Abstract
Objective
To determine adulthood functioning following chronic iron deficiency in infancy.
Study design
At 25 years, we compared 33 participants with chronic iron deficiency in infancy to 89 who were iron-sufficient before and/or after iron therapy. Outcomes included education, employment, marital status, physical and mental health.
Results
Adjusting for sex and SES, a higher proportion of the chronic iron-deficient group did not complete secondary school (58.1% vs.19.8% in iron-sufficient group, Wald-value = 8.74, p = .003), were not pursuing further education/training (76.1% vs. 31.5%, Wald-value = 3.01, p = .08; suggestive trend), and were single (83.9% vs. 23.7%, Wald-value = 4.49, p = .03). They reported poorer emotional health and more negative emotions and feelings of dissociation/detachment. Results were similar in secondary analyses comparing the chronic iron-deficient group to participants in the iron-sufficient group who had been iron-deficient before treatment in infancy. Path analysis showed direct paths for chronic iron deficiency in infancy and being single and more detachment/dissociation at 25 years. There were indirect paths for chronic iron deficiency and not completing secondary school via poorer cognitive functioning in early adolescence and more negative emotions via behavior problems in adolescence, indicating a cascade of adverse outcomes.
Conclusion
The observational nature of the study limits causal inference, despite control for background factors. Nonetheless, the results indicate substantial loss of human potential. There may be broader societal implications, because many adults worldwide had chronic iron deficiency in infancy. Iron deficiency can be prevented or treated before it becomes chronic or severe.
Keywords: anemia, infant, adult, developmental origins of health and disease
Iron deficiency is most prevalent in developing countries1 but remains a problem elsewhere, especially among infants and pregnant women.2,3 Iron deficiency anemia (or other indication of chronic, severe iron deficiency) in infancy is associated with poorer cognitive, motor, socialemotional, and neurophysiologic outcomes.4,5 Most studies report lower scores despite iron treatment and correction of anemia (see reviews6,7). Available longitudinal studies find persisting differences (see reviews5,8,9).
Most information on outcome beyond early childhood comes from our longitudinal study in Costa Rica. By early adolescence, children with chronic, severe iron deficiency in infancy had not caught up in motor performance to their peers who were iron-sufficient before and/or after iron treatment in infancy.10 More of them had repeated a grade in school and/or been referred for special services.11 Their mothers and teachers reported them to have more anxiety/depression, social problems, and inattention, along with a corresponding increase in summary measures of internalizing problems and total problems.11 At 19 years, they did worse on neurocognitive tests of executive function and recognition memory.12 They did not catch-up in overall cognitive performance; the gap in cognitive test scores widened for those from families with lower socioeconomic status (SES).13
We report here functional outcomes at 25 years related to educational attainment, employment, health, and close relationships. We expected that poorer adult outcome would result from a cascade of effects, e.g., disadvantaged background combining with chronic iron deficiency in infancy to affect cognitive performance and socio-emotional behavior in childhood and adolescence and subsequent functional outcomes.11,13,14
Methods
The original study was conducted in a predominantly working class urban community near San Jose, Costa Rica.15 Enrollment (July 1983 through February 1985) entailed door-to-door screening of all 12- to 23-month-old infants with birth weight > 2.5 kg and singleton term uncomplicated birth, who were free of acute or chronic medical problems and had normal physical examinations.15 Iron status varied from iron sufficiency to marked iron deficiency anemia (see below). All iron-deficient infants, regardless of hemoglobin (HB) concentration, and those with low ferritin received iron therapy for 3 months (6 mg/kg/day).
The mean age at study entry was 17 months. Iron deficiency was thus likely to have lasted for months, especially because unmodified cow milk was often introduced early. Of the 191 infants in the initial study, 6 lacked information about iron status after iron therapy, resulting in a potential sample size of 185. Participants were reassessed at 5 years,16 early adolescence (11–14 years),11 mid-adolescence (15–17 years),13 and 19 years.13 Participants provided signed informed consent for the 25-year follow-up. The protocol was approved by ethics committees of University of Michigan and Universidad Ciencias Médicas, Costa Rica.
