Abstract
The survival advantage of women over men with cutaneous melanoma and the reports of accelerated progression of melanoma during pregnancy have led to studies of the effect of hormones and hormone receptors on the development and progression of melanoma. However, the results are inconclusive. We therefore evaluated the expression of estrogen receptor α, estrogen receptor β, and androgen receptor in melanomas of stage- and age-matched pregnant women, non-pregnant women, and men by immunohistochemical analysis of formalin-fixed, paraffin-embedded archival tissues. In addition, we also assessed the mitotic rate using the anti-phospho-histone H3 antibody by immunohistochemistry. Our data showed a trend of more frequent expression of estrogen receptor β in the melanomas of pregnant patients than in the melanomas of male patients, without a significant difference observed between pregnant and non-pregnant women. However, no association between the expression of estrogen receptor β and survival was observed. The small cohort may have limited the statistical power of the study, and larger scale studies are needed to elucidate the potential role of estrogen receptor β as a prognostic marker of melanoma.
Keywords: estrogen receptor, androgen receptor, melanoma, pregnancy, pHH3
Introduction
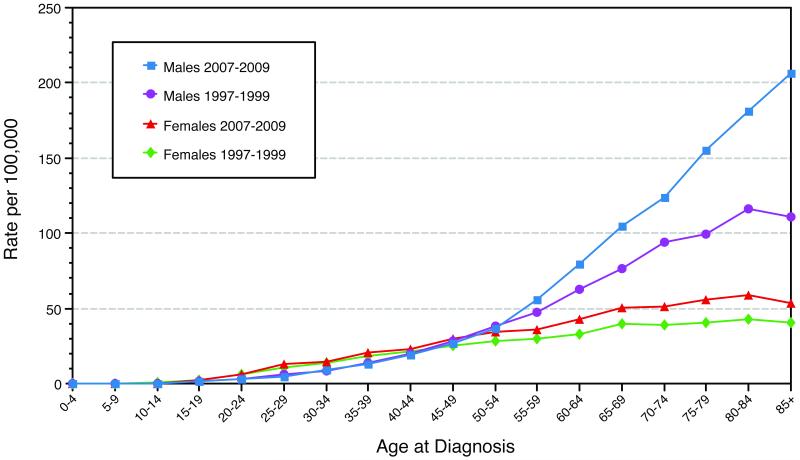
Cutaneous melanoma, one of the most frequently encountered malignancies in pregnant women, accounts for 25% of all cancers diagnosed during pregnancy (1) and is diagnosed up to 1 in 1000 gestations (2). The incidence of melanoma during pregnancy is expected to rise due to the increased incidence of melanoma in the general population and the relatively high incidence of melanoma in women of child-bearing age (Figure 1, Surveillance Epidemiology and End Results data). Although most agree that women have a significant survival advantage over men in cutaneous melanoma(3), the effects of pregnancy, sex hormones, and hormone receptors on the survival of melanoma patients are still controversial (4, 5). di Giorgi et al. showed that melanoma expressed detectable estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) mRNA by reverse transcriptase polymerase chain reaction (6). They also demonstrated that ERβ protein was expressed in melanomas using immunohistochemical analysis. However, no study has been performed to compare the immunohistochemical expressions of estrogen and androgen receptors in the melanomas of pregnant women, non-pregnant women, and men. Therefore, we conducted this retrospective study to assess the expression of hormone receptors in the melanomas of stage- and age-matched pregnant women, non-pregnant women, and men. We hypothesized that the melanomas of pregnant women have a higher prevalence of expression of hormone receptors than those of non-pregnant women and men, and we assessed the possible role of hormone receptor expression as a prognostic marker. In addition, we evaluated the tumor mitotic rate in stage III and IV melanomas by immunohistochemical analysis using anti-phospho-histone H3 (pHH3) antibody and assessed its association with prognosis.
Figure 1.
Surveillance Epidemiology and End Results incidence of cutaneous melanoma by age and sex 1997-1999 vs. 2007-2009.
Materials and Methods
This study was approved with waived informed consent by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. The electronic medical records of the patients included in the study were reviewed to record relevant data (age, race, pregnancy status, gestational age, tumor site, tumor type, Breslow thickness of tumor, ulceration, primary tumor vs. metastasis, disease stage, metastatic site, treatment received, date of initial diagnosis, date of disease progression, date of last follow-up, date of death).
The Pregnant Women Study Patients
We identified 41 women who were diagnosed with melanoma during pregnancy or within 6 months after delivery from January 1996 to December 2011 via database searches at the Departments of Melanoma Medical Oncology and Pathology at MD Anderson Cancer Center (MDACC). Paraffin-embedded tissue-blocks were available for 20 patients. Two of the 20 patients were excluded because their primary melanoma was from a non-cutaneous site. The remaining 18 patients were included in the study group.
