Abstract
Aim
The purpose of the current study was to determine if long term treatment with an endothelin-A (ETA) receptor antagonist attenuates the progression of coronary plaques in patients with coronary endothelial dysfunction.
Methods
Thirty-five patients with non-obstructive coronary disease and coronary endothelial dysfunction were randomized in a double blind manner to treatment with placebo or ETA receptor antagonist Atrasentan (10 mg) for six months. Endothelial function was assessed by the change in coronary blood flow and coronary artery diameter in response to intracoronary acetylcholine. Normalized mean total atheroma volume (TAVMEAN), percent atheroma volume (PAV) and changes of atheroma volume were assessed by intravascular ultrasound (IVUS) at baseline and 6-month follow-up.
Results
In segments with coronary endothelial dysfunction, there was a significant decrease in normalized TAVMEAN and PAV at six months from baseline in the Atrasentan group compared to the placebo group median (IQR) −2.00 mm3 (−7.28, 2.53.) vs 9.11 mm3 (1.23, 14.05), p=0.0024 and 0.955% (−3.43, 1.70) vs 3.85% (−0.39, 14.59) p=0.010. There was no change in normalized TAV or PAV in the segments with normal endothelial function.
Conclusion
This study demonstrates that 6-month treatment with Atrasentan attenuates progression of coronary plaque in segments with endothelial dysfunction.
Keywords: endothelial function, endothelin receptor (ETA) antagonist, Normalized mean total atheroma volume (TAVMEAN), percent atheroma volume (PAV), intravascular ultrasound (IVUS)
Introduction
Endothelial dysfunction, an early stage of atherosclerosis, is characterized by an impairment of endothelial-dependent vasodilatory function and altered anti-inflammatory and anticoagulant properties of endothelium[1] and is associated with myocardial ischemia[2, 3], increased coronary plaque vulnerability[4] and cardiovascular events.[5, 6] Endothelin-1 (ET-1) via smooth muscle endothelin receptor (ETA) activation has both mitogenic and vasoconstricting properties at pathophysiological levels and is increased in the coronary circulation of patients with endothelial dysfunction.[7, 8] ET-1 plays an important role in the development of endothelial dysfunction and progression of atherosclerosis.[8–10]
ETA antagonism attenuates the histological changes associated with atherosclerosis in experimental animals.[11]. We have also previously shown that 6-month administration of ETA receptor antagonist improves coronary microvascular endothelial function in humans.[12] However, the effect of long term therapy with an ETA receptor antagonist on the coronary plaque progressions is unknown. Thus the purpose of the present study was to test the hypothesis that long-term treatment with Atrasentan - a highly selective antagonist of the ETA receptor in humans attenuates the progression of segmental coronary plaques as measured by IVUS in patients with early atherosclerosis and endothelial dysfunction.
Methods
This study is a single-center, double blinded, randomized controlled trial sponsored by the National Institutes of Health. The design and demographics of the study have been previously published.[12, 13] The study protocol was approved by the Mayo Foundation Institutional Review Board and all patients included in the study signed the consent forms.
Study Population
Consecutive patients referred to the cardiac catheterization laboratory for evaluation of coronary artery disease between July 2001 and December 2006 and found to have non-obstructive disease were screened for inclusion in the study. These Patients had a comprehensive coronary physiology study including the assessment of endothelial function and non-endothelium-independent coronary flow reserve per clinical practice protocol in our institution[12, 13] were included in this study if they had both coronary microvascular and epicardial endothelial dysfunction. According to our previous studies, we defined microvascular endothelial dysfunction as ≤ 50% increase in coronary blood flow (CBF) in response to the maximal dose of acetylcholine (ACh) compared with baseline CBF. [2, 12] Epicardial endothelial dysfunction was defined as epicardial vasoconstriction to ACh (more than 20% decrease in coronary artery diameter in response to ACh). Exclusion criteria for the study have been previously reported [2, 12] and included ≥50% diameter stenosis of any coronary artery, prior myocardial infarction,[14] unstable angina pectoris,[15] uncontrolled hypertension, peripheral vascular disease, ejection fraction <55%, left ventricular hypertrophy,, and significant endocrine, hepatic, renal, or inflammatory disease.
Protocol for Evaluation of Endothelial Function
After diagnostic coronary angiography and exclusion of significant obstructive coronary artery disease, coronary endothelial function was assessed as previously described. [2, 5, 12] Coronary endothelial function was assessed by measuring the coronary vasoreactivity and CBF of left anterior descending coronary artery (LAD) in response to selective ACh infusion.[2, 5, 12] Hemodynamic data, Doppler measurements and coronary angiography were obtained after each infusion of ACh.[16] Segments with epicardial endothelial dysfunction were defined as those with a decrease of more than 20% in coronary artery diameter in response to 10−4 mol/l of ACh.[2, 4, 5, 16] Endothelium-independent coronary function was assessed by intracoronary bolus injection of incremental doses (18–60 µg) of adenosine (Fujisawa Pharmaceutical Co, Osaka, Japan) and coronary flow reserve was calculated.
