Abstract
Diazoxide has been identified over the past 50 years to have a number of physiological effects, including lowering the blood pressure and rectifying hypoglycemia. Today it is used clinically to treat these conditions. More recently, another important mode of action emerged: diazoxide has powerful protective properties against cardiac ischemia. The heart has intrinsic protective mechanisms against ischemia injury; one of which is ischemic preconditioning. Diazoxide mimics ischemic preconditioning. The purpose of this treatise is to review the literature in an attempt to identify the many effectors of diazoxide and discuss how they may contribute diazoxide’s cardioprotective properties. Particular emphasis is placed on the concentration ranges in which diazoxide affects its different targets and how this compares with the concentrations commonly used to study cardioprotection. It is concluded that diazoxide may have several potential effectors that may potentially contribute to cardioprotection, including KATP channels in the pancreas, smooth muscle, endothelium, neurons and the mitochondrial inner membrane. Diazoxide may also affect other ion channels and ATPases and may directly regulate mitochondrial energetics. It is possible that the success of diazoxide lies in this promiscuity and that the compound acts to rebalance multiple physiological processes during cardiac ischemia.
Keywords: Cardioprotection, Ischemia, Ischemic preconditioning, Diazoxide, KATP channels, Mitochondria
Introduction
Diazoxide (CAS Number: 364-98-7; 7-chloro-3-methyl-2H-1,2,4-benzothiadiazine 1,1-dioxide; Figure 1) has a molecular weight of 230.7 and a molecular formula of C8H7ClN2O2S. It is a white powder insoluble in water, but soluble in organic solvents (e.g. 10 mg/ml in DMSO). The dogma has arisen in recent years (particularly in the cardioprotection literature) that diazoxide is an agent with a unique molecular target. This is not the case and the purpose of this literature review is to highlight the multiplicity of diazoxide effectors to assist in a better understanding of mechanisms involved in the established cardioprotective effects of this compound.
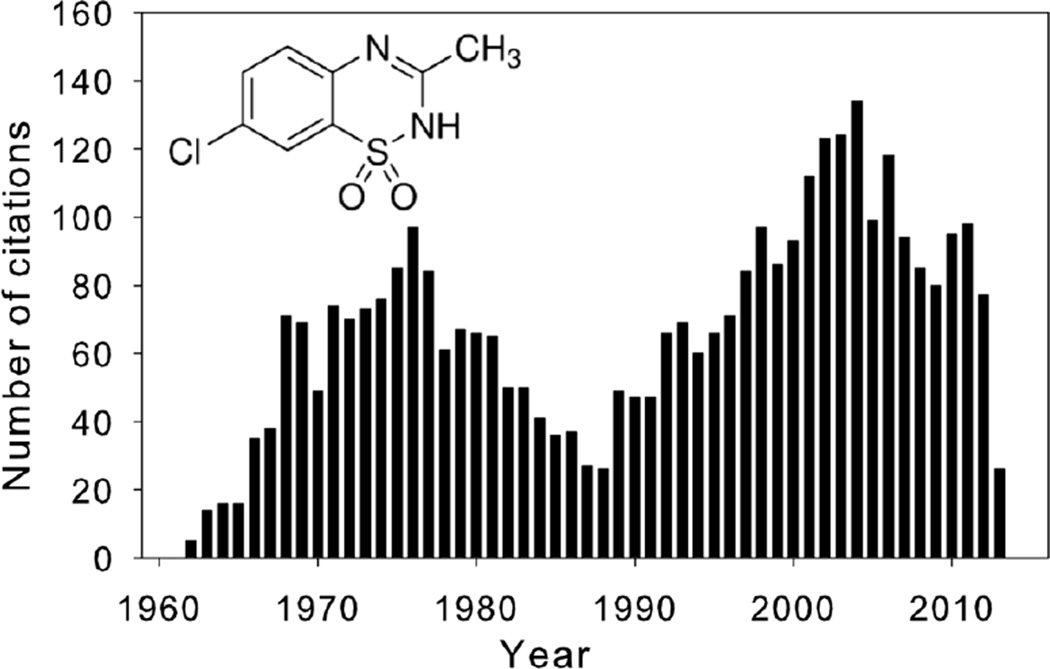
Figure 1.
The number of PubMed citations returned when searching with the keyword “diazoxide”. The data are binned over the time period 1960–2013. The inset shows the structural formula for diazoxide.
History
In the early 1960’s, a study was designed to examine possible non-diuretic mechanisms by which benzothiadiazines lower blood pressure - diazoxide was found to directly cause vasodilation of blood vessels independent of diuretic actions (Rubin, et al., 1962). Early reports, however, also demonstrated that some hypotensive drugs such as diazoxide led to elevated blood glucose levels (hyperglycemia) (Okun, et al., 1964; F. Wolff, 1964). The following years saw a large rise in publications, mostly related to the hypotensive and hyperglycemic effects of diazoxide (Figure 1). Other actions, including effects on renal excretory function, also started to emerge (Johnson, 1971; Rubin, et al., 1968). Nevertheless, the compound became accepted for its oral use in the management of intractable hypoglycemia and intravenously in the management of hypertensive emergencies. The publication rate waned in the mid-1980’s. Following the identification of diazoxide molecular effectors in pancreatic β-cells (Henquin & Meissner, 1982; Trube, et al., 1986) and vascular smooth muscle cells (Standen, et al., 1989), a secondary rise in diazoxide-related publications occurred (Figure 1), which was further stimulated by the mid-1990’s findings that diazoxide has powerful cardioprotective properties (Garlid, et al., 1997; Nakai & Ichihara, 1994).
Clinical use
In tablet form (e.g. Proglycem, FDA approved in 1976) diazoxide is prescribed orally (usually 2 to 3 times daily) for the management of symptomatic hypoglycemia. Side effects include shortness of breath, swelling in extremities, tachycardia, chest pain, blurred vision, bruising or bleeding, unusual weakness; and decreased frequency of urination. Intravenously (e.g. Hyperstat) diazoxide is indicated as a peripheral vasodilator for short-term use in the emergency reduction of blood pressure in severe, nonmalignant and malignant hypertension in hospitalized adults; and in acute severe hypertension in hospitalized children, when prompt and urgent decrease of diastolic pressure is required. Diazoxide is also used to treat hypoglycemia that results from congenital hyperinsulinism of infancy (HI) (Hussain, et al., 2004). The mechanisms of diazoxide’s clinical action relate predominantly to opening of pancreatic and smooth muscle KATP channels, as will be discussed in subsequent sections.
Diazoxide is cardioprotective against ischemic insults
During the treatment of patients with hypotension, early studies suggested an increase in myocardial injury with diazoxide (e.g. chest pain and ST elevation) (Kanada, et al., 1976; O’Brien, et al., 1975). These effects may have been related to the hypotensive action of the drug. Most controlled animal studies to date, however, as well as in vitro studies with human cardiac tissues, suggest that diazoxide has cardioprotective properties (Garlid, et al., 1997; Nakai & Ichihara, 1994; Y. Wang, et al., 1999). Intrinsic adaptive physiological processes within the myocardium render the heart more resistant to potentially lethal ischemic injury. One of the protective phenomena is ischemic preconditioning (IPC) - the most powerful means of delaying myocardial injury that has been identified to date (Yellon & Downey, 2003). Diazoxide is powerfully anti-ischemic and/or recapitulates the cardioprotective effects of IPC. A full review of these studies is beyond the scope of this review and some of these studies are highlighted in Table 1. The cardioprotective effects are observed in a variety of species (rat, rabbit, dog and human), with ex vivo and in vivo methods and over a concentration range of ∼10–100 µM (or 1–10 mg/kg intravenously). Most studies utilize a single dose of diazoxide. In one study, the EC25 for diazoxide’s protective effect (measured as an increased the time to onset of contracture) in isolated rat hearts subjected to 30 min global ischemia was reported to be ∼10 µM (Garlid, et al., 1997). The K1/2 is likely to be higher. In another study, the optimal protective concentration in isolated rat hearts subjected to ischemia/reperfusion was reported to be 80 µM (Y. Wang, et al., 1999). With instrumented dogs, 80 µM (but not 8 µM) diazoxide was reported to provide partial protection against the development of a post-ischemic infarct (Sanada, et al., 2001). The protective effect of diazoxide is equivalent to that of ischemic preconditioning (IPC) and diazoxide is often used as a pharmacological means to induce preconditioning. Moreover, both the IPC- and diazoxide-induced protection is minimized by tolbutamide, HMR-1883 or glibenclamide (sulfonylurea compounds that block various types of KATP channels (Escande, 1989; Faivre & Findlay, 1989; Gogelein, et al., 1998; Quayle, et al., 1995; Trube, et al., 1986)) or 5HD (often used as a mitochondrial KATP blocker, but which also has other off-target effects; see later) (Birincioglu, et al., 1999), suggesting a causative link between the diazoxide effector(s) and mechanism(s) involved in IPC. The purpose of this mini-review is to highlight the multiplicity of diazoxide effectors.
Table 1.
