Abstract
Purpose
In this prospective National Cancer Institute–funded American College of Radiology Imaging Network/Radiation Therapy Oncology Group cooperative group trial, we hypothesized that standardized uptake value (SUV) on post-treatment [18F]fluorodeoxyglucose positron emission tomography (FDG-PET) correlates with survival in stage III non–small-cell lung cancer (NSCLC).
Patients and Methods
Patients received conventional concurrent platinum-based chemoradiotherapy without surgery; postradiotherapy consolidation chemotherapy was allowed. Post-treatment FDG-PET was performed at approximately 14 weeks after radiotherapy. SUVs were analyzed both as peak SUV (SUVpeak) and maximum SUV (SUVmax; both institutional and central review readings), with institutional SUVpeak as the primary end point. Relationships between the continuous and categorical (cutoff) SUVs and survival were analyzed using Cox proportional hazards multivariate models.
Results
Of 250 enrolled patients (226 were evaluable for pretreatment SUV), 173 patients were evaluable for post-treatment SUV analyses. The 2-year survival rate for the entire population was 42.5%. Pretreatment SUVpeak and SUVmax (mean, 10.3 and 13.1, respectively) were not associated with survival. Mean post-treatment SUVpeak and SUVmax were 3.2 and 4.0, respectively. Post-treatment SUVpeak was associated with survival in a continuous variable model (hazard ratio, 1.087; 95% CI, 1.014 to 1.166; P = .020). When analyzed as a prespecified binary value (≤ v > 3.5), there was no association with survival. However, in exploratory analyses, significant results for survival were found using an SUVpeak cutoff of 5.0 (P = .041) or 7.0 (P < .001). All results were similar when SUVmax was used in univariate and multivariate models in place of SUVpeak.
Conclusion
Higher post-treatment tumor SUV (SUVpeak or SUVmax) is associated with worse survival in stage III NSCLC, although a clear cutoff value for routine clinical use as a prognostic factor is uncertain at this time.
INTRODUCTION
Stage III, nonoperative non–small-cell lung cancer (NSCLC) is common and, despite improvements in treatment, still has a poor prognosis.1 With modern staging and treatment (definitive concurrent chemoradiotherapy, with radiation dose of approximately 60 Gy), median and 2-year survival rates for medically fit patients are about 20 months and 40%, respectively.2,3
One challenge with concurrent chemoradiotherapy is difficulty ascertaining disease status soon after treatment. Computed tomography (CT) of the chest is commonly obtained after treatment but is difficult to interpret after radiotherapy. Many patients with stable or even responding disease after chemoradiotherapy, based on CT, have viable active malignancy on pathologic assessment.4
It has been suggested that positron emission tomography (PET) is superior to CT for post-treatment evaluation of NSCLC.5 PET has efficacy for staging of NSCLC, with improved sensitivity and specificity for detection of regional or distant metastatic disease compared with conventional staging.6 In patients with presumed stage III disease, PET has value by detecting patients with otherwise occult stage IV disease.7 PET is also useful in defining gross tumor volume for treatment planning, particularly distinguishing regions of atelectasis from viable, metabolically active tumor mass and identifying involvement of regional lymph nodes.8,9 Given the value of PET in other settings, we hypothesized that it would be useful as a biomarker to assess response after chemoradiotherapy, as suggested by a series of retrospective studies from Australia.10 We hypothesized in this prospective study that [18F]fluorodeoxyglucose (FDG) PET obtained relatively soon after chemoradiotherapy could predict long-term prognosis. Specifically, we sought to determine whether the post-treatment, primary tumor FDG standardized uptake value (SUV) could serve as a useful prognostic imaging biomarker.
PATIENTS AND METHODS
This multicenter, National Cancer Institute–funded prospective study (ClinicalTrials.gov identifier: NCT00083083) was conducted jointly by the American College of Radiology Imaging Network (ACRIN), the primary cooperative group, and the Radiation Therapy Oncology Group (RTOG). Each participating institution (Appendix Table A1, online only) obtained institutional review board approval before accrual, and all patients provided written study-specific informed consent.
Study Patients
Eligible patients had inoperable stage III (or selected inoperable stage II) NSCLC, with no evidence of stage IV disease by conventional imaging (CT of chest/upper abdomen, bone scintigraphy, and CT/magnetic resonance imaging of the brain). Patients who had PET before being enrolled were eligible, as long as it was recent (≤ 6 weeks) and performed on an ACRIN-qualified scanner. This technically allowed patients who had evidence of stage IV disease by pre-enrollment PET to register on study. However, patients had to be considered candidates for definitive concurrent chemoradiotherapy, and this was intended to exclude patients with confirmed stage IV disease by pre-enrollment PET. A Zubrod performance status 0 to 1 was required, along with medical suitability for concurrent chemoradiotherapy. Patients had to be capable of undergoing FDG-PET, with fasting glucose ≤ 200 mg/dL. Patients who had prior thoracic radiotherapy or were planned for thoracic surgery were not eligible.
Treatment
The protocol did not specify the details of chemoradiotherapy, as long as it included immediate concurrent, platinum-based doublet chemotherapy with radiotherapy. Radiotherapy fields had to include all gross disease seen on pretreatment PET, with dose ≥ 60 Gy. Because of logistical constraints, central review of radiotherapy fields/portals was not performed. In addition to cisplatin (or carboplatin), a second concurrent nonplatinum agent was required (eg, paclitaxel, etoposide). Adjuvant chemotherapy was allowed for up to 16 weeks after radiotherapy but was not mandatory. Patients could be treated on separate, institutional review board–approved therapeutic clinical trials as long as the general eligibility for this study was met.
FDG-PET
It was judged impractical to exclude patients who already had a pre-enrollment PET, as long as the scan was completed ≤ 6 weeks before enrollment. Because the primary end point of this study was post-treatment PET, the protocol did not mandate a new PET after enrollment if a high-quality PET was already done on an ACRIN-qualified scanner. The details of the scanner qualification process have been described previously.12
Conventional modern equipment/techniques for FDG-PET (with or without PET/CT) were used in this study. Patients had to fast for ≥ 4 hours and have a blood glucose level less than 200 mg/dL before FDG injection. The FDG dose was not mandated; the recommended dose was 0.14 to 0.21 mCi/kg (approximately 10 to 20 mCi) for scanners with bismuth germanate, lutetium oxyorthosilicate, or gadolinium oxyorthosilicate detectors. Sodium iodide detector scanners were not allowed. Emission scanning began 50 to 70 minutes after FDG injection and included the body from upper/mid neck to proximal femurs. Acquisition times for emission and transmission scans were in accordance with the manufacturer's recommendations.
Post-treatment PET was required at approximately 14 weeks (12 to 16 weeks) after radiotherapy (and at least 4 weeks after the completion of adjuvant chemotherapy, if applicable). The protocol required that both pre- and post-treatment scans be done on the same scanner.
PET Image Interpretation and SUV Measurement
PET scans were interpreted qualitatively and quantitatively by nuclear medicine physicians/radiologists at each institution, using standardized reporting forms to record the FDG uptake in the primary tumor, regional lymph nodes, and common sites of distant metastasis (ie, bones, adrenals, liver, contralateral lung). These local reviewers were provided with educational materials on image interpretation, specifically describing how to measure peak SUV (SUVpeak). However, formal demonstration of expertise was not mandated. SUVs for regions of interest (ROIs) were determined using two different metrics, maximum SUV (SUVmax) and SUVpeak. SUVmax represents the highest single-voxel SUV within the ROI. SUVpeak, in contrast, represents the mean SUV within a small circular ROI (0.75 to 1.5 cm in diameter) that encompasses the SUVmax. (Thus, SUVpeak will always be lower than SUVmax.) A detailed discussion of the potential advantages and disadvantages of studying SUVmax versus SUVpeak is beyond the scope of this article, although briefly addressed in the Discussion.
