Abstract
E-cadherin, a cell-cell adhesion glycoprotein, is frequently down-regulated with tumorigenic progression. The extracellular domain of E-cadherin is cleaved by proteases to generate a soluble ectodomain fragment, termed sEcad, which is elevated in the urine or serum of cancer patients. In this study, we explored the functional role of sEcad in the progression of skin squamous cell carcinomas. We found that full length E-cadherin (FL-Ecad) expression was decreased and sEcad increased in human clinical tumor samples as well as in UV-induced squamous cell carcinomas (SCCs) in mice. Interestingly, sEcad associated with members of the human epidermal growth factor receptor (HER) and insulin growth factor-1 receptor (IGF-1R) family of receptors in human and UV-induced mouse tumors. Moreover, in both E-cadherin-positive (E-cadherin+ ) and -negative (E-cadherin− ) cells in vitro, sEcad activated downstream mitogen-activated protein (MAP) kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling and enhanced tumor growth, motility and invasion, the latter via activation of matrix metalloproteinase-2 (MMP-2) and MMP-9. To this end, HER, PI3K or MEK inhibitors suppressed sEcad’s tumorigenic effects, including proliferation, migration and invasion. Taken together, our data suggest that sEcad contributes to skin carcinogenesis via association with the HER/IGF-1R-family of receptors and subsequent activation of the MAPK and PI3K/Akt/mTOR pathways, thereby implicating sEcad as a putative therapeutic target in cutaneous SCCs.
Keywords: Squamous cell carcinomas, UV-irradiation, soluble E-cadherin, HER/ErbB receptors, IGF-1R, proliferation, migration, invasion
INTRODUCTION
Full length E-cadherin (FL-Ecad), a transmembrane glycoprotein that mediates cell-cell adhesion, contains an extracellular, a transmembrane and a highly conserved cytoplasmic domain. In most types of epithelial cancers, FL-Ecad downregulation correlates with tumor grade1–2. We previously showed that FL-Ecad decreases as normal skin epithelium progresses from dysplastic lesions, to papillomas and to overt SCCs in a chronic skin photocarcinogenesis model 3. Similarly, studies using SCC cell lines, 3-dimensional human SCC tissue constructs and reports examining human skin tumors demonstrate that down-regulation of FL-Ecad contributes to skin tumorigenesis4–5.
FL-Ecad down-regulation, mediated by MMPs and members of the membrane-anchored family of metalloproteinases (ADAMs), leads to the release of an 80 kDa sEcad fragment into the tumor microenvironment6. sEcad is constitutively shed at low levels in normal epithelial cells, but is significantly elevated in primary prostate tumor sites and metastatic foci 7. A correlation between heightened sEcad levels in urine, serum, and tumors and histopathological grade, metastasis recurrence and decreased survival has been described 8,9. In prostate and ovarian cancers, protease-induced sEcad facilitates tumor cell migration and invasion, whereas depletion of sEcad from the conditioned media reversed these effects 10–12. Thus, it is clear that sEcad exerts pro-oncogenic effects in prostate, lung and ovarian cancers, but whether sEcad plays a role in skin SCCs and the mechanisms involved, have yet to be elucidated.
The human epidermal growth factor receptor (HER) and insulin-like growth factor receptor (IGF-1R) families provide strong mitogenic and pro-survival signals in skin tumors 13–17. The HER family consists of four tyrosine kinase receptors, including HER1 (EGFR), HER2, HER3 and HER4. Ligand binding leads to the activation of multiple signaling pathways, including the MAPK and PI3K/Akt/mTOR pathways, which promote cancer cell proliferation, reduce apoptosis, and enhance invasiveness and angiogenesis18. To this end, genetic ablation or inhibitors against HER1 reduced skin tumor growth19,20, whereas overexpression of HER1 ligands resulted in spontaneous skin tumor formation21. Transgenic K5-HER2 mice display epidermal hyperplasia and develop spontaneous skin tumors22, while overexpression of IGF-1R led to enhanced activation of IGF-1R, MAPK, PI3K and Akt signaling 17,23 and increased skin tumor burden13,17. Dual inhibition of IGF-1R and HER1 using the monoclonal antibodies A12 (IGF-1R) and Cetuximab (HER1) significantly reduced skin tumor volume and prolonged survival in a SCC orthotopic xenograft model24, demonstrating that HER1 and IGF-1R signaling play an important role in skin tumorigenesis.
Recent literature suggests that sEcad acts as a ligand for specific HER family members25,26. Specifically, Najy and colleagues (2008) demonstrated endogenous sEcad binding preferentially to HER2 and HER3 in the MCF-7 breast cancer cell line25. In addition, exogenous sEcad or recombinant human E-cadherin/Fc chimeric protein (rhEcad/Fc) was determined to bind HER2 and HER3, enhance HER2-HER3 heterodimerization, HER3 phosphorylation and activate downstream ERK1/2 in breast cancer cells without affecting HER2 phosphorylation25. In normal Madin-Darby canine kidney (MDCK) cells, exogenous rhEcad/Fc promoted cell survival via activation of HER1, PI3K, Akt and ERK1/2 signaling26. Overall, these studies suggest that sEcad may promote mitogenic, migratory and invasive properties in skin SCCs through HER receptors, enhancing MAPK and PI3K/Akt signaling and potentially inducing the activation of pro-invasive MMPs.
In this report, we used both in vivo and in vitro assays to investigate the role of sEcad in skin carcinogenesis. Here, we present evidence that FL-Ecad is down-regulated concomitantly with increased shedding of sEcad during the progression of skin cancers. Furthermore, we demonstrate that sEcad associates and activates the HER/IGF-1 receptor families, via MAPK and PI3K/Akt/mTOR signaling pathways, promoting cancer cell proliferation, migration and invasion facilitated by MMPs.
RESULTS
Down-regulation of FL E-cad corresponds with enhanced sEcad shedding
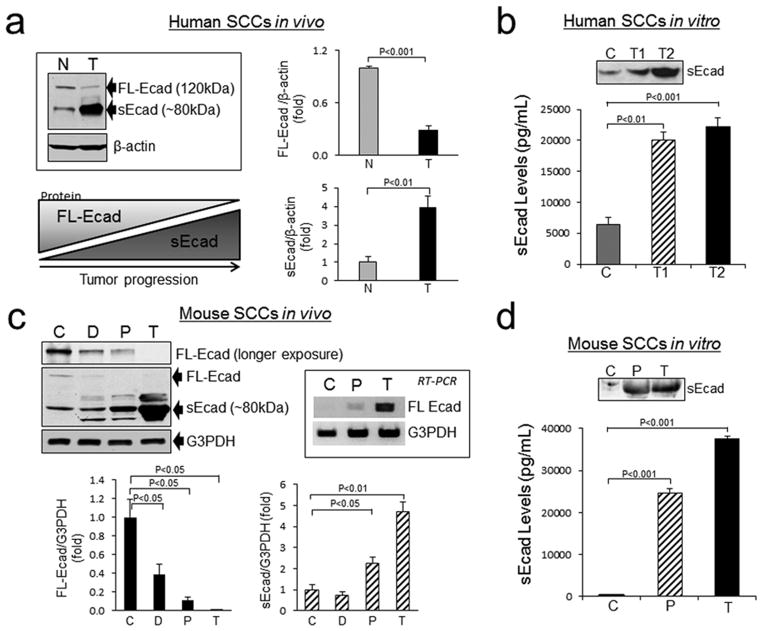
To address whether an inverse correlation between FL-Ecad and sEcad levels exists during the progression of skin cancers, we first analyzed the protein expression of FL-Ecad and sEcad in resected human skin SCCs and human and mouse cell culture systems. Western blot analysis showed a statistically significant decrease in FL-Ecad and a parallel increase of the 80kDa sEcad fragment in SCCs versus normal skin epidermal samples from human subjects (Fig. 1a). Human SCC cell lines demonstrated a statistically significant increase in sEcad shedding versus controls, as assessed by ELISA and immunoblot analyses (Fig. 1b). This inverse FL-Ecad / sEcad relationship was also confirmed in a chronic photocarcinogenesis skin cancer mouse model comprised of areas of dysplasia, resected papillomas and SCCs (Fig. 1c). Interestingly, E-cadherin mRNA levels increased with UV-induced skin cancer progression (Fig. 1c). Using an in vitro SCC skin cancer progression model, we further validated that sEcad secreted levels increased in the conditioned media of cells with progression from normal skin keratinocytes to papillomas and SCCs (Fig. 1d).
