Abstract
Transplanted bone marrow-derived mononuclear cells (BMMNCs) can promote arteriogenesis and angiogenesis by incorporating into vascular walls and differentiating into smooth muscle cells (SMCs) and endothelial cells (ECs). Here, we explored whether BMMNCs can enhance arteriogenesis and angiogenesis and promote long-term functional recovery in a rat model of permanent middle cerebral artery occlusion (pMCAO). Sprague-Dawley rats were injected with vehicle or 1×107 BMMNCs labeled with BrdU via femoral vein 24 h after induction of pMCAO. Functional deficits were assessed weekly through day 42 after pMCAO, and infarct volume was assessed on day 7. We visualized the angioarchitecture by latex perfusion on days 14 and 42. BMMNC transplantation significantly reduced infarct volume and neurologic functional deficits compared with untreated or vehicle-treated ischemic groups. In BMMNC-treated rats, BrdU-positive cells were widely distributed in the infarct boundary zone, were incorporated into vessel walls, and enhanced the growth of leptomeningeal anastomoses, the circle of Willis, and basilar arteries. BMMNCs were shown to differentiate into SMCs and ECs from day 14 after stroke and preserved vascular repair function for at least 6 weeks. Our data indicate that BMMNCs can significantly enhance arteriogenesis and angiogenesis, reduce infarct volume, and promote long-term functional recovery after pMCAO in rats.
Keywords: angiogenesis, arteriogenesis, BMMNCs, cerebral ischemia, functional recovery, infarct volume
1. Introduction
After ischemic stroke, blood supply to the injured brain is decreased, leading to dysfunction of the brain tissue in that area. This type of stroke is usually precipitated by progressive atherosclerosis or an embolus from the heart or neck vessels (Shuaib et al., 2011). The only treatment currently available for ischemic stroke is thrombolytic therapy. However, it would be very beneficial to be able to restore anterograde perfusion of the ischemic territory through arterial revascularization (Bang et al., 2011; Shuaib et al., 2011).
The circle of Willis, leptomeningeal anastomoses, and microvasculature in the peri-infarction zone are important paths of collateral perfusion for the injured brain tissue (Carmeliet and Jain, 2011; Shuaib et al., 2011). Promoting additional collateral circulation may represent a potential treatment for ischemic stroke (Carmeliet and Jain, 2011; Shuaib et al., 2011). Collateral vessels bring bulk flow to ischemic cerebral tissues and may be sufficient to replace the stroke-induced loss of perfusion (Bang et al., 2011; Shuaib et al., 2011). Establishment of collateral circulation and new capillaries can be achieved by neovasculogenesis, which refers to the development of a new vascular network and comprises two different processes: arteriogenesis and angiogenesis (Ruiz-Salmeron et al., 2011; Troidl and Schaper, 2012). Arteriogenesis occurs when preexisting collateral arterioles transform into functional collateral arteries, whereas angiogenesis is the expansion of the vascular network via sprouting in the ischemic border (Carmeliet and Jain, 2011; Sugiyama et al., 2011). Evidence suggests that therapeutic arteriogenesis and angiogenesis can be stimulated by cell transplantation (Hao et al., 2011; Imada et al., 2005; Li et al., 2011; Ruiz-Salmeron et al., 2011; Yoon et al., 2010).
One cell type that might be useful for promoting collateral vessel growth through autologous or allogeneic transplantation is the bone marrow mononuclear cell (BMMNC). The arteriogenic effects of BMMNCs have been confirmed in limb ischemia and myocardial infarction (Imada et al., 2005; Li et al., 2011; Ruiz-Salmeron et al., 2011; Yoon et al., 2010). Transplanted BMMNCs also have been shown to cross the blood-brain barrier and to convert into neuronal and glial cell types in a cerebral ischemic animal model (Iihoshi et al., 2004; Zhang et al., 2011). Additionally, BMMNCs can promote functional recovery by decreasing neurodegeneration, promoting angiogenesis, reducing the level of proinflammatory cytokines such as tumor necrosis factor-α, and releasing numerous trophic factors such as nerve growth factor (Brenneman et al., 2010; Giraldi-Guimaraes et al., 2009; Hao et al., 2011; Ribeiro-Resende et al., 2009).
Although bone marrow harvest and reinfusion of autologous BMMNCs has been shown to be feasible and safe in patients with acute ischemic stroke (Savitz et al., 2011), no study has examined whether transplanted BMMNCs can promote arteriogenesis (especially for leptomeningeal anastomoses) and whether they can incorporate into the adult vasculature. We hypothesized that BMMNCs would promote neovasculogenesis by differentiating into vessel cells [smooth muscle cells (SMCs) and endothelial cells (ECs)] in a rat model of ischemic stroke. In the current study, we focused on testing the arteriogenic effects of BMMNCs and their direct impact on the prognosis of ischemic stroke. We found that transplantation of BMMNCs induced arteriogenesis and angiogenesis, alleviated ischemic brain damage, and promoted long-term functional recovery. To the best of our knowledge, this is the first report of using BMMNCs to promote cerebral arteriogenesis in a rat model of ischemic stroke.
2. Materials and methods
2.1. Animals and experimental groups
Adult male Sprague-Dawley rats (300–310 g, 3 months old; supplied by the Animal Experimental Center of Zhengzhou University) were randomly divided into four groups: sham-operated group (n=26), untreated ischemic group (n=34), vehicle-treated ischemic group (n=36), and BMMNC-treated ischemic group (n=54). Rats were maintained in accordance with the guidelines of the NIH (Guide for the Care and Use of Laboratory Animals, 1976). All protocols were approved by the Animal Care and Use Committee of Zhengzhou University. All rats were given free access to food and water throughout the study.
