Abstract
Individuals with seasonal affective disorder (SAD) may have a decreased retinal sensitivity in the non-image forming light-input pathway. We examined the post illumination pupil response (PIPR) among individuals with SAD and healthy controls to identify possible differences in the melanopsin signaling pathway. We also investigated whether melanopsin gene (OPN4) variations would predict variability in the PIPR. Fifteen SAD and 15 control participants (80% women, mean age 36.7 years, SD = 14.5) were assessed in the fall/winter. Participants were diagnosed based on DSM-IV-TR criteria. Infrared pupillometry was used to measure pupil diameter prior to, during, and after red and blue stimuli. In response to blue light, the SAD group had a reduced PIPR and a lower PIPR percent change relative to controls. The PIPR after the blue stimulus also varied on the basis of OPN4 I394T genotype, but not OPN4 P10L genotype. These findings may indicate that individuals with SAD have a less sensitive light input pathway as measured by the PIPR, leading to differences in neurobiological and behavioral responses such as alertness, circadian photoentrainment, and melatonin release. In addition, this sensitivity may vary based on sequence variations in OPN4, although a larger sample and replication is needed.
Keywords: Seasonal affective disorder, melanopsin, pupillometry
1. Introduction
Seasonal affective disorder (SAD) accounts for approximately 10–20% of outpatients with recurrent depression (Magnusson and Partonen, 2005). Treatment of this large segment of depressed individuals may be improved with a better understanding of the etiology of SAD. Although the pathophysiologic mechanisms underlying SAD remain unknown, one hypothesis postulates a seasonal variation in retinal subsensitivity (Reme et al., 1990; Hebert et al., 2002). Retinal subsensitivity to light is suggested by many of the phenomena observed in SAD including: the regular winter timing of depressive episodes, a lengthened melatonin release profile during longer winter nights (Wehr et al., 2001), the utility of light therapy as an antidepressant treatment (Rohan, et al., 2009), and retinal electrophysiology research (reviewed below). Individual differences in retinal signaling may explain retinal subsensitivity (Wehr et al., 2001). Specifically, retinal subsensitivity in SAD may result from the absence of a seasonal change in retinal sensitivity found in healthy controls, such that the retina fails to become more sensitive to the low light levels of winter. In contrast to previous studies, the pupillometry method in the present study is designed to isolate the response of melanopsin-containing, intrinsically photosensitive retinal ganglion cells (ipRGC), which are the primary class of photoreceptors entraining the circadian clock. A polymorphism in the melanopsin gene has been associated with increased risk of SAD, suggesting that the melanopsin gene may be etiologically significant in SAD (Roecklein et al., 2009).
In addition, melanopsin-driven processes have significant overlap with SAD symptomatology. Melanopsin cells in the retina project to multiple areas in mammals (Do and Yau, 2010), and if these projections are conserved in humans, they would include areas involved in circadian regulation (suprachiasmatic nuclei, intergeniculate leaflet), energy homeostasis (lateral hypothalamus), and sleep regulation (ventral suparaventricular zone, preoptic nucleus; Hattar et al., 2002; Hannibal and Fahrenkrug, 2004; Hattar et al., 2006). Circadian, energy, and sleep processes are either involved in the symptoms of SAD (e.g., fatigue), or hypothesized to be etiologically significant in SAD.
Given the overlap between SAD symptomatology and melanopsin-driven processes, we hypothesized that retinal subsensitivity in SAD may be mediated at least in part by melanopsin containing ipRGCs. Theoretically the ipRGC sensitivity in SAD could be lower than that of healthy individuals, such that the environmental irradiance levels in winter may fall below this threshold, leading to depression. The present study measures melanopsin cell sensitivity in individuals with SAD compared to non-depressed controls.
Melanopsin containing ipRGCs project from the retina to the suprachiasmatic nucleus, which serves as a circadian clock, and have a peak sensitivity of ~482 nm when measured in vitro (Provencio et al., 2001; Lucas et al., 2001; Hattar et al., 2002; Berson et al., 2002; Gooley et al., 2003; Dacey et al., 2005) and peak sensitivity of 482 nm when measured in vivo (Gamlin et al., 2007; Markwell et al., 2010). In addition to entraining the circadian clock, ipRGCs are involved in the pupil light reflex, in which the pupil constricts and redilates in response to light. The pupil area in melanopsin knockout mice after 1 min of blue light is much larger (i.e., less constricted) than that of wild-type mice (Hattar et al., 2003; Lucas et al., 2003). Using primate cell recording techniques, Gamlin et al. (2007) and Dacey et al. (2005) demonstrated that, unlike rods and cones, ipRGCs have a sustained response after removal of the light source. This results in continuing constriction of the pupil after light off and is called the post-illumination pupil response (PIPR). The PIPR is intrinsic to melanopsin-containing ipRGCs (Gamlin et al., 2007). Therefore, the magnitude of the PIPR response to blue light reflects the sensitivity of the melanopsin pathway (Gamlin et al., 2007). The PIPR then provides an elegant way to isolate the contributions of ipRGCs from those of rods and cones. The PIPR has been measured in a range of healthy and clinical populations (Gamlin et al., 2007; Kawasaki et al., 2007; Kardon et al., 2009; Kankipati et al., 2010; McDougal et al., 2010; Kardon et al., 2011; Kankipati et al., 2011; Feigl et al., 2011; Park et al., 2011; Kawasaki et al., 2012; Feigl et al., 2012), and the present design is based in part on the methods in these studies. However, the PIPR has yet to be measured in SAD.
Electroretinography (ERG) is another tool that has been used to assess retinal sensitivity in SAD. ERG records electrical responses at the corneal surface to measure the function of retinal cells such as rods (scoptic) or cones (photopic), but not retinal ganglion cells. Photoreceptor (and bipolar cell) sensitivity is assessed by the measure log K, which is a calculation of the intensity of light that evokes half of the maximum (Vmax) rod or cone response. Using this measure, Lavoie et al. (2009) found that rods were less sensitive in SAD patients compared to controls during winter measurements. They also showed that the Vmax amplitudes of both rods and cones were reduced in SAD patients compared to controls. Most interestingly, all three of these measures were normalized in SAD following light therapy treatment (Lavoie et al., 2009). Decreased rod sensitivity seems to be a state marker for depression in SAD, as Gagne and Hebert (2011) also found that rods had decreased sensitivity in SAD during winter, which normalized during the summer. While studying the effects of light history on retinal sensitivity, Gagne and Hebert (2011) identified a possible trait marker for SAD. In participants with SAD, exposure to bright light for 1 hour prior to scoptic measurement caused a decrease in Vmax during winter and summer measurements (Gagne and Hebert, 2011). Because ipRGCs are most sensitive to blue light, Gagne et al., (2011) compared retinal responses of SAD and control participants after exposure to red or blue light. In response to blue light, both groups had a decrease in an ERG measure that is driven by ON-bipolar cells and Muller cells. These data reviewed here support a role for retinal subsensitivity in SAD, but do not assess the specific contribution of ipRGCs. Given the relationship between SAD, retinal subsensitivity, and gene polymorphisms in melanopsin, we hypothesize that ipRGCs specifically will be less sensitive in SAD participants than in controls when measured using a unique pupillometry measure specific to ipRGCs.