Figure 1.
Down-regulation of FL-Ecad correlates with enhanced sEcad shedding. (a) Representative immunoblots and analyses of FL-Ecad and sEcad in normal human skin epidermal curettings and human SCC tumors. Insert: schematic of FL-Ecad and sEcad levels with tumor progression (b) Expression of sEcad in the conditioned media of normal (D033; C), SCC12b (T1) and SCC13 (T2) cells by ELISA after normalization for cell number. Insert: Representative immunoblot of sEcad in 24 h conditioned media from 1×106 cells (c) Western immunoblot analyses of FL-Ecad and sEcad protein and semiquantitative RT-PCR of FL-Ecad mRNA levels in tissue lysates prepared from SKH-1 age-matched non-irradiated (C), chronically UV-irradiated epidermal curettings containing dysplastic foci (D), and resected UV-induced papillomas (P) and skin tumors (T). (d) sEcad in conditioned media from PMK (C), papillomas (SP308) and tumor (PAM212) cells normalized to cell number. Insert: Representative immunoblot of sEcad in 24h conditioned media from 1×106 cells. Protein loading was normalized by probing with β-actin (Santa Cruz Biotechnology) or anti-glyceraldehyde-3-phosphate dehydrogenase (G3PDH; 1:10,000, Ambion, Austin, TX) antibodies. Band intensity was quantified using NIH Scion Image and normalized to G3PDH or β-actin. All error bars, ± SEM. All quantitative data were generated from a minimum of three replicates.
sEcad enhances pro-oncogenic properties in mouse SCCs
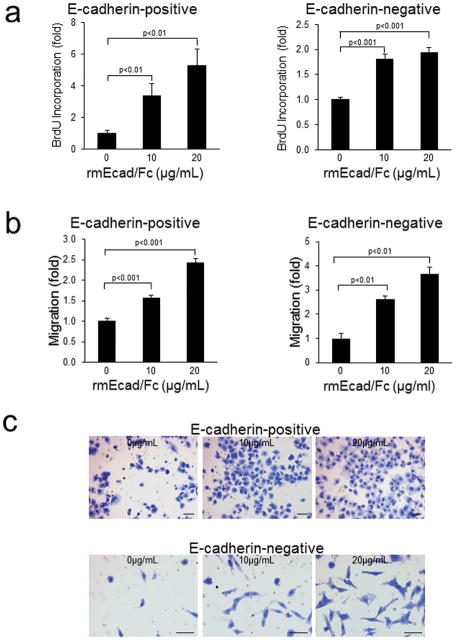
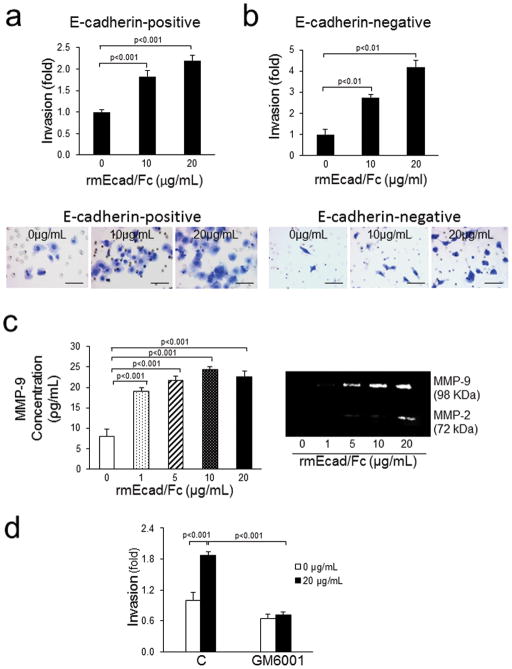
The effect of sEcad on cellular proliferation, migration and invasion was evaluated in the E-cadherin+ PAM212 and E-cadherin− CC4A cell lines. Increasing concentrations of the rmEcad/Fc resulted in a dose-dependent increase in cell proliferation in both cell lines tested (Fig. 2a). A significant increase in sEcad-induced migration of cancer cells through uncoated porous filters was also noted (Fig. 2b and c). To examine the effect of sEcad on invasion, PAM212 and CC4A cells were seeded into Transwell chambers overlaid with Matrigel to provide a three-dimensional protein-rich barrier to invasion. Cells treated with rmEcad/Fc exhibited a 2 to 4 fold increase in invasion compared to untreated controls (Fig. 3a and b). Because matrix metalloproteinases, particularly MMP-9 and MMP-2, are key players in tumor dissemination, we evaluated the levels of pro-and -active MMP-2 and MMP-9 in rmEcad/Fc-treated PAM212 cells. ELISA demonstrated a dose-dependent increase in MMP-9 secretion (Fig. 3c) and gelatin zymography demonstrated an enhanced activation of MMP-9 and MMP-2 in the conditioned media of rmEcad/Fc treated cells. To confirm that sEcad enhances invasion via MMPs, we examined the invasive potential of PAM212 cells treated with 20μg/mL rmEcad/Fc in the presence or absence of GM6001 (Ilomastat; BIOMOL, Plymouth, PA), a potent MMP inhibitor that reduces the activity of collagenases and gelatinases, including MMP-2 and MMP-927. sEcad-stimulated invasion was completely blocked in the presence of GM6001 (Fig. 3d), providing further evidence for the role of sEcad-induced MMP activity in skin cancer invasion.
Figure 2.
sEcad promotes PAM212 (FL-Ecad+) and CC4A (FL-Ecad−) proliferation and migration. (a) BrdU incorporation in PAM212 or CC4A cells by BrdU ELISA. (b) Transwell migration of PAM212 and CC4A cells in response to rmEcad/Fc administration. (c) Representative images of 0.5% crystal violet stained migrating PAM212 and CC4A cells in transwell assay. Scale bars, 250 μm. All error bars, ± SEM. All quantitative data were generated from a minimum of three replicates.