The Non-pregnant Women and Men Control Patients
Using the search criteria “metastatic melanoma, age range 20–45 years, from January 1996 to December 2011,” in the database of the Department of Pathology at MDACC we identified 18 women who were not pregnant at the time of or within 1 year of diagnosis and 18 men as the control patients. These non-pregnant women and men were stage- and age-matched to the pregnant study patients. For the patients with stage III or IV disease, the first order of match was the clinical stage. The metastatic site in patients with stage IV disease was also matched. The second order of match was the Breslow thickness of the tumor by the pT stage of the 7th edition of the AJCC staging manual. Additional database searches were performed to identify cases that matched the patients who had stage I disease, in which the Breslow thickness of the tumor was matched as close to that of the study patients as possible.
Immunohistochemistry
The appropriate formalin-fixed paraffin-embedded tissue blocks were obtained after reviewing the hematoxylin and eosin stained slides. The immunohistochemical reaction was performed using a BOND MAX automated system (Leica Microsystems, Buffalo Grove, IL) according to the manufacturer’s protocol. Briefly, 4-μm tissue sections were dewaxed, washed, and incubated with antibodies against ERα (clone 6F11, Novocastra, Newcastle Upon Tyne/UK; dilution 1:35), ERβ (clone EMR02, Leica Microsystems, Buffalo Grove, IL; dilution 1:100), Androgen Receptor (clone AR441, Dako, Carpinteria, CA; dilution 1:30), and pHH3 (Upstate Millipore, Temecula, CA; dilution 1:400) at room temperature for 15 minutes. The slides then were treated with Epitope Retrieval (Leica Microsystems) in citrate buffer for 5 minutes after washing. Endogenous peroxidase activity was blocked by Peroxide Block (Leica Microsystems) for 5 minutes. After washing, the sections were incubated with Post Primary immunoglobulin G linker reagent (polymer enhancer; Leica Microsystems)) for 8 minutes and then incubated with Poly-Horseradish Peroxidase immunoglobulin G polymer (Leica Microsystems) for 8 minutes after washing. The immunoreaction was visualized using 3, 3′-diaminobenzidine and hematoxylin counterstaining. Appropriate positive and negative controls for each antibody were performed simultaneously with the samples.
Hormone Receptor Expression and Mitotic Rate
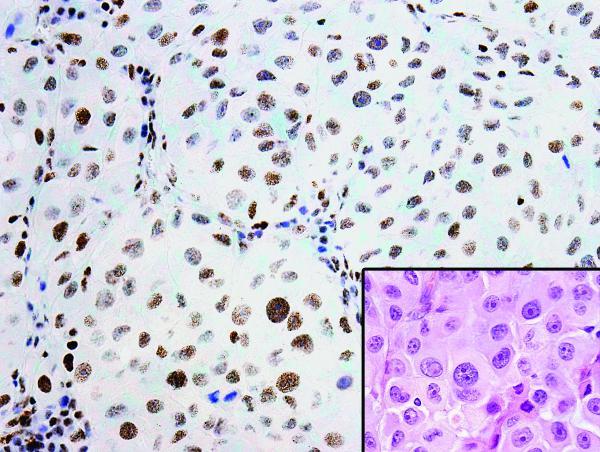
We defined the positive expression of hormone receptors as 10% or more of the tumor cells having positive nuclear staining (Fig. 2). The expression was recorded as both binary and continuous (percent positive) variables. The mitotic rate was defined as the number of pHH3-positive tumor cell nuclei per square millimeter (mm2), corresponding to 4.5 consecutive fields at 40× magnification on an Olympus BX41 microscope. The consecutive fields were determined by scanning the slides to find the “hotspot” area with the highest numbers of pHH3-positive tumor cell nuclei as the first field to start the counting.
Figure 2.
Positive ERβ expression by immunohistochemical nuclear staining of ERβ antibody (20× magnification). Inset: The corresponding section of the tumor stained with hematoxylin and eosin (20× magnification).
Evaluation of the Immunohistochemical Expression
The immunohistochemically stained slides were examined by two pathologists (JHZ and VGP) using the definition above for the expression of hormone receptors. The pHH3 counts were performed together by the two pathologists with a double-headed microscope and the results were scored as a consensus between the two pathologists.
Follow-Up and Survival
The follow-up time was calculated from the date each specimen was collected at MDACC until the patient’s death or the last follow-up at MDACC through the end of July 2012. The survival time was calculated from the date each specimen was collected at MDACC until the patient’s death or the last follow-up at MDACC through the end of July 2012 using the Kaplan-Meier method taking into account censoring.