The present study was designed to specifically test the sensitivity of ipRGCs in SAD using a melanopsin-specific pupillometry test. M1-type melanopsin cells are directly involved in the pupillary light reflex as well as in circadian photoentrainment (Schmidt et al., 2011). Hence, impairment in one process (i.e., pupil constriction) may reflect impairment in the other processes mediated by M1 cells (i.e., photoentrainment). Although rods and cones also contribute to circadian photoentrainment (Provencio, 2011), melanopsin cells drive 40–50% of the response (Do and Yau, 2010). Because melanopsin cells are such a large component of the pathway implicated in retinal subsensitivity in SAD, it is important to investigate whether there may be a decrease in melanopsin cell sensitivity. Therefore, in the present study, we compared individuals with SAD and controls on the melanopsin-driven PIPR. We also examined differences in the PIPR by melanopsin gene (OPN4) sequence variations. Although others (i.e., Higuchi et al., 2013) have found that the steady-state pupil diameter varies as a function of melanopsin gene variation I394T, this will be the first test of the melanopsin-specific PIPR in SAD with both the P10L and I394T melanopsin gene variations.
2. Methods
2.1 Participants
Participants age 18–65 were recruited from the greater Pittsburgh, Pennsylvania metropolitan area through community advertising (latitude 40°26′N). The institutional review board of the University of Pittsburgh approved this study, and participants gave informed consent and received compensation. None of the participants reported a history of psychotic or bipolar disorder, sleep disordered breathing, narcolepsy, or current substance use disorder. All participants were studied during the fall or winter months in 2011 and 2012 while individuals in the SAD group were in a Major Depressive Episode.
2.2 Clinical Assessments
SAD group participants met Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; APA, 2000) criteria for unipolar Major Depressive Disorder With a Seasonal Pattern according to the Structured Clinical Interview for DSM-IV Axis I Disorders - Research Version (SCID-I; First et al., 2002). Participants also completed the Modified Seasonal Pattern Assessment Questionnaire (M-SPAQ; Blouin et al., 1992; Rosenthal et al., 1984), the Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorder version (SIGH-SAD; Williams et al., 1992), and the Beck Depression Inventory, Second Edition (BDI-II; Beck et al., 1996). Inclusion criteria for the SAD group were (1) a diagnosis of MDD-SP based on the SCID, (2) a SIGH-SAD score of > 19 total, > 9 on the HAM-D subscale, and > 4 on the atypical subscale which defines an SAD episode (Terman et al., 1990), and (3) a Global Seasonality Score (GSS) from the M-SPAQ of > 11, endorsing “feeling worst” in January and/or February (with or without other months) but not July and/or August, and perceiving seasonal changes as at least a “moderate” problem (Kasper et al., 1989). Inclusion criteria for the control group were (1) no history of mood disorder based on the SCID, (2) scores below episode criteria listed above on the SIGH-SAD, (3) a GSS of 8 or 9 and endorsing “no problems” with seasonal changes on the M-SPAQ, or GSS < 8 (Kasper et al., 1989), and (4) BDI-II score < 10.
2.3 Stimuli
Lights of equal corneal irradiance (13.7 log Photons/cm2/s) were presented for 30s ON, and 120s OFF. Retinal irradiance was calculated to account for age-related decreases in lens transmission and pupil diameter during the stimulus. Using age and pupil diameter during stimulus presentation, retinal irradiance was calculated for each participant. Stimuli were turned on and off electronically using a commercially available remote receiver and E-Prime 2 software (Psychology Software Tools, Inc., Pittsburgh, PA). Round light emitting diode bulbs (Super Bright LEDs; St. Louis, MO) were placed behind an 8.5″ diameter mylar diffuser (Speedotron, Chicago, IL) at a distance of 17″ from the eye (~29° of the visual field; lenses were not used to focus the stimulus on a point in the retina). The LED lights were presented to both eyes, and pupil responses were measured in the left eye in all subjects, so the consensual response was not used. Stimuli are 22.68 nm (467.7 nm blue light) and 15.78 nm (632.9nm red light) full-width half-maximum (FWHM), and absolute corneal irradiance was measured at the approximate location of the participants’ eye in the headrest with a 50-micron width aperture spectrophotometer (USB4000 Fiber Optic Spectrometer, Ocean Optics, Dunedin, FL). Due to findings that ipRGCs may be bistable and responses to blue light could be potentiated by red light (Melyan et al., 2005; Mure et al., 2009; Mure et al., 2007), we did not randomize the order of stimuli presentation. Instead, the blue stimulus was presented first, followed by red. This pattern was repeated twice.
Calculated retinal irradiance (Er) was averaged across all subjects for the blue and red stimuli (Blue Er=12.3 log Photons/cm2/s; Red Er=12.5 log Photons/cm2/s; Pokorny et al., 1987). We calculated the retinal irradiance (Er) based on the measured corneal irradiance (Ec), the lens transmittance for the given wavelength (T(λ, age) =10−D; where D(λ, age) is the optical density from Pokorny 1987), the solid angular size of the light screen (Ωscreen=0.378 steradians; from the diameter of the screen, d=6.30 cm, which was at a distance ds=8.66 cm from the subject’s pupil), and the solid angular size of the image on the retina (Ωeye; which was calculated based on the size of the pupil aperture diameter, a=3--5 mm, and the typical focal length of the adult human eye, f=17.0 mm,).
Ωscreen = 2π (1-cos(arctan(d/2) / ds) = 0.378 steradians
Ωeye = 2π (1-cos(arctan((a/2) / f ) = 0.03 -- 0.05 steradians
Er = (Ωeye/Ωscreen) T(λ, age) Ec
The minimum pupil diameter during stimulus presentation was used for the individual pupil aperture, a, for each participant, and was M = 0.35 mm for the blue stimulus and M = 0.34 mm for the red stimulus. However, individual diameter and age were used to calculate retinal irradiance for each participant.
2.4 Absence of blue light hazard
Blue wavelength light can lead to photochemical injury to the retina between 400nm – 550nm (Sliney, 1994). To ensure the safety of the light stimuli, the time-integrated (60s) hazard-weighted irradiance was calculated and found to be lower than the limit established by the American Conference of Governmental Industrial Hygienists (ACGIH, 2011) for a large angle light source by a factor of 36,000.
2.5 Pupillometry
Pupil area was measured with near-infrared illumination and solid-state video pupillometry with the EyeTrac 6 at 60 Hz using an EYE-TRAC (R) 6000 processing unit (Applied Science Laboratories, Inc.; Bedford, MA). The focal length was not changed from factory setting, which was 27mm with an F-Number of 4.5 for the EyeStart camera. Testing was constrained to between 2:00 pm and 5:30 pm, as data suggest that the magnitude of the blue PIPR (Zele et al., 2011) and iris constriction varies across the day (Figueiro et al., 2005). An 11 min dark adaptation period was used prior to the first blue stimulus, although level of dark or light adaptation is unlikely to diminish the target response because melanopsin cells show resistance to bleaching and little adaptation under continuous light (Zhu et al., 2007). Participants were tested alone in a dark room, but could communicate with experimentors during the study by intercom. Due to significant autonomic modulation of the pupil and resultant potential effects of noise or surprise on pupil constriction, experimenters refrained from contacting participants by intercom during testing. Participants were instructed to maintain fixation at a point projected on a computer screen that was visible when the stimuli were either on or off. Participants rested in a table-mounted adjustable forehead and chinrest to minimize head movement. Data were processed using EyeNal Analysis Software for the ASL EyeTrac 6 pupillometry system (Applied Science Laboratories, Inc.; Bedford, MA), and blinks were removed using custom scripts in MATLAB software (MathWorks, Inc.; Natick, MA). Prior to removing blinks, the percentage of data lost to blinks and fixation loss was calculated. Calibration of the pupillometer with known size standard stimuli yielded a scaling factor of 11.9 and an intercept of 0.181. These values were used to convert the arbitrary units of pupil diameter recorded by the ASL system to millimeters.
