Abstract
Fibrosis is increasingly appreciated as a major player in adipose tissue dysfunction. In rapidly expanding adipose tissue, pervasive hypoxia leads to an induction of HIF1α that in turn leads to a potent pro-fibrotic transcriptional program. The pathophysiological impact of adipose tissue fibrosis is likely to play an equally important role on systemic metabolic alterations as fibrotic conditions play in the liver, heart and kidney. Here, we discuss recent advances in our understanding of the genesis, modulation and systemic impact of excessive extracellular matrix (ECM) accumulation in adipose tissue of both rodents and humans and the ensuing impact on metabolic dysfunction.
Introduction
Obesity is a primary cause of type-2 diabetes mellitus (T2DM), characterized by diminished or inappropriate insulin secretion, insulin insensitivity and additional metabolic disturbances such as dyslipidemia and impaired liver functions. A key step during the progression from the lean to the obese state is the rapid expansion of adipose tissue. Adipocytes can rapidly reach the diffusional limit of oxygen due to the inability of the neo-vasculature to keep pace with rapid tissue expansion. Hypoxia is therefore an early determinant for adipose tissue dysfunction (Sun et al., 2011). Hypoxic adipose tissue (AT) has unique alterations that are likely contributors towards the link of obesity with its comorbidities. Fibrosis is a hallmark of metabolically dysfunctional AT. Adipocytes are surrounded by a network of extracellular matrix proteins that serve as mechanical support and that respond to different signaling events (Khan et al., 2009). Maintaining a high degree of flexibility of the ECM allows adipose tissue to expand in a healthy manner, without adverse metabolic consequences. Over the course of the developments of obesity, increased interstitial fibrosis in white AT (WAT) may decrease ECM flexibility and reduce the tissue plasticity, which ultimately leads to adipocyte dysfunction. Of note, abnormal collagen deposition, a hallmark of fibrosis development in adipose tissue, is tightly associated with tissue inflammation characterized by infiltration of macrophages and many other immune cells (Sun et al., 2011). The pathological impact of fibrosis and inflammation on obesity and obesity-related metabolic disorders has been extensively investigated in the past several years. Despite many unresolved questions, studies from our laboratories and others with different models have elucidated some molecular mechanisms underlying the dysregulation of adipose tissue ECM remodeling and its impact on metabolic dysfunction, both in rodents and in the clinical setting. The accumulation of fibrosis is the culmination of several pathological processes and can affect different organs, such as the liver, heart, and kidney (Wynn, 2007). Fibrosis can also be the result of local inflammation. Fibrosis is usually defined as an excessive accumulation of ECM components, which can result from an imbalance between excess synthesis of fibrillar components, such as collagens I, III and VI, and an impairment in degradation of these proteins. The generation of ECM components is part of a regenerative step, fundamental to the replacement of dead or injured cells during the repair process, in response to inflammation. However, if the damage persists, pivotal cellular actors, such as myofibroblasts remain activated and fibrillar components initially produced to replace the normal parenchymal tissue can persist and accumulate in the tissue, giving rise to the characteristic fibrotic appearance. Thus, while tissue remodeling and ECM synthesis are initially a physiological response and beneficial for the tissue, excessive accumulation of fibrosis in the absence of resolution of inflammation can be highly deleterious for organ tissue homeostasis and function.
Components of adipose ECM
Since the function of the ECM depends tightly on its molecular assembly, it is important to know which components are involved in the formation of adipose ECM. Studies indicate that fibronectin and collagens are the most abundant proteins of interstitial fibers and pericellular basement membranes in AT. Among these components, Type I collagens provide the major ECM framework necessary to sustain the structure and function of mesenchymal tissues. While this type of collagen has been considered to be resistant to proteolysis, several matrix metalloproteinases (MMPs) have been reported to digest it under certain circumstances during development or chronic disease progression (Stamenkovic, 2003). Another major ECM protein in adipose tissue is collagen VI, whose subunits are highly regulated in adipocytes, both at the gene expression level and at the post-translational level. Due to its pivotal contributions to ECM stability, collagen VI has been extensively studied by our laboratory with different mouse models (Khan et al., 2009; Park and Scherer, 2012). Mature collagen VI is composed of three subunits: α1, α2, and α3. All these subunits are required for stable formation of the protein. Upon challenging mice that lack collagen VI with either a high fat diet or with an ob/ob mutation, we found that these mice have impaired ECM stability and hence reduced adipose tissue fibrosis. Also, the local and systemic metabolic parameters are improved, despite an increase in adipocyte size, both in the context of the high fat diet challenge or in the background of the ob/ob mutation. This suggests that AT fibrosis caused by an excess of collagen VI is an important determinant of metabolic dysregulation. Reduced rates of adipocyte necrosis and a concomitant reduction of macrophage infiltration were also observed in the absence of collagen VI. These improved metabolic phenotypes can be rationalized with a model in which the destabilized ECM leads to reduced mechanical stress on the rapidly expanding adipocytes. Importantly, we recently reported that the carboxyl-terminal C5 domain of the α3 chain is proteolytically cleaved from the collagen VI (Col6) microfibrils in obese fat tissue (Park and Scherer, 2012). While the details of this cleavage event and the participating proteolytic enzymes still remain to be identified, the cleaved C5 domain that we refer to as endotrophin, effectively augment s fibrosis, angiogenesis and inflammation through activation of the TGFβ pathway and recruitment of macrophages and endothelial cells in tumor tissue. As a result, endotrophin is not only a potent contributor to adipose tissue dysfunction in the context of high fat diets, but also contributes to more effective growth of breast cancer cells that infiltrate the fatty stroma of the mammary gland. In addition, it is intimately involved in causing enhanced resistance to chemotherapeutic agents (Park, 2013).