Statistical Methods
McNemar’s exact test was used to assess the differences in hormone receptor expression between pregnant patients and non-pregnant control patients and male control patients. Fisher’s exact and Wilcoxon rank-sum tests were used to assess the association of hormone receptor expression with Breslow thickness of tumor, primary tumor site, primary tumor or metastasis, and disease stage. Kaplan-Meier survival curves were used to estimate survival, and the log-rank test was used to assess the differences in survival between the pregnant group and the two control groups. Fixed effects Cox regression was performed to assess the expression of ERβ on overall survival taking into account matching. The Kruskal-Wallis test was used to evaluate the differences in follow-up and survival times among the three patient groups. Spearman’s rank-order correlation was used to assess the association of pHH3 with Breslow thickness and ERβ expression. A p value <0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.2 for Windows (SAS Institute Inc., Cary, NC).
Results
Patient and Tumor Characteristics
Table 1 summarizes patient and tumor characteristics. The median and range of age for the pregnant patients, the non-pregnant women patients and the male patients were 30/21-44, 31/20-43 and 30/26-43 years old, respectively. There were 3 stage I, 3 stage III and 12 stage IV patients in each group.
Table 1.
Summary of Patient and Tumor Characteristics
| Pregnant Patients (n=18) |
Non-Pregnant Control Patients (n=18) |
Male Control Patients (n=18) |
|
|---|---|---|---|
| Age (year) | |||
| Median/Range | 30/21-44 | 31/20-43 | 30/26-43 |
| Primary tumor site | |||
| Head/Neck | 3 | 5 | 3 |
| Trunk | 4 | 2 | 6 |
| Extremities | 7 | 7 | 7 |
| Unknown | 4 | 4 | 2 |
| Breslow thickness (mm) | |||
| Median/Range | 1.63/0.4-16 | 2.0/0.48-12 | 3.75/0.65-22 |
| pHH3 count/mm2 | |||
| Median/Range | 9.5/1-42 | 11/0-18 | 10/1-42 |
| Ulceration | |||
| Yes | 3 | 3 | 6 |
| No | 10 | 7 | 7 |
| Not available for review | 1 | 4 | 3 |
| Clinical stage | |||
| I | 3 | 3 | 3 |
| II | 0 | 0 | 0 |
| III | 3 | 3 | 3 |
| IV | 12 | 12 | 12 |
Hormone Receptor Expression
The results of the immunohistochemical analyses are summarized in Table 2. Only two cases expressed ERα. One was from a pregnant patient, and the other was from a male control patient. Both patients had acral lentiginous type melanoma of the toe. Of 22 cases that expressed ERβ, 10 (56%) were from pregnant patients, 7 (39%) were from non-pregnant female control patients, and 5 (29%) were from male control patients. The percentage of ERβ-positive cells ranged from 30% to more than 90%. A trend of more frequent ERβ expression was observed in pregnant patients than in male patients (p=0.07). No significant difference of ERβ expression was observed between pregnant and non-pregnant female patients (p=0.54). ERβ expression was not associated with Breslow thickness of tumor (p=0.51), primary tumor site (p=0.94), primary tumor or metastasis (p=0.40), or disease stage at diagnosis (p=0.79). ERβ expression did not correlate with the survival time from the dates the specimens were collected (hazard ratio, 1.215; 95% confidence interval for hazard ratio, 0.472-3.131; p=0.69).
Table 2.
Hormone Receptor Expression, Follow-Up, and Survival Times
| Pregnant Patients (n=18) |
Non-Pregnant Control Patients (n=18) |
Male Control Patients (n=18) |
|
|---|---|---|---|
|
| |||
| ERα | Positive 1 Negative 17 |
Positive 0 Negative 18 |
Positive 1 Negative 17 |
|
| |||
| ERβ | Positive 10 Negative 8 |
Positive 7 Negative 11 |
Positive 5 Negative 12 |
|
| |||
| AR | Positive 0 Negative 18 |
Positive 0 Negative 18 |
Positive 0 Negative 18 |
|
| |||
| Follow-up | |||
| Died of disease | 8 | 6 | 5 |
| Died, unknown cause | 0 | 4 | 6 |
| Alive | 10 | 6 | 4 |
| Lost | 0 | 2 | 3 |
|
| |||
| Survival time (months) Median/Range |
37.6/3.8-96.5 | 28.8/3.7-126 | 27.7/0-99.1 |
|
| |||
| Survival time subset to Stage IV disease (months) Median/Range |
13.6/3.9-96.3 | 22.5/3.7-97.4 | 11/0-74.2 |
Note: ERα = estrogen receptor α; ERβ = estrogen receptor β; AR = androgen receptor.
