Abstract
The hematopoietic system is dynamic during development and in adulthood, undergoing countless spatial and temporal transitions during the course of one’s life. Microenvironmental cues in the many unique hematopoietic niches differ, characterized by distinct soluble molecules, membrane-bound factors, and biophysical features that meet the changing needs of the blood system. Research from the last decade has revealed the importance of substrate elasticity and biomechanical force in determination of stem cell fate. Our understanding of the role of these factors in hematopoiesis is still relatively poor; however, the developmental origin of blood cells from the endothelium promts a model for comparison. Many endothelial mechanical sensors and second messenger systems may also determine hematopoietic stem cell fate, self renewal, and homing behaviors. Further, the intimate contact of hematopoietic cells with mechanosensitive cell types, including osteoblasts, endothelial cells, mesenchymal stem cells, and pericytes, places them in close proximity to paracrine signaling downstream of mechanical signals. The objective of this review is to present an overview of the sensors and intracellular signaling pathways activated by mechanical cues and highlight the role of mechanotransductive pathways in hematopoiesis.
Keywords: hematopoietic stem cells, hemogenic endothelium, biomechanical force, shear stress, mechanotransduction, cellular microenvironment
INTRODUCTION
An extensive body of work has contributed to our appreciation for cellular plasticity in the last two decades. In the context of development and cancer biology, we have learned that a cell can differentiate, dedifferentiate, and even transdifferentiate, resulting in entirely new functions and cellular identities. These capacities are defined by intrinsic properties inherited by developmental origins and by signals from the microenvironment. There is increasing need for a better understanding of the private in vivo lives of stem cells (Sanchez Alvarado, 2008). In the last decade, it has become apparent that regulatory pathways which define cellular behavior such as cell cycle entry and differentiation can have vastly distinct roles in vitro and in stem and progenitor cells of the developing embryo (Chen et al., 2009a; Chong et al., 2009; Wenzel et al., 2011). This highlights the need to study the native stem cell environment and to accurately model niche components that determine stem cell potential.
The blood system, and arguably the skin, gut, and neural system, have contributed the most to our understanding of mammalian stem cell niches, in terms of the many complex cellular interactions and regulatory pathways that determine quiescence, self-renewal, and differentiation. There is a diverse array of specialized cell types that form the niche. Many of these are terminally differentiated and some remain in a relatively plastic state, while contributing essential factors to promote development and/or maintain homeostasis. Over 300 years ago, researchers were fascinated by the fundamentals of cellular mechanics (Pelling and Horton, 2008), but only recently has the field of stem cell biology acknowledged the roles of intracellular tension and extracellular biophysical factors as critical determinants of differentiation (Engler et al., 2006). Important physical properties of the extracellular matrix (ECM) include elasticity, nanotopography, and spatial distribution of adhesive substrates and ligands. Biomechanical forces include mechanical loading or pressure, friction, and stretching. Together, these mechanical cues determine the activity of critical regulatory pathways that modulate differentiation, cell division, cell survival, and motility. The physical environment impacts cell-intrinsic signaling, as well as paracrine signaling that can dictate cellular potential and behavior of nearby neighbors.
Advances in chemical engineering and materials science have broadened our biological understanding of stem cell biology through the use of lithography, including soft lithography and capillary force lithography, microcontact printing, microfluidics, and microassembly. For review of advances in engineering and materials-based approaches, the reader is referred elsewhere (Jakab et al., 2010; Kim et al., 2012; McNamara et al., 2010; Whitesides et al., 2001). With further exploration, this interdisciplinary field has potential to impact not only classically defined areas of regenerative medicine, such as reconstruction of bone and cartilage, but also other cellular therapies used for treatment of disorders and injuries complicated by dysregulation of the immune system, bone marrow failure syndromes, autoimmunity, autoinflammation, and hematological malignancy. In this review, we describe recent advances in our understanding of how biophysical cues and mechanosensory pathways determine blood development and homeostasis.
HEMATOPOIETIC ONTOGENY
The first clues to hematopoietic ontogeny were reported at the turn of the century in several vertebrate species, including man, mouse, and chick (Dantschakoff, 1907; Emmel, 1916; Jordan, 1916; Maximov, 1909; Minot, 1912; Sabin, 1917; Stricht, 1899). In the past 30 years, our understanding of the developmental origins of blood has matured, aided by the study of humans, as well as diverse model systems such as the quail and chicken, zebrafish, mouse, fly, and frog (Ciau-Uitz et al., 2010; Dieterlen-Lievre et al., 2006; Evans et al., 2003; Medvinsky et al., 2011; Orkin and Zon, 2008; Tavian and Peault, 2005). In particular, the accessibility of the chick embryo has permitted interspecies transplantation that has enabled tracing of the regions of the early embryo that contribute to the blood system. Zebrafish have allowed for transparent viewing of blood emergence and migration and have proved a powerful tool for large-scale pharmacological identification of critical regulatory pathways in hematopoiesis. Importantly, the mouse has provided genetic tractability and embryonic stem cell based in vitro modeling of blood development.
During embryogenesis, the first hematopoietic cells to populate the vasculature emerge from yolk sac blood islands. “Primitive” blood consists primarily of megakaryocytes, macrophage progenitors, and nucleated erythrocytes that express embryonic and fetal globins (ε and (βH1) and do not contribute to the adult blood system (Silver and Palis, 1997). By embryonic day 9.5 (E9.5), and even E8.0, cells arise with greater multipotency and, when delivered pre- or peri-natally, can contribute to adult hematopoiesis (Weissman et al., 1978; Yoder et al., 1997). The identity of these cells can be characterized by their requirement for the Runxl/AML1/Cbfa2 master regulator of hematopoiesis (Cai et al., 2000; North et al., 1999; Okuda et al., 1996; Samokhvalov et al., 2007; Tanaka et al., 2012). Indeed, lineage tracing experiments have revealed that cells derived from the E6.5 to E7.5 extraembryonic mesoderm of the yolk sac are a source for intraembryonic sites of hematopoiesis that later account for the majority of the downstream descendants in the adult blood system (Samokhvalov et al., 2007; Tanaka et al., 2012). Because of their ability to contribute to adult hematopoiesis via a neonatal intermediate, yolk sac derived cells could be considered akin to pre-hematopoietic stem cells (pre-HSCs) most recently defined as the precursor to the adult-type definitive HSC that arises within the embryo proper (Medvinsky et al., 2011). It is important to note, however, that the origin(s) of the hematopoietic stem cell is still strongly debated and continues to polarize the field of hematopoiesis.
Definitive hematopoietic stem cells (HSCs) replenish the blood supply throughout life, and their precursors are known to arise during embryogenesis at E10.5 in the region of the embryo containing the dorsal aorta, gonads, and mesonephroi (AGM) (Medvinsky and Dzierzak, 1996; Muller et al., 1994). Evidence from both zebrafish and mouse now implicate a subpopulation of endothelial cells with hemogenic potential as a de novo source of HSCs that contribute to life-long hematopoiesis (Bertrand et al., 2010; Boisset et al., 2010; Chen et al., 2009b; Eilken et al., 2009; Kissa and Herbomel, 2010; Lam et al., 2010; Lancrin et al., 2009; Zovein et al., 2008). Indeed, endothelial cells and nascent hematopoietic cells share several of the same cell surface markers, and both are derived from an angioblast population that expresses Brachyury and Flk-1 (Huber et al., 2004; Kabrun et al., 1997; Millauer et al., 1993; Shalaby et al., 1997; Shalaby et al., 1995; Yamaguchi et al., 1993). Hemogenic endothelial cells line the dorsal aorta and are enriched near the vitelline artery (Yokomizo and Dzierzak, 2010). In the zebrafish, nascent hematopoietic cells emerge from the lumen of the vessel in a carefully choreographed exit that maintains vessel integrity while allowing entry into the blood stream (Kissa and Herbomel, 2010). On the wall of the dorsal aorta in mice and chick, intra-aortic clusters of varying sizes extend into the blood stream. Labeling of these hematopoietic clusters shows gradual transition from endothelial phenotypes on the vessel wall to hematopoietic at the distal edges of the cellular aggregate (Jaffredo et al., 1998; Yokomizo and Dzierzak, 2010). This endothelial to hematopoietic transition is dependent upon the expression of Runxl, which is thought to mediate suppression of endothelial genes, including VE-Cadherin/CD144, CD31, and Flk-1/KDR/ VEGFR2, and upregulation of hematopoietic genes, CD41, CD45, Gfi1B, Ikaros, and PU.1 (Iacovino et al., 2011; Mizuochi et al., 2012). Late stage E11.5 AGM regions contain peak numbers of definitive HSCs, as measured by careful assessment of somite stage and transplantation assays for determination of long-term, transferable multipotency of blood lineages (Taylor et al., 2010). Beginning around E11.0, hematopoietic stem and progenitor cells (HSPCs) emigrate from the AGM to the fetal liver for expansion and maturation (Morrison et al., 1995). The placenta is also a significant reservoir for HSCs and blood progenitors around this time (Gekas et al., 2005; Ottersbach and Dzierzak, 2005). After E14.5, HSPC egress begins for colonization of the fetal spleen, thymus, and bone (Ciriza et al., 2013; Godin and Cumano, 2002; Morrison et al., 1995; Samokhvalov et al., 2007). The bone marrow becomes the primary site of hematopoiesis in adult life, and provides a niche suitable for HSC homeostasis and progenitor maturation.
BIOMECHANICAL FORCES IN THE HEMATOPOIETIC NICHE
Blood flow is a critical determinant of arterial lineage specification, vascular remodeling, and hematopoiesis, and begins at E8.25 in the murine embryo (Adamo et al., 2009; Chouinard-Pelletier et al., 2013; Lucitti et al., 2007; North et al., 2009). Three types of hemodynamic forces influence vascular and hematopoietic cells, including shear stress [friction), circumferential strain [stretching], and hydrostatic pressure. We have found that shear stress, specifically, has important implications for hematopoietic signaling (Adamo et al., 2009) and changes during development as a function of blood viscosity, cardiac output (i.e. blood velocity), and vessel dimensions/location. For this reason, entry of erythroblasts into the early circulation has been found to significantly increase blood viscosity and consequently shear stress with important implications for angiogenesis (Chouinard-Pelletier et al., 2013). Blood flow in the murine yolk sac vasculature has been measured at peak values of 1 to 2 dyne/cm2 (highest in the yolk sac at 16 somites) (Chouinard-Pelletier et al., 2013) and in the E10.5 dorsal aorta at 5 dyne/cm2 (Adamo et al., 2009). The fetal liver is also highly vascularized, but patterns of shear stress in this organ at the time of HSPC colonization are poorly understood. Migrating HSPCs survey microsites in the liver prior to settling, akin to the process of rolling and arrest observed in mature leukocytes. Later in development, long after blood production has shifted to the bone marrow, some areas of the mouse aorta can experience instantaneous wall shear stress that exceeds 600 dyne/cm2, an order of magnitude higher than values found in the human (Suo et al., 2007).