The dynamics of fibrosis are tightly regulated by MMPs, a family of neutral endopeptidases that cleave collagenous proteins, thereby enabling remodeling of ECM. Among 28 members in the MMP superfamily, the membrane type 1 MMP (MT1-MMP, also known as MMP14) plays an important role in ECM remodeling of AT (Chun et al., 2006). MT1-MMP is required for the modulation of tight pericellular collagens to allow preadipocytes to grow out of the stroma. The congenital absence of MT1-MMP impairs WAT development, thereby causing a lipodystrophic phenotype in the adult. Several other MMPs, including MMP-3, -9, -11, -12, -13, -16 and -24 are also highly expressed in AT and their levels are modulated under different physiological conditions. While there are various models of genetic null mutations of MMPs in the mouse leading to obesity or insulin resistance, their specific functions in obese AT remain to be further elucidated.
Underlying mechanisms leading to excess ECM accumulation
The role of HIF1α
What is the source of this prevailing ECM stress? Evidence from our and other laboratories suggests that hypoxia is a major initiating factor for ECM induction. We have observed in rodent models that within just a few days of high fat diet feeding, a doubling of the fat cell area can be observed in some fat pads, rapidly creating a local hypoxic state (Halberg et al., 2009a). As a result, HIF1α is induced in AT. Unlike its action in tumor tissues, HIF1α fails to solicit an effective pro-angiogenic response in adipose tissue. This is the case even if a transgenic cassette is supplied that drives the expression of a dominant-active HIF1α in adipose tissue. Despite these conditions, HIF1α is still unable to induce an angiogenic program in adipocytes. Instead, the presence of HIF1α induces an alternative transcriptional program, mainly entailing enhanced synthesis of ECM components, leading eventually to the development of fibrosis. HIF1α may also induce a change in cellular redox status, which in turn affects enzymes involved in collagen cross-linking and stabilization, such as lysyl oxidase (LOX) and prolyl-4-hydroxylase (Mariman and Wang, 2010).
Recently, we further demonstrated that this fibrotic response in obese fat pads can effectively be prevented by either the use of a chemical HIF1α inhibitor (PX-478), or by overexpression of a dominant-negative HIF1α mutant (Sun et al., 2013). Inhibition of HIF1α is associated with metabolic improvements, even under a high fat diet challenge. These data further highlight the critical role of HIF1α in increasing the fibrotic response, an integral aspect of obesity-associated adipose tissue dysfunction.
HIF1 proteins regulate the response to oxygen availability at the transcriptional level in human adipose tissue as well (Karpe et al., 2002). As such, the gene expression of HIF1α is up-regulated in subcutaneous AT of obese subjects (Cancello et al., 2005). Similar dysfunctional HIF1 signaling as seen in rodents seems to prevail in human AT as well (Halberg et al., 2009a; Jiang et al., 2011; Krishnan et al., 2012). Overall, these observations suggest an important role of the local oxygen supply and consumption for the overall health of adipose tissue. Furthermore, they suggest an involvement of HIF-driven molecular processes aimed at maintaining tissue homeostasis in human AT as well (Goossens et al., 2011).
Other models of excessive ECM accumulation
The functions of ECM proteins and their modifying enzymes (proteinases) in AT have also been investigated in other mouse models. In db/db mice, a variety of collagens (mainly type I, IV and VI) are found to abnormally accumulate in the epididymal WAT (Liu et al., 2009). On the other hand, genetic ablation of MT1-MMP causes the formation of a rigid network of collagen fibrils and impairs the genesis of adipocytes (Chun et al., 2006). Hypotrophic adipocytes have also been observed in mice that are null for a glycoprotein called SPARC (secreted acidic cysteine-rich glycoprotein) which is critically involved in ECM synthesis. Moreover, the issue of obesity-associated ECM accumulation has also been addressed pharmacologically. Further supporting the role of ECM in the pathogenesis of obesity development, we demonstrated that the effective anti-diabetic actions of PPARγ agonists, such as thiazolidinediones, are mediated at least partially by taking advantage of the potent anti-fibrotic actions of PPARγ in adipocytes and macrophages (Khan et al., 2009) through suppression of the transcription of collagens. All these data from different models highlight that the excessive accumulation of ECM is a hallmark of “unhealthy”, pathologically challenged fat pad expansion.
Correlations with other pathological features of adipose tissue
Adipocytes that are encapsulated in a tight ECM “shell” lose their proper functions in fat pads and undergo necrosis at increased frequency. After cell death, remnant large lipid droplets remain in the tissue for several weeks. Dysfunctional live adipocytes surrounding the lipid droplets prompt the infiltration of macrophages, neutrophils, lymphocytes and mast cells, initiating a local pro-inflammatory environment. Macrophages in adipose tissue are differentiated into two broad functional subtypes: M1 and M2 macrophages. The M1 class refers to the classical, activated, pro-inflammatory subtype induced by TH1 cytokines, such as IFN-γ, LPS and TNF-α, ultimately leading to cytotoxicity and tissue injury; in contrast, M2 is an inactivated subclass of cells geared for immune suppression. These cells are alternatively activated by TH2 cytokines, such as IL-4, IL-13, IL-10 and TGF-β. Lipid droplets in fibrotic AT are surrounded predominantly by M1-type macrophages. Indeed, M1 macrophages have been frequently shown to aggregate and form crown-like structures (CLS) surrounding the necrotic adipocytes in advanced stages of tissue dysfunction (Sun et al., 2011) (Fig. 1). In further support of this notion, several reports highlight significant correlations between collagen VIα3 and chronic inflammation, based on increased M1 macrophage infiltration (Pasarica et al., 2009). The phenomenon of reduced macrophage infiltration in AT in collagen VI−/− mice further highlights the causal relationship between ECM, adipocyte survival and inflammation (Khan et al., 2009).