In addition to the institutional interpretations, pre- and post-treatment PET scans were centrally reviewed at ACRIN by an expert nuclear medicine physician with extensive experience in FDG-PET. A single dedicated workstation was used for this purpose, and SUVpeak was measured with an automated program in a circular ROI 1.5 cm in diameter. The central reader was blinded to clinical data and the institutional SUV measurements.
Follow-Up
Patients were observed for a minimum of 2 years (or until death) after completion of treatment in accordance with standard clinical practice. Nonprotocol PET was allowed but not mandated.
Statistical Analysis
The primary objective of this study was to determine the relationship between institutional-determined post-treatment SUVpeak and overall survival (SUVmax analysis was a secondary end point). Several ways of analyzing the relationship between SUV and survival were prespecified. On the basis of a small study by Rosenzweig et al,12 our primary end point was to associate survival using Cox proportional hazards modeling with post-treatment SUVpeak as a binary predictor, with a cutoff SUV of 3.5. However, in addition to the binary predictor, the following prespecified analyses were planned: a four-category model (SUV ≤ 2, 2 to 3.5, 3.5 to 7, and > 7), and a continuous model of SUV measurements. Prespecified secondary analyses using pretreatment SUV also were performed.
Exploratory evaluation of other potential SUV cutoffs was also performed, as a post hoc, non-prespecified analysis. The power calculation and detailed statistical analyses can be found in the Appendix (online only).
RESULTS
Patient Population
Accrual began in June 2005 and ended in May 2009. Thirty-seven institutions accrued 250 patients to the study. Sixteen patients were ineligible, and eight patients did not have evaluable pretreatment PET, leaving 226 patients. Of these 226 patients, 173 had evaluable post-treatment PET, representing the analysis cohort for the primary end point. Details on eligibility and evaluability are listed in Appendix Tables A2, A3, and A4 (online only); the most common reason for ineligibility was failure to perform one or more clinical staging tests within protocol-specified time periods before enrollment. The most common reason for subsequent inevaluability for post-treatment analysis was early death. Approximately 89% of PET scans were PET/CT studies. Details regarding the patient population are listed in Table 1.
Table 1.
Patient Demographics and Clinical Characteristics, Both for the Original Population of All Registered Patients and the Final Population of Patients Evaluable for the Primary End Point of Post-Treatment SUV Analyses
| Demographic or Clinical Characteristic | All Patients Registered (N = 250) |
Subgroup of Patients Evaluable for the Primary End Point (n = 173) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 65.5 | 65 | ||
| Range | 36-85 | 36-84 | ||
| Race* | ||||
| White | 184 | 73.6 | 135 | 78.0 |
| African American | 29 | 11.6 | 15 | 8.7 |
| Asian | 32 | 12.8 | 22 | 12.7 |
| Other/unknown | 9 | 3.6 | 3 | 1.7 |
| Clinical stage† | ||||
| IIB | 9 | 3.6 | 7 | 4.1 |
| IIIA | 123 | 49.2 | 96 | 55.5 |
| IIIB | 114 | 45.6 | 70 | 40.5 |
| IV‡ | 1 | 0.4 | 0 | 0.0 |
| Data not available | 3 | 1.2 | 0 | 0.0 |
| Sex | ||||
| Male | 162 | 64.8 | 112 | 64.7 |
| Female | 88 | 35.2 | 61 | 35.3 |
| Performance status | ||||
| 0 (fully active) | 111 | 44.4 | 84 | 48.6 |
| 1 (ambulatory, capable of light work) | 137 | 54.8 | 89 | 51.5 |
| Data not available | 2 | 0.8 | 0 | 0.0 |
| Chemotherapy regimen | ||||
| Carboplatin/paclitaxel | 102 | 40.8 | 73 | 42.2 |
| Cisplatin/etoposide | 36 | 14.4 | 29 | 16.8 |
| Other | 93 | 37.2 | 71 | 41.0 |
| Data not available | 19 | 7.6 | 0 | 0 |
| Adjuvant (postradiotherapy) chemotherapy given | ||||
| Yes | 100 | 40.0 | 78 | 45.1 |
| No | 129 | 51.6 | 94 | 54.3 |
| Data not available (unknown) | 21 | 8.4 | 1 | 0.6 |
| Radiation dose, Gy | ||||
| < 50 | 10 | 4.0 | 0 | 0 |
| 50-60 Gy | 11 | 4.4 | 8 | 4.6 |
| 60-70 Gy | 153 | 61.2 | 124 | 71.7 |
| ≥ 70 Gy | 56 | 22.4 | 39 | 22.5 |
| Data not available | 20 | 8.0 | 2 | 1.2 |
Abbreviation: SUV, standardized uptake value.
Multiple races may be endorsed by a single participant, such that the total over all options may sum to greater than 100%.
One patient with clinical stage recorded only as stage III was grouped into the stage IIIB row for both columns.
One patient was enrolled with oligometastatic stage IV disease.
SUV Measurements
The SUV results (SUVpeak and SUVmax) are listed in Table 2. The median and mean pretreatment values vary between 9.45 and 13.96. (As expected, there is a difference of approximately 20% between SUVpeak and SUVmax.) Median and mean post-treatment SUVs vary between 2.50 and 3.95, again with SUVmax values being higher than SUVpeak.
Table 2.
Distributional Summary of SUV Data Based on Timing of PET and Location of the Reading
| Variable | No. of Evaluable Patients | SUV |
||
|---|---|---|---|---|
| Median | Mean | SD | ||
| Pretreatment PET | ||||
| SUVpeak | ||||
| Institutional review | 226 | 9.45 | 10.28 | 6.24 |
| Central review | 225 | 10.40 | 11.40 | 6.39 |
| SUVmax | ||||
| Institutional review | 226 | 12.10 | 13.09 | 7.24 |
| Central review | 225 | 12.80 | 13.96 | 7.99 |
| Post-treatment PET | ||||
| SUVpeak | ||||
| Institutional review | 173 | 2.50 | 3.22 | 2.59 |
| Central review | 174 | 2.50 | 3.20 | 2.53 |
| SUVmax | ||||
| Institutional review | 170 | 2.90 | 3.95 | 3.33 |
| Central review | 174 | 2.90 | 3.89 | 3.21 |
Abbreviations: PET, positron emission tomography; SD, standard deviation; SUV, standardized uptake value; SUVmax, maximum standardized uptake value; SUVpeak, peak standardized uptake value.
Although on a population analysis, the mean and median SUVs for institutional versus central reads do not differ greatly, there were some cases with significant discrepancies. As shown in Figure 1, the concordance correlation coefficients for pre- and post-treatment SUVpeak were 0.72 (95% CI, 0.65 to 0.78) and 0.80 (95% CI, 0.74 to 0.85), respectively. The corresponding values for pre- and post-treatment SUVmax were better than those for SUVpeak, at 0.90 (95% CI, 0.88 to 0.92) and 0.87 (95% CI, 0.83 to 0.90), respectively.
Fig 1.