Iron status in infancy was based on venous HB and 3 measures of iron status. Anemic was defined as HB ≤ 105 g/L and non-anemic as HB ≥ 120 g/L, with HB concentrations in between considered intermediate.15 Iron-deficient was defined as serum ferritin < 12 ng/mL and free erythrocyte protoporphyrin ≥ 1.77 µmol/L (100 µg/dL) red blood cells and/or transferrin saturation < 10%.15 After iron therapy, HB increased 37 g/L in iron-deficient anemic infants.15 None remained iron-deficient anemic, although many still had altered iron measures.16 Following the approach since the 5-year follow-up,10–13,16 the chronic-iron deficiency group consisted of participants with marked iron deficiency anemia in infancy (HB ≤ 100 g/l) or iron deficiency with higher HB that did not become iron-sufficient after iron therapy (their higher erythrocyte protoporphyrin concentrations indicated more chronic, severe iron deficiency).16 The primary analyses compared participants who had chronic, severe iron deficiency in infancy with those who were iron-sufficient before and/or after iron therapy, defined as non-anemic and no more than 1 abnormal iron measure.
No participant had iron deficiency anemia on subsequent blood testing (5, 11–14, and 19 years)11,13,16 with the exception of 4 women at 19 years. Iron deficiency was present in less than 5%. Thus, dietary iron was apparently sufficient to correct iron measures that remained altered after treatment in infancy and support good iron status subsequently.
Adult assessment
The follow-up was planned as a 2-hour assessment. However, due to work obligations, only 51 participants were able to come for testing between February 2007 and July 2008. We therefore collected information on functional outcomes for the rest of the sample using the questions that could be obtained by telephone in 20–30 minutes (February to June 2009). The information (in person or by telephone) was obtained by an experienced physician (SG) who had examined the participants at 19 years and remained unaware of their iron status or response to treatment in infancy.
Education and academic achievement were measured by questions from the Status Questionnaire.17 Employment was measured by questions from Monitoring the Future.18 Questions from a SES measure that has been sensitive to differences at the lower end of the spectrum in Latin American populations (Graffar19) assessed the type of home and material goods in the home. Close relationships were measured by portions of the Monitoring the Future survey.18 Physical and mental health were measured by questions from the Status Questionnaire17 and the Young Adult Health Survey.20 Mental health was assessed further by the Beck Depression Inventory21 and a short form of the State-Trait Anxiety (STAI) scale.22 Life stressors were measured by a modified Social Readjustment Rating Scale.23 Because our goal was to identify group differences in functional outcome, we considered it reasonable to use these measures even though they had not been standardized in Costa Rica. The project’s experienced psychologists and physicians deemed the questions to be relevant and appropriate in this context.
Statistical analyses
Initial comparisons of the chronic iron-deficient and iron-sufficient groups on each of the outcomes entailed t-tests. We then tested each significant difference with covariates using multiple regression for continuous outcomes and logistic regression for dichotomous ones and retaining covariates that contributed significantly to a given model. On conceptual grounds, we considered sex and SES of family of origin (based on parental education and occupation24 and dichotomized as lower- or middle-class) as covariates. We also considered other preexisting group differences and disadvantages, which often co-occur with iron deficiency, such as slightly lower birth weight, earlier weaning from the breast, more father absence, less supportive home environment, etc.15 To consider them in a parsimonious fashion, we created a propensity score10 and considered it as a covariate. A propensity score indexes the risk for having a condition of interest based on relevant preexisting factors and allows consideration of multiple factors in a single control variable in analyses of outcome.25 The other covariate considered was lead level (available only in infancy and early adolescence), given the well-established relations to long-term cognitive and behavioral outcomes. Secondary analyses divided the iron-sufficient group, comparing the chronic iron-deficient group to participants who became iron-sufficient after iron therapy in infancy (separate from those who were iron-sufficient at enrollment).
