, Ramos ME. Predictors of HIV-related stigma among young people living with HIV. Health Psychol. 2006;25:501–509. doi: 10.1037/0278-6133.25.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goffman E. Stigma: notes on the management of spoiled identity. Englewood Cliffs: Prentice Hall; 1963. [Google Scholar]
- 29.Jones EE, Farina A, Hastorf AH, Markus H, Miller DT, Scott RA. Social stigma: the psychology of marked relationships. New York: W.H. Freeman & Company; 1984. [Google Scholar]
- 30.Pennebaker JW. Confession, inhibition, and disease. In: Berkowitz L, editor. Advances in experimental social psychology. vol 22. Orlando: Academic Press; 1989. pp. 211–244. [Google Scholar]
- 31.Alonzo AA, Reynolds NR. Stigma, HIV and AIDS: an exploration and elaboration of a stigma trajectory. Soc Sci Med. 1995;41:303–315. doi: 10.1016/0277-9536(94)00384-6. [DOI] [PubMed] [Google Scholar]
- 32.Norman A, Chopra M, Kadiyala S. Factors related to HIV disclosure in 2 South African communities. Am J Public Health. 2007;97:1775–1781. doi: 10.2105/AJPH.2005.082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vu L, Andrinopoulos K, Mathews C, Chopra M, Kendall C, Eisele TP. Disclosure of HIV status to sex partners among HIV-infected men and women in Cape Town, South Africa. AIDS Behav. 2012;16:132–138. doi: 10.1007/s10461-010-9873-y. [DOI] [PubMed] [Google Scholar]
- 34.Patel R, Ratner J, Gore-Felton C, Kadzirange G, Woelk G, Katzenstein D. HIV disclosure patterns, predictors, and psychosocial correlates among HIV positive women in Zimbabwe. AIDS Care. 2012;24:358–368. doi: 10.1080/09540121.2011.608786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansergh G, Marks G, Simoni JM. Self-disclosure of HIV infection among men who vary in time since seropositive diagnosis and symptomatic status. AIDS. 1995;9:639–644. doi: 10.1097/00002030-199506000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Hays RB, McKusick L, Pollack L, Hilliard R, Hoff C, Coates TJ. Disclosing HIV seropositivity to significant others. AIDS. 1993;7:425–431. doi: 10.1097/00002030-199303000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Bogardus ES. Measurement of personal-group relations. Sociometry. 1947;10:306–311. [Google Scholar]
- 38.Karakayali N. Social distance and affective orientations. Sociol Forum. 2009;24:538–562. [Google Scholar]
- 39.Granovetter M. The strength of weak ties. Am J Sociol. 1973;78:1360–1380. [Google Scholar]
- 40.Mayfield Arnold E, Rice E, Flannery D, Rotheram-Borus MJ. HIV disclosure among adults living with HIV. AIDS Care. 2008;20:80–92. doi: 10.1080/09540120701449138. [DOI] [PubMed] [Google Scholar]
- 41.Weiser SD, Tsai AC, Gupta R, et al. Food insecurity is associated with morbidity and patterns of healthcare utilization among HIV-infected individuals in rural Uganda AIDS. 2012;26:67–75. doi: 10.1097/QAD.0b013e32834cad37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai AC, Bangsberg DR, Frongillo EA, et al. Food insecurity, depression and the modifying role of social support among people living with HIV/AIDS in rural Uganda. Soc Sci Med. 2012;74:2012–2019. doi: 10.1016/j.socscimed.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyrod R. Masculinity and the persistence of AIDS stigma. Cult Health Sex. 2011;13:443–456. doi: 10.1080/13691058.2010.542565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nattabi B, Li J, Thompson SC, Orach CG, Earnest J. Between a rock and a hard place: stigma and the desire to have children among people living with HIV in northern Uganda. J Int AIDS Soc. 2012;15:17421. doi: 10.7448/IAS.15.2.17421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGrath JW, Ankrah EM, Schumann DA, Nkumbi S, Lubega M. AIDS and the urban family: its impact in Kampala, Uganda. AIDS Care. 1993;5:55–70. doi: 10.1080/09540129308258584. [DOI] [PubMed] [Google Scholar]