None of the cases expressed androgen receptor.
Mitotic rate by pHH3
The mitotic rate by pHH3 labeling ranged from 1 to 42/mm2 (median 9.5/mm2) for the pregnant patients, 0 to 18/mm2 (median 11/mm2) for the non-pregnant female control patients, and 1 to 42/mm2 (median 10/mm2) for the male control patients. The pHH3 count was significantly higher in stage IV tumors than in stage I or III tumors (p=0.0001) and was significantly higher in metastatic tumors than in primary tumors (p=0.0003). However, the pHH3 count was not associated with the survival time (p=0.09). PHH3 count was not significantly associated with Breslow thickness of tumor (p=0.09) or primary tumor site (p=0.34). No association between pHH3 count and ERβ expression was observed (p=0.53).
Follow-Up
From the dates the specimens were collected at MDACC, the median follow-up times for the pregnant patients, non-pregnant female control patients, and male control patients were 15.8 months (range, 3.8–96.5 months), 28.5 months (range, 3.7–126 months), and 25.8 months (range, 0.03–99.1 months), respectively (Table 2). The differences in the follow-up times among the three groups were not statistically significant (p=0.46).
Survival Time
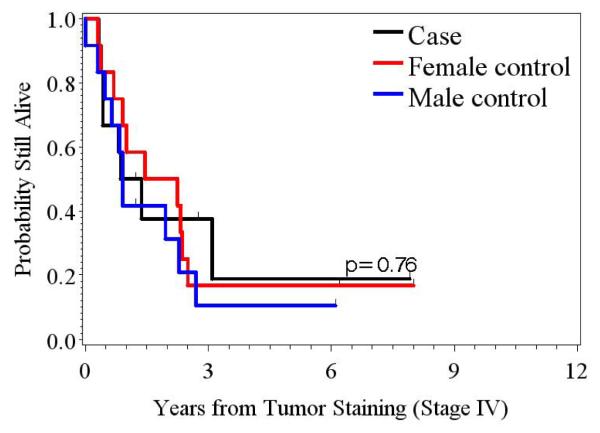
From the dates the specimens were collected at MDACC, the median survival time for the pregnant patients, non-pregnant female control patients, and male control patients were 37.6 months (range, 3.8–96.5 months), 28.8 months (range, 3.7–126 months), and 27.7 months (range, 0.03–99.1 months), respectively. The difference in survival time among the three groups was not statistically significant (p=0.87). The survival time subset only to the stage IV patients for the pregnant patients, the non-pregnant female control patients, and the male control patients were 13.6 months (range, 3.9–96.3 months), 22.5 months (range, 3.7–97.4 months) and 11 months (range, 0.03–74.2 months), respectively (Table 2). The difference in the survival times among the three groups was not statistically significant (p=0.76) (Figure 3).
Figure 3.
Kaplan-Meier survival curve demonstrates no difference in survival in patients with stage IV disease among the pregnant group, the non-pregnant female control group, and the male control group.
Treatment Received
All the patients with primary tumors in both the study and control groups underwent wide local excision and sentinel lymph node dissection after biopsy confirmation of melanoma. When the sentinel lymph node was positive for melanoma, regional lymphadenectomy was performed and systemic staging procedures were carried out. Patients with stage I disease were monitored without adjuvant therapy. A total of 5 patients with stage III disease (2 pregnant patients, 2 male control patients, and 1 female control patient) refused adjuvant therapy. Two patients with stage IV disease (both in the male control group) refused adjuvant therapy. The remaining patients received at least one type of adjuvant therapy, including interferon, biochemotherapy, chemotherapy alone, chemotherapy plus radiation therapy, bio-immunotherapy, anti-CTLA4, granulocyte macrophage colony-stimulating factor, or tumor-infiltrating lymphocytes and interleukin-2.
One patient received BRAF inhibitor, and another patient received BRAF plus MEK inhibitors.
Discussion
Although our study did not reveal a statistically significant difference in ERβ expression, a trend in our findings indicated that ERβ was more frequently expressed in the melanomas of pregnant women than in men, but no difference in ERβ expression was found between pregnant and non-pregnant women. These findings warrant further study. To our knowledge, this is the first study to evaluate hormone receptor expression in the melanomas of stage- and age-matched patients of pregnant women, non-pregnant women, and men.