Diazoxide is cardioprotective
| Preparation | Procedure | Result | Concentration |
|---|---|---|---|
| Isolated rat hearts | 25 minutes of global ischemia and 30 minutes of reperfusion | Diazoxide and cromakalim increased the time to onset of contracture and improved post-ischemic functional recovery | 11–30 µM (Garlid, et al., 1997) |
| In vivo rabbits | 30 min of regional ischemia and 3 h of reperfusion | Diazoxide administered before ischemia reduces infarct size | 10 mg/kg (Baines, et al., 1999) |
| Langendorff-perfused rat hearts | Diazoxide pretreatment prior to 40 minutes ischemia and 30 minutes of reperfusion | Diazoxide improved left ventricular end-diastolic pressure, LDH release and coronary flow after I/R | 1–100 µM (80 µM is optimal) (Y. Wang, et al., 1999) |
| Langendorff-perfused rabbit hearts | Diazoxide pretreatment prior to 30 minutes ischemia and 60 minutes of reperfusion | Reduction in infarct size and improved mitochondrial function | 100 µM (Miura, et al., 2000) |
| In vivo rabbits | 30 min of regional ischemia and 3 h of reperfusion | Diazoxide administered before ischemia reduces infarct size | 1 mg/kg (Ockaili, et al., 1999) |
| In vivo rats | 30 min of regional ischemia and 2 h of reperfusion | Diazoxide administered before ischemia reduces infarct size | 10 mg/kg iv (Fryer, et al., 2000) |
| Human right atrial specimens | 90 min ‘ischemia’ and 2h ‘reperfusion’ | Less CK leakage in diazoxide group | 100 µM (Ghosh, et al., 2000) |
| Isolated rabbit hearts | 30 min of regional ischemia and 2 h of reperfusion | Diazoxide administered before ischemia reduces infarct size | 100 µM (S. Wang, et al., 2001) |
| Isolated mouse hearts | 20 min of global ischemia and 1 h of reperfusion | Improved post-ischemic functional recovery | 100 µM (Suzuki, et al., 2003) |
| Isolated mouse hearts | 30 min of global ischemia and 1 h of reperfusion | Diazoxide administered before ischemia improved functional recovery and reduced infarct size. The protection was blunted in Kir6.2(−/−) mice | 30 µM (Wojtovich, et al., 2013) |
| Isolated mouse hearts | 40 min of global ischemia and 30 minutes of reperfusion | Diazoxide IV administration 24 h prior to ischemia improves post-ischemic functional recovery | 7 mg/kg (Y. Wang, et al., 2001) |
| Open-chest pig | 30 min of regional ischemia and 3 h of reperfusion | Diazoxide administered before ischemia reduces infarct size | 3.5 mg/kg (Schwartz, et al., 2002) |
| Open-chest beagle dogs | 90 min of regional ischemia and 6 h of reperfusion | Diazoxide partially decreased infarct size | 80 µM (Sanada, et al., 2001) |
Diazoxide activates pancreatic β-cell KATP channels
Diazoxide has long been recognized to be hyperglycemic by inhibiting insulin release from pancreatic β-cells (Loubatieres, et al., 1966; Okun, et al., 1964; F. W. Wolff, et al., 1963). The mechanism was found to be an increased membrane K+ permeability (measured with Rb+ flux assays), leading to membrane hyperpolarization, inhibition of Ca2+ influx (Henquin & Meissner, 1982) and diminished insulin secretory release. Shortly after the discovery of cardiac KATP channels (Noma, 1983; Trube & Hescheler, 1984), similar channels in the pancreatic β-cell were found to be responsible for the diazoxide-induced increase of K+ permeability (Trube, et al., 1986). The diazoxide-sensitivity of the pancreatic β-cell KATP channel is high (K1/2 of 7–20 µM; Table 2), which is in the same concentration range as the drug’s cardioprotective benefit (Table 1). Pancreatic β-cell KATP channels are unlikely to be involved in diazoxide’s cardioprotective effects when using ex vivo preparations (e.g. isolated hearts). In patients, however, or when using diazoxide with in vivo experimental approaches, the possibility must be considered that diazoxide may elevate blood glucose levels, which in turn may influence the ischemic outcome. Provision of glucose, together with insulin and potassium (GIK), has clear beneficial effects during ischemia (Opie, 1975) but the benefit of increasing glucose levels alone is questionable (LaDisa, et al., 2004; Spath, et al., 1976).
Table 2.
Effectors of diazoxide
| Target | Effect | Concentration (K1/2) |
|---|---|---|
| Mitochondrial KATP channel | Opens | 2–27 µM (Garlid, et al., 1993; Y. Liu, et al., 2001; Wojtovich, et al., 2013) |
| Pancreatic β-cell KATP channel | Opens | 7–20 µM (Trube, et al., 1986; Zunkler, et al., 1988) |
| Kir6.1/SUR1 or Kir6.2/SUR1 channels | Opens | 10 µM (Y. Liu, et al., 2001) |
| Smooth muscle | Relaxation | 7–20 µM (Mahmoudian & Mirkhani, 1998; Newgreen, et al., 1990) |
| Rabbit mesenteric KATP channel | Opens | 37 µM (Quayle, et al., 1995) |
| Kir6.1/SUR2B channels | Opens | 60 µM (Inagaki, et al., 1995) |
| Ventricular KATP channels | Cytosolic ADP is needed (D’Hahan, et al., 1999) | |
| Kir6.2/SUR2A channels | Cytosolic ADP is needed (D’Hahan, et al., 1999; Matsuoka, et al., 2000) | |
| Succinate dehydrogenase | Inhibition | 32–49 µM (Dzeja, et al., 2003; Schafer, et al., 1969) |
Diazoxide activates smooth muscle KATP channels
The hypotensive properties of diazoxide have been described over 50 years ago (Rubin, et al., 1962). As is the case for other KATP channel openers (Gross, et al., 1989; Quayle, et al., 1997; S. Sakamoto, et al., 1989), the vasodilatory effects of diazoxide in vascular smooth muscle is due to opening of vascular KATP (or KNDP) channels (Standen, et al., 1989), which leads to local relaxation in smooth muscle due to an increased membrane K+ permeability, subsequent inhibition of excitability, lowered cytosolic Ca2+ and relaxation. Patch clamp data demonstrated that the diazoxide sensitivity of smooth muscle KATP/KNDP channels is in the same concentration range as that of pancreatic β-cell KATP channels (Table 2). KATP channels have a pronounced role in controlling coronary blood flow and the coronary reserve, particularly in the resistance arterioles (Akai, et al., 1995; Sato, et al., 1994). At 2.5mg/kg (within the cardioprotective concentration range; Table 1) intravenous diazoxide causes a 180% increase in coronary flow in anesthetized dogs (Scott & Cowley, 1969). In some studies diazoxide was noted to improve coronary flow, which is associated with cardioprotection, in perfused hearts (Feng, et al., 2002; Garlid, et al., 1997; Suzuki, et al., 2003), despite the fact that the coronary flow reserve is low in crystalloid-perfused hearts (Deng, et al., 1995; Masuda, et al., 1994). In a study with barbiturate-anesthetized dogs, administration of diazoxide (2.5mg/kg) prior to 30 min of regional ischemia and 1h reperfusion almost doubled the transmural myocardial perfusion in the ischemic region (measured with labeled microspheres) (Alcindor, et al., 2004). Thus, improved cardiac perfusion (local or global) may possibly account for at least some of the cardioprotective effects of diazoxide.
Does diazoxide activate ‘cardiac’ sarcolemmal KATP channels?
Before addressing this question, it would be instructive to examine the mechanism by which diazoxide opens KATP channels. KATP channels are heterooctamers of four Kir6.1 and/or Kir6.2 subunits in complex with SURx subunits (Nichols, 2006). Two genes (ABCC8 and ABCC9) respectively give rise to SUR1 and SUR2 subunits, each of which may be alternatively spliced. The most commonly described splice variants studied are SUR1, SUR2A and SUR2B (Nichols, 2006). When comparing the effects of diazoxide on Kir6.2/SUR1, Kir6.2/SUR2A or Kir6.2/SUR2B channels, it was found that the effects of diazoxide depend on the cytosolic ADP levels (Matsuoka, et al., 2000). Each of the subunit combinations were activated by diazoxide, but the activation of Kir6.2/SUR2A was only observed at elevated ADP levels. Thus, under basal conditions and when intracellular ADP levels are low, diazoxide potently activates SUR1-containing channels (such as the pancreatic β-cell KATP channel) or channels with SUR2B (smooth muscle KATP channel), which would explain the drug’s history of acting on these channels. The inability of diazoxide to activate Kir6.2/SUR2A (the prototypic cardiac KATP channel combination (Babenko, et al., 1998)) under basal conditions has also been observed by others (Garlid, et al., 1993), which led to the erroneous consensus that cardiac KATP channels are insensitive to diazoxide. At an elevated cytosolic ADP level of 100 µM, the SUR2A/Kir6.2 channel attains the same diazoxide sensitivity as the Kir6.2/SUR1 channel. Moreover, cardiac KATP channels are readily activated by diazoxide in the presence of a creatine kinase inhibitor (which elevates cytosolic ADP) (D’Hahan, et al., 1999). Since cytosolic ADP levels are substantially elevated during early ischemia (Ramani, et al., 1996), the possibility must be considered that cardiac KATP channels are de facto effectors of diazoxide during the ischemic period and that they may participate in the drug’s cardioprotective actions. Indeed, diazoxide leads to more pronounced shortening of the action potential during the first 10 min of ischemia in an isolated rat heart (Garlid, et al., 1997; Suzuki, et al., 2003) and KATP channels open more readily during metabolic inhibition in myocytes pre-incubated with diazoxide (Rodrigo, et al., 2004). Moreover, the preconditioning-like protection of diazoxide is prevented by HMR-1883 (Birincioglu, et al., 1999; Suzuki, et al., 2003; Tanno, et al., 2001), a blocker with putative selectivity for ‘the cardiac sarcolemmal KATP channel’ (Gogelein, et al., 1998) (but see (H. X. Zhang, et al., 2011)). Also, the ability of diazoxide to improve post-ischemic functional recovery is lost in Kir6.2 deficient mice (Suzuki, et al., 2003; Wojtovich, et al., 2013), which lack sarcolemmal KATP channels (Suzuki, et al., 2001). These arguments provide strong evidence that sarcolemmal KATP channels are diazoxide effectors. Apart from the fact that diazoxide may affect Kir6.2/SUR2A channels (under ischemic conditions), recent progress has questioned the contention that ‘the’ cardiac KATP channel is necessarily comprised of these two subunits. There is a report of a novel diazoxide-sensitive 22 pS conductance KATP channel in rat ventricular myocytes (Wu, et al., 2007). KATP channels in rodent atria (Baron, et al., 1999; Flagg, et al., 2008), human ventricle (Fedorov, et al., 2011) and the rodent cardiac conduction system (Bao, et al., 2011) all have a high sensitivity to diazoxide (presumably due to the presence of SUR1 or SUR2B) and the contribution of these channels to cardioprotection and arrhythmias should be considered when assessing effectors of diazoxide.