Figure 3.
sEcad facilitates SCC cell invasion via MMP-2 and MMP-9. (a) Serum-starved PAM212 and (b) CC4A cell invasion through Matrigel in response to rmEcad/Fc administration. Insert: Representative 0.5% crystal violet staining of rmEcad/Fc treated PAM212 or CC4A cells invading through Matrigel. Scale bars, 250 μm. (c) MMP levels and activities in the presence or absence of rmEcad/Fc were quantified by ELISA and gelatin zymography, respectively. (d) Invasive potential of PAM212 cells treated with rmEcad/Fc was determined after pretreatment with 20μM GM6001 for 1 h, and invasion though Matrigel analyzed in the presence of rmEcad/Fc (20μg/mL) and 10μM GM6001 for an additional 22h. All error bars, ± SEM. All quantitative data were generated from a minimum of three replicates.
sEcad associates with HER/IGF-1R receptors and activates downstream signaling
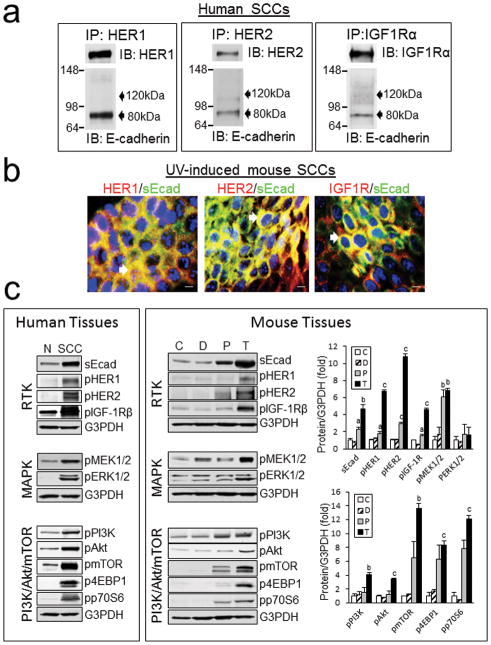
To determine if sEcad exerts its effects via the HER/IGF-1R family of receptors, extracts from human SCCs were immunoprecipitated with HER1, HER2 or IGF-1Rα specific antibodies. Immunoblotting revealed association of endogenous sEcad with HER1, HER2 and IGF-1R in human SCCs, whereas, no appreciable association of FL-Ecad with the above receptors was noted (Fig. 4a). Similarly, double immunofluorescence labeling of cryostat sectioned UV-induced tumors using an E-cadherin ectodomain-specific antibody, which recognizes both FL-Ecad and sEcad, demonstrated co-localization of E-cadherin with HER1, HER2 and IGF-1Rα (Fig. 4b). However, given the minimal levels of FL-Ecad in tumors by immunoblotting (Fig. 1c), it is most likely that the immunostaining is primarily derived from sEcad. Concomitant with increased sEcad, we observed a parallel increase in the levels of HER1, HER2 and IGF-1R phosphorylation along with activation of downstream pro-oncogenic signaling in human and UV-induced mouse SCCs compared with normal human or mouse epidermal skin samples (Fig. 4c). Specifically, we observed heightened expression of phosphorylated MEK, as well as PI3K, Akt, mTOR and its downstream effectors 4EBP1 and p70S6K in papillomas and SCCs relative to control tissues, albeit to varying levels (Fig. 4c).
Figure 4.
sEcad associates with activated HER and IGF-1R signaling pathways in human and mouse skin tumors. (a) Extracts prepared from human SCC tumor samples were immunoprecipitated with HER1, HER2 or IGF-1Rα-specific antibodies, followed by western blotting with antibodies against E-cadherin (ectodomain specific), HER1, HER2 or IGF-IRα. (b) Double immunofluorescence labeling of sEcad (extracellular domain specific antibody, green) with HER1, HER2 and IGF-1R (red). Hoechst was used to stain nuclei (blue). Arrows depict areas of overlap. Scale bars, 50 μm. (c) Immunoblots of sEcad, phospho-HER1, HER2, IGF-1R and phospho-MEK, ERK1/2, PI3K, Akt, mTOR, 4EBP1 and p70S6K in normal human skin tissue (N) and human SCC samples (SCC). Immunoblot and analyses of sEcad, phospho-HER1, HER2, IGF-1R and phospho-MEK, ERK1/2, PI3K, Akt, mTOR, 4EBP1 and p70S6K in SKH-1 age-matched non-irradiated (C), chronically UV-irradiated epidermal skin containing dysplastic foci (D), resected UV-induced papillomas (P) and tumors (T). Protein loading was normalized by probing with G3PDH. Band intensity was quantified using NIH Scion Image and normalized to G3PDH. Results are presented as mean ± SEM. n=3. Statistical significance is shown as following: aP<0.05, bP<0.01, cP<0.001 vs. corresponding controls (C).
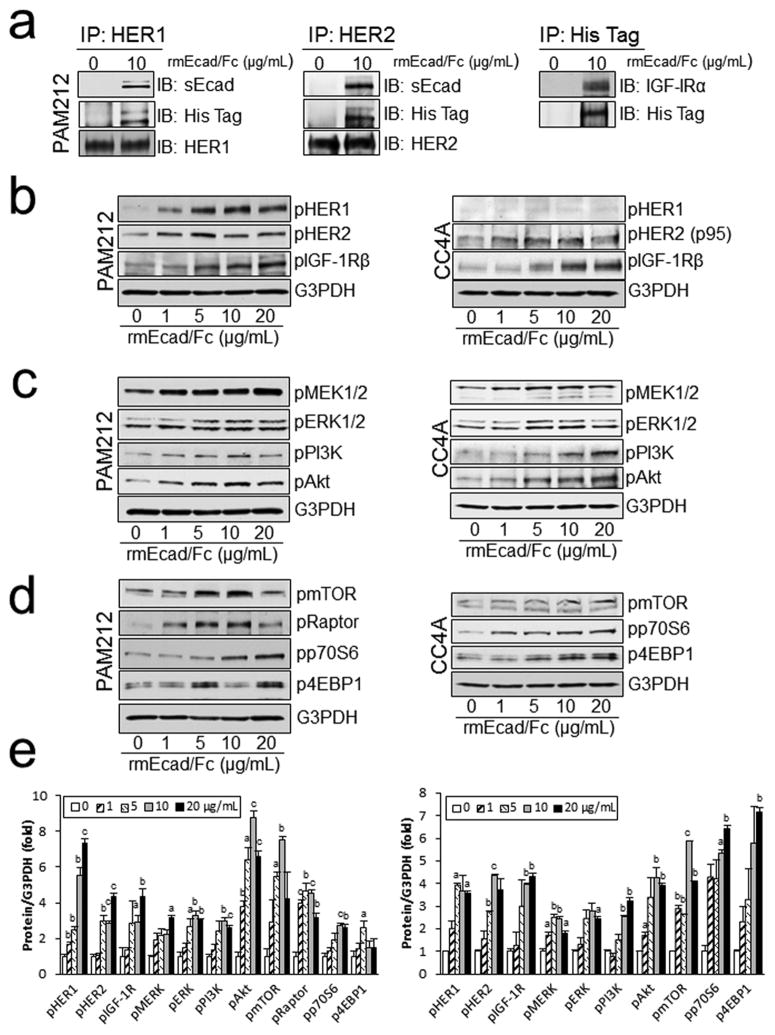
The association of sEcad with HER1, HER2 and IGF-1R was also confirmed by in vitro studies. Lysates from control and rmEcad/Fc treated PAM212 (E-cadherin+) were immunoprecipitated with HER1, HER2 or IGF-1R specific antibodies and analyzed by western blotting using a His-tagged and E-cadherin extracellular domain specific antibody for the presence of rmEcad/Fc. Our results show that sEcad co-immunoprecipitates with HER1, HER2 or IGF-1R in PAM212 cells (Fig. 5a). Next, HER/IGF-1 receptor activation was evaluated in PAM212 and CC4A cells treated with increasing concentrations of rmEcad/Fc. HER1, HER2 and IGF-1 receptor phosphorylation was notably increased by rmEcad/Fc administration in PAM212 cells (Fig. 5b). In separate experiments, the more invasive CC4A cells expressed IGF-1R and HER2 (p95), which were also dose dependently activated after rmEcad/Fc treatment (Fig. 5b). Furthermore, rmEcad/Fc induced MEK, ERK, PI3K and Akt phosphorylation (Fig. 5c) and activated mTOR and its substrates p70S6K and 4EBP1 in both PAM212 and CC4A cells (Fig. 5d). Collectively, these results demonstrate that exogenous sEcad stimulates the MAPK and PI3K/Akt/mTOR axis by interacting and forming a complex with the HER and IGF-1 receptor tyrosine kinases in both E-cadherin+ and E-cadherin− cells in vitro.