Assessment of Coronary Blood Flow
The assessment of coronary blood flow has been previously described[12]. Briefly doppler flow velocity spectra were analyzed online to determine the time-averaged peak velocity. Volumetric CBF was determined from the following relation: CBF=cross-sectional area xaverage peak velocity x 0.5.[16] Endothelium-dependent coronary flow reserve was calculated as percent change in CBF (% Δ CBF) in response to ACh as previously described[17]. The endothelium-independent coronary flow reserve ratio was calculated by dividing the average peak velocity after adenosine injection by the baseline average peak velocity.[2] Basal NO activity was evaluated by measuring the change in CAD and CBF (% Δ CAD and % Δ CBF, respectively) in response to L-NMMA. Coronary vascular resistance (CVR) was estimated as mean arterial blood pressure/CBF.[18]
Quantitative Coronary Angiography
Quantitative measurements of coronary arteries were obtained with a computer-based image-analysis system using end-diastolic cine frames with best shown segments and measured off-line by an independent investigator.[19]
IVUS Image Acquisition and Analysis
In a subgroup of patients found to have at least one segment with epicardial endothelial dysfunction an IVUS study was performed at the end in the physiologic study for evaluation of endothelial function. A 20-MHz, 2.9-F IVUS imaging catheter (Eagle Eye, Volcano Corp., Roncho Cordova, CA, USA) was advanced to the distal LAD artery after intracoronary administration of 100–200 µg nitroglycerin. Automated pullback at 0.5 mm/s was then performed to the ostium of LAD. The location of the catheter seen on the cine film of each segment was used to correlate the identified IVUS image with the angiographic segment. The IVUS images were recorded throughout the LAD artery on a 0.5-in videotape for later offline analysis.[4] Coronary artery sections with vasoconstriction in the IVUS image using distance from anatomical landmarks that are seen on angiography and IVUS such as side branches were identified. The IVUS images from each section were saved and afterwards the grey scale and tissue characteristics were analysed by investigators who were blind to the vasoconstriction results.
IVUS Image Analysis
An investigator blinded to the original allocation selected a distal branch site as a landmark for analysis. Subsequently, every 10th image was analyzed, representing cross-sections spaced exactly 0.5mm apart. IVUS measurements were performed in accordance with the standards of the American College of Cardiology and the European Society of Cardiology.[20] The investigator performed a calibration by measuring 1-mm grid marks in the image. Manual planimetry was used to trace the leading edges of the luminal and external elastic membrane borders. Percentage of plaque burden was calculated as plaque plus media cross sectional area divided by external elastic membrane cross sectional area (EEMCSA) multiplied by 100. Efficacy parameter of percent atheroma volume (PAV) was calculated as Σ(EEMCSA – LUMENCSA)/ΣEEEMCSA × 100, where LUMENCSA is the luminal cross sectional area. For each patient, the change in PAV was computed as PAV (follow-up) – PAV (baseline). The mean total atheroma volume (TAVMEAN) was calculated as Σ(EEMCSA – LUMENCSA)/n, where n is the number of evaluable cross-sections in the pullback. Normalized TAVMEAN for each patient was calculated as the TAVMEAN multiplied by 20 of comparable cross-sections in pullbacks for all patients completing the trial. The change in normalized TAVMEAN was calculated as normalized TAVMEAN (follow-up) – normalized TAVMEAN (baseline). Separate analysis was done for segments with normal and abnormal endothelial function.
Follow-Up
After the patients were enrolled in the study they were randomly assigned and treated in a double-blind fashion according to a computer-generated code with either Atrasentan (ABT-627, A-147627; trade name Xinlay; Abbott Laboratories, Abbott Park, III - a highly selective antagonist of the ETA receptor in humans) at the dose of 10 mg orally once a day or placebo for six months, in addition to standard medical therapy as previously reported[13]. Treatment assignments were concealed from participants and study staff except for the pharmacist technician. Study and placebo tablets (provided by Abbott Laboratory) were distributed in bottles and were identical in appearance.
Six month follow-up coronary artery angiogram including coronary endothelial function and IVUS studies were performed by an independent investigator blinded to treatment allocation. The pre-specified primary endpoint was the change at six months from baseline in PAV computed as PAV (follow-up) – PAV (baseline) and the change at six months from baseline in normalized TAVMEAN calculated as normalized TAVMEAN (follow-up) - normalized TAVMEAN (baseline).
Statistical Analysis
Data are displayed as means ± SD if normally distributed or count and percentage as appropriate. If data were not normally distributed, values are expressed as medians with first and third quartiles in parentheses. Analysis to compare continuous data was performed with paired or unpaired Student t test or anova as appropriate. If the data distribution was heavily skewed differences between the groups were compared with Wilcoxon rank sum test. Categorical variables were presented as frequencies and compared using Pearson X2 statistics or Fisher exact test as appropriate. Multiple linear regression was used to estimate the treatment effect adjusted for other covariates. All statistical tests were 2-sided and a value of p < 0.05 was considered to be statistically significant.
Results
Characteristics of the Patients
35 patients had a baseline and follow-up IVUS images and were included in the study. Baseline demographic and clinical characteristics were similar between the groups (Table 1). The groups were well matched to age, gender, coronary risk factors and medical treatment.
Table 1.