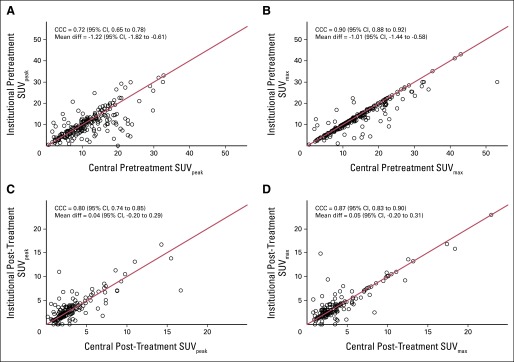
Scatter plots demonstrating institutional versus central read results for standardized uptake value (SUV) within the primary lung tumor. Pretreatment (A) peak SUV (SUVpeak) and (B) maximum SUV (SUVmax). Post-treatment (C) SUVpeak and (D) SUVmax. CCC, concordance correlation coefficient; Mean diff, mean difference between institutional SUV and central SUV.
Post-Treatment SUV and Survival
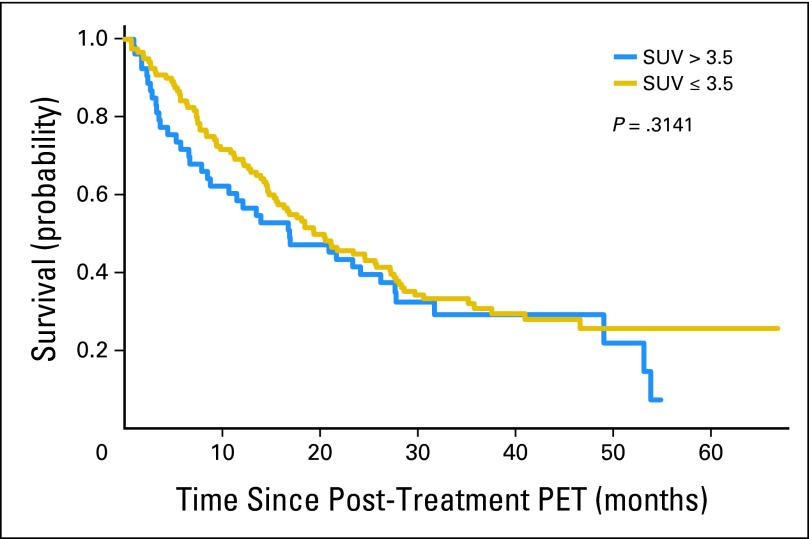
Overall survival based on the study-prespecified SUVpeak cutoff of 3.5 is shown in Figure 2. Measured from the date of enrollment, the 2-year actuarial survival rate for patients with institutional post-treatment SUVpeak ≤ 3.5 was 51% (95% CI, 41% to 59%) compared with 47% (95% CI, 33% to 60%) for patients with an SUVpeak greater than 3.5 (P = .29). Measured from the date of post-treatment PET, the corresponding 2-year survival rates were 45% (95% CI, 36% to 53%) versus 40% (95% CI, 26% to 52%), respectively (P = .31).
Fig 2.
Overall survival as a function of post-treatment peak standardized uptake value (SUV; ≤ v > 3.5). The results are not statistically significantly different. PET, positron emission tomography.
Similarly, separating the study population using quartiles for institutional-measured post-treatment SUVpeak was not significant (P = .15, type III test). However, the prespecified subgroup analysis for SUVpeak greater than 7 was highly significant (P < .001).
When SUVpeak was analyzed as a continuous variable (prespecified), it was significantly associated with survival (hazard ratio [HR], 1.086; P = .011). The HR in the multivariate model was 1.087 (P = .020; ie, an increase of 1.0 point in post-treatment SUVpeak translated into a 9% increase in the risk of death).
When these analyses were repeated using central SUVs (prespecified), as opposed to the institutional SUVs, the multivariate model incorporating central-review SUVpeak as a continuous variable showed a slightly stronger association with survival (HR, 1.125; P ≤ .001) than when institutional values were used.
As shown in Table 3, the results were not substantially different when SUVmax was used in place of SUVpeak. Table 3 summarizes the association between post-treatment SUV measurements and survival using various cutoff values of SUV, for both institutional and central reads (P values not adjusted for multiple comparisons). Appendix Tables A5 and A6 (online only) display the results of multivariate analyses of prognostic factors, showing that post-treatment SUV was the only statistically significant prognostic factor (P values for age and performance status were between .05 and .15).
Table 3.
Summary of the Results of Univariate and Multivariate (Cox regression) Models for Overall Survival as a Function of SUV
| Type of Analysis* and SUV Cutoff Values | Institutional Read |
Central Read |
||
|---|---|---|---|---|
| Hazard Ratio | P | Hazard Ratio | P | |
| SUVpeak | ||||
| Continuous | ||||
| Univariate | 1.086 | .011† | 1.125 | < .001† |
| Multivariate | 1.087 | .020† | 1.125 | < .001† |
| Categorical | ||||
| Univariate, ≤ v > 3.5 | 1.215 | .315 | 1.317 | .177 |
| Multivariate, ≤ v > 3.5 | 1.197 | .379 | 1.308 | .201 |
| Univariate, ≤ v > 5‡ | 1.713 | .021† | 2.145 | .001† |
| Multivariate, ≤ v > 5‡ | 1.667 | .041† | 2.148 | .002† |
| Univariate§ | ||||
| 2-3.5 (v < 2) | 1.182 | .446 | 1.126 | .578 |
| 3.5-7 (v < 2) | 0.983 | .946 | 1.012 | .965 |
| > 7 (v < 2) | 4.138 | < .001† | 2.938 | < .001† |
| Multivariate§ | ||||
| 2-3.5 (v < 2) | 1.190 | .455 | 1.150 | .543 |
| 3.5-7 (v < 2) | 0.968 | .903 | 1.016 | .956 |
| > 7 (v < 2) | 4.389 | < .001† | 3.051 | < .001† |
| Univariate§‖ | ||||
| Q2 v Q1 | 1.369 | .208 | 1.189 | .483 |
| Q3 v Q1 | 0.914 | .736 | 0.922 | .765 |
| Q4 v Q1 | 1.405 | .182 | 1.367 | .205 |
| Multivariate§‖ | ||||
| Q2 v Q1 | 1.501 | .126 | 1.075 | .780 |
| Q3 v Q1 | 0.875 | .635 | 0.879 | .653 |
| Q4 v Q1 | 1.351 | .263 | 1.289 | .322 |
| SUVmax | ||||
| Continuous | ||||
| Univariate | 1.089 | .002† | 1.101 | < .001† |
| Multivariate | 1.084 | .005† | 1.098 | < .001† |
| Categorical | ||||
| Univariate, ≤ v > 3.5 | 0.942 | .751 | 1.149 | .459 |
| Multivariate, ≤ v > 3.5 | 0.863 | .461 | 1.182 | .389 |
| Univariate, ≤ v > 5‡ | 1.410 | .112 | 1.597 | .035† |
| Multivariate, ≤ v > 5‡ | 1.377 | .159 | 1.596 | .038† |
| Univariate§ | ||||
| 2-3.5 (v < 2) | 1.569 | .061 | 1.224 | .401 |
| 3.5-7 (v < 2) | 0.916 | .752 | 0.946 | .841 |
| > 7 (v < 2) | 2.272 | .007† | 2.382 | .003† |
| Multivariate§ | ||||
| 2-3.5 (v < 2) | 1.906 | .014† | 1.133 | .621 |
| 3.5-7 (v < 2) | 0.950 | .860 | 0.953 | .866 |
| > 7 (v < 2) | 2.273 | .012† | 2.260 | .010† |
| Univariate§‖ | ||||
| Q2 v Q1 | 1.416 | .170 | 1.257 | .364 |
| Q3 v Q1 | 1.034 | .898 | 0.969 | .909 |
| Q4 v Q1 | 1.345 | .248 | 1.454 | .143 |
| Multivariate§‖ | ||||
| Q2 v Q1 | 1.578 | .090 | 1.165 | .560 |
| Q3 v Q1 | 1.105 | .716 | 0.940 | .831 |
| Q4 v Q1 | 1.348 | .267 | 1.383 | .215 |
Abbreviations: Q, quartile; SUV, standardized uptake value; SUVmax, maximum standardized uptake value; SUVpeak, peak standardized uptake value.