Figure 5.
sEcad associates with HER and IGF-1 receptors and activates downstream signaling pathways in PAM212 and CC4A cells. (a) Lysates from PAM212 cells in the presence or absence of rmEcad/Fc were immunoprecipitated with HER1, HER2 or His-tag specific antibodies, followed by immunoblotting with E-cadherin (ectodomain), His-tag, HER1, HER2, or IGF-IRα specific antibodies. (b), Western blotting showing enhanced rmEcad/Fc-induced phosphorylation of HER1, HER2 and IGF-1R 26 h following rmEcad/Fc stimulation. (c) Phosphorylation of MEK, ERK1/2, PI3K, Akt and (d) mTOR, Raptor, p70S6K and 4EBP1 are increased with rmEcad/Fc treatment. An antibody to G3PDH was used as a loading control in all panels. (e) phospho-HER1, HER2, IGF-1R, MEK, ERK1/2, PI3K, Akt, mTOR, 4EBP1 and p70S6K band intensity relative to G3PDH in rmEcad/Fc (0–20 μg/mL) treated PAM212 and CC4A cells as quantified by NIH Scion Image. Results are present as mean ± SEM. n=2~3. Statistical significance is shown as following: aP<0.05, bP<0.01, cP<0.001 vs. corresponding controls (0μg/mL).
HER inhibitors block sEcad-induced pro-oncogenic signaling
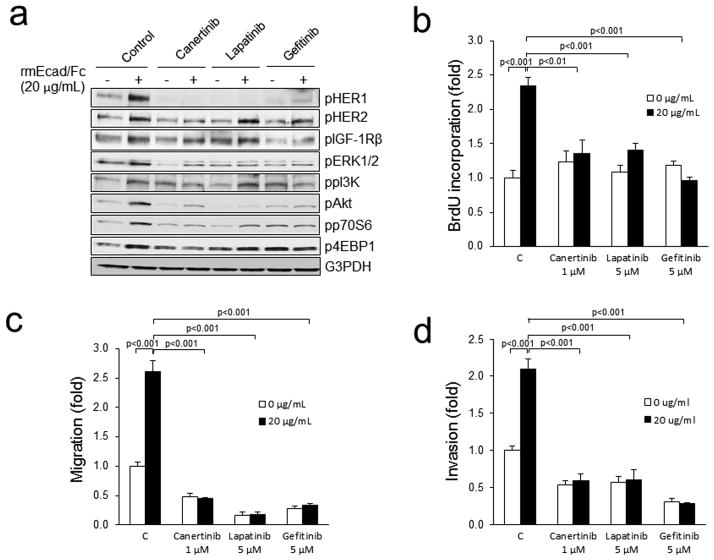
To validate that sEcad signals via the HER family of receptors, we treated PAM212 cells with various HER inhibitors in the presence or absence of rmEcad/Fc. Canertinib, a pan-HER inhibitor, completely abrogated sEcad-induced HER1 and HER2 phosphorylation in PAM212 cells (Fig. 6a). Lapatinib, a HER1/2 inhibitor at 5 μM, significantly down-regulated the sEcad-induced phosphorylation of HER1, ERK1/2, pAkt and p70S6K, but exhibited minimal changes on pHER2, PI3K and 4EBP1. Gefitinib, a HER1 specific inhibitor decreased the sEcad-induced phosphorylation of HER1, ERK1/2, Akt, p70S6K and PI3K, but had only minimal effects on pHER2 and p4EBP1. Canertinib, Lapatinib and Gefitinib, in the presence of sEcad, did not alter phospho-IGF-1R levels compared to their respective corresponding controls (Fig. 6a). Next, we evaluated the effects of these inhibitors on the mitogenic, migratory and invasive capabilities in sEcad-stimulated PAM212 cells. Interestingly, all three inhibitors effectively inhibited sEcad-induced proliferation (Fig. 6b), migration (Fig. 6c) and invasion (Fig. 6d).
Figure 6.
HER inhibitors block sEcad-induced signaling and tumorigenicity. (a) PAM212 cells were pre-incubated with or without Canertinib (1 μM), Lapatinib (5 μM) or Gefitinib (5 μM) for 2 h then were treated with 20μg/mL of rmEcad/Fc for 26h. Cells were lysed, and the indicated proteins were detected by immunoblotting. (b) Proliferation in rmEcad/Fc treated PAM212 cells in the presence or absence of the indicated RTK inhibitors for 24h as assessed by the BrdU ELISA assay. (c) Migration of rmEcad/Fc-treated PAM212 cells with or without pre-incubation of 1 μM of Canertinib, 5 μM of Lapatinib or 5 μM of Gefitinib. (d) Invasion of rmEcad/Fc-treated PAM212 cells with or without pre-incubation of 1 μM of Canertinib, 5 μM of Lapatinib or 5 μM of Gefitinib. Cells that migrated through the control insert or invaded the Matrigel layer were fixed, stained, photographed and counted in ten random high-power fields per insert. Results are presented as mean ± SEM of fold change in rmEcad/Fc or inhibitor treated cells compared with control cells. All quantitative data were generated from a minimum of three replicates.
MEK and PI3K inhibitors block sEcad-induced ERK and Akt/mTOR activation and oncogenic signaling
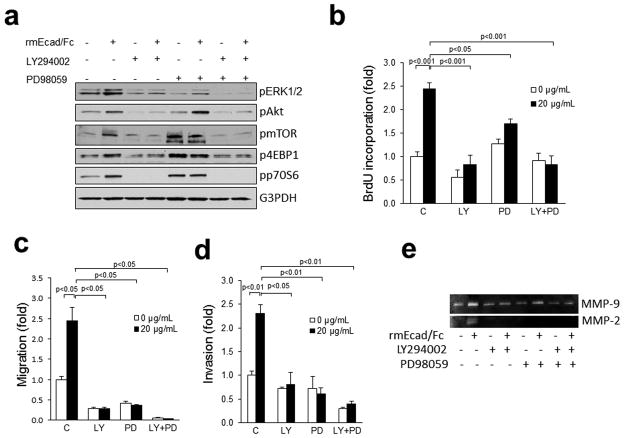
Signal transduction by the HER/IGF-1R family of receptors is mediated by the MAPK and PI3K/Akt pathways. Treatment of PAM212 cells with rmEcad/Fc increased phosphorylation of ERK1/2, Akt, mTOR, 4EBP1 and p70S6K (Fig. 7a). The PI3K inhibitor LY294002 and the MEK inhibitor PD98059, alone or together, blocked the pERK1/2 induction. Activation of Akt, mTOR, 4EBP1 and p70S6K by rmEcad/Fc was also inhibited by LY294002, but insensitive to PD98059. Moreover, treatment of PAM212 cells with the PI3K inhibitor LY294002, alone or in combination with the MEK inhibitor PD98059 reduced rmEcad/Fc-induced proliferation, migration, invasion, as well as MMP-2 and MMP-9 activation (Fig. 7, b and c and d and e). PD98059 alone had less of an effect on rmEcad/Fc-induced proliferation, but more marked effects on migration and invasion. Interestingly, MEK and PI3K inhibitors, in combination with rmEcad/Fc induced synergistic inhibition of migration and invasion with a less pronounced inhibition of proliferation. These results provide evidence that sEcad acts via the PI3K and MEK pathways and its substrates Akt/mTOR and ERK1/2 to facilitate pro-oncogenic properties in skin cancer cells.