Both ERα and ERβ proteins are expressed in the normal skin (7). However, previous studies showed that ERβ protein is frequently expressed in cutaneous melanoma, whereas ERα protein is rarely expressed (7, 8). Our findings confirm this observation. Studies (9-11) of estrogen-sensitive cancers such as breast and ovarian cancers showed that loss or decreasing levels of ERβ or increased ERα to ERβ ratio may be involved in carcinogenesis, thereby suggesting that ERβ has a tumor-suppressive function. Studies of estrogen receptors in prostate and colon cancers yielded similar results (12-15) . Omoto et al. (16) performed a survival analysis of patients with invasive breast cancer and found that 5-year disease-free survival was longer in patients with ERβ-positive tumors than in those with ERβ-negative tumors. We speculate that ERβ might explain the generally favorable prognosis of melanoma in women, although we could not statistically confirm this possibility based on our data. This could potentially be due to the small cohort, which probably limited the statistical power of the study. Fuqua et al. (17) studied ERβ expression by immunohistochemical analysis in 242 breast cancer patients and found that ERβ expression was not associated with clinical and biological parameters but correlated with aneuploidy, thereby suggesting that ERβ expression could be an independent prognostic marker. Likewise, we did not find any association of ERβ expression with clinical or pathologic parameters such as Breslow thickness, mitotic rate as assessed by pHH3 immunohistochemical analysis, tumor site, and primary or metastatic tumors. Schmidt et al. (8) evaluated the immunohistochemical expression of ERα and ERβ in various cutaneous melanocytic lesions and found that ERβ expression was most prominent in lesions close to the epidermis, such as severely dysplastic nevus or melanoma in situ, and that the expression diminished in deeper and thicker lesions, thereby suggesting that ERβ may serve as a prognostic marker in melanoma. Studies by di Giorgi et al (6) on ERβ in cutaneous melanoma revealed similar findings. We were not able to confirm these findings, probably because of the relative predominance of metastatic tumors in our study.
Reports of the development and accelerated progression of melanoma in pregnancy have led to several retrospective case-control studies of the effect of pregnancy on melanoma survival. Some reported poorer outcome when melanoma developed during pregnancy (18-20), whereas others showed that pregnancy did not significantly affect survival in patients with stage I or II disease (21-25). However, no such studies have included patients with stage III or IV disease. Most of our patients had stage IV disease, and our data did not show any significant difference in survival between the pregnant patients and the non-pregnant female and male control patients.
Little is known on the expression of androgen receptor in human melanoma. Briton et al. (26) assessed cancer risk among infertile women with androgen excess disorders and found that the standardized incidence ratio was statistically significant for breast cancer, uterine cancer, and melanoma (standardized incidence ratio, 1.96; 95% CI, 1.12-3.18). Apolipoprotein D, an androgen-regulated protein, has been found in cutaneous melanoma (27). An animal study by Hsueh et al. (28) showed that androgen blockade enhanced the response to melanoma vaccine in male mice. Richardson et al. (29) found that 17β estrodiol and estrone inhibited invasion and dehydroepiandrosterone enhanced invasion in melanoma cell lines. The same authors also found that the serum estrogen/androgen index was decreased in female patients with stage IV disease, although this finding was not statistically significant. We did not find any expression of androgen receptor in the specimens included in our study.
Mitotic rate has been found to be a prognostic factor in primary cutaneous melanoma (30-34). The conventional method of counting the mitotic rate on slides stained with hematoxylin and eosin is time consuming and could demonstrate considerable intra- and inter-observer variations. PHH3 is produced by the phosphorylation of Histone 3 during mitotic chromatin condensation in the late G2 and M phases of the cell cycle. PHH3 immunohistochemical staining has been shown to be both sensitive and highly specific for the detection of mitosis since it does not label apoptotic cells (35-37). Therefore, assessing pHH3 nuclear staining of the tumor cells is more efficient and reliable than the conventional method (38, 39). Furthermore, pHH3 staining has been reported to be very useful in facilitating objective grading in meningiomas (40) and astrocytomas (41), in differentiating melanoma from nevi (42), and in evaluating thin melanomas (43). Recently, Ladstein et al. (44) reported that the pHH3 count is a stronger prognostic indicator than the mitotic rate as assessed by hematoxylin and eosin staining for disease-specific survival in nodular melanomas with a Breslow thickness of 0.7-44.0 mm. Our data indicate that the pHH3 count is significantly higher in patients with stage IV disease and in metastatic tumors than in stage I or III disease or primary tumors. However, we did not discern any association of pHH3 with the survival time, which was most likely attributable to the relatively small cohort.
In summary, we observed a trend that ERβ was more frequently expressed in the melanomas of pregnant women than in those of men, but not in those of non-pregnant women. The small cohort may have limited the statistical power of the study. Larger scale studies are needed to elucidate the potential role of ERβ as a prognostic marker of melanoma.
Acknowledgement
We thank Markeda Wade in the Department of Scientific Publications at MD Anderson Cancer Center for critically reviewing the manuscript.
