Abstract
Haematopoietic cell transplantation (HCT) survivors are at increased risk for developing congestive heart failure (CHF), primarily due to pre-HCT exposure to anthracyclines. We examined the association between the development of CHF after HCT and polymorphisms in 16 candidate genes involved in anthracycline metabolism, iron homeostasis, anti-oxidant defence, and myocardial remodelling. A nested case-control study design was used. Cases (post-HCT CHF) were identified from 2,950 patients who underwent HCT between 1988 and 2007 at City of Hope and had survived ≥1 year. This cohort formed the sampling frame for selecting controls (without CHF) matched on: age, race/ethnicity, cumulative anthracycline exposure, stem cell source (allogeneic, autologous), and length of follow-up. Seventy-seven cases with pre-HCT germline DNA and 178 controls were genotyped. Multivariate analysis revealed that the odds of CHF was higher in females (Odds Ratio [OR]=2.9, p<0.01), individuals with pre-HCT chest radiation (OR=4.7, p=0.05), hypertension (OR=2.9, p=0.01), and with variants of genes coding for the NAD(P)H-oxidase subunit RAC2 (rs13058338, 7508T→A; OR=2.8, p<0.01), HFE (rs1799945, 63C→G; OR=2.5, p=0.05) or the doxorubicin efflux transporter ABCC2 (rs8187710, 1515G→A; OR=4.3, p<0.01). A combined (clinical and genetic) CHF predictive model performed better (area under the curve [AUC], 0.79) than the genetic (AUC=0.67) or the clinical (AUC=0.69) models alone.
Keywords: Anthracyclines, congestive heart failure, genetic susceptibility, haematopoietic cell transplantation, late effects
INTRODUCTION
Haematopoietic cell transplantation (HCT) is the treatment of choice for many haematological malignancies.(Copelan 2006) Advances in HCT strategies and supportive care have contributed to a growing population of long-term HCT survivors.(Wingard, et al 2002) These survivors are at risk of developing treatment-related complications that significantly impact quantity and quality of survival.(Baker, et al 2007, Bhatia, et al 1996, Syrjala, et al 2005) One of the more serious complications is the development of congestive heart failure (CHF), occurring several years following completion of therapy.(Armenian, et al 2008, Armenian, et al 2011, Tichelli, et al 2008) Outcome is especially poor; less than 50% survive five years after diagnosis of CHF.(Armenian, et al 2008, Armenian, et al 2011) Previous studies have demonstrated that the risk of post-HCT CHF is primarily due to pre-HCT exposure to anthracyclines.(Armenian, et al 2008, Armenian, et al 2011) Anthracycline-related cardiotoxicity is dose-dependent, and there are well-established factors that modify this association, such as young age at exposure to anthracyclines, being female, and radiation to the chest. Furthermore, the risk of anthracycline-related cardiotoxicity increases significantly among those who develop conventional cardiovascular risk factors (CVRFs: hypertension, diabetes mellitus, and dyslipidaemia).(Armenian and Bhatia 2008, Armenian, et al 2011, Armenian, et al 2012, Barry, et al 2008) However, these modifiers fail to explain the inter-individual variability in the risk of anthracycline-related CHF. The pathogenesis of anthracycline-related CHF includes oxidative stress and intracardiac metabolic derangements induced by anthracycline metabolites that are known to be cardiotoxic.(Minotti, et al 2004a, Minotti, et al 2004b) Susceptibility due to inherited genetic variation in these pathways could explain the inter-individual variability in risk of anthracycline-related CHF – an area that is understudied in this population.
The current study evaluated the role of key genes on the risk of anthracycline-related CHF in long-term survivors of HCT. Genes targeted in this study include those involved in free radical metabolism, regulation of renin-angiotensin and beta-adrenergic systems, as well as those impacting the pharmacodynamics of anthracyclines, selecting known functional polymorphisms within these genes (Supplementary Figure 1).
METHODS
A nested case-control study design was used. Cases were identified from a cohort of 2,950 consecutive patients who underwent HCT at City Hope (COH) between 1988 and 2007 for a haematological malignancy, and survived one or more years. These patients were followed as part of the COH Long-term Follow-up Program.
Cases
To be eligible, patients were required to be free of cardiac disease before HCT and to have developed symptomatic heart failure (CHF) after HCT, using the criteria established by the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for the diagnosis of clinical heart failure.(Hunt, et al 2005) These criteria included presence of symptoms (dyspnea and fatigue) and signs (oedema and rales) consistent with CHF. Diagnostic echocardiograms were used to document the extent of cardiac compromise. Individuals with asymptomatic cardiomyopathy (depressed ejection fraction or shortening fraction, but no symptoms), or those who developed transient cardiac dysfunction due to a potentially reversible acute complication, such as sepsis, and who subsequently had no evidence of cardiac dysfunction during follow-up were excluded.
Controls
Controls consisted of HCT survivors from the same cohort of 1+ year survivors who had no clinical evidence of CHF. Random selection of multiple controls (one to three) utilized the following matching criteria: age at HCT (± 5 years), race/ethnicity, cumulative anthracycline dose (in 100 mg/m2 strata), stem cell donor source (autologous, allogeneic), and length of follow-up to exceed the latency between HCT and the index case. The Institutional Review Board at COH approved the study.
Demographic and Clinical Variables
For both cases and controls, medical records from within COH and outside (if needed) were used to abstract clinical and therapeutic information regarding the pre-transplantation period, transplantation conditioning, and post-HCT period, using an established protocol described previously.(Armenian, et al 2008, Armenian, et al 2010, Armenian, et al 2011) The following data were collected: demographics, disease characteristics (diagnosis, date of diagnosis), treatment before HCT (chemotherapy: protocols/regimens, including cumulative dose of anthracyclines; radiation therapy: total dose, field, and dose per fraction), conditioning regimens used (chemotherapeutic agents, radiation [total body irradiation (TBI), number of fractions, and total dose]). Therapeutic exposures were summarized for cases and controls. Anthracycline cardiotoxicity risk factor score was calculated by multiplying cumulative dose by a factor that reflects the cardiotoxic potential of each drug (Supplemental Table 1).(Shankar, et al 2008, van Dalen, et al 2009) Chest irradiation included radiation to the mantle or mediastinal field.
Information regarding CVRFs was abstracted from the medical record; these included: hypertension, diabetes, and dyslipidaemia. To be included in this analysis, the CVRF had to be present after HCT, but before the onset of CHF. Furthermore, the condition had to be active at the time of the event (cases) or for a comparable period of follow-up (controls), as defined previously.(Armenian, et al 2012)
DNA isolation and genotyping
Genomic DNA was isolated from pre-HCT biospecimens and, depending upon availability, the following prioritization strategy was used: peripheral blood stem cells (PBSCs) > formalin-fixed paraffin-embedded (FFPE) bone marrow (BM) core biopsies > unstained slides of BM smears. Flexigene DNA Kit (Qiagen, Valencia, CA) was used for DNA extraction from PBSCs; QuickExtract DNA extraction solution (Epicenter, Illumina; Madison, WI) was used for FFPE and BM smears. Concentration of amplifiable DNA from FFPE or BM smear samples was measured at 1/100 dilution using 2 SYBR Green-based quantitative polymerase chain reaction (qPCR) assays (rs9024 and rs1883112) referencing to standards of a good quality DNA, NA11831, obtained from the National Institute of General Medical Sciences Human Genetic Cell Repository (Coriell Cell Repository, Camden, New Jersey). A minimum concentration of 100 pg/μl was required for a sample to be qualified for the study. Genotyping was via Sequenom iPLEX single nucleotide polymorphism (SNP) chemistry on a MassArray system. Pre-amplification prior to genotyping was performed for samples available at a lower concentration (100 pg/μl to 1 ng/μl).
When necessary, individual SNPs were sequenced using BigDye chemistry (Applied Biosystems; Foster City, CA) for allelic discrimination in order to obtain failed genotypes or to validate ambiguous genotypes. We were unable to obtain genotypes for ABCC1 (rs45511401) and ADRB1 (rs1801253) in more than 40% of cases and controls; as a result, they were not included in our analyses. Genotype call rates were 99.0% overall and above 98.6% for the 3 SNPs associated with CHF. There were 94 individuals with multiple sources of biospecimens. Paired discrepancy testing for genotyped data (PBSCs: BM FFPE or BM smear slides) revealed >99% consistency between high quality DNA (PBSC) and FFPE or BM smear DNA. The 16 genes and 17 SNPs are detailed in Supplemental Table II. Laboratory personnel were blinded to case-control status.
Statistical analysis
Cases and controls were compared with respect to demographics, pre-HCT therapeutic exposures, CVRFs and genetic variants using univariate conditional logistic regression for dichotomous outcomes, and linear regression adjusting for matching set for continuous outcomes. Genetic ancestry was estimated for cases and controls using STRUCTURE 2.0 (Pritchard, et al 2000) based on 42 ancestry informative markers and used to confirm matching of genetic background between cases and controls (Supplemental Table III).(Ding, et al 2012) Deviation of the genotype distributions from Hardy-Weinberg equilibrium (HWE) was tested in control patients using the Pearson goodness-of-fit χ2 test. Lack of deviation of genotype distributions was demonstrated in all SNPs except rs7412 in APOE; this gene was excluded from the analysis. The remaining 14 polymorphisms were not in linkage disequilibrium (r2<0.05) and therefore, no haplotypes were calculated.
Multivariate conditional logistic regression was used to identify variables that were significantly associated with CHF (dependent variable). Variables included in the model were genetic variants and clinical variables significantly associated with CHF in the univariate analysis at p=0.05 level. Receiver operating characteristic analyses were performed in order to assess the predictive utility of clinical or genetic variables alone, or clinical variables combined with genetic variables for assessing the risk of anthracycline-related CHF. Data were analysed using SPSS Version 18.0 (IBM, Armonk, NY) and SAS 9.2 (SAS institute, Cary, NC). All statistical tests were 2-sided. A false discovery rate method(Sabatti, et al 2003) was used to compute adjusted p-values to account for multiple comparisons. P values < 0.05 were considered statistically significant.
RESULTS
Demographic and Clinical Characteristics
Table I summarizes the demographic and clinical characteristics of the 77 cases and 178 matched controls included in this study. Cases were more likely to be female (55.8% vs. 33.1%, p<0.01), to have received chest radiation prior to HCT (14.3% vs. 5.1%, p=0.01), and to have hypertension (28.6% vs. 14.6%, p<0.01). Cases and controls were comparable with respect to primary cancer diagnosis, time from primary cancer diagnosis to HCT, conditioning chemotherapy or radiation exposure, body mass index, and presence of diabetes and dyslipidaemia.
Table I.
Patient and Treatment Characteristics
| Characteristics | Cases (N=77) | Controls (N=178) | Odds Ratio | 95% CI | P-Value |
|---|---|---|---|---|---|
| Sex | |||||
| Female, n (%) | 43 (55.8) | 59 (33.1) | 2.82 | 1.53–5.18 | <0.01 |
| Ethnicity/race, n (%) * | |||||
| Non-Hispanic White | 46 (59.7) | 115 (64.6) | - | - | - |
| Hispanic | 21 (27.3) | 38 (21.3) | - | - | |
| Black | 7 (9.1) | 18 (10.1) | - | - | |
| Other | 3 (3.9) | 7 (3.9) | - | - | |
| Diagnosis, n (%) ** | |||||
| Non-Lymphoma | 27 (35.1) | 67 (37.6) | 1.00 | ||
| Leukaemia | 17 (10.2) | 42 (23.6) | |||
| Multiple myeloma | 10 (13.0) | 25 (14.0) | |||
| Lymphoma | 50 (64.9) | 111 (62.4) | 1.12 | 0.62–2.25 | 0.63 |
| Non-Hodgkin lymphoma | 36 (46.8) | 94 (52.8) | |||
| Hodgkin lymphoma | 14 (18.2) | 17 (9.6) | |||
| Age at primary cancer diagnosis, years | |||||
| Median (range) | 46.8 (14.0–68.3) | 48.2 (3–72) | 0.99 | 0.97–1.02 | 0.65 |
| Time from diagnosis to HCT, years | |||||
| Median (range) | 1.3 (0.2–15.0) | 1.0 (0.2–15.9) | 1.08 | 0.98–1.21 | 0.14 |
| Age at HCT * , years | |||||
| Median (range) | 49.2 (16.0–68.6) | 51.0 (6.4–72.6) | - | - | - |
| Pre-HCT treatment * | |||||
| Anthracycline, mg/m2 | |||||
| Median (range) | 300 (60–650) | 300 (40–600) | - | - | - |
| Chest radiation, n (%) | 11 (14.3) | 9 (5.1) | 0.01 | ||
| HCT Type, n (%) * | |||||
| Autologous | 63 (81.8) | 153 (86.0) | - | - | - |
| Allogeneic | 14 (18.2) | 25 (14.0) | - | - | |
| Conditioning regimen, n (%) | |||||
| Cyclophosphamide | 58 (75.3) | 123 (69.1) | 1.51 | 0.74–3.08 | 0.25 |
| Etoposide | 61 (79.2) | 133 (74.7) | 1.25 | 0.60–2.60 | 0.55 |
| Total-body Irradiation | 39 (50.6) | 102 (57.3) | 0.74 | 0.44–1.38 | 0.39 |
| BMI at HCT, n (%) | |||||
| ≥30 Kg/m2 | 17 (22.1) | 47 (26.4) | 0.71 | 0.38–1.35 | 0.29 |
| Post-HCT cardiovascular risk factors, n (%) | |||||
| Hypertension | 22 (28.6) | 26 (14.6) | 2.65 | 1.32–5.30 | <0.01 |
| Diabetes | 12 (15.6) | 18 (10.1) | 2.16 | 0.95–4.92 | 0.08 |
| Dyslipidaemia | 7 (9.1) | 24 (13.5) | 0.70 | 0.28–1.70 | 0.32 |
Matching criteria
Analysed as lymphoma vs. non-lymphoma.
HCT, haematopoietic cell transplantation; BMI, body mass index; 95% CI, 95% confidence interval.
Median time from cancer diagnosis to onset of CHF was 4.3 years (range, 1–30 years); time from HCT to CHF was 2.5 years (range, 0.1–13 years) (Table II). A diagnostic history and physical examination report performed by a clinician confirming diagnosis of CHF was available for all 77 cases, and all were classified as having stage C or D heart failure – structural heart disease with symptoms of heart failure. Dyspnea on exertion was the most common presenting symptom (92%), followed by fatigue (90%), and orthopnea (55%). Confirmatory echocardiogram or multi-gated acquisition scan reports were available for 66 (86%) cases; median ejection fraction (EF) was 39% (range, 13–52%), and all had greater than 10% reduction of EF from their pre-HCT baseline. Of the 77 cases genotyped, 56 (73%) have died. The most common cause of death was relapse/progression of primary disease (48%); heart failure accounted for 17% of the deaths.
Table II.
Clinical Presentation at CHF Diagnosis
| Time to CHF onset, years, range | ||
| Cancer diagnosis to CHF, Median | 5.2 | 1.0–30.2 |
| HCT to CHF, Median | 2.5 | 0.1–13.0 |
| Reported symptoms, n % | ||
| Dyspnea with exertion | 71 | 92.2 |
| Fatigue | 69 | 89.6 |
| Orthopnea | 42 | 54.5 |
| Dyspnea at rest | 32 | 41.6 |
| Weight gain | 29 | 37.7 |
| ≥2 of above | 72 | 93.5 |
| Ejection fraction, n % * | ||
| ≤25 | 15 | 19.5 |
| 26–40 | 27 | 35.1 |
| 41–49 | 21 | 27.3 |
| ≥50 | 3 | 3.9 |
Echocardiogram or multiple gate acquisition scan reports from the time of CHF diagnosis were available for 66 cases.
CHF, congestive heart failure; HCT, haematopoietic cell transplantation.
Genetic variants and risk of CHF
The distribution of the genetic variants among cases and controls is presented in Table III. In the univariate analysis, there was an over-representation of the high-risk variant of NAD(P)H oxidase subunit RAC2 (rs13058338, 7508T→A; Odds Ratio [OR]: 2.6, p<0.05), HFE (rs1799945, 63C→G; OR: 2.6, p<0.05) and the doxorubicin efflux transporter ABCC2 (rs8187710, 1515G→A; OR: 5.2, p<0.05) among the cases. There was no difference in time from anthracycline exposure to CHF onset among individuals with the high-risk variants compared to wild type (data not shown). Finally, there was no association between cumulative anthracycline dose and risk of CHF by genotype (data not shown).
Table III.
Risk variants for anthracycline-related CHF
| Gene | SNP rs-ID | Chr. | Position* | At risk genotype | Cases (n=77) | Controls (n=178) | OR** (95% CI) | FDR-adjusted P -Value |
|---|---|---|---|---|---|---|---|---|
| AGT | rs699 | 1 | 230845794 | GA/AA | 54 (70.1) | 122 (68.5) | 1.15 (0.57–2.30) | 0.88 |
| AGTR1 | rs5186 | 3 | 148459988 | CA/AA | 26 (33.7) | 79 (44.4) | 0.68 (0.37–1.25) | 0.88 |
| ADRB2 | rs1042713 | 5 | 148206440 | GG | 31 (40.3) | 54 (30.3) | 0.60 (0.31–1.20) | 0.35 |
| HFE | rs1800562 | 6 | 26093141 | GA/AA | 2 (2.6) | 15 (8.4) | 0.30 (0.05–1.23) | 0.28 |
| rs1799945 | 6 | 26091179 | CG/GG | 23 (29.9) | 27 (15.2) | 2.58 (1.27–5.20) | 0.03 | |
| SOD2 | rs4880 | 6 | 160113872 | GA/AA | 65 (84.4) | 132 (74.2) | 1.79 (0.90–3.38) | 0.28 |
| ABCC2 | rs8187710 | 10 | 101611294 | GA/AA | 16 (20.8) | 13 (7.3) | 5.22 (1.92–13.84) | 0.02 |
| NQO1 | rs1800566 | 16 | 69745145 | CT/CC | 73 (94.8) | 168 (94.4) | 0.88 (0.23–3.42) | 0.88 |
| CYBA | rs4673 | 16 | 88713236 | GA/AA | 39 (50.6) | 92 (51.7) | 1.29 (0.72–2.44) | 0.65 |
| ACE | rs4343 | 17 | 61566031 | AG/GG | 57 (74.0) | 122 (68.6) | 1.28 (0.67–2.45) | 0.72 |
| CBR1 | rs9024 | 21 | 37445313 | GG | 60 (77.9) | 129 (72.4) | 1.51 (0.76–3.03) | 0.46 |
| CBR3 | rs1056892 | 21 | 37518706 | GG | 35 (45.5) | 74 (41.6) | 1.08 (0.59–1.87) | 0.88 |
| NCF4 | rs1883112 | 22 | 37256846 | AA | 18 (23.3) | 36 (20.2) | 1.06 (0.54–2.13) | 0.88 |
| RAC2 | rs13058338 | 22 | 37632770 | TA/AA | 42 (54.5) | 58 (32.6) | 2.61 (1.46–4.69) | 0.02 |
Chromosomal position in the GRCh37.p5
Univariate model
CHF, congestive heart failure; SNP, single nucleotide polymorphism;rs ID, rs identification; Chr, chromosome; OR, odds ratio; 95% CI, 95% confidence interval; FDR, false discovery rate.
Risk factors for anthracycline-related CHF
Multivariate conditional logistic regression revealed that the odds of CHF was higher among females (OR= 2.9, 95% confidence interval [CI], 1.4 to 6.0), individuals with pre-HCT exposure to chest radiation (OR= 4.7, 95% CI, 1.0–16.5), those with hypertension (OR= 2.9, 95% CI, 1.3–6.7), and those with RAC2 (rs13058338; 7508T→A) (OR= 2.8, 95% CI, 1.4–5.6), HFE (rs1799945; 63C→G) (OR= 2.5, 95% CI, 1.0–6.3), and ABCC2 (rs8187710; 1515G→A) (OR= 4.3, 95% CI, 1.5–12.5), genotypes (Table IV).
Table IV.
Multivariate analysis of risk factors associated with anthracycline-related CHF*
| Odds Ratio | 95% CI | P-value | |
|---|---|---|---|
| Sex | |||
| Male | 1.00 | ||
| Female | 2.92 | 1.43–5.97 | <0.01 |
| Chest radiation | |||
| None | 1.00 | ||
| Any | 4.73 | 1.00–16.46 | 0.05 |
| Hypertension | |||
| None | 1.00 | ||
| Hypertension | 2.92 | 1.27–6.72 | 0.01 |
| At risk genotypes | |||
| HFE (rs1799945), CC | 1.00 | ||
| HFE (rs1799945), GC/GG | 2.53 | 1.02–6.31 | 0.05 |
| RAC2 (rs13058338), TT | 1.00 | ||
| RAC2 (rs13058338), TA/AA | 2.83 | 1.42–5.64 | <0.01 |
| ABCC2 (rs8187710), GG | 1.00 | ||
| ABCC2 (rs8187710), GA/AA | 4.33 | 1.50–12.50 | <0.01 |
Cases and controls matched on: age, race/ethnicity, haematopoetic cell transplantation source (autologous, allogeneic), cumulative anthracycline dose, length of follow-up.
CHF, congestive heart failure; 95% CI, 95% confidence interval.
A separate multivariable regression model was developed to understand the interaction between sex and genetic susceptibility. An ordinal variable was created as follows: Referent - males with <2 high-risk variants (RAC2 rs13058338, 7508T→A; HFE rs1799945, 63C→G; or ABCC2 rs8187710, 1515G→A); males with ≥2 high-risk variants; females with <2 high-risk variants; females with ≥2 high-risk variants. Compared to the referent group, the odds of CHF were increased among females with ≥2 high-risk variants (OR=17.1, 95% CI: 3.7–58.8, p<0.01). The odds of CHF were also increased among males carrying ≥2 high-risk variants (OR=5.0, 95% CI: 1.1–16.0, p=0.04), and among females with <2 high-risk variants (OR=2.4, 95% CI: 1.1–4.4, p=0.03).
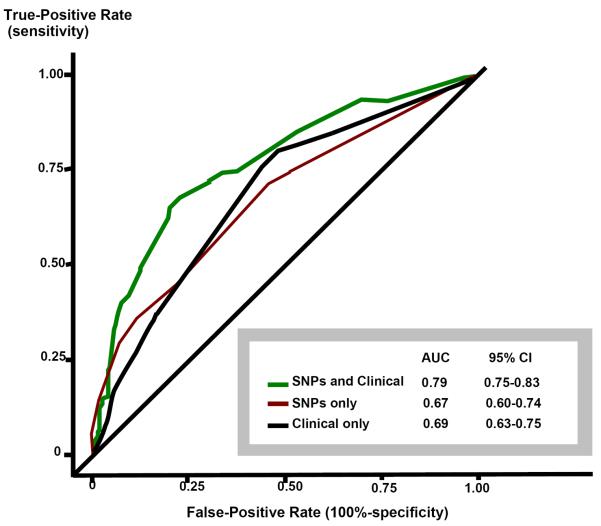
Finally, receiver operating characteristic analyses were conducted to compare three models: clinical variables alone (female sex, pre-HCT chest radiation, hypertension), genetic risk factors alone (HFE [rs1799945; 63C→G], RAC2 [rs13058338; 7508T→A], ABCC2 [rs8187710; 1515G→A]), and a combined (clinical and genetic) model. The combined model performed better (area under the curve [AUC], 0.79 [95% CI: 75–0.83]) than the genetic (AUC=0.67 [95% CI: 0.60–0.74) or the clinical (AUC=0.69 [95% CI: 63–0.75) models alone, (Figure 1).
Figure I. Receiver operating characteristic (ROC) curves.
ROC curves of three different models. Clinical model includes female sex, pre-haematopoietic cell transplantation (HCT) chest irradiation and post-HCT hypertension. The single nucleotide polymorphism (SNP) model includes at-risk variants of RAC2, HFE and ABCC2. The SNP and Clinical model includes all six variables.
DISCUSSION
The overall goal of this study was to determine the role of key candidate genes involved in anthracycline metabolism, iron homeostasis, anti-oxidant defence and myocardial remodelling in the development of anthracycline-related cardiomyopathy, and to understand the contribution of these genes to the observed inter-individual variability in cardiomyopathy risk. In a previously published study, we demonstrated that the risk of CHF was increased, especially among autologous HCT recipients, exceeding previously described estimates for conventionally-treated cancer survivors. (Armenian, et al 2008, Armenian, et al 2011) In addition, time between cardiotoxic exposure and clinically apparent cardiovascular disease was shorter than that reported in other populations, such as childhood cancer survivors.(Lipshultz, et al 1991, Mulrooney, et al 2009, van der Pal, et al 2012) The higher risk and shorter latency among HCT survivors is ascribed to the high cumulative anthracycline exposure and high prevalence of CVRFs, such as hypertension or diabetes. (Armenian, et al 2011, Armenian, et al 2012) Importantly, conditioning-related therapeutic exposures do not appear to modify this risk. (Armenian, et al 2011, Armenian, et al 2012) However, these clinical risk factors do not fully explain the inter-individual variability in CHF risk after exposure to anthracyclines. Using a nested case-control design, we identified variants in genes involved in free radical generation (RAC2), iron homeostasis (HFE), and anthracycline metabolism (ABCC2) to be associated with anthracycline-related CHF.
The protein encoded by ABCC2 is a member of the superfamily of ATP-binding cassette (ABC) transporters that transport molecules across cell membranes.(Konig, et al 1999) As a member of the multidrug resistant protein subfamily, this protein is expressed in the liver and kidney, but not in the heart.(Cui, et al 1999, Konig, et al 1999) In rat models, inhibition of ABCC2 protein expression by a bacterial toxin has resulted in a reduced biliary clearance of anthracyclines; biliary clearance is responsible for 50% of anthracycline disposition.(Hidemura, et al 2003) The high-risk variant of the ABCC2 gene (rs8187710; GA/AA) has been associated with a 2.3-fold risk of anthracycline-related CHF in conventionally-treated cancer patients. (Wojnowski, et al 2005) The current study demonstrates the association of this genetic variant with the development of CHF after HCT; the odds of CHF were increased 4 times in patients with GA/AA variant compared to individuals with the ABCC2 GG genotype. The observed association may be attributed to the altered pharmacokinetics of anthracyclines, resulting in delayed drug clearance, higher peak plasma levels, and greater myocyte injury and subsequent cardiac functional impairment, demonstrating again, that the risk of CHF after HCT is contributed to a significant extent by the pre-HCT anthracycline exposure.
RAC2 encodes for a GTPase required for the NAD(P)H oxidase activity. (Deng and Wojnowski 2007) The NAD(P)H oxidase multienzyme complex catalyses 1-electron reduction of oxygen with NADH or NAD(P)H used as an electron donor. (Deng and Wojnowski 2007, Wojnowski, et al 2005) In the myocardium, this complex is thought to be an important source of reactive oxygen species (ROS).(Raddatz, et al 2011) One-electron reduction of anthracyclines results in the formation of a semiquinone. (Minotti, et al 2004a) This semiquinone quickly regenerates its parent quinone by reducing ROS, such as superoxide anion and hydrogen peroxide, via the NAD(P)H oxidase multienzyme complex. (Minotti, et al 2004a, Vasquez-Vivar, et al 1997) The ROS generated from this cycle are cardiotoxic to the myocardium. (Minotti, et al 2004a) In a conventionally-treated cohort of lymphoma survivors, (Wojnowski, et al 2005) the high-risk variant of RAC2 (7508T→A) conferred a 2.6-fold risk of CHF in an unadjusted analysis. We demonstrated that the odds of developing CHF after HCT was increased nearly 3 times among those with the high-risk variant of RAC2 (7508T→A), after adjusting for the relevant clinical and genetic factors.
One of the main routes for anthracycline metabolism is the two-electron reduction of the C-13 carbonyl group in the anthracycline side chain, resulting in the formation of alcohol metabolites.(Minotti, et al 2004a, Mordente, et al 2003) These alcohol substrates convert aconitase/iron regulatory protein-1 (IRP1) into a “null protein”, disrupting intracellular iron homeostasis.(Minotti, et al 2004a, Minotti, et al 2004b) The HFE gene encodes a major histocompatibility complex class I-like protein that regulates iron transport and metabolism.(Feder, et al 1996) Homozygosity for high-risk variants of HFE is often seen in individuals with hereditary haemochromatosis, resulting in excess iron deposition in major tissues, including the heart.(Feder, et al 1996, Minotti, et al 2004b) Individuals with high-risk HFE variants may be especially susceptible to intracellular iron dysregulation and myocyte injury that result from IRP-1 inactivation due to anthracycline alcohol metabolites. In a study evaluating the functional role of the HFE in anthracycline-mediated cardiotoxicity, Hfe knock-out (Hfe−/−) mice treated with anthracycline had higher iron concentrations in the heart than Hfe−/− mice treated with saline; importantly, Hfe−/− mice were significantly more likely to have cardiotoxicity after anthracycline exposure when compared to wild-type mice treated with the drug.(Miranda, et al 2003) In the current study, the high-risk variant of HFE (63C→G) was associated with a 2.5-fold risk of CHF among HCT survivors when compared to HFE CC genotype. Due to the overall poor outcomes of our patient cases (>70% deceased at the time of genotyping), we were unable to evaluate the myocardial iron burden of cases and controls vis-à-vis HFE genotype. Previous studies have reported that HCT survivors are at an especially high risk of transfusion-dependent iron overload, and individuals with high-risk variants of HFE may be at highest risk.(Armand, et al 2011, Majhail, et al 2009, Majhail, et al 2008) To date, no study has described the association of HFE genotype with risk of anthracycline-related CHF in cancer survivors.
The mechanism of sex-specific association with anthracyclines and CHF seen in the current study needs to be further elucidated. It has been proposed that differences in body fat composition between men and women could alter the pharmacokinetics of the drug.(Barry, et al 2007) With the exception of idarubicin (used in a very small proportion of patients in the current study), anthracyclines do not reach high concentration in adipose tissue.(Lee, et al 1982, Lipshultz, et al 1995) If women have a higher percentage of body fat for the same body-surface area as compared with men, equivalent doses of the drug could lead to greater concentrations in non-adipose tissues, such as the heart, and lead to more cardiotoxicity. The current study is the first one to demonstrate that the odds of CHF in females with multiple (≥2) high-risk variants of genes involved in anthracycline metabolism, free radical generation, and iron homeostasis were 17 times higher when compared to males with <2 high-risk genotype, identifying a subgroup of survivors who were at highest risk of CHF. In addition, concomitant presence of hypertension, and previous exposure to chest radiation remained independent predictors of post-HCT CHF, emphasizing the importance of risk-prediction models that incorporate genetic as well as clinical factors in CHF risk assessment. In fact, the AUC for the combined model (clinical and genetic) was better (AUC=0.79) than the genetic (AUC=0.67) or the clinical (AUC=0.69) models alone.
We have previously demonstrated an association between CBR3 (Blanco, et al 2008, Blanco, et al 2012) and anthracycline-related cardiomyopathy in childhood cancer survivors, restricted to those exposed to low-to-moderate dose anthracyclines. The current study, with a primarily adult patient population, did not find an association between CBR3 and CHF (p=0.9). These differences could be explained by the significantly higher proportion of childhood cancer survivors that received low-to-moderate dose anthracyclines (1–250 mg/m2), as opposed to the adults in the current study and the fact that the controls were matched to cases on anthracycline dose in the current study. Finally, there is some evidence that carbonyl reductase activity declines with increasing age, thus contributing to the lack of association between CBR3 and CHF in the current study.(Gonzalez-Covarrubias, et al 2009) A previous study also demonstrated an association between SLC28A3 (intracellular drug transport) and CHF risk in childhood cancer survivors.(Visscher, et al 2012) While we did not genotype variants of the SLC28A3 gene, another study in survivors of adult-onset malignancy(Wojnowski, et al 2005) failed to demonstrate an association with functionally-similar genes involved in intracellular drug transport (SLC8A1, SLC22A1 SLC22A4, SLC22A16). Of note, patient characteristics in the current study were comparable to those reported by Wojnowski, et al (2005); nearly all were exposed to anthracyclines as adults, with comparable anthracycline exposures between cases and controls. The current study demonstrates a novel association between the high risk variant for HFE and CHF, as well as an interaction between female sex and high-risk genetic variants. Importantly, we have developed a comprehensive predictive model that relies on clinically validated outcomes and incorporates both clinical (demographics, cardiotoxic therapy, comorbidities) and genetic risk factors in the evaluation of CHF risk in long-term survivors of anthracycline-based cancer therapy. In fact, the combined clinical and genetic model was able to accurately predict the likelihood of CHF occurrence in up to 80% of HCT survivors in the study.
Any retrospective outcome assessment is limited by the amount of information available for review and biospecimens available for analysis. Genomic DNA was isolated from a variety of pre-HCT biospecimens, prioritized as follows: PBSC, FFPE BM core biopsies, and unstained slides of BM smears. Recognizing the potential for differences in quality of the DNA, we genotyped 94 paired samples, revealing excellent consistency between high quality DNA (e.g.: peripheral blood and FFPE or DNA smear. For the current study, all cases met the definition of clinical heart failure, as described by the ACC/AHA (Hunt et al 2005), and we were able to obtain source documentation on most patients to demonstrate reduced cardiac function from baseline. Our cases did not include individuals with diminished cardiac function who were asymptomatic, allowing us to describe prevalence of clinical and genetic risk factors across a well-characterized disease phenotype. For patients with haematological malignancies who are treated with HCT, documentation of normal cardiac function prior to HCT is a requirement. While transplant-related exposures are not associated with an increased risk of CHF, of the high prevalence of CVRFs, such as hypertension and diabetes, among the HCT recipients exacerbate the risk of anthracycline-related CHF in these patients. Pathogenetically, findings in the current study are directly linked to anthracycline-related cardiotoxicity, hence demonstrating that these genetic variants play a role in anthracycline-related cardiotoxicity in the non-HCT population.
In summary, we found that the risk of anthracycline-related cardiotoxicity is significantly modified by high-risk variants of RAC2 (free radical generation), HFE (dysregulation of iron homeostasis), and ABCC2 (intracellular accumulation of cardiotoxic anthracyclines), and that the risk is independent of previously established clinical risk factors, such as chest radiation exposure, cardiovascular risk factors, such as hypertension, and female sex. These data, when confirmed in an independent prospective cohort, could form the basis for novel approaches for prevention in anthracycline-exposed individuals; these would include targeted screening (e.g. female sex, pre-HCT chest radiation exposure, presence of at risk genotype), behavior modification (e.g. adoption of healthy lifestyle, aggressive management of CVRFs, such as hypertension), and early pharmacological intervention (angiotensin converting enzyme inhibitors or beta blockers) for high risk survivors with early evidence of cardiac dysfunction after HCT.
Supplementary Material
Acknowledgments
Funding/support: This study was supported, in part, by grants from the National Institutes of Health (2 K12 CA001727-14, P30 CA33572, P01 CA30206), ASBMT HistoGenetics New Investigator Award.
Footnotes
Author contributions:
S.H. Armenian had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Study concept and design: S.H. Armenian, S. Bhatia.
Acquisition of data: S.H. Armenian, G. Mills, Y. Ding, K. Venkataraman, F. L. Wong, D. Senitzer, S. Wang
Analysis and interpretation of data: S. H. Armenian, Y. Ding, C.L. Sun, F.L Wong, S.L. Neuhausen, S. Bhatia.
Drafting of the manuscript: S. H. Armenian, S. Bhatia
Critical revision of the manuscript and important intellectual content: S.H. Armenian, Y. Ding, F.L. Wong, S.L. Neuhausen, S.J. Forman, S. Bhatia
Procurement of funding: S.H. Armenian, S.J. Forman, S. Bhatia
The authors have no relevant financial conflicts of interest to disclose.
Presented, in part, at the 54th annual meeting of the American Society of Hematology, Atlanta.
References
- Armand P, Kim HT, Rhodes J, Sainvil MM, Cutler C, Ho VT, Koreth J, Alyea EP, Hearsey D, Neufeld EJ, Fleming MD, Steen H, Anderson D, Kwong RY, Soiffer RJ, Antin JH. Iron overload in patients with acute leukemia or MDS undergoing myeloablative stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:852–860. doi: 10.1016/j.bbmt.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenian SH, Bhatia S. Cardiovascular disease after hematopoietic cell transplantation--lessons learned. Haematologica. 2008;93:1132–1136. doi: 10.3324/haematol.13514. [DOI] [PubMed] [Google Scholar]
- Armenian SH, Sun CL, Francisco L, Steinberger J, Kurian S, Wong FL, Sharp J, Sposto R, Forman SJ, Bhatia S. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol. 2008;26:5537–5543. doi: 10.1200/JCO.2008.17.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenian SH, Sun CL, Mills G, Teh JB, Francisco L, Durand JB, Wong FL, Forman SJ, Bhatia S. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:1138–1144. doi: 10.1016/j.bbmt.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenian SH, Sun CL, Shannon T, Mills G, Francisco L, Venkataraman K, Wong FL, Forman SJ, Bhatia S. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood. 2011;118:6023–6029. doi: 10.1182/blood-2011-06-358226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenian SH, Sun CL, Vase T, Ness KK, Blum E, Francisco L, Venkataraman K, Samoa R, Wong FL, Forman SJ, Bhatia S. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120:4505–4512. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KS, Ness KK, Steinberger J, Carter A, Francisco L, Burns LJ, Sklar C, Forman S, Weisdorf D, Gurney JG, Bhatia S. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry E, Alvarez JA, Scully RE, Miller TL, Lipshultz SE. Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin Pharmacother. 2007;8:1039–1058. doi: 10.1517/14656566.8.8.1039. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Ramsay NK, Steinbuch M, Dusenbery KE, Shapiro RS, Weisdorf DJ, Robison LL, Miller JS, Neglia JP. Malignant neoplasms following bone marrow transplantation. Blood. 1996;87:3633–3639. [PubMed] [Google Scholar]
- Blanco JG, Leisenring WM, Gonzalez-Covarrubias VM, Kawashima TI, Davies SM, Relling MV, Robison LL, Sklar CA, Stovall M, Bhatia S. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H:quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer. 2008;112:2789–2795. doi: 10.1002/cncr.23534. [DOI] [PubMed] [Google Scholar]
- Blanco JG, Sun CL, Landier W, Chen L, Esparza-Duran D, Leisenring W, Mays A, Friedman DL, Ginsberg JP, Hudson MM, Neglia JP, Oeffinger KC, Ritchey AK, Villaluna D, Relling MV, Bhatia S. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children's Oncology Group. J Clin Oncol. 2012;30:1415–1421. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- Cui Y, Konig J, Buchholz JK, Spring H, Leier I, Keppler D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol Pharmacol. 1999;55:929–937. [PubMed] [Google Scholar]
- Deng S, Wojnowski L. Genotyping the risk of anthracycline-induced cardiotoxicity. Cardiovasc Toxicol. 2007;7:129–134. doi: 10.1007/s12012-007-0024-2. [DOI] [PubMed] [Google Scholar]
- Ding Y, Sun CL, Li L, Li M, Francisco L, Sabado M, Hahn B, Gyorffy J, Noe J, Larson GP, Forman SJ, Bhatia R, Bhatia S. Genetic susceptibility to therapy-related leukemia after Hodgkin lymphoma or non-Hodgkin lymphoma: role of drug metabolism, apoptosis and DNA repair. Blood Cancer J. 2012;2:e58. doi: 10.1038/bcj.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R, Jr., Ellis MC, Fullan A, Hinton LM, Jones NL, Kimmel BE, Kronmal GS, Lauer P, Lee VK, Loeb DB, Mapa FA, McClelland E, Meyer NC, Mintier GA, Moeller N, Moore T, Morikang E, Prass CE, Quintana L, Starnes SM, Schatzman RC, Brunke KJ, Drayna DT, Risch NJ, Bacon BR, Wolff RK. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Covarrubias V, Zhang J, Kalabus JL, Relling MV, Blanco JG. Pharmacogenetics of human carbonyl reductase 1 (CBR1) in livers from black and white donors. Drug Metab Dispos. 2009;37:400–407. doi: 10.1124/dmd.108.024547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidemura K, Zhao YL, Ito K, Nakao A, Tatsumi Y, Kanazawa H, Takagi K, Ohta M, Hasegawa T. Shiga-like toxin II impairs hepatobiliary transport of doxorubicin in rats by down-regulation of hepatic P glycoprotein and multidrug resistance-associated protein Mrp2. Antimicrob Agents Chemother. 2003;47:1636–1642. doi: 10.1128/AAC.47.5.1636-1642.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr., Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- Konig J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim Biophys Acta. 1999;1461:377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Lee YT, Chan KK, Harris PA. Tissue disposition of doxorubicin in experimental animals. Med Pediatr Oncol. 1982;10:259–267. doi: 10.1002/mpo.2950100306. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, Orav EJ, Gelber RD, Colan SD. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- Majhail NS, Lazarus HM, Burns LJ. Iron overload in hematopoietic cell transplantation. Bone Marrow Transplant. 2008;41:997–1003. doi: 10.1038/bmt.2008.99. [DOI] [PubMed] [Google Scholar]
- Majhail NS, DeFor TE, Lazarus HM, Burns LJ. Iron-overload after autologous hematopoietic cell transplantation. Leuk Res. 2009;33:578–579. doi: 10.1016/j.leukres.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004a;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- Minotti G, Recalcati S, Menna P, Salvatorelli E, Corna G, Cairo G. Doxorubicin cardiotoxicity and the control of iron metabolism: quinone-dependent and independent mechanisms. Methods Enzymol. 2004b;378:340–361. doi: 10.1016/S0076-6879(04)78025-8. [DOI] [PubMed] [Google Scholar]
- Miranda CJ, Makui H, Soares RJ, Bilodeau M, Mui J, Vali H, Bertrand R, Andrews NC, Santos MM. Hfe deficiency increases susceptibility to cardiotoxicity and exacerbates changes in iron metabolism induced by doxorubicin. Blood. 2003;102:2574–2580. doi: 10.1182/blood-2003-03-0869. [DOI] [PubMed] [Google Scholar]
- Mordente A, Minotti G, Martorana GE, Silvestrini A, Giardina B, Meucci E. Anthracycline secondary alcohol metabolite formation in human or rabbit heart: biochemical aspects and pharmacologic implications. Biochem Pharmacol. 2003;66:989–998. doi: 10.1016/s0006-2952(03)00442-8. [DOI] [PubMed] [Google Scholar]
- Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, Donaldson SS, Green DM, Sklar CA, Robison LL, Leisenring WM. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raddatz E, Thomas AC, Sarre A, Benathan M. Differential contribution of mitochondria, NADPH oxidases, and glycolysis to region-specific oxidant stress in the anoxic-reoxygenated embryonic heart. Am J Physiol Heart Circ Physiol. 2011;300:H820–835. doi: 10.1152/ajpheart.00827.2010. [DOI] [PubMed] [Google Scholar]
- Sabatti C, Service S, Freimer N. False discovery rate in linkage and association genome screens for complex disorders. Genetics. 2003;164:829–833. doi: 10.1093/genetics/164.2.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar SM, Marina N, Hudson MM, Hodgson DC, Adams MJ, Landier W, Bhatia S, Meeske K, Chen MH, Kinahan KE, Steinberger J, Rosenthal D. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics. 2008;121:e387–396. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol. 2005;23:6596–6606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- Tichelli A, Bhatia S, Socie G. Cardiac and cardiovascular consequences after haematopoietic stem cell transplantation. Br J Haematol. 2008;142:11–26. doi: 10.1111/j.1365-2141.2008.07165.x. [DOI] [PubMed] [Google Scholar]
- van Dalen EC, van der Pal HJ, Caron HN, Kremer LC. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database Syst Rev. 2009:CD005008. doi: 10.1002/14651858.CD005008.pub2. [DOI] [PubMed] [Google Scholar]
- van der Pal HJ, van Dalen EC, van Delden E, van Dijk IW, Kok WE, Geskus RB, Sieswerda E, Oldenburger F, Koning CC, van Leeuwen FE, Caron HN, Kremer LC. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30:1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Martasek P, Hogg N, Masters BS, Pritchard KA, Jr., Kalyanaraman B. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry. 1997;36:11293–11297. doi: 10.1021/bi971475e. [DOI] [PubMed] [Google Scholar]
- Visscher H, Ross CJ, Rassekh SR, Barhdadi A, Dube MP, Al-Saloos H, Sandor GS, Caron HN, van Dalen EC, Kremer LC, van der Pal HJ, Brown AM, Rogers PC, Phillips MS, Rieder MJ, Carleton BC, Hayden MR. Pharmacogenomic Prediction of Anthracycline-Induced Cardiotoxicity in Children. J Clin Oncol. 2012;30:1422–1428. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- Wingard JR, Vogelsang GB, Deeg HJ. Stem cell transplantation: supportive care and long-term complications. Hematology Am Soc Hematol Educ Program. 2002:422–444. doi: 10.1182/asheducation-2002.1.422. [DOI] [PubMed] [Google Scholar]
- Wojnowski L, Kulle B, Schirmer M, Schluter G, Schmidt A, Rosenberger A, Vonhof S, Bickeboller H, Toliat MR, Suk EK, Tzvetkov M, Kruger A, Seifert S, Kloess M, Hahn H, Loeffler M, Nurnberg P, Pfreundschuh M, Trumper L, Brockmoller J, Hasenfuss G. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.