We conducted path analyses using Mplus (www.StatModel.com) to identify pathways to poorer adult outcome. We included factors from infancy (family SES, iron status) and early adolescent behavior problems and cognitive function. Cognitive function was indexed by a composite of standardized cognitive test scores at 11–14 years13 and behavior problems by the total problem score of the Child Behavior Checklist.26 There was continuity in these measures between 5 years and early adolescence, but the relations between early adolescence and outcome at 25 years were stronger than at 5 years. We therefore used data from adolescence in the path analyses.
As a preliminary step for depressive symptoms, we determined how Beck Depression Inventory items clustered together in this Costa Rican sample using principal components factor analysis with varimax rotation. Three factors emerged (z-scored), related to negative emotions, feelings of detachment or dissociation from normal activities, and physical symptoms of depression.
Results
Of the 185 potential subjects for longitudinal follow-up, 122 (65.9%) participated in the adult assessment; 2 declined and 3 were living outside Costa Rica. The remaining 58 subjects could not be located. The only statistically significant background difference between those who participated at 25 years and those who did not was that their childhood home environments, as measured by the Home Observation for Measurement of the Environment-Revised,27 had been more supportive of child development (p < .05). Demographic characteristics of the sample are shown in Table I. The mean age at assessment was 25 years (range, 22.8 to 27.0 years). Most individuals (n = 86, 70.5%) had participated in all previous assessments; an additional 29 (23.8%) had been part of the early adolescent assessment (11–14 years) and at least one other follow-up before 25 years. Males and females were about equally represented. Approximately 70% were employed full-time in the past year. About a third were pursuing higher education or further training. About a third were married or engaged.
Table 1.
Characteristics of the 122 participants in the 25-year follow-up*
| % (n)† | |
|---|---|
| Female | 49.2 (60) |
| Chronically iron-deficient in infancy | 27.0 (33) |
| Did not complete secondary school | 41.8 (51) |
| Not currently enrolled in school or training | 67.5 (81) |
| Not employed ≥ 20 hours/week in past month | 30.4 (34) |
| Not employed full time ≥ 6 months in past year | 29.8 (28) |
| Single | 65.5 (76) |
| Has children | 39.0 (46) |
| Age at testing, years | 25.0 (1.0) |
The number of participants with data varies slightly depending on the variable.
Values are % (n) for all variables except age at testing, which is shown as mean (SE).
Of the 122 participants, 33 (27.0%) had chronic iron deficiency as infants, and 89 (73.0%) had been iron-sufficient before and/or after iron therapy (41 became iron-sufficient after treatment). There was no differential attrition; individuals with chronic iron deficiency in infancy comprised 31.7% of those not assessed in the 25-year follow-up vs. 27.0% of those assessed (χ2 = 0.45, p = .50). As in previous follow-ups, a higher proportion of the chronic iron-deficient group was male (69.7% vs. 43.8% of the iron-sufficient group, χ2 = 6.45, p = .01). The age at assessment was similar (24.8 ± 1.0 years in the iron-deficient group vs. 25.1 ± 1.0 years in the iron-sufficient group, t = 1.03, p = .31). There were no differences in the distribution of functional outcome responses obtained in person or by telephone with the exception of being in school or obtaining further training. A higher proportion of participants who were interviewed in person reported in the affirmative, regardless of iron status group in infancy, compared with those interviewed by telephone (52.0% vs. 20.0%, χ2 = 13.25, p < .001).
Table II compares 25-year functional outcomes in chronic iron-deficient and iron-sufficient groups. Propensity score and lead levels did not contribute significantly to the models relating iron status in infancy to adult outcomes and were not retained as covariates. However, sex and SES of family of origin were significant covariates for some outcomes and therefore considered in all comparisons.