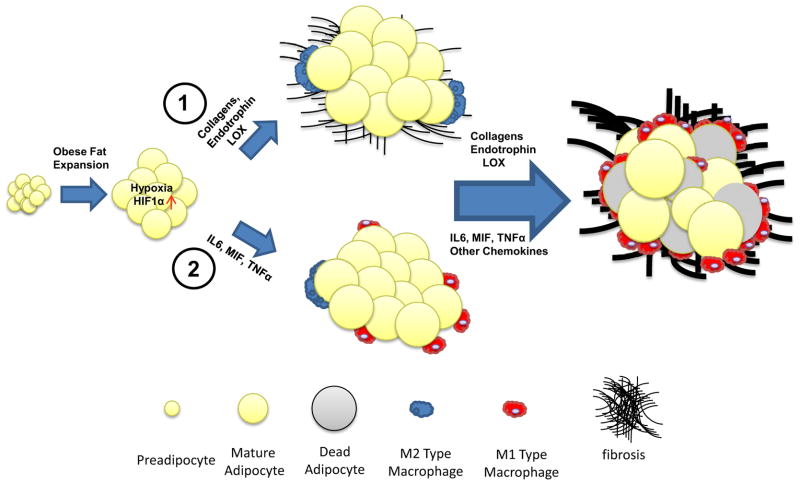
Figure 1. Proposed models for the sequential steps leading to adipose tissue fibrosis and metabolic dysfunction.
Obese fat pad expansion quickly leads to a hypoxic state. As a result, HIF1α is induced. (1). A whole set of “fibrotic response” genes, including collagens and their biosynthetic enzymes, such as LOX, are dramatically up-regulated under these conditions. This regulation results in the abnormal development of ECM, leading to local fibrosis, which triggers necrosis of adipocytes. The dead adipocytes then attract classically activated pro-inflammatory M1 macrophages which ultimately lead to inflammation and metabolic dysfunction. (2). Other models suggest that HIF1α may also directly induce pro-inflammatory factors, such as IL6 and MIF, which in turn causes M1 macrophage infiltration. The preadipocytes, macrophages and the interactions between these cell types ultimately produce fibrotic components, which finally lead cause pathological expansion of fat pads.
In addition, inflammation in obese AT caused by hypoxia can lead to further, more excessive local ECM accumulation (Fig. 1), leading to a feed-forward mechanism for ECM synthesis. Hypoxia up-regulates key inflammatory genes, including IL-6 and macrophage inflammation factor 1 (MIF1) through HIF1α induction. Low-grade inflammation in obese WAT can further stimulate accumulation of interstitial fibrosis (Henegar et al., 2008). In inflamed fat tissue, both adipocytes (mainly pre-adipocytes) and infiltrated macrophages express and secrete collagens. The secreted levels are tightly regulated by crosstalk between macrophages and preadipocytes under different physiological and pathological conditions (Keophiphath et al., 2009).
While the strong relationship linking inflammatory processes to ECM remodeling components is well-established, a causal relationship between inflammation and fibrosis in the progress of the pathophysiology of obesity remains to be defined in the context of obesity and lipodystrophy. A detailed time course analysis over 20 days of high fat diet feeding showed that the fibrotic program is induced shortly after high fat diet challenge, while infiltration of macrophages and the associated inflammation appear much later in the process (Halberg et al., 2009a). Based on this data and a thorough review of the literature, we favor a model in which inflammation is becoming a driving phenomenon only at later stages in the course of adipose tissue dysfunction, in response to a more fibrotic environment (Fig. 1). Regardless of its exact timing of initiation, an excessive chronic increase of the local inflammatory tone is a key mediator for the main downstream sequelae of metabolic dysfunction in adipose tissue.
Abnormal fibrosis in AT is also linked directly or indirectly to many other pathological changes locally, events that eventually have a systemic impact as well. For example, pathologic accumulation of ECM contributes to an increased level of signaling via integrins, contributing to the metabolically unfavorable environment generally observed in fibrotic fat pads; increased interstitial fibrosis in WAT also disrupts cell-cell communication, a critical mediator of fat pad expansion in response to caloric overload. The rigid nature of these fibrotic fat pads prompts limited physical and metabolic flexibility, resulting in ectopic lipid deposition in peripheral tissues, such as the muscle and in the liver (Savage et al., 2007).