- 46.Zuch M, Lurie M. 'A virus and nothing else': the effect of ART on HIV-related stigma in rural South Africa. AIDS Behav. 2012;16:564–570. doi: 10.1007/s10461-011-0089-6. [DOI] [PubMed] [Google Scholar]
- 47.Campbell C, Skovdal M, Madanhire C, Mugurungi O, Gregson S, Nyamukapa C. "We, the AIDS people.…": how antiretroviral therapy enables Zimbabweans living with HIV/AIDS to cope with stigma. Am J Public Health. 2011;101:1004–1010. doi: 10.2105/AJPH.2010.202838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalichman SC, Simbayi LC, Cloete A, Mthembu PP, Mkhonta RN, Ginindza T. Measuring AIDS stigmas in people living with HIV/AIDS: the Internalized AIDS-Related Stigma Scale. AIDS Care. 2009;21:87–93. doi: 10.1080/09540120802032627. [DOI] [PubMed] [Google Scholar]
- 49.Tsai AC, Weiser SD, Steward WT, et al. Evidence for the reliability and validity of the internalized AIDS-related stigma scale in rural Uganda. AIDS Behav. 2013;17:427–433. doi: 10.1007/s10461-012-0281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kling JR, Liebman JB, Katz LF. Experimental analysis of neighborhood effects. Econometrica. 2007;75:83–119. [Google Scholar]
- 51.Wu AW, Rubin HR, Mathews WC, et al. A health status questionnaire using 30 items from the Medical Outcomes Study. Preliminary validation in persons with early HIV infection. Med Care. 1991;29:786–798. doi: 10.1097/00005650-199108000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Obermeyer CM, Baijal P, Pegurri E. Facilitating HIV disclosure across diverse settings: a review. Am J Public Health. 2011;101:1011–1023. doi: 10.2105/AJPH.2010.300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gourieroux C, Monfort A, Trognon A. Pseudo maximum likelihood methods: Applications to Poisson models. Econometrica. 1984;52:701–720. [Google Scholar]
- 54.Carter RE, Lipsitz SR, Tilley BC. Quasi-likelihood estimation for relative risk regression models. Biostatistics. 2005;6:39–44. doi: 10.1093/biostatistics/kxh016. [DOI] [PubMed] [Google Scholar]
- 55.Brant R. Assessing proportionality in the proportional odds model for ordinal logistic regression. Biometrics. 1990;46:1171–1178. [PubMed] [Google Scholar]
- 56.Peterson BL, Harrell FE. Partial proportional dods models for ordinal response variables. Appl Statist. 1990;39:205–217. [Google Scholar]
- 57.Antelman G, Smith Fawzi MC, Kaaya S, et al. Predictors of HIV-1 serostatus disclosure: a prospective study among HIV-infected pregnant women in Dar es Salaam, Tanzania. AIDS. 2001;15:1865–1874. doi: 10.1097/00002030-200109280-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olley BO, Seedat S, Stein DJ. Self-disclosure of HIV serostatus in recently diagnosed patients with HIV in South Africa. Afr J Reprod Health. 2004;8:71–76. [PubMed] [Google Scholar]
- 59.Suzan-Monti M, Blanche J, Bile P, et al. Individual and structural factors associated with HIV status disclosure to main partner in Cameroon: ANRS 12–116 EVAL survey, 2006–2007. J Acquir Immune Defic Syndr. 2011;57(Suppl 1):S22–S26. doi: 10.1097/QAI.0b013e31821fcfa8. [DOI] [PubMed] [Google Scholar]
- 60.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 61.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data -- or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 62.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 63.Froot KA. Consistent covariance matrix estimation with cross-sectional dependence and heteroskedasticity in financial data. J Fin Quant Analysis. 1989;24:333–355. [Google Scholar]
- 64.Rogers WH. Regression standard errors in clustered samples. Stata Tech Bull. 1993;13:19–23. [Google Scholar]
- 65.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 66.Bartus T. Estimation of marginal effects using margeff. Stata J. 2005;5:309–329. [Google Scholar]