Laboratory Follow-up Results
| Variable | Atrasentan (n=18) | Placebo(n=17) | P value |
|---|---|---|---|
| Age, y | 50 ± 9 | 48 ± 13 | 0.656 |
| Male gender, n (%) | 6 (33) | 5 (29) | 1.000 |
| Diabetes mellitus, n (%) | 1 (6) | 2 (12) | 0.6 |
| Hypercholesterolemia, n (%) | 11 (61) | 10 (59) | 1.000 |
| Smoker, n (%) | 2 (11) | 1 (6) | 1.000 |
| Previous smoker, n (%) | 8 (44) | 6 (35) | 0.73 |
| Family history, n (%) | 11 (61) | 10 (63) | 1.000 |
| Body mass index | 30.7 ± 5.0 (1.18) | 29.1 ± 4.7 (1.13) | 0.35 |
| Mean arterial pressure, mmHg | |||
| Baseline | 93 ± 12 | 95 ± 13 | 0.67 |
| 6-month F/U | 84 ± 12 | 96 ± 11 | 0.003 |
| p value | 0.017 | 0.781 | |
| Medication use, n (%) | |||
| ACE inhibitor | 4 (22) | 4 (24) | 1.000 |
| β-blocker | 6 (33) | 5 (29) | 1.000 |
| Aspirin | 9 (50) | 10 (59) | 0.73 |
| Calcium channel blocker | 7 (39) | 10 (59) | 0.32 |
| Statin | 8 (44) | 8 (47) | 1.000 |
| Oral hypoglycemic agent | 0 (0) | 1 (6) | 0.49 |
| Total Cholesterol, mg/dL | |||
| Baseline | 197 ± 38 | 197 ± 42 | 0.99 |
| 6-Month Follow-up | 161 ± 45 | 164 ± 33 | 0.85 |
| P value | 0.002 | 0.004 | |
| Triglycerides, mg/dL | |||
| Baseline | 204 ± 264 | 123 ± 50 | 0.22 |
| 6-Month Follow-up | 130 ± 165 | 105 ± 52 | 0.56 |
| P value | 0.01 | 0.26 | |
| HDL, mg/dL | |||
| Baseline | 52 ± 18 | 59 ± 17 | 0.27 |
| 6-Month Follow-up | 50 ± 15 | 55 ± 15 | 0.36 |
| P value | 0.78 | 0.04 | |
| LDL, mg/dL | |||
| Baseline | 114 ± 39 | 113 ± 42 | 0.97 |
| 6-Month Follow-up | 86 ± 29 | 88 ± 24 | 0.84 |
| P value | <0.001 | 0.016 | |
| Lipoprotein (a), mg/dL | |||
| Baseline | 28.78 ± 32.02 | 20.76 ± 27.22 | 0.43 |
| 6-Month Follow-up | 21.00 ± 23.15 | 20.71 ± 29.81 | 0.84 |
| P value | 0.13 | 0.97 | |
| Glucose, mg/dL | |||
| Baseline | 99 ± 13 | 95 ± 27 | 0.60 |
| 6-Month Follow-up | 99 ± 34 | 104 ± 29 | 0.65 |
| P value | 0.99 | 0.06 | |
| Glycosylated hemoglobin, % | |||
| Baseline | 5.59 ± 0.83 | 5.51 ± 0.80 | 0.77 |
| 6-Month Follow-up | 5.59 ± 0.87 | 5.52 ± 0.64 | 0.78 |
| P value | 1.000 | 0.72 | |
| Creatinine, mg/dL | |||
| Baseline | 1.00 ± 0.16 | 0.97 ± 0.12 | 0.55 |
| 6-Month Follow-up | 0.89 ± 0.12 | 0.92 ± 0.13 | 0.57 |
| P value | 0.24 | 0.25 |
LVEF indicates left ventricular ejection fraction; ACE, angiotensin-converting enzyme; HDL, high-density lipoprotein; and LDL, low-density lipoprotein.
Plus-minus values are mean±SD.
Comparison of Serial IVUS Data between Placebo and Atrasentan Group
Segments with normal and abnormal endothelial function were identified in each subject. Analysis was done based on whether the segments had endothelial dysfunction at baseline.
Segments with Endothelial dysfunction
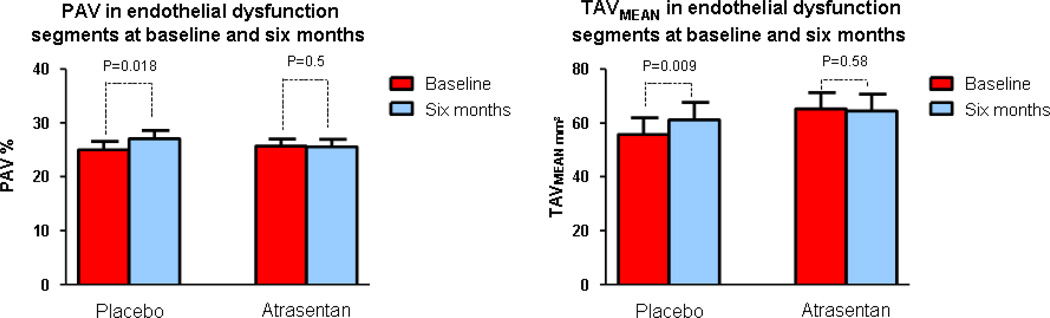
Normalized volume area (VA), normalized lumen area (LA), normalized TAVMEAN and PAV were similar between the two groups at baseline in the segments with endothelial dysfunction. In these segments with endothelial dysfunction in the placebo group normalized TAVMEAN and PAV increased significantly at six compared to baseline median (IQR) 63.0 mm3 (31.8, 79.55) vs 54.0 mm3 (30.0, 73.4), p=0.009 and 28.48 % (21.045, 30.220) vs 21.79 % (20.535, 29.72), p=0.018 respectively but not in the Atrasentan group 56.9 mm3 (43.75, 78.25) vs 59.4 mm3 (44.75, 78.54) p= 0.581 and 23.855 % (21.763, 27.013) vs 24.605 % (22.36, 26.455) p=0.545 respectively (Figure 1)(Table 2).
Figure 1.
Comparison of normalized TAVMEAN and PAV in the segments with endothelial dysfunction between baseline and 6-month follow-up in the Atrasentan and placebo groups. TAVMEAN, mean total atheroma volume; PAV, percent atheroma volume
Table 2.