Bone provides a number of distinct mechanical environments associated with sinusoids, blood vessels, and mineralized bone matrix. Several of these regions of the bone are complex but well characterized niches comprised of osteocytes, osteoblasts, osteoclasts, endothelial cells, mesenchymal stromal cells, pericytes, nerve cells, as well as mature blood lineages such as macrophages (Bianco, 2011; Ehninger and Trumpp, 2011). HSPCs can experience a wide array of elasticities in the done marrow, notably the range covering adipose (Esubstrate<1KPa, cell memdranes (Esubstrate=1–3KPa), and collagenous bone (Esubstrate=100KPa) (Discher et al., 2009; Engler et al., 2006). Bone is a vascularized organ and must provide passage in and out of the marrow to circulating endothelial progenitors, hematopoietic cells, nutrients, and signaling molecules. Fluid flow is expected to be slow in the vasculature that supplies blood to the medullary cavity of the bone, but fluid movement in the lacunar-canalicular network of the bone can expose osteocytic processes to shear stresses of 6–50 dyne/cm2 associated with mechanical loading and ambulation (Piekarski and Munro, 1977; Price et al., 2011; Weinbaum et al., 1994; Zeng et al., 1994). Not only does this have important implications for mechanical stimulation, this fluid flow around osteocytes is necessary for delivery of nutrients and other signaling molecules, including glucose, lactic acid, nitric oxide, estradiol, testosterone, prostaglandin, adenosine-5’-triphosphate (ATP), vitamin D, and corticosteroids (Price et al., 2011). This suggests that the hematopoietic cells in the endosteum would not be directly exposed to fluid forces of these magnitudes, but could be impacted by paracrine signaling downstream of osteocyte mechanotransduction and biochemical signaling. Either directly or indirectly, endothelial cells and pericytes are also likely to interface with fluid forces and provide signals to HSPCs that tightly regulate cell cycling and egress.
MECHANOSENSORS & SIGNAL TRANSDUCTION
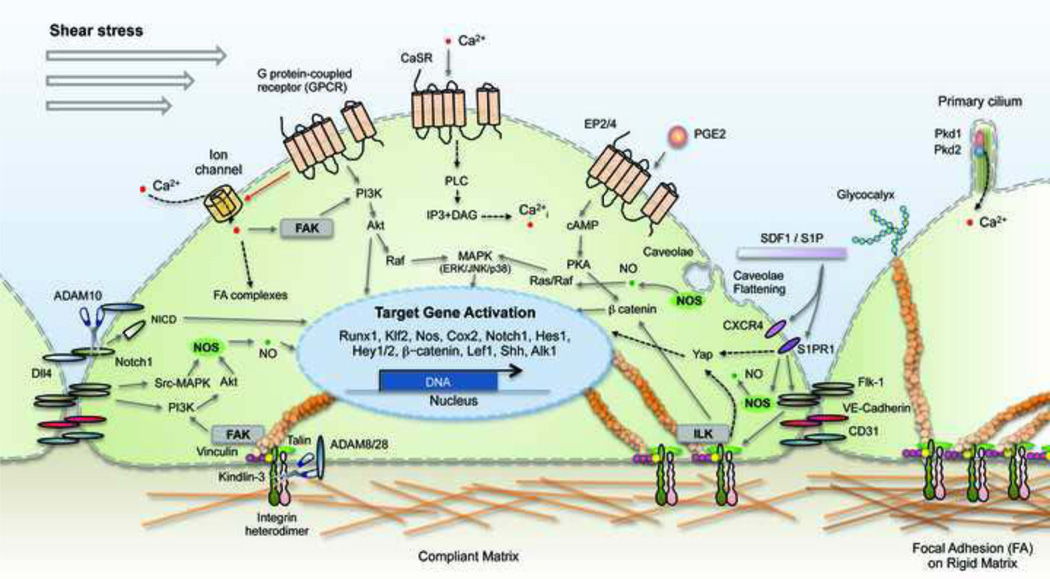
Little is known regarding the mechanisms by which biophysical cues in the microenvironment may affect cellular functions or indeed how the sensing apparatus and transduction of mechanical signals operates in HSCs. The most likely mechanism by which cells monitor substrate elasticity involves two-way interactions with the actin-myosin cytoskeleton, coupled through membrane receptors such as integrins (Even-Ram et al., 2006). But, as outlined in Table 1, there are numerous cellular components and mechanosensitive molecules that transmit mechanical information into the cell and to the nucleus, including integrins (Chen et al., 1999; Wang et al., 1993), adherens junction proteins (Shay-Salit et al., 2002), G protein-coupled receptors (GPCR) (Chachisvilis et al., 2006; Gudi et al., 1996; Lehoux et al., 2006; Makino et al., 2006), ion channels (Brayden et al., 2008; Olesen et al., 1988; Owsianik et al., 2006), glycocalyx (Weinbaum et al., 2003), myosin motors, cytoskeletal filaments, caveolae (Park et al., 2000; Park et al., 1998; Rizzo et al., 1998b) and other signaling molecules. As depicted in Figure 1, mechanoreceptors are organized on and throughout the cell according to cell type and mechanical environment. In endothelial cells, for example, several membrane-associated structures reside on the apical (luminal) surface, including ion channels (Barakat, 2001; Olesen et al., 1988; Schwarz et al., 1992; Traub et al., 1999), caveolae (Park et al., 2000; Park et al., 1998), and G proteins (Gudi et al., 1998). By contrast, a number of other proteins and molecules are enriched on the basolateral surfaces and throughout the cells such as integrins (Gloe et al., 2002), cell-cell adhesion molecules (Fujiwara et al., 2001), adherens junctions (Shay-Salit et al., 2002), and the cytoskeleton. This arrangement of sensors provides polarity and impacts cell division, shape, function, and motility.
Table 1.
General Mechanosensors and Mechanosensitive Signaling Pathways
| Mechanosensors | Functions in hematopoiesis |
Interactions | Downstream | References |
|---|---|---|---|---|
| Cell Adhesion Molecules | ||||
| α2β1 Integrin | Homing and homeostasis | Extracellular matrix | p38 MAPK | (Klekotka et al., 2001) |
| α4β1(VLA-4) | Homeostasis | Fibronectin (CS-1) | (Craddock et al., 1997; Papayannopoulou et al., 1995; Potocnik et al., 2000; Williams et al., 1991) | |
| β1 Integrin | Migration | ILK-GSK3β, Wnt, Notch | (Hirsch et al., 1996; Potocnik et al., 2000; Rallis et al., 2010) | |
| VE-cadherin | Proliferation and maintenance | (Fraser et al., 2002; Oberlin et al., 2010) | ||
| N-cadherin | Differentiation | (Hosokawa et al., 2010; Puch et al., 2001) | ||
| Flk- 1/KDR/VEGF2 | Endothelial and hematoendothelial specification | Cell adhesion Shear stress | Src-MAPK, PI3K-Akt eNOS, integrins, small GTPase | (Jalali et al., 1998; Jin et al., 2003; Tzima et al., 2005) |
| G-protein coupled receptors (GPCR) | ||||
| Gαi2 | Erythropoietin modulation | Fluid shear stress | Ca2+ influx, ERK, JNK | (Jo et al., 1997; Miller et al., 1996) |
| EP2 and EP4 Gαs | HSPC self renewal and expansion | PGE2 | cAMP, Wnt/β- catenin, intracellular Ca2+, NOS | (Goessling et al., 2009; Reich and Frangos, 1991; Smalt et al., 1997; Williams et al., 1994) |
| S1PR/EDG1 | Chemoattractant for HSCs and progenitors | Fluid shear stress, Flk- 1/KDR/VEGFR2, protein kinase C | eNOS, Rac GTPase- associated protein CrkII, YAP | (Hughes et al., 2005; Igarashi et al., 2003; Takada et al., 1997; Tanimoto et al., 2002; Venkataraman et al., 2008) |
| CXCR4 | HSC homing and engraftment to the marrow | SDF1-α | Rac1 | (del Pozo et al., 2004; Gu et al., 2003; Ratajczak et al., 2010) |
| CaSR | Cell adhesion and lodgement | Ca2+, Integrins | Integrins | (Adams et al., 2006; Lam et al., 2011; Tharmalingam et al., 2011) |
| Ion channels | ||||
| TRPV4 | Membrane stretching, Polycystin-2 | Ca2+, NO, endothelial reorientation | (Köttgen et al., 2008; Mendoza et al., 2010; Thodeti et al., 2009) | |
| PKD1, PKD2 | Fluid shear stress | Ca2+, NO, HH signaling | (Abou Alaiwi et al., 2009; Hierck et al., 2008; Nauli et al., 2008) | |
| A Disintegrin | ||||
| And Metalloprotease (ADAM) Family | ||||
| ADAM28 | Lymphocyte emigration | α4β1 integrin | Degradation of ECM | (Weber and Saftig, 2012) |
| ADAM8 | Releasing first erythrocytes into the blood stream | Fluid shear stress | Degradation of ECM | (Fourie et al., 2003; Iida et al., 2010) |
| ADAM10 | HSC specification | Notch, Fluid shear stress | NICD | (Gibb et al., 2010; Gibb et al., 2011; Yoda et al., 2011) |
| Nitric Oxide (NO) signaling | ||||
| NOS | Regulation of vascular tone, angiogenesis, endothelial migration and hematopoiesis | Fluid shear stress, Klf2a, PI3K, Notch, Wnt | NO | (Adamo et al., 2009; Davies, 1995; Lucitti et al., 2007; Michurina et al., 2004; North et al., 2009) |
| Other sensors | ||||
| Glycocalyx | HSPS maintenance, differentiation, homing, mobilization | Fluid shear stress | NO, reorganization of the cytoskeleton | (Florian et al., 2003; Hemmorantaa et al., 2007; Thi et al., 2004) |
| Caveolae | Integrins, laminar shear stress | Ca 2+, NO, Ras/Raf/ERK1/2 | (Drab et al., 2001; Rizzo et al., 1998; Wary et al., 1998) | |
| FAK | Hematopoietic lodgment and lineage development | Fluid shear stress | Akt, eNOS | (Glodek et al., 2007; Koshida et al., 2005) |
Abbreviations: Flk/KDR/VEGF2 (Fetal liver kinase/Kinase insert domain receptor/Vascular endothelial growth factor), S1PR/EDG1 (Sphingosine 1-phosphate receptor), CXCR4 (chemokine C-X-C motif receptor), CaSR (Calcium sensing receptor), TRPV4 (Transient receptor potential Vallinoid Type 4), PKD (Polycystin), NOS (Nitric oxide synthase), FAK (Focal adhesion kinase)
Figure 1. Putative mechanosensors of the hemogenic endothelium.
Biomechanical stimuli such as shear stress and substrate rigidity can be sensed by integrins and other cell adhesion molecules, ADAMs, GPCR, ion channels, caveolae, glycocalyx, and primary cilia. Mechanical cues detected by these sensors are converted into biochemical signals that regulate self renewal, differentiation, and homing behaviors. Intracellular signaling mechanisms demonstrated to lie downstream of biophysical cues in hematopoietic or hemogenic endothelial cells are represented by solid lines. Pathways found in other cell types are represented by dashed lines. ADAM, a disintegrin and metalloprotease; CaSR, Calcium Sensing Receptor; DAG, diacylglycerol; FA, focal adhesion; FAK, focal adhesion kinase;Flk-1, fetal liver kinase-1; GPCR, G protein-coupled receptor; ILK, integrin linked kinase; IP3, inositol triphosphate; NICD, Notch intracellular domain; NO, nitric oxide; NOS, nitric oxide synthase; PGE2, prostaglandin E2; Pkd1, polycystin-1; Pkd2, polycystin-2; PKA/ PKC, protein kinase A/ C; PLC, phospholipase C; SIP, sphingosine-1 phosphate; SDF1, stromal derived factor 1.