Under the univariate setting, the only covariate in the Cox regression model is the corresponding SUV. Under the multivariate setting, the SUV and other prespecified covariates were included in the model.
Significant.
The analysis using a cutoff SUV of 5 was not a study-prespecified end point. This was performed as an exploratory secondary analysis.
Multiple comparisons were adjusted for in these comparisons via the Bonferroni correction (ie, the cutoff for declaring significance changed to be .05/3, or P = .0167).
Cutoffs correspond to the quartiles of the SUV distribution, where Q1 = 25% quartile, Q2 = median, and Q3 = 75% quartile.
Protocol-Specified Analyses of Pretreatment SUV and Survival
Pretreatment SUV, analyzed as either a continuous or categorical variable, was not associated with survival on univariate or multivariable analyses.
Exploratory Analyses of Post-Treatment SUV and Survival
We explored other potential cutoff values for SUV. When using a binary cutoff of 5.0 for post-treatment SUVpeak, a highly significant association with survival was noted (Fig 3). This was true with both institutional (HR, 1.667; P = .041) and central reads (HR, 2.148; P = .002). Using central reads, the 2-year actuarial survival (measured from enrollment) for patients with SUVpeak ≤ 5.0 was 53% (95% CI, 44% to 60%), compared with 38% (95% CI, 19% to 56%) for patients with SUVpeak greater than 5.0 (P = .001). The corresponding rates from dates of the post-treatment PET were 47% (95% CI 39% to 55%) versus 25% (95% CI 10% to 43%), respectively (P = .001).
Fig 3.
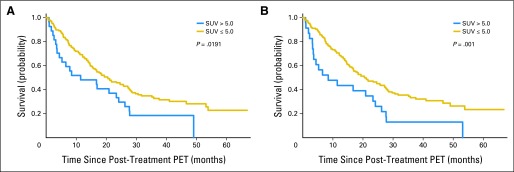
Overall survival based on a peak standardized uptake value (SUV) cutoff of 5.0. (A) Results based on local institutional read. (B) Results based on central review read. PET, positron emission tomography.
Data from several additional exploratory analyses are shown in the Appendix and Appendix Tables A7 to A9 (online only). These include analyses in which various subpopulations of patients with certain protocol violations or uncommon features were excluded (eg, excluding patients with clinical stage IIB disease). An evaluation of the relative change in SUV [ie, (posttreatment SUV – pretreatment SUV)/pretreatment SUV] as a potential predictor of outcome was also performed (this showed no significant associations).
DISCUSSION
To our knowledge, this is the largest prospective study to date evaluating the prognostic value of post-treatment PET findings in patients with stage III NSCLC. Our specific aim was partially met; post-treatment SUVpeak was significantly associated with survival based on a continuous variable statistical model. However, our primary prespecified statistical analysis (binary division of the population into groups with post-treatment SUVpeak ≤ v > 3.5) was not significant; we conclude that using a simple post-treatment SUVpeak (or SUVmax) cutoff of 3.5 after chemoradiotherapy is not valuable for clinical decision making. Our study cannot in fact identify any other cutoff value for routine clinical use as a predictor for survival.
Nonetheless, intriguing exploratory results were found. Patients with exceptionally high SUVs after chemoradiotherapy have poor outcomes—there were no long-term survivors among patients with post-treatment SUVpeak greater than 7. This subpopulation could be considered for early additional treatment. We also identified potential value of a post-treatment SUVpeak cutoff value of 5.0 (Fig 3). We felt it was necessary to explore another binary cutoff for post-treatment SUV (other than 7.0), because the small number of patients with SUV greater than 7.0 after chemoradiotherapy limits its applicability. We caution that the analysis of a post-treatment SUV cutoff of 5.0 is highly exploratory, post hoc, and would need validation in future studies.
A similar single-institution study by Lopez Guerra et al14 yielded greater risk stratification than we observed. In their modest-sized series (49 patients), the median post-treatment SUVmax was 3.7; patients with values less than the median had a 2-year survival rate of approximately 50%, compared with a rate of approximately 20% for patients with post-treatment SUVmax greater than the median (P = .0112).
Qualitative analyses of PET after (chemo)radiotherapy also show positive results.10,15,16 Our study does not refute the value of qualitative analyses or suggest that quantitative post-treatment (SUV-based) measurements are superior. However, we do point out that our study is, to our knowledge, the first to show a relationship between post-treatment PET and survival in a multicenter setting, with many readers of variable experience and expertise. Figure 1 shows moderate variability between individual institution readers and central review. We note with interest that the statistical results for post-treatment SUVpeak are stronger when using central review analysis compared with local institutional review analysis, and this may be related to a learning curve with respect to definition of the ROI, which in turn has been shown to be an important factor in the precise determination of SUVpeak.17 Our study results do not differ greatly when post-treatment SUVmax (which is more reproducible than SUVpeak) is used instead of SUVpeak. We emphasize SUVpeak data throughout the Results because it was the prespecified primary end point of the study when it was designed, written, and approved. However, our analyses do not show major differences when using SUVpeak or SUVmax. Given the better interobserver reliability of SUVmax, it is possible that SUVmax may ultimately be more clinically useful for post-treatment analyses. However, we remain concerned that SUVmax is subject to noise because it is based on a single voxel in the tumor. We suggest that in the future, the use of automated programs for SUVpeak measurement, as we did for the central reads, may increase the reliability of this metric. Such automated programs are now available on most modern PET/CT workstations.
Of note, neither pretreatment SUVmax nor SUVpeak correlated with outcomes. This differs from a recent meta-analysis on the topic18 in which many of the patients had early-stage disease treated surgically. In stage III NSCLC, the Ontario Clinical Oncology Group showed a relationship between pretreatment SUV and survival.19 We do not have an explanation for the discordance between our pretreatment SUV data and others. It has indeed been difficult to identify reproducible pretreatment prognostic factors for stage III NSCLC.20
There are limitations of our study. These mainly reflect the need for flexibility in a multicenter study of complex, ill, heterogeneous patients. First, the exact treatment given to patients in this study was not strictly regimented with regard to radiation dose or chemotherapy agents. Our sample size is not large enough to perform subgroup analyses among different exact treatments.
A second limitation is heterogeneity in the timing of the post-treatment PET. The study mandated that post-treatment PET be done 12 to 16 weeks after completion of radiotherapy, although patients with violations were still considered evaluable for this analysis. Postradiotherapy inflammation (pneumonitis) is associated with increased FDG uptake and is a highly dynamic and complex process.21 Accordingly, our results may have differed with rigid timing for post-treatment PET; however, this might have reduced accrual and general applicability of our study. Unfortunately, we could not collect clinical data on radiation pneumonitis to attempt to correlate it with post-treatment PET.