Diazoxide activates mitochondrial KATP channels
A K+ selective ion channel, blocked by ATP with an IC50 of 800 µM, was initially described in the inner membrane of fused giant mitoplasts prepared from rat liver mitochondria using patch clamp methods (Inoue, et al., 1991). A similar mitochondrial KATP (mKATP) channel was recorded when purified membranes of rat liver or bovine heart were reconstituted in lipid bilayer membranes (Bednarczyk, et al., 2005; Nakae, et al., 2003; Paucek, et al., 1992; D. X. Zhang, et al., 2001). Patch clamping of the inner mitochondrial membrane of human T-lymphocytes also demonstrated the existence of a mKATP channel that is blocked by millimolar concentrations of ATP (Dahlem, et al., 2004). Some studies, however, failed to find mKATP channels in the mitochondrial inner membrane (Antonenko, et al., 1994; Brustovetsky, et al., 2005). Despite initial reports suggesting the presence of KATP channel subunits (Kir6.x and SURx) in mitochondria, the current consensus appears to be that these subunits do not comprise the mKATP channel (Wojtovich, et al., 2013; H. Zhang, et al., 2009). Recent developments implicate novel, short-form, SUR2 splice variants and Kir1.1 subunits as potential subunit candidates (Foster, et al., 2012; Ye, et al., 2009). The mKATP channel is activated by the cromakalim analog EMD60480 in the nanomolar range (EC50 of 6 nM) and diazoxide in the micromolar range (2.3–27 µM; Table 2; also see (Nakae, et al., 2003)). Other patch clamp data, however, failed to demonstrate activation of mKATP channels by 50 µM diazoxide (Dahlem, et al., 2004). Diazoxide (10 µM) directly increases K+ fluxes in isolated mitochondria, as measured with the surrogate monovalent flux carrier, thallium (Tl+) (Wojtovich, et al., 2013). The diazoxide concentrations needed to open mKATP channels are within the cardioprotective range (Table 1) and are similar to that needed to open other KATP channels (Table 2). The activation of KATP channel opening by diazoxide can be antagonized with 5-hydroxydecanoate (5HD) (Nakae, et al., 2003; D. X. Zhang, et al., 2001), which is therefore often employed as a mKATP channel-selective blocker (Garlid, et al., 1997). However, care should be employed when using this compound since some studies report no (or an unusually slow and irreversible) block of mKATP channel by 5HD (Choma, et al., 2009; Dahlem, et al., 2004). Moreover, 5HD may have other off-target effects (e.g., cardiac mitochondria can directly metabolize 5HD (Hanley, et al., 2002; Suleiman, et al., 2001)). The selectivity of diazoxide to affect only mKATP channels is increasingly being questioned (Jaggar, et al., 1993; Li, et al., 2010; Moritani, et al., 1994; Notsu, Ohhashi, et al., 1992; Notsu, Tanaka, et al., 1992; K. Sakamoto, et al., 1998; Szewczyk, et al., 2010). Given the electrogenic nature of the mKATP channel, the expectation is that the mitochondrial membrane potential (ΔΨm) may be affected by its opening. Mixed results exist in this regard, with some results indicting no effect, with others suggesting depolarization of the ΔΨm (Table 3). Interestingly, the depolarizing effect of 10 µM, but not 100 µM diazoxide, is blocked by 5-hydroxydecanoic acid (5HD), which the authors interpreted as non-specific effects at the higher diazoxide concentrations (Murata, et al., 2001). A similar conclusion was reached by others, suggesting that at >50 µM, diazoxide has mitochondrial effects that are independent of mKATP channels (Kowaltowski, et al., 2001). Interestingly, ΔΨm depolarization induced by anoxia/reoxygenation or reactive oxidant species is prevented by diazoxide (Table 3). Since maintenance of ΔΨm is essential for ATP synthesis, this may be a potential protective or anti-ischemic effect.
Table 3.
Effects of diazoxide on mitochondrial membrane potential (Δψm)
| Preparation | Concentration | Method | Result |
|---|---|---|---|
| Isolated rat cardiac myocytes | 200 µM | TMRE fluorescence | No effect on Δψm (Lawrence, et al., 2001) |
| Isolated guinea-pig ventricular myocytes | 100 µM | TMRE fluorescence | No effect on Δψm (Hanley, et al., 2002) |
| Cultured human atrial-derived cardiocytes | 30 µM | JC-1 fluorescence | No effect on Δψm (Carroll, et al., 2001) |
| Cultured hippocampal neurons | 30 µM | TMRE fluorescence | Depolarize Δψm (D. Liu, et al., 2002) |
| Isolated mitochondria from piglet brain | 50–500 µM | TMRE fluorescence | Depolarize Δψm (Busija, et al., 2005) |
| Isolated rat cardiac mitochondria | 500 µM | TMRE fluorescence | Depolarize Δψm (Katakam, et al., 2007) |
| Permeabilized rabbit ventricular myocytes | 10–100 µM | TMRE fluorescence | Depolarize Δψm (Murata, et al., 2001) |
| Rat heart mitochondria | >50 µM | TPP+-selective electrode | Depolarize Δψm(Kowaltowski, et al., 2001) |
| Isolated rat cardiac myocytes | 100 µM | TMRE fluorescence | aPrevents ROS-mediated Δψm depolarization (Jin, et al., 2012) |
| Neonatal rat cardiac myocytes | 20–200 µM | TMRE fluorescence | Prevents H2O2-mediated Δψm depolarization (Akao, et al., 2001) |
| Cultured neonatal rat cardiac myocytes | 100 µM | JC-1 fluorescence | Protects against Δψm depolarization induced by anxoxia/reoxygenation (Xu, et al., 2001) |
| Cultured H9c2 cells | 50 µM | TMRE fluorescence | Prevents Δψm depolarization induced by hypoxia/reoxygenation (Mykytenko, et al., 2008) |
The intervention used was intermittent laser illumination of a 15 min period.
Diazoxide improves mitochondrial function
As early as 1969, diazoxide has been recognized as an inhibitor of the mitochondrial complex II protein, succinate dehydrogenase (SDH) (Schafer, et al., 1969). This inhibition, which is also observed in heart (Hanley & Daut, 2005; Hanley, et al., 2002), occurs at concentrations often used to study cardioprotection (K1/2=32 µM; Table 1) and similar to those affecting other diazoxide effectors (Table 2). As expected, SDH inhibition by diazoxide leads to increased flavoprotein fluorescence (Schafer, et al., 1971) (but see (Lawrence, et al., 2001)) with a K1/2 of 27 µM (Y. Liu, et al., 1998). It is possible that the cardioprotective effect of diazoxide is due at least partly to SDH inhibition since other SDH inhibitors (e.g. thenoyltrifluoroacetone) are also cardioprotective (Duda, et al., 2007). The mechanisms by which SDH inhibition is cardioprotective are under active investigation and may relate to partial uncoupling of oxidative phosphorylation (Kopustinskiene, et al., 2003),53. Indeed, short-term administration of 2,4-dinitrophenol (DNP), an uncoupler of oxidative phosphorylation, induces preconditioning-like cardioprotection (Minners, et al., 2000), an effect that is independent of KATP channel activation (Rodrigo, et al., 2002). Since the mitochondrial effects of diazoxide are dependent on the metabolic substrate used and do not persist in the absence of K+ (B. Liu, et al., 2010; Riess, et al., 2008), this also argues against a KATP channel-mediated response. Experiments with other approaches further demonstrated that transient (or partial) inhibition of the oxidative phosphorylation may serve as a mechanism to reduce I/R injury (Stottrup, et al., 2010). The activity of SDH, coupled with the coenzyme Q cycle, is a site of generation of reactive oxygen species (Demin, et al., 1998) and the possibility has been raised that diazoxide and other SDH inhibitors mediate (at least some of) their cardioprotective effects via modulation of ROS production (Carroll, et al., 2001; Drose, et al., 2011; Dzeja, et al., 2003; Pain, et al., 2000; Pasdois, et al., 2008; Terzic, et al., 2000). Additionally, diazoxide has been demonstrated to protect mitochondrial energetic function and prevent swelling during metabolic stress (Dos Santos, et al., 2002; Iwai, et al., 2000; Ozcan, et al., 2001), partly by preventing Ca2+-dependent inhibition of oxidative phosphorylation (Holmuhamedov, et al., 2012). Whatever the underlying molecular mechanism(s), it is clear that improved mitochondrial function must be considered as an important diazoxide effector in cardioprotection.
Are endothelial KATP channels involved?
Although diazoxide can cause vasodilation in the absence of an intact endothelium (Antoine, et al., 1992), several reports indicate the endothelium to be another diazoxide target (Feleder & Adler-Graschinsky, 1997). In humans, plethysmography recordings of forearm blood flow demonstrated that endothelial function is impaired by ischemia (induced by blood pressure cuff inflation), which is prevented by IPC or diazoxide pre-administration (800 µg/min for 20 min) (Broadhead, et al., 2004). Post-ischemic endothelial dysfunction also occurs in animal models, and can be minimized by IPC or diazoxide-preconditioning (Duda, et al., 2006; Pagliaro, et al., 2003). Although excessively high diazoxide concentrations were used in some of these studies, the coronary endothelium is in fact exquisitely sensitive to diazoxide: it causes endothelial hyperpolarization in the sub-micromolar concentration range and Ca2+ oscillations have been reported to occur with concentrations as low as 100 nM (Langheinrich, et al., 1998; White & Hiley, 2000). KATP channels in the endothelium are established to contribute significantly to coronary function (Ishizaka & Kuo, 1997; Janigro, et al., 1993; Kuo & Chancellor, 1995; Q. Liu & Flavahan, 1997; Malester, et al., 2007; Schnitzler, et al., 2000; von Beckerath, et al., 1996). The endothelial KATP channels are thought to be composed of Kir6.1 and Kir6.2 in combination with the diazoxide-sensitive SUR2B subunit (Jansen-Olesen, et al., 2005; Schnitzler, et al., 2000; Yoshida, et al., 2004) (Table 2). Activation of these channels by diazoxide (Schnitzler, et al., 2000) may be partly responsible for the observed electrophysiological effects. It is unclear at present how diazoxide leads to protection against endothelial dysfunction. Mitochondrial energetics and mKATP channel activation may contribute, but the changes in membrane potential and cytosolic Ca2+ suggest that other ion channels (particularly endothelial KATP channels) may also be involved. KATP channel-mediated endothelial hyperpolarization may be propagated to the coronary smooth muscle through connexin-mediated myo-endothelial electrical communication (Murai, et al., 1999). It is also possible that endothelial KATP channel opening (and associated changes in cytosolic Ca2+) may benefit the secretion of vasoactive substances such as nitric oxide (Luckhoff & Busse, 1990; H. Wang, et al., 2007), which has an established cardioprotective role (Downey, et al., 2007). It should be a priority of future experiments to investigate these possibilities.