Baseline and follow-up IVUS findings according to endothelial function
| Variable | Atrasentan (n=18) | Placebo (n=17) | P |
|---|---|---|---|
| At the Segments with Endothelial Dysfunction | |||
| Normalized VA, mm3 | |||
| Baseline, median (IQR) | 244.1 (200.65, 289.80) | 235 (140.1, 268.53) | 0.220 |
| Follow-up, median (IQR) | 243.3 (196.9, 297.9) | 244.6 (147.9, 263.04) | 0.3221 |
| Difference, median (IQR) | −0.10 (−14.6, 3.9) | 7.8 (−0.65, 11.1) | 0.0694 |
| Normalized LA, mm3 | |||
| Baseline, median (IQR) | 171.7 (151.7, 217.15) | 165.2 (110.1, 186.33) | 0.1760 |
| Follow-up, median (IQR) | 178.7 (142.55, 226.1) | 160.2 (116.3, 184.89) | 0.1464 |
| Difference, median (IQR) | −0.1 (−11.55, 4.5) | 4.0 (−6.43, 7.86) | 0.3638 |
| Normalized TAVMEAN, mm3 | |||
| Baseline, median (IQR) | 59.4 (44.75, 78.54) | 54.0 (30.0, 73.4) | 0.2760 |
| Follow-up, median (IQR) | 56.9 (43.75, 78.25) | 63 (31.8, 79.55) | 0.8430 |
| Difference, median (IQR) | −0.8 (−4.25, 1.45) | 2.6 (0.4, 9.45) | 0.0050 |
| PAV, mm3 | |||
| Baseline, median (IQR) | 24.605 (22.36, 26.455) | 21.79 (20.535, 29.72) | 0.3385 |
| Follow-up, median (IQR) | 23.855 (21.763, 27.013) | 28.48 (21.045, 30.220) | 0.4283 |
| Difference, median (IQR) | −4.5 (−16.5, 9.15) | 15.5 (−1.7, 71.8) | 0.0121 |
| At the Segments with Normal Endothelial Function | |||
| Normalized VA, mm3 | |||
| Baseline, median (IQR) | 321.3 (282.25, 364.6) | 281.67 (187.7, 333.2) | 0.0577 |
| Follow-up, median (IQR) | 313.4 (266.55, 367.3) | 265.2 (185.1, 335.2) | 0.0556 |
| Difference, median (IQR) | 1.6 (−8.9, 9.35) | 1.67 (−8.3, 9.93) | 0.6440 |
| Normalized LA, mm3 | |||
| Baseline, median (IQR) | 218.9 (198.25, 258.95) | 186.33 (144.2, 246.5) | 0.1058 |
| Follow-up, median (IQR) | 214.6 (203.4, 257.95) | 193.14 (147.0, 249.9) | 0.0861 |
| Difference, median (IQR) | 1.0 (−10.4, 9.2) | −0.2 (−8.3, 11.2) | 0.8689 |
| Normalized TAVMEAN, mm3 | |||
| Baseline, median (IQR) | 92.5 (67.75, 127.65) | 67.0 (35.6, 98.07) | 0.0477 |
| Follow-up, median (IQR) | 91.8 (62.65, 119.2) | 65.4 (36.50, 100.07) | 0.0477 |
| Difference, median (IQR) | −1.1 (−5.3, 4.4) | 1.0 (−1.665, 4.3) | 0.2687 |
| PAV, mm3 | |||
| Baseline, median (IQR) | 29.055 (21.913, 35.438) | 24.5 (19.815, 29.99) | 0.1375 |
| Follow-up, median (IQR) | 28.15 (21.165, 35.818) | 25.14 (19.64, 28.67) | 0.1375 |
| Difference, median (IQR) | 0.16 (−1.073, 0.855) | 0.49 (−1.275, 1.53) | 0.5414 |
IVUS indicates intravascular ultrasound; VA, vessel area; IQR, interquartile range; LA, lumen area; TAVMEAN, mean total atheroma volume; PAV, percent atheroma volume.
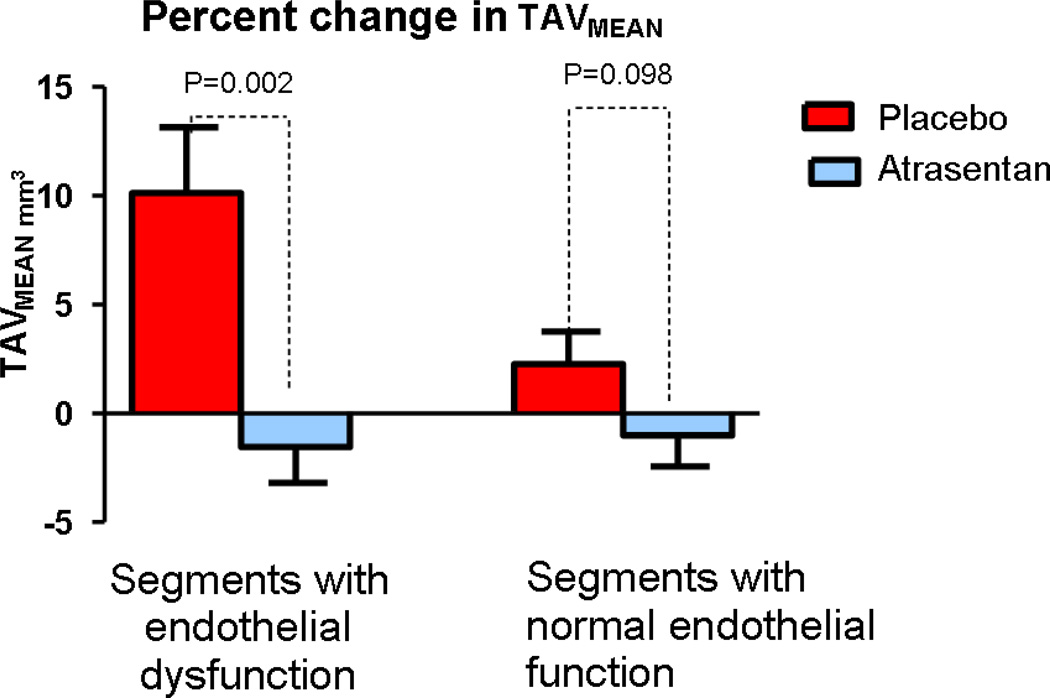