Source of Funding: This study was funded by the Division of Pathology and Laboratory Medicine for Research Projects by Fellows at MD Anderson Cancer Center and was supported in part by the National Institutes of Health via MD Anderson’s Cancer Center Support Grant (NCI Grant P30 CA016672) for the statistical analysis. Jane H. Zhou, MD was funded by T32CA163185-02.
Footnotes
Conflict of Interests: None declared.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jhaveri MB, Driscoll MS, Grant-Kels JM. Melanoma in pregnancy. Clin Obstet Gynecol. 2011;54(4):537–45. doi: 10.1097/GRF.0b013e318236e18b. Epub 2011/10/28. [DOI] [PubMed] [Google Scholar]
- 2.Pentheroudakis G, Orecchia R, Hoekstra HJ, Pavlidis N. Cancer, fertility and pregnancy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v266–73. doi: 10.1093/annonc/mdq198. Epub 2010/06/29. [DOI] [PubMed] [Google Scholar]
- 3.Joosse A, Collette S, Suciu S, et al. Superior Outcome of Women With Stage I/II Cutaneous Melanoma: Pooled Analysis of Four European Organisation for Research and Treatment of Cancer Phase III Trials. J Clin Oncol. 2012;30(18):2240–7. doi: 10.1200/JCO.2011.38.0584. Epub 2012/05/02. [DOI] [PubMed] [Google Scholar]
- 4.de Giorgi V, Gori A, Grazzini M, et al. Estrogens, estrogen receptors and melanoma. Expert Rev Anticancer Ther. 2011;11(5):739–47. doi: 10.1586/era.11.42. Epub 2011/05/11. [DOI] [PubMed] [Google Scholar]
- 5.Joosse A, de Vries E, Eckel R, et al. Gender differences in melanoma survival: female patients have a decreased risk of metastasis. J Invest Dermatol. 2011;131(3):719–26. doi: 10.1038/jid.2010.354. Epub 2010/12/15. [DOI] [PubMed] [Google Scholar]
- 6.de Giorgi V, Mavilia C, Massi D, et al. Estrogen receptor expression in cutaneous melanoma: a real-time reverse transcriptase-polymerase chain reaction and immunohistochemical study. Arch Dermatol. 2009;145(1):30–6. doi: 10.1001/archdermatol.2008.537. Epub 2009/01/21. [DOI] [PubMed] [Google Scholar]
- 7.Ohata C, Tadokoro T, Itami S. Expression of estrogen receptor beta in normal skin, melanocytic nevi and malignant melanomas. The Journal of dermatology. 2008;35(4):215–21. doi: 10.1111/j.1346-8138.2008.00447.x. Epub 2008/04/19. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt AN, Nanney LB, Boyd AS, King LE, Jr., Ellis DL. Oestrogen receptor-beta expression in melanocytic lesions. Exp Dermatol. 2006;15(12):971–80. doi: 10.1111/j.1600-0625.2006.00502.x. Epub 2006/11/07. [DOI] [PubMed] [Google Scholar]
- 9.Roger P. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. 2001. [PubMed]
- 10.Shaw JA, Udokang K, Mosquera JM, Chauhan H, Jones JL, Walker RA. Oestrogen receptors alpha and beta differ in normal human breast and breast carcinomas. The Journal of pathology. 2002;198(4):450–7. doi: 10.1002/path.1230. Epub 2002/11/16. [DOI] [PubMed] [Google Scholar]
- 11.Park BW, Kim KS, Heo MK, et al. Expression of estrogen receptor-beta in normal mammary and tumor tissues: is it protective in breast carcinogenesis? Breast cancer research and treatment. 2003;80(1):79–85. doi: 10.1023/A:1024406223619. Epub 2003/08/02. [DOI] [PubMed] [Google Scholar]
- 12.Latil A, Bieche I, Vidaud D, et al. Evaluation of androgen, estrogen (ER alpha and ER beta), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res. 2001;61(5):1919–26. Epub 2001/03/31. [PubMed] [Google Scholar]
- 13.Pasquali D, Rossi V, Esposito D, et al. Loss of estrogen receptor beta expression in malignant human prostate cells in primary cultures and in prostate cancer tissues. The Journal of clinical endocrinology and metabolism. 2001;86(5):2051–5. doi: 10.1210/jcem.86.5.7441. Epub 2001/05/10. [DOI] [PubMed] [Google Scholar]
- 14.Pasquali D, Staibano S, Prezioso D, et al. Estrogen receptor beta expression in human prostate tissue. Molecular and cellular endocrinology. 2001;178(1-2):47–50. doi: 10.1016/s0303-7207(01)00418-x. Epub 2001/06/19. [DOI] [PubMed] [Google Scholar]