Table 2.
Functional outcomes at 25 years by iron status in infancy.
| Outcome | Chronic iron-deficient* |
Iron-sufficient* | Log Odds Ratio (95% CI) |
Test statistic† |
p-value |
|---|---|---|---|---|---|
| Did not complete secondary school | 66.7 (22) | 19.2 (17) | −1.44 (−2.34, −0.54) | 10.21 | 0.001 |
| Adjusted for gender and SES | 58.1 (19) | 19.8 (17) | −1.40 (−2.35, −0.45) | 8.74 | 0.003 |
| Not currently enrolled in school or training | 83.9 (28) | 27.4 (24) | −1.12 (−2.20, −0.04) | 4.29 | 0.04 |
| Adjusted for gender and SES | 76.1 (25) | 31.5 (28) | −0.88 (−1.90, 0.14) | 3.01 | 0.08 |
| Not employed ≥ 20 hours/week in past month | 21.9 (7) | 39.0 (35) | 0.58 (−0.40, 1.46) | 1.41 | 0.24 |
| Not employed full time ≥ 6 months in past year | 26.9 (9) | 31.9 (28) | 0.17 (−0.85, 1.19) | 0.11 | 0.74 |
| Single | 83.3 (27) | 22.5 (20) | −1.27 (−2.35, −0.19) | 5.55 | 0.02 |
| Adjusted for gender and SES | 83.9 (28) | 23.7 (21) | −1.17 (−2.27, −0.07) | 4.49 | 0.03 |
| Has children | 32.3 (11) | 47.3 (42) | 0.38 (−0.48, 1.24) | 0.79 | 0.37 |
| Beck Depression Inventory (total score) ‡ | 8.25 (1.42) | 6.12 (0.75) | 2.13 (−1.15, 5.41) | 1.65 | 0.20 |
| Negative emotions (z-score) | 0.53 (0.25) | −0.12 (0.08) | 0.65 (0.23, 1.07) | 9.62 | 0.002 |
| Adjusted for gender and SES | 0.52 (0.19) | −0.20 (0.11) | 0.72 (0.29, 1.16) | 9.3 | 0.003 |
| Detached/dissociated (z-score) | 0.68 (0.22) | −0.20 (0.09) | 0.88 (0.49, 1.26) | 9.06 | 0.004 |
| Adjusted for gender and SES | 0.62 (0.19) | −0.20 (0.11) | 0.82 (0.39, 1.26) | 9.69 | 0.002 |
| Physical symptoms of depression (z-score) | 0.05 (0.18) | −0.01 (0.11) | 0.06 (−0.37, 0.48) | 0.10 | 0.75 |
| Self-rating of emotional health (z-score) | −0.36 (0.22) | 0.15 (0.09) | −0.51 (−0.91, −0.11) | 4.58 | 0.04 |
| Adjusted for gender and SES | −0.41 (0.18) | 0.19 (0.11) | −0.59 (−1.02, −0.17) | 4.41 | 0.04 |
| Self-rating of overall health (z-score) | −0.04 (0.17) | 0.02 (0.11) | −0.06 (−0.48, 0.36) | 0.09 | 0.79 |
| Self-reported physical health problems (z-score) | −0.09 (0.19) | 0.01 (0.11) | −0.10 (−0.52, 0.31) | 0.22 | 0.64 |
| Anxiety (State-Trait Anxiety Inventory) § | 13.49 (1.02) | 12.87 (0.53) | 0.62 (−1.52, 2.76) | 0.33 | 0.59 |
| Life stressors in past year (number) □ | 2.15 (0.41) | 2.69 (0.23) | −0.54 (−1.43, 0.36) | 1.39 | 0.24 |
| Housing and material goods ¶ | 16.21 (0.81) | 17.36 (0.42) | −1.15 (−2.85, 0.55) | 1.58 | 0.22 |
Values are % (n) for categorical variables and mean (SE) for continuous variables. The number of participants with data varies slightly depending on the variable.