Cell Adhesion Molecules
Cell adhesion molecules play central roles in regulating vascular development, hematopoiesis, hemostasis, and immune and inflammatory response (Ye et al., 2012). The stem cell microenvironment is particularly dependent upon the ligand-binding properties of supportive niche cells to control quiescence and trafficking of stem and progenitor cells (Chen et al., 2013; Ehninger and Trumpp, 2011). In addition to mediating attachment to ECM and/or neighboring cells, some adhesion molecules transmit information bidirectionally across the cell membrane. Some of these include integrins, cadherins, selectins, connexins, CD44, sialomucins, intercellular adhesion molecule 1 (Icaml/CD54), and vascular cell adhesion molecule 1 (Vcaml/CD106) (Imai et al., 1999; Osawa et al., 1996; Simmons et al., 2001; Uchida et al., 1998).
Early support for the notion that anchorage sites might be important for HSC homeostasis came from studies of integrin α4(β1 (VLA-4) (Anderson et al., 1990; Martin et al., 1990; Williams et al., 1991; Zsebo et al., 1990). Integrins are heterodirmeric glycoproteins that consist of α and β peptide chains, and each subunit contains a large extracellular domain, a single-pass transmembrane domain, and a short cytoplasmic tail. Antibodies against the β1 subunit were shown to inhibit reconstitution activity from bone marrow and to efficiently mobilize CD34+ hematopoietic progenitors into the bloodstream (Papayannopoulou and Nakamoto, 1993). Since these first reports, it has become evident that α4β1 integrin is indispensible for definitive hematopoiesis both during embryogenesis and in adulthood, due almost exclusively to its role in adhesion to supportive stroma. It is expressed highly on HSCs and progenitors, and ablation of (31 integrin function genetically or by neutralizing antibodies renders HSPCs unable to engraft the bone marrow or spleen (Craddock et al., 1997; Papayannopoulou et al., 1995; Potocnik et al., 2000; Williams et al., 1991). In embryogenesis, ItgB1-null hematopoietic progenitor cells are specified normally, accumulate in the peripheral blood, but fail to populate the fetal liver (Hirsch et al., 1996; Potocnik et al., 2000). This defect has been characterized as an inability to anchor to the endosteal surface and less so a problem of finding the medullary space, as measured by optical trap and in vivo fluorescent imaging of the adult femur (Askenasy et al., 2003). Presumably with similar functions, the α4, α6 and α9 integrins have also been found to be critical for HSC-microenvironment interactions (Grassinger et al., 2009; Qian et al., 2006; Schreiber et al., 2009). Notably, hemogenic endothelium is specifically enriched with a4 integrins (Ogawa et al., 1999), and HSPCs express high levels of α4, α6, α7, α9 and β1 (Grassinger et al., 2009; Potocnik et al., 2000; Schreiber et al., 2009; Voura et al., 1997). Genetic pathways downstream of integrin signaling include Ras/MAPK, phosphatidylinositide 3-kinase (PI3K)/Akt, RhoA/ROCK, Wnt/β-catenin, TGF-β (Sun et al., 2012), but those most relevant to hematopoietic development will depend upon the integrin heterodimers present on the cell surface and the composition of the ECM in the niche. Whereas integrins can bind cell-surface molecules and components of the ECM, such as fibronectin, collagen, and laminin, and provide the cell with information about ECM rigidity, nanotopography, microgeometry, and mechanical stress (Barczyk et al., 2010; Hynes, 2002), cadherins mediate cell-cell contact.
Cadherins were originally identified as cell surface glycoproteins responsible for Ca2+-dependent hemophilic cell-cell adhesion during mouse embryo and chick development (Yoshida and Takeich, 1982) and their function extends to multiple aspects of tissue morphogenesis, including cell recognition and sorting, boundary formation and maintenance, coordinated cell movements, and the induction and maintenance of structural and functional cell and tissue polarity in the three decades since their discovery. In particular, VE-cadherin is important in the hematopoietic population. Fraser et al. were the first to show that the VE-cadherin+ population from different stages of embryonic development in mice generated more hematopoietic cells ex vivo relative to the Flk-1+ VE-cadherin- population(Fraser et al., 2002). Moreover, VE-cadherin expressing cells in the human embryonic liver showed outstanding self-renewal, proliferation and differentiation potential, suggesting that VE-cadherin marks a primitive HSC population (Oberlin et al., 2010). The function of N-cadherin in hematopoiesis is still controversial. N-cadherin deficiency in mice causes no detectable defects in HSC maintenance or hematopoiesis(Kiel et al., 2009) but does contribute to the long-term engraftment of HSCs(Hosokawa et al., 2010). Further, disruption of N-cadherin with blocking antibodies significantly diminished colony formation from CD34+ progenitor cells, implicating N-cadherin in the differentiation program of early hematopoietic progenitors (Puch et al., 2001). Another classical cadherin, E-cadherin also has been shown to function during normal erythropoiesis. Inhibition of E-cadherin with anti-human E-cadherin antibodies decreased the differentiation of erythropoietic progenitors into erythroblast, suggesting that E-cadherin is involved in the differentiation and maturation of the erythroid lineage(Armeanu et al., 1995). However, its role during myeloid and lymphoid differentiation has not been studied. Corn et al. reported that E-cadherin was expressed in mature PBMCs and normal lymphoblastoid cell line. These suggest that E-cadherin is involved in multi-lineage hematopoiesis. Moreover, they found E-cadherin was aberrantly methylated in leukemia cell lines(Corn, 2000), indicating that the epigenetic regulation of E-cadherin is important during leukemogenesis.
A Disintegrin And Metalloprotease (ADAM) Family
The ADAM family is a class of proteases that cleave and/or modulate binding affinity of adhesion molecules, chemokines, cytokines, growth factors, and transmembrane receptors (Weber and Saftig, 2012). The proteolytic activity of ADAMs can be triggered by factors such as mechanical and osmotic stress, G protein-coupled receptors (GPCRs), protein kinase C, intracellular calcium, serum factors and growth factors (Huovila et al., 2005). Several members of the ADAM family have been implicated in regulation of cellular adhesion of blood cells. For example, ADAM28 binds α4βl integrin and can modulate lymphocyte emigration (Weber and Saftig, 2012). Another family member, ADAM8, is expressed primarily in cells of the immune system, and was demonstrated to be essential for the release of primitive erythrocytes into the blood stream of the zebrafish embryo (Fourie et al., 2003; Iida et al., 2010). Prior to emergence of primitive erythrocytes, “bloodless” plasma flows through the vasculature. Newly specified erythrocytes first aggregate in and adhere to the lumen of the dorsal aorta and posterior cardinal vein. A unified and sudden release of erythrocytes from the vessel walls occurs at 27 hours post-fertilization, allowing the blood cells to enter circulation (Iida et al., 2010). Inactivation of ADAM8 or use of metalloprotease inhibitors was found to cause erythroid retention on the vessel walls, stagnation, and presumably subsequent cell death. The trigger for this synchronized detachment is unclear, but the authors speculate that plasma flow (i.e., shear stress) could play a role, as tnnt2 morphants which lack a heartbeat exhibited a similar defect. Further, they suggest the substrate for ADAM8 is likely to be an adhesion molecule, such as CD41 or a related integrin. Alternatively, degradation of the ECM may serve to either remove the matrix as a barrier to cell motility (Werb et al., 1999) or result in the release of biologically active fragments of ECM molecules that regulate motility, proliferation, and cell survival (Larsen et al., 2006). It is interesting to speculate that a similar event could occur later in development during definitive hematopoiesis as HSPCs leave the aorta for the fetal liver and could coincide with downregulation of endothelial-type cell adhesion molecules such as VE-Cadherin/CD144 and CD31 (Mizuochi et al., 2012; Yokomizo and Dzierzak, 2010).
There is also evidence to suggest that ADAMs play a critical role in HSC specification (Gibb et al., 2010; Gibb et al., 2011; Yoda et al., 2011). ADAM10 is required for S2 cleavage of the Notch receptor extracellular domain. Only after removal of the extracellular domain can S3 cleavage occur by the γ-secretase complex, allowing the NICD to translocate to the nucleus and activate Notch target genes. It has also been suggested that ADAMs may be involved in ectodomain shedding of membrane-bound Delta ligand. Interestingly, blood flow is required for activation of Notch in the vasculature. One potential mechanism for Notch activation is direct modulation of ADAM10 by shear stress, though this idea remains untested. Alternatively, the shearing forces caused by blood flow may amplify mechanical forces exerted by ligation to clustered or immobilized Delta ligand thereby exposing the Notch cleavage site to ADAM10 (Gordon et al., 2007). In culture systems, immobilization of Delta on culture substrates or beads is necessary for downstream signaling (Varnum-Finney et al., 2000), T cell differentiation from HSCs (Taqvi et al., 2006), and expansion of hematopoietic cord blood progenitor cells (Delaney et al., 2005). This highlights the importance of spatial presentation of regulatory factors and may be indicative of a role for Notch1 in concert with ADAMs in detection of tension and cytoskeletal rearrangements that occur as a result of blood flow.
Nitric Oxide Signaling
Nitric oxide (NO) is an important endocrine and paracrine signaling molecule that plays a key role in the regulation of vascular tone, angiogenesis, endothelial migration, and hematopoiesis (Adamo et al., 2009; Davies, 1995; Lucitti et al., 2007; Michurina et al., 2004; North et al., 2009). Many studies have demonstrated that hemodynamic flow is an important biophysical cue that regulates nitric oxide synthase (NOS) activity and NO release from vascular endothelium (Fukumura et al., 2001; Ranjan et al., 1995; Uematsu et al., 1995). Indeed, pulsatile flow typical of a normal cardiac cycle has been shown to trigger rapid and sustained NO production (White and Frangos, 2007). There are three NOS genes in the mammalian genome, each of which has distinct modes of regulation and cell type specificities (Alderton et al., 2001). In the hematopoietic lineages, the neuronal isoform (nNOS) is detectable in neutrophils (Chen and Mehta, 1996; Forstermann et al., 1998; Greenberg et al., 1998; Wallerath et al., 1997), whereas the endothelial isoform (eNOS) is found in lymphocytes, megakaryocytes, and platelets (Chen and Mehta, 1996; Sase and Michel, 1995). Inducible NOS (iNOS) is expressed in megakaryocytes, eosinophils, and monocytes (Amin et al., 1995; Wallerath et al., 1997). Additionally, both the nNOS and eNOS isoforms are detectable at high levels in the fetal liver and in the bone marrow stroma (Krasnov et al., 2008).
NO production is coordinated by multiple mechanotransductive elements including ECM, cell-ECM adhesion, cell-cell adhesion complexes, cytoskeleton, and membrane components. Although fluid shear stress is strictly applied to luminal surfaces of endothelial cells, shear forces are transmitted to cell-cell junctions and the ECM. Several studies demonstrate that inhibition of FAK signaling by interfering with autophosphorylation at Tyr-397 impairs flow-induced phosphorylation of Akt (Ser473) and eNOS (Serll79). This not only points to the FA complex and FAK as critical components of the mechanotransduction machinery in endothelium but also reveals a potential mechanism of shear-induced regulation of NO signaling (Koshida et al., 2005). Other flow-based studies have suggested that phosphorylation of eNOS at Serll79 occurs via the PI3K-Akt-eNOS signaling pathway in human umbilical vein endothelial cells (HUVECs) and enhances enzyme activity in a Ca2+-independent manner (Dimmeler et al., 1999; Fisslthaler et al., 2000). Interestingly, shear stress appears to promote packaging of eNOS in caveolar vesicles, and this has been suggested to contribute to the regulated activity of NOS (Rizzo et al., 2003).