- 15.Konstantinopoulos PA, Kominea A, Vandoros G, et al. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour’s dedifferentiation. European journal of cancer. 2003;39(9):1251–8. doi: 10.1016/s0959-8049(03)00239-9. Epub 2003/05/24. [DOI] [PubMed] [Google Scholar]
- 16.Omoto Y, Inoue S, Ogawa S, et al. Clinical value of the wild-type estrogen receptor beta expression in breast cancer. Cancer letters. 2001;163(2):207–12. doi: 10.1016/s0304-3835(00)00680-7. Epub 2001/02/13. [DOI] [PubMed] [Google Scholar]
- 17.Fuqua SA, Schiff R, Parra I, et al. Estrogen receptor beta protein in human breast cancer: correlation with clinical tumor parameters. Cancer Res. 2003;63(10):2434–9. Epub 2003/05/17. [PMC free article] [PubMed] [Google Scholar]
- 18.Reintgen DS, McCarty KS, Jr., Vollmer R, Cox E, Seigler HF. Malignant melanoma and pregnancy. Cancer. 1985;55(6):1340–4. doi: 10.1002/1097-0142(19850315)55:6<1340::aid-cncr2820550630>3.0.co;2-t. Epub 1985/03/15. [DOI] [PubMed] [Google Scholar]
- 19.Slingluff CL, Jr., Reintgen DS, Vollmer RT, Seigler HF. Malignant melanoma arising during pregnancy. A study of 100 patients. Annals of surgery. 1990;211(5):552–7. doi: 10.1097/00000658-199005000-00005. discussion 8-9. Epub 1990/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller E, Barnea Y, Gur E, et al. Malignant melanoma and pregnancy: second thoughts. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2010;63(7):1163–8. doi: 10.1016/j.bjps.2009.05.050. Epub 2009/07/14. [DOI] [PubMed] [Google Scholar]
- 21.McManamny DS, Moss AL, Pocock PV, Briggs JC. Melanoma and pregnancy: a long-term follow-up. British journal of obstetrics and gynaecology. 1989;96(12):1419–23. doi: 10.1111/j.1471-0528.1989.tb06306.x. Epub 1989/12/01. [DOI] [PubMed] [Google Scholar]
- 22.Wong JH, Sterns EE, Kopald KH, Nizze JA, Morton DL. Prognostic significance of pregnancy in stage I melanoma. Archives of surgery. 1989;124(10):1227–30. doi: 10.1001/archsurg.1989.01410100133023. discussion 30-1. Epub 1989/10/01. [DOI] [PubMed] [Google Scholar]
- 23.MacKie RM, Bufalino R, Morabito A, Sutherland C, Cascinelli N. Lack of effect of pregnancy on outcome of melanoma. For The World Health Organisation Melanoma Programme. Lancet. 1991;337(8742):653–5. doi: 10.1016/0140-6736(91)92462-b. Epub 1991/03/16. [DOI] [PubMed] [Google Scholar]
- 24.Lens MB, Rosdahl I, Ahlbom A, et al. Effect of pregnancy on survival in women with cutaneous malignant melanoma. J Clin Oncol. 2004;22(21):4369–75. doi: 10.1200/JCO.2004.02.096. Epub 2004/10/30. [DOI] [PubMed] [Google Scholar]
- 25.O’Meara AT, Cress R, Xing G, Danielsen B, Smith LH. Malignant melanoma in pregnancy. A population-based evaluation. Cancer. 2005;103(6):1217–26. doi: 10.1002/cncr.20925. Epub 2005/02/16. [DOI] [PubMed] [Google Scholar]
- 26.Brinton LA, Moghissi KS, Westhoff CL, Lamb EJ, Scoccia B. Cancer risk among infertile women with androgen excess or menstrual disorders (including polycystic ovary syndrome) Fertil Steril. 2010;94(5):1787–92. doi: 10.1016/j.fertnstert.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda E, Vizoso F, Martin A, et al. Apolipoprotein D expression in cutaneous malignant melanoma. Journal of surgical oncology. 2003;83(2):99–105. doi: 10.1002/jso.10245. Epub 2003/05/29. [DOI] [PubMed] [Google Scholar]
- 28.Hsueh EC, Gupta RK, Lefor A, Reyzin G, Ye W, Morton DL. Androgen blockade enhances response to melanoma vaccine. The Journal of surgical research. 2003;110(2):393–8. doi: 10.1016/s0022-4804(03)00005-2. Epub 2003/06/06. [DOI] [PubMed] [Google Scholar]