Test statistics are Wald-value for dichotomous variables and F-value for continuous variables.
Maximum possible score = 63; higher values indicate more symptoms of depression.
Maximum possible score = 40; higher values indicate more symptoms of anxiety.
29 possible stressors.
Maximum possible score = 24; higher values indicate better housing and more material goods.
Individuals with chronic iron deficiency in infancy completed one year less of schooling, on average, than the iron-sufficient group (11.5 ± 0.3 vs. 12.5 ± 0.4 years, t = 2.03, p = .04). The year turned out to be critical for finishing secondary school and receiving a “bachillerato” (roughly equivalent to a US high school diploma). Much higher proportions of the chronic iron-deficient than iron-sufficient groups did not complete secondary school and were not pursuing further education or training. Controlling for family SES and sex did not substantially alter the findings: the approximately 3-fold difference in secondary school completion remained statistically significant and the approximately 2-fold difference in pursuit of further education/training still showed a suggestive trend (Table II). There were no differences in employment outcomes (working ≥ 20 hours/week in the past month; working full-time at least 6 months in the past year).
Over 80% of the chronic iron-deficient group was single, that is, never married or engaged, compared with approximately 24% of the iron-sufficient group (Table II). There was no effect of sex or SES of family of origin. There were no differences in the proportion with children. The chronic iron-deficient group rated their emotional health17 as worse than that of the iron-sufficient group and reported more symptoms than the iron-sufficient group for 2 factors derived from the Beck Depression Inventory (negative emotions and feelings of detachment/dissociation). There were no group differences in self-reported anxiety, physical symptoms of depression, problems related to substance abuse, physical health, or life stresses. We did not have anthropometry at 25 years since most information was collected by telephone. However, at 19 years there were no group differences in body mass index (25.8 ± 6.6 in the iron-deficient group vs. 23.8 ± 4.1 years in the iron-sufficient group, t = 1.54, p = .13).
Secondary analyses that considered participants who became iron-sufficient following treatment (separately from those who were iron-sufficient at enrollment) yielded the same statistically significant and suggestive differences compared to the chronic iron-deficient group. For instance, much like the entire iron-sufficient group (Table II), the proportions among those who became iron-sufficient were 24.4% not completing secondary school, 33.8% not being in school or further training, and 23.7% being single, again controlling for SES and sex, compared with 58.1%, 76.1%, and 83.9%, respectively, in the chronic iron-deficient group (details of secondary analyses available on request).
To determine whether the major adult outcomes that showed differences depending on infant iron status were interrelated, we examined the correlations among them. There was only one statistically significant correlation. Not completing secondary school was negatively correlated with self-reported emotional health (r = −.29, p = .001).