Accumulating evidence has shown that NO signaling plays a critical role in definitive hematopoiesis. In particular, NOS activity has been found to regulate quiescence of hematopoietic progentors in the bone marrow and spleen (Krasnov et al., 2008). Despite detectable levels of NOS isoforms in hematopoietic cells, these effects on cell cycling were found to be mediated exclusively by paracrine NO signaling from stromal cells of the hematopoietic niche (Krasnov et al., 2008). Importantly, nNOS is the primary isoform responsible for production of NO in mesenchymal stromal cells of the bone marrow and fetal liver. As HSCs are derived from hemogenic endothelial cells within the dorsal aorta, NO produced locally in endothelial cells could direct HSC emergence and control self renewal. We and others have shown that ectopic NO enhances hematopoiesis and can rescue defects caused by cardiac dysfunction, while inhibition of NO signaling results in significant loss of blood stem and progenitor cells (Adamo et al., 2009; North et al., 2009). NO appears to be absolutely required for maintenance of HSC in the AGM region and is activated downstream of Klf2a in zebrafish (Wang et al., 2011). This upregulation of the Klf2-NO axis is consistent with signaling through PI3K, but this remains to be definitively demonstrated. Further, there is evidence to suggest that NO could lie downstream of Notch and Wnt signaling, based upon the ability of NO donors and antagonists to control HSC numbers in Notch and Wnt mutants (North et al., 2009).
G Protein-Coupled Receptors (GPCR)
The GPCR superfamily is perhaps the largest and most diverse group of membrane proteins, encoded by over 800 genes in the human genome (Fredriksson et al., 2003). GPCRs share in common a seven-pass transmembrane domain that functions by binding a wide spectrum of extracellular signals, including photons, ions, small organic molecules, and proteins (Venkatakrishnan et al., 2013). GPCRs undergo conformational changes upon ligand binding, resulting in coordinated activation of cytosolic signaling networks and cellular response (Venkatakrishnan et al., 2013). GPCRs are diverse in structure and function but can be phylogenetically categorized into 5 primary receptor families: rhodopsin, glutamate, frizzled/taste2, adhesion, and secretin (Fredriksson et al., 2003). GPCRs of particular relevance to hematopoietic development can be found in the rhodopsin family (chemokine C-X-C motif receptor 4 (CXCR4) and sphingosine 1-phosphate receptor (S1PR1/EDG1)), glutamate family (calcium sensing receptor (CaSR)), and frizzled family (Wnt receptors Smoothened (SMOH), Frizzled 4 (FZD4), and FZD9) (Adams et al., 2006; Corrigan et al., 2009; Gering and Patient, 2005; Ranheim et al., 2005; Seitz et al., 2005; Zou et al., 1998). Functions of these receptors in hematopoiesis include regulation of lineage specification and homing.
A number of GPCR modulated by shear stress may protect the self renewal capacity of the HSPC as it prepares to travel to the fetal liver for expansion, such as the EP2 and EP4 Gαs protein-coupled receptors of prostaglandin E2 (PGE2). Stimulation by shear stress has been shown to induce prostaglandin synthesis in arterial endothelial cells (Roller et al., 1993). Importantly, in addition to roles in vasodilation, PGE2 has been found to inhibit macrophage maturation (Zaslona et al., 2012) and promote cell survival and expansion of HSPCs (Goessling et al., 2011; Goessling et al., 2009; North et al., 2007). Upon binding to PGE2, the EP2 and EP4 Gαs protein-coupled receptors trigger an increase in intracellular cAMP levels (Regan et al., 1994) and amplify Wnt signaling activity via PKA-mediated stabilization of β-catenin (Goessling et al., 2009). In human cells, and perhaps at sufficiently high doses in rhesus macaque, PGE2 may regulate HSC cell survival and proliferation by impacting major regulators of HSC development, including RUNX1, LM02, LY6A, HOXA9, CXCR4, FLT3, JAK1, CCR1, and CD8a, HHEX, JUNB, LCK, and TF (Goessling et al., 2011; Goessling et al., 2009). Interestingly, Wnt signaling can also be activated directly downstream of (β1-integrin in an ILK-GSK3b dependent pathway, which further leads to Notch activation (Rallis et al., 2010). Notably, osteoblasts collected from the adult bone marrow activate many of these same pathways that modulate HSPC self renewal and cycling in response to shear stress, including PGE2 production, intracellular Ca2+ release, and NOS activity (Reich and Frangos, 1991; Smalt et al., 1997; Williams et al., 1994).
Sphingosine 1-phosphate (S1P) is a sphingolipid messenger molecule produced in response to laminar shear stress which is known to be a potent chemoattractant for HSCs and progenitors in the adult (Massberg et al., 2007; Seitz et al., 2005). The majority of circulating plasma S1P is produced by erythrocytes and endothelial cells through the activity of sphingosine kinases, but is also known to be released by platelets (Pappu et al., 2007; Venkataraman et al., 2008). S1P levels are high in the blood at micromolar concentrations, but are extremely low in tissues due to degradation by S1P lyase (Hanel et al., 2007; Hla et al., 2008; Okajima, 2002; Schwab et al., 2005). Production of S1P and its GPCR S1PR1/EDG1 has been found to be induced in vascular endothelial cells by laminar shear stress typical of arterial vessels and can activate eNOS activity (Hughes et al., 2005; Igarashi et al., 2003; Takada et al., 1997; Tanimoto et al., 2002; Venkataraman et al., 2008). HSPCs express S1P receptors, undergo chemotaxis in response to S1P, and have been found to utilize S1P gradients to emigrate from peripheral tissues to the lymphatic system (Kimura et al., 2004; Massberg et al., 2007). The same signals that provide direction to emigrating HSPC from the bone marrow could also be linked with instructions for homing and adhesion in the fetal liver. S1PR1, via Flk-1/KDR/VEGFR2 and possibly also protein kinase C, phosphorylates the Rac1 GTPase-associated protein CrkII (Endo et al., 2002; Igarashi et al., 2003; Tanimoto et al., 2002). S1P also potently induces yes-activated protein (YAP) dephosphorylation, nuclear localization, and transcriptional activity of cell proliferation related genes such as CTGF and Cyr61 (Miller et al, 2012). As S1P lyase inhibitors have been reported to disrupt CXCR4 antagonist-based mobilization (Ratajczak et al., 2010), it is intriguing to speculate that newly emergent HSCs from the AGM could use the S1P chemokine as a guide for entry into the blood stream and that this might occur in connection with Yap activation and associated cell division.
Genetically engineered mice have implicated stromal derived factor 1-α (SDF1-α] and its GPCR receptor, CXCR4 in HSC homing and engraftment to the marrow (Avigdor et al., 2004; Barker, 1997; Ding et al., 2012; Nagasawa et al., 1996; Papayannopoulou, 2003; Zou et al., 1998). Notably, SDFl-α −/− and CXCR4−/− embryos have greater impairment of myelopoiesis in bone marrow as compared to the fetal liver, suggesting that SDFl-α and CXCR4 are involved in colonization of bone marrow by hematopoietic progenitors during embryogenesis but not as critical for seeding of newly specified HSCs from the AGM [Ma et al., 1998; Nagasawa et al., 1996; Tachibana et al., 1998; Zou et al., 1998). SDFl-α is expressed by developing stroma in fetal bones, and its receptor CXCR4 is expressed on HSPCs (Ara et al., 2003). Interestingly, actively signaling CXCR4 is associated with lipid rafts [Lee et al., 2009; Ratajczak et al., 2004). As the CXCR4 receptor is a lipid raft-associated protein, its signaling sensitivity can be modulated by colocalization with other signaling molecules, including Rac1 (del Pozo et al., 2004; Gu et al., 2003). Packaging of CXCR4 together with Racl in lipid rafts facilitates GTP binding and activation of Rac1 (Ratajczak et al., 2010).
Calcium Sensing Receptor (CaSR], an ion-sensing G protein-coupled receptor, functionally couples to integrins and, in conjunction with intracellular calcium release, promotes cellular adhesion and migration in tumor cells (Tharmalingam et al., 2011). Other studies have suggested that extracellular Ca2+ sources, such as those found in the bone, can influence HSC lodgement in the endosteal niche via the CaSR (Adams et al., 2006; Lam et al., 2011). Activation of the CaSR on HSCs by extracellular Ca2+ allows HSC to adhere to ECM components like collagen I, thus retaining them in close proximity to osteoblasts (Adams et al., 2006; Lam et al., 2011).
Other Mechanosensors
The type III receptor kinase known as Flk-1/KDR/VEGFR2 is renowned as a mesodermal surface marker expressed on cells with hematopoietic potential. It also plays a critical role in mechanosensation in complex with the cell adhesion molecules PECAM and VE-Cadherin (Tzima et al., 2005). Flk-1 initiates a cascade of intracellular signaling events through activation of Src-MAPK, PI3K-Akt, and eNOS downstream of shear stress (Jalali et al., 1998; Jin et al., 2003; Tzima et al., 2005). These signaling pathways can activate integrins and small GTPases (Rho, Rac and Cdc42) to orchestrate cytoskeletal reorganization [Tzima, 2006). There is evidence from embryonic stem cell based-models of hematoendothelial development that shear stress promotes endothelial and hematopoietic specification from the Flk-1+ fraction of differentiated embryonic stem cells (Adamo et al., 2009; Wolfe and Ahsan, 2013; Yamamoto et al., 2005). Consistent with the Flk-1−/− phenotypes in mice, pharmacological treatment with the Flk-1 kinase inhibitor SU1498 blocks differentiation of endothelial cells and their downstream hematopoietic progeny (Shalaby et al., 1997; Shalaby et al., 1995; Wolfe and Ahsan, 2013; Yamamoto et al., 2005). SU1498 inhibits both Flk-1 and ERK kinase activity, implicating PI3K and MAPK signaling in these shear-induced commitment events (Boguslawski et al., 2004).
The relevance of other mechanosensors in the hematopoietic system remains less well described, yet there are hints that ion channels (Brayden et al., 2008; Olesen et al., 1988; Owsianik et al., 2006) and caveolae (Park et al., 2000; Park et al., 1998; Rizzo> et al., 1998b) may regulate intracellular signaling critical for self renewal and survival. Recently, Barbosa et al. reported that ATP was effective at inducing differentiation of hematopoietic cells via activation of Ca2+ influx in mice (Barbosa et al., 2011), implicating Ca2+ oscillation in the regulation of HSPC quiescence and differentiation. Other studies suggest that Ca2+ signaling could be controlled by channels linked to the cytoskeleton or ECM. The increase in intracellular Ca2+ is localized to FA complexes associated with β1 integrin, and is detectable milliseconds after mechanical stimulation (Kobayashi and Sokabe, 2010). Ca2+ influx can also be altered in response to ECM rigidity. Endothelial cells cultured on stiff substrate show spontaneous Ca2+ oscillations of larger amplitude than those cultured on soft substrate (Kobayashi and Sokabe, 2010). Thus, mechanosensitive ion channels can be directly activated by externally applied mechanical stimuli as well as internal cytoskeletal rearrangement. Ca2+ influx can also be triggered by stimulation of primary cilia. Nearly all interphase and nondividing cells have a single, nonmotile cilium that protrudes from the cell surface and in some cell types acts as a mechanosensor (Eggenschwiler and Anderson, 2007). In embryonic aortic and cardiac endothelium, these hair-like structures can be approximately 2 µm in length (Nauli et al., 2008; Poelmann et al., 2008).and serve as low shear sensors. Interestingly, mature hematopoietic cells lack primary cilia (Pazour and Witman, 2003) but, some human lymphoid and myeloid cells have been found to express intraflagellar transport (IFT) 20, an essential molecule in ciliary assembly (Finetti et al.,2009).