2.6 Genotyping
In past studies, two coding variations in OPN4 have been tested for increased risk of SAD (Roecklein et al., 2009). These two variations are the only coding variations in OPN4, and are referred to as P10L (rs2675703) and I394T (rs1079610). Both single nucleotide polymorphisms (SNPs) were genotyped from saliva samples for the present study using high-resolution melt analysis based on the melting behavior of fluorescently labeled DNA (Reed et al., 2007). Due to variations in the frequency of OPN4 alleles across ethnicities, only those of Caucasian ancestry were assessed in the present study.
2.7 Visual Information
Although not confirmed by eye or medical examination, all participants reported no history of any retinopathy, glaucoma, cataracts, amblyopia, macular degeneration, congenital color vision deficiencies, or any type of blindness (e.g., scotoma, night blindness, etc.). Individuals with corrected vision were not excluded from the study, but were encouraged to wear contact lenses during testing if possible to minimize interference during recording from glasses frames.
2.8 Statistical Analysis
Prior to data analysis, participants with more than 10% data loss were excluded from analyses (n = 2). Data analysis included (1) blink removal, (2) averaging pupil diameter (mm) across epochs of testing, and (3) diagnostic and genotype group comparisons. Groups were compared on the frequency of fixation losses and blinks. We used linear interpolation to define and replace blink data points previously described by Steinhauer et al. (2000; MATLAB, Natick, MA). The baseline was defined as the average pupil diameter during the 7 seconds prior to each stimulus. Constriction during lights on was measured as the mean pupil diameter during the 30-second stimulus (i.e., mean diameter from stimulus onset to 30 seconds after onset). Sustained response was defined as the mean of the diameter starting 10 seconds after stimulus offset for 30 seconds (i.e., 10 seconds after stimulus offset to 40 seconds after stimulus offset). The re-adaptation period in dark after stimulus presentation was 2 minutes, although shorter durations have been used (Herbst et al., 2011). We calculated the following values consistent with Kankipati et al. (2010, 2011):
PIPR (mm) = [baseline pupil diameter mm – Sustained pupil diameter mm]
PIPR Change (%) = [(PIPR X 100)/Baseline]
Net PIPR (mm) = (Blue PIPR – Red PIPR)
Net PIPR Change (%) = [Blue PIPR Change (%) – Red PIPR Change (%)]
Analysis of covariance (ANCOVA) was used to compare groups on these outcome variables, including the diameter during lights on. The Composite Scale of Morningness (CSM; Smith, Reilly, & Midkiff, 1989) was used to estimate chronotype for each person. Variables that may be associated with the PIPR or diagnosis such as chronotype (CSM score), time of awakening, calculated retinal irradiance, time of day at assessment, age, gender, and calculated retinal irradiance were tested for correlation with outcome variables, group association, and were included as covariates in ANCOVA if significantly associated (only age and gender were ultimately retained as covariates). All ANCOVAs were conducted using SPSS Statistics for Macintosh, version 19.0 (SPSS Inc., Chicago, IL). Summary data are reported as mean (M) ± standard deviation (SD). All tests were two-tailed, and a P value of 0.05 was used.
Our first study hypothesis was that groups would not differ on variables expected to influence retinal irradiance including age, baseline pupil diameter, pupil diameter during the stimulus presentation, and calculated retinal irradiance. Second, we tested to be sure groups did not differ on factors that could affect the sensitivity of the melanopsin system such as time of assessment, daylength on the day of testing, and individual chronotype. Assuming that those factors were equally distributed across the SAD and control groups, our third and main, hypothesis was that individuals in the SAD group would demonstrate a less robust PIPR in response to the blue test stimulus compared to the control group, but that no differences between groups in response to the red control stimulus would be observed.
3. Results
Groups did not differ based on age (F1,29 = 0.058, P = 0.81), or gender (X21,29 = 0.995, P = 0.32). Participants ranged from age 20 to 60, and the mean and standard deviation of age by group are reported in Table 1. In addition, age, gender, daylength on the testing day, time of testing, and calculated retinal irradiance were not significantly correlated with the main outcome variable, the blue PIPR (all P’s > 0.05; see Table 1) or associated with group membership (see Table 1). Age and gender were retained as covariates in the main analyses. The percentage of data lost due to fixation losses and blinks was calculated, and did not differ between groups (SAD: M = 12%, SD = 8%; Control: M = 10%, SD = 7%; F (1, 29) = .42, p = .53).
Table 1.
Demographic Characteristics and Baseline Measures Compared Between SAD Participants and Controls; M(SD).
| SAD (n = 15) | Controls (n = 15) | |
|---|---|---|
| Age (y) | 36.1(14.8) | 37.4(14.7) |
| % Female | 86.8% | 73.4% |
| Baseline pupil diameter (mm) | 5.73(1.14) | 5.99(1.03) |
| MEQa | 48.4(12.8) | 54.5(8.8) |
| Daylength on assessment (hh:mm) | 11:03(0:34) | 11:18(0:33) |
| Time of assessment (hh:mm) | 15:33(1:06) | 15:31(1:37) |
| Calculated retinal irradiance | ||
| Blue (log photons/cm2/s) | 12.27(.22) | 12.25(.18) |
| Red (log photons/cm2/s) | 12.49(.18) | 12.44(.14) |
All P’s were greater than 0.05 (ns).
MEQ - Morningness Eveningness Questionnaire measure of chronotype.
Although chronotype as measured by the CSM was not associated with the PIPR or with group membership (p’s > .05), individuals in the SAD group had lower scores indicating a trend towards evening chronotype (M = 32.3) and controls had a higher mean indicating a relative trend towards morning chronotype (M = 36.9). However, both groups fall in the “intermediate chronotype” scoring range (i.e., 27–41) on the CSM. Individuals did differ based on self-reported time of awakening (F1,29 = 4.29, P = 0.048), with the SAD group getting up later on average (M = 8:09 AM) compared to the control group (M = 6:37 AM). However, time of awakening was not associated with the main outcome variable, the PIPR.
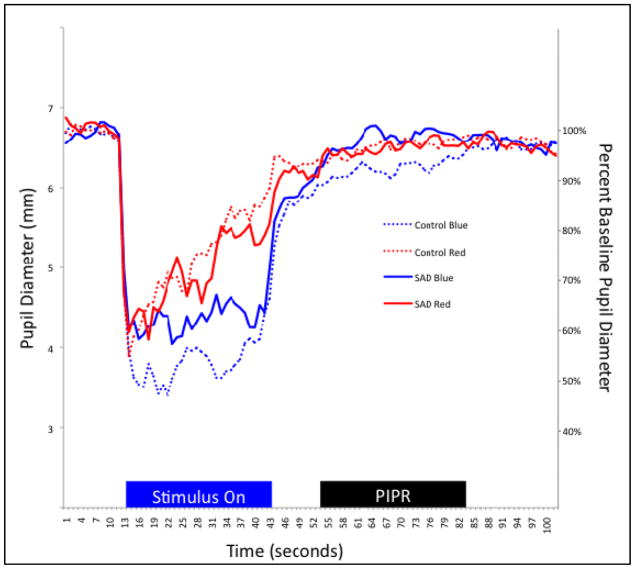
Pupil diameter in response to the blue and red stimuli was plotted against time for representative SAD and control group participants in Figure 1. Means and standard deviations for the 7-second baseline pupil diameter, 30-second sustained response after light offset, PIPR (mm), and PIPR Change (%), Net PIPR, and Net PIPR Change (%) are shown in Table 2. Diagnostic groups did not differ on the measure of baseline pupil diameter prior to the first test stimulus (F1,29 = 0.09, ns), or on the measure of pupil diameter during the blue stimulus duration (F1,29 = 1.46, ns) or red stimulus duration (F1,29 = 2.16, ns). There was no relationship between baseline pupil diameter and net PIPR change (r2 = −0.16, ns).