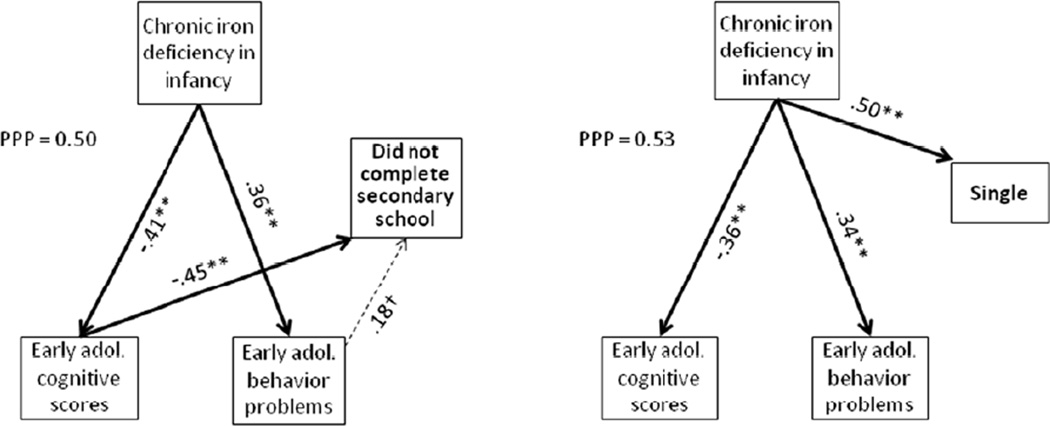
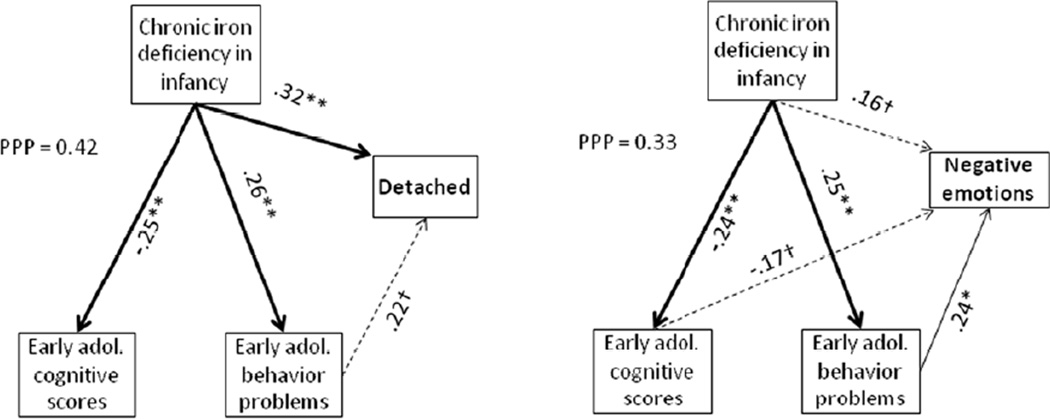
Our analyses that considered pathways leading to poorer adult outcome focused on educational attainment and marital status and mental health (Figures 1 and 2). The path analysis for low educational attainment (not completing secondary school) showed indirect paths for chronic iron deficiency in infancy via lower cognitive functioning and more behavior problems (suggestive trend) in early adolescence. In contrast, for being single, there was only a direct path for iron deficiency in infancy. In the path analyses for mental health outcomes, there was a direct path between iron deficiency in infancy and feelings of detachment/dissociation and an indirect path via early adolescent behavior problems (suggestive trend). There was an indirect path for iron deficiency in infancy and negative emotions via early adolescent behavior problems and suggestive trends for a direct path and another indirect path via early adolescent cognitive test scores (negative relation).
Figure 1.
Path analyses for not completing secondary school and being single. Goodness of fit under the Bayesian modeling was assessed by the Posterior Predictive P-value (PPP); values close to 0.5 in either direction are considered to indicate a good fit, whereas values less than 0.10 or greater than 0.90 indicate a poor fit. The path coefficients are provided for all statistically significant or suggestive paths. The strongest paths are shown as thick lines, other significant ones as regular lines, and those with suggestive trends as dashed lines. Statistical significance of individuals paths is indicated by * p < .05, ** p < .01, and † p < .10.
Figure 2.
Path analyses for mental health outcomes (negative emotions, feelings of detachment/dissociation). Goodness of fit under the Bayesian modeling was assessed by the Posterior Predictive P-value (PPP); values close to 0.5 in either direction are considered to indicate a good fit, , whereas values less than 0.10 or greater than 0.90 indicate a poor fit. The path coefficients are provided for all statistically significant or suggestive paths. The strongest paths are shown as thick lines, other significant ones as regular lines, and those with suggestive trends as dashed lines. Statistical significance of individuals paths isare indicated by * p < .05, ** p < .01, and † p < .10.