A third limitation is that post-treatment biopsies were rarely performed. Thus, there are no conclusive data in our study to confirm or refute whether an abnormal post-treatment PET represented viable tumor versus radiation-induced inflammation. This is a ubiquitous problem in thoracic radiation oncology that is hardly unique to our study and is unlikely to be overcome soon. This is why we elected to use overall survival (rather than local control) as the primary end point of our study.
It is possible that quantitative analysis of post-treatment PET is indeed useful but that SUV is not the best metric. Other, more sophisticated tools for analyzing PET images are becoming more widely available. One technique under investigation is to study SUVs at multiple time points—it has been suggested that a further increase in SUV at 90 minutes after injection (compared with the SUV at 60 minutes) may predict prognosis.22
Another option is to study metabolic tumor volume or total lesion glycolysis, which take into account not only the intensity of tracer uptake, but also the size of the residual lesion.23–25 However, metabolic tumor volume or total lesion glycolysis is also likely to be confounded by post-treatment inflammatory responses. A different potential solution is to evaluate FDG-PET during radiotherapy, before anticipated induction of radiation pneumonitis.26–28 A third area of research is the use of alternative tracers, such as 18F-fluorothymidine29 for response assessment or 18F-fluoromisonidazole for detection of hypoxia.30 RTOG and ACRIN have just activated a new prospective trial (RTOG 1106/ACRIN 6697) to explore the prognostic value of baseline hypoxia imaging with 18F-fluoromisonidazole in a patient population similar to ours.
In conclusion, SUV measured on post-treatment FDG-PET in patients with locally advanced NSCLC has some value as a prognostic factor. A statistically significant association between post-treatment SUV and survival was identified on univariate and multivariate continuous-variable modeling. However, we could not identify a clinically significant cutoff value for post-treatment SUV at this time. Our data do suggest that high post-treatment SUV portends a poor outcome.
Although post-treatment FDG-PET seems to provide prognostic information, it is not yet known whether subsequent treatment decisions based on this information can improve clinical outcomes. Further investigation of the role of post-treatment FDG-PET in therapeutic decision making in this clinical setting is warranted.
Acknowledgment
We gratefully acknowledge the participating institutions and the principal investigators at each site (see Appendix Table A1). We also thank the many radiologists, radiation and medical oncologists, nuclear medicine technologists, and research coordinators at the participating institutions, and the American College of Radiology Imaging Network (ACRIN) and Radiation Therapy Oncology Group (RTOG) staff who supported the ACRIN 6668/RTOG 0235 trial at both ACRIN and RTOG headquarters, in Philadelphia, PA, and the Biostatistical and Data Management Center at Brown University in Providence, RI. Without the diligent efforts of these many individuals, this study would not have been possible. The authors also thank Denise Moore and Shawnta Ringold-Donaldson for assistance in preparation of this article.
Appendix
Statistical Considerations
The sample size was based on the hypothesis that patients with peak standardized uptake value (SUVpeak) ≤ 3.5 (representing 45% of evaluable patients) would have a 2-year survival of 50% (measured from the date of enrollment), whereas patients with an SUVpeak more than 3.5 would have a 2-year survival of 30%. This resulted in 169 evaluable patients to achieve 90% power. We estimated the need for 250 enrolled patients to obtain 169 patients with evaluable post-treatment positron emission tomography (PET) scans.
For each standardized uptake value (SUV) measurement, a univariate Cox regression model was first fit using SUV as the sole predictor. Then, a multivariate Cox regression model was fit to include several other prespecified covariates, including age, sex, baseline Zubrod performance status, baseline clinical stage, radiotherapy dose (Gy), and chemotherapy regimen, which was broken into three groups (cisplatin and etoposide, carboplatin and paclitaxel, and other). Hazard ratios (HRs) were reported. In addition, for categorical SUVs, Kaplan-Meier curves were plotted to show the difference in survivorship among the categories. For the models with four groups of SUV (quartiles), the Bonferroni adjustment was made to correct for multiple comparisons within the analysis (ie, the significance level was set to be P = .0167). No adjustment of multiple comparisons was made across analyses.
To reflect routine clinical practice, institutional SUV measurements were used for the primary statistical end points. However, a plan to repeat these analyses using centrally determined SUVpeak and maximum SUV (SUVmax) was also prespecified. The concordance correlation coefficient was also calculated between institutional and central SUVs to assess agreement (Lin LI: Biometrics 45:255-268, 1989).
The main secondary goals include determining the ability of institutional/central post-treatment SUVmax to predict long-term survival; determining the ability of institutional/central post-treatment SUVpeak, as well as institutional/central post-treatment SUVmax, to predict local control; determining the ability of institutional/central pretreatment SUVpeak, as well as institutional/central pretreatment SUVmax, to predict long-term survival and local control; and determining the reliability of these measurements between the institution and a central review facility.
In addition to the Cox regression, the concordance measure (C statistic) was calculated to assess the overall predictive performance of the developed models (Uno H, et al: Stat Med 30:1105-1117, 2011). Data were analyzed using SAS 9.2 (SAS Institute, Cary, NC) and R v2.13.1 (R Project, http://www.r-project.org/), with P < .05 considered statistically significant.
Additional Exploratory Analyses
The first exploratory subset analysis examined whether the study results would differ when restricted to stage III patients (ie, excluding the seven patients with an evaluable post-treatment PET who had stage IIB disease), leaving 166 evaluable patients. This analysis had no significant effect on the study findings and did not change the overall conclusion for any of the cutoff values under consideration (3.5, 5.0, and 7.0: HR, 1.227, P = .33; HR, 1.831, P = .018; and HR, 4.554, P < .001, respectively). The results of the multivariate model using institutional continuous post-treatment SUVpeak for the remaining subset of stage IIIA/IIIB patients are listed in Appendix Table A7.
A second exploratory subset analysis excluded patients who had distant metastatic disease diagnosed on the post-treatment PET and confirmed by follow-up. This reduced the evaluable sample size to 145 patients. With this reduced sample size and stricter criteria for evaluability, the strength of the association between SUVpeak and survival decreased (continuous model: HR, 1.069, P = .09 by univariate analysis; and HR, 1.061, P = .18 by multivariate analysis). The SUVpeak cutoff of 7.0 remained strongly significant (HR, 4.279; P < .001), but the cutoff of 5.0 was no longer significant (HR, 1.394; P = .26). The results of the multivariate model using institutional continuous post-treatment SUVpeak for the remaining subset of nonmetastatic patients are listed in Appendix Table A8.
A final exploratory subset analysis addressed whether there was any effect on study results from heterogeneity in the time interval from completion of radiotherapy to post-treatment PET. Among patients with an evaluable post-treatment PET, 27 patients (15.6%) had their scans done less than 12 weeks after radiotherapy, and 34 patients (19.7%) had their scans done more than 16 weeks after radiotherapy. Excluding these patients (leaving only 112 evaluable patients) does not change the overall conclusions with respect to cutoff values of 3.5 or 7.0 (HR, 1.265, P = .39; and HR, 4.641, P = .001, respectively). The exploratory results with respect to an SUVpeak cutoff of 5.0 are no longer statistically significant with this reduced sample size (HR, 1.804; P = .08). The results of the multivariate model using institutional continuous post-treatment SUVpeak for the remaining subset of patients with PET scans done 12 to 16 weeks after radiotherapy are listed in Appendix Table A9.