2.2. Permanent middle cerebral artery (MCA) occlusion (pMCAO)
We induced pMCAO by intraluminal vascular occlusion (Wang et al., 2010) and used laser Doppler to assess regional cerebral blood flow (CBF) as described previously (Chen et al., 2009). Briefly, rats were anesthetized with an intraperitoneal injection of 4% (350 mg/kg) chloral hydrate. Rectal temperature was maintained at 37±0.5°C throughout the surgical procedure by means of a feedback-regulated water-heating system. An incision was made in the scalp to expose the skull. For detection of CBF on the MCAO side, the fiber optic probe (tip diameter, 0.5 mm) of a computer-controlled laser Doppler flowmeter (moorVMS-LDF, Moor Instruments Ltd., UK) was fixed to the surface of the skull on the left side (2 mm posterior, 6 mm lateral to bregma). Then, the left common carotid artery, external carotid artery, and internal carotid artery were isolated through a midline incision. The ipsilateral internal carotid artery was occluded with Heifetz aneurysm clips. To block the origin of the MCA, we inserted a nylon wire (0.22–0.24 mm in diameter, 18.0–19.5 mm in length) with a rounded tip from the left common carotid artery into the lumen of the internal carotid artery through a small incision. Successful MCAO was defined as ≥80% decrease in CBF and was confirmed by laser-Doppler flowmetry. Sham-operated rats were subjected to the same surgical procedure except that the wire was not inserted. Untreated rats were subjected to pMCAO only. Twenty-four hours after pMCAO, cell-treated rats were anesthetized with 4% (350 mg/kg) chloral hydrate, and blunt dissection was used to isolate the femoral vein. A 26-gauge needle was inserted into the lumen of the vein, and approximately 10 million BMMNCs in 300 μL total fluid volume of phosphate-buffered saline (PBS) were delivered over a 3-min period based on a previous study (Yang et al., 2011). Vehicle-treated rats were injected with an equal volume of PBS. After completion of the injection, the syringe was withdrawn and pressure was applied to the femoral vein for approximately 1 min to stop bleeding. Skin was sutured after thorough disinfection.
2.3. BMMNC preparation
5-Bromo-2′-deoxyuridine (BrdU, B5002, Sigma-Aldrich, St Louis, MO, USA) was used as a tracer for newly formed DNA of bone marrow cells. We injected adult male Sprague-Dawley rats intraperitoneally with BrdU (50 mg/kg) once daily for 14 days before harvesting the bone marrow (Li et al., 2001). BMMNCs were isolated as previously described (Giraldi-Guimaraes et al., 2009; Ribeiro-Resende et al., 2009). The rats were anesthetized by an intraperitoneal injection of 4% chloral hydrate and then sacrificed. Their femurs were aseptically dissected and both ends cut; bone marrow was extruded with serum-free DMEM F12. The extracted bone marrow was subjected to density-gradient centrifugation (260g, 25 min) in 1.083 g/mL Histopaque 1083 (Sigma-Aldrich). The mononuclear cell layer was recovered from the gradient interface and washed with DMEM F12 in three consecutive steps of centrifugation (5 min) and resuspension. After cells were counted and the viability checked, BMMNCs were resuspended in cold, sterile PBS to a final concentration of 3×107 cells/mL and used immediately for implantation. This process required approximately 2 h.
2.4. Fluorescence-activated cell sorting (FACS) analysis
To evaluate the purity of the isolated bone marrow cells as putative BMMNCs, we subjected a portion of the cell pellet to FACS analysis to determine the expression of various immunophenotypic markers. Thus, approximately 1×107 cells of each rat (n=5) were incubated in 2% fetal bovine serum in PBS at 4°C for 30 min with 1 μL of monoclonal antibody specific for CD34 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), CD45, CD90 (BD Biosciences, San Jose, CA, USA), and CD117 (Abcam, Cambridge, MA, USA). To confirm the labeling rate, we incubated some cells with 2 M HCl to depurinate the DNA. Cells were washed with PBS containing 0.1% fetal bovine serum albumin and 0.2% Tween-20 and incubated with 1 μL of anti-BrdU-fluorescein isothiocyanate (BD Biosciences) for 30 min (Montes et al., 2011). As a negative control, some cells were incubated in buffer without primary antibodies.
2.5. Tests of neurologic function and body weight
An investigator blinded to treatment group tested the rats on days 1, 7, 14, 21, 28, 35, and 42 after pMCAO or sham surgery with the modified neurological severity score (mNSS; n=12/group) (Narantuya et al., 2010). The mNSS comprises tests of motor function, sensory function, balance, reflex, and general movement on a scale of 0 to 22 (0 = no deficit, 22 = maximal deficit). In motor function tests, the rats are raised by their tail to evaluate the flexion of limbs and head movements in the vertical axis, or they are placed on a flat surface for gait analysis. In sensory function tests, the rats are examined for visual and tactile functions, and a proprioception test is performed by pushing the paw against the table edge to stimulate limb muscles. Balance is tested by placing the rats on a slim beam and counting the number of limbs that fall from the beam and the time before the rat falls off the beam. Reflexes tested were the pinna reflex, corneal reflex, and startle reflex. The pinna reflex is tested by touching the auditory meatus and observing whether the rat shakes his head. The corneal reflex is tested by lightly stimulating the cornea with cotton and observing whether the rat blinks. The startle reflex is tested by observing whether the rat has a motor response to a sudden loud noise. Movement is assessed by recording the presence of seizures, myoclonus, or myodystony. Body weights were measured on the same days. Body weight was expressed as percent change according to the formula: percent change in body weight = (body weight at each time point – body weight before surgery)/body weight before surgery × 100% (Wu et al., 2011).
2.6. Measurement of infarct volume
Rat brains (n=6/group) were excised and cut into five 2-mm-thick coronal sections from the frontal pole. Sections were quickly stained with 2% 2,3,5-triphenyltetrazolium-chloride (TTC) in normal saline at 37°C for 30 min and then stored in phosphate-buffered 4% paraformaldehyde (PFA) overnight before analysis. These brain sections were rinsed in 4% PFA and imaged by a digital camera (Canon E40D, Tokyo, Japan) as described previously (Zhang et al., 2011). The area of damaged parenchyma (unstained tissue) was measured on the anterior surface of each slice with Sigma Scan Pro 5.0 (SPSS Science). Each infarct area was then multiplied by the ratio of the surface of the infarcted (ipsilateral) hemisphere to the intact (contralateral) hemisphere at the same level to correct for brain swelling. The lesion volumes were calculated by the following equation: percent hemispheric lesion volume = [total infarct volume − (left hemisphere volume − right hemisphere volume)]/right hemisphere volume × 100% (Wang et al., 2010).