Figure 1.
Time trace plot of the pupillary response to the blue and red stimuli for one representative SAD participant and one Control participant.
Notes: Stimulus duration, shown in the blue “Stimulus On” block, was for 30 seconds, following 7 seconds of baseline recording. PIPR, shown in the black “PIPR” block, was measured starting 10 seconds after light offset for 30 seconds. Oscillations evident in the individual traces are attributed to the lack of midriasis and stimulus presentation to both eyes, including the eye being measured.
Table 2.
Diagnostic group comparisons on PIPR outcome variables.
| SAD
|
Controls
|
|||||
|---|---|---|---|---|---|---|
| R | B | B-R (Net) | R | B | B-R (Net) | |
| Baseline pupil (mm) | 5.59(1.15) | 5.73(1.14) | 0.15(0.35) | 5.80(1.14) | 5.99(1.03) | 0.19(0.40) |
|
| ||||||
| Stimulus (mm) | 4.01(1.06) | 3.99(1.04) | −0.03(0.31) | 3.79(0.72) | 3.79(0.60) | −0.01(0.25) |
| Sustained (mm) | 5.65(1.05) | 5.86(1.05) | 0.21(0.29) | 5.78(0.96) | 5.76(1.05) | −0.02(0.32) |
| PIPR (mm) | −0.063(0.68) | 0.01(0.28)* | −0.07(0.27) | 0.02(0.47) | 0.23(0.34)* | 0.21(0.60) |
| PIPR Change (%) | −2.29(12.3) | −0.79(5.56)* | −0.86(5.73) | −0.50(8.72) | 3.85(5.88)* | 4.35(11.22) |
Mean values are shown in mm or percentages, followed by the Standard Deviation in parentheses; Baseline - Average pupil diameter 7 seconds prior to light onset; Stimulus – Average diameter over 30 seconds during stimulus on; Sustained - Average diameter over 30 seconds, starting 10 seconds after light offset; B - blue stimulus; R - red stimulus; B-R - Net difference between values of either PIPR or PIPR percent change for the blue and red stimuli.
P < 0.05
3.1 Diagnostic group comparisons
The control group showed a significantly larger PIPR in response to the blue stimulus than the SAD group (F1,29 = 4.28, P < 0.05), when controlling for age and gender (Table 2). Similarly, the percent change from baseline in response to the blue stimulus was significantly higher in controls compared to SAD participants (F1,29 = 4.93, P < 0.05). Groups did not differ on other pupil metrics (Table 2). Although means were in the hypothesized direction for net PIPR percent change, this difference was not statistically significant (P = 0.12). This is the first piece of evidence suggesting a diminished melanopsin-specific sensitivity in SAD.
3.2 Genotype comparisons
The mean and standard deviation for pupil values by genotype are shown in Table 3, along with genotype frequency by group. The blue PIPR was significantly different across genotypes of I394T, as the rare CC genotype had the largest blue PIPR (0.54 mm), the CT was intermediate (0.16 mm), and the TT genotype was lowest (−0.03 mm). Specific contrasts indicated that the TT and CT groups were significantly different from one another. When I394T C allele carriers (CC and CT genotypes; n = 19) were compared to non-carriers (TT genotypes; n = 11), the C allele carriers had a significantly higher mean blue PIPR compared to those without the C allele (F1,29 = 6.21, P < 0.05). We can only speculate that genetic variants may affect melanopsin sensitivity given that our sample size is small for typical genetic association tests.
Table 3.
Melanopsin genotype groups comparisons; M(SD) or n(%).
| P10L
|
I394T
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | P | CC | CT | TT | P | |
| N | 17 | 9 | 3 | 2 | 17 | 11 | ||
| Baseline pupil (mm) | 5.65(1.04) | 6.16(1.33) | 6.14(0.42) | ns | 5.75(0.09) | 5.92(1.24) | 5.78(0.94) | ns |
| blue PIPR (mm) | 0.12(0.34) | 0.17(0.33) | −0.10(0.32) | ns | 0.54(0.56) | 0.16(0.30) | −0.03(0.27) | <0.05 |
| red PIPR (mm) | −0.11(0.64) | 0.22(0.50) | −0.20(0.32) | ns | −0.31(0.94) | 0.04(0.65) | −0.08(0.40) | ns |
| Net PIPR (mm) | −0.01(0.60) | 0.00(0.57) | 1.00(1.39) | ns | 0.43(1.10) | −0.05(0.55) | 0.24(0.91) | ns |
| Net PIPR Change (%) | 2.37(11.4) | 0.03(5.28) | 2.17(5.26) | ns | 17.17(29.34) | −0.05(7.01) | 1.71(5.02) | ns |
|
| ||||||||
| SAD group | 8(50%) | 6(38%) | 2(12%) | ns | 8(50%) | 7(44%) | 1(6%) | ns |
| Control group | 10(71.5%) | 3(21.5%) | 1(7%) | 3(20%) | 11(73%) | 1(7%) | ||
Notes: ns > 0.05; P10L – rs2675703; I394T – rs107961
4. Discussion
Our main study hypothesis, that the PIPR is diminished in SAD, was supported by the finding that the SAD group had a significantly smaller magnitude PIPR in response to the blue test stimulus compared to the control group. In addition, the PIPR percent change in response to the blue stimulus was significantly higher in the control group compared to the SAD group. However, the net PIPR and the net PIPR percent change were not significantly different, although group means were in the predicted direction. These results are unlikely to be due to differences in retinal irradiance or melanopsin sensitivity as diagnostic groups and outcome variables did not vary based on variables expected to influence retinal irradiance including age, baseline pupil diameter, pupil diameter during the stimulus presentation, or retinal irradiance calculated post-hoc based on age and pupil diameter during the stimulus. In addition, diagnostic groups and outcome variables did not vary by factors that could affect the sensitivity of the melanopsin system such as time of assessment, photoperiod on the day of testing, and chronotype.
The I394T genotype was associated with the blue PIPR, but the P10L genotype was not associated with pupil measures. Because only 2 individuals had the rare I394T (CC) genotype and 3 individuals had the rare P10L (TT) genotype, these analyses are preliminary and likely underpowered. Others recently reported that I394T genotype interacts with stimulus intensity to predict the steady-state pupil diameter in healthy college students (Higuchi et al., 2013), which may be consistent with our findings here. However, Higuchi et al. (2003) did not measure the PIPR but rather measured steady state pupil diameter during stimulus presentation at various intensities, and did not genotype P10L, the variation found to be previously associated with SAD (Roecklein et al., 2009).
Both group means for chronotype fell in the intermediate range, although the SAD group mean trended towards relative eveningness compared to the control group. This is consistent with misalignment between the circadian clock and sleep/wake cycle seen in SAD (Lewy, 2007; Lewy et al., 2006). Time of awakening was consistent in that the SAD group reported waking later than controls. However, neither chronotype nor time of awakening were associated with the PIPR, so these variables are unlikely to explain the group difference found here on the PIPR.