Table A1.
Participating Institutions and Their Lead Physician Investigators
| Institution | Lead Nuclear Medicine Physician/Radiologist | Lead Radiation Oncologist |
|---|---|---|
| Akron General Medical Center | Eve A. Echt, MD | Mitchel Fromm, MD |
| Brown University | Don Yoo, MD | Thomas DiPetrillo, MD |
| Cleveland Clinic | Donald R. Neumann, MD, PhD | Gregory Videtic, MD |
| Community Medical Center | Joseph Triolo, MD | Bong Chang, MD |
| Dartmouth-Hitchcock Medical Center | — | Alan Hartford, MD |
| Florida Radiation Oncology Group/Baptist Medical Center | Larry Wilf, MD | Douglas Johnson, MD |
| Fox Chase Cancer Center | Michael Yu, MD | Steven Feigenberg, MD |
| Holy Name Hospital | Jacqueline C. Brunetti, MD | Charles Vialotti, MD |
| Hospital of St Raphael | Vincente Caride, MD | Joseph Cardinale, MD |
| Lankenau Hospital | Nancy Sherwin, MD | Albert DeNittis, MD |
| Medical College of Wisconsin | Robert Hellman, MD | Elizabeth Gore, MD |
| Medical University of South Carolina | James Ravenel, MD | Anand Sharma, MD |
| Memorial and St Elizabeth's Health Care Services, LLP | Charles DuMontier, MD | Susan Laduzinsky, MD |
| National Cancer Center of Korea | Seok-ki Kim, MD | Kwan Ho Cho, MD |
| North Broward Hospital | Carlos Muhletaler, MD | Kenneth Monson, MD |
| Radiological Associates of Sacramento | Richard Myers, MD | Seth Rosenthal, MD |
| Regional Cancer Center–Waukesha & Oconomowoc | Gregory Francken, MD | Wingate Clapper, MD |
| Renown Health and Nevada Cancer Research Foundation | Richard Hodge, MD | Lawrence Dardick, MD |
| Scottsdale Medical Imaging, LTD | Ronald Korn, MD, PhD | Farley Yang, MD |
| South Shore Hospital | James Strain, MD | Joseph Barthold, MD |
| St Mary's Hospital | Karen Killeen, MD | George Trivette, MD |
| St Vincent Anderson Regional Hospital (St John's Health System) | — | Darrel L. Ross, MD |
| Tallahassee Memorial Hospital | William Yaakob, MD | Tim Bolek, MD |
| Thomas Jefferson University Hospital | Charles Intenzo, MD | Mitchell Machtay, MD |
| Tom Baker Cancer Centre | Reinhard Kloiber, MD | Colum Smith, MD |
| University of Alabama at Birmingham | Janis O. Malley, MD | Ruby Meredith, MD |
| University of Colorado Hospital | Adrienne Sage-El, MD | Laurie Gaspar, MD |
| University of Iowa Hospital | Michael Graham, MD | Geraldine Jacobson, MD |
| University of Pittsburgh | James Mountz, MD | David Stefanik, MD |
| University of Southern California | Shahram Bonyadlou, MD | Oscar Streeter, MD |
| University of Texas MD Anderson Cancer Center | Homer Macapinlac, MD | Ritsuko Komaki, MD |
| University of Texas Southwestern | Dana Matthew, MD, PhD | Hak Choy, MD |
| University of Utah Health Science Center | John M. Hoffman, MD | Ying Hitchcock, MD |
| Vanderbilt University Medical Center | John Worrell, MD | Bo Lu, MD |
| Washington University School of Medicine | Barry A. Siegel, MD | Jeffrey D. Bradley, MD |
| West Michigan Cancer Center Community Clinical Oncology Program | Robert Davis, MD | Raymond Lord, MD |
| William Beaumont Hospital | — | John Robertson, MD |
Table A2.
Eligibility Status of Patients Accrued to the Study
| Eligibility Status | No. of Patients | % |
|---|---|---|
| Total registrations | 250 | 100.0 |
| Ineligible patients | 16 | 6.4 |
| CT scan performed > 6 weeks before registration (section 5.1.2.3) | 5 | 2.0 |
| Cancer stage criteria not met (section 5.1.2) | 3 | 1.2 |
| Pretreatment PET scan performed > 6 weeks before registration (section 5.1.8.1) | 2 | 0.8 |
| Study activity began before consent (section 5.1.6) | 2 | 0.8 |
| Alkaline phosphatase level was not done within 4 weeks of registration (section 5.1.2.2) | 1 | 0.4 |
| MRI scan performed > 8 weeks before registration (section 5.1.2.4) | 1 | 0.4 |
| Participant not receiving concurrent chemoradiotherapy per protocol (section 5.1.7) | 1 | 0.4 |
| Pretreatment PET/CT not done (section 5.1.8) | 1 | 0.4 |
| Eligible patients | 234 | 93.6 |
Abbreviation: CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
Table A3.
Pretreatment PET Evaluability Status of Patients Accrued to the Study
| Evaluability Status | No. of Patients | % |
|---|---|---|
| Total eligible patients | 234 | 100.0 |
| Inevaluable patients | 8 | 3.4 |
| Pretreatment PET not performed | 1 | 0.4 |
| Pretreatment SUVpeak not provided | 7 | 3.0 |
| Evaluable patients | 226 | 96.6 |
Abbreviations: PET, positron emission tomography; SUVpeak, peak standardized uptake value.
Table A4.
Post-Treatment PET Evaluability Status of Patients Accrued to the Study
| Evaluability Status | No. of Patients | % |
|---|---|---|
| Total eligible patients | 234 | 100.0 |
| Inevaluable patients | 61 | 26.1 |
| Death | 26 | 11.1 |
| Medical comorbidity | 4 | 1.7 |
| Patient discontinued protocol treatment | 2 | 0.9 |
| Patient refused post-treatment scan | 9 | 3.8 |
| Patient explicitly withdraws from further study participation | 5 | 2.1 |
| Patient relocated 2.5 hours away from study center | 1 | 0.4 |
| Patient started nonprotocol therapy | 2 | 0.9 |
| Patient transferred to hospice | 1 | 0.4 |
| Post-treatment image not done on ACRIN-qualified scanner | 1 | 0.4 |
| Patient admitted to hospital; CT scan showed metastatic disease | 1 | 0.4 |
| Scheduling problem | 1 | 0.4 |
| SUVpeak not evaluable because of poor image quality | 2 | 0.9 |
| SUVpeak not evaluable because of patient having eaten | 1 | 0.4 |
| SUVpeak not provided by the institution for unknown reason. | 5 | 2.1 |
| Evaluable patients | 173 | 73.9 |
Abbreviations: ACRIN, American College of Radiology Imaging Network; CT, computed tomography; PET, positron emission tomography; SUVpeak, peak standardized uptake value.
Table A5.