- 67.Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12:308–331. [Google Scholar]
- 68.Dunkle KL, Stephenson R, Karita E, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371:2183–2191. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 69.Chemaitelly H, Cremin I, Shelton J, Hallett TB, Abu-Raddad LJ. Distinct HIV discordancy patterns by epidemic size in stable sexual partnerships in sub-Saharan Africa. Sex Transm Infect. 2012;88:51–57. doi: 10.1136/sextrans-2011-050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berkman LF, Glass T, Brissette I, Seeman TE. From social integration to health: Durkheim in the new millennium. Soc Sci Med. 2000;51:843–857. doi: 10.1016/s0277-9536(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 72.Granovetter M. Getting a job. Cambridge: Harvard University Press; 1974. [Google Scholar]
- 73.Wolitski RJ, Gomez CA, Parsons JT. Effects of a peer-led behavioral intervention to reduce HIV transmission and promote serostatus disclosure among HIV-seropositive gay and bisexual men. AIDS. 2005;19(Suppl 1):S99–S109. doi: 10.1097/01.aids.0000167356.94664.59. [DOI] [PubMed] [Google Scholar]
- 74.Link BG, Phelan JC. Conceptualizing stigma. Annu Rev Sociol. 2001;27:363–385. [Google Scholar]
- 75.Castro A, Farmer P. Understanding and addressing AIDS-related stigma: from anthropological theory to clinical practice in Haiti. Am J Public Health. 2005;95:53–59. doi: 10.2105/AJPH.2003.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fafchamps M. Solidarity networks in preindustrial societies: rational peasants with a moral economy. Econ Dev Cult Change. 1992;41:147–174. [Google Scholar]
- 77.Fafchamps M, Lund S. Risk-sharing networks in rural Philippines. J Dev Econ. 2003;71:261–287. [Google Scholar]
- 78.de Weerdt J, Dercon S. Risk-sharing networks and insurance against illness. J Dev Econ. 2006;81:337–356. [Google Scholar]
- 79.Reidpath DD, Chan KY, Gifford SM, Allotey P. 'He hath the French pox': stigma, social value and social exclusion. Sociol Health Illn. 2005;27:468–489. doi: 10.1111/j.1467-9566.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- 80.Neuberg SL, Smith SM, Asther T. Why people stigmatize: toward a biocultural framework. In: Heatherton TF, Kleck RE, Hebl MR, Hull JG, editors. The social psychology of stigma. New York: The Guilford Press; 2000. pp. 31–61. [Google Scholar]
- 81.Pryor JB, Reeder GD, Vinacco R, Kott TL. The instrumental and symbolic functions of attitudes towards persons with AIDS. J Appl Soc Psychol. 1989;19:377–404. [Google Scholar]
- 82.Katz D. The functional approach to the study of attitudes. Public Opin Quart. 1960;24:163–204. [Google Scholar]
- 83.Sears DO, Hensler CP, Speer LK. Whites' opposition to "busing": self-interest or symbolic politics? Am Pol Sci Rev. 1979;73:369–384. [Google Scholar]
- 84.McConahay JB, Hough JC. Symbolic racism. J Soc Issues. 1976;32:23–45. [Google Scholar]
- 85.Goudge J, Ngoma B, Manderson L, Schneider H. Stigma, identity and resistance among people living with HIV in South Africa. SAHARA J. 2009;6:94–104. doi: 10.1080/17290376.2009.9724937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simoni JM, Mason HR, Marks G, Ruiz MS, Reed D, Richardson JL. Women's self-disclosure of HIV infection: rates, reasons, and reactions. J Consult Clin Psychol. 1995;63:474–478. doi: 10.1037//0022-006x.63.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marsden PV, Campbell KE. Measuring tie strength. Soc Forces. 1984;63:482–501. [Google Scholar]
- 88.Marsden PV, Campbell KE. Reflections on conceptualizing and measuring tie strength. Soc Forces. 2012;91:17–23. [Google Scholar]
- 89.Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24:518–529. doi: 10.1002/nur.10011. [DOI] [PubMed] [Google Scholar]