To our knowledge this is the first study to test and report a diminished ipRGC-mediated blue PIPR in patients with SAD compared to controls with no history of depression. The implications and next steps for this line of research are detailed below.
4.1 Limitations
The magnitude of the PIPR to blue light observed in our study is lower (i.e., .01 mm in SAD and .23 mm in controls) than the values observed by others (e.g., .7 mm in controls; Gamlin et al., 2007). Although we used a light source of 13.7 log photons/cm2/s at the cornea, it was not presented in Maxwellian view, but rather was a diffuse light source presented without pharmacological pupil dilation. The calculated effective retinal irradiance was 12.3–12.5 log photons/cm2/s due to age dependent light absorption by the lens and due to pupil constriction during stimulus presentation. However, age, minimum pupil diameter during the stimulus, and calculated retinal irradiance were not associated with the magnitude of the blue PIPR, nor were those variables different between diagnostic groups, indicating that individual differences in retinal irradiance are unlikely to explain our findings. Because a threshold retinal irradiance of at least 12 log photons/cm2/s is required to produce the PIPR (Gamlin et al., 2007), our calculated retinal irradiance of between 12.3 and 12.5 log photons/cm2/s was on the very low end of irradiance needed to elicit the PIPR. Using higher irradiance, and either pupil dilation or a stimulus presented in Maxwellian view would further increase and standardize retinal irradiance across participants in future research. Future studies could also adjust stimuli before testing based on age-predicted lens absorption to better equate retinal irradiance across participants (Coren, 1987; Pokorny et al., 1987; Gamlin et al., 2007), although the standard deviations between calculated retinal irradiances in our study was only 0.19 log photons/cm2/s for the blue stimulus, and 0.16 log photons/cm2/s for the red stimulus.
An additional limitation may result from the current study’s measurement of circadian time utilizing the MEQ, which is a strong but imperfect predictor of objective measures of circadian time such as dim-light melatonin onset (DLMO; Bernert et al., 2006). However, exact identification of circadian time may not be crucial for the current sample. Data from Zele et al. (2011) indicate that the magnitude of the PIPR is highest and relatively stable between CT3 and CT11, and in the evening the amplitude of the PIPR decreases about 2.4 hours prior to DLMO, an hour or so prior to bedtime. Therefore, since our participants were all studied during the predicted afternoon plateau of the maximum PIPR amplitude, and prior to the period of decrease in PIPR, it is unlikely that significant variation due to the timing of assessment or circadian phase is present in our study. However, others have found a similar peak PIPR during this time, but not a plateau (Munch et al., 2012). Therefore, future studies could measure DLMO to confirm that any effects of circadian variation on the PIPR are removed or controlled in group comparisons, ensuring that the results here, indicating that the diagnostic group difference is not due to circadian time, are indeed valid.
Recent light history can alter the sensitivity of melanopsin containing cells (Wong, et al., 2005), which may have an unknown effect on our measures. Importantly, circadian responses are more sensitivie to light during short photoperiods when tested in Syrian hamsters (Glickman et al., 2012). Determining how photoperiod alters circadian responses to light (Glickman et al., 2012), including the effect on the PIPR, will be an important step for future studies with seasonal comparisons between human subjects. In our study, we attempted to control for light history by including photoperiod on the day of assessment as a covariate. No variance in dependent variables was accounted for by photoperiod and groups did not differ based on the photoperiod on the day of testing. A more accurate measurement of light received by a given individual prior to pupillometry could be collected in future studies with the use of light-sensitive actigraphy.
Subjects were tested with freely reactive pupils, while not under midriasis. A freely reactive pupil initially constricts in response to a stimulus, decreasing the light reaching the retina, leading to redilation until a steady state is achieved under constant illumination. The pupil diameter at steady state differs between individuals, therefore allowing differing amounts of light to reach the retina. Although differing steady state diameter could have affected the current results, pupil diameter and retinal irradiance based on pupil diameter were not associated with outcome variables or group membership in the present study and are therefore unlikely to have confounded our tests. Further, individuals under pharmacologically induced midriasis may also have different overall pupil sizes, and would also therefore differ in the amounts of light at the retina unless a Maxwellian presentation is used. Our use of freely reactive pupils and non-Maxwellian presentation are more similar to the naturalistic responses of this circadian photoreceptive system in humans. In addition, other authors have presented stimuli unilaterally, and measured the consensual response in the non-dominant eye to control for differences in retinal irradiance. In the present case, stimuli were presented bilaterally, and measured in the left eye while dominance was not assessed. It is likely that bilateral presentation caused the diameter during stimulus presentation to be smaller than it would be with midriasis, thereby reducing the retinal irradiance. However, retinal irradiance was calculated post-hoc, taking pupil diameter during stimulus presentation, and age, into account. Although this may introduce noise into measurement, lack of midriasis is a more naturalistic measurement setting. In addition, the comparison here of red and blue light is confounded by the difference in retinal irradiance due to selective lens absorption of blue wavelengths. In future studies, the retinal irradiance should be equated (ours were 12.3 blue vs. 12.3 red log photons/cm2/s) allowing for a comparison of the red and blue responses.
The age of participants ranged from 20 to 60 years, and was relatively evenly distributed across this range, with no evidence of skewness or kurtosis (e.g., there were not more older than younger participants). However, lens density increases with increasing age. In the present study, we did not have the ability to increase the stimulus intensity for older individuals to ensure equal retinal irradiance across participants, but age and gender were covaried in our analyses, and age-dependent retinal irradiance was calculated and found to not differ between diagnostic groups. Since age is evenly distributed across old and younger individuals in this study, and participants were matched by age, the effect of age on retinal irradiance is not expected to explain the group difference found in this study.
Nine participants in the SAD group, and two participants in the control group reported taking medications or legal drugs that could potentially affect pupil diameter. Pupil responsiveness can be affected by drugs that affect both the sympathetic (e.g., nicotine, buproprion) and parasympathetic (e.g., caffeine, clomipramine) autonomic nervous system either agonistically or antagonistically. In the present study, participants were not excluded on the basis of medications, but this could be done in future studies to further control the potential effects of these medications and prevent any potential group differences. Although more individuals in the SAD group were taking medications compared to the control group, post hoc analyses confirmed that medication status was not associated with PIPR magnitude (data not shown). Including individuals taking medications increases the external validity of our results, but medication use will continue to be measured and controlled for in future studies.
The retinal subsensitivity hypothesis of SAD proposes that a seasonal variation in retinal sensitivity, with winter subsensitivity and normal functioning in summer, may explain SAD. Future studies should measure the PIPR in SAD and control participants in both summer and winter to determine if one of two situations exist: (1) retinal sensitivity may not increase in winter in SAD as predicted by the retinal subsensitivity hypothesis, or (2) individuals with SAD have lower melanopsin-specific sensitivity all year, which is only problematic when winter light levels fall below a given threshold.
Finally, we did not record the refraction or prescription for participants with corrected vision, or covary for whether or not participants wore contact lenses, although we did not have any participants who used glasses. Because some contact lenses are tinted, and tinting could cause attenuation of specific wavelengths of light, this will be recorded in future studies.