Correlations with insulin resistance
While excessive local inflammation in AT is related to systemic insulin resistance at least in rodents, the temporal and mechanistic connections initiating fibrosis prior to inflammation have not yet been worked out in detail as of yet. Moreover, while the obesity-associated infiltration of monocytes and macrophages into AT has been extensively studied (Sun et al., 2011; Weisberg et al., 2003), it remains unclear whether this infiltration of immune cells is strictly secondary to a concomitant necrosis of adipocytes during fat expansion, or whether it is the result of an abnormal profile of chemokine secretion by ECM-laden adipocytes, or a combination of the two. Late-stage processes related to the interaction between adipose tissue inflammation and insulin sensitivity are better understood. Mechanistically, several components of inflammatory pathways have been implicated in reducing insulin sensitivity, such as TNF-α (Hotamisligil et al., 1993), c-jun N-terminal kinase (Hirosumi et al., 2002), and various activators NF-κB (Yuan et al., 2001). Specifically, TNF-α induces serine phosphorylation of insulin receptor substrate 1 to inhibit insulin signaling pathways, which ultimately results in insulin resistance (Hotamisligil et al., 1993). Of note, Toll-like receptor 4 (TLR4), expressed in both macrophages and adipocytes, is a key player leading to the induction or suppression of genes orchestrating the inflammatory response (Shi et al., 2006). There are many additional levels at which an adipocyte can experience cellular stress caused by a more prominent ECM. For example, as active endocrine cells, adipocytes secrete many factors that play critical roles in systemic insulin sensitivity. Disturbances within this “secretory fingerprint” of adipocyte-derived factors as a consequence of a more prominent ECM, can lead to a further reduction in beneficial adipokines, such as adiponectin, with an ensuing secondary impact on insulin sensitivity (Halberg et al., 2009b).
Cellular origin of the ECM: adipocytes versus macrophages?
The quantitative contributions of different cell types towards fibrotic deposits in adipose tissue are difficult to establish. It is clear though that there is no single predominant cell type responsible for ECM synthesis. Oversized adipocytes are the predominant cell type by mass in hypoxic adipose tissue. However, preadipocytes and macrophages are central players as well and are likely to outnumber adipocytes. In vitro studies suggest that preadipocytes in contact to inflammatory cells, such as macrophages, can be the high level “producers” of selective ECM molecules, such as collagen I, tenascin-C and fibronectin and its receptor α5-integrin, as well as activin A as seen in WAT from obese individuals (Keophiphath et al., 2009). Meanwhile, macrophages are found to be the master “regulators” of fibrosis. They produce soluble mediators including transforming growth factor-β1 (TGF-β1) and platelet-derived growth factor (PDGF) that directly activate fibroblasts, and control ECM dynamics by regulating the balance of various MMPs and their inhibitors (Song et al., 2000). Macrophages also regulate fibrogenesis by releasing chemokines that attract fibroblasts and other, inflammatory cells. With their potential to act in both a pro- and anti-fibrotic capacity, as well as their ability to regulate the activation of myofibroblasts, macrophages are fully integrated into all stages of the fibrotic process. The observation that the level of mast cell accumulation is associated with collagen accumulation in obese AT suggests that these cells contribute to adipose remodeling (Divoux et al., 2012). Whether mast cells promote or degrade collagens in adipose depots is unknown. Finally, we cannot exclude that adipocytes per se could contribute to the production of fibrosis as illustrated by the intricate relationship between adipocyte and pericellular collagen in obese adipose parenchyma (Divoux et al., 2010). Of note in this context are similarities and differences to the fibrotic response in the liver. The major source for ECM components in the liver is the stellate cell. Surprisingly, the stellate cell, in its quiescent, inactive state, expresses a transcriptional profile similar to the adipocyte. Both adiponectin and PPARγ, two archetypical adipocyte markers, are important to clamp stellate cells in their inactive “adipocyte-like” state (Shafiei et al., 2011). Upon activation by various insults, these stellate cells turn into myofibroblasts and unleash their full fibrotic program. The specific cell types involved and the step-wise progression from a non-fibrotic to a fibrotic state therefore differ significantly between adipose tissue and the liver.
Relationship to angiogenesis
Angiogenesis plays a critical role in healthy AT expansion (Sun et al., 2012). Excessive deposition of ECM can affect the angiogenic properties of AT. In addition to generating enhanced rigidity to the vasculature, the ECM can either promote or inhibit angiogenesis directly. For example, Collagen IV, a major basement membrane protein of blood vessels, inhibits the initial sprouting of vessels during angiogenesis. Many other ECM components also play a role in this process. These ECM components are expected to make the tissue stiffer and less accommodating for adipocyte expansion and capillary proliferation. As the result, the capillary density in adipose tissue of the obese is reduced and is accompanied by larger, dysfunctional vessels. The regulated expression of the targets of HIF1α (hypoxia inducible factor-1α), the major transcriptional factor mediating hypoxic responses, remains controversial in obese adipose tissue. Some studies in mice and human models described up or down regulation of VEGF during adipose tissue expansion, whereas some other groups report an increase in HIF1α targets, such as thrombospondin- 1 and PAI-1 (Miranda et al., 2010; Voros et al., 2005)
Paradoxically, we found that endotrophin, can stimulate angiogenesis in tumors through recruitment of endothelial precursor cells, while at the same time having potent pro-fibrotic properties (Park and Scherer, 2012).