2.7. Visualization of cerebral vessels by latex perfusion
We perfused the rats with latex to visualize the leptomeningeal anastomoses, the circle of Willis, and basilar artery on days 14 and 42 after stroke (n=6/group/time point) as previously reported (Sugiyama et al., 2011). The rats were anesthetized with 4% chloral hydrate, and the right atrium of the heart was incised to allow venous outflow. The aorta was cannulated and injected at 150 mmHg first with 5 mL of saline and then with 5 mL of latex compound (Zhengzhou Latex Product Inc, Zhengzhou, China), which was a mixture of white latex and a small amount of carbon black (50 μL/mL, Boss Inc, Zhengzhou, China). Then the brain was removed carefully from the skull, and photographs were taken of the dorsal and ventral brain surfaces. The distal MCA was identified from its peripheral branch angle. We counted the number of vessels and measured the diameter of the leptomeningeal anastomoses at the point of confluence where the distal MCA joins the distal anterior cerebral artery or posterior cerebral artery. The vessel diameter of the circle of Willis was measured just proximal to the point at which the left anterior cerebral artery diverges from the olfactory artery (Sugiyama et al., 2011). All measurements were made with ImageJ 1.46 software by an investigator blinded to experimental groups.
2.8. Immunofluorescence double staining
Because transplanted allogeneic cells are eliminated from the heart by day 35 after implantation (Huang et al., 2010), we sacrificed the rats treated with allogeneic BMMNCs on days 7, 14, 28, and 42 after pMCAO to explore the long-term fate and therapeutic effects of BMMNCs after stroke. Brain specimens (n=6/time point) were cut into 12-μm sections via cryoultramicrotomy and then were treated with 2 M HCl for 30 min and washed before being incubated with blocking buffer. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 5 min, and nonspecific binding was blocked in blocking buffer (5% normal goat serum, 0.3% Triton X in PBS) for 1 h at room temperature. Mouse anti-BrdU monoclonal antibody (BBI, Shanghai, China), rabbit anti-rat polyclonal antibody against smooth muscle actin (α-SMA; BBI), and rabbit anti-rat polyclonal antibody against CD31 (BBI) were used as primary antibodies at a dilution of 1:250. Sections were incubated in primary antibodies overnight at 4°C and then with FITC-conjugated goat anti-mouse antibody (Zhongshan Inc., Beijing, China) or TRITC-conjugated goat anti-rabbit antibody (Zhongshan Inc.) at 1:250 dilution for 2 h at room temperature for visualization of antibody binding. PBS was used as a negative control for immunohistochemical stains (Shimada et al., 2010). Photomicrographs were obtained with an epifluorescence deconvolution microscope (Olympus CKX41, Olympus, Japan).
2.9. Microvessel density and BrdU-positive cell measurement
We double stained tissues for BrdU and α-SMA or CD31 to measure microvessel density and assess transplanted BMMNC differentiation (Zacharek et al., 2009). Briefly, microvessels that had a diameter < 10 μm, were CD31-positive, and were located in the infarct boundary zone were defined as capillaries; microvessels that had a diameter > 10 μm, were α-SMA-positive, and were located in the infarct boundary zone and cortical surface of the ipsilateral side in each section were defined as arterioles. Cells that were positive for BrdU and α-SMA were counted as SMCs, and cells that were positive for BrdU and CD31 were counted as ECs. An investigator blinded to treatment group counted cells in three sections per rat and four fields per section. Data are presented as cells/mm2.
2.10. Statistical analysis
Rats that did not have at least 80% decrease in CBF, that had an mNSS score <12 or >16 on day 1 after pMCAO, or that did not survive for a sufficient time were excluded from the study. SPSS 12.0 was used for statistical analysis of the data. All data are presented as mean ± standard error of mean (SEM). The neurologic deficit score and body weight changes were analyzed by two-way analysis of variance (ANOVA) for repeated measures. A Chi-square test was used for analyzing mortality. Other measurements were analyzed by one-way ANOVA followed by the LSD test. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Characterization of the BMMNC population
The BMMNCs were composed of different cellular populations. The means ± SEM for the immunophenotypic markers of BMMNCs were CD34 (14.9±0.5%), CD45 (84.4±0.5%), CD90 (10.0±0.28%), and CD117 (13.5±0.3%) (n=5 rats). The BrdU incorporation assays revealed that 65.5±2.8% of cells were BrdU-positive.
3.2. Mortality, neurologic functional recovery, and body weight changes
The mortality on day 42 was 0 (0/26) in the sham group, 23.5% (8/34) in the untreated pMCAO group, 27.8% (10/36) in the vehicle-treated pMCAO group, and 22.2% (12/54) in the BMMNC-treated pMCAO group. Mortality rate did not differ significantly among the three pMCAO groups (p>0.05). Most deaths occurred within the first 72 h after pMCAO. Three rats died within 24 h and two died between 48 and 72 h in the untreated group; four rats died within 24 h and two died between 24 and 48 h in the vehicle-treated group; five rats died within 24 h and three died between 48 and 72 h in the BMMNC-treated group. Before ischemia, rats exhibited no neurologic deficits. All rats showed significant decline in neurologic function after pMCAO and an increase in neurologic deficit score. After 7 days, the neurologic deficit scores of rats treated with BMMNCs were significantly lower than those of untreated and vehicle-treated rats (F = 4.154, p<0.05, n=12 rats/group; Fig. 1A). The body weight of rats that underwent pMCAO decreased rapidly during the first 7 days and then increased slowly. The body weight of BMMNC-treated rats recovered to baseline level at 14 days after pMCAO, whereas the body weight of untreated and vehicle-treated rats did not recover to baseline until 28 days. However, the difference among the three groups was not statistically significant (F = 1.837, p>0.05; Fig. 1B).
Fig. 1.
BMMNC transplantation promotes neurologic functional recovery and ameliorates brain damage but does not affect body weight changes. (A) Neurologic deficit as assessed by modified neurological severity score (mNSS) was not significantly different between vehicle-treated and BMMNC-treated rats on day 1 after pMCAO. However, beginning on day 7 after stroke, neurologic function was significantly better in the BMMNC-treated group. *p<0.05 vs. vehicle treatment; n=12/group. (B) Rat body weight decreased rapidly during the first 7 days after pMCAO before beginning to increase. Changes in body weight did not differ significantly among the BMMNC-treated, vehicle-treated, and untreated groups; n=12/group. (C) Representative images of brain slices stained with TTC. Absence of red stain represents infarct area. (D) Brain infarct volume was significantly smaller in BMMNC-treated rats than in untreated and vehicle-treated rats on day 7 after pMCAO. *p<0.05 vs. untreated and vehicle-treated groups; n=6/group.