Figure 7.
sEcad signals through PI3K and MAPK pathways to promote oncogenicity. (a) PAM212 cells were pre-incubated with or without LY294002 (20 μM, PI3K inhibitor) and PD98059 (20 μM, MEK inhibitor) either alone or in combination (20 μM +20 μM) for 1 h, then treated with rmEcadh/Fc (20 μg/mL) for 26h. Cells were lysed, and the indicated proteins were detected by immunoblotting. (b) Proliferation in rmEcad/Fc treated PAM212 cells in the presence or absence of 20 μM PD98059 and 20 μM LY294002, alone or in combination. (c) Migration of rmEcad/Fc-treated PAM212 cells in the presence or absence of LY294002 and PD98059, alone or in combination. (d) Invasion of rmEcad/Fc-treated PAM212 cells with or without pre-incubation of LY294002 and PD98059 alone or in combination. Cells that migrated through the control insert or invaded the Matrigel were fixed, stained, photographed and counted in ten random high-power fields per insert. Results are presented as mean ± SEM of fold change in treated cells compared with control cells. All quantitative data were generated from a minimum of three replicates. (e) Gelatin zymography showing MMP-2 and MMP-9 activities of rmEcad/Fc-treated PAM212 cells with or without pre-incubation of LY294002 and PD98059, alone or in combination.
DISCUSSION
Down-regulation of membrane-bound E-cadherin is well known to play a pivotal role in the maintenance of cell-cell adhesion of epithelial cells, suppress cellular transformation and negatively regulate neoplastic growth in lung, colon and mammary tumors28–31. This down-regulation may be at the level of gene transcription, mRNA translation or as a result of protein post-translational processing.
In skin carcinogenesis, we previously demonstrated that FL-Ecad protein levels significantly decrease during the progression of UV-induced skin SCCs through prostaglandin E2-induced internalization and degradation via the lysosome and proteasome pathways3. Proteolytic cleavage of transmembrane proteins, also termed ectodomain shedding, is an additional post-translational process which reduces membrane protein levels, and in some cases releases soluble ectodomain fragments into the extracellular milieu. Our current study demonstrates a novel inverse correlation between FL-Ecad and sEcad protein levels from normal tissues to UV-induced tumors, concomitant with increasing E-cadherin transcript levels, suggesting that ectodomain shedding contributes to FL-Ecad down-regulation. This mechanism may also explain the paradox of increased levels of sEcad and low levels of membrane FL-Ecad in most cancers. Intriguingly, reports have shown that FL-Ecad is found in lymph nodes containing metastatic foci and expressed in bone, liver and other metastatic lesions when proteases are abundant 30–32. Independently, others report enhanced sEcad expression in liver, brain and lung metastatic tissues from prostate cancer patients 7. Yang et al (2005) demonstrated that primary gastric tumors, metastatic lymph nodes and peritoneal lavage fluid all contained E-cadherin mRNA, albeit at lower levels in primary tumors33. These reports, and our current data, suggest that FL-Ecad is being synthesized and subsequently down-regulated via post-translational processing, which may include shedding and/or internalization and degradation.
Our data further demonstrate that sEcad has the ability to induce skin tumor cell proliferation and migration. This sEcad-induced mitogenic effect was also demonstrated by Najy et al (2008) in the SKBR3 breast cancer cell line25. The migratory characteristics are in concordance with studies in breast, ovarian and prostate cancer cell lines showing that recombinant sEcad chimeric protein enhances migration, whereas immunodepletion of sEcad from the conditioned media reversed these effects8, 10–12. Besides regulating proliferation and migration, we also found that sEcad influences SCC invasion via enhancing secretion and activation of pro-invasive MMP-2 and MMP-9, and confirmed that this invasive property was dependent on MMPs by using the MMP inhibitor GM6001. This is consistent with a previous report demonstrating enhanced sEcad-induced MT1-MMP, MMP-2 and MMP-9 secretion in transformed lung cancer cells11.
Several observations demonstrate that increased receptor tyrosine kinase activity and down-regulation of FL-Ecad function are associated with human breast and skin tumor progression34,35. Specifically, FL-Ecad mediated cell-cell aggregation was inhibited by HER1/EGFR treatment, whereas inactivation of HER1/EGFR promoted E-cadherin-dependent adhesion35. Similarly, overexpression of FL-Ecad down-regulated HER1 and reversed the invasive phenotype of human papillomavirus-transfected keratinocytes36. Here, our data demonstrates a previously unrecognized interaction of sEcad, but minimal association of FL-Ecad, with HER1/HER2 and IGF-1R in human SCC specimens in vivo. We further demonstrate co-localization of E-cadherin with these receptors in UV-induced mouse SCCs. Because membrane-bound FL-Ecad is minimally expressed in these UV-induced tumors, and we used an E-cadherin ectodomain-specific antibody that recognizes both FL-Ecad and sEcad, it is likely that the immunostaining seen on the UV-induced SCC sections is primarily from the shed ectodomain sEcad fragment. Moreover, in the human and UV-induced mouse tumors in which sEcad expression was amplified, we simultaneously found a parallel increase in HER1, HER2 and IGF-1R activation and this correlated with enhanced MAPK and PI3K/Akt/mTOR signaling. To confirm that sEcad interacts with the HER and IGF-1R receptors, and induce their activation, we exogenously treated skin cancer cells with His-tagged recombinant sEcad. To the best of our knowledge, this is the first report to demonstrate that exogenous sEcad physically interacts and activates the HER and IGF-1R family of receptors in cutaneous SCCs in vitro. Indeed, we show sEcad-induced HER and IGF-1R phosphorylation resulting in activation of several intracellular signaling pathways, including the pro-oncogenic MAPK and PI3K/Akt pathways. We also provide novel evidence that sEcad signals via mTOR and its substrates 4EBP1 and p70S6K. Our results are in accordance with some of the work by Najy et al (2008), who demonstrated that exogenous sEcad bound preferentially to HER2 and HER3, enhancing HER2-HER3 heterodimerization and activation of downstream ERK1/2 in breast cancer cells25.
To validate that sEcad promotes oncogenicity via HER signaling in skin SCCs, we treated PAM212 cells exposed to sEcad with selective HER inhibitors. Our data demonstrated that sEcad signals through the HER receptors to activate downstream MAPK and PI3K/Akt/mTOR signaling. In support of this, we are the first to demonstrate that sEcad-induced HER transactivation mediates pro-oncogenic functions. Specifically, Canertinib, Lapatinib and Gefitinib inhibited sEcad-induced proliferation, migration and invasion. Interestingly, Canertinib at the same dose, did not affect sEcad-induced IGF-1R phosphorylation, suggesting that in our system, binding and activation of IGF-1R by sEcad is not via HER1/2 heterodimerization with IGF-1R, as previously shown in Trastuzumab-resistant cells by Nahta et al (2005)37. However, sEcad-induced cellular functions were abrogated by Canertinib, suggesting the existence of possible alternative downstream signaling pathways for IGF-1R and/or alternative functions. This will be explored in future studies.