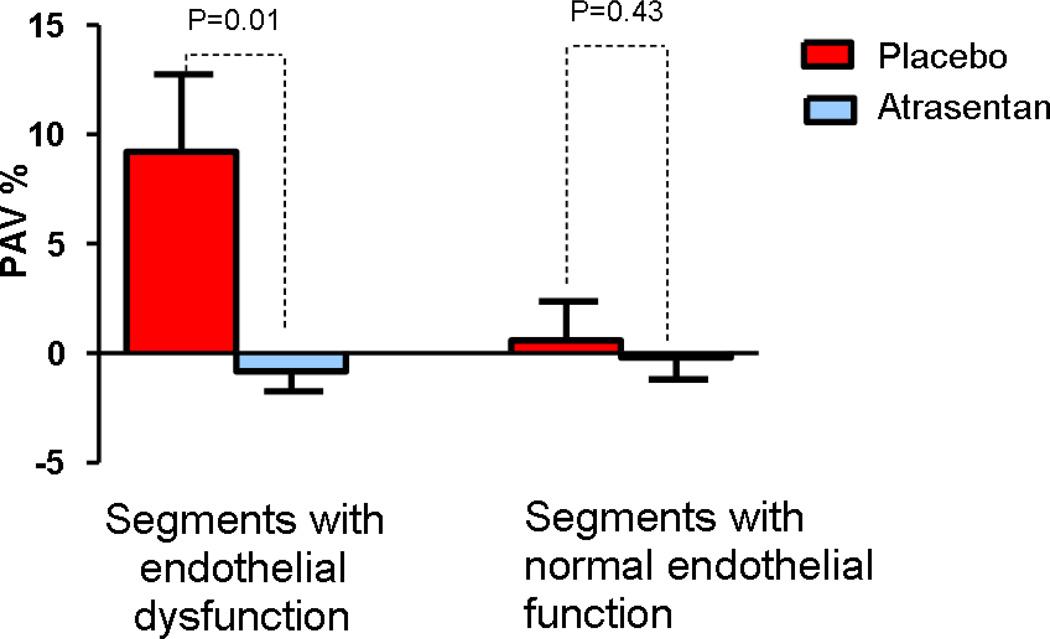
In these segments with endothelial dysfunction there was a significant decrease in normalized TAVMEAN and PAV at six months from baseline in the Atrasentan group compared to the placebo group median (IQR) −2.00 mm3 (−7.28, 2.53.) vs 9.11 mm3 (1.23, 14.05), p=0.0024 and 0.955% (−3.43, 1.70) vs 3.85% (−0.39, 14.59) p=0.010 (Figure 2 and 3)(Table 2). After adjusting for baseline characteristics and medication use including treatment with statins, Atrasentan was associated with significant attenuation of both TAVMEAN and PAV compared to placebo.
Figure 2.
Comparison of percent change at six months from baseline in normalized TAVMEAN between the Atrasentan and placebo group according to endothelial function. TAVMEAN, mean total atheroma volume
Figure 3.
Comparison of percent change at six months from baseline in normalized PAV between the Atrasentan and placebo group according to endothelial function. PAV, percent atheroma volume
There however was no difference at six months from baseline in either normalized VA or normalized LA between the Atrasentan and placebo group −0.10 mm3 (−14.6, 3.9) vs 7.8 mm3 (−0.65, 11.1), p=0.0694 and −1. mm3 (−11.55, 4.5) vs 4.0 mm3 (−6.43, 7.86) p=0.3638 respectively.
Segments with normal endothelial function
Normalized volume area (VA), normalized lumen area (LA), normalized TAVMEAN and PAV were similar the two groups at baseline. There were no significant changes within and between the two groups at six months compared to baseline in the normalized VA, normalized LA, normalized TAVMEAN or PAV (Table2).
Coronary epicardial endothelial function
There was no significant difference in percent change in coronary artery diameter to ACh at segments with endothelial dysfunction and normal endothelial function between the two groups at six months follow-up compared to baseline in any of the groups (Table 3)
Table 3.
Baseline and follow-up percent change in coronary artery diameter in response to acetylcholine
| Segments with Endothelial Dysfunction | |||
|---|---|---|---|
| Atrasentan | Placebo | p Value | |
| Baseline | −33.33 (−40.51, −13.10) | −20.0 (−23.53, −10.53) | 0.2126 |
| At 6 month | −20.69 (−48.33, −0.91) | −14.81 (−33.54, −7.14) | 0.7197 |
| Difference | 0.29 (−15.68, 19.42) | 7.84 (−21.47, 20.00) | 0.7482 |
| Segments with normal endothelial function | |||
| Atrasentan | Placebo | p Value | |
| Baseline | −8.20 (−13.20, 0.00) | −4.35 (−10.00, 0.00) | 0.2851 |
| At 6 month | −4.01 (−11.78, 0.00) | −5.56 (−10.00, −3.70) | 0.5724 |
| Difference | 4.47 (−3.17, 12.03) | −0.32 (−3.85, 3.75) | 0.0923 |
CAD indicates coronary artery diameter.