Diazoxide regulates neurotransmitter release
A novel role for KATP channels has been described in the sympathetic nervous system, where it was found that norepinephrine (NE) release is inhibited by active KATP channels (Oe, et al., 1999). The data supporting this finding are that KATP channel openers (including 100 µM diazoxide) inhibit NE release as well as the increase in atrial rate induced by electrical stimulation of the sympathetic ganglion (Mohan & Paterson, 2000; Oe, et al., 1999). In contrast, KATP channel blockers had the opposite effects. KATP channels also are involved in neurotransmitter release from parasympathetic nerves. Acetylcholine (Ach) is synthesized and released in the central nervous system as well as from autonomic ganglia in the peripheral nervous system and in postganglionic parasympathetic neurons. In the heart, vagal nerve stimulation causes Ach release, which slows the heart rate by G-protein-mediated activation of Kir3.x channels (Coetzee, et al., 1999). It was found that Ach-release evoked by electrical stimulation of isolated guinea pig atria was stimulated by KATP channel blockers, suggesting a role for negative feedback of KATP channels in exocytotic processes in these neurons (Kilbinger, et al., 2002). Importantly, 30 µM diazoxide significantly reduced the stimulation-evoked release of [3H]acetylcholine. Interestingly, Ach release of neurons in the ileum is affected similarly by KATP channel activity (Zini, et al., 1991), whereas mesenteric neurons are not affected by KATP channel modulation (Kilbinger, et al., 2002; Schworer & Kilbinger, 1989). These observations suggest diversity of KATP channels in various neuronal compartments, which remains to be studied. The KATP channels in dorsal vagal neurons have been suggested to be composed of Kir6.2/SUR1 channels (Karschin, et al., 1998) (as in the pancreas), which raises issues of potential cardiovascular side-effects of sulphonylurea treatment in the setting of diabetes. The study of the molecular composition of the KATP channels in sympathetic and parasympathetic neurons and the molecular mechanisms responsible for coupling KATP channel activity to exocytotic release await more refined tools (such as mice lacking KATP channels in specific tissue compartments). Moreover, the pathophysiological consequences of modulating neurotransmitter release by diazoxide in the context of ischemia and cardioprotection remain a fertile area for future investigation. Interestingly, depletion of presynaptic nerve terminals of their NE stores with reserpine (a monoamine transporter blocker) results in the failure of ischemic preconditioning protocols to be cardioprotective (Toombs, et al., 1993), suggesting that the NE release is critical to preconditioning-mediated protection. The ability of diazoxide to stimulate NE release from sympathetic nerve endings must therefore be considered when evaluating the cardioprotective actions of the compound.
Other diazoxide effectors
Other than those discussed above, diazoxide affects the functions of several other proteins and biological processes. For example, in the cardioprotective range, 100 µM diazoxide causes a significant increase in ATPase activity in purified cardiac membranes isolated from guinea pig hearts (Bienengraeber, et al., 2000). Stimulation of mitochondrial ATPase activity has also been reported (Dzeja, et al., 2003; Portenhauser, et al., 1971), which is due to stimulation of the F0F1 ATP synthase (Belisle & Kowaltowski, 2002). The mechanism appears to be that diazoxide stabilizes Mg-ADP bound in the catalytic site (possibly within the nucleotide binding fold) of the β subunit of the mitochondrial ATP synthase (Contessi, et al., 2004). A similar mechanism has been reported for activation of KATP channels by diazoxide (Bienengraeber, et al., 2000). Thus, the possibility should be considered that stabilization of Mg-ADP complexes at nucleotide binding folds might be a general action of diazoxide.
Diazoxide also affects other ion channels. For example, inhibition of voltage gated inward and outward currents has been reported in CA1 hippocampal neurons (Erdemli & Krnjevic, 1995). Direct effects of diazoxide on cardiovascular ion channels have not yet been reported. Interestingly, however, pharmacological preconditioning ith 100 µM diazoxide strongly reduces the cardiac L-type Ca2+ channel density and the amplitude of cytosolic Ca2+ transients (Gonzalez, et al., 2010). It remains to be determined to what extent cardiovascular ion channels (other than KATP channels) are affected by diazoxide-induced preconditioning. There are also reports that diazoxide may act to open the mitochondrial permeability transition pore (Katoh, et al., 2002). Non-channel mediated actions of diazoxide have also been reported. For example, 100 µM diazoxide was reported to induce translocation of PKC-ε from the cytosol to mitochondria in H9c2 cells. Moreover, diazoxide-induced flavoprotein oxidation was inhibited by PKC-ε inhibition and transfection with wild type PKC-ε mimicked the flavoprotein-oxidizing effect of diazoxide). These data led the authors to conclude that the primary effect of diazoxide is PKC-ε activation (Kim, et al., 2006), which has known cardioprotective properties (Steinberg, 2012). Diazoxide also attenuates swelling of isolated rabbit ventricular myocytes during metabolic inhibition (Al-Dadah, et al., 2007).
Conclusion
A drug does not have to be selective in order to be effective. It has been argued that an ideal drug may be one whose efficacy is not solely based on a single target, but rather on rebalancing several biological effectors/process that contribute to the etiology, pathogeneses, and progression of diseases (i.e. a promiscuous drug) (Mencher & Wang, 2005). Diazoxide appears to be such as a drug: while it is clear that diazoxide has potent cardioprotective properties, a review of the literature shows that diazoxide has multiple effectors that may synergistically contribute to its cardioprotective properties (Figure 2). Diazoxide is, however, neither specific nor selective. The specificity of diazoxide (defined here as the capacity of a drug to have a unique action) is questionable since it affects blood pressure, blood glucose levels and contributes to cardioprotection. Diazoxide also lacks selectivity (defined here as the ability of a drug to affect a particular molecular target in preference to others) since it affects several types of KATP channels and also have other off-target effects. Moreover, the diazoxide sensitivities of these various effectors are similar (and within the range of concentrations used for most studies when examining cardioprotection). Care should be taken when referring to diazoxide as having specificity or selectivity to a particular biological process or effector (e.g. an ion channel) and the reader is urged to consider the multiplicity of diazoxide’s effectors when evaluating the cardioprotection literature.
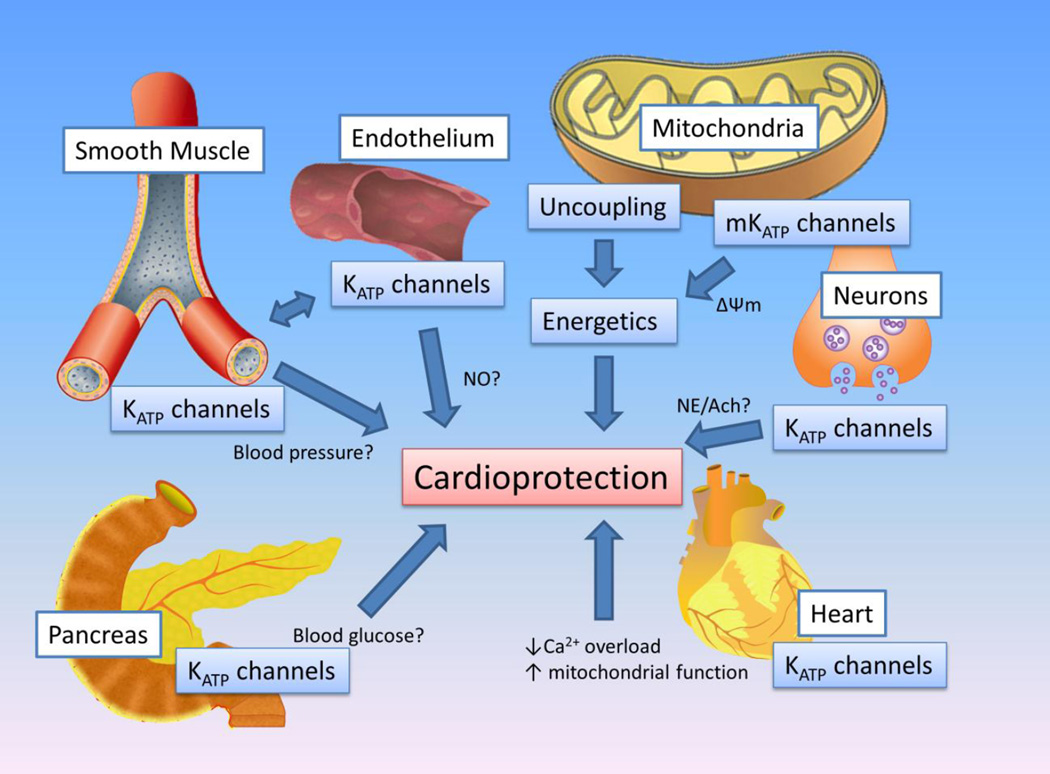
Figure 2.
A cartoon summary of the main potential effectors of diazoxide, depicting some of the potential mechanisms by which they may contribute to cardioprotection. NO is nitric oxide, NE is norepinephrine and Ach is acetylcholine. For details and concentrations at which diazoxide affects these targets, please refer to the text and the Tables.
Acknowledgments
Acknowledgments
The author is grateful to Dr Kathleen C Kinnally, NYU College of Dentistry, for reading the manuscript and for making useful suggestions.
Funding
This work was supported by National Institutes of Health grant HL093563.