3.3. BMMNCs reduce infarct volume
Fig. 1C shows representative brain slices from vehicle- and BMMNC-treated rats stained with TTC on day 7 after stroke. The total infarct volume was significantly less in the BMMNC-treated rats than in the untreated or vehicle-treated rats on day 7 after pMCAO (n=6/group, p<0.05; Fig. 1D).
3.4. BMMNCs enhance leptomeningeal anastomoses growth and enlarge diameter of circle of Willis and basilar arteries
On day 14 after pMCAO, latex perfusion revealed that BMMNC treatment had significantly increased the vessel diameter of leptomeningeal anastomoses as compared with vehicle treatment (Fig. 2A–C; BMMNC: 53.2 ± 5.3 μm; vehicle: 33.3 ± 2.8 μm, p<0.05; n=6). In contrast, vessel diameter did not differ significantly among the vehicle-treated, untreated (31.7 ± 3.6 μm), and sham-operated (30.2 ± 4.4 μm) groups (Fig. 2C). The number of leptomeningeal anastomoses was significantly greater in the BMMNC-treated group than in the vehicle-treated group (Fig. 2D; BMMNC: 47.2 ± 4.7; vehicle: 30.8 ± 2.9, p<0.05; n=6). Moreover, the number of leptomeningeal anastomoses also increased as a result of the pMCAO procedure (sham: 20.0 ± 2.6 vs. untreated: 31.7 ± 3.4, p<0.05; n=6; Fig. 2A and D). BMMNC transplantation also led to a significant increase in the diameter of the circle of Willis and basilar arteries as measured by latex infusion (Figs. 3 and 4). Compared with the vehicle-treated group (14 days, circle of Willis: 209±14.8 μm; basilar arteries: 291.8±11.9 μm; 42 days, circle of Willis: 213±10.2 μm; basilar arteries: 304.3±7.2 μm), there was a marked increase in vessel diameter on day 14 (circle of Willis: 287.8±11.0 μm, p<0.05; basilar arteries: 491.2±16.6 μm, p<0.05; n=6) and day 42 (circle of Willis: 292.3±5.4 μm, p<0.05; basilar arteries: 501.2±9.2 μm, p<0.05; n=6) after BMMNC treatment (Figs. 3C and 4C). However, the vessel diameter of the circle of Willis and basilar arteries was not influenced by pMCAO surgery on day 14 (circle of Willis: sham, 202.5±10.8 μm vs. untreated, 203.8±12.4 μm, p>0.05; basilar arteries: sham, 286.0±10.0 μm vs. untreated, 289.2±12.6 μm, p>0.05; n=6) or day 42 (circle of Willis: sham, 206.7±7.0 μm vs. untreated, 216.0±8.0 μm, p>0.05; n=6; basilar arteries: sham, 289.3±9.0 μm vs. untreated, 297.5±7.4 μm, p>0.05; n=6; Figs. 3C and 4C).
Fig. 2.
BMMNCs enhance leptomeningeal anastomoses (LA). (A) Superficial angioarchitecture of rat brains was visualized by latex perfusion on day 14 after pMCAO. The boxed regions in the upper panels are shown at higher magnification in the lower panels. The arrows indicate vessels of leptomeningeal anastomoses (scale bar = 50 μm). (B) Representative images of α-SMA immunostaining in the dorsal superficial arterioles (scale bar = 50 μm). (C) The mean diameter of the leptomeningeal anastomoses was significantly greater in the BMMNC-treated pMCAO rats than in sham-operated, untreated pMCAO, and vehicle-treated pMCAO rats. *p<0.05 vs. vehicle treatment; n=6/group. (D) The number of leptomeningeal anastomoses after pMCAO was also greater in the BMMNC-treated rats than in the other groups. *p<0.05 vs. vehicle treatment; #p<0.05 vs. sham group; n=6/group.
Fig. 3.
BMMNCs enhance the vessel diameter of the circle of Willis. (A–B) Superficial brain angioarchitecture was visualized by latex perfusion on days 14 (A) and 42 (B) after stroke or sham surgery. The arrows indicate the measurement point of the circle of Willis (scale bar = 500 μm). (C) Quantification of the circle of Willis vessel diameter. #p<0.05 vs. the vehicle-treated group on day 14, *p<0.05 vs. the vehicle-treated group on day 42.
Fig. 4.
BMMNCs enhance the vessel diameters of basilar arteries. (A–B) Superficial brain angioarchitecture was visualized by latex perfusion on days 14 (A) and 42 (B) after pMCAO or sham surgery (scale bar = 500 μm). (C) Quantification showed that the vessel diameters of the basilar arteries were enhanced by transplanted BMMNCs. #p<0.05 vs. the vehicle-treated group on day 14; *p<0.05 vs. the vehicle-treated group on day 42.
3.5. Transplanted BMMNCs incorporate into brain vessels and promote arteriogenesis and angiogenesis
On day 7 after stroke, the transplanted cells appeared to remain undifferentiated (no α-SMA or CD31 expression; Fig. 5D–F). By 14 days, double staining of the blood vessels with anti-BrdU and anti-α-SMA revealed that BMMNCs had differentiated into vascular SMCs in the leptomeningeal and intracranial arterioles (Fig. 5G–I). Similar results were also observed on days 28 and 42 after pMCAO (Fig. 5J–O). The number of BrdU-positive SMCs increased on day 14 (15.1±1.6 per mm2), peaked on day 28 (23.1±2.3 per mm2), and decreased on day 42 (11.7±1.3 per mm2) (F = 10.7, p=0.000; both p<0.05 vs. day 14 or 42, Fig. 6N). In addition, arteriogenesis of intracranial arterioles was maintained for at least 42 days after treatment with BMMNCs (Fig. 5P–R). Colocalization of BrdU with the endothelial marker CD31 confirmed that BMMNCs also differentiated into endothelial-like cells in the leptomeningeal arterioles and intracranial microvessels on days 14, 28, and 42 (Fig. 6A–I). The number of BrdU-positive ECs increased on day 14 (37.4±4.6 per mm2), peaked on day 28 (49.6±5.2 per mm2), and decreased on day 42 (30.1±2.1 per mm2) (F = 5.5, p=0.007; both p<0.05 vs. day 14 or 42, n=6/time point; Fig. 6M). Although the number of BrdU-positive ECs and SMCs tended to be lower on day 42 than on day 14, the difference was not significant (p>0.05). Double staining for BrdU and CD31 on day 42 showed that BMMNCs had differentiated into endothelial–like cells in the intracranial parenchymal microvessels after stroke (Fig. 6J–L).