Coronary microvascular endothelial function
At follow-up, percent change in CBF after infusion of ACh was significantly improved in the Atrasentan group compared to the placebo [44.99 (28.0, 91.5) vs 0.42 (−35.05, 16.73), p=0.0002] as was reported previously (12). There was no significant difference in coronary flow reserve at six month from baseline between the Atrasentan and placebo group.
Comparison of Baseline and Follow-Up Clinical and Laboratory Characteristics
Lipid profiles were significantly improved in both group at six months compared to baseline and follow-up lipid profiles were not significantly different between the two groups. Fasting blood glucose and glycosylated hemoglobin were not changed between baseline and follow-up in both groups.
Discussion
The present study demonstrates for the first time that long-term ETA receptor antagonism attenuates progression of coronary plaque in segments with epicardial endothelial dysfunction in patients with early coronary atherosclerosis and non obstructive coronary artery disease. ET-1 mediated ETA receptor activation however did not have any significant effect in the plaque progression in segments with normal epicardial endothelial function. There was no change in overall or segmental epicardial endothelial function. In the current study significant progression of the coronary plaque volume occurred only in the segments with endothelial dysfunction in patients within the placebo group at 6-month follow-up from baseline but not in the Atrasentan group. There also was no significant change in coronary plaque volumes in the segments with normal endothelial function at 6-month follow-up from baseline in either the placebo or Atrasentan group. These results demonstrate that endogenous ET-1 pathway plays a role in atherosclerosis and long-term ETA receptor antagonism has a protective effect in the progression of coronary plaque only in segments with endothelial dysfunction which are more prone to development and progression of atherosclerosis. Thus, the current study further supports a role for endothelial dysfunction as an early stage of atherosclerosis and underscores the role of the endogenous endothelin pathway as a participant and potential therapeutic target for plaque regression in coronary atherosclerosis.
The coronary segments with endothelial dysfunction represent a potential initiating event in vascular injury and abnormal vascular repair which is associated with local inflammation,[21] plaque vulnerability and progression.[4, 21, 22] The current study demonstrates that segments with endothelial dysfunction in the coronary artery are characterized by an increased rate in plaque progression compared to adjacent segments with normal endothelial function in the same vascular territory and in the same individuals and further supports the role of endothelial dysfunction as site of abnormal vascular repair and an early stage of coronary atherosclerosis.
The ability to identify segments with endothelial dysfunction that are associated with coronary plaque development and the ability to attenuate coronary plaque progression is critically important because plaque burden is one of the major risk factor for future cardiovascular events.[23] In addition we have recently demonstrated that segments with coronary endothelial dysfunction and minimal atherosclerosis are associated with plaque characteristics that are typical of vulnerable plaques[4]. Moreover, in the recent PROSPECT study, features of coronary plaque vulnerability (thin (-cap) fibroatheroma, small lumen diameter and large plaque burden) have been shown to be significantly associated with the development of future adverse cardiovascular events and mainly plaque progression in patients with lesions that were thought angiographically mild at baseline[23]. Thus, the presence of segments with endothelial dysfunction may signify underlying vulnerable plaques which are prone to rapid plaque progression.
Potential Mechanisms
Several potential mechanisms may account for the attenuation in plaque progression with long term ETA receptor antagonism.
Improvement in Endothelial function
Endothelial function assessed in coronary vessels using ACh as the stimulus measures NO-induced vasodilation and by association the ability of the endothelium to protect against the initiation and progression of atherosclerosis. Thus, vascular reactivity is one of the measurable functions of the endothelium and serves as an index and a window to the underlying process of vascular injury and early atherosclerosis in endothelial dysfunction.[24]
Endothelial dysfunction is a systemic disorder characterized not only by abnormal tonic vascular constriction but also by a proinflammatory, proliferative and procoagulatory milieu that favors all stages of atherogenesis.[25] ET-1 through the ETA receptor may contributes locally to the development of endothelial dysfunction and atherogenesis[26–30] through regulation of tonic vascular constriction and its mitogenic properties[31]. ET-1 levels are elevated in conditions associated with vascular endothelial dysfunction[32] and in patients with advanced atherosclerosis.[7, 8] Moreover, ET-1 is highly expressed in the atherosclerotic plaques in humans[2] and is released form the plaque in response to mechanical injury.[2]
Previous studies have demonstrated that acute blockade of ETA receptor improves epicardial endothelial function [26] and we have recently shown that chronic medication with ETA receptors antagonism improves coronary microvascular endothelial function.[12] However, we did not show any improvement in the epicardial endothelial function with Atrasentan in the previous and the current study. The attenuation or stabilization in plaque progression observed in current study in the segments with endothelial dysfunction occurred without improvement in vasoreactivity component in the respective segments with endothelial dysfunction. Indeed impaired vasoreactivity and conversely its improvement only partially account for endothelial dysfunction and may not necessarily correlate with the onset or recovery of the other characteristics of epicardial endothelial dysfunction. Thus, the relatively short period of time of the study may have allowed demonstration of only the effect on the microcirculation and a longer duration of treatment may be needed to show the beneficial effect on the epicardial arteries.
Attenuation of inflammation
Inflammatory mechanisms are closely associated with atherosclerosis and plaque vulnerability. ETA receptors are expressed on inflammatory cells such as neutrophiles and macrophages. These cells play a key role in vascular dysfunction and inflammation via the possible formation of an autocrine loop between endothelin-1 and ETA receptor.[33]
ET-1 promotes macrophage foam cell formation possibly via increased degradation of ATP-binding cassette transporter G1 (ABC G1) which mediate the net mass efflux of cholesterol to mature high density lipoprotein[34]. ET receptor antagonist was shown to substantially inhibited the development of atherosclerosis in a genetic model of hyperlipidemia by inhibiting macrophage foam-cell formation[35].