List of abbreviations
- Δψm
mitochondrial membrane potential
- 5HD
5-hydroxydecanoate
- DNP
2,4-dinitrophenol
- I/R injury
Ischemia/reperfusion injury
- IPC
Ischemic preconditioning
- KATP channel
ATP-sensitive K+ channel
- ROS
Reactive oxygen species
- SDH
succinate dehydrogenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement
The author declares that there are no conflicts of interest
References
- Akai K, Wang Y, Sato K, Sekiguchi N, Sugimura A, Kumagai T, Komaru T, Kanatsuka H, Shirato K. Vasodilatory effect of nicorandil on coronary arterial microvessels: its dependency on vessel size and the involvement of the ATP-sensitive potassium channels. Journal of Cardiovascular Pharmacology. 1995;26:541–547. doi: 10.1097/00005344-199510000-00006. [DOI] [PubMed] [Google Scholar]
- Akao M, Ohler A, O’Rourke B, Marban E. Mitochondrial ATP-sensitive potassium channels inhibit apoptosis induced by oxidative stress in cardiac cells. Circulation Research. 2001;88:1267–1275. doi: 10.1161/hh1201.092094. [DOI] [PubMed] [Google Scholar]
- Al-Dadah AS, Voeller RK, Schuessler RB, Damiano RJ, Jr., Lawton JS. Maintenance of myocyte volume homeostasis during stress by diazoxide is cardioprotective. Annals of Thoracic Surgery. 2007;84:857–862. doi: 10.1016/j.athoracsur.2007.04.103. [DOI] [PubMed] [Google Scholar]
- Alcindor D, Krolikowski JG, Pagel PS, Warltier DC, Kersten JR. Cyclooxygenase-2 mediates ischemic, anesthetic, and pharmacologic preconditioning in vivo. Anesthesiology. 2004;100:547–554. doi: 10.1097/00000542-200403000-00013. [DOI] [PubMed] [Google Scholar]
- Antoine MH, Berkenboom G, Fang ZY, Fontaine J, Herchuelz A, Lebrun P. Mechanical and ionic response of rat aorta to diazoxide. European Journal of Pharmacology. 1992;216:299–306. doi: 10.1016/0014-2999(92)90374-d. [DOI] [PubMed] [Google Scholar]
- Antonenko YN, Smith D, Kinnally KW, Tedeschi H. Single-channel activity induced in mitoplasts by alkaline pH. Biochimica et Biophysica Acta. 1994;1194:247–254. doi: 10.1016/0005-2736(94)90306-9. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Reconstituted human cardiac KATP channels: functional identity with the native channels from the sarcolemma of human ventricular cells. Circulation Research. 1998;83:1132–1143. doi: 10.1161/01.res.83.11.1132. [DOI] [PubMed] [Google Scholar]
- Baines CP, Liu GS, Birincioglu M, Critz SD, Cohen MV, Downey JM. Ischemic preconditioning depends on interaction between mitochondrial KATP channels and actin cytoskeleton. American Journal of Physiology. 1999;276:H1361–H1368. doi: 10.1152/ajpheart.1999.276.4.H1361. [DOI] [PubMed] [Google Scholar]
- Bao L, Kefaloyianni E, Lader J, Hong M, Morley G, Fishman GI, Sobie EA, Coetzee WA. Unique properties of the ATP-sensitive K(+) channel in the mouse ventricular cardiac conduction system. Circulation. Arrhythmia and Electrophysiology. 2011;4:926–935. doi: 10.1161/CIRCEP.111.964643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A, van Bever L, Monnier D, Roatti A, Baertschi AJ. A novel K(ATP) current in cultured neonatal rat atrial appendage cardiomyocytes. Circulation Research. 1999;85:707–715. doi: 10.1161/01.res.85.8.707. [DOI] [PubMed] [Google Scholar]
- Bednarczyk P, Dolowy K, Szewczyk A. Matrix Mg2+ regulates mitochondrial ATP-dependent potassium channel from heart. FEBS Letters. 2005;579:1625–1632. doi: 10.1016/j.febslet.2005.01.077. [DOI] [PubMed] [Google Scholar]
- Belisle E, Kowaltowski AJ. Opening of mitochondrial K+ channels increases ischemic ATP levels by preventing hydrolysis. Journal of Bioenergetics and Biomembranes. 2002;34:285–298. doi: 10.1023/a:1020256502583. [DOI] [PubMed] [Google Scholar]
- Bienengraeber M, Alekseev AE, Abraham MR, Carrasco AJ, Moreau C, Vivaudou M, Dzeja PP, Terzic A. ATPase activity of the sulfonylurea receptor: a catalytic function for the KATP channel complex. FASEB Journal. 2000;14:1943–1952. doi: 10.1096/fj.00-0027com. [DOI] [PubMed] [Google Scholar]
- Birincioglu M, Yang XM, Critz SD, Cohen MV, Downey JM. S-T segment voltage during sequential coronary occlusions is an unreliable marker of preconditioning. American Journal of Physiology. 1999;277:H2435–H2441. doi: 10.1152/ajpheart.1999.277.6.H2435. [DOI] [PubMed] [Google Scholar]
- Broadhead MW, Kharbanda RK, Peters MJ, MacAllister RJ. KATP channel activation induces ischemic preconditioning of the endothelium in humans in vivo. Circulation. 2004;110:2077–2082. doi: 10.1161/01.CIR.0000144304.91010.F0. [DOI] [PubMed] [Google Scholar]
- Brustovetsky T, Shalbuyeva N, Brustovetsky N. Lack of manifestations of diazoxide/5-hydroxydecanoate-sensitive KATP channel in rat brain nonsynaptosomal mitochondria. Journal of Physiology. 2005;568:47–59. doi: 10.1113/jphysiol.2005.091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busija DW, Katakam P, Rajapakse NC, Kis B, Grover G, Domoki F, Bari F. Effects of ATP-sensitive potassium channel activators diazoxide and BMS-191095 on membrane potential and reactive oxygen species production in isolated piglet mitochondria. Brain Research Bulletin. 2005;66:85–90. doi: 10.1016/j.brainresbull.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Carroll R, Gant VA, Yellon DM. Mitochondrial K(ATP) channel opening protects a human atrial-derived cell line by a mechanism involving free radical generation. Cardiovascular Research. 2001;51:691–700. doi: 10.1016/s0008-6363(01)00330-3. [DOI] [PubMed] [Google Scholar]
- Choma K, Bednarczyk P, Koszela-Piotrowska I, Kulawiak B, Kudin A, Kunz WS, Dolowy K, Szewczyk A. Single channel studies of the ATP-regulated potassium channel in brain mitochondria. Journal of Bioenergetics and Biomembranes. 2009;41:323–334. doi: 10.1007/s10863-009-9233-7. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Annals of the New York Academy of Sciences. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Contessi S, Metelli G, Mavelli I, Lippe G. Diazoxide affects the IF1 inhibitor protein binding to F1 sector of beef heart F0F1ATPsynthase. Biochemical Pharmacology. 2004;67:1843–1851. doi: 10.1016/j.bcp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- D’Hahan N, Moreau C, Prost AL, Jacquet H, Alekseev AE, Terzic A, Vivaudou M. Pharmacological plasticity of cardiac ATP-sensitive potassium channels toward diazoxide revealed by ADP. Proc.Natl.Acad.Sci.U.S.A. 1999;96:12162–12167. doi: 10.1073/pnas.96.21.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlem YA, Horn TF, Buntinas L, Gonoi T, Wolf G, Siemen D. The human mitochondrial KATP channel is modulated by calcium and nitric oxide: a patch-clamp approach. Biochimica et Biophysica Acta. 2004;1656:46–56. doi: 10.1016/j.bbabio.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Demin OV, Kholodenko BN, Skulachev VP. A model of O2.-generation in the complex III of the electron transport chain. Molecular and Cellular Biochemistry. 1998;184:21–33. [PubMed] [Google Scholar]
- Deng Q, Scicli AG, Lawton C, Silverman NA. Coronary flow reserve after ischemia and reperfusion of the isolated heart. Divergent results with crystalloid versus blood perfusion. Journal of Thoracic and Cardiovascular Surgery. 1995;109:466–472. doi: 10.1016/S0022-5223(95)70277-6. [DOI] [PubMed] [Google Scholar]
- Dos Santos P, Kowaltowski AJ, Laclau MN, Seetharaman S, Paucek P, Boudina S, Thambo JB, Tariosse L, Garlid KD. Mechanisms by which opening the mitochondrial ATP-sensitive K(+) channel protects the ischemic heart. American Journal of Physiology. Heart and Circulatory Physiology. 2002;283:H284–H295. doi: 10.1152/ajpheart.00034.2002. [DOI] [PubMed] [Google Scholar]
- Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Failure Reviews. 2007;12:181–188. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- Drose S, Bleier L, Brandt U. A common mechanism links differently acting complex II inhibitors to cardioprotection: modulation of mitochondrial reactive oxygen species production. Molecular Pharmacology. 2011;79:814–822. doi: 10.1124/mol.110.070342. [DOI] [PubMed] [Google Scholar]
- Duda M, Czarnowska E, Kurzelewski M, Konior A, Beresewicz A. Ischemic preconditioning prevents endothelial dysfunction, P-selectin expression, and neutrophil adhesion by preventing endothelin and O2- generation in the post-ischemic guinea-pig heart. Journal of Physiology and Pharmacology. 2006;57:553–569. [PubMed] [Google Scholar]
- Duda M, Konior A, Klemenska E, Beresewicz A. Preconditioning protects endothelium by preventing ET-1-induced activation of NADPH oxidase and xanthine oxidase in post-ischemic heart. Journal of Molecular and Cellular Cardiology. 2007;42:400–410. doi: 10.1016/j.yjmcc.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Bast P, Ozcan C, Valverde A, Holmuhamedov EL, Van Wylen DG, Terzic A. Targeting nucleotide-requiring enzymes: implications for diazoxide-induced cardioprotection. American Journal of Physiology. Heart and Circulatory Physiology. 2003;284:H1048–H1056. doi: 10.1152/ajpheart.00847.2002. [DOI] [PubMed] [Google Scholar]
- Erdemli G, Krnjevic K. Actions of diazoxide on CA1 neurons in hippocampal slices from rats. Canadian Journal of Physiology and Pharmacology. 1995;73:608–618. doi: 10.1139/y95-077. [DOI] [PubMed] [Google Scholar]
- Escande D. The pharmacology of ATP-sensitive K+ channels in the heart. Pflugers Archives-European Journal of Physiology. 1989;414:S93–S98. doi: 10.1007/BF00582255. [DOI] [PubMed] [Google Scholar]
- Faivre JF, Findlay I. Effects of tolbutamide, glibenclamide and diazoxide upon action potentials recorded from rat ventricular muscle. Biochimica et Biophysica Acta. 1989;984:1–5. doi: 10.1016/0005-2736(89)90334-9. [DOI] [PubMed] [Google Scholar]
- Fedorov VV, Glukhov AV, Ambrosi CM, Kostecki G, Chang R, Janks D, Schuessler RB, Moazami N, Nichols CG, Efimov IR. Effects of K(ATP) channel openers diazoxide and pinacidil in coronary-perfused atria and ventricles from failing and non-failing human hearts. Journal of Molecular and Cellular Cardiology. 2011 doi: 10.1016/j.yjmcc.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feleder EC, Adler-Graschinsky E. Endothelium-mediated and N omega-nitro-L-arginine methyl ester-sensitive responses to cromakalim and diazoxide in the rat mesenteric bed. European Journal of Pharmacology. 1997;319:229–238. doi: 10.1016/s0014-2999(96)00843-6. [DOI] [PubMed] [Google Scholar]
- Feng J, Li H, Rosenkranz ER. Diazoxide protects the rabbit heart following cardioplegic ischemia. Molecular and Cellular Biochemistry. 2002;233:133–138. doi: 10.1023/a:1015554211010. [DOI] [PubMed] [Google Scholar]