To understand whether activation of MEK and PI3K are both necessary for sEcad-mediated cell proliferation, migration and invasion, the PI3K inhibitor (LY294002) and MEK inhibitor (PD98059) were used either alone or in combination. Both ERK1/2 and Akt/p70S6K phosphorylation were decreased after PI3K inhibition. Similarly, MEK inhibition resulted in decreased ERK1/2 phosphorylation. Interestingly, mTOR/p70S6K/4EBP1 phosphorylation increased in the presence of the MEK inhibitor PD98059, even in the absence of rmEcad/Fc. This is not surprising, since it has become increasingly apparent that a complex network of horizontal and vertical signaling crosstalk and feedback loops exist between the MAPK axis and the mTOR axis, whereby inhibition of components of one axis results in a compensatory upregulation of the alternate pathway38. This may explain why Akt phosphorylation was enhanced by sEcad in the presence of the MEK inhibitor. In addition, since we have shown that sEcad can activate both HER and IGF-1R family members, it is plausible that this sEcad-induced Akt/ mTOR/p70S6K/4EBP1 activation may occur via HER1–4 or IGF-1R signaling. Functionally, our data further show significant reductions in sEcad-induced PAM212 proliferation in the presence of the PI3K inhibitor LY294002, but less significant effects with MEK inhibitor PD98059. Because the mTOR/p70S6K/4EBP1 signaling branch plays a central role in regulating cell growth and proliferation39,40, it is feasible that the enhanced sEcad-induced mTOR/p70 S6-kinase/4EBP1 expression levels seen with PD98059 may mediate these effects. Furthermore, our data suggest that sEcad-induced proliferation, migration and invasion are PI3K and MEK-dependent, since irrespective of which inhibitor is utilized, these functional effects are blocked. This conclusion is supported by our results, wherein the PI3K and MEK inhibitors, either alone or in combination, significantly inhibited rmEcad-induced activation of MMP-2 and MMP-9.
Additionally, in this study we provide novel findings that these sEcad-induced pro-oncogenic effects were evident in both E-cadherin+ and E-cadherin− SCC cell lines. In support of our data, Najy et al (2008) showed that exogenous sEcad stimulated HER2-HER3 heterodimerization, ERK activation and cellular proliferation in the E-cadherin− SKBR3 breast cancer cell line25. Moreover, it is well known that the tumor microenvironment, in vivo, is a dynamic system that consists of interactions between many cell types (stromal, endothelial and inflammatory cells etc.), soluble factors, signaling molecules, extracellular matrix and mechanical cues. Importantly, FL-Ecad is expressed on epithelial cells, platelets and a variety of inflammatory cells, including leukocytes and tumor associated macrophages (TAMs)41–44. Because the tumor microenvironment contains an abundance of proteinases (MMPs, ADAM etc.), and is enriched with inflammatory cells and possibly platelets with metastatic spread, it would be tempting to speculate that in E-cadherin− tumors, sEcad may be derived from inflammatory or protease-mediated shedding of the E-cadherin ectodomain from these cell types. Indeed, Fong et al (2011) demonstrated that sEcad is shed from platelets42 and Maretzky et al (2008) demonstrated that pro-inflammatory cytokines and TGF-β have been shown to induce the release of sEcad in cultured keratinocytes45. Therefore, one cannot exclude the possibility that in the in vivo setting, enhanced pro-inflammatory cytokines, TGF-β and/or MMPs in the tumor milieu, may act in an autocrine/paracrine fashion to stimulate the release of sEcad from adjacent normal epithelial cells and/or other cell types that contain FL-Ecad.
In conclusion, the present study has described how FL-Ecad down-regulation coincides with enhanced sEcad shedding in skin cancers, the latter, which binds HER/IGF-1R and activates downstream pro-oncogenic signaling to induce tumor cell proliferation, migration and invasion. Because the HER/IGF-1R pathways are central regulators of tumor growth and survival, with dual inhibition reducing angiogenesis and cutaneous squamous cell carcinomas24, our findings may provide a strong rationale to study the clinical efficacy of anti-sEcad therapies for patients with primary or metastatic skin cancers.
MATERIALS AND METHODS
Cell culture, isolation and reagents
The E-cadherin+ PAM212 cells were a generous gift of Dr. Yuspa (NCI, Bethesda, MD), whereas the E-cadherin− CC4A cells were kindly provided by Dr. Klein-Szanto (Fox Chase Cancer Center, Philadelphia, PA). PAM212 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Lonza) with 10% FBS, CC4A cells in SMEM medium (Gibco Life Technologies) supplemented with 10% FBS and 2 mM L-glutamine and DO33, SCC12b, SCC13 cells were cultured in the presence of 3T3 feeder cells, as described previously46. Cell isolation and culture of primary mouse keratinocytes (PMK) were performed as previously described3.
Drug and inhibitor treatments
PAM212 were seeded in 6-well plates 24 hours prior to any treatment. Cells were pretreated for 2h with the following inhibitors from LC Laboratories (Woburn, MA): 1μM of pan-HER (Canertinib), 5μM of HER1/HER2 (Lapatinib) and 5μM of HER1 (Gefitinib) inhibitors. PI3K (LY294002, 20 μM) and MEK (PD98059, 20 μM) inhibitors were purchased from EMD (Billerica, MA). Recombinant mouse sEcad (rmEcad/Fc) was purchased from R & D Systems. GM6001 was purchased from Biomol (Ann Arbor, MI).
Gelatin zymography
Conditioned media from control or rmEcad/Fc treated cells were concentrated 10-fold using Centricon devices (Millipore; Bedford, MA) according to the manufacturer’s instructions and applied to SDS-PAGE gels containing gelatin (0.1% w/v). Gels were washed twice in renaturing buffer (Invitrogen) and incubated at 37°C for 24 h in developing buffer (Invitrogen). Gels were stained with Coomassie blue and destained with methanol: acetic acid (50:10). Zymogram is representative of triplicate experiments.
Western blot analysis
Whole-cell extracts from normal human epidermal samples, human SCCs, curetted mouse epidermis, resected papillomas, resected mouse cutaneous SCCs and cells were processed for western blots, as described previously3. Immunoreactive bands were developed by enhanced chemiluminescence (ECL) (Santa Cruz Biotechnology, Santa Cruz, CA USA) and visualized by autoradiography (Kodak, Rochester, NY). Relative levels of total and phosphorylated proteins were determined using the following antibodies: HER1, HER2, IGF-1R, MEK1/2, ERK1/2, PI3K, Akt, mTOR, Raptor, 70S6K (Thr389), 4EBP1 (Cell Signaling Technologies). Antibody to the extracellular domain of E-cadherin was obtained from Santa Cruz Biotechnology (H108). Equal protein sample loading was monitored using anti-G3PDH (Ambion) or anti-actin (Santa Cruz Biotechnology) antibodies. Primary human SCC samples and adjacent normal epidermal skin samples were obtained from the Cooperative Human Tissue network (CHTN; NCI) after receipt of approval from the Institutional IRB at Stony Brook University.
Immunoprecipitation
Human skin tumors and cells were homogenized and lysed in lysis buffer (20 mM Tris-HCl, pH 7.5; 137 mM NaCl; 100 mM NaF; 10% glycerol (vol/vol); 1.0 % (vol/vol) Nonidet P-40; 1mM PMSF and protease inhibitor cocktail (Sigma). Lysates were cleared by centrifugation at 10,000 rpm for 10 min at 4 °C. Supernatants were incubated with HER1/EGFR (Ab-15), HER2 (Ab-17) and IGF-1Rα (Ab4)-specific antibodies from Thermo Fisher Scientific for 4 h and protein A/G plus agarose beads (Santa Cruz, sc-2003) for 2 h at 4 °C. The immunocomplexes were washed three times, boiled in sample buffer and loaded on SDS-PAGE for protein analysis.
Semi-Quantitative RT-PCR
Total cellular RNA from normal curetted mouse epidermis, resected papillomas and SCCs (N=2) were extracted and the level of E-cadherin mRNA was determined using a semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR), as previously described47, using the following mouse E-cadherin primers: Upper primer 5′-GGACTACGATTATCTGAACG-3′, Lower primer 5′-AACACACACACTATCCAGC-3′.