Several studies have suggested an abnormal oxygen supply in obese AT in humans as well. Adipose Tissue Blood Flow (ATBF) or the AT oxygen partial pressures have been measured. Using radioactive xenon, a first report in 1966 demonstrated a reduction in the radioisotope clearance in obese subcutaneous AT (Larsen et al., 1966). A study using a polarographic electrode to measure the subcutaneous oxygen tension has shown that obese subcutaneous AT is hypoxic (Kabon et al., 2004). In humans, the ATBF reduction correlates with the state of insulin-sensitivity of the subjects (Jansson et al., 1998). On the other hand, it was also recently proposed that obese adipose may be hyperoxic under some conditions and the aberrations of oxygen delivery are related to anomalies in oxygen consumption (Goossens et al., 2011). Another recent paper suggests that with increasing BMI, there is a global reduction in oxygen delivery to adipose tissue. For a given BMI, there was at least a 5% variation of arterial blood oxygen saturation. However, the authors could not find obvious hypoxic-related metabolic signatures in this group of subjects composed of a majority of lean or at most merely overweight individuals (Hodson et al., 2013). Furthermore, in these cases, adipose tissue structure was not explored in detail. Unlike in rodents, a direct link between the measurements of local AT hypoxia and human AT fibrosis has not been established. An indirect link has been proposed by Pasarica et al., where measures of AT oxygen pressure in lean/overweight and obese subjects through a direct insertion of a polarographic Clark electrode, negatively correlates with mRNA levels for collagen VI (Pasarica et al., 2009). Only one study evaluated macrophage accumulation in AT and liver injury in obese subjects with chronic intermittent hypoxia resulting from chronic obstructive sleep apnea. Sleep apnea is an independent factor for insulin-resistance and dyslipidemia. Whereas systemic hypoxia was associated with liver fibro-inflammation, it did not worsen obesity-induced macrophage accumulation in AT depots in over one hundred morbidly obese subjects (Aron-Wisnewsky et al., 2012), though the link with depot fibrosis was not explored in this study. Future studies will need to establish better potential links between adipose tissue fibrosis accumulation and altered oxygen delivery to the adipose tissue in human fat.
Structural and clinical manifestations of fibrosis in human adipose tissue
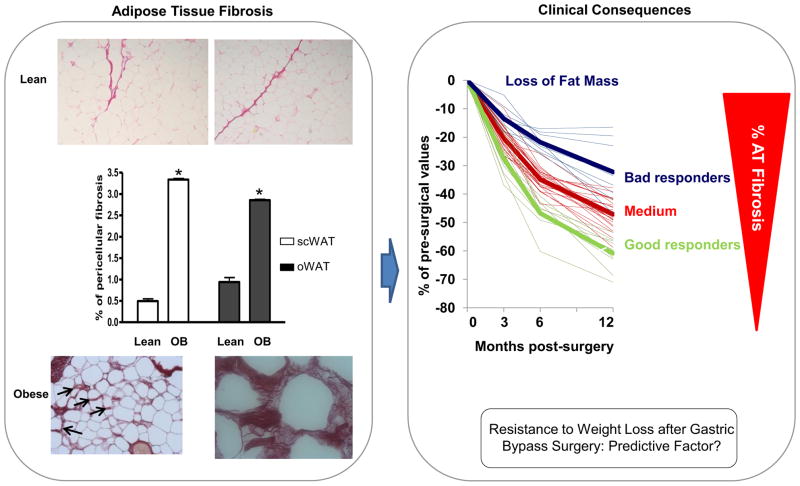
The fundamental processes leading to fibrosis are similar in rodent and human fat tissue. In case of excessive development of AT in human obesity, conditions are geared towards a state favoring fibrosis deposition. A chronic positive energy balance promoting obesity development induces AT modifications with adipocyte hypertrophy and hyperplasia, accumulation of immune cells and neovascularization (Rutkowski et al., 2009). These morphological modifications lead to ECM remodeling with degradation of the existing ECM and the production of new ECM components. The discovery that such process could be altered in human adipose tissue of obese subjects initially stemmed from the examination of transcriptomic signatures of AT depots from lean and obese subjects experiencing weight variation. There is a dysregulation of many ECM components in AT of obese compared to lean subjects (Henegar et al., 2008; Mutch et al., 2009). ECM gene networks are tightly associated with inflammatory gene networks. Overfeeding experiments in humans suggest that early changes in ECM genes occur in moderate weight gain with trends towards collagen deposition and neovascularization in subcutaneous AT (Alligier et al., 2012). Further immunohistochemistry experiments confirmed that fibrosis accumulation is a structural hallmark of obese human AT and highlighted a close spatial relationship between fibrosis and immune cells accumulation. The quantification of total fibrillar collagens by picrosirius red staining revealed higher amounts in both subcutaneous and visceral AT in obese versus lean subjects (Fig. 2). The amount of collagen accumulation in these two depots is positively linked and moribund adipocytes sometimes appear to be trapped in these collagen depots, as they are negative for a perilipin stain (Divoux et al., 2010). Whereas large bundles of collagens are observed through the adipose parenchyma, the accumulation of collagen around the adipocyte (i.e. “periadipocyte collagens”) was particularly characteristic of obesity. Relationships were found between collagen accumulation and adipocyte size in adipose tissue of children and adults. Collagen I, III and VI appeared particularly abundant in obese AT (Divoux et al., 2010). Collagens also accumulate around AT vessels (Divoux et al., 2010; Spencer et al., 2011) (Fig. 2). A genetic contribution to the development of pro-fibrotic conditions in obese AT cannot be excluded. GWAs approaches in other fibrotic-prone tissues, such as liver fibro-inflammation, have led to the discovery of the PLNA3 variant (Sookoian and Pirola, 2011). However, no current GWAs studies in obesity have provided convincing evidence that pro-fibrotic genes contribute (Sandholt et al., 2012). One reason may be due to phenotyping employed in these studies, since most GWAS efforts have investigated the genetic contribution of BMI or visceral adiposity in the cohorts examined, but did not focus on specific AT phenotypes, such as inflammation and obesity-associated fibrosis.