Fig. 5.
Transplanted BMMNCs incorporate into brain vessels and promote arteriogenesis. (A–B) Hematoxylin and eosin (HE) staining for the subtle anatomical structure of the normal leptomeningeal anastomoses (A: scale bar = 50 μm; B: scale bar = 10 μm). (C) Fluorescent staining of BrdU (green) on day 14 after pMCAO shows BrdU adjacent to the infarct boundary zone (scale bar = 100 μm). Representative images of leptomeningeal anastomoses close to the infarct boundary zone on days 7, 14, 28, and 42 (D–O) and intracranial arterioles on day 42 (P–R) stained with anti-BrdU antibody (green) and anti-α-SMA antibody (red) (scale bar = 50 μm).
Fig. 6.
Transplanted BMMNCs incorporate into brain vessels and promote angiogenesis. Representative images of leptomeningeal anastomoses on days 14, 28 and 42 (A–I) and intracranial parenchymal microvessels on day 42 (J–L) stained with anti-BrdU antibody (green) and anti-CD31 antibody (red) (scale bar = 50 μm). (M–N) Quantification showed an increase in the number of BrdU-positive endothelial cells (ECs, M) and smooth muscle cells (SMCs, N). *p<0.05 vs. the number of BrdU-positive ECs or SMCs on day 14 or 42; n=6/time point.
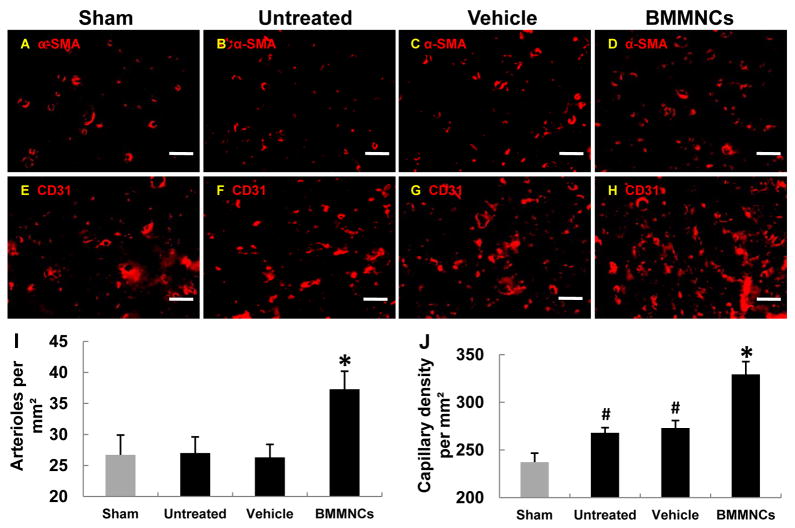
On day 42, the number of cerebral arterioles in the brain parenchyma was significantly greater in the BMMNC-treated group than in the vehicle-treated, untreated, and sham-operated groups. However, no significant difference was observed between the untreated ischemic group and the sham-operated group (pMCAO+BMMNCs, 37.3±2.9 vs. pMCAO+vehicle, 23.2±2.5, p<0.05; untreated+pMCAO, 27.0±2.6 vs. sham, 26.7±3.2, p>0.05; n=6 rats/group; Fig. 7A–D and 7I). The capillary density was markedly increased in the BMMNC-treated group compared to that in the vehicle-treated, untreated, and sham-operated groups (pMCAO+BMMNCs, 329.3±13.4 vs. pMCAO+vehicle, 273.0±7.8, p<0.05; untreated+pMCAO, 268.0±5.4 vs. sham, 237.2±9.4, p<0.05; n=6 rats/group; Fig. 7E–H and 7J).
Fig. 7.
Transplanted BMMNCs incorporate into brain microvessels and promote angiogenesis and arteriogenesis. (A–H) Fluorescent staining shows the density of capillaries and arterioles in the region adjacent to the infarct border in each group on day 42 (scale bar = 50 μm). The number of arterioles per square millimeter (I) and density of microvessels per square millimeter (J) were each greater in the BMMNC-treated pMCAO group than in the sham group, untreated pMCAO group, or vehicle-treated pMCAO group. *p<0.05 vs. vehicle-treated and untreated groups; #p<0.05 vs. the sham group; n=6/group.
4. Discussion
The results of this study show that BMMNCs can enhance cerebral collateral formation by promoting arteriogenesis and angiogenesis after pMCAO in rats. Although it has already been reported that BMMNCs might be a promising option for cell therapy, to our knowledge, this study is the first to demonstrate the arteriogenic actions of BMMNCs in the ischemic brain. Previously it has been reported that BMMNCs exert neuroprotective effects by differentiating into neurons and glia (Iihoshi et al., 2004; Zhang et al., 2011). It has also been shown that BMMNCs have anti-inflammatory effects (Brenneman et al., 2010). However, these actions only partially explain the contribution of BMMNCs to reduction in cerebral infarct volume and promotion of functional recovery after ischemia.