Discussion
In this long-term follow-up, individuals who had chronic iron deficiency in infancy had indications of poorer adult functioning in all domains except physical health and employment. Our framework of cascading effects was partially borne out. In path analyses, prior cognitive test scores and behavior problems contributed to some outcomes. Not completing secondary school was the only outcome where an indirect path for chronic iron deficiency via early adolescent outcomes was stronger than the direct effect for iron deficiency in infancy. However, contrary to expectation, there were no direct or indirect paths for SES of family of origin. For secondary school graduation or further training, this seems to conflict with our previous report of effects of both SES and iron deficiency in infancy on cognitive test scores.13 However, the studies had different foci – moderating effects in the longitudinal analysis of cognitive scores13 and mediators of functional outcome in the present study.
As for other early insults, the mechanisms by which chronic iron deficiency in infancy could contribute to adverse adult outcomes are undoubtedly complex. From animal models, it is clear that early-life iron deficiency adversely impacts the developing brain. Short- and long-term effects include impaired myelination and dendritogenesis, altered neurotransmitter functioning, changes in neurometabolism in important brain regions such as the hippocampus, striatum, and cortex, and altered gene and protein profiles, with associated behavioral changes.8 Neurophysiological and behavioral alterations in human infants are consistent with central nervous system effects in animal models.5,8,28
Cascading effects seem likely, regardless of limitations in our ability to characterize them. For example, early brain effects of chronic, severe iron deficiency in infancy may disrupt fundamental neural processes underlying sensory, cognitive, socio-emotional, and motor development and contribute to diverging developmental trajectories. Early behavioral differences, such as wariness, hesitance, and lack of positive affect,14,28 might contribute to long-lasting effects through their impact on caregivers – the chronically iron-deficient infant “might be less likely to seek, receive, or benefit from developmentally supportive interactions with the physical and social environment.”29 Our findings of more feelings of detachment and dissociation in adulthood seem particularly relevant in light of the pattern of behavioral differences in infancy14 and more internalizing problems in early adolescence.11
It is noteworthy that adult functional outcome was good among individuals with HB > 100 g/l and iron deficiency in infancy who became iron-sufficient with 3 months of iron therapy. This observation suggests that poor long-term outcome, at least on measures of overall functioning, may be prevented if iron treatment is given before iron deficiency becomes chronic and severe. Good functional outcome in individuals who became iron-sufficient following treatment in infancy also means that potential adverse effects of iron therapy30 are unlikely to be a factor in the poor outcome among individuals with chronic iron deficiency in infancy. This is a consideration because the dose of iron was at the high end of the currently recommended range. Subsequent iron deficiency is also unlikely to be a major contributor to outcome, because less than 5% of participants were iron-deficient at childhood and adolescent follow-ups.11,13,16
Albeit limited by small sample size, the Costa Rica study provides the only available data on adult outcome following iron deficiency in infancy. Like any other observational study, it cannot support causal inferences, despite control for family SES and consideration of other background differences. Another limitation is that we have no information about mothers’ iron status during pregnancy, and infant iron status was determined at 12 to 23 months postnatal age. Thus, infant iron deficiency could have started prenatally or in the first year of life. Consequently, this study cannot address the issue of differential effects of iron deficiency depending on when it occurs during early development. However, the circumstances of the Costa Rica study are similar to those in many settings worldwide, where programs of iron supplementation in pregnancy and infancy are either absent or less than optimal and infants may be exposed to insufficient iron pre-and/or postnatally. Thus, the findings may pertain to the real-life experiences of many infants. Other limitations include attrition and interview method. Although there was no differential attrition based on iron status in infancy or almost all background characteristics, a third of the cohort could not be located at 25 years. Obtaining information from participants in person yielded a higher proportion reporting further training/education than by telephone. These participants may have had more flexible schedules, allowing them to come in person, or face-to-face contact may have facilitated more detailed responses. Although the higher proportion reporting further training was observed regardless of iron status group and thus does not appear to have affected the findings, uniform in-person data collection would have been stronger.