Immunofluorescence
Paraffin-embedded UV-induced tumor sections were processed for immunofluorescence (IF) staining using E-cadherin ectodomain (Sigma, DECMA-1) and HER1, HER2 and IGF-1R-specific antibodies, as previously described3. Staining was detected using Alexa-488 and Alexa-594-conjugated secondary antibodies (Invitrogen) followed by Hoechst nuclear counterstaining.
ELISA Assays
Conditioned media from human and mouse cells were analyzed for sEcad and MMP-9 using human and mouse E-cadherin Quantikine ELISA Kits and mouse MMP-9 ELISA kit (R&D Systems, Minneapolis, MN), according to the manufacturer’s specifications, respectively. For measuring mouse MMP-9, serum-free medium was added to cell cultures and cells were exposed to increasing concentrations of rmEcad/Fc (R&D Systems) for 24 hours. The culture media were collected and concentrated using Centrifugal Ultra Filters (Millipore, Billerica, MA) and MMP-9 levels were measured by ELISA. Each experiment was performed in triplicate.
Cell proliferation assays
rmEcad/Fc stimulated PAM212 or CC4A cells, cultured in the presence or absence of inhibitors, were assayed for cell proliferation using an ELISA BrdU kit (Roche Molecular Biochemicals; Mannheim, Germany), as per the manufacturer’s instructions. Results are expressed as the mean ± SEM, N=3.
Cell Migration and Invasion Assays
Migration and invasion of PAM212 or CC4A cells were measured using 8.0μm pore BD BioCoat Control Insert 24-well plates and Matrigel Invasion Chamber 24-well Plates, respectively (BD Bioscience). Cells were collected, washed, and 2×105 cells were plated in 0.4% FBS medium in the top chamber, with 0–20μg/mL of rmEcad/Fc in the bottom chamber. After 22 h, cells on the top were removed using a cotton swab. Migrated or invaded cells on the lower surface were fixed with methanol, stained with 0.5% crystal violet, examined by bright field microscopy and photographed. In the inhibitor experiments, cells were pre-incubated with the inhibitors in the top and lower chambers for 1 or 2h. The lower chambers were changed to the same conditions of inhibitors with rmEcad/Fc (20 μg/mL) for an additional 22 h. In some experiments, cells were pretreated with 20 μM GM6001 for 1 h, then placed in the invasion chamber in the presence or absence of rmEcad/Fc together with 10 μM GM6001. Migration or invasion was quantitated by counting migrated or invaded cells in at least ten random high-power fields per insert and expressed as averages for triplicate experiments. Results are presented as fold change of the number of migrated/invaded cells to the untreated controls in triplicate experiments.
Mice and UV Irradiation
SKH-1 mice (Charles River Laboratories) between 8 and 14 weeks of age, and typically weighing between 20 and 30 g, were allowed free access to water and standard mouse feed. All experimental procedures were approved by the Institutional Laboratory Animal Care and Use Committee of the investigators. For the chronic photocarcinogenesis model, the dorsal skin of SKH-1 mice were exposed to 180mJ/cm2 UVB twice per week for 35 weeks, as previously described27.
Statistical analysis
Data are presented as means with standard errors (SEM). Independent two-sample t-tests compare differences between two groups. A p-value below 0.05 indicated statistical significance. Statistics were analyzed with SPSS 15.0 software (SPSS Inc, Chicago, IL, USA).
Acknowledgments
The authors would like to thank Dr. Yuspa (NCI, Bethesda, MD) and Dr. Klein-Szanto (Fox Chase Cancer Center, Philadelphia, PA) for the PAM212 and CC4A cells, respectively. This work was supported by NIH grants K08CA133910 (NCI) and R21ES015832 (NIEHS) to SB as well as NIH grant R01CA138998 (NCI) to MA.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Gamallo C, Palacios J, Suarez A, Pizarro A, Navarro P, Quintanilla M, et al. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol. 1993;142:987–993. [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer B, Johnson JP, Leitl F, Jauch KW, Heiss MM, Schildberg FW, et al. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53:1690–1695. [PubMed] [Google Scholar]
- 3.Brouxhon S, Kyrkanides S, O’Banion MK, Johnson R, Pearce DA, Centola GM, et al. Sequential down-regulation of E-cadherin with squamous cell carcinoma progression: loss of E-cadherin via a prostaglandin E2-EP2 dependent posttranslational mechanism. Cancer Res. 2007;67:7654–7664. doi: 10.1158/0008-5472.CAN-06-4415. [DOI] [PubMed] [Google Scholar]
- 4.Cavallaro U, Christofori G. Cell adhesion and signaling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 5.Margulis A, Zhang W, Alt-Holland A, Crawford HC, Fusenig NE, Garlick JA. E-cadherin suppression accelerates squamous cell carcinoma progression in three-dimensional, human tissue constructs. Cancer Res. 2005;65:1783–1791. doi: 10.1158/0008-5472.CAN-04-3399. [DOI] [PubMed] [Google Scholar]
- 6.Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell–cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci USA. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuefer R, Hofer MD, Gschwend JE, Pienta KJ, Sanda MG, Chinnajyan AM, et al. The role of an 80 kDa fragment of E-cadherin in the metastatic progression of prostate cancer. Clin Cancer Res. 2003;9:6447–6452. [PubMed] [Google Scholar]
- 8.Chan AO, Chu KM, Lam SK, Wong BC, Kwok KF, Law S, et al. Soluble E-cadherin is an independent pretherapeutic factor for long-term survival in gastric cancer. J Clin Oncol. 2003;21:2288–2293. doi: 10.1200/JCO.2003.08.078. [DOI] [PubMed] [Google Scholar]
- 9.Katayama M, Hirai S, Kamihagi K, Nakagawa K, Yasumoto M, Kato I. Soluble E-cadherin fragments increased in circulation of cancer patients. Br J Cancer. 1994;69:580–585. doi: 10.1038/bjc.1994.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies G, Jiang WG, Mason MD. Matrilysin mediates extracellular cleavage of E-cadherin from prostate cancer cells: A key mechanism in hepatocyte growth factor/scatter factor-induced cell-cell dissociation and in vitro invasion. Clin Cancer Res. 2001;7:3289–3297. [PubMed] [Google Scholar]
- 11.Nawrocki-Raby B, Gilles C, Polette M, Bruyneel E, Laronze JY, Bonnet N, et al. Upregulation of MMPs by soluble E-cadherin in human lung tumor cells. International J Cancer. 2003;105:790–795. doi: 10.1002/ijc.11168. [DOI] [PubMed] [Google Scholar]
- 12.Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, et al. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007;67:2030–2039. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- 13.Bol D, Kiguchi K, Gimenez-Conti I, Rupp T, DiGiovanni J. Overexpression of insulin-like growth factor-1 induces hyperplasia, dermal abnormalities, and spontaneous tumor formation in transgenic mice. Oncogene. 1997;14:1725–1734. doi: 10.1038/sj.onc.1201011. [DOI] [PubMed] [Google Scholar]
- 14.El-Abaseri TB, Putta S, Hansen LA. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis. 2006;27:225–231. doi: 10.1093/carcin/bgi220. [DOI] [PubMed] [Google Scholar]
- 15.Madson JG, Hansen LA. Multiple mechanisms of Erbb2 action after ultraviolet irradiation of the skin. Mol Carcinog. 2007;4:624–628. doi: 10.1002/mc.20335. [DOI] [PubMed] [Google Scholar]