- Flagg TP, Kurata HT, Masia R, Caputa G, Magnuson MA, Lefer DJ, Coetzee WA, Nichols CG. Differential structure of atrial and ventricular KATP: atrial KATP channels require SUR1. Circulation Research. 2008;103:1458–1465. doi: 10.1161/CIRCRESAHA.108.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DB, Ho AS, Rucker J, Garlid AO, Chen L, Sidor A, Garlid KD, O’Rourke B. Mitochondrial ROMK Channel Is a Molecular Component of MitoKATP. Circulation Research. 2012;111:446–454. doi: 10.1161/CIRCRESAHA.112.266445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer RM, Eells JT, Hsu AK, Henry MM, Gross GJ. Ischemic preconditioning in rats: role of mitochondrial K(ATP) channel in preservation of mitochondrial function. American Journal of Physiology. Heart and Circulatory Physiology. 2000;278:H305–H312. doi: 10.1152/ajpheart.2000.278.1.H305. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Paucek P, Pikula S, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial KATP channel as a receptor for potassium channel openers. J.Biol.Chem. 1993;271:8796–8799. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D’Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circulation Research. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Standen NB, Galinanes M. Evidence for mitochondrial K ATP channels as effectors of human myocardial preconditioning. Cardiovascular Research. 2000;45:934–940. doi: 10.1016/s0008-6363(99)00407-1. [DOI] [PubMed] [Google Scholar]
- Gogelein H, Hartung J, Englert HC, Scholkens BA. HMR 1883, a novel cardioselective inhibitor of the ATP-sensitive potassium channel. Part I: effects on cardiomyocytes, coronary flow and pancreatic beta-cells. Journal of Pharmacology and Experimental Therapeutics. 1998;286:1453–1464. [PubMed] [Google Scholar]
- Gonzalez G, Zaldivar D, Carrillo E, Hernandez A, Garcia M, Sanchez J. Pharmacological preconditioning by diazoxide downregulates cardiac L-type Ca2+ channels. British Journal of Pharmacology. 2010;161:1172–1185. doi: 10.1111/j.1476-5381.2010.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GJ, Pieper GM, Farber NE, Warltier DC, Hardman H. Effects of nicorandil on coronary circulation and myocardial ischemia. The American Journal of Cardiology. 1989;63:11J–17J. doi: 10.1016/0002-9149(89)90199-9. [DOI] [PubMed] [Google Scholar]
- Hanley PJ, Daut J. K(ATP) channels and preconditioning: A re-examination of the role of mitochondrial K(ATP) channels and an overview of alternative mechanisms. J.Mol.Cell Cardiol. 2005 May; doi: 10.1016/j.yjmcc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. K(ATP) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. Journal of Physiology. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin JC, Meissner HP. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochemical Pharmacology. 1982;31:1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Holmuhamedov EL, Oberlin A, Short K, Terzic A, Jahangir A. Cardiac subsarcolemmal and interfibrillar mitochondria display distinct responsiveness to protection by diazoxide. PloS ONE. 2012;7:e44667. doi: 10.1371/journal.pone.0044667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain K, Aynsley-Green A, Stanley CA. Medications used in the treatment of hypoglycemia due to congenital hyperinsulinism of infancy (HI) Pediatric Endocrinology Reviews. 2004;(2 Suppl 1):163–167. [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JPt, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor [see comments] Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- Ishizaka H, Kuo L. Endothelial ATP-sensitive potassium channels mediate coronary microvascular dilation to hyperosmolarity. American Journal of Physiology. 1997;273:H104–H112. doi: 10.1152/ajpheart.1997.273.1.H104. [DOI] [PubMed] [Google Scholar]
- Iwai T, Tanonaka K, Koshimizu M, Takeo S. Preservation of mitochondrial function by diazoxide during sustained ischaemia in the rat heart. British Journal of Pharmacology. 2000;129:1219–1227. doi: 10.1038/sj.bjp.0703148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH, Squires PE, Dunne MJ. Effects of 5-hydroxydecanoate on ATP-regulated potassium ion channels in insulin secreting cells. Biochemical Society Transactions. 1993;21:427S. doi: 10.1042/bst021427s. [DOI] [PubMed] [Google Scholar]
- Janigro D, West GA, Gordon EL, Winn HR. ATP-sensitive K+ channels in rat aorta and brain microvascular endothelial cells. Am. J. Physiol. 1993;265:C812–C821. doi: 10.1152/ajpcell.1993.265.3.C812. [DOI] [PubMed] [Google Scholar]
- Jansen-Olesen I, Mortensen CH, El-Bariaki N, Ploug KB. Characterization of K(ATP)-channels in rat basilar and middle cerebral arteries: studies of vasomotor responses and mRNA expression. European Journal of Pharmacology. 2005;523:109–118. doi: 10.1016/j.ejphar.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Jin C, Wu J, Watanabe M, Okada T, Iesaki T. Mitochondrial K+ channels are involved in ischemic postconditioning in rat hearts. Journal of Physiological Sciences. 2012;62:325–332. doi: 10.1007/s12576-012-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BF. Diazoxide and renal function in man. Clinical Pharmacology and Therapeutics. 1971;12:815–824. doi: 10.1002/cpt1971125815. [DOI] [PubMed] [Google Scholar]
- Kanada SA, Kanada DJ, Hutchinson RA, Wu D. Angina-like syndrome with diazoxide therapy for hypertensive crisis. Annals of Internal Medicine. 1976;84:696–699. doi: 10.7326/0003-4819-84-6-696. [DOI] [PubMed] [Google Scholar]
- Karschin A, Brockhaus J, Ballanyi K. KATP channel formation by the sulphonylurea receptors SUR1 with Kir6.2 subunits in rat dorsal vagal neurons in situ. J Physiol. 1998;509:339–346. doi: 10.1111/j.1469-7793.1998.339bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakam PV, Jordan JE, Snipes JA, Tulbert CD, Miller AW, Busija DW. Myocardial preconditioning against ischemia-reperfusion injury is abolished in Zucker obese rats with insulin resistance. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2007;292:R920–R926. doi: 10.1152/ajpregu.00520.2006. [DOI] [PubMed] [Google Scholar]
- Katoh H, Nishigaki N, Hayashi H. Diazoxide opens the mitochondrial permeability transition pore and alters Ca2+ transients in rat ventricular myocytes. Circulation. 2002;105:2666–2671. doi: 10.1161/01.cir.0000016831.41648.04. [DOI] [PubMed] [Google Scholar]
- Kilbinger H, Krause A, Mang CF, Englert H, Wirth K. Effects of K(ATP) channel modulators on acetylcholine release from guinea-pig isolated atria and small intestine. Naunyn Schmiedebergs Arch. Pharmacol. 2002;365:371–377. doi: 10.1007/s00210-002-0539-9. [DOI] [PubMed] [Google Scholar]
- Kim MY, Kim MJ, Yoon IS, Ahn JH, Lee SH, Baik EJ, Moon CH, Jung YS. Diazoxide acts more as a PKC-epsilon activator, and indirectly activates the mitochondrial K(ATP) channel conferring cardioprotection against hypoxic injury. British Journal of Pharmacology. 2006;149:1059–1070. doi: 10.1038/sj.bjp.0706922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopustinskiene DM, Toleikis A, Saris NE. Adenine nucleotide translocase mediates the K(ATP)-channel-openers-induced proton and potassium flux to the mitochondrial matrix. Journal of Bioenergetics and Biomembranes. 2003;35:141–148. doi: 10.1023/a:1023746103401. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K(+) channel of heart mitochondria. American Journal of Physiology. Heart and Circulatory Physiology. 2001;280:H649–H657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- Kuo L, Chancellor JD. Adenosine potentiates flow-induced dilation of coronary arterioles by activating KATP channels in endothelium. American Journal of Physiology. 1995;269:H541–H549. doi: 10.1152/ajpheart.1995.269.2.H541. [DOI] [PubMed] [Google Scholar]
- LaDisa JF, Jr., Krolikowski JG, Pagel PS, Warltier DC, Kersten JR. Cardioprotection by glucose-insulin-potassium: dependence on KATP channel opening and blood glucose concentration before ischemia. American Journal of Physiology. Heart and Circulatory Physiology. 2004;287:H601–H607. doi: 10.1152/ajpheart.00122.2004. [DOI] [PubMed] [Google Scholar]
- Langheinrich U, Schnitzler M, Daut J. Ca2+ transients induced by K+ channel openers in isolated coronary capillaries. Pflugers Archiv (European Journal of Physiology) 1998;435:435–438. doi: 10.1007/s004240050536. [DOI] [PubMed] [Google Scholar]
- Lawrence CL, Billups B, Rodrigo GC, Standen NB. The KATP channel opener diazoxide protects cardiac myocytes during metabolic inhibition without causing mitochondrial depolarization or flavoprotein oxidation. British Journal of Pharmacology. 2001;134:535–542. doi: 10.1038/sj.bjp.0704289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rapedius M, Baukrowitz T, Liu GX, Srivastava DK, Daut J, Hanley PJ. 5-Hydroxydecanoate and coenzyme A are inhibitors of native sarcolemmal KATP channels in inside-out patches. Biochimica et Biophysica Acta. 2010;1800:385–391. doi: 10.1016/j.bbagen.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Liu B, Zhu X, Chen CL, Hu K, Swartz HM, Chen YR, He G. Opening of the mitoKATP channel and decoupling of mitochondrial complex II and III contribute to the suppression of myocardial reperfusion hyperoxygenation. Molecular and Cellular Biochemistry. 2010;337:25–38. doi: 10.1007/s11010-009-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. Journal of Cerebral Blood Flow and Metabolism. 2002;22:431–443. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Liu Q, Flavahan NA. Hypoxic dilatation of porcine small coronary arteries: role of endothelium and KATP-channels. British Journal of Pharmacology. 1997;120:728–734. doi: 10.1038/sj.bjp.0700939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ren G, O’Rourke B, Marban E, Seharaseyon J. Pharmacological comparison of native mitochondrial K(ATP) channels with molecularly defined surface K(ATP) channels. Molecular Pharmacology. 2001;59:225–230. [PubMed] [Google Scholar]
- Liu Y, Sato T, O’Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- Loubatieres A, Alric R, Mariani MM, de Malbosc H, Ribes G. [Diazoxide experimentally exercises an inhibitory action on the basal secretion of insulin] Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. 1966;160:168–171. [PubMed] [Google Scholar]