Figure 2. Higher levels of fibrosis in obese versus lean subjects in both subcutaneous and visceral AT.
Morbidly obese subjects with more fibrosis in their subcutaneous adipose tissue lose less fat mass at 3, 6 and 12 months post bypass, suggesting that fibrosis could be used as a good and independent predictor of weight loss after surgical interventions. Arrows indicate pericellular fibrosis. Data are represented as mean +/− SEM.
Clinical consequences of AT fibrosis
The study of causal impact of AT fibrosis accumulation on obesity-related complications is difficult to evaluate because of the correlative nature of investigation studies in human studies. Bioinformatics analyses of the AT transcriptome have demonstrated a strong positive correlation between BMI, AT inflammation and ECM genes in morbid obesity (Mutch et al., 2009).
The relationship between AT fibrosis and insulin-resistance was documented in a human study in which collagen VI expression strongly correlated with glucose metabolism impairment (Pasarica et al., 2009). Those data were further supported by a more recent study demonstrating an increase in ECM components as well as large vessels and capillaries in subcutaneous AT of obese insulin-resistant subjects compared to a lean control group (Spencer et al., 2011).
The discovery that the rapid induction of insulin resistance by acute overfeeding leads to the transcriptional induction of ECM genes (Tam et al., 2010) suggests that collagen remodeling and insulin resistance are likely to be linked in human AT as well. Minimally, the ECM induction could be a result of AT adaptation in this specific clinical context.
In morbid obesity, no association between fibrosis accumulation and surrogate markers of insulin resistance were found. Furthermore, the accumulation of collagen in visceral and subcutaneous depots may have a differential impact on ensuing complications. The accumulation of fibrosis in visceral depots was negatively associated with circulating triglycerides. Multivariate association showed that serum triglyceride, visceral tissue fibrosis and adipocyte size were closely related, suggesting that visceral fibrosis may impact circulating triglycerides levels by limiting adipocyte size. Obese patients with smaller average adipocyte cell size manifest a better plasma lipid profile. This is in agreement with recent observations by Arner and colleagues showing that women with adipocyte hypertrophy have a more adverse metabolic profile than women with cellular hyperplasia at a comparable BMI levels (Arner et al., 2010). The generation of visceral fibrosis could contribute to limit adipocyte expansion, thus acting as an adaptation mechanism that contributes to slowing down the negative effect of adipocyte hypertrophy. However, these relationships could not be established in subcutaneous AT. The mechanisms and kinetics of fibrotic depot accumulations are unknown and difficult to approach in humans. Clinically, fibrosis is more abundant in subcutaneous than in visceral depots where inflammatory cell accumulation is greater, though the nature of the fibrosis in these two depots is quite different. Interestingly, fibrosis accumulation in subcutaneous AT could impact weight loss outcomes after bariatric surgery. Patients undergoing gastric bypass surgery experience a situation leading to drastic fat mass loss and shrinking adipocytes. Subjects with more fibrosis in their AT lose less fat mass during the subsequent 12 months post surgery. While this surgical model is extremely efficient for rapid fat mass loss in obese patients, there is significant individual variability in the response to the intervention (Buchwald et al., 2004). Classically, subjects with higher BMI pre-surgery lose more weight and fat mass post bariatric surgery (Flancbaum et al., 1997). Age and metabolic situation also explain the different weight loss profiles (Campos et al., 2008). Strikingly, in this study, while the patients had higher BMI, they had a less favorable fat loss profile. This group also displays high levels of IL-6. Whether subcutaneous fibrosis could be used as good and independent predictor of weight loss in surgical or dietary intervention studies deserves obviously more attention in larger groups of individuals and with alternative nutritional challenges. Further exploration is also needed to follow the evolution of fibrotic depots after weight loss and to evaluate the relationship between fibrosis and AT stiffness (Fig. 2).
Therapeutic interventions: lifestyle, surgery and pharmacological
The question of the reversibility of fibrosis is crucial in several disease states and initiated efforts into new pharmacological or environmental interventions. Many studies evaluated the improvements or the worsening of fibrosis in the liver post bariatric surgery. The results are ambiguous. Some groups have described a slight increase in fibrosis in patients that were poor responders to bariatric surgery (Mathurin et al., 2006; Mathurin et al., 2009). Others described a decrease in the ECM gene expression, supporting the beneficial effects of current surgical procedures (Barker et al., 2006; Dixon et al., 2006; Klein et al., 2006).
With regards to the reversibility of AT fibrosis during weight loss, the question is not fully resolved. Transcriptomic analysis and histological evaluation of subcutaneous AT fibrosis on obese patients prior to bariatric surgery and the same patients 2 years after bariatric surgery have suggested that fibrosis is not reverted after weight loss (Cancello et al., 2013)and is likely to take a very long time to reach baseline levels.
Anti-fibrotic therapies are a major focus in liver disease. Studies on anti-TGF-β and activation of PPARγ pathways are ongoing. The process of turn-over of AT fibrosis needs to be better understood in order to develop pharmacological interventions to prevent or to inhibit fibrosis in human obesity.