Caveolae are specialized vesicular organelles of the plasma membrane that are involved in transmembrane signaling, adhesion, differentiation, endocytosis, and transport of large and small molecules like cholesterol [Anderson, 1998; Okamoto et al., 1998). Caveolae are a type of lipid raft present on most cell types, including endothelial cells, smooth muscle cells, fibroblasts, and adipocytes (Fernandez-Hernando et al., 2010; Rothberg et al., 1992). In immune cells, caveolae appear in cells of the myeloid lineage but not the lymphoid lineage, with the exception of some T cell leukemia cell lines and bovine lymphocytes (Harris et al., 2002a; Harris et al., 2002b). Trafficking of caveolae to the membrane has been found to be induced by laminar shear stress and is dependent both on integrins and the microtubule network. β1 integrin or ILK ablation in mice results in dramatically reduced numbers of caveolae due to impaired transport of caveolin-1-enriched vesicles along microtubules to the plasma membrane (Wickstrom et al., 2010). Recently, Sinha and colleagues showed that flattening of caveolae allow endothelial cells to compensate rapidly for membrane tension changes associated with mechanical stress (Sinha et al., 2011). This instantaneous remodeling of the membrane surface provides the cell with reservoirs of signaling receptors and ligands available for immediate deployment (Freund et al., 2012), as occurs during the NO signaling response to changes in biophysical cues (Feron et al., 1996; Garcia-Cardena et al., 1996; Shaul et al., 1996; Venema et al., 1997). Early work demonstrated in vasculature of the lung that fluid flow and hydrostatic pressure could stimulate protein tyrosine phosphorylation and release of activated eNOS at the luminal endothelial plasma membrane (Rizzo et al., 1998a; Rizzo et al., 1998b). This activity was localized within caveolae and resulted in a Ras/Raf/ERK 1/2 signaling cascade (Rizzo et al., 1998b). In light of strong evidence for regulation of NO signaling by caveolae in the context of shear stress, it will be important to evaluate whether caveolae play a role in modulating NO production within embryonic vasculature of the AGM during HSC specification
CONCLUSIONS
The hematopoietic system is dynamic in adulthood, but perhaps more so during development, in terms of spatial and temporal transitions. Cells migrate, self renew, and differentiate into a complex array of immune cells with very distinct functions and cellular morphologies. Specific developmental history (Dieterlen-Lievre et al., 2006), trophic effects of neighboring tissues (Peeters et al., 2009), and biophysical cues (Adamo et al.,2009; Hoist et al., 2010; North et al., 2009) determine the potential of hematopoietic cells. Indeed, it is a combination of intrinsic and extrinsic factors that must interface to regulate the development of the blood system. The path to producing transplantable HSPCs from pluripotent sources is gaining momentum. Importantly, studies designed to test the capacity of human pluripotent stem cells to support definitive hematopoiesis have demonstrated the value of extensive knowledge of developmental signals critical to achieving this goal (Amabile et al., 2013; Kennedy et al., 2012). Recent research detailing the complexities of the blood system and its biophysical requirements highlight the need for a more complete concept of the niche. Future work aimed at interrogating the roles of elasticity, nanotopography, and hemodynamic force will broaden our understanding of the types of signals, soluble and mechanical, that define the hematopoietic microenvironment and will advance the field toward establishing alternative, high quality sources of hematopoietic cells that can be used for cellular therapies.
Highlights.
Mechanical cues in the niche evolve during hematopoietic ontogeny to accommodate changing needs of the blood system
Vascular forces contribute to several stages of hematopoiesis, including definitive HSC emergence
Shear stress activates intracellular signaling critical for blood development
Current methodologies for in vitro generation of hematopoietic cells can be improved by a better understanding of regulatory biophysical cues
Acknowledgements
We acknowledge valuable comments and suggestions on the manuscript by anonymous reviewers and group members of the Center for Stem Cell and Regenerative Medicine, Brown Foundation Institute of Molecular Medicine. P.L.W. was supported by NIH grant 1K01DK092365, the American Society of Hematology Scholar Award, and the State of Texas Emerging Technology Fund.
Abbreviations
- ADAM
a disintegrin and metalloprotease
- AGM
aorta-gonad-mesonephros
- CaSR
calcium sensing receptor
- CXCR
chemokine C-X-C motif receptor
- DAG
diacylglycerol
- Dll
Delta-like
- ECM
extracellular matrix
- FA
focal adhesion
- FAK
focal adhesion kinase
- Flk-1
Fetal liver kinase-1
- FZD
Frizzled
- GPCR
G protein-coupled receptor
- HSC
hematopoietic stem cell
- HSPC
hematopoietic stem and progenitor cells
- HUVEC
human umbilical vein endothelial cells
- ILK
integrin linked kinase
- IP3
inositol triphosphate
- MAPK
mitogen activated protein kinase
- NICD
Notch intracellular domain
- NOS
nitric oxide synthase
- PGE2
prostaglandin E2
- PI3K
phosphatidylinositide 3-kinase
- PKA
protein kinase A
- PKD
Polycystin
- PLC
phospholipase C
- ROCK
Rho-associated protein kinase
- S1P
sphingosine 1-phosphate
- S1PR/EDG1
Sphingosine 1-phosphate receptor
- SDF1
stromal derived factor 1
- TRPV4
Transient receptor potential Vallinoid Type 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou Alaiwi WA, Lo ST, Nauli SM. Primary cilia: highly sophisticated biological sensors. Sensors (Basel) 2009;9:7003–7020. doi: 10.3390/s90907003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia-Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, Yoder MC, Garcia-Cardena G, Daley GQ. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. The Biochemical journal. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amabile G, Welner RS, Nombela-Arrieta C, D'Alise AM, Di Ruscio A, Ebralidze AK, Kraytsberg Y, Ye M, Kocher O, Neuberg DS, Khrapko K, Silberstein LE, Tenen DG. in vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood. 2013;121:1255–1264. doi: 10.1182/blood-2012-06-434407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin AR, Attur M, Vyas P, Leszczynska-Piziak J, Levartovsky D, Rediske J, Clancy RM, Vora KA, Abramson SB. Expression of nitric oxide synthase in human peripheral blood mononuclear cells and neutrophils. Journal of inflammation. 1995;47:190–205. [PubMed] [Google Scholar]
- Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Rauch C, March CJ, Boswell HS, Gimpel SD, Cosman D, et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990;63:235–243. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- Anderson RG. The caveolae membrane system. Annual review of biochemistry. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- Ando J, Komatsuda T, Kamiya A. Cytoplasmic calcium response to fluid shear stress in cultured vascular endothelial cells. In Vitro Cell Dev Biol. 1988;24:871–877. doi: 10.1007/BF02623896. [DOI] [PubMed] [Google Scholar]
- Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19:257–267. doi: 10.1016/s1074-7613(03)00201-2. [DOI] [PubMed] [Google Scholar]
- Armeanu S, Bühring HJ, Reuss-Borst M, Müller CA, Klein G. E-Cadherin is Functionally Involved in the Maturation of the Erythroid Lineage. The Journal of cell biology. 1995;131:243–249. doi: 10.1083/jcb.131.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askenasy N, Yolcu ES, Shirwan H, Stein J, Yaniv I, Farkas DL. Characterization of adhesion and viability of early seeding hematopoietic cells in the host bone marrow in vivo and in situ. Experimental hematology. 2003;31:1292–1300. doi: 10.1016/j.exphem.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, Ben-Hur H, Naor D, Nagler A, Lapidot T. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- Barakat AI. A model for shear stress-induced deformation of a flow sensor on the surface of vascular endothelial cells. Journal of theoretical biology. 2001;210:221–236. doi: 10.1006/jtbi.2001.2290. [DOI] [PubMed] [Google Scholar]
- Barbosa CMV, Leon CMMP, Nogueira-Pedro A, Wasinsk F, Araújo RC, Miranda A, Ferreira AT, Paredes-Gamero EJ. Differentiation of hematopoietic stem cell and myeloid populations by ATP is modulated by cytokines. Cell Death and Disease. 2011;2:el65. doi: 10.1038/cddis.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell and tissue research. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JE. Early transplantation to a normal microenvironment prevents the development of Steel hematopoietic stem cell defects. Experimental hematology. 1997;25:542–547. [PubMed] [Google Scholar]
- Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011;117:5281–5288. doi: 10.1182/blood-2011-01-315069. [DOI] [PubMed] [Google Scholar]
- Boguslawski G, McGlynn PW, Harvey KA, Kovala AT. SU1498, an inhibitor of vascular endothelial growth factor receptor 2, causes accumulation of phosphorylated ERK kinases and inhibits their activity in vivo and in vitro. The Journal of biological chemistry. 2004;279:5716–5724. doi: 10.1074/jbc.M308625200. [DOI] [PubMed] [Google Scholar]
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- Brakemeier S, Eichler I, Hopp H, Kohler R, Hoyer J. Up-regulation of endothelial stretch-activated cation channels by fluid shear stress. Cardiovasc Res. 2002;53:209–218. doi: 10.1016/s0008-6363(01)00476-x. [DOI] [PubMed] [Google Scholar]
- Brayden JE, Earley S, Nelson MT, Reading S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clinical and experimental pharmacology & physiology. 2008;35:1116–1120. doi: 10.1111/j.1440-1681.2007.04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, de Bruijn M, Ma X, Dortland B, Luteijn T, Downing RJ, Dzierzak E. Haploinsufficiency of AML1 affects the temporal and spatial generation of hematopoietic stem cells in the mouse embryo. Immunity. 2000;13:423–431. doi: 10.1016/s1074-7613(00)00042-x. [DOI] [PubMed] [Google Scholar]
- Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15463–15468. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Pacal M, Wenzel P, Knoepfler PS, Leone G, Bremner R. Division and apoptosis of E2f-deficient retinal progenitors. Nature. 2009a;462:925–929. doi: 10.1038/nature08544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. The Journal of biological chemistry. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- Chen LY, Mehta JL. Variable effects of L-arginine analogs on L-arginine-nitric oxide pathway in human neutrophils and platelets may relate to different nitric oxide synthase isoforms. The Journal of pharmacology and experimental therapeutics. 1996;276:253–257. [PubMed] [Google Scholar]
- Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009b;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Lewallen M, Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development. 2013;140:255–265. doi: 10.1242/dev.083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JL, Wenzel PL, Saenz-Robles MT, Nair V, Ferrey A, Hagan JP, Gomez YM, Sharma N, Chen HZ, Ouseph M, Wang SH, Trikha P, Culp B, Mezache L, Winton DJ, Sansom OJ, Chen D, Bremner R, Cantalupo PG, Robinson ML, Pipas JM, Leone G. E2fl-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature. 2009;462:930–934. doi: 10.1038/nature08677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard-Pelletier G, Jahnsen ED, Jones EA. Increased shear stress inhibits angiogenesis in veins and not arteries during vascular development. Angiogenesis. 2013;16:71–83. doi: 10.1007/s10456-012-9300-2. [DOI] [PubMed] [Google Scholar]
- Ciau-Uitz A, Liu F, Patient R. Genetic control of hematopoietic development in Xenopus and zebrafish. The International journal of developmental biology. 2010;54:1139–1149. doi: 10.1387/ijdb.093055ac. [DOI] [PubMed] [Google Scholar]