Rapid recovery of CBF is critical for the maintenance of neural function (Bang et al., 2011; Liebeskind, 2012). To some extent, collaterals can compensate for the loss of blood flow in the occluded vessel by acting as an alternate delivery route for oxygen and nutrients (Liebeskind, 2012; Sugiyama et al., 2011; Zacharek et al., 2009). After cerebral ischemia, leptomeningeal anastomoses are the most important collateral pathways and could be a potential therapeutic target (Shuaib et al., 2011; Sugiyama et al., 2011). However, the spontaneous proliferation of collateral circulation cannot prevent, or can only partially prevent, the detrimental effects of vascular occlusion because arteriogenesis is slow and self-limiting (Busch et al., 2003; Buschmann et al., 2003; Troidl and Schaper, 2012). Our study showed that the hemodynamic changes that follow pMCAO can increase the number of leptomeningeal anastomoses, but cannot increase the diameter of arterioles or arteries. Because of their small diameter, the new vessels have high resistance and cannot efficiently compensate for the loss of a major occluded artery (Busch et al., 2003; Buschmann et al., 2003; Troidl and Schaper, 2012). Thus, stimulation of collateral growth and expansion holds potential as a therapeutic route for ischemic stroke (Buschmann et al., 2003; Liebeskind, 2012).
BMMNCs are a heterogeneous mixture of cells that include endothelial progenitor cells, hematopoietic stem cells, mesenchymal stem cells, and multipotent adult progenitor cells, among others (Seeger et al., 2009). In in vitro experiments, the differentiation capacity of BMMNCs from peripheral blood into SMCs or smooth muscle-like cells was first described by Caplice and colleagues (Simper et al., 2002). Additional studies that used reverse transcription PCR confirmed that isolated CD14/CD105 (endoglin) double-positive BMMNCs can express α-SMA and SM-22α genes and differentiate into smooth muscle-like cells after several days of cell culture and stimulation with platelet-derived growth factor, basic fibroblast growth factor, and transforming growth factor (Sugiyama et al., 2006). Yoon et al. (Yoon et al., 2010) found that some BMMNC cell types can differentiate into SMCs and ECs in vitro. Although some research has failed to show a link between transplanted BMMNCs and arteriogenesis and angiogenesis in vivo (Ziegelhoeffer et al., 2004), many other researchers have suggested that BMMNCs do contribute to arteriogenesis and angiogenesis and that some cell components of BMMNCs can incorporate into vessel walls (Imada et al., 2005; Kuliszewski et al., 2011; Li et al., 2011; Yoon et al., 2010). For example, in animal models of limb ischemia and myocardial infarction, bone marrow-derived endothelial progenitor cells contribute to vascular remodeling (Kuliszewski et al., 2011). Research also has revealed that hematopoietic stem cells, mesenchymal stem cells, and multipotent adult progenitor cells can not only incorporate directly into the adult growing vasculature and differentiate into ECs and SMCs, but also can produce large numbers of cytokines and trophic factors that promote ischemic tissue repair (Aranguren et al., 2008; Huang et al., 2010; Yeh et al., 2003). In addition, subpopulations of BMMNCs, such as CD34+/M-cadherin+ cells, can promote arteriogenesis and angiogenesis by differentiating into SMCs and ECs in ischemic hindlimbs (Terry et al., 2011). Some researchers also have demonstrated the neovascularization efficacy of BMMNCs in diabetic patients (Ruiz-Salmeron et al., 2011). These findings illustrate that BMMNCs have the capacity to differentiate into SMCs and ECs and are involved in the progress of arteriogenesis and angiogenesis, which may contribute to the enhancement of blood flow restoration in ischemic tissue. Although it has been reported that BMMNCs are neuroprotective after stroke (Hao et al., 2011; Savitz et al., 2011) and that BMMNC treatment can enhance CBF in the early phase of cerebral infarction (6 h after administration) (Fujita et al., 2010), to date, no specific data have shown whether BMMNCs can promote arteriogenesis after stroke. Our data show that BMMNCs have the capacity to differentiate into SMCs and ECs and incorporate into growing cerebral vessel walls after ischemic stroke, similar to their actions in coronary artery disease and peripheral artery disease (Imada et al., 2005; Seeger et al., 2009; Terry et al., 2011; Yoon et al., 2010). Rats injected with BMMNCs developed an enhanced collateral circulation that included leptomeningeal arterioles, parenchyma arterioles, and basal arteries, which altered the distribution of blood flow after pMCAO. Similar to previous findings (Hao et al., 2011), our study revealed that BMMNCs can significantly reduce infarct volume and enhance long-term neurologic functional recovery after pMCAO.
Although BMMNCs have been shown to promote angiogenesis (Hao et al., 2011), until now the effects of BMMNCs on arteriogenesis have not been well studied. With arteriogenesis, functional and larger collateral arteries are formed from preexisting arterioles. These arteries not only feed areas deprived of their normal blood supply but also supply new capillaries (angiogenesis) (Wei et al., 2001). Thus, the collaterals formed by arteriogenesis are potentially able to fully replace an occluded artery, whereas new capillaries formed via angiogenesis cannot (Buschmann et al., 2003; Troidl and Schaper, 2012). Furthermore, arteriogenesis proceeds much faster than angiogenesis. Because the process of arteriogenesis starts with preexisting collateral vessels, restoration of blood flow can be accomplished within 3 days; however, in angiogenesis, new blood vessels are initiated through vascular sprouts and form connections with intact microvessels, a process that has been shown to require a week after cerebral ischemia (Troidl and Schaper, 2012). Hence, arteriogenesis is more rapid and more efficacious than angiogenesis for restoring blood flow to ischemic brain.
Huang et al. (Huang et al., 2010) reported that transplanted allogeneic cells (mesenchymal stem cells) were eliminated from the heart by day 35 after implantation; in our data, the differentiated cells survived for at least 42 days. The difference in the cell types and internal environments between myocardial infarction and cerebral infarction may account for the disparity.
Although arteriogenesis has been shown to enhance recovery of blood flow to ischemic tissue (Ruiz-Salmeron et al., 2011; Sugiyama et al., 2011; Yoon et al., 2010), the underlying molecular basis is undefined. Studies have shown that the differentiation of vascular SMCs requires Notch signaling after myocardial infarction (Li et al., 2011), that endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impaired activation of the cell cycle gene network (Dai and Faber, 2010), and that platelet endothelial cell adhesion molecule-1 knockout significantly increases tissue perfusion and collateral-dependent blood flow in an animal model of hindlimb ischemia (Chen et al., 2010). Additional research is needed to define which of these intracellular molecular pathways is activated in arteriogenesis and angiogenesis after BMMNC transplantation.