- 16.Wan YS, Wang ZQ, Shao Y, Voorhees JJ, Fisher GJ. Ultraviolet irradiation activates PI 3-kinase/AKT survival pathway via EGF receptors in human skin in vivo. Int J Oncol. 2001;18:461–466. doi: 10.3892/ijo.18.3.461. [DOI] [PubMed] [Google Scholar]
- 17.Wilker E, Lu J, Rho O, Carbajal S, Beltrán L, DiGiovanni J. Role of PI3K/Akt Signaling in Insulin-Like Growth Factor-1 (IGF-1) Skin Tumor Promotion. Mol Carcinog. 2005;44:137–145. doi: 10.1002/mc.20132. [DOI] [PubMed] [Google Scholar]
- 18.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 19.Dlugosz AA, Hansen L, Cheng C, Alexander N, Denning MF, Threadgill DW, et al. Targeted disruption of the epidermal growth factor receptor impairs growth of squamous papillomas expressing the v-ras(Ha) oncogene but does not block in vitro keratinocyte responses to oncogenic ras. Cancer Res. 1997;57:3180–3188. [PubMed] [Google Scholar]
- 20.El-Abaseri TB, Fuhrman J, Trempus C, Shendrik I, Tennant RW, Hansen LA. Chemoprevention of UV light-induced skin tumorigenesis by inhibition of the epidermal growth factor receptor. Cancer Res. 2005;65:3958–3965. doi: 10.1158/0008-5472.CAN-04-2204. [DOI] [PubMed] [Google Scholar]
- 21.Vassar R, Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991;5:714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- 22.Bol D, Kiguchi K, Beltrán L, Rupp T, Moats S, Gimenez-Conti I, et al. Severe follicular hyperplasia and spontaneous papilloma formation in transgenic mice expressing the neu oncogene under the control of the bovine keratin 5 promoter. Mol Carcinog. 1998;21:2–12. [PubMed] [Google Scholar]
- 23.DiGiovanni J, Bol DK, Wilker E, Beltrán L, Carbajal S, Moats S, et al. Constitutive Expression of Insulin-like Growth Factor-1 in Epidermal Basal Cells of Transgenic Mice Leads to Spontaneous Tumor Promotion. Cancer Res. 2000;60:1561–1570. [PubMed] [Google Scholar]
- 24.Galer CE, Corey CL, Wang Z, Younes MN, Gomez-Rivera F, Jasser SA, et al. Dual inhibition of epidermal growth factor receptor and insulin-like growth factor receptor I: reduction of angiogenesis and tumor growth in cutaneous squamous cell carcinoma. Head Neck. 2011;33:189–198. doi: 10.1002/hed.21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Najy AJ, Day KC, Day ML. The ectodomain shedding of E-cadherin by ADAM15 supports ErbB receptor activation. J Biol Chem. 2008;283:18393–18401. doi: 10.1074/jbc.M801329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inge LJ, Barwe SP, D’Ambrosio J, Gopal J, Lu K, Ryazantsev S, et al. Soluble E-cadherin promotes cell survival by activating epidermal growth factor receptor. Exp Cell Res. 2011;317:838–848. doi: 10.1016/j.yexcr.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Kim AL, Athar M, Bickers DR, Gautier J. Stage-specific alterations of cyclin expression during UVB-induced murine skin tumor development. Photochem Photobiol. 2002;75:58–67. doi: 10.1562/0031-8655(2002)075<0058:ssaoce>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Moll R, Mitze M, Frixen UH, Birchmeier W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am J Pathol. 1993;143:1731–1742. [PMC free article] [PubMed] [Google Scholar]
- 29.Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- 30.Batistatou A, Charalabopoulos AK, Scopa CD, Nakanishi Y, Kappas A, Hirohashi S, et al. Expression patterns of dysadherin and E-cadherin in lymph node metastases of colorectal carcinoma. Virchows Arch. 2006;448:763–767. doi: 10.1007/s00428-006-0183-8. [DOI] [PubMed] [Google Scholar]
- 31.Bongiorno PF, al-Kasspooles M, Lee SW, Rachwal WJ, Moore JH, Whyte RI, et al. E-cadherin expression in primary and metastatic thoracic neoplasms and in Barrett’s oesophagus. Br J Cancer. 1995;71:166–172. doi: 10.1038/bjc.1995.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saha B, Chaiwun B, Imam SS, Tsao-Wei DD, Groshen S, Naritoku WY, et al. Overexpression of E-cadherin protein in metastatic breast cancer cells in bone. Anticancer Res. 2007;27:3903–3908. [PubMed] [Google Scholar]
- 33.Yang J, Dai DQ. A comparative study of E-cadherin mRNA expression in primary tumors and metastatic foci of gastric cancer. Zhonghua Zhong Liu Za Zhi. 2005;27:25–28. [PubMed] [Google Scholar]
- 34.Fujii K, Furukawa F, Matsuyoshi N. Ligand activation of overexpressed epidermal growth factor receptor results in colony dissociation and disturbed E-cadherin function in HSC-1 human cutaneous squamous carcinoma cells. Exp Cell Res. 1996;223:50–62. doi: 10.1006/excr.1996.0057. [DOI] [PubMed] [Google Scholar]
- 35.Hazan RB, Norton L. The epidermal growth factor receptor modulates the interaction of E-cadherin with the actin cytoskeleton. J Biol Chem. 1998;273:9078–9084. doi: 10.1074/jbc.273.15.9078. [DOI] [PubMed] [Google Scholar]
- 36.Wilding J, Vousden KH, Soutter WP, McCrea PD, Del Buono R, Pignatelli M. E-cadherin transfection down-regulates the epidermal growth factor receptor and reverses the invasive phenotype of human papilloma virus-transfected keratinocytes. Cancer Res. 1996;56:5285–5292. [PubMed] [Google Scholar]
- 37.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 38.Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest. 2011;121:3797–3803. doi: 10.1172/JCI57152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 40.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 41.Esch TR, Jonsson MV, Levanos VA, Poveromo JD, Sorkin BC. Leukocytes infiltrating the submandibular glands of NOD mice express E-cadherin. J Autoimmun. 2000;15:387–393. doi: 10.1006/jaut.2000.0451. [DOI] [PubMed] [Google Scholar]
- 42.Fong KP, Barry C, Tran AN, Traxler EA, Wannemacher KM, Tang HY. Deciphering the human platelet sheddome. Blood. 2011;117:e15–e26. doi: 10.1182/blood-2010-05-283838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uchida Y, Kawai K, Ibusuki A, Kanekura T. Role for E-cadherin as an inhibitory receptor on epidermal gamma delta T cells. J Immunol. 2011;186:6945–6954. doi: 10.4049/jimmunol.1003853. [DOI] [PubMed] [Google Scholar]
- 44.Van den Bossche J, Malissen B, Mantovani A, De Baetselier P, Van Ginderachter JA. Regulation and function of the E-cadherin/catenin complex in cells of the monocyte-macrophage lineage and DCs. Blood. 2012;119:1623–1633. doi: 10.1182/blood-2011-10-384289. [DOI] [PubMed] [Google Scholar]
- 45.Maretzky T, Scholz F, Köten B, Proksch E, Saftig P, Reiss K. ADAM10-mediated E-cadherin release is regulated by proinflammatory cytokines and modulates keratinocyte cohesion in eczematous dermatitis. J Invest Dermatol. 2008;128:1737–1746. doi: 10.1038/sj.jid.5701242. [DOI] [PubMed] [Google Scholar]
- 46.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–344. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 47.Kyrkanides S, Miller JH, Tallents R, Brouxhon S, Centola G, Olschowka J. Intraperitoneal inoculation of Sandhoff mouse neonates with an HIV-1 based lentiviral vector exacerbates the attendant neuroinflammation and disease phenotype. J Neuroimmunol. 2007;188:39–47. doi: 10.1016/j.jneuroim.2007.05.010. [DOI] [PubMed] [Google Scholar]