- 29.Richardson B, Price A, Wagner M, et al. Investigation of female survival benefit in metastatic melanoma. British journal of cancer. 1999;80(12):2025–33. doi: 10.1038/sj.bjc.6690637. Epub 1999/09/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azzola MF, Shaw HM, Thompson JF, et al. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: an analysis of 3661 patients from a single center. Cancer. 2003;97(6):1488–98. doi: 10.1002/cncr.11196. Epub 2003/03/11. [DOI] [PubMed] [Google Scholar]
- 31.Busam KJ. The prognostic importance of tumor mitotic rate for patients with primary cutaneous melanoma. Annals of surgical oncology. 2004;11(4):360–1. doi: 10.1245/ASO.2004.02.910. Epub 2004/04/09. [DOI] [PubMed] [Google Scholar]
- 32.Francken AB, Shaw HM, Thompson JF, et al. The prognostic importance of tumor mitotic rate confirmed in 1317 patients with primary cutaneous melanoma and long follow-up. Annals of surgical oncology. 2004;11(4):426–33. doi: 10.1245/ASO.2004.07.014. Epub 2004/04/09. [DOI] [PubMed] [Google Scholar]
- 33.Barnhill RL, Katzen J, Spatz A, Fine J, Berwick M. The importance of mitotic rate as a prognostic factor for localized cutaneous melanoma. Journal of cutaneous pathology. 2005;32(4):268–73. doi: 10.1111/j.0303-6987.2005.00310.x. Epub 2005/03/17. [DOI] [PubMed] [Google Scholar]
- 34.Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28(14):2452–9. doi: 10.1200/JCO.2009.27.1627. Epub 2010/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendzel MJ, Wei Y, Mancini MA, et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106(6):348–60. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- 36.Hendzel MJ, Nishioka WK, Raymond Y, Allis CD, Bazett-Jones DP, Th’ng JPH. Chromatin condensation is not associated with apoptosis. J Biol Chem. 1998;273(38):24470–8. doi: 10.1074/jbc.273.38.24470. [DOI] [PubMed] [Google Scholar]
- 37.Juan G, Traganos F, James WM, et al. Histone H3 phosphorylation and expression of cyclins A and B1 measured in individual cells during their progression through G(2) and mitosis. Cytometry. 1998;32(2):71–7. doi: 10.1002/(sici)1097-0320(19980601)32:2<71::aid-cyto1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 38.Angi M, Damato B, Kalirai H, Dodson A, Taktak A, Coupland SE. Immunohistochemical assessment of mitotic count in uveal melanoma. Acta Ophthalmol. 2011;89(2):E155–E60. doi: 10.1111/j.1755-3768.2009.01769.x. [DOI] [PubMed] [Google Scholar]
- 39.Ikenberg K, Pfaltz M, Rakozy C, Kempf W. Immunohistochemical dual staining as an adjunct in assessment of mitotic activity in melanoma. Journal of cutaneous pathology. 2012;39(3):324–30. doi: 10.1111/j.1600-0560.2011.01858.x. [DOI] [PubMed] [Google Scholar]
- 40.Ribalta T, McCutcheon AE, Aldape KD, Bruner JM, Fuller GN. The mitosis-specific antibody anti-phosphohistone-H3 (PHH3) facilitates rapid reliable grading of meningiomas according to WHO 2000 criteria. American Journal of Surgical Pathology. 2004;28(11):1532–6. doi: 10.1097/01.pas.0000141389.06925.d5. [DOI] [PubMed] [Google Scholar]
- 41.Colman H, Giannini C, Huang L, et al. Assessment and prognostic significance of mitotic index using the mitosis marker phospho-histone H3 in low and intermediate-grade infiltrating astrocytomas. American Journal of Surgical Pathology. 2006;30(5):657–64. doi: 10.1097/01.pas.0000202048.28203.25. [DOI] [PubMed] [Google Scholar]
- 42.Nasr MR, El-Zammar O. Comparison of pHH3, Ki-67, and survivin immunoreactivity in benign and malignant melanocytic lesions. Am J Dermatopath. 2008;30(2):117–22. doi: 10.1097/DAD.0b013e3181624054. [DOI] [PubMed] [Google Scholar]
- 43.Casper DJ, Ross KI, Messina JL, et al. Use of Anti-phosphohistone H3 Immunohistochemistry to Determine Mitotic Rate in Thin Melanoma. Am J Dermatopath. 2010;32(7):650–4. doi: 10.1097/DAD.0b013e3181cf7cc1. [DOI] [PubMed] [Google Scholar]
- 44.Ladstein RG, Bachmann IM, Straume O, Akslen LA. Prognostic Importance of the Mitotic Marker Phosphohistone H3 in Cutaneous Nodular Melanoma. Journal of Investigative Dermatology. 2012;132(4):1247–52. doi: 10.1038/jid.2011.464. [DOI] [PubMed] [Google Scholar]