In summary, transplanted BMMNCs have the capacity to differentiate into SMCs and ECs after pMCAO. These results are consistent with those reported for coronary and peripheral artery disease. The differentiated cells enhanced arteriogenesis (especially for leptomeningeal anastomoses) and angiogenesis by incorporating directly into the collateral vessel walls and provided powerful neuroprotective effects against ischemic stroke. Previous research has shown that BMMNCs can protect neurons; modulate microglia; and increase the expression of insulin-like growth factor-1, interleukin-10, vascular endothelial growth factor, and stromal cell-derived factor-1α in cell culture models of ischemic stroke (Sharma et al., 2010). Additionally, it has been reported that nitric oxide generation in response to BMMNCs may contribute to the mechanism by which BMMNCs enter the brain, reduce lesion size, and improve outcome in ischemic stroke (Kasam et al., 2012). However, no research has been carried out to determine how BMMNCs differentiate into SMCs and ECs and which cell components of BMMNCs play important roles in this process. These intracellular molecular mechanisms may underlie the neuroprotective effects of BMMNCS after ischemic stroke and warrant further exploration. Nevertheless, our results show that cell therapy with BMMNCs could be a promising option for the treatment of ischemic stroke.
Highlight.
BMMNCs can enhance arteriogenesis (especially for leptomeningeal arterioles) and angiogenesis, and promote long-term functional recovery after stroke.
Acknowledgments
This work was supported by grants from NSFC (81271284), Medical Science and Technology Research Programs of Henan Province (WKJ2010-2-016), The Overseas Training Program of Henan Province Medical Academic Leaders (2011023), and NIH (K01AG031926, R01AT007317, R01NS078026). We thank Claire Levine for assistance with this manuscript.
Footnotes
Declaration of Conflicting Interests
No potential conflicts of interest were disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aranguren XL, McCue JD, Hendrickx B, Zhu XH, Du F, Chen E, Pelacho B, Penuelas I, Abizanda G, Uriz M, Frommer SA, Ross JJ, Schroeder BA, Seaborn MS, Adney JR, Hagenbrock J, Harris NH, Zhang Y, Zhang X, Nelson-Holte MH, Jiang Y, Billiau AD, Chen W, Prosper F, Verfaillie CM, Luttun A. Multipotent adult progenitor cells sustain function of ischemic limbs in mice. J Clin Invest. 2008;118:505–514. doi: 10.1172/JCI31153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, Lee KH, Liebeskind DS. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman M, Sharma S, Harting M, Strong R, Cox CS, Jr, Aronowski J, Grotta JC, Savitz SI. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30:140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch HJ, Buschmann IR, Mies G, Bode C, Hossmann KA. Arteriogenesis in hypoperfused rat brain. J Cereb Blood Flow Metab. 2003;23:621–628. doi: 10.1097/01.WCB.0000057741.00152.E4. [DOI] [PubMed] [Google Scholar]
- Buschmann IR, Busch HJ, Mies G, Hossmann KA. Therapeutic induction of arteriogenesis in hypoperfused rat brain via granulocyte-macrophage colony-stimulating factor. Circulation. 2003;108:610–615. doi: 10.1161/01.CIR.0000074209.17561.99. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Li G, Zhang W, Wang J, Sigmund CD, Olson JE, Chen Y. Ischemia-induced brain damage is enhanced in human renin and angiotensinogen double-transgenic mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2009;297:R1526–R1531. doi: 10.1152/ajpregu.91040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Rubin J, Tzima E. Role of PECAM-1 in arteriogenesis and specification of preexisting collaterals. Circ Res. 2010;107:1355–1363. doi: 10.1161/CIRCRESAHA.110.229955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res. 2010;106:1870–1881. doi: 10.1161/CIRCRESAHA.109.212746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Ihara M, Ushiki T, Hirai H, Kizaka-Kondoh S, Hiraoka M, Ito H, Takahashi R. Early protective effect of bone marrow mononuclear cells against ischemic white matter damage through augmentation of cerebral blood flow. Stroke. 2010;41:2938–2943. doi: 10.1161/STROKEAHA.110.596379. [DOI] [PubMed] [Google Scholar]
- Giraldi-Guimaraes A, Rezende-Lima M, Bruno FP, Mendez-Otero R. Treatment with bone marrow mononuclear cells induces functional recovery and decreases neurodegeneration after sensorimotor cortical ischemia in rats. Brain Res. 2009;1266:108–120. doi: 10.1016/j.brainres.2009.01.062. [DOI] [PubMed] [Google Scholar]
- Hao Q, Su H, Palmer D, Sun B, Gao P, Yang GY, Young WL. Bone marrow-derived cells contribute to vascular endothelial growth factor-induced angiogenesis in the adult mouse brain by supplying matrix metalloproteinase-9. Stroke. 2011;42:453–458. doi: 10.1161/STROKEAHA.110.596452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XP, Sun Z, Miyagi Y, McDonald Kinkaid H, Zhang L, Weisel RD, Li RK. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007:1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- Imada T, Tatsumi T, Mori Y, Nishiue T, Yoshida M, Masaki H, Okigaki M, Kojima H, Nozawa Y, Nishiwaki Y, Nitta N, Iwasaka T, Matsubara H. Targeted delivery of bone marrow mononuclear cells by ultrasound destruction of microbubbles induces both angiogenesis and arteriogenesis response. Arterioscler Thromb Vasc Biol. 2005;25:2128–2134. doi: 10.1161/01.ATV.0000179768.06206.cb. [DOI] [PubMed] [Google Scholar]
- Kasam M, Yang B, Strong R, Schaar K, Misra V, Xi X, Grotta JC, Aronowski J, Savitz SI. Nitric oxide facilitates delivery and mediates improved outcome of autologous bone marrow mononuclear cells in a rodent stroke model. PLoS One. 2012;7:e32793. doi: 10.1371/journal.pone.0032793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliszewski MA, Kobulnik J, Lindner JR, Stewart DJ, Leong-Poi H. Vascular gene transfer of SDF-1 promotes endothelial progenitor cell engraftment and enhances angiogenesis in ischemic muscle. Mol Ther. 2011;19:895–902. doi: 10.1038/mt.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen J, Chopp M. Adult bone marrow transplantation after stroke in adult rats. Cell Transplant. 2001;10:31–40. [PubMed] [Google Scholar]