Multivariate Analysis Including Post-Treatment SUVpeak (institutional read) as a Variable
| Parameter | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Age (years): continuous | 1.021 | 0.998 to 1.044 | .079 |
| Sex: female v male | 1.164 | 0.781 to 1.735 | .456 |
| Performance status: ambulatory, capable of light work v fully active | 1.328 | 0.911 to 1.937 | .140 |
| Clinical stage | |||
| IIIA v IIB | 0.828 | 0.339 to 2.026 | .680 |
| IIIB v IIB | 0.985 | 0.395 to 2.455 | .974 |
| Radiotherapy dose (Gy): continuous | 0.990 | 0.949 to 1.033 | .642 |
| Chemotherapy regimen | |||
| Cisplatin + etoposide v carboplatin + paclitaxel | 1.062 | 0.594 to 1.898 | .839 |
| Other v carboplatin + paclitaxel | 1.138 | 0.749 to 1.729 | .544 |
| Post-treatment SUVpeak: continuous | 1.087 | 1.014 to 1.166 | .020 |
NOTE. To assess the overall performance of the model, the C statistic was computed using the approach developed by Uno et al (Uno H, et al: Stat Med 30:1105-1117, 2011). It is 0.579 under the above model. For comparison, the C statistic is 0.573 when post-treatment SUVpeak is excluded from the model.
Abbreviation: SUVpeak, peak standardized uptake value.
Table A6.
Multivariate Analysis Including Post-Treatment SUVpeak (central read) as a Variable
| Parameter | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Age (years): continuous | 1.018 | 0.996 to 1.042 | .112 |
| Sex: female v male | 1.163 | 0.791 to 1.710 | .443 |
| Performance status: ambulatory, capable of light work v fully active | 1.433 | 0.990 to 2.073 | .057 |
| Clinical stage | |||
| IIIA v IIB | 0.884 | 0.338 to 2.314 | .802 |
| IIIB v IIB | 0.987 | 0.370 to 2.631 | .979 |
| Radiotherapy dose (Gy): continuous | 0.968 | 0.925 to 1.013 | .163 |
| Chemotherapy regimen | |||
| Cisplatin + etoposide v carboplatin + paclitaxel | 1.041 | 0.583 to 1.861 | .892 |
| Other v carboplatin + paclitaxel | 1.103 | 0.722 to 1.688 | .650 |
| Post-treatment SUVpeak: continuous | 1.125 | 1.049 to 1.206 | < .001 |
Abbreviation: SUVpeak, peak standardized uptake value.
Table A7.
Multivariate Analysis Including Post-Treatment SUVpeak (institutional read) as a Variable, After Excluding Patients With Stage IIB Disease (n = 7)
| Parameter | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Age (years): continuous | 1.020 | 0.997 to 1.044 | .091 |
| Sex: female v male | 1.141 | 0.763 to 1.706 | .521 |
| Performance status: ambulatory, capable of light work v fully active | 1.340 | 0.911 to 1.970 | .137 |
| Clinical stage: IIIB v IIIA | 1.197 | 0.819 to 1.750 | .353 |
| Radiotherapy dose (Gy): continuous | 0.996 | 0.954 to 1.040 | .870 |
| Chemotherapy regimen | |||
| Cisplatin + etoposide v carboplatin + paclitaxel | 1.020 | 0.571 to 1.820 | .947 |
| Other v carboplatin + paclitaxel | 1.048 | 0.687 to 1.599 | .827 |
| Post-treatment SUVpeak: continuous | 1.095 | 1.022 to 1.174 | .010 |
Abbreviation: SUVpeak, peak standardized uptake value.
Table A8.
Multivariate Analysis Including Post-Treatment SUVpeak (institutional read) as a Variable, After Excluding Patients With Distant Metastatic Disease Diagnosed on the Post-Treatment PET and Confirmed by Follow-Up (n = 28)
| Parameter | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Age (years): continuous | 1.013 | 0.987 to 1.039 | .337 |
| Sex: female v male | 1.001 | 0.632 to 1.585 | .997 |
| Performance status: ambulatory, capable of light work v fully active | 1.147 | 0.749 to 1.757 | .528 |
| Clinical stage | |||
| IIIA v IIB | 0.744 | 0.277 to 2.002 | .559 |
| IIIB v IIB | 0.848 | 0.308 to 2.336 | .750 |
| Radiotherapy dose (Gy): continuous | 0.976 | 0.930 to 1.024 | .320 |
| Chemotherapy regimen | |||
| Cisplatin + etoposide v carboplatin + paclitaxel | 1.131 | 0.592 to 2.160 | .710 |
| Other v carboplatin + paclitaxel | 1.325 | 0.821 to 2.138 | .250 |
| Post-treatment SUVpeak: continuous | 1.061 | 0.974 to 1.156 | .176 |
Abbreviations: PET, positron emission tomography; SUVpeak, peak standardized uptake value.
Table A9.
Multivariate Analysis Including Post-Treatment SUVpeak (institutional read) as a Variable, After Excluding Patients With Post-Treatment PET Scans Done < 12 or > 16 Weeks After Radiotherapy (n = 61)
| Parameter | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Age (years): continuous | 1.008 | 0.980 to 1.037 | .585 |
| Sex: female v male | 1.024 | 0.611 to 1.717 | .927 |
| Performance status: ambulatory, capable of light work v fully active | 1.460 | 0.889 to 2.396 | .135 |
| Clinical stage | |||
| IIIA v IIB | 1.161 | 0.267 to 5.059 | .842 |
| IIIB v IIB | 1.539 | 0.343 to 6.911 | .574 |
| Radiotherapy dose (Gy): continuous | 0.982 | 0.932 to 1.034 | .494 |
| Chemotherapy regimen | |||
| Cisplatin + etoposide v carboplatin + paclitaxel | 0.778 | 0.369 to 1.640 | .510 |
| Other v carboplatin + paclitaxel | 1.240 | 0.714 to 2.152 | .445 |
| Post-treatment SUVpeak: continuous | 1.092 | 0.973 to 1.225 | .134 |
Abbreviations: PET, positron emission tomography; SUVpeak, peak standardized uptake value.