Furthermore, links between lipodystrophy and fibrosis accumulation have been described previously. For example, mice overexpressing a constitutively active human TGF-β1 developed a pro-fibrotic phenotype in the liver, kidney, and adipose tissue, and exhibited in that case a severe reduction in body fat mass accumulation with a phenotype reminiscent of lipodystrophy (Clouthier et al., 1997). In patients with lipodystrophy (due to either genetic mutations or an anti-HIV therapy), enhanced fibrosis, combined with small-sized adipocytes, has been described, without any evidence of an increase in angiogenesis. Whereas a precise description of the fibrotic depot composition or the kinetics of fibrosis deposition in the context of these lipodystrophies has yet to be described, this represents another example of an association of fibrosis with pathological conditions (Bereziat et al., 2011).
Concluding remarks
Fibrosis is increasingly appreciated as a major hallmark of dysfunctional adipose tissue. Assessed either biochemically as total tissue hydroxyproline, or histologically as a trichrome or picrosirius red stain, we find the levels routinely to be associated with overall metabolic fitness of the fat pad, i.e. the ability of adipose tissue to adapt to changing nutrient conditions. As with many other tissues, powerful anti-fibrotic agents that not only prevent the formation of fibrosis, but to some extent have the potential to reverse it, promise to drive significant metabolic improvements, but are unavailable to date. Furthermore, a better understanding of what the critical driving forces are for the induction of the ECM in adipose tissue promises to be a fruitful area of diabetes research in the near future.
Acknowledgments
The authors thank colleagues in Touchstone Diabetes Center, especially Stephen Spurgin and Michael Neinast for help. Authors were supported by the National Institutes of Health (grants R01-DK55758 and P01DK088761-01 to P.E.S.) and the Juvenile Diabetes Research Foundation (JDRF 17-2012-36 to P.E.S.), as well as by French National Agency of Research (ANR-Adipofib), Fondation pour la Recherche Medicale (FRM) and Assistance Publique Hôpitaux de Paris, APHP (Clinical Research Contract, CRC fibrota) to K.C.
Footnotes
Conflict of interest: The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alligier M, Meugnier E, Debard C, Lambert-Porcheron S, Chanseaume E, Sothier M, Loizon E, Hssain AA, Brozek J, Scoazec JY, et al. Subcutaneous adipose tissue remodeling during the initial phase of weight gain induced by overfeeding in humans. J Clin Endocrinol Metab. 2012;97:E183–192. doi: 10.1210/jc.2011-2314. [DOI] [PubMed] [Google Scholar]
- Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, Bernard S, Arner P. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron-Wisnewsky J, Minville C, Tordjman J, Levy P, Bouillot JL, Basdevant A, Bedossa P, Clement K, Pepin JL. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J Hepatol. 2012;56:225–233. doi: 10.1016/j.jhep.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Barker KB, Palekar NA, Bowers SP, Goldberg JE, Pulcini JP, Harrison SA. Non-alcoholic steatohepatitis: effect of Roux-en-Y gastric bypass surgery. The American journal of gastroenterology. 2006;101:368–373. doi: 10.1111/j.1572-0241.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- Bereziat V, Cervera P, Le Dour C, Verpont MC, Dumont S, Vantyghem MC, Capeau J, Vigouroux C. LMNA mutations induce a non-inflammatory fibrosis and a brown fat-like dystrophy of enlarged cervical adipose tissue. Am J Pathol. 2011;179:2443–2453. doi: 10.1016/j.ajpath.2011.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. Jama. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- Campos GM, Rabl C, Mulligan K, Posselt A, Rogers SJ, Westphalen AC, Lin F, Vittinghoff E. Factors associated with weight loss after gastric bypass. Arch Surg. 2008;143:877–883. doi: 10.1001/archsurg.143.9.877. discussion 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- Cancello R, Zulian A, Gentilini D, Mencarelli M, Della Barba A, Maffei M, Vitti P, Invitti C, Liuzzi A, Di Blasio AM. Permanence of molecular features of obesity in subcutaneous adipose tissue of ex-obese subjects. International journal of obesity. 2013 doi: 10.1038/ijo.2013.7. (2005) [DOI] [PubMed] [Google Scholar]
- Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Comerford SA, Hammer RE. Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-beta1 transgenic mice. J Clin Invest. 1997;100:2697–2713. doi: 10.1172/JCI119815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divoux A, Moutel S, Poitou C, Lacasa D, Veyrie N, Aissat A, Arock M, Guerre-Millo M, Clement K. Mast cells in human adipose tissue: link with morbid obesity, inflammatory status, and diabetes. J Clin Endocrinol Metab. 2012;97:E1677–1685. doi: 10.1210/jc.2012-1532. [DOI] [PubMed] [Google Scholar]
- Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817–2825. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obesity surgery. 2006;16:1278–1286. doi: 10.1381/096089206778663805. [DOI] [PubMed] [Google Scholar]
- Flancbaum L, Choban PS, Bradley LR, Burge JC. Changes in measured resting energy expenditure after Roux-en-Y gastric bypass for clinically severe obesity. Surgery. 1997;122:943–949. doi: 10.1016/s0039-6060(97)90336-6. [DOI] [PubMed] [Google Scholar]
- Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clement K, et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009a;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg N, Schraw TD, Wang ZV, Kim JY, Yi J, Hamilton MP, Luby-Phelps K, Scherer PE. Systemic fate of the adipocyte-derived factor adiponectin. Diabetes. 2009b;58:1961–1970. doi: 10.2337/db08-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M, Poitou C, Basdevant A, Stich V, Viguerie N, et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008;9:R14. doi: 10.1186/gb-2008-9-1-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Hodson L, Humphreys SM, Karpe F, Frayn KN. Metabolic signatures of human adipose tissue hypoxia in obesity. Diabetes. 2013;62:1417–1425. doi: 10.2337/db12-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Jansson PA, Larsson A, Lonnroth PN. Relationship between blood pressure, metabolic variables and blood flow in obese subjects with or without non-insulin-dependent diabetes mellitus. Eur J Clin Invest. 1998;28:813–818. doi: 10.1046/j.1365-2362.1998.00360.x. [DOI] [PubMed] [Google Scholar]
- Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, Gonzalez FJ. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes. 2011;60:2484–2495. doi: 10.2337/db11-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabon B, Nagele A, Reddy D, Eagon C, Fleshman JW, Sessler DI, Kurz A. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100:274–280. doi: 10.1097/00000542-200402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpe F, Fielding BA, Ilic V, Macdonald IA, Summers LK, Frayn KN. Impaired postprandial adipose tissue blood flow response is related to aspects of insulin sensitivity. Diabetes. 2002;51:2467–2473. doi: 10.2337/diabetes.51.8.2467. [DOI] [PubMed] [Google Scholar]
- Keophiphath M, Achard V, Henegar C, Rouault C, Clement K, Lacasa D. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol. 2009;23:11–24. doi: 10.1210/me.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, Seki E, Brenner D, Korenblat K, McCrea J. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1564–1572. doi: 10.1053/j.gastro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Krishnan J, Danzer C, Simka T, Ukropec J, Walter KM, Kumpf S, Mirtschink P, Ukropcova B, Gasperikova D, Pedrazzini T, et al. Dietary obesity-associated Hif1alpha activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes & development. 2012;26:259–270. doi: 10.1101/gad.180406.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen OA, Lassen NA, Quaade F. Blood flow through human adipose tissue determined with radioactive xenon. Acta Physiol Scand. 1966;66:337–345. doi: 10.1111/j.1748-1716.1966.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariman EC, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67:1277–1292. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathurin P, Gonzalez F, Kerdraon O, Leteurtre E, Arnalsteen L, Hollebecque A, Louvet A, Dharancy S, Cocq P, Jany T, et al. The evolution of severe steatosis after bariatric surgery is related to insulin resistance. Gastroenterology. 2006;130:1617–1624. doi: 10.1053/j.gastro.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, Pigeyre M, Verkindt H, Dharancy S, Louvet A, et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532–540. doi: 10.1053/j.gastro.2009.04.052. [DOI] [PubMed] [Google Scholar]
- Miranda M, Escote X, Ceperuelo-Mallafre V, Megia A, Caubet E, Naf S, Gomez JM, Gonzalez-Clemente JM, Vicente V, Vendrell J. Relation between human LPIN1, hypoxia and endoplasmic reticulum stress genes in subcutaneous and visceral adipose tissue. International journal of obesity (2005) 2010;34:679–686. doi: 10.1038/ijo.2009.290. [DOI] [PubMed] [Google Scholar]
- Mutch DM, Tordjman J, Pelloux V, Hanczar B, Henegar C, Poitou C, Veyrie N, Zucker JD, Clement K. Needle and surgical biopsy techniques differentially affect adipose tissue gene expression profiles. Am J Clin Nutr. 2009;89:51–57. doi: 10.3945/ajcn.2008.26802. [DOI] [PubMed] [Google Scholar]
- Park J, Morley TS, Scherer PE. Inhibition of Endotrophin, a Cleavage Product of Collagen VI, Confers Cisplatin Sensitivity to Tumors. EMBO Mol Med. 2013 doi: 10.1002/emmm.201202006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Invest. 2012;122:4243–4256. doi: 10.1172/JCI63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, Ravussin E, Bray GA, Smith SR. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94:5155–5162. doi: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. The FEBS journal. 2009;276:5738–5746. doi: 10.1111/j.1742-4658.2009.07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandholt CH, Hansen T, Pedersen O. Beyond the fourth wave of genome-wide obesity association studies. Nutr Diabetes. 2012;2:e37. doi: 10.1038/nutd.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiei MS, Shetty S, Scherer PE, Rockey DC. Adiponectin regulation of stellate cell activation via PPARgamma-dependent and -independent mechanisms. Am J Pathol. 2011;178:2690–2699. doi: 10.1016/j.ajpath.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Ouyang N, Horbelt M, Antus B, Wang M, Exton MS. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol. 2000;204:19–28. doi: 10.1006/cimm.2000.1687. [DOI] [PubMed] [Google Scholar]
- Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE, Jr, Peterson CA, Kern PA. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab. 2011;96:E1990–1998. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE. Selective inhibition of hypoxia-inducible factor 1alpha ameliorates adipose tissue dysfunction. Mol Cell Biol. 2013;33:904–917. doi: 10.1128/MCB.00951-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci U S A. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam CS, Viardot A, Clement K, Tordjman J, Tonks K, Greenfield JR, Campbell LV, Samocha-Bonet D, Heilbronn LK. Short-term overfeeding may induce peripheral insulin resistance without altering subcutaneous adipose tissue macrophages in humans. Diabetes. 2010;59:2164–2170. doi: 10.2337/db10-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology. 2005;146:4545–4554. doi: 10.1210/en.2005-0532. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]