In addition, ET-1 receptor stimulates the release of inflammatory mediators in macrophages such as prostaglandin E2 and TNF[34] possibly via ET-1-induced NF-kappa B activation. In experimental studies this ET-1 effect was blocked by the ET-A-receptor antagonist, a specific inhibitor of the IkappaB-alpha-degrading proteasome complex and also prevented NF-kappa B activation thus demonstrating the ability of ET-1 to activate inflammatory pathways in human macrophages[36]. .Previous studies have also demonstrated that ETA receptor antagonist block the effect of endothelin-1 on kinin B1 receptor expression which is associated with oxidative stress[37]. Thus blocking the inflammatory process and oxidative stress may be a potential mechanism contributing to the attenuation of plaque progression with ETA receptor antagonism in the study.
We have previously reported that in a larger group of patients that long term treatment with Atrasentan resulted in a reduction of blood pressure, improvement in glucose and lipid metabolism. [13] These effects were however not observed in this smaller group. Moreover, the differential and beneficial effect of ETA receptor antagonist occurred in separate and adjacent coronary segments in the same coronary tree. Thus, the beneficial effect of ETA receptor antagonism in this study was achieved without a significant effect on systemic risk factors which underscores the more specific role of endothelin in the progression of atherosclerosis
Limitations
There are several limitations to the present study. First, a significant number of patients who were randomized in the study did not have adequate IVUS data at baseline and follow-up to be included in the final analysis. However, the baseline characteristics of the patients who were included in the final analysis did not differ significantly from those who were not. Nevertheless, there still remains a risk of bias in the results.
Second, the current study is focused on patients with early atherosclerosis and non-obstructive coronary artery disease. The results of the study may thus not be generalizable to patients with significant atherosclerosis or unstable coronary artery disease.
Third, the study focused on the effects of 6-month administration of selective ETA receptor antagonists on coronary plaque progression over a relatively short period. Longer observation periods may be necessary to adequately evaluate the effects of selective ETA receptor antagonists.
Fourth, we did not evaluate for effect of ETA receptor antagonists on specific plaque characteristics. For example, previous studies using more novel imaging techniques have demonstrated that coronary endothelial dysfunction is associated with necrotic core of coronary plaque.[4]. Further studies using more sophisticated imaging modalities are needed to evaluate the effect of ETA receptor antagonism on plaque characteristics.
Conclusions
This study demonstrates for the first time that 6-month treatment with Atrasentan attenuates plaque progression in coronary segments with endothelial dysfunction. This study extends previous observations that endothelin-1 and segmental endothelial dysfunction plays an important role in plaque progression and vulnerability. The present study suggests that the endogenous endothelin pathway may play an important role in the pathogenesis and potentially as a therapeutic target in patients with early coronary atherosclerosis.
Supplementary Material
Flow chart of random assignment to treatment, completion of the trial, and reasons for not completing it.
Acknowledgments
Sources of Funding
The study was supported by grants from the NIH: NIH K24 HL-69840, NIH R01 HL-63911, HL-77131, HL 92954, HL 085307, DK 73608, DK 77013 and Mayo Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 2.Hasdai D, Gibbons RJ, Holmes DR, Jr., et al. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–3395. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 3.Zeiher AM, Krause T, Schachinger V, et al. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circulation. 1995;91:2345–2352. doi: 10.1161/01.cir.91.9.2345. [DOI] [PubMed] [Google Scholar]
- 4.Lavi S, Bae JH, Rihal CS, et al. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart. 2009;95:1525–1530. doi: 10.1136/hrt.2009.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 6.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 7.Lerman A, Edwards BS, Hallett JW, et al. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991;325:997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- 8.Lerman A, Holmes DR, Jr., Bell MR, et al. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation. 1995;92:2426–2431. doi: 10.1161/01.cir.92.9.2426. [DOI] [PubMed] [Google Scholar]
- 9.Best PJ, McKenna CJ, Hasdai D, et al. Chronic endothelin receptor antagonism preserves coronary endothelial function in experimental hypercholesterolemia. Circulation. 1999;99:1747–1752. doi: 10.1161/01.cir.99.13.1747. [DOI] [PubMed] [Google Scholar]
- 10.Hasdai D, Lerman A. The atherogenic potential of endothelin. Coron Artery Dis. 1995;6:901–904. [PubMed] [Google Scholar]
- 11.Kowala MC, Rose PM, Stein PD, et al. Selective blockade of the endothelin subtype A receptor decreases early atherosclerosis in hamsters fed cholesterol. Am J Pathol. 1995;146:819–826. [PMC free article] [PubMed] [Google Scholar]