- Li Y, Hiroi Y, Ngoy S, Okamoto R, Noma K, Wang CY, Wang HW, Zhou Q, Radtke F, Liao R, Liao JK. Notch1 in bone marrow-derived cells mediates cardiac repair after myocardial infarction. Circulation. 2011;123:866–876. doi: 10.1161/CIRCULATIONAHA.110.947531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebeskind DS. Collateral perfusion: time for novel paradigms in cerebral ischemia. Int J Stroke. 2012;7:309–310. doi: 10.1111/j.1747-4949.2012.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes R, Ayllón V, Gutierrez-Aranda I, Prat I, Hernández-Lamas MC, Ponce L, Bresolin S, te Kronnie G, Greaves M, Bueno C, Menendez P. Enforced expression of MLL-AF4 fusion in cord blood CD34+ cells enhances the hematopoietic repopulating cell function and clonogenic potential but is not sufficient to initiate leukemia. Blood. 2011;117:4746–4758. doi: 10.1182/blood-2010-12-322230. [DOI] [PubMed] [Google Scholar]
- Narantuya D, Nagai A, Sheikh AM, Masuda J, Kobayashi S, Yamaguchi S, Kim SU. Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement. PLoS One. 2010;5:e11746. doi: 10.1371/journal.pone.0011746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Resende VT, Pimentel-Coelho PM, Mesentier-Louro LA, Mendez RM, Mello-Silva JP, Cabral-da-Silva MC, de Mello FG, de Melo Reis RA, Mendez-Otero R. Trophic activity derived from bone marrow mononuclear cells increases peripheral nerve regeneration by acting on both neuronal and glial cell populations. Neuroscience. 2009;159:540–549. doi: 10.1016/j.neuroscience.2008.12.059. [DOI] [PubMed] [Google Scholar]
- Ruiz-Salmeron R, de la Cuesta-Diaz A, Constantino-Bermejo M, Perez-Camacho I, Marcos-Sanchez F, Hmadcha A, Soria B. Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant. 2011;20:1629–1639. doi: 10.3727/096368910X0177. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Misra V, Kasam M, Juneja H, Cox CS, Jr, Alderman S, Aisiku I, Kar S, Gee A, Grotta JC. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- Seeger FH, Rasper T, Koyanagi M, Fox H, Zeiher AM, Dimmeler S. CXCR4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arterioscler Thromb Vasc Biol. 2009;29:1802–1809. doi: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- Sharma S, Yang B, Strong R, Xi X, Brenneman M, Grotta JC, Aronowski J, Savitz SI. Bone marrow mononuclear cells protect neurons and modulate microglia in cell culture models of ischemic stroke. Journal of neuroscience research. 2010;88:2869–2876. doi: 10.1002/jnr.22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada IS, Peterson BM, Spees JL. Isolation of locally derived stem/progenitor cells from the peri-infarct area that do not migrate from the lateral ventricle after cortical stroke. Stroke. 2010;41:e552–e560. doi: 10.1161/STROKEAHA.110.589010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- Simper D, Stalboerger PG, Panetta CJ, Wang S, Caplice NM. Smooth muscle progenitor cells in human blood. Circulation. 2002;106:1199–1204. doi: 10.1161/01.cir.0000031525.61826.a8. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Kugiyama K, Nakamura S, Kataoka K, Aikawa M, Shimizu K, Koide S, Mitchell RN, Ogawa H, Libby P. Characterization of smooth muscle-like cells in circulating human peripheral blood. Atherosclerosis. 2006;187:351–362. doi: 10.1016/j.atherosclerosis.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Yagita Y, Oyama N, Terasaki Y, Omura-Matsuoka E, Sasaki T, Kitagawa K. Granulocyte colony-stimulating factor enhances arteriogenesis and ameliorates cerebral damage in a mouse model of ischemic stroke. Stroke. 2011;42:770–775. doi: 10.1161/STROKEAHA.110.597799. [DOI] [PubMed] [Google Scholar]
- Terry T, Chen Z, Dixon RAF, Vanderslice P, Zoldhelyi P, Willerson JT, Liu Q. CD34+/M-cadherin+bone marrow progenitor cells promote arteriogenesis in ischemic hindlimbs of ApoE−/− mice. PLoS One. 2011;6:e20673. doi: 10.1371/journal.pone.0020673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troidl K, Schaper W. Arteriogenesis versus angiogenesis in peripheral artery disease. Diabetes Metab Res Rev. 2012;28(Suppl 1):27–29. doi: 10.1002/dmrr.2232. [DOI] [PubMed] [Google Scholar]
- Wang J, Jiang C, Liu C, Li X, Chen N, Hao Y. Neuroprotective effects of progesterone following stroke in aged rats. Behav Brain Res. 2010;209:119–122. doi: 10.1016/j.bbr.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–2184. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- Wu H, Wu T, Xu X, Wang J, Wang J. Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. J Cereb Blood Flow Metab. 2011;31:1243–1250. doi: 10.1038/jcbfm.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Strong R, Sharma S, Brenneman M, Mallikarjunarao K, Xi X, Grotta JC, Aronowski J, Savitz SI. Therapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic stroke. J Neurosci Res. 2011;89:833–839. doi: 10.1002/jnr.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- Yoon CH, Koyanagi M, Iekushi K, Seeger F, Urbich C, Zeiher AM, Dimmeler S. Mechanism of improved cardiac function after bone marrow mononuclear cell therapy: role of cardiovascular lineage commitment. Circulation. 2010;121:2001–2011. doi: 10.1161/CIRCULATIONAHA.109.909291. [DOI] [PubMed] [Google Scholar]
- Zacharek A, Chen J, Cui X, Yang Y, Chopp M. Simvastatin increases notch signaling activity and promotes arteriogenesis after stroke. Stroke. 2009;40:254–260. doi: 10.1161/STROKEAHA.108.524116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Du F, Yang D, Wang R, Yu CJ, Huang XN, Hu HY, Liu W, Fu J. Granulocyte colony-stimulating factor increases the therapeutic efficacy of bone marrow mononuclear cell transplantation in cerebral ischemia in mice. BMC Neurosci. 2011;12:61. doi: 10.1186/1471-2202-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]