- Luckhoff A, Busse R. Activators of potassium channels enhance calcium influx into endothelial cells as a consequence of potassium currents. Naunyn Schmiedebergs Arch. Pharmacol. 1990;342:94–99. doi: 10.1007/BF00178979. [DOI] [PubMed] [Google Scholar]
- Mahmoudian M, Mirkhani H. Effect of diazoxide, an ATP-dependent potassium-channel opener, on isolated aortae of normal and diabetic rats. General Pharmacology. 1998;31:569–571. doi: 10.1016/s0306-3623(98)00030-5. [DOI] [PubMed] [Google Scholar]
- Malester B, Tong X, Ghiu IA, Kontogeorgis A, Gutstein DE, Xu J, Hendricks-Munoz K, Coetzee WA. Transgenic Expression of A Dominant Negative KATP Channel Subunit in the Mouse Endothelium: Effects on Coronary Flow and Endothelin-1 Secretion. FASEB Journal. 2007;21:2162–2172. doi: 10.1096/fj.06-7821com. [DOI] [PubMed] [Google Scholar]
- Masuda M, Chang-Chun C, Cho BC, Flameng W. Coronary reserve and contractile reserve in crystalloid- and blood-perfused rabbit hearts. Heart and Vessels. 1994;9:175–182. doi: 10.1007/BF01746061. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Matsushita K, Katayama Y, Fujita A, Inageda K, Tanemoto M, Inanobe A, Yamashita S, Matsuzawa Y, Kurachi Y. C-terminal tails of sulfonylurea receptors control ADP-induced activation and diazoxide modulation of ATP-sensitive K(+) channels. Circulation Research. 2000;87:873–880. doi: 10.1161/01.res.87.10.873. [DOI] [PubMed] [Google Scholar]
- Mencher SK, Wang LG. Promiscuous drugs compared to selective drugs (promiscuity can be a virtue) BMC Clinical Pharmacology. 2005;5:3. doi: 10.1186/1472-6904-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minners J, van den Bos EJ, Yellon DM, Schwalb H, Opie LH, Sack MN. Dinitrophenol, cyclosporin A, and trimetazidine modulate preconditioning in the isolated rat heart: support for a mitochondrial role in cardioprotection. Cardiovascular Research. 2000;47:68–73. doi: 10.1016/s0008-6363(00)00069-9. [DOI] [PubMed] [Google Scholar]
- Miura T, Liu Y, Kita H, Ogawa T, Shimamoto K. Roles of mitochondrial ATP-sensitive K channels and PKC in anti-infarct tolerance afforded by adenosine A1 receptor activation. Journal of the American College of Cardiology. 2000;35:238–245. doi: 10.1016/s0735-1097(99)00493-3. [DOI] [PubMed] [Google Scholar]
- Mohan RM, Paterson DJ. Activation of sulphonylurea-sensitive channels and the NO-cGMP pathway decreases the heart rate response to sympathetic nerve stimulation. Cardiovasc Res. 2000;47:81–89. doi: 10.1016/s0008-6363(00)00057-2. [DOI] [PubMed] [Google Scholar]
- Moritani K, Miyazaki T, Miyoshi S, Asanagi M, Zhao LS, Mitamura H, Ogawa S. Blockade of ATP-sensitive potassium channels by 5-hydroxydecanoate suppresses monophasic action potential shortening during regional myocardial ischemia. Cardiovascular Drugs and Therapy. 1994;8:749–756. doi: 10.1007/BF00877122. [DOI] [PubMed] [Google Scholar]
- Murai T, Muraki K, Imaizumi Y, Watanabe M. Levcromakalim causes indirect endothelial hyperpolarization via a myo-endothelial pathway. British Journal of Pharmacology. 1999;128:1491–1496. doi: 10.1038/sj.bjp.0702956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M, Akao M, O’Rourke B, Marban E. Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca(2+) overload during simulated ischemia and reperfusion: possible mechanism of cardioprotection. Circulation Research. 2001;89:891–898. doi: 10.1161/hh2201.100205. [DOI] [PubMed] [Google Scholar]
- Mykytenko J, Reeves JG, Kin H, Wang NP, Zatta AJ, Jiang R, Guyton RA, Vinten-Johansen J, Zhao ZQ. Persistent beneficial effect of postconditioning against infarct size: role of mitochondrial K(ATP) channels during reperfusion. Basic Research in Cardiology. 2008;103:472–484. doi: 10.1007/s00395-008-0731-2. [DOI] [PubMed] [Google Scholar]
- Nakae Y, Kwok WM, Bosnjak ZJ, Jiang MT. Isoflurane activates rat mitochondrial ATP-sensitive K+ channels reconstituted in lipid bilayers. American Journal of Physiology. Heart and Circulatory Physiology. 2003;284:H1865–H1871. doi: 10.1152/ajpheart.01031.2002. [DOI] [PubMed] [Google Scholar]
- Nakai T, Ichihara K. Effects of diazoxide on norepinephrine-induced vasocontraction and ischemic myocardium in rats. Biological and Pharmaceutical Bulletin. 1994;17:1341–1344. doi: 10.1248/bpb.17.1341. [DOI] [PubMed] [Google Scholar]
- Newgreen DT, Bray KM, McHarg AD, Weston AH, Duty S, Brown BS, Kay PB, Edwards G, Longmore J, Southerton JS. The action of diazoxide and minoxidil sulphate on rat blood vessels: a comparison with cromakalim. British Journal of Pharmacology. 1990;100:605–613. doi: 10.1111/j.1476-5381.1990.tb15854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Notsu T, Ohhashi K, Tanaka I, Ishikawa H, Niho T, Fukutake K, Mizota M. 5-Hydroxydecanoate Inhibits ATP-Sensitive K+ Channel Currents in Guinea-Pig Single Ventricular Myocytes. European Journal of Pharmacology. 1992;220:35–41. doi: 10.1016/0014-2999(92)90008-r. [DOI] [PubMed] [Google Scholar]
- Notsu T, Tanaka I, Takano M, Noma A. Blockade of the ATP-sensitive K+ channel by 5-hydroxydecanoate in guinea pig ventricular myocytes. The Journal of Pharmacology and Experimental Therapeutics. 1992;260:702–708. [PubMed] [Google Scholar]
- O’Brien KP, Grigor RR, Taylor PM. Intravenous diazoxide in treatment of hypertension associated with recent myocardial infarction. British Medical Journal. 1975;4:74–77. doi: 10.1136/bmj.4.5988.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockaili R, Emani VR, Okubo S, Brown M, Krottapalli K, Kukreja RC. Opening of mitochondrial KATP channel induces early and delayed cardioprotective effect: role of nitric oxide. American Journal of Physiology. 1999;277:H2425–H2434. doi: 10.1152/ajpheart.1999.277.6.H2425. [DOI] [PubMed] [Google Scholar]
- Oe K, Sperlagh B, Santha E, Matko I, Nagashima H, Foldes FF, Vizi ES. Modulation of norepinephrine release by ATP-dependent K(+)-channel activators and inhibitors in guinea-pig and human isolated right atrium. Cardiovasc Res. 1999;43:125–134. doi: 10.1016/s0008-6363(99)00052-8. [DOI] [PubMed] [Google Scholar]
- Okun R, Wilson WR, Gelfand MD. The Hyperglycemic Effect of Hypotensive Drugs. Journal of Chronic Diseases. 1964;17:31–39. doi: 10.1016/0021-9681(64)90037-2. [DOI] [PubMed] [Google Scholar]
- Opie LH. Metabolism of free fatty acids, glucose and catecholamines in acute myocardial infarction. Relation to myocardial ischemia and infarct size. American Journal of Cardiology. 1975;36:938–953. doi: 10.1016/0002-9149(75)90086-7. [DOI] [PubMed] [Google Scholar]
- Ozcan C, Holmuhamedov EL, Jahangir A, Terzic A. Diazoxide protects mitochondria from anoxic injury: implications for myopreservation. Journal of Thoracic and Cardiovascular Surgery. 2001;121:298–306. doi: 10.1067/mtc.2001.111421. [DOI] [PubMed] [Google Scholar]
- Pagliaro P, Chiribiri A, Mancardi D, Rastaldo R, Gattullo D, Losano G. Coronary endothelial dysfunction after ischemia and reperfusion and its prevention by ischemic preconditioning. Ital.Heart J. 2003;4:383–394. [PubMed] [Google Scholar]
- Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, Cohen MV, Downey JM. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circulation Research. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- Pasdois P, Beauvoit B, Tariosse L, Vinassa B, Bonoron-Adele S, Dos Santos P. Effect of diazoxide on flavoprotein oxidation and reactive oxygen species generation during ischemia-reperfusion: a study on Langendorff-perfused rat hearts using optic fibers. American Journal of Physiology. Heart and Circulatory Physiology. 2008;294:H2088–H2097. doi: 10.1152/ajpheart.01345.2007. [DOI] [PubMed] [Google Scholar]
- Paucek P, Mironova G, Mahdi F, Beavis AD, Woldegiorgis G, Garlid KD. Reconstitution and partial purification of the glibenclamide- sensitive, ATP-dependent K+ channel from rat liver and beef heart mitochondria. J.Biol.Chem. 1992;267:26062–26069. [PubMed] [Google Scholar]
- Portenhauser R, Schafer G, Trolp R. Inhibition of mitochondrial metabolism by the diabetogenic thiadiazine diazoxide. II. Interaction with energy conservation and ion transport. Biochemical Pharmacology. 1971;20:2623–2632. doi: 10.1016/0006-2952(71)90171-7. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Bonev AD, Brayden JE, Nelson MT. Pharmacology of ATP-sensitive K+ currents in smooth muscle cells from rabbit mesenteric artery. Am.J Physiol. 1995;269:C1112–C1118. doi: 10.1152/ajpcell.1995.269.5.C1112. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiological Reviews. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Ramani K, Lust WD, Whittingham TS, Lesnefsky EJ. ATP catabolism and adenosine generation during ischemia in the aging heart. Mechanisms of Ageing and Development. 1996;89:113–124. doi: 10.1016/0047-6374(96)01732-0. [DOI] [PubMed] [Google Scholar]
- Riess ML, Camara AK, Heinen A, Eells JT, Henry MM, Stowe DF. KATP channel openers have opposite effects on mitochondrial respiration under different energetic conditions. Journal of Cardiovascular Pharmacology. 2008;51:483–491. doi: 10.1097/FJC.0b013e31816bf4a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo GC, Davies NW, Standen NB. Diazoxide causes early activation of cardiac sarcolemmal KATP channels during metabolic inhibition by an indirect mechanism. Cardiovascular Research. 2004;61:570–579. doi: 10.1016/j.cardiores.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Rodrigo GC, Lawrence CL, Standen NB. Dinitrophenol pretreatment of rat ventricular myocytes protects against damage by metabolic inhibition and reperfusion. Journal of Molecular and Cellular Cardiology. 2002;34:555–569. doi: 10.1006/jmcc.2002.1536. [DOI] [PubMed] [Google Scholar]
- Rubin AA, Roth FE, Taylor RM, Rosenkilde H. Pharmacology of diazoxide, an antihypertensive, nondiuretic benzothiadiazine. Journal of Pharmacology and Experimental Therapeutics. 1962;136:344–352. [PubMed] [Google Scholar]
- Rubin AA, Taylor RM, Roth FE. A brief review of the development of diazoxide as an antihypertensive agent. Annals of the New York Academy of Sciences. 1968;150:457–460. doi: 10.1111/j.1749-6632.1968.tb19070.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Yamazaki J, Nagao T. 5-hydroxydecanoate selectively reduces the initial increase in extracellular K+ in ischemic guinea-pig heart. European Journal of Pharmacology. 1998;348:31–35. doi: 10.1016/s0014-2999(98)00238-6. [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Liang CS, Stone CK, Hood WB. Effects of pinacidil on myocardial blood flow and infarct size after acute left anterior decending coronary artery occlusion and reperfusion in awake dogs with and without a coexisting left circumflex coronary artery stenosis. Journal of Cardiovascular Pharmacology. 1989;14:747–755. doi: 10.1097/00005344-198911000-00011. [DOI] [PubMed] [Google Scholar]