- 12.Reriani M, Raichlin E, Prasad A, et al. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation. 122:958–966. doi: 10.1161/CIRCULATIONAHA.110.967406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raichlin E, Prasad A, Mathew V, et al. Efficacy and safety of atrasentan in patients with cardiovascular risk and early atherosclerosis. Hypertension. 2008;52:522–528. doi: 10.1161/HYPERTENSIONAHA.108.113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crow RS, Prineas RJ, Jacobs DR, Jr., et al. A new epidemiologic classification system for interim myocardial infarction from serial electrocardiographic changes Unstable angina. A classification. Am J Cardiol. 1989;64:454–461. doi: 10.1016/0002-9149(89)90420-7. [DOI] [PubMed] [Google Scholar]
- 15.Braunwald E. Unstable angina. A classification. Circulation. 1989;80:410–414. doi: 10.1161/01.cir.80.2.410. [DOI] [PubMed] [Google Scholar]
- 16.Doucette JW, Corl PD, Payne HM, et al. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85:1899–1911. doi: 10.1161/01.cir.85.5.1899. [DOI] [PubMed] [Google Scholar]
- 17.Ofili EO, Labovitz AJ, Kern MJ. Coronary flow velocity dynamics in normal and diseased arteries. Am J Cardiol. 1993;71:3D–9D. doi: 10.1016/0002-9149(93)90128-y. [DOI] [PubMed] [Google Scholar]
- 18.Quyyumi AA, Dakak N, Andrews NP, et al. Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995;92:320–326. doi: 10.1161/01.cir.92.3.320. [DOI] [PubMed] [Google Scholar]
- 19.Al Suwaidi J, Higano ST, Holmes DR, Jr., et al. Measuring maximal percent area stenosis poststent placement with intracoronary Doppler and the continuity equation and correlation with intracoronary ultrasound and angiography. Am J Cardiol. 1999;84:650–654. doi: 10.1016/s0002-9149(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 20.Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 21.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 22.Crauwels HM, Van Hove CE, Holvoet P, et al. Plaque-associated endothelial dysfunction in apolipoprotein E-deficient mice on a regular diet. Effect of human apolipoprotein AI. Cardiovasc Res. 2003;59:189–199. doi: 10.1016/s0008-6363(03)00353-5. [DOI] [PubMed] [Google Scholar]
- 23.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 24.Reriani MK, Lerman LO, Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomark Med. 4:351–360. doi: 10.2217/bmm.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson TJ. Assessment and treatment of endothelial dysfunction in humans. J Am Coll Cardiol. 1999;34:631–638. doi: 10.1016/s0735-1097(99)00259-4. [DOI] [PubMed] [Google Scholar]
- 26.Halcox JP, Nour KR, Zalos G, Quyyumi AA. Coronary vasodilation and improvement in endothelial dysfunction with endothelin ET(A) receptor blockade. Circ Res. 2001;89:969–976. doi: 10.1161/hh2301.100980. [DOI] [PubMed] [Google Scholar]
- 27.MacCarthy PA, Pegge NC, Prendergast BD, et al. The physiological role of endogenous endothelin in the regulation of human coronary vasomotor tone. J Am Coll Cardiol. 2001;37:137–143. doi: 10.1016/s0735-1097(00)01042-1. [DOI] [PubMed] [Google Scholar]
- 28.Kyriakides ZS, Kremastinos DT, Bofilis E, et al. Endogenous endothelin maintains coronary artery tone by endothelin type A receptor stimulation in patients undergoing coronary arteriography. Heart. 2000;84:176–182. doi: 10.1136/heart.84.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li JS, Lariviere R, Schiffrin EL. Effect of a nonselective endothelin antagonist on vascular remodeling in deoxycorticosterone acetate-salt hypertensive rats. Evidence for a role of endothelin in vascular hypertrophy. Hypertension. 1994;24:183–188. doi: 10.1161/01.hyp.24.2.183. [DOI] [PubMed] [Google Scholar]
- 30.Dashwood MR, Barker SG, Muddle JR, et al. [125I]-endothelin-1 binding to vasa vasorum and regions of neovascularization in human and porcine blood vessels: a possible role for endothelin in intimal hyperplasia and atherosclerosis. J Cardiovasc Pharmacol. 1993;22(Suppl 8):S343–S347. doi: 10.1097/00005344-199322008-00090. [DOI] [PubMed] [Google Scholar]
- 31.Mathew V, Hasdai D, Lerman A. The role of endothelin in coronary atherosclerosis. Mayo Clin Proc. 1996;71:769–777. doi: 10.1016/S0025-6196(11)64842-8. [DOI] [PubMed] [Google Scholar]
- 32.Kiowski W, Linder L, Stoschitzky K, et al. Diminished vascular response to inhibition of endothelium-derived nitric oxide and enhanced vasoconstriction to exogenously administered endothelin-1 in clinically healthy smokers. Circulation. 1994;90:27–34. doi: 10.1161/01.cir.90.1.27. [DOI] [PubMed] [Google Scholar]
- 33.Mencarelli M, Pecorelli A, Carbotti P, et al. Endothelin receptor A expression in human inflammatory cells. Regul Pept. 2009;158:1–5. doi: 10.1016/j.regpep.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Lin CY, Lee TS, Chen CC, et al. Endothelin-1 exacerbates lipid accumulation by increasing the protein degradation of the ATP-binding cassette transporter G1 in macrophages. J Cell Physiol. 2010 doi: 10.1002/jcp.22556. [DOI] [PubMed] [Google Scholar]
- 35.Babaei S, Picard P, Ravandi A, et al. Blockade of endothelin receptors markedly reduces atherosclerosis in LDL receptor deficient mice: role of endothelin in macrophage foam cell formation. Cardiovasc Res. 2000;48:158–167. doi: 10.1016/s0008-6363(00)00169-3. [DOI] [PubMed] [Google Scholar]
- 36.Browatzki M, Pfeiffer CA, Schmidt J, Kranzhofer R. Endothelin-1 induces CD40 but not IL-6 in human monocytes via the proinflammatory transcription factor NF-kappaB. Eur J Med Res. 2005;10:197–201. [PubMed] [Google Scholar]
- 37.Morand-Contant M, Anand-Srivastava MB, Couture R. Kinin B1 receptor upregulation by angiotensin II and endothelin-1 in rat vascular smooth muscle cells: receptors and mechanisms. Am J Physiol Heart Circ Physiol. 299:H1625–H1632. doi: 10.1152/ajpheart.00735.2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow chart of random assignment to treatment, completion of the trial, and reasons for not completing it.