4.2 Conclusions
The present study indicates that the melanopsin-specific PIPR is less pronounced in SAD during winter compared to controls. If the reduced PIPR is confirmed to be due to SAD group membership rather than diabetes, glaucoma, retinitis pigmentosa or other systemic diseases, then the PIPR may represent a biomarker of SAD. In the present study, participants in both groups reported being free of diabetes, diabetic retinopathy, glaucoma and retinitis pigmentosa, but these and other systemic diseases will need to be more rigorously assessed in future studies. If retinal subsensitivity in SAD is mediated at least in part by reduced melanopsin sensitivity, a few implications are possible. Given the role of melanopsin in both circadian and acute behavioral effects of light, it is possible that reduced melanopsin sensitivity would lead to impaired photoentrainment of the circadian clock, reduced alertness, or even changes in sleep homeostasis, all of which may be etiologically significant in SAD (Stephenson et al., 2011). For example, men with SAD have a longer duration of melatonin release in winter compared to summer (Wehr et al., 2001). In humans, short wavelength light is the most effective for acute suppression of melatonin, indicating a primary role for melanopsin in melatonin suppression (Brainard et al., 2001; Thapan et al., 2001; Revell et al., 2010). Reduced melanopsin sensitivity, if confirmed upon replication, is likely to be only one of multiple risk factors in SAD, with others including other photoreceptors, or serotonergically mediated reduced sensitivity of the central clock to light (Stephenson et al., 2011).
In the future, it will be important to evaluate stimulus irradiance levels and threshold effects, control for recent light history and circadian time, and then determine the clinical significance of the PIPR if these results are replicated. Clinical challenges remaining in SAD treatment include that only about 53% of individuals with SAD respond to light therapy (Terman et al., 1989) and only 41% of those adhere to the treatment in future winters (Schwartz et al., 1996), possibly because the treatment as prescribed is not fully effective. Treatment response can also be incomplete when compared to summer spontaneous remission (Postolache et al., 1998). Further understanding of melanopsin’s etiological impact on SAD may improve treatment. If ipRGCs are hypofunctional in some or even most individuals with SAD, two possibilities exist: (1) light therapy may not work at all for these individuals, or (2) light therapy may need to be a higher intensity or longer duration than for other individuals with SAD. Until these two options are explored empirically, the mechanism behind melanopsins’ potential role in light therapy would be speculative. Studies have established that blue or blue-enriched lights at significantly lower light irradiances or durations, yield similar rates of improvement in SAD as traditional bright white light therapy units (Glickman et al., 2006; Anderson et al., 2009; Meesters et al., 2011), and are able to phase advance rhythms (Smith et al., 2009). Outstanding questions include whether the melanopsin cells are primary in mediating light therapy’s effects, and whether it may be possible to potentiate the effects of light therapy by using long wavelength light first (Rollag, 2008; Mure et al., 2009; Gooley et al, 2010). Furthermore, it is possible that the PIPR could be used to predict the timing, duration, and potential benefit of light therapy, opening the door to individualized treatment prescriptions in SAD. For example, given that some individuals with SAD don’t respond to light therapy, the PIPR might help predict which individuals should try antidepressants (e.g., Moscovitch et al., 2004; Modell et al., 2005; Lam et al., 2006) or cognitive behavioral therapy for SAD (Rohan et al., 2004; 2007; 2009). It could be, however, that subgroups of individuals with a diminished PIPR could change the light therapy time of administration, spectral composition, light intensity, or duration to achieve better results. Our work is preliminary, based on the limitations listed above, but this line of research has the potential to identify a simple and non-invasive neurological test for psychopathology.
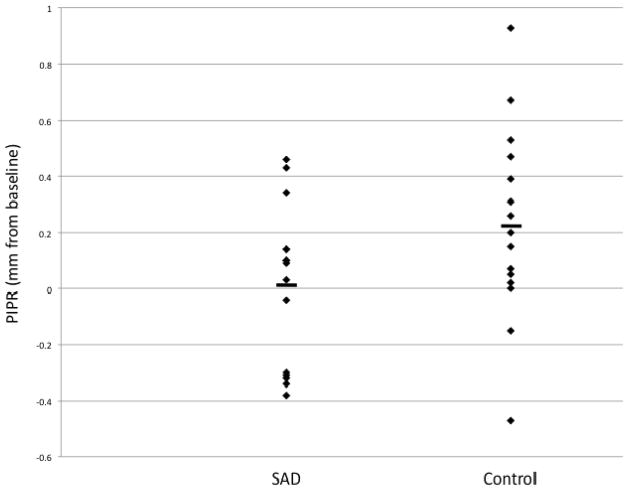
Figure 2.
Post-Illumination Pupil Response (PIPR) values expressed in mm as change from baseline pupil diameter, between SAD and control groups.
Note: The mean post-illumination pupil response (PIPR) is marked with a horizontal bar for each group. PIPR was measured from 10–40 seconds after stimulus off, and is presented here as a change from baseline. Baseline was calculated for the seven seconds prior to stimulus on.
Acknowledgments
We are grateful to Paul D. Gamlin, Ph.D., for assistance in the design of the study and comments on an early draft of the manuscript. Development of this manuscript was primarily supported by 1 R03 MH096119-01A1 to Kathryn A. Roecklein, Ph.D. as well as grant numbers UL1 RR024153 and UL1TR000005 to the University of Pittsburgh Clinical and Translational Science Institute (CTSI) from the National Institutes of Health.