The Costa Rica sample consisted of healthy infants who were growing normally by US standards. The observed outcomes might not generalize to settings where infectious diseases and growth faltering are common. The results might also not pertain to developed countries. However, prior to widespread use of iron-fortified infant products in the 1970s iron deficiency anemia was common in US infants, much like what we observed in the Costa Rica sample. Thus, many adults over the age of 40 years in the US today are likely to have had iron deficiency anemia in infancy. The situation is similar in Europe and Canada. Because iron therapy in clinical settings is often for a shorter period and less carefully supervised than in the Costa Rica study, iron deficiency was probably not fully corrected for many such infants in North America and Europe. Our findings may thus be relevant to them in adulthood. The adverse effects that we observed in adulthood are substantial. Outcomes such as not completing secondary school or pursuing further training or education may provide a good foundation for estimating the economic impact of early-life iron deficiency, because they are likely to influence career paths and income over time. However, it is unclear whether long-term outcomes would be as problematic if iron deficiency had been detected and treated earlier.
Compared with individuals who had been iron-sufficient in infancy, adults who had chronic iron deficiency as infants were less likely to complete secondary school or pursue further training and more likely to be single, experience negative emotions, and feel detached or dissociated. If replicated in larger samples, the adverse outcomes represent a substantial loss of human potential that is sad for the individual and detrimental for the population. But a cause for optimism is that participants with less chronic iron deficiency (with or without anemia) who became iron-sufficient with iron therapy in infancy were functioning well. The findings may help guide practice and policy, because iron deficiency is a condition that can be prevented or treated before it becomes chronic or severe.
Acknowledgments
We are grateful to study participants for their continued commitment and the skilled psychologists who carefully collected data at each follow-up. Feyza Corapci, PhD (post-doctoral fellowship supported by the same NIH grant as the project [R37 HD31606]), helped select functional outcome measures for the 25-year follow-up.
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The 25-year follow-up was supported by MERIT (R37 HD31606 to B.L.). The infancy study and other follow-ups were supported by the National Institutes of Health (R01 HD14122 and R01 HD31606). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- HB
hemoglobin
- SES
socioeconomic status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Portions of the study were presented as a platform presentation at the American Academic Societies’ meeting, Boston, MA, April 28-May 2, 2012.
References
- 1.Stoltzfus RJ. Iron interventions for women and children in low-income countries. J Nutr. 2011;141:756S–762S. doi: 10.3945/jn.110.128793. [DOI] [PubMed] [Google Scholar]
- 2.Brotanek JM, Gosz J, Weitzman M, Flores G. Secular trends in the prevalence of iron deficiency among US toddlers, 1976–2002. Arch Pediatr Adolesc Med. 2008;162:374–381. doi: 10.1001/archpedi.162.4.374. [DOI] [PubMed] [Google Scholar]
- 3.Bodnar LM, Cogswell ME, McDonald T. Have we forgotten the significance of postpartum iron deficiency? Am J Obstet Gynecol. 2005;193:36–44. doi: 10.1016/j.ajog.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Lozoff B. Iron deficiency and child development. Food Nutr Bull. 2007;28:S560–S571. doi: 10.1177/15648265070284S409. [DOI] [PubMed] [Google Scholar]
- 5.Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69:S43–S48. doi: 10.1111/j.1753-4887.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logan S, Martins S, Gilbert R. Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia. Cochrane Database Syst Rev. 2001;2 doi: 10.1002/14651858.CD001444. CD001444. [DOI] [PubMed] [Google Scholar]
- 7.Szajewska H, Ruszczynski M, Chmielewska A. Effects of iron supplementation in nonanemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: a systematic review of randomized controlled trials. Am J Clin Nutr. 2010;91:1684–1690. doi: 10.3945/ajcn.2010.29191. [DOI] [PubMed] [Google Scholar]
- 8.Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peirano PD, Algarin DR, Chamorro R, Reyes S, Garrido MI, