Abstract
Biomedical applications of porous silicon include drug delivery, imaging, diagnostics and immunotherapy. This review summarizes new silicon particle fabrication techniques, dynamics of cellular transport, advances in the multistage vector approach to drug delivery, and the use of porous silicon as immune adjuvants. Recent findings support superior therapeutic efficacy of the multistage vector approach over single particle drug delivery systems in mouse models of ovarian and breast cancer. With respect to vaccine development, multivalent presentation of pathogen-associated molecular patterns on the particle surface creates powerful platforms for immunotherapy, with the porous matrix able to carry both antigens and immune modulators.
Keywords: porous silicon, vaccine, immunotherapy, chemotherapeutics, drug delivery
Porous nanostructures used in biomedical applications include titanium oxide, aluminum oxide, and silicon (Si).[1] Porosification of Si was discovered by the Uhlir husband and wife team in the 1950s,[2] but biomedical applications didn’t rise in popularity until the early 1990s when Leigh Canham and Ulrich Gosele reported red-orange photoluminescence and quantum confinement effects in the absorption spectrum of porous Si (pSi), respectively. [3,4] Researchers sought to use the visible light emission from pSi to drive optoelectronic devices.[5] By the mid-1990s, the chemical instability and low electroluminescence efficiency of pSi redirected interest towards biomedical applications. pSi was found to completely dissociate in aqueous solutions with pH greater than 7 to silicic acid, a natural compound found in the human body, with clearance of the compound in urine. [6,7] Abundant silicon microfabrication technologies permitted particle fabrication in diverse sizes and geometries, with tunable pore sizes that could be optimized to fit the diverse cargo.
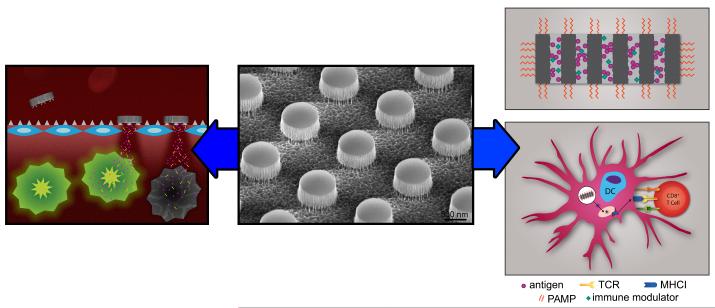
Functionalization, adsorption of drugs, and loading of diverse molecules or nanoparticles into pSi make it attractive for the delivery of chemotherapeutics, genetic material, and immune modulators. Exciting findings in the last couple of years, showing complete tumor growth blockade using hybrid particle constructs called multistage vectors (MSV; Figure 1, left), and advances in particle engineering that make it possible to create devices that mislead the host into recognizing particles as pathogens for vaccine development (Figure 1, right), are presented in this review, in addition to details on particle fabrication (Figure 1, center), degradation, and cellular transport.
Figure 1. Schematic overview presenting porous silicon microparticle fabrication and applications in drug delivery and immunotherapy.
The central image is a scanning electron micrograph showing a patterned pSi wafer prior to sonication-based release of the patterned particles. The multistage vector concept of drug delivery is shown to the left, with intravascular administration of pSi particles resulting in tumor-associated vascular accumulation based on geometric properties, charge or targeting ligands. Secondary nanoparticles, released from the porous matrix, migrate into the tumor tissue to deliver the therapeutic payload. Utilization of pSi for vaccine development is shown to the right. Particles, loaded with antigens and immune modulators, are surface-coated with pathogen-associated molecular patterns (PAMPs), leading to enhanced dendritic cell activation and particle internalization. Once internalized, the antigen cargo is processed, both within the endosomal pathway and within the proteasome, for presentation to lymphocytes in association with major histocompatibility class I and II molecules. Graphical images created by Mr. Matthew Landry, graphic artist in the Department of Nanomedicine at The Methodist Hospital Research Institute.
Novel techniques for pSi particle fabrication
pSi is typically produced through top-down porosification methods. The most commonly used method is electrochemical etching of single crystalline silicon wafers in an aqueous hydrofluoric (HF) acid solution.[8] The porous structure is controlled by the density of the etching current, the resistivity of silicon, and the concentration of the etching solution. pSi particles with defined size and shape have been fabricated using conventional photolithography,[9-11] nanoparticle lithography [12] or microdroplet patterning.[13] Recently Ryckman et al. [14] reported a rapid and low-cost approach for patterning pSi by direct imprinting of micro- and nanoscale features.
Over the last decade, the Ferrari research team has reported a series of combinatorial manufacturing protocols to fabricate pSi particles using top-down electrochemical porosification in combination with the industry standard photolithographic patterning [9-11]. The fabrication protocols consist of two major steps, formation of a pSi film and patterning of the particles. The shapes and sizes of the particles are precisely controlled and can be adjusted using a photomask. Using an i-line contact aligner, these protocols allow the production of monodisperse particles with a wide range of particle sizes (400 nm and greater), exclusively limited by the size of the lithographic pattern, with nanopore sizes ranging between 5-150 nm and porosity between 30%-90%. The fabricated particles feature non-spherical shapes with aligned cylindrical nanopores.[9,10] By altering the process flow of porosification and lithography, both hemispherical and discoidal pSi particles have been fabricated in a variety of geometrical configurations (Figure 2).
Figure 2. Porous silicon microparticles in varying geometries.
The pseudo-colored scanning electron micrograph shows a variety of pSi microparticles, fabricated in diverse sizes, shapes, and porosities. Image taken at 17,500x magnification using the FEI Nova NanoSEM housed in the Methodist Hospital Research Institute SEM/AFM Imaging Core.
As detailed by Chiappini et al.,[10] hemispherical pSi particles are fabricated by first patterning an array of circles onto a silicon nitride layer on a silicon wafer using photolithography. The array is transferred to the nitride layer by Reactive Ion Etch (RIE). Subsequently, pSi particles are formed by controlled electrochemical etch in a HF based solution.[10] A highly porous layer is formed underneath the particles to function as a release layer, retaining the particles on the substrate to allow for extensive on-wafer cleaning and treatment. Monodisperse hemispherical particles are released in isopropyl alcohol by sonication breakdown of the fragile release layer.
A novel fabrication strategy for discoidal pSi particles was described by Godin et al. [11]. pSi films were formed by electrochemical etching of Si in HF, followed by deposition of a silicon dioxide layer known as Low Temperature Oxide (LTO). This enabled direct photolithographic patterning of discoidal pSi particles on the pSi films, rendering precise and independent control over particle size, shape and porous structure. A CF4 RIE was then performed to etch through the LTO and the pSi layer to form discoidal pSi particles. Using this strategy, discoidal particles with diameters ranging from 400 to 2600 nm; heights from 200 to 700 nm; pore sizes from 5 to 150 nm; and porosities from 40 to 90% were fabricated.[11] This versatile fabrication method can be directly ported to large scale production within the framework of established semiconductor processes.
Recently, metal assisted chemical synthesis of pSi nanowires was reported,[15-17] providing a simple and cost-efficient alternative for preparing pSi particles. The morphology and porous structure of pSi nanowires are a function of metal nanoparticles, H2O2 concentration, surfactant, and silicon resistivity. By tuning the H2O2 concentration in the etching solution during synthesis, barcode pSi nanowires with multiple segments of different porosities were synthesized. Control over the dimension, shape and array density of the nanowires by means of nanoscale lithography were also demonstrated.[15] Endothelial associations with nanowires and hemispherical pSi particles are supported by confocal and scanning electron micrographs,. Nanowires, shown in Figure 3a, are imaged based on light reflectance and appear as white rods, while hemispherical particles, shown in Figure 3b, are pseudo-colored in blue.
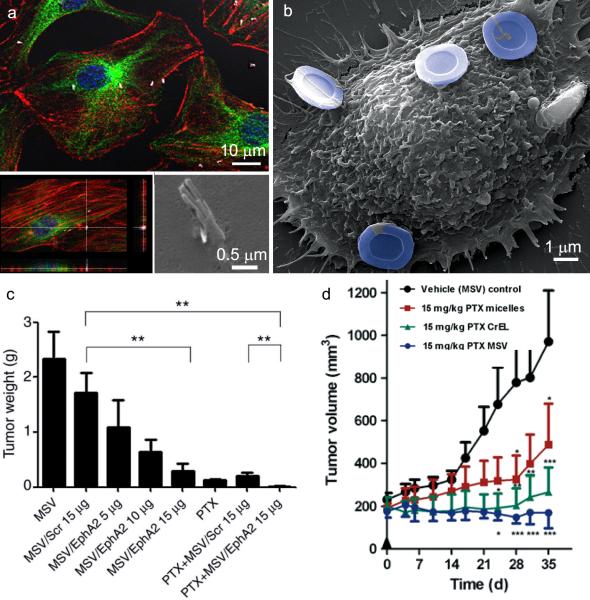
Figure 3. In vitro cellular association of pSi particles with endothelial cells and in vivo therapeutic efficacy of multistage vector delivery of EphA2 siRNA and paclitaxel.
A. Confocal (single plane and z-stacked) and an electron micrograph show 2 μm pSi nanowires located within or in the process of being internalized by endothelial cells. Cells in the confocal images are labeled with nuclear (blue) and cytoskeleton (red actin and green microtubules) dyes. B. The scanning electron micrograph shows an endothelial cell with 3 μm hemispherical pSi particles associated with the cell surface. Particles are pseudo-colored in blue. Reproduced by permission of The Royal Society of Chemistry, Nanoscale, Serda et al.[50] C. Tumor weight 6 weeks after initiation of biweekly multistage vector (MSV) /EphA2 siRNA injections (intravascular) in combination with paxlitaxel (PTX; weekly; intraperitoneal) or monotherapy in a mouse model of ovarian cancer (2 mg/kg PTX; scr = scrambled siRNA; **p<0.01). Reprinted with permission from the American Association of Cancer Research, Clinical Cancer Research, Shen H, et al. [33]. D. Antitumor efficacy of MSV loaded with PTX in female nude mice bearing MDA-MB-468 breast cancer xenografts. Treatment groups included: (i) control micelles; (ii) PTX micelles; (iii) PTX Cremophor EL (CrEL, a polyethoxylated castor oil formulation); and (iv) PTX MSV (all were treated with a single dose at Day 0; PTX 15 mg/kg). Asterisks denote results in which the difference was statistically significant compared to the control group (*p<0.05; **p<0.01; ***p<0.001). Reproduced with permission from Elsevier, Cancer Letters, Blanco E, et al. [35].
Dissolution of porous silicon
The lifespan of particles influences the rate of release of their therapeutic payload. pSi is oxidized in ambient air and at physiological pH and temperature creating an oxide monolayer.[5] While SiO2 is insoluble in acidic solutions, in basic solutions, hydroxide ions solubilize the silicon oxide layer (equation 1), driving further oxidation and dissolution. The end product is a soluble form of silicon, orthosilicic acid [Si(OH)4].
| (equation 1) |
While large pore Si particles degrade with half-lives of less than 8 hoursrsin vitro in phosphate buffered saline (PBS; pH 7.2), and serum due to their high surface area, coating increases their stability. Aminopropyl-triethoxysilane (APTES) silylation and coating of particles with short chain polyethylene glycols (PEG) has been shown to reduce rates of in vitro dissolution up to 6-fold.[7] PEGs of different lengths extend the pSi particle life-time with length being positively correlated with lifespan. However, while PEG was initially thought to be non-immunogenic,[18] reports of anti-PEG antibody production have recently been reported, with rapid particle clearance upon repeat injections.[19]
Canham and colleagues [20] first reported that pSi pore size impacts the rate of degradation, with larger pores leading to faster degradation rates. This was confirmed by Godin et al. [7] who showed that in vitro pSi particles with small pores (10 nm) solubilize slower than those with large pores (30-50 nm). While 80% of the large pore particles eroded within 24 hours in PBS at 37°C, less than 10% of the small pore particles eroded within the same time span.
Impact of serum on cellular associations with pSi particles
Both cationic and anionic particles spontaneously and selectively bind serum proteins. In vivo it is the blood plasma-derived corona that a cell sees when it encounters a particle.[21,22] While both cationic and anionic pSi particles bind to albumin and antibody, cationic particles selectively bind higher levels of fibrinogen and anionic particles absorb more apolipoproteins when incubated with plasma in vitro.[21] Interestingly, pSi particles degrade faster in serum than in PBS, indicating that the protein coat does not slow the rate of dissolution of pSi, in contrast to the protective effect seen with PEGylation of the particle surface..[7]
Serum opsonization selectively impacts cellular associations and particle internalization.[21,23] In vitro endothelial uptake of oxidized pSi microparticles is inhibited in the presence of serum; however, APTES-modified particles continue to be internalized [24]. Conversely, macrophages internalize both cationic and anionic particles, in the presence and absence of serum. Following internalization, acidification of the endosome further impacts the rate of pSi degradation, slowing the process which may provide for a slower more sustained intracellular release of the therapeutic cargo. While both cationic and anionic pSi microparticles have high rates of accumulation in the liver and spleen, cationic particles preferentially accumulate in the spleen.[21]
Cells are highly dynamic, and once internalized, pSi particles do not serve as static depots. Serda and colleagues [25] have demonstrated the transfer of pSi particles between endothelial cells, both by means of direct cell-to-cell transfer and through particle release and reuptake. Confocal and electron microscopy reveals that endothelial cells incubated with oxidized, APTES or dylight-modified pSi particles (the latter two following uptake in serum-free media) support the existence of similar transfer events.These events are enhanced by environmental triggers, such as serum deprivation, and may also be influenced by the particle’s therapeutic payload.
Porous silicon delivery of cancer therapeutics
pSi has successfully been used to deliver conventional chemotherapy agents, siRNA, and imaging agents to cancer cells. While the porous matrix enables loading with therapeutic and imaging payloads, delivery is influenced by geometry, surface modifications, and tunable degradation.[26] Based on theoretical analysis, for non-spherical particles in particular, the volume and width-to-height aspect ratio dictates the margination behavior of the particles within the blood, influencing interactions with luminal endothelial cells.[27] Godin et al. [11] reported a five-fold increase in tumor accumulation for discoidal silicon over similar diameter spherical silica beads in a mouse model of breast cancer.
Early work with pSi particles included loading with non-therapeutic payloads to study cellular uptake, loading and release kinetics.[9,28] The MSV consisted of primary pSi microparticles loaded with secondary quantum dots, single-walled carbon nanotubes, or superparamagnetic iron oxide nanoparticles. Loading and release of nanoparticles from the porous matrix was dependent on pore size and the rate of pSi degradation.[29] Later, it was demonstrated that secondary nanoparticles could be surface-modified with chitosan to enhance endosomal escape and intracellular targeting of the various particle stages to diverse locations.[30] More recently, silicon particles have been surface-modified with small peptides, such as arginine-glycine-aspartate, [31] and thioaptamers specific for E-selectin to enhance accumulation in the tumor vasculature.[32]
Silencing RNA delivery has been used in the payload-specific characterization of pSi as a therapeutic carrier. Tanaka and colleagues [33] demonstrated that hemispherical pSi particles can be used for the delivery of siRNA specific for the EphA2 oncogene, a known contributor to many types of cancer including ovarian. This work demonstrated that pSi could be loaded with of dioleoyl phosphatidylcholine (DOPC) nanoliposomes containing siRNA. A single administration of the MSV yielded downregulation of EphA2 for up to three weeks, whereas lipid-based gene delivery with DOPC alone required twice weekly injections for continuous gene silencing. These findings were reinforced by Shen and colleagues [34] who created MSV constructs using discoidal particles (Figure 3c). Biweekly MSV-EphA2 therapy induced a dose-dependent response with respect to tumor mass. Combination therapy using MSV-EphA2 and paxlitaxel (PTX) caused complete elimination of the tumor mass. These studies support the use of pSi for controlled release and protected shuttling of siRNA to provide long-term therapeutic benefits for EphA2-regulated cancer.
Several groups have used particles to enhance the delivery of conventional chemotherapeutics. Commercial and custom chemotherapy formulations include liposomes, micelles, polymers, and crystals.[35] Blanco and colleagues [36] recently demonstrated that micelles containing PTX can be loaded into discoidal pSi particles for multistage delivery. While single particle therapy with one dose of PTX micelles was not sufficient to control tumor growth, MSV delivery of PTX micelles successfully suppressed growth to levels equivalent to PTX Cremephor EL (CrEL) (Figure 3d). However, while PTX CrEL caused respiratory distress and abnormal gait, no acute responses was seen with MSV based delivery, demonstrating that MSVs can maintain drug efficacy while decreasing toxicity. Thus, while early liposome and micelle formulations have been used to make chemotherapeutics more soluble and less toxic, the MSV approach adds an additional layer of controlled delivery and release.
Other novel applications of pSi include covalent binding of chemotherapeutics like daunorubicin for sustained release,[37] multi-functional agents that deliver both chemotherapeutics and agents that heat in infrared fields to induce hyperthermic cancer cell death [38], coated agents that release chemotherapeutics when exposed to magnetic fields [39,40], combined loading of siRNA and chemotherapeutics,[41] and particles which release doxorubicin payloads based upon pH and temperature sensitivity.[42] Such strategies have demonstrated rich flexibility in pSi nanodelivery, and foreshadow many future opportunities to gain additional therapeutic benefit at lower doses from conventional anti-cancer agents.[43]
Porous silicon immunotherapy applications
A novel application of pSi is the development of adjuvants and vaccines for cancer therapy. Porous silica and silicon particles offer a means of loading antigens in aqueous media by immersion or capillary action, without the use of organic solvents.[44] Cell mediators of sustained immune responses are antigen presenting cells (APC), with dendritic cells (DCs) being recognized as potent activators of adaptive immunity. DCs engulf foreign objects by fluid-phase pinocytosis, receptor-mediated endocytosis, and phagocytosis, and secrete pro-inflammatory cytokines and chemokines. Proposed mechanisms of action for the classical adjuvant alum include sustained antigen release (depot effect) and enhanced antigen uptake by APC. However, alum-mediated immune responses are predominately humoral (antibody-based), limiting their utility for cancer therapy. Particle-based delivery systems provide opportunities to enhance cellular T helper (Th)-1 immune responses.[45]
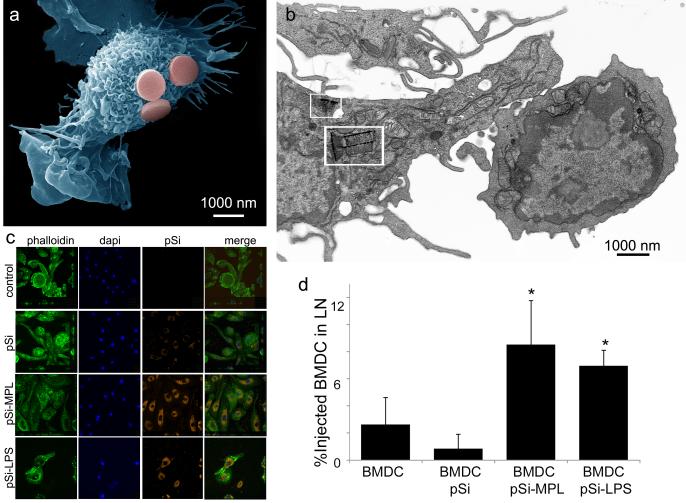
Serda and colleagues [44] have demonstrated that pSi microparticles presenting pathogen-associated molecular patterns (PAMPs) mimic pathogens, leading to enhanced ex vivo internalization of the microparticles by murine bone marrow-derived DCs (Figure 4a-c). Presentation of the toll-like receptor (TLR)-4 ligands monophosphoryl lipid A (MPL) or lipopolysaccharide (LPS) on pSi increased DC uptake of the particles (Figure 4c). However, treatment of THP-1 macrophages with cytochalasin B blocked particle uptake, but did not block IL-1β secretion, indicating that signaling through TLR-4 receptors was sufficient for activation of antigen presenting cells. TLR-4 ligands-bound pSi stimulated DCs to secrete IL-1β, IL-6, and TNF-α; increased surface expression of co-stimulatory and major histocompatibility molecules (MHC) on DC; and enhanced migration of injected BMDC to the draining lymph nodes (Figure 4d). Loading of the pSi microparticles with a peptide antigen (ovalbumin SIINFEKL) resulted in specific in vivo association of the carrier DC with T cells carrying a transgenic T cell receptor that specifically recognized the peptide in association of MHC class I molecules (Th1 immunity).
Figure 4. In vitro association of pSi microparticles with dendritic cells and in vivo migration of the particle-loaded cells to the draining lymph node.
A. The scanning electron micrograph shows a murine bone marrow-derived dendritic cell (BMDC) associating with three pSi microparticles, pseudo-colored in pink (cell false-colored blue). B. The transmission electron micrograph shows a BMDC with multiple internalized pSi particles (white boxes), associating with a T cell. C. Confocal micrographs of BMDC following 3 hr incubation with pSi microparticles. Particles are labeled with DyLight 594 and TLR-4 ligands, while cells are labeled with Oregon Green 488 phalloidin and DAPI for actin and nuclear visualization, respectively. D. In vivo percent of hock-injected CellTracker Orange-labeled BMDC migrating to the draining lymph nodes (LN) following stimulation by pSi microparticles with discrete surface functionalization (*p<0.001 compared to BMDC).Reproduced with permission from Molecular Pharmaceutics, Volume 8, Meraz et al, Activation of the Inflammasome and Enhanced Migration of Microparticle-Stimulated Dendritic Cells to the Draining Lymph Node, pages 1683-1696. Copyright 2012 American Chemical Society.
Activation of DCs with monoclonal antibodies against CD40 (CD40 mAb) have anti-tumor efficacy, however, therapeutically effective doses can cause severe side effects. Sailor and colleagues [46] demonstrated that attachment of CD40 mAb to pSi nanoparticles amplified the antibody’s activation potency with regard to B cells to levels achieved using free antibody at 30-40 fold larger concentrations. Greater nanoparticle uptake was reported to be due to engagement of either CD40 or Fc gamma receptors. Treatment of APCs with nanoparticles stimulated a dose-dependent increase in expression of co-stimulatory and MHC molecules.
Multivalent presentation of PAMPs by particles mimics repetitive presentation by live pathogens, leading to enhanced antigenicity through receptor crosslinking and immune cell activation.[47,48] It is hoped that the increase in DC activation and engulfment of particles, combined with sustained release of the antigen cargo, will enable cancer-specific immune responses and induce potent protective immunity, without the need for repeated doses of the vaccine.
Conclusions
The versatility in creating pSi particles with diverse geometries, controlled porosity, and modifiable surface functionalities create attractive devices for biomedical applications. Degradation of pSi in physiological fluids with pH greater than 7, and the ability to tune the rate of particle degradation through pore size and surface modification, thus controlling the rate of drug release, makes these particles ideal of drug and antigen delivery. Successful blockade of tumor growth with MSVs and activation of APC with pSi adjuvants support the effective use of pSi as delivery vehicles and vaccines for cancer therapy.
Future perspectives
pSi is included in the group of inorganic thermal coupling nanomaterials, which includes gold nanoparticles and single wall carbon nanotubes. Using a near-infrared light source, pSi has been shown to have heat generation abilities, attributed to high absorbance and large surface-to-volume ratio.[49] Future applications include the use of pSi particles to enhance radiofrequency-induced thermal ablation of cancer cells, expanding the versatility of pSi.
Highlights.
Porous silicon particles can be fabricated in diverse geometrical configurations and sizes
Multistage delivery of therapeutics for cancer is superior to single particle therapy
pSi microparticles presenting pathogen-associated molecular patterns activate immune cells
Acknowledgements
This research was supported by the National Institute of Health Grant U54CA151668 and U54CA143837.
Footnotes
Disclosures The multistage vector system is licensed to Leonardo Biosystems, Inc., a company founded and directed by Dr. Mauro Ferrari. Xuewu Liu has ownership interest, including patents, in Leonardo Biosystems.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsang CK, Kelly TL, Sailor MJ, Li YY. Highly stable porous silicon-carbon composites as label-free optical biosensors. Acs Nano. 2012;6(12):10546–10554. doi: 10.1021/nn304131d. [DOI] [PubMed] [Google Scholar]
- 2.Uhlir A JBSTJ, 35: 2. Electrolytic Shaping of Germanium and Silicon. Mar, 1956. pp. 333–347.
- 3.Canham LT. Silicon quantum wire array fabrication by electrochemcal and chemical dissolutions of wafers. appl Phys Lett. 1990;57(1046-1048) [Google Scholar]
- 4.Lehmann V, Gosele U. Porous silicon formation - a quantum wire effect. Applied Physics Letters. 1991;58(8):856–858. [Google Scholar]
- 5.Sailor MFopspPsipp, characterization, and applications. First Ed Wiley-VCH Verlag GmbH & Co. KGaA; 2012. pp. 1–42. [Google Scholar]
- 6.Park JH, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater. 2009;8(4):331–336. doi: 10.1038/nmat2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godin B, Gu J, Serda RE, Bhavane R, Tasciotti E, Chiappini C, Liu X, Tanaka T, Decuzzi P, Ferrari M. Tailoring the degradation kinetics of mesoporous silicon structures through pegylation. Journal of biomedical materials research Part A. 2010;94(4):1236–1243. doi: 10.1002/jbm.a.32807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canham LT, INSPEC (Information service) Properties of porous silicon. INSPEC; London: 1987. [Google Scholar]
- 9.Tasciotti E, Liu X, Bhavane R, Plant K, Leonard AD, Price BK, Cheng MM, Decuzzi P, Tour JM, Robertson F, Ferrari M. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol. 2008;3(3):151–157. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 10.Chiappini C, Tasciotti E, Fakhoury J, Fine D, Pullan L, Wang Y, Fu L, Liu X, Ferrari M. Tailored porous silicon microparticles: Fabrication and properties. ChemPhysChem. 2010;11(5):1029–1035. doi: 10.1002/cphc.200900914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godin B, Chiappini C, Srinivasan S, Alexander J, Yokoi K, Ferrari M, Decuzzi P, Liu X. Discoidal porous silicon particles: Fabrication and biodistribution in breast cancer bearing mice. Advanced Functional Materials. 2012;22(20):4225–4235. doi: 10.1002/adfm.201200869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JS, Meade SO, Segal E, Sailor MJ. Porous silicon-based polymer replicas formed by bead patterning. physica status solidi (a) 2007;204(5):1383–1387. [Google Scholar]
- 13.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into tgf-beta-secreting cells inducing cd4+cd25+ regulatory t cell proliferation. J Exp Med. 2005;202(7):919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryckman JD, Liscidini M, Sipe JE, Weiss SM. Direct imprinting of porous substrates: A rapid and low-cost approach for patterning porous materials. Nano Lett. 2010;11(5):1857–1862. doi: 10.1021/nl1028073. [DOI] [PubMed] [Google Scholar]
- 15.Chiappini C, Liu X, Fakhoury JR, Ferrari M. Biodegradable porous silicon barcode nanowires with defined geometry. Advanced Functional Materials. 2010;20(14):2231–2239. doi: 10.1002/adfm.201000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hochbaum AI, Gargas D, Hwang YJ, Yang P. Single crystalline mesoporous silicon nanowires. Nano Lett. 2009;9(10):3550–3554. doi: 10.1021/nl9017594. [DOI] [PubMed] [Google Scholar]
- 17.Qu Y, Liao L, Li Y, Zhang H, Huang Y, Duan X. Electrically conductive and optically active porous silicon nanowires. Nano Lett. 2009;9(12):4539–4543. doi: 10.1021/nl903030h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wattendorf U, Merkle HP. Pegylation as a tool for the biomedical engineering of surface modified microparticles. J Pharm Sci. 2008;97(11):4655–4669. doi: 10.1002/jps.21350. [DOI] [PubMed] [Google Scholar]
- 19.Ishihara T, Takeda M, Sakamoto H, Kimoto A, Kobayashi C, Takasaki N, Yuki K, Tanaka KI, Takenaga M, Igarashi R, Maeda T, et al. Accelerated blood clearance phenomenon upon repeated injection of peg-modified pla-nanoparticles. Pharm Res-Dord. 2009;26(10):2270–2279. doi: 10.1007/s11095-009-9943-x. [DOI] [PubMed] [Google Scholar]
- 20.Canham LT. Bioactive silicon structure fabrication through nanoetching techniques. Adv Mater. 1995;7(12):1033. &. [Google Scholar]
- 21.Serda RE, Blanco E, Mack A, Stafford SJ, Amra S, Li Q, van de Ven A, Tanaka T, Torchilin VP, Wiktorowicz JE, Ferrari M. Proteomic analysis of serum opsonins impacting biodistribution and cellular association of porous silicon microparticles. Mol Imaging. 2011;10(1):43–55. [PMC free article] [PubMed] [Google Scholar]
- 22.Walczyk D, Bombelli FB, Monopoli MP, Lynch I, Dawson KA. What the cell “sees” in bionanoscience. Journal of the American Chemical Society. 2010;132(16):5761–5768. doi: 10.1021/ja910675v. [DOI] [PubMed] [Google Scholar]
- 23.Serda RE, Gu J, Bhavane RC, Liu X, Chiappini C, Decuzzi P, Ferrari M. The association of silicon microparticles with endothelial cells in drug delivery to the vasculature. Biomaterials. 2009;30(13):2440–2448. doi: 10.1016/j.biomaterials.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Serda RE, Gu J, Burks JK, Ferrari K, Ferrari C, Ferrari M. Quantitative mechanics of endothelial phagocytosis of silicon microparticles. Cytometry Part A : the journal of the International Society for Analytical Cytology. 2009;75(9):752–760. doi: 10.1002/cyto.a.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Ferrati S, Shamsudeen S, Summers HD, Rees P, Abbey JV, Schmulen J, Liu X, Wong ST, Bean AJ, Ferrari M, Serda RE. Inter-endothelial transport of microvectors using cellular shuttles and tunneling nanotubes. Small. 2012;8(20):3151–3160. doi: 10.1002/smll.201200472. [DOI] [PubMed] [Google Scholar]
- 26.Serda RE, Godin B, Blanco E, Chiappini C, Ferrari M. Multi-stage delivery nano-particle systems for therapeutic applications. Biochim Biophys Acta. 2011;1810(3):317–329. doi: 10.1016/j.bbagen.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decuzzi P, Ferrari M. The receptor-mediated endocytosis of nonspherical particles. Biophysical journal. 2008;94(10):3790–3797. doi: 10.1529/biophysj.107.120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serda RE, Mack A, Pulikkathara M, Zaske AM, Chiappini C, Fakhoury JR, Webb D, Godin B, Conyers JL, Liu XW, Bankson JA, et al. Cellular association and assembly of a multistage delivery system. Small. 2010;6(12):1329–1340. doi: 10.1002/smll.201000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godin B, Tasciotti E, Liu X, Serda RE, Ferrari M. Multistage nanovectors: From concept to novel imaging contrast agents and therapeutics. Accounts of chemical research. 2011;44(10):979–989. doi: 10.1021/ar200077p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serda RE, Mack A, van de Ven AL, Ferrati S, Dunner K, Jr., Godin B, Chiappini C, Landry M, Brousseau L, Liu X, Bean AJ, et al. Logic-embedded vectors for intracellular partitioning, endosomal escape, and exocytosis of nanoparticles. Small. 2010;6(23):2691–2700. doi: 10.1002/smll.201000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Ven AL, Kim P, Haley O, Fakhoury JR, Adriani G, Schmulen J, Moloney P, Hussain F, Ferrari M, Liu X, Yun SH, et al. Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution. J Control Release. 2012;158(1):148–155. doi: 10.1016/j.jconrel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mann AP, Bhavane RC, Somasunderam A, Liz Montalvo-Ortiz B, Ghaghada KB, Volk D, Nieves-Alicea R, Suh KS, Ferrari M, Annapragada A, Gorenstein DG, et al. Thioaptamer conjugated liposomes for tumor vasculature targeting. Oncotarget. 2011;2(4):298–304. doi: 10.18632/oncotarget.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka T, Mangala LS, Vivas-Mejia PE, Nieves-Alicea R, Mann AP, Mora E, Han HD, Shahzad MM, Liu X, Bhavane R, Gu J, et al. Sustained small interfering rna delivery by mesoporous silicon particles. Cancer Res. 2010;70(9):3687–3696. doi: 10.1158/0008-5472.CAN-09-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Shen H, Rodriguez-Aguayo C, Xu R, Gonzalez-Villasana V, Mai J, Huang Y, Zhang G, Guo X, Bai L, Qin G, Deng X, et al. Enhancing chemotherapy response with sustained epha2 silencing using multistage vector delivery. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(7):1806–1815. doi: 10.1158/1078-0432.CCR-12-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Molecular pharmaceutics. 2011;8(6):2101–2141. doi: 10.1021/mp200394t. [DOI] [PubMed] [Google Scholar]
- 36**.Blanco E, Sangai T, Hsiao A, Ferrati S, Bai L, Liu X, Meric-Bernstam F, Ferrari M. Multistage delivery of chemotherapeutic nanoparticles for breast cancer treatment. Cancer letters. 2012 doi: 10.1016/j.canlet.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Chhablani J, Nieto A, Hou H, Wu EC, Freeman WR, Sailor MJ, Cheng L. Oxidized porous silicon particles covalently grafted with daunorubicin as a sustained intraocular drug delivery system. Investigative ophthalmology & visual science. 2013;54(2):1268–1279. doi: 10.1167/iovs.12-11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma M, Chen H, Chen Y, Wang X, Chen F, Cui X, Shi J. Au capped magnetic core/mesoporous silica shell nanoparticles for combined photothermo-/chemo-therapy and multimodal imaging. Biomaterials. 2012;33(3):989–998. doi: 10.1016/j.biomaterials.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 39.Thomas CR, Ferris DP, Lee JH, Choi E, Cho MH, Kim ES, Stoddart JF, Shin JS, Cheon J, Zink JI. Noninvasive remote-controlled release of drug molecules in vitro using magnetic actuation of mechanized nanoparticles. Journal of the American Chemical Society. 2010;132(31):10623–10625. doi: 10.1021/ja1022267. [DOI] [PubMed] [Google Scholar]
- 40.Liu Q, Zhang J, Xia W, Gu H. Towards magnetic-enhanced cellular uptake, mri and chemotherapeutics delivery by magnetic mesoporous silica nanoparticles. Journal of nanoscience and nanotechnology. 2012;12(10):7709–7715. doi: 10.1166/jnn.2012.6618. [DOI] [PubMed] [Google Scholar]
- 41.Chen AM, Zhang M, Wei D, Stueber D, Taratula O, Minko T, He H. Co-delivery of doxorubicin and bcl-2 sirna by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small. 2009;5(23):2673–2677. doi: 10.1002/smll.200900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu X, Hao X, Wu Y, Zhang J, Zhang X, Wang PC, Zou G, Liang XJ. Multifunctional hybrid silica nanoparticles for controlled doxorubicin loading and release with thermal and ph dually response. Journal of materials chemistry B, Materials for biology and medicine. 2013;1(8):1109–1118. doi: 10.1039/C2TB00223J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanco E, Hsiao A, Ruiz-Esparza GU, Landry MG, Meric-Bernstam F, Ferrari M. Molecular-targeted nanotherapies in cancer: Enabling treatment specificity. Molecular oncology. 2011;5(6):492–503. doi: 10.1016/j.molonc.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Meraz IM, Melendez B, Gu J, Wong ST, Liu X, Andersson HA, Serda RE. Activation of the inflammasome and enhanced migration of microparticle-stimulated dendritic cells to the draining lymph node. Mol Pharm. 2012 doi: 10.1021/mp3001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Serda RE. Particle platforms for cancer immunotherapy. International journal of nanomedicine. 2013;8:1–14. doi: 10.2147/IJN.S31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu L, Ruff LE, Qin Z, Corr M, Hedrick SM, Sailor MJ. Multivalent porous silicon nanoparticles enhance the immune activation potency of agonistic cd40 antibody. Adv Mater. 2012;24(29):3981–3987. doi: 10.1002/adma.201200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Little SR. Reorienting our view of particle-based adjuvants for subunit vaccines. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(4):999–1000. doi: 10.1073/pnas.1120993109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baumgartner CK, Malherbe LP. Regulation of cd4 t-cell receptor diversity by vaccine adjuvants. Immunology. 2010;130(1):16–22. doi: 10.1111/j.1365-2567.2010.03265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong C, Lee J, Zheng H, Hong SS, Lee C. Porous silicon nanoparticles for cancer photothermotherapy. Nanoscale research letters. 2011;6(1):321. doi: 10.1186/1556-276X-6-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serda RE, Ferrati S, Godin B, Tasciotti E, Liu X, Ferrari M. Mitotic trafficking of silicon microparticles. Nanoscale. 2009;1(2):250–259. doi: 10.1039/b9nr00138g. [DOI] [PMC free article] [PubMed] [Google Scholar]