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Conference of Governmental Industrial Hygienists. Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. American Conference of Governmental industrial Hygienists; Cincinnati, Ohio: 2011. Nonionizing radiation and fields; pp. 136–140. [Google Scholar]
- Anderson JL, Glod CA, Dai J, Cao Y, Lockley SW. Lux vs. wavelength in light treatment of Seasonal Affective Disorder. Acta Psychiatrica Scandinavica. 2009;120:203–212. doi: 10.1111/j.1600-0447.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- APA. The diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory - 2nd Edition Manual. The Psychological Corporation; San Antonio, Texas: 1996. [Google Scholar]
- Bernert RA, Hasler BP, Cromer KR, Joiner TE. SLEEP 2006 Annual Meeting of the Associated Professional Sleep Societies. 2006. Diurnal preferences and circadian phase: A meta-analysis; pp. A54–A55. [Google Scholar]
- Berson D, Dunn F, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Blouin A, Blouin J, Aubin P, Carter J, Goldstein C, Boyer H, Perez E. Seasonal patterns of bulimia nervosa. The American Journal of Psychiatry. 1992;149:73–81. doi: 10.1176/ajp.149.1.73. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. Journal of Neuroscience. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coren S. A rapid method to assess crystalline lens pigment density in vivo. Acta Ophthalmologica (Copenhagen) 1987;65:575–578. doi: 10.1111/j.1755-3768.1987.tb07043.x. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiological Reviews. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigl B, Mattes D, Thomas R, Zele AJ. Intrinsically photosensitive (melanopsin) retinal ganglion cell function in glaucoma Investigative ophthalmology and visual science. 2011;52:4362–4367. doi: 10.1167/iovs.10-7069. [DOI] [PubMed] [Google Scholar]
- Feigl B, Zele AJ, Fader SM, Howes AN, Hughes CE, Jones KA, Jones R. The post-illumination pupil response of melanopsin-expressing intrinsically photosensitive retinal ganglion cells in diabetes. Acta Ophthalmologica. 2012;90:e230–234. doi: 10.1111/j.1755-3768.2011.02226.x. [DOI] [PubMed] [Google Scholar]
- Figueiro MG, Bullough JD, Parsons RH, Rea MS. Preliminary evidence for a change in spectral sensitivity of the circadian system at night. Journal of Circadian Rhythms. 2005;3:14. doi: 10.1186/1740-3391-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/PSY SCREEN) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Gagne AM, Hebert M. Atypical pattern of rod electroretinogram modulation by recent light history: a possible biomarker of seasonal affective disorder. Psychiatry Research. 2011;187:370–374. doi: 10.1016/j.psychres.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Gagne AM, Levesque F, Gagne P, Hebert M. Impact of blue vs red light on retinal response of patients with seasonal affective disorder and healthy controls. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2011;35:227–231. doi: 10.1016/j.pnpbp.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Research. 2007;47:946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman G, Byrne B, Pineda C, Hauck WW, Brainard GC. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs) Biological Psychiatry. 2006;59:502–507. doi: 10.1016/j.biopsych.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Glickman G, Webb IC, Elliott JA, Baltazar RM, Reale ME, Lehman MN, Gorman MR. Photic sensitivity for circadian response to light varies with photoperiod. Journal of Biological Rhythms. 2012;27:308–318. doi: 10.1177/0748730412450826. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. Journal of Neuroscience. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Science Translational Medicine. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Fahrenkrug J. Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell and Tissue Research. 2004;316:99–113. doi: 10.1007/s00441-004-0858-x. [DOI] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. Journal of Comparative Neurology. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao H, Takao M, Berson D, Yau K. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert M, Beattie CW, Tam EM, Yatham LN, Lam RW. Electroretinography in patients with winter seasonal affective disorder. Psychiatry Research. 2004;127:27–34. doi: 10.1016/j.psychres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Hebert M, Dumont M, Lachapelle P. Electrophysiological evidence suggesting a seasonal modulation of retinal sensitivity in subsyndromal winter depression. Journal of Affective Disorders. 2002;68:191–202. doi: 10.1016/s0165-0327(00)00192-0. [DOI] [PubMed] [Google Scholar]
- Herbst K, Sander B, Milea D, Lund-Andersen H, Kawasaki A. Test-retest repeatability of the pupil light response to blue and red light stimuli in normal human eyes using a novel pupillometer. Frontiers in Neurology. 2011;2:10. doi: 10.3389/fneur.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi, et al. Melanopsin gene polymorphism I394T is associated with pupillary light responses in a dose-dependent manner. PLoS ONE. 2012;x:xx–xx. doi: 10.1371/journal.pone.0060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankipati L, Girkin CA, Gamlin PD. Post-illumination pupil response in subjects without ocular disease. Investigative Ophthalmology and Visual Science. 2010;51:2764–2769. doi: 10.1167/iovs.09-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankipati L, Girkin CA, Gamlin PD. The post-illumination pupil response is reduced in glaucoma patients. Investigative Ophthalmology and Visual Science. 2011;52:2287–2292. doi: 10.1167/iovs.10-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology. 2009;116:1564–1573. doi: 10.1016/j.ophtha.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic pupillometry in patients with retinitis pigmentosa. Ophthalmology. 2011;118:376–381. doi: 10.1016/j.ophtha.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Kasper S, Wehr TA, Bartko JJ, Gaist PA, Rosenthal NE. Epidemiological findings of seasonal changes in mood and behavior. A telephone survey of Montgomery County, Maryland. Archives of General Psychiatry. 1989;46:823–833. doi: 10.1001/archpsyc.1989.01810090065010. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Kardon RH. Intrinsically photosensitive retinal ganglion cells. Journal of Neuroophthalmology. 2007;27:195–204. doi: 10.1097/WNO.0b013e31814b1df9. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Crippa SV, Kardon R, Leon L, Hamel C. Characterization of pupil responses to blue and red light stimuli in autosomal dominant retinitis pigmentosa due to NR2E3 mutation. Investigative Ophthalmology and Visual Science. 2012;53:5562–5569. doi: 10.1167/iovs.12-10230. [DOI] [PubMed] [Google Scholar]
- Lam RW, Beattie CW, Buchanan A, Mador JA. Electroretinography in seasonal affective disorder. Psychiatry Research. 1992;43:55–63. doi: 10.1016/0165-1781(92)90141-o. [DOI] [PubMed] [Google Scholar]
- Lam RW, Beattie CW, Buchanan A, Remick RA, Zis AP. Low electrooculographic ratios in patients with seasonal affective disorder. The American Journal of Psychiatry. 1991;148:1526–1529. doi: 10.1176/ajp.148.11.1526. [DOI] [PubMed] [Google Scholar]
- Lam RW, Levitt AJ, Levitan RD, Enns MW, Morehouse R, Michalak EE, Tam EM. The Can-SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. The American Journal of Psychiatry. 2006;163:805–812. doi: 10.1176/ajp.2006.163.5.805. [DOI] [PubMed] [Google Scholar]
- Lavoie MP, Lam RW, Bouchard G, Sasseville A, Charron MC, Gagne AM, Tremblay P, Filteau MJ, Hebert M. Evidence of a biological effect of light therapy on the retina of patients with seasonal affective disorder. Biological Psychiatry. 2009;66:253–258. doi: 10.1016/j.biopsych.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Lewy AJ. Melatonin and human chronobiology. Cold Spring Harbor Symposium in Quantitative Biology. 2007;72:623–636. doi: 10.1101/sqb.2007.72.055. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proceedings of the National Academy of Science U S A. 2006;103(19):7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nature Neuroscience. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Magnusson A, Partonen T. The diagnosis, symptomatology, and epidemiology of seasonal affective disorder. CNS Spectrums. 2005;10:625–634. doi: 10.1017/s1092852900019593. quiz 621–614. [DOI] [PubMed] [Google Scholar]