- Ciriza J, Thompson H, Petrosian R, Manilay JO, Garcia-Ojeda ME. The Migration of Hematopoietic Progenitors from the Fetal Liver to the Fetal Bone Marrow: Lessons Learned and Possible Clinical Applications. Experimental hematology. 2013 doi: 10.1016/j.exphem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Corn PGSBP, Ruckdeschel ES, Douglas D, Baylin SB, Herman JG. E-Cadherin expression is silenced by 5' CpG Island Methylation in Acute Leukemia. Clin Cancer Res. 2000;6:4243–4248. [PubMed] [Google Scholar]
- Corrigan PM, Dobbin E, Freeburn RW, Wheadon H. Patterns of Wnt/Fzd/LRP gene expression during embryonic hematopoiesis. Stem cells and development. 2009;18:759–772. doi: 10.1089/scd.2008.0270. [DOI] [PubMed] [Google Scholar]
- Craddock CF, Nakamoto B, Andrews RG, Priestley GV, Papayannopoulou T. Antibodies to VLA4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. Blood. 1997;90:4779–4788. [PubMed] [Google Scholar]
- Dantschakoff W. Uber das erste Aufretem der Blutelements im Huhnerembryo. Folia Hematol. 1907:159. [Google Scholar]
- Davies PF. Flow-mediated endothelial mechanotransduction. Physiological reviews. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- Delaney C, Varnum-Finney B, Aoyama K, Brashem-Stein C, Bernstein ID. Dose-dependent effects of the Notch ligand Deltal on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106:2693–2699. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterlen-Lievre F, Pouget C, Bollerot K, Jaffredo T. Are intra-aortic hemopoietic cells derived from endothelial cells during ontogeny? Trends in cardiovascular medicine. 2006;16:128–139. doi: 10.1016/j.tcm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and Macrophages move in. The Journal of experimental medicine. 2011;208:421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- Emmel VE. The cell clusters in the dorsal aorta of mammalian embryos. Am. J. Anat. 1916:401–421. [Google Scholar]
- Endo A, Nagashima K, Kurose H, Mochizuki S, Matsuda M, Mochizuki N. Sphingosine 1-phosphate induces membrane ruffling and increases motility of human umbilical vein endothelial cells via vascular endothelial growth factor receptor and Crkll. The Journal of biological chemistry. 2002;277:23747–23754. doi: 10.1074/jbc.M111794200. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Developmental cell. 2003;5:673–690. doi: 10.1016/s1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- Even-Ram S, Artym V, Yamada KM. Matrix control of stem cell fate. Cell. 2006;126:645–647. doi: 10.1016/j.cell.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Yu J, Davalos A, Prendergast J, Sessa WC. Endothelial-specific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. The American journal of pathology. 2010;177:998–1003. doi: 10.2353/ajpath.2010.091287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. The Journal of biological chemistry. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, Pazour GJ, Rosenbaum JL, Baldari CT. Intraflagella transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nature cell biology. 2009;11:1332–1339. doi: 10.1038/ncb1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisslthaler B, Dimmeler S, Hermann C, Busse R, Fleming I. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta physiologica Scandinavica. 2000;168:81–88. doi: 10.1046/j.1365-201x.2000.00627.x. [DOI] [PubMed] [Google Scholar]
- Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circulation research. 2003;93:el36–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Boissel JP, Kleinert H. Expressional control of the 'constitutive' isoforms of nitric oxide synthase (NOS I and NOS III) FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1998;12:773–790. [PubMed] [Google Scholar]
- Fourie AM, Coles F, Moreno V, Karlsson L. Catalytic activity of ADAM8, ADAM15, and MDC-L (ADAM28) on synthetic peptide substrates and in ectodomain cleavage of CD23. The Journal of biological chemistry. 2003;278:30469–30477. doi: 10.1074/jbc.M213157200. [DOI] [PubMed] [Google Scholar]
- Fraser ST, Ogawa M, Yu RT, Nishikawa S, Yoder MC, Nishikawa S. Definitive hematopoietic commitment within the embryonic vascular endothelial-cadherin+ population. Experimental hematology. 2002;30:1070–1078. doi: 10.1016/s0301-472x(02)00887-1. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Molecular pharmacology. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Freund JB, Goetz JG, Hill KL, Vermot J. Fluid flows and forces in development: functions, features and biophysical principles. Development. 2012;139:1229–1245. doi: 10.1242/dev.073593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K, Masuda M, Osawa M, Kano Y, Katoh K. Is PECAM-1 a mechanoresponsive molecule? Cell structure and function. 2001;26:11–17. doi: 10.1247/csf.26.11. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Developmental cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Developmental cell. 2005;8:389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Gibb DR, El Shikh M, Kang DJ, Rowe WJ, El Sayed R, Cichy J, Yagita H, Tew JG, Dempsey PJ, Crawford HC, Conrad DH. ADAM10 is essential for Notch2-dependent marginal zone B cell development and CD23 cleavage in vivo. The Journal of experimental medicine. 2010;207:623–635. doi: 10.1084/jem.20091990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb DR, Saleem SJ, Kang DJ, Subler MA, Conrad DH. ADAM10 overexpression shifts lympho- and myelopoiesis by dysregulating site 2/site 3 cleavage products of Notch. J Immunol. 2011;186:4244–4252. doi: 10.4049/jimmunol.1003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glodek AM, Le Y, Dykxhoorn DM, Park SY, Mostoslavsky G, Mulligan R, Lieberman J, Beggs HE, Honczarenko M, Silberstein LE. Focal adhesion kinase is required for CXCL12-induced chemtactic and pro-adhesive response in hematopoietic precursor cells. Leukemia. 2007;21:1723–1732. doi: 10.1038/sj.leu.2404769. [DOI] [PubMed] [Google Scholar]
- Gloe T, Sohn HY, Meininger GA, Pohl U. Shear stress-induced release of basic fibroblast growth factor from endothelial cells is mediated by matrix interaction via integrin alpha(v)beta3. The Journal of biological chemistry. 2002;277:23453–23458. doi: 10.1074/jbc.M203889200. [DOI] [PubMed] [Google Scholar]
- Godin I, Cumano A. The hare and the tortoise: an embryonic haematopoietic race. Nature reviews. Immunology. 2002;2:593–604. doi: 10.1038/nri857. [DOI] [PubMed] [Google Scholar]
- Goessling W, Allen RS, Guan X, Jin P, Uchida N, Dovey M, Harris JM, Metzger ME, Bonifacino AC, Stroncek D, Stegner J, Armant M, Schlaeger T, Tisdale JF, Zon LI, Donahue RE, North TE. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell stem cell. 2011;8:445–458. doi: 10.1016/j.stem.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, Zon LI. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC. Structural basis for autoinhibition of Notch. Nature structural & molecular biology. 2007;14:295–300. doi: 10.1038/nsmb1227. [DOI] [PubMed] [Google Scholar]
- Grassinger J, Haylock DN, Storan MJ, Haines GO, Williams B, Whitty GA, Vinson AR, Be CL, Li S, Sorensen ES, Tarn PP, Denhardt DT, Sheppard D, Choong PF, Nilsson SK. Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with alpha9betal and alpha4betal integrins. Blood. 2009;114:49–59. doi: 10.1182/blood-2009-01-197988. [DOI] [PubMed] [Google Scholar]
- Greenberg SS, Ouyang J, Zhao X, Giles TD. Human and rat neutrophils constitutively express neural nitric oxide synthase mRNA. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 1998;2:203–212. doi: 10.1006/niox.1998.0176. [DOI] [PubMed] [Google Scholar]
- Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- Gudi SR, Clark CB, Frangos JA. Fluid flow rapidly activates G proteins in human endothelial cells. Involvement of G proteins in mechanochemical signal transduction. Circulation research. 1996;79:834–839. doi: 10.1161/01.res.79.4.834. [DOI] [PubMed] [Google Scholar]
- Gudi SR, Lee AA, Clark CB, Frangos JA. Equibiaxial strain and strain rate stimulate early activation of G proteins in cardiac fibroblasts. The American journal of physiology. 1998;274:C1424–C1428. doi: 10.1152/ajpcell.1998.274.5.C1424. [DOI] [PubMed] [Google Scholar]
- Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- Hemmorantaa H, Satomaab T, Blomqvistb M, Heiskanenb A, Aitioc O, Saarinenb J, Natunenb J, Partanena J, Lainea J, Jaatinen T. N-glycan structures and associated gene expression reflect the characteristic N-glycosylation pattern of human hematopoietic stem and progenitor cells. Experimental hematology. 2007;35:1279–1292. doi: 10.1016/j.exphem.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Hierck BP, Van der Heiden K, Alkemade FE, Van de Pas S, Van Thienen JV, Groenendijk BC, Bax WH, Van der Laarse A, Deruiter MC, Horrevoets AJ, Poelmann RE. Primary cilia sensitize endothelial cells for fluid shear stress. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:725–735. doi: 10.1002/dvdy.21472. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Iglesias A, Potocnik AJ, Hartmann U, Fassler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of betal integrins. Nature. 1996;380:171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- Hla T, Venkataraman K, Michaud J. The vascular SIP gradient-cellular sources and biological significance. Biochimica et biophysica acta. 2008;1781:477–482. doi: 10.1016/j.bbalip.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoist J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, Kondyurin A, Ma L, Oberhauser AF, Weiss AS, Rasko JE. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nature biotechnology. 2010;28:1123–1128. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- Hosokawa K, Arai F, Yoshihara H, Iwasaki H, Nakamura Y, Gomei Y, Suda T. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood. 2010;116:554–563. doi: 10.1182/blood-2009-05-224857. [DOI] [PubMed] [Google Scholar]
- Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. The Journal of clinical investigation. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SK, Wacker BK, Kaneda MM, Elbert DL. Fluid shear stress modulates cell migration induced by sphingosine 1-phosphate and vascular endothelial growth factor. Annals of biomedical engineering. 2005;33:1003–1014. doi: 10.1007/s10439-005-5756-1. [DOI] [PubMed] [Google Scholar]
- Huovila AP, Turner AJ, Pelto-Huikko M, Karkkainen I, Ortiz RM. Shedding light on ADAM metalloproteinases. Trends in biochemical sciences. 2005;30:413–422. doi: 10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Iacovino M, Chong D, Szatmari I, Hartweck L, Rux D, Caprioli A, Cleaver O, Kyba M. HoxA3 is an apical regulator of haemogenic endothelium. Nature cell biology. 2011;13:72–78. doi: 10.1038/ncb2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T. VEGF induces S1P1 receptors in endothelial cells: Implications for cross-talk between sphingolipid and growth factor receptors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10664–10669. doi: 10.1073/pnas.1934494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida A, Sakaguchi K, Sato K, Sakurai H, Nishimura D, Iwaki A, Takeuchi M, Kobayashi M, Misaki K, Yonemura S, Kawahara A, Sehara-Fujisawa A. Metalloprotease-dependent onset of blood circulation in zebrafish. Current biology :CB. 2010;20:1110–1116. doi: 10.1016/j.cub.2010.04.052. [DOI] [PubMed] [Google Scholar]
- Imai K, Kobayashi M, Wang J, Ohiro Y, Hamada J, Cho Y, Imamura M, Musashi M, Kondo T, Hosokawa M, Asaka M. Selective transendothelial migration of hematopoietic progenitor cells: a role in homing of progenitor cells. Blood. 1999;93:149–156. [PubMed] [Google Scholar]
- Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;2:022001. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali S, Li YS, Sotoudeh M, Yuan S, Li S, Chien S, Shyy JY. Shear stress activates p60src-Ras-MAPK signaling pathways in vascular endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 1998;18:227–234. doi: 10.1161/01.atv.18.2.227. [DOI] [PubMed] [Google Scholar]
- Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circulation research. 2003;93:354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- Jo H, Sipos K, Go YM, Law R, Rong J, McDonald JM. Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gbeta/gamma-dependent signaling pathways. The Journal of biological chemistry. 1997;272:1395–1401. doi: 10.1074/jbc.272.2.1395. [DOI] [PubMed] [Google Scholar]
- Jordan H. Evidence of hemogenic capacity of the endothelium. Anat. Rec. 1916:417–420. [Google Scholar]
- Kabrun N, Buhring HJ, Choi K, Ullrich A, Risau W, Keller G. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development. 1997;124:2039–2048. doi: 10.1242/dev.124.10.2039. [DOI] [PubMed] [Google Scholar]
- Kennedy M, Awong G, Sturgeon CM, Ditadi A, LaMotte-Mohs R, Zuniga-Pflucker JC, Keller G. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell reports. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Acar M, Radice GL, Morrison SJ. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell stem cell. 2009;4:170–179. doi: 10.1016/j.stem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HN, Jiao A, Hwang NS, Kim MS, Kang DH, Kim DH, Suh KY. Nanotopography-guided tissue engineering and regenerative medicine. Advanced drug delivery reviews. 2012 doi: 10.1016/j.addr.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Boehmler AM, Seitz G, Kuci S, Wiesner T, Brinkmann V, Kanz L, Mohle R. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 2004;103:4478–4486. doi: 10.1182/blood-2003-03-0875. [DOI] [PubMed] [Google Scholar]
- Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- Klekotka PA, Santoro SA, Zutter MM. alpha 2 integrin subunit cytoplasmic domain-dependent cellular migration requires p38 MAPK. The Journal of biological chemistry. 2001;276:9503–9511. doi: 10.1074/jbc.M006286200. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Sokabe M. Sensing substrate rigidity by mechanosensitive ion channels with stress fibers and focal adhesions. Current opinion in cell biology. 2010;22:669–676. doi: 10.1016/j.ceb.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Roller A, Sun D, Kaley G. Role of shear stress and endothelial prostaglandins in flow- and viscosity-induced dilation of arterioles in vitro. Circulation research. 1993;72:1276–1284. doi: 10.1161/01.res.72.6.1276. [DOI] [PubMed] [Google Scholar]
- Koshida R, Rocic P, Saito S, Kiyooka T, Zhang C, Chilian WM. Role of focal adhesion kinase in flow-induced dilation of coronary arterioles. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:2548–2553. doi: 10.1161/01.ATV.0000188511.84138.9b. [DOI] [PubMed] [Google Scholar]
- Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffi D, Tauber R, Wegierski T, Nitschke R, Suzull M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. The Journal of cell biology. 2008;182:437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnov P, Michurina T, Packer MA, Stasiv Y, Nakaya N, Moore KA, Drazan KE, Enikolopov G. Neuronal nitric oxide synthase contributes to the regulation of hematopoiesis. Molecular medicine. 2008;14:141–149. doi: 10.2119/2007-00011.Krasnov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam BS, Cunningham C, Adams GB. Pharmacologic modulation of the calcium-sensing receptor enhances hematopoietic stem cell lodgment in the adult bone marrow. Blood. 2011;117:1167–1175. doi: 10.1182/blood-2010-05-286294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam EY, Hall CJ, Crosier PS, Crosier KE, Flores MV. Live imaging of Runxl expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood. 2010;116:909–914. doi: 10.1182/blood-2010-01-264382. [DOI] [PubMed] [Google Scholar]
- Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Current opinion in cell biology. 2006;18:463–471. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Lee HM, Wu W, Wysoczynski M, Liu R, Zuba-Surma EK, Kucia M, Ratajczak J, Ratajczak MZ. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052–2062. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. Journal of internal medicine. 2006;259:381–392. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Prossnitz ER, Bunemann M, Wang JM, Yao W, Schmid-Schonbein GW. G protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. American journal of physiology. Cell physiology. 2006;290:C1633–C1639. doi: 10.1152/ajpcell.00576.2005. [DOI] [PubMed] [Google Scholar]
- Martin FH, Suggs SV, Langley KE, Lu HS, Ting J, Okino KH, Morris CF, McNiece IK, Jacobsen FW, Mendiaz EA, et al. Primary structure and functional expression of rat and human stem cell factor DNAs. Cell. 1990;63:203–211. doi: 10.1016/0092-8674(90)90301-t. [DOI] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov AA. Untersuchungen uber Blut und Bindegewebe. I. Die Fruhesten Entwicklongsstadien der Blut- und Bindewegebezellen beim Saugetierembryo, bis zum Anfang der Blutbildung in der Leber. Arch. Mikr. Anat. 1909:444. [Google Scholar]
- McNamara LE, McMurray RJ, Biggs MJ, Kantawong F, Oreffo RO, Dalby MJ. Nanotopographical control of stem cell differentiation. Journal of tissue engineering. 2010;2010:120623. doi: 10.4061/2010/120623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. American journal of physiology. Heart and circulatory physiology. 2010;298:H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michurina T, Krasnov P, Balazs A, Nakaya N, Vasilieva T, Kuzin B, Khrushchov N, Mulligan RC, Enikolopov G. Nitric oxide is a regulator of hematopoietic stem cell activity. Molecular therapy : the journal of the American Society of Gene Therapy. 2004;10:241–248. doi: 10.1016/j.ymthe.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Miller BA, Bell L, Hansen CA, Robinshaw JD, Linder ME, Cheung JY. G-protein alpha subunit Gi(alpha)2 mediates erythropoietin signal transduction in human erythroid precursors. The Journal of clinical investigation. 1996;98:1728–1736. doi: 10.1172/JCI118971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Yang J, DeRan M, Wu C, Su AI, Bonamy GM, Liu J, Peters EC, Wu X. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chemistry & biology. 2012;19:955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Minot CS. Development of the blood, the vascular system, the spleen: Human Embryology. Keibel-Mall. 1912;2:525. [Google Scholar]
- Mizuochi C, Fraser ST, Biasch K, Horio Y, Kikushige Y, Tani K, Akashi K, Tavian M, Sugiyama D. Intra-aortic clusters undergo endothelial to hematopoietic phenotypic transition during early embryogenesis. PloS one. 2012;7:e35763. doi: 10.1371/journal.pone.0035763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marin-Padilla M, Speck NA. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM, Weber GJ, Harris J, Cutting CC, Huang P, Dzierzak E, Zon LI. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, Fitzgerald GA, Daley GQ, Orkin SH, Zon LI. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin E, Fleury M, Clay D, Petit-Cocault L, Candelier JJ, Mennesson B, Jaffredo TMS. VE-cadherin expression allows identification of a new class of hematopoietic stem cells within human embryonic liver. Blood. 2010;116:4444–4455. doi: 10.1182/blood-2010-03-272625. [DOI] [PubMed] [Google Scholar]
- Okajima F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an antiatherogenic mediator? Biochimica et biophysica acta. 2002;1582:132–137. doi: 10.1016/s1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. The Journal of biological chemistry. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Developmental cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annual review of physiology. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T. Bone marrow homing: the players, the playfield, and their evolving roles. Current opinion in hematology. 2003;10:214–219. doi: 10.1097/00062752-200305000-00004. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T, Nakamoto B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:9374–9378. doi: 10.1073/pnas.90.20.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- Park H, Go YM, Darji R, Choi JW, Lisanti MP, Maland MC, Jo H. Caveolin-1 regulates shear stress-dependent activation of extracellular signal-regulated kinase. American journal of physiology. Heart and circulatory physiology. 2000;278:H1285–H1293. doi: 10.1152/ajpheart.2000.278.4.H1285. [DOI] [PubMed] [Google Scholar]
- Park H, Go YM, St John PL, Maland MC, Lisanti MP, Abrahamson DR, Jo H. Plasma membrane cholesterol is a key molecule in shear stress-dependent activation of extracellular signal-regulated kinase. The Journal of biological chemistry. 1998;273:32304–32311. doi: 10.1074/jbc.273.48.32304. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Current opinion in cell biology. 2003;15:105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- Peeters M, Ottersbach K, Bollerot K, Orelio C, de Bruijn M, Wijgerde M, Dzierzak E. Ventral embryonic tissues and Hedgehog proteins induce early AGM hematopoietic stem cell development. Development. 2009;136:2613–2621. doi: 10.1242/dev.034728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelling AE, Horton MA. An historical perspective on cell mechanics. Pflugers Archiv : European journal of physiology. 2008;456:3–12. doi: 10.1007/s00424-007-0405-1. [DOI] [PubMed] [Google Scholar]
- Piekarski K, Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269:80–82. doi: 10.1038/269080a0. [DOI] [PubMed] [Google Scholar]
- Potocnik AJ, Brakebusch C, Fassler R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 2000;12:653–663. doi: 10.1016/s1074-7613(00)80216-2. [DOI] [PubMed] [Google Scholar]
- Price C, Zhou X, Li W, Wang L. Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: direct evidence for load-induced fluid flow. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:277–285. doi: 10.1002/jbmr.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puch S, Armeanu S, Kibler C, Johnson KR, Muller CA, Wheelock MJ, Klein G. N-cadherin is developmentally regulated and functionally involved in early hematopoietic cell differentiation. Journal of cell science. 2001;114:1567–1577. doi: 10.1242/jcs.114.8.1567. [DOI] [PubMed] [Google Scholar]
- Qian H, Tryggvason K, Jacobsen SE, Ekblom M. Contribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrins. Blood. 2006;107:3503–3510. doi: 10.1182/blood-2005-10-3932. [DOI] [PubMed] [Google Scholar]
- Rallis C, Pinchin SM, Ish-Horowicz D. Cell-autonomous integrin control of Wnt and Notch signalling during somitogenesis. Development. 2010;137:3591–3601. doi: 10.1242/dev.050070. [DOI] [PubMed] [Google Scholar]
- Ranheim EA, Kwan HC, Reya T, Wang YK, Weissman IL, Francke U. Frizzled 9 knock-out mice have abnormal B-cell development. Blood. 2005;105:2487–2494. doi: 10.1182/blood-2004-06-2334. [DOI] [PubMed] [Google Scholar]
- Ranjan V, Xiao Z, Diamond SL. Constitutive NOS expression in cultured endothelial cells is elevated by fluid shear stress. The American journal of physiology. 1995;269:H550–H555. doi: 10.1152/ajpheart.1995.269.2.H550. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, Kucia M, Janowska-Wieczorek A, Ratajczak J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Reca R, Wysoczynski M, Kucia M, Baran JT, Allendorf DJ, Ratajczak J, Ross GD. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18:1482–1490. doi: 10.1038/sj.leu.2403446. [DOI] [PubMed] [Google Scholar]
- Regan JW, Bailey TJ, Pepperl DJ, Pierce KL, Bogardus AM, Donello JE, Fairbairn CE, Kedzie KM, Woodward DF, Gil DW. Cloning of a novel human prostaglandin receptor with characteristics of the pharmacologically defined EP2 subtype. Molecular pharmacology. 1994;46:213–220. [PubMed] [Google Scholar]