- Sanada S, Kitakaze M, Asanuma H, Harada K, Ogita H, Node K, Takashima S, Sakata Y, Asakura M, Shinozaki Y, Mori H, Kuzuya T, Hori M. Role of mitochondrial and sarcolemmal K(ATP) channels in ischemic preconditioning of the canine heart. American Journal of Physiology. Heart and Circulatory Physiology. 2001;280:H256–H263. doi: 10.1152/ajpheart.2001.280.1.H256. [DOI] [PubMed] [Google Scholar]
- Sato K, Kanatsuka H, Sekiguchi N, Akai K, Wang Y, Sugimura A, Kumagai T, Komaru T, Shirato K. Effect of an ATP sensitive potassium channel opener, levcromakalim, on coronary arterial microvessels in the beating canine heart. Cardiovasc.Res. 1994;28:1780–1786. doi: 10.1093/cvr/28.12.1780. [DOI] [PubMed] [Google Scholar]
- Schafer G, Portenhauser R, Trolp R. Inhibition of mitochondrial metabolism by the diabetogenic thiadiazine diazoxide. I. Action on succinate dehydrogenase and TCA-cycle oxidations. Biochemical Pharmacology. 1971;20:1271–1280. doi: 10.1016/0006-2952(71)90358-3. [DOI] [PubMed] [Google Scholar]
- Schafer G, Wegener C, Portenhauser R, Bojanovski D. Diazoxide, an inhibitor of succinate oxidation. Biochemical Pharmacology. 1969;18:2678–2681. [PubMed] [Google Scholar]
- Schnitzler MM, Derst C, Daut J, Preisig-Muller R. ATP-sensitive potassium channels in capillaries isolated from guinea-pig heart. Journal of Physiology. 2000;525:307–317. doi: 10.1111/j.1469-7793.2000.t01-1-00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz LM, Welch TS, Crago MS. Cardioprotection by multiple preconditioning cycles does not require mitochondrial K(ATP) channels in pigs. American Journal of Physiology. Heart and Circulatory Physiology. 2002;283:H1538–H1544. doi: 10.1152/ajpheart.00040.2002. [DOI] [PubMed] [Google Scholar]
- Schworer H, Kilbinger H. Effects of cromakalim on acetylcholine release and smooth muscle contraction in guinea-pig small intestine. Naunyn Schmiedebergs Arch.Pharmacol. 1989;339:706–708. doi: 10.1007/BF00168666. [DOI] [PubMed] [Google Scholar]
- Scott JC, Cowley AW., Jr. The effect of diazoxide on coronary blood flow. American Journal of Cardiology. 1969;24:865–869. doi: 10.1016/0002-9149(69)90477-9. [DOI] [PubMed] [Google Scholar]
- Spath JA, Ogletree ML, Lefer AM. Lack of a significant protective effect of augmented circulating glucose on the ischemic myocardium.BJ. Canadian Journal of Physiology and Pharmacology. 1976;54:423–429. doi: 10.1139/y76-060. [DOI] [PubMed] [Google Scholar]
- Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Steinberg SF. Cardiac actions of protein kinase C isoforms. Physiology (Bethesda) 2012;27:130–139. doi: 10.1152/physiol.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stottrup NB, Lofgren B, Birkler RD, Nielsen JM, Wang L, Caldarone CA, Kristiansen SB, Contractor H, Johannsen M, Botker HE, Nielsen TT. Inhibition of the malate-aspartate shuttle by pre-ischaemic aminooxyacetate loading of the heart induces cardioprotection. Cardiovascular Research. 2010;88:257–266. doi: 10.1093/cvr/cvq205. [DOI] [PubMed] [Google Scholar]
- Suleiman MS, Halestrap AP, Griffiths EJ. Mitochondria: a target for myocardial protection. Pharmacology and Therapeutics. 2001;89:29–46. doi: 10.1016/s0163-7258(00)00102-9. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Ogura T, Seino S, Marban E, Nakaya H. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circulation Research. 2001;88:570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Saito T, Sato T, Tamagawa M, Miki T, Seino S, Nakaya H. Cardioprotective effect of diazoxide is mediated by activation of sarcolemmal but not mitochondrial ATP-sensitive potassium channels in mice. Circulation. 2003;107:682–685. doi: 10.1161/01.cir.0000055187.67365.81. [DOI] [PubMed] [Google Scholar]
- Szewczyk A, Kajma A, Malinska D, Wrzosek A, Bednarczyk P, Zablocka B, Dolowy K. Pharmacology of mitochondrial potassium channels: dark side of the field. FEBS Letters. 2010;584:2063–2069. doi: 10.1016/j.febslet.2010.02.048. [DOI] [PubMed] [Google Scholar]
- Tanno M, Miura T, Tsuchida A, Miki T, Nishino Y, Ohnuma Y, Shimamoto K. Contribution of both the sarcolemmal K(ATP) and mitochondrial K(ATP) channels to infarct size limitation by K(ATP) channel openers: differences from preconditioning in the role of sarcolemmal K(ATP) channels. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2001;364:226–232. doi: 10.1007/s002100100448. [DOI] [PubMed] [Google Scholar]
- Terzic A, Dzeja PP, Holmuhamedov EL. Mitochondrial K(ATP) channels: probing molecular identity and pharmacology. Journal of Molecular and Cellular Cardiology. 2000;32:1911–1915. doi: 10.1006/jmcc.2000.1256. [DOI] [PubMed] [Google Scholar]
- Toombs CF, Wiltse AL, Shebuski RJ. Ischemic preconditioning fails to limit infarct size in reserpinized rabbit myocardium. Implication of norepinephrine release in the preconditioning effect. Circulation. 1993;88:2351–2358. doi: 10.1161/01.cir.88.5.2351. [DOI] [PubMed] [Google Scholar]
- Trube G, Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Archives-European Journal of Physiology. 1984;401:178–184. doi: 10.1007/BF00583879. [DOI] [PubMed] [Google Scholar]
- Trube G, Rorsman P, Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Archives-European Journal of Physiology. 1986;407:493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- von Beckerath N, Dittrich M, Klieber HG, Daut J. Inwardly rectifying K+ channels in freshly dissociated coronary endothelial cells from guinea-pig heart. J.Physiol. 1996;491:357–365. doi: 10.1113/jphysiol.1996.sp021221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Long C, Duan Z, Shi C, Jia G, Zhang Y. A new ATP-sensitive potassium channel opener protects endothelial function in cultured aortic endothelial cells. Cardiovascular Research. 2007;73:497–503. doi: 10.1016/j.cardiores.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Wang S, Cone J, Liu Y. Dual roles of mitochondrial K(ATP) channels in diazoxide-mediated protection in isolated rabbit hearts. American Journal of Physiology. Heart and Circulatory Physiology. 2001;280:H246–H255. doi: 10.1152/ajpheart.2001.280.1.H246. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirai K, Ashraf M. Activation of mitochondrial ATP-sensitive K(+) channel for cardiac protection against ischemic injury is dependent on protein kinase C activity. Circulation Research. 1999;85:731–741. doi: 10.1161/01.res.85.8.731. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kudo M, Xu M, Ayub A, Ashraf M. Mitochondrial K(ATP) channel as an end effector of cardioprotection during late preconditioning: triggering role of nitric oxide. Journal of Molecular and Cellular Cardiology. 2001;33:2037–2046. doi: 10.1006/jmcc.2001.1468. [DOI] [PubMed] [Google Scholar]
- White R, Hiley CR. Hyperpolarisation of rat mesenteric endothelial cells by ATP-sensitive K(+) channel openers. European Journal of Pharmacology. 2000;397:279–290. doi: 10.1016/s0014-2999(00)00271-5. [DOI] [PubMed] [Google Scholar]
- Wojtovich AP, Urciuoli WR, Chatterjee S, Fisher AB, Nehrke K, Brookes PS. KIR 6.2 is not the mitochondrial KATP channel, but is required for cardioprotection by ischemic preconditioning. American Journal of Physiology. Heart and Circulatory Physiology. 2013 doi: 10.1152/ajpheart.00972.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff F. Diazoxide Hyperglycaemia and Its Continued Relief by Tolbutamide. Lancet. 1964;1:309–310. doi: 10.1016/s0140-6736(64)92414-6. [DOI] [PubMed] [Google Scholar]
- Wolff FW, Langdon RG, Ruebner BH, Hollander C, Skoglund RD. A New Form of Experimental Diabetes. Diabetes. 1963;12:335–338. doi: 10.2337/diab.12.4.335. [DOI] [PubMed] [Google Scholar]
- Wu SN, Wu AZ, Sung RJ. Identification of two types of ATP-sensitive K+ channels in rat ventricular myocytes. Life Sciences. 2007;80:378–387. doi: 10.1016/j.lfs.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Xu M, Wang Y, Ayub A, Ashraf M. Mitochondrial K(ATP) channel activation reduces anoxic injury by restoring mitochondrial membrane potential. American Journal of Physiology. Heart and Circulatory Physiology. 2001;281:H1295–H1303. doi: 10.1152/ajpheart.2001.281.3.H1295. [DOI] [PubMed] [Google Scholar]
- Ye B, Kroboth SL, Pu JL, Sims JJ, Aggarwal NT, McNally EM, Makielski JC, Shi NQ. Molecular identification and functional characterization of a mitochondrial sulfonylurea receptor 2 splice variant generated by intraexonic splicing. Circulation Research. 2009;105:1083–1093. doi: 10.1161/CIRCRESAHA.109.195040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Feig JE, Morrissey A, Ghiu IA, Artman M, Coetzee WA. KATP channels of primary human coronary artery endothelial cells consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. Journal of Molecular and Cellular Cardiology. 2004;37:857–869. doi: 10.1016/j.yjmcc.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Zhang DX, Chen YF, Campbell WB, Zou AP, Gross GJ, Li PL. Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circulation Research. 2001;89:1177–1183. doi: 10.1161/hh2401.101752. [DOI] [PubMed] [Google Scholar]
- Zhang H, Flagg TP, Nichols CG. Cardiac sarcolemmal K(ATP) channels: Latest twists in a questing tale! Journal of Molecular and Cellular Cardiology. 2009 doi: 10.1016/j.yjmcc.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HX, Akrouh A, Kurata HT, Remedi MS, Lawton JS, Nichols CG. HMR 1098 is not an SUR isotype specific inhibitor of heterologous or sarcolemmal K ATP channels. Journal of Molecular and Cellular Cardiology. 2011;50:552–560. doi: 10.1016/j.yjmcc.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zini S, Ben-Ari Y, Ashford ML. Characterization of sulfonylurea receptors and the action of potassium channel openers on cholinergic neurotransmission in guinea pig isolated small intestine. J Pharmacol.Exp.Ther. 1991;259:566–573. [PubMed] [Google Scholar]
- Zunkler BJ, Lenzen S, Manner K, Panten U, Trube G. Concentration-dependent effects of tolbutamide, meglitinide, glipizide, glibenclamide and diazoxide on ATP-regulated K+ currents in pancreatic B-cells. Naunyn Schmiedebergs Arch. Pharmacol. 1988;337:225–230. doi: 10.1007/BF00169252. [DOI] [PubMed] [Google Scholar]