- Meesters Y, Dekker V, Schlangen LJ, Bos EH, Ruiter MJ. Low-intensity blue-enriched white light (750 lux) and standard bright light (10 000 lux) are equally effective in treating SAD. A randomized controlled study. BMC Psychiatry. 2011;11:17. doi: 10.1186/1471-244X-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- Modell JG, Rosenthal NE, Harriett AE, Krishen A, Asgharian A, Foster VJ, Metz A, Rockett CB, Wightman DS. Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biological Psychiatry. 2005;58:658–667. doi: 10.1016/j.biopsych.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Moscovitch A, Blashko CA, Eagles JM, Darcourt G, Thompson C, Kasper S, Lane RM. A placebo-controlled study of sertraline in the treatment of outpatients with seasonal affective disorder. Psychopharmacology (Berlin) 2004;171:390–397. doi: 10.1007/s00213-003-1594-8. [DOI] [PubMed] [Google Scholar]
- Munch M, Leon L, Crippa SV, Kawasaki A. Circadian and wake-dependent effects on the pupil light reflex in response to narrow-bandwidth light pulses. Investigations in Ophthalmology and Visual Science. 2012;53:4546–4555. doi: 10.1167/iovs.12-9494. [DOI] [PubMed] [Google Scholar]
- Mure LS, Cornut PL, Rieux C, Drouyer E, Denis P, Gronfier C, Cooper HM. Melanopsin bistability: a fly’s eye technology in the human retina. PLoS One. 2009;4:e5991. doi: 10.1371/journal.pone.0005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mure LS, Rieux C, Hattar S, Cooper HM. Melanopsin-dependent nonvisual responses: evidence for photopigment bistability in vivo. Journal of Biological Rhythms. 2007;22:411–424. doi: 10.1177/0748730407306043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Rosenthal NE, Moul DE, Schwartz PJ, Oren DA. Effects of phototherapy on electrooculographic ratio in winter seasonal affective disorder. Psychiatry Research. 1993;49:99–107. doi: 10.1016/0165-1781(93)90098-2. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Rosenthal NE, Myers F, Schwartz PJ, Oren DA. Effects of season on electro-oculographic ratio in winter seasonal affective disorder. Psychiatry Research. 1995;59:151–155. doi: 10.1016/0165-1781(95)02788-2. [DOI] [PubMed] [Google Scholar]
- Park JC, Moura AL, Raza AS, Rhee DW, Kardon RH, Hood DC. Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Investigations in Ophthalmology and Visual Science. 2011;52:6624–6635. doi: 10.1167/iovs.11-7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny J, Smith VC, Lutze M. Aging of the human lens. Applied Optics. 1987;26:1437–1440. doi: 10.1364/AO.26.001437. [DOI] [PubMed] [Google Scholar]
- Postolache TT, Hardin TA, Myers FS, Turner EH, Yi L, Barnett R, Matthews J, Rosenthal NE. Greater improvement in summer than with light treatment in winter in patients with seasonal affective disorder. The American Journal of Psychiatry. 1998;155:1614–1616. doi: 10.1176/ajp.155.11.1614. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. Journal of Neuroscience. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I. The hidden organ in your eyes. Scientific American. 2011;304:54–59. doi: 10.1038/scientificamerican0511-54. [DOI] [PubMed] [Google Scholar]
- Reed GH, Kent JO, Wittwer CT. High-resolution DNA melting analysis for simple and efficient molecular diagnostics. Pharmacogenomics. 2007;8:597–608. doi: 10.2217/14622416.8.6.597. [DOI] [PubMed] [Google Scholar]
- Reme C, Terman M, Wirz-Justice A. Are deficient retinal photoreceptor renewal mechanisms involved in the pathogenesis of winter depression? Archives of General Psychiatry. 1990;47:878–879. doi: 10.1001/archpsyc.1990.01810210086016. [DOI] [PubMed] [Google Scholar]
- Revell VL, Barrett DC, Schlangen LJ, Skene DJ. Predicting human nocturnal nonvisual responses to monochromatic and polychromatic light with a melanopsin photosensitivity function. Chronobiology International. 2010;27:1762–1777. doi: 10.3109/07420528.2010.516048. [DOI] [PubMed] [Google Scholar]
- Roecklein KA, Rohan KJ, Duncan WC, Rollag MD, Rosenthal NE, Lipsky RH, Provencio I. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. Journal of Affective Disorders. 2009;114:279–285. doi: 10.1016/j.jad.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan KJ, Roecklein KA, Haaga DAF. Biological and psychological mechanisms of seasonal affective disorder: A review and integration. Current Psychiatry Reviews. 2009;5:37–47. [Google Scholar]
- Rohan KJ, Roecklein KA, Tierney Lindsey K, Johnson LG, Lippy RD, Lacy TJ, Barton FB. A randomized controlled trial of cognitive-behavioral therapy, light therapy, and their combination for seasonal affective disorder. Journal of Consulting and Clinical Psychology. 2007;75:489–500. doi: 10.1037/0022-006X.75.3.489. [DOI] [PubMed] [Google Scholar]
- Rohan KJ, Tierney Lindsey K, Roecklein KA, Lacy TJ. Cognitive-behavioral therapy, light therapy, and their combination in treating seasonal affective disorder. Journal of Affective Disorders. 2004;80:273–283. doi: 10.1016/S0165-0327(03)00098-3. [DOI] [PubMed] [Google Scholar]
- Rollag MD. Does melanopsin bistability have physiological consequences? Journal of Biological Rhythms. 2008;23:396–399. doi: 10.1177/0748730408323067. [DOI] [PubMed] [Google Scholar]
- Rosenthal NE, Bradt GH, Wehr TA. Seasonal Pattern Assessment Questionnaire. National Institute of Mental Health; Bethesda, Maryland: 1984. [Google Scholar]
- Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends in Neuroscience. 2011;34:572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz PJ, Brown C, Wehr TA, Rosenthal NE. Winter seasonal affective disorder: a follow-up study of the first 59 patients of the National Institute of Mental Health Seasonal Studies Program. The American Journal of Psychiatry. 1996;153:1028–1036. doi: 10.1176/ajp.153.8.1028. [DOI] [PubMed] [Google Scholar]
- Sliney DH, Wolbarsht M. Safety with Lasers and Other Optical Sources: A Comprehensive Handbook. Plenum Press; New York: 1980. pp. 1–1035. [Google Scholar]
- Sliney DH. UV radiation ocular exposure dosimetry. Documenta Ophthalmologica. 1994;88:243–254. doi: 10.1007/BF01203678. [DOI] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. Journal of Applied Psychology. 1989;74:728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Medicine. 2009;10:287–294. doi: 10.1016/j.sleep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer SR, Condray R, Kasparek A. Cognitive modulation of midbrain function: task-induced reduction of the pupillary light reflex. International Journal of Psychophysiology. 2000;39:21–30. doi: 10.1016/s0167-8760(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Stephenson KM, Schroder CM, Bertschy G, Bourgin P. Complex interaction of circadian and non-circadian effects of light on mood: Shedding new light on an old story. Sleep Medicine Reviews [Epub], 1–10. 2011 doi: 10.1016/j.smrv.2011.09.002. doi.org/10.1016/j.smrv.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Terman MA, Terman JS, Quitkin FM, McGrath PJ, Stewart JW, Rafferty B. Light therapy for seasonal affective disorder. A review of efficacy. Neuropsychopharmacology. 1989;2:1–22. doi: 10.1016/0893-133x(89)90002-x. [DOI] [PubMed] [Google Scholar]
- Terman M, Terman J, Rafferty B. Experimental design and measures of success in the treatment of winter depression by bright light. Psychopharmacology Bulletin. 1990;26:505–510. [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. Journal of Physiology. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr TA, Duncan WC, Jr, Sher L, Aeschbach D, Schwartz PJ, Turner EH, Postolache TT, Rosenthal NE. A circadian signal of change of season in patients with seasonal affective disorder. Archives of General Psychiatry. 2001;58:1108–1114. doi: 10.1001/archpsyc.58.12.1108. [DOI] [PubMed] [Google Scholar]
- West KE, Jablonski MR, Warfield B, Cecil KS, James M, Ayers MA, Maida J, Bowen C, Sliney DH, Rollag MD, Hanifin JP, Brainard GC. Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. Journal of Applied Physiology. 2011;110:619–626. doi: 10.1152/japplphysiol.01413.2009. [DOI] [PubMed] [Google Scholar]
- Williams JB, Link MJ, Rosenthal NE, Amira L, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale - Seasonal Affective Disorder Version (SIGH-SAD) New York State Psychiatric Institute; New York: 1992. [Google Scholar]
- Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Zele AJ, Feigl B, Smith SS, Markwell EL. The circadian response of intrinsically photosensitive retinal ganglion cells. PLoS One. 2011;6:e17860. doi: 10.1371/journal.pone.0017860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tu DC, Denner D, Shane T, Fitzgerald CM, Van Gelder RN. Melanopsin-dependent persistence and photopotentiation of murine pupillary light responses. Investigative Ophthalmology & Visual Science. 2007;48:1268–1275. doi: 10.1167/iovs.06-0925. [DOI] [PubMed] [Google Scholar]