Footnotes
Listen to the podcast by Dr Salama at www.jco.org/podcasts
Supported by the American College of Radiology Imaging Network, which receives funding from the National Cancer Institute through Grants No. U01 CA079778 and U01 CA080098, and by the Radiation Therapy Oncology Group, which receives funding from the National Cancer Institute through the Grants No. U01 CA21661 and 32115.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00083083.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Fenghai Duan, WorldCare Clinical (C); Barry A. Siegel, Siemens Healthcare (C), GE Healthcare (C) Stock Ownership: Barry A. Siegel, Radiology Corporation of America Honoraria: Barry A. Siegel, Siemens Medical Solutions, GE Healthcare, Philips Healthcare; Homer Macapinlac, Siemens Medical Solutions Research Funding: None Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Mitchell Machtay, Fenghai Duan, Barry A. Siegel, Janet S. Reddin, Reginald Munden, Albert DeNittis, Abass Alavi
Provision of study materials or patients: Barry A. Siegel, Douglas W. Johnson, Jeffrey D. Bradley
Collection and assembly of data: Mitchell Machtay, Fenghai Duan, Barry A. Siegel, Janet S. Reddin, Douglas W. Johnson, Larry H. Wilf, Nancy Sherwin, Seok-ki Kim, Gregory Videtic, Donald R. Neumann, Ritsuko Komaki, Jeffrey D. Bradley, Abass Alavi
Data analysis and interpretation: Mitchell Machtay, Fenghai Duan, Barry A. Siegel, Bradley S. Snyder, Jeremy J. Gorelick, Janet S. Reddin, Reginald Munden, Kwan Ho Cho, Homer Macapinlac, Abass Alavi
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 2.Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small cell lung cancer: The Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008;26:5755–5760. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto N, Nakagawa K, Nishimura Y, et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol. 2010;28:3739–3745. doi: 10.1200/JCO.2009.24.5050. [DOI] [PubMed] [Google Scholar]
- 4.Le Chevalier T, Arriagada R, Quoix E, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in unresectable non-small cell lung carcinoma. Lung Cancer. 1994;10(suppl 1):S239–S244. doi: 10.1016/0169-5002(94)91687-x. [DOI] [PubMed] [Google Scholar]
- 5.Mac Manus MP, Hicks RJ, Matthews JP, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–1292. doi: 10.1200/JCO.2003.07.054. [DOI] [PubMed] [Google Scholar]
- 6.Truong MT, Viswanathan C, Erasmus JJ. Positron emission tomography/computed tomography in lung cancer staging, prognosis, and assessment of therapeutic response. J Thorac Imaging. 2011;26:132–146. doi: 10.1097/RTI.0b013e3182128704. [DOI] [PubMed] [Google Scholar]
- 7.MacManus MP, Hicks RJ, Matthews JP, et al. High rate of detection of unsuspected distant metastases by pet in apparent stage III non-small-cell lung cancer: Implications for radical radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:287–293. doi: 10.1016/s0360-3016(01)01477-8. [DOI] [PubMed] [Google Scholar]
- 8.Bradley J, Bae K, Choi N, et al. A phase II comparative study of gross tumor volume definition with or without PET/CT fusion in dosimetric planning for non-small-cell lung cancer (NSCLC): Primary analysis of Radiation Therapy Oncology Group (RTOG) 0515. Int J Radiat Oncol Biol Phys. 2012;82:435–441.e1. doi: 10.1016/j.ijrobp.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Ruysscher D, Nestle U, Jeraj R, et al. PET scans in radiotherapy planning of lung cancer. Lung Cancer. 2012;75:141–145. doi: 10.1016/j.lungcan.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 10.MacManus MP, Hicks RJ, Matthews JP, et al. Metabolic (FDG-PET) response after radical radiotherapy/chemoradiotherapy for non-small cell lung cancer correlates with patterns of failure. Lung Cancer. 2005;49:95–108. doi: 10.1016/j.lungcan.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 11. Reference deleted.
- 12.Scheuermann JS, Saffer JR, Karp JS, et al. Qualification of PET scanners for use in multicenter cancer clinical trials: The American College of Radiology Imaging Network experience. J Nucl Med. 2009;50:1187–1193. doi: 10.2967/jnumed.108.057455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenzweig RE, Erdi Y, Schoter H, et al. Positron emission tomography after 3D conformal radiation therapy for NSCLC. Int J Radiat Oncol Biol Phys. 2001;89(suppl):159. abstr. [Google Scholar]
- 14.Lopez Guerra JL, Gladish G, Komaki R, et al. Large decreases in standardized uptake values after definitive radiation are associated with better survival of patients with locally advanced non-small cell lung cancer. J Nucl Med. 2012;53:225–233. doi: 10.2967/jnumed.111.096305. [DOI] [PubMed] [Google Scholar]
- 15.Velazquez ER, Aerts HJ, Oberije C, et al. Prediction of residual metabolic activity after treatment in NSCLC patients. Acta Oncol. 2010;49:1033–1039. doi: 10.3109/0284186X.2010.498441. [DOI] [PubMed] [Google Scholar]
- 16.Aerts HJ, van Baardwijk AA, Petit SF, et al. Identification of residual metabolic-active areas within individual NSCLC tumours using a pre-radiotherapy (18)fluorodeoxyglucose-PET-CT scan. Radiother Oncol. 2009;91:386–392. doi: 10.1016/j.radonc.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanderhoek M, Perlman SB, Jeraj R. Impact of the definition of peak standardized uptake value on quantification of treatment response. J Nucl Med. 2012;53:4–11. doi: 10.2967/jnumed.111.093443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paesmans M, Berghmans T, Dusart M, et al. Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: Update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol. 2010;5:612–619. doi: 10.1097/JTO.0b013e3181d0a4f5. [DOI] [PubMed] [Google Scholar]
- 19.Ung Y, Gu K, Cline A, et al. An Ontario Clinical Oncology Group (OCOG) randomized trial (PET START) of FDG PET/CT in patients with stage III NSCLC: Predictors of overall survival. J Clin Oncol. 2011;29(suppl):456s. abstr 7018. [Google Scholar]
- 20.Berghmans T, Paesmans M, Sculier JP. Prognostic factors in stage III non-small cell lung cancer: A review of conventional, metabolic and new biological variables. Ther Adv Med Oncol. 2011;3:127–138. doi: 10.1177/1758834011401951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacManus MP, Ding Z, Hogg A, et al. Association between pulmonary uptake of fluorodeoxyglucose detected by positron emission tomography scanning after radiation therapy for non-small-cell lung cancer and radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2011;80:1365–1371. doi: 10.1016/j.ijrobp.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Houseni M, Chamroonrat W, Zhuang J, et al. Prognostic implication of dual-phase PET in adenocarcinoma of the lung. J Nucl Med. 2010;51:535–542. doi: 10.2967/jnumed.109.068643. [DOI] [PubMed] [Google Scholar]
- 23.Liao S, Penney BC, Zhang H, et al. Prognostic value of the quantitative metabolic volumetric measurement on 18F-FDG PET/CT in stage IV nonsurgical small-cell lung cancer. Acad Radiol. 2012;19:69–77. doi: 10.1016/j.acra.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Lee P, Weerasuriya DK, Lavori PW, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys. 2007;69:328–333. doi: 10.1016/j.ijrobp.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 25.Chen HH, Chiu NT, Su WC, et al. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology. 2012;264:559–566. doi: 10.1148/radiol.12111148. [DOI] [PubMed] [Google Scholar]
- 26.Kong FM, Frey KA, Quint LE, et al. A pilot study of [18F]fluorodeoxyglucose positron emission tomography scans during and after radiation-based therapy in patients with non–small-cell lung cancer. J Clin Oncol. 2007;25:3116–3123. doi: 10.1200/JCO.2006.10.3747. [DOI] [PubMed] [Google Scholar]
- 27.Massaccesi M, Calcagni ML, Spitilli MG, et al. 18F-FDG PET-CT during chemo-radiotherapy in patients with non-small cell lung cancer: The early metabolic response correlates with the delivered radiation dose. Radiat Oncol. 2012;7:106. doi: 10.1186/1748-717X-7-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Elmpt W, Ollers M, Dingemans AM, et al. Response assessment using 18F-FDG PET early in the course of radiotherapy correlates with survival in advanced-stage non-small cell lung cancer. J Nucl Med. 2012;53:1514–1520. doi: 10.2967/jnumed.111.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Everitt S, Hicks RJ, Ball D, et al. Imaging cellular proliferation during chemo-radiotherapy: A pilot study of serial 18F-FLT positron emission tomography/computed tomography imaging for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;75:1098–1104. doi: 10.1016/j.ijrobp.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 30.Vera P, Bohn P, Edet-Sanson A, et al. Simultaneous positron emission tomography (PET) assessment of metabolism with 1F-fluoro-2-deoxy-d-glucose (FDG), proliferation with 1F-fluoro-thymidine (FLT), and hypoxia with 1F-fluoro-misonidazole (F-miso) before and during radiotherapy in patients with non-small-cell lung cancer (NSCLC): A pilot study. Radiother Oncol. 2011;98:109–116. doi: 10.1016/j.radonc.2010.10.011. [DOI] [PubMed] [Google Scholar]