- Reich KM, Frangos JA. Effect of flow on prostaglandin E2 and inositol trisphosphate levels in osteoblasts. The American journal of physiology. 1991;261:C428–C432. doi: 10.1152/ajpcell.1991.261.3.C428. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Mcintosh DP, Oh P, Schnitzer JE. In situ flow activates endothelial nitric oxide synthase in luminal caveolae of endothelium with rapid caveolin dissociation and calmodulin association. The Journal of biological chemistry. 1998a;273:34724–34729. doi: 10.1074/jbc.273.52.34724. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Morton C, DePaola N, Schnitzer JE, Davies PF. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. American journal of physiology. Heart and circulatory physiology. 2003;285:H1720–H1729. doi: 10.1152/ajpheart.00344.2002. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Sung A, Oh P, Schnitzer JE. Rapid mechanotransduction in situ at the luminal cell surface of vascular endothelium and its caveolae. The Journal of biological chemistry. 1998b;273:26323–26329. doi: 10.1074/jbc.273.41.26323. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Sabin FR. Preliminary note on the difeferentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in th eliving chick. Anantomical Rec. 1917:199–204. doi: 10.1089/152581602753448496. [DOI] [PubMed] [Google Scholar]
- Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- Sanchez Alvarado A. Stem cells: time to check our premises. Cell stem cell. 2008;3:25–29. doi: 10.1016/j.stem.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Sase K, Michel T. Expression of constitutive endothelial nitric oxide synthase in human blood platelets. Life sciences. 1995;57:2049–2055. doi: 10.1016/0024-3205(95)02191-k. [DOI] [PubMed] [Google Scholar]
- Schreiber TD, Steinl C, Essl M, Abele H, Geiger K, Muller CA, Aicher WK, Klein G. The integrin alpha9betal on hematopoietic stem and progenitor cells: involvement in cell adhesion, proliferation and differentiation. Haematologica. 2009;94:1493–1501. doi: 10.3324/haematol.2009.006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through SIP lyase inhibition and disruption of SIP gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Schwarz G, Callewaert G, Droogmans G, Nilius B. Shear stress-induced calcium transients in endothelial cells from human umbilical cord veins. The Journal of physiology. 1992;458:527–538. doi: 10.1113/jphysiol.1992.sp019432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz G, Boehmler AM, Kanz L, Mohle R. The role of sphingosine 1-phosphate receptors in the trafficking of hematopoietic progenitor cells. Annals of the New York Academy of Sciences. 2005;1044:84–89. doi: 10.1196/annals.1349.011. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficientmice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. The Journal of biological chemistry. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9462–9467. doi: 10.1073/pnas.142224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Luscinskas FW, Connolly A, Dewey CF, Jr., Gimbrone MA., Jr. Fluid shear stress modulates cytosolic free calcium in vascular endothelial cells. The American journal of physiology. 1992;262:C384–C390. doi: 10.1152/ajpcell.1992.262.2.C384. [DOI] [PubMed] [Google Scholar]
- Silver L, Palis J. Initiation of murine embryonic erythropoiesis: a spatial analysis. Blood. 1997;89:1154–1164. [PubMed] [Google Scholar]
- Simmons PJ, Levesque JP, Haylock DN. Mucin-like molecules as modulators of the survival and proliferation of primitive hematopoietic cells. Annals of the New York Academy of Sciences. 2001;938:196–206. doi: 10.1111/j.1749-6632.2001.tb03590.x. discussion 206-197. [DOI] [PubMed] [Google Scholar]
- Sinha B, Koster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–413. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalt R, Mitchell FT, Howard RL, Chambers TJ. Induction of NO and prostaglandin E2 in osteoblasts by wall-shear stress but not mechanical strain. The American journal of physiology. 1997;273:E751–E758. doi: 10.1152/ajpendo.1997.273.4.E751. [DOI] [PubMed] [Google Scholar]
- Stricht MVD. Lorigine des premieres cellules sanguines et des premiers vaisvaissaux sanguins dans l'aire vasculaire de chauves-souris. Bull. Acad. R. Med. Belg. 1899:336–351. [Google Scholar]
- Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:346–351. doi: 10.1161/01.ATV.0000253492.45717.46. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochemical and biophysical research communications. 1997;240:737–741. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Hayashi M, Kubota Y, Nagai H, Sheng G, Nishikawa S, Samokhvalov IM. Early ontogenic origin of the hematopoietic stem cell lineage. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4515–4520. doi: 10.1073/pnas.1115828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto T, Jin ZG, Berk BC. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS) The Journal of biological chemistry. 2002;277:42997–43001. doi: 10.1074/jbc.M204764200. [DOI] [PubMed] [Google Scholar]
- Taqvi S, Dixit L, Roy K. Biomaterial-based notch signaling for the differentiation of hematopoietic stem cells into T cells. Journal of biomedical materials research. Part A. 2006;79:689–697. doi: 10.1002/jbm.a.30916. [DOI] [PubMed] [Google Scholar]
- Tavian M, Peault B. Embryonic development of the human hematopoietic system. The International journal of developmental biology. 2005;49:243–250. doi: 10.1387/ijdb.041957mt. [DOI] [PubMed] [Google Scholar]
- Taylor E, Taoudi S, Medvinsky A. Hematopoietic stem cell activity in the aorta-gonad-mesonephros region enhances after mid-day 11 of mouse development. The International journal of developmental biology. 2010;54:1055–1060. doi: 10.1387/ijdb.103152et. [DOI] [PubMed] [Google Scholar]
- Tharmalingam S, Daulat AM, Antflick JE, Ahmed SM, Nemeth EF, Angers S, Conigrave AD, Hampson DR. Calcium-sensing receptor modulates cell adhesion and migration via integrins. The Journal of biological chemistry. 2011;286:40922–40933. doi: 10.1074/jbc.M111.265454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circulation research. 2009;104:1123–1130. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub O, Ishida T, Ishida M, Tupper JC, Berk BC. Shear stress-mediated extracellular signal-regulated kinase activation is regulated by sodium in endothelial cells. Potential role for a voltage-dependent sodium channel. The Journal of biological chemistry. 1999;274:20144–20150. doi: 10.1074/jbc.274.29.20144. [DOI] [PubMed] [Google Scholar]
- Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circulation research. 2006;98:176–185. doi: 10.1161/01.RES.0000200162.94463.d7. [DOI] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- Uchida N, Tsukamoto A, He D, Friera AM, Scollay R, Weissman IL. High doses of purified stem cells cause early hematopoietic recovery in syngeneic and allogeneic hosts. The Journal of clinical investigation. 1998;101:961–966. doi: 10.1172/JCI1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, Nerem RM, Harrison DG. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. The American journal of physiology. 1995;269:C1371–H1378. doi: 10.1152/ajpcell.1995.269.6.C1371. [DOI] [PubMed] [Google Scholar]
- Varnum-Finney B, Wu L, Yu M, Brashem-Stein C, Staats S, Flowers D, Griffin JD, Bernstein ID. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. Journal of cell science 113 Pt. 2000;23:4313–4318. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- Venema VJ, Zou R, Ju H, Marrero MB, Venema RC. Caveolin-1 detergent solubility and association with endothelial nitric oxide synthase is modulated by tyrosine phosphorylation. Biochemical and biophysical research communications. 1997;236:155–161. doi: 10.1006/bbrc.1997.6921. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circulation research. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallerath T, Gath I, Aulitzky WE, Pollock JS, Kleinert H, Forstermann U. Identification of the NO synthase isoforms expressed in human neutrophil granulocytes, megakaryocytes and platelets. Thrombosis and haemostasis. 1997;77:163–167. [PubMed] [Google Scholar]
- Wang L, Zhang P, Wei Y, Gao Y, Patient R, Liu F. A blood flow-dependent klf2a-NO signaling cascade is required for stabilization of hematopoietic stem cell programming in zebrafish embryos. Blood. 2011;118:4102–4110. doi: 10.1182/blood-2011-05-353235. [DOI] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- Weber S, Saftig P. Ectodomain shedding and ADAMs in development. Development. 2012;139:3693–3709. doi: 10.1242/dev.076398. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. Journal of biomechanics. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman I, Papaioannou V, Gardner R. Fetal Hematopoietic origins of the adult hematolymphoid system. Differentiation of Normal and Neoplatic Hematopoietic Cells Cold Spring Harbor Laboratory. 1978:33–47. [Google Scholar]
- Wenzel PL, Chong JL, Saenz-Robles MT, Ferrey A, Hagan JP, Gomez YM, Rajmohan R, Sharma N, Chen HZ, Pipas JM, Robinson ML, Leone G. Cell proliferation in the absence of E2F1-3. Developmental biology. 2011;351:35–45. doi: 10.1016/j.ydbio.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z, Vu TH, Rinkenberger JL, Coussens LM. Matrix-degrading proteases and angiogenesis during development and tumor formation. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 1999;107:11–18. doi: 10.1111/j.1699-0463.1999.tb01521.x. [DOI] [PubMed] [Google Scholar]
- White CR, Frangos JA. The shear stress of it all: the cell membrane and mechanochemical transduction. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2007;362:1459–1467. doi: 10.1098/rstb.2007.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annual review of biomedical engineering. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- Williams DA, Rios M, Stephens C, Patel VP. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature. 1991;352:438–441. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]
- Williams JL, Iannotti JP, Ham A, Bleuit J, Chen JH. Effects of fluid shear stress on bone cells. Biorheology. 1994;31:163–170. doi: 10.3233/bir-1994-31204. [DOI] [PubMed] [Google Scholar]
- Wolfe RP, Ahsan T. Shear stress during early embryonic stem cell differentiation promotes hematopoietic and endothelial phenotypes. Biotechnology and bioengineering. 2013;110:1231–1242. doi: 10.1002/bit.24782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an fit-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sokabe T, Watabe T, Miyazono K, Yamashita JK, Obi S, Ohura N, Matsushita A, Kamiya A, Ando J. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. American journal of physiology. Heart and circulatory physiology. 2005;288:H1915–H1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- Ye F, Kim C, Ginsberg MH. Reconstruction of integrin activation. Blood. 2012;119:26–33. doi: 10.1182/blood-2011-04-292128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda M, Kimura T, Tohmonda T, Uchikawa S, Koba T, Takito J, Morioka H, Matsumoto M, Link DC, Chiba K, Okada Y, Toyama Y, Horiuchi K. Dual functions of cell-autonomous and non-cell-autonomous ADAM10 activity in granulopoiesis. Blood. 2011;118:6939–6942. doi: 10.1182/blood-2011-06-357210. [DOI] [PubMed] [Google Scholar]
- Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM, Orlic D. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7:335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- Yokomizo T, Dzierzak E. Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development. 2010;137:3651–3661. doi: 10.1242/dev.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida C, Takeich M. Teratocarcinoma cell adhesion: Identification of a cell-surface protein involved in calcium-dependent cell aggregation. Cell. 1982;28:217–224. doi: 10.1016/0092-8674(82)90339-7. [DOI] [PubMed] [Google Scholar]
- Zaslona Z, Serezani CH, Okunishi K, Aronoff DM, Peters-Golden M. Prostaglandin E2 restrains macrophage maturation via E prostanoid receptor 2/protein kinase A signaling. Blood. 2012;119:2358–2367. doi: 10.1182/blood-2011-08-374207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Cowin SC, Weinbaum S. A fiber matrix model for fluid flow and streaming potentials in the canaliculi of an osteon. Annals of biomedical engineering. 1994;22:280–292. doi: 10.1007/BF02368235. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe ML. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell stem cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu RY, Birkett NC, Okino KH, Murdock DC, et al. Stem cell factor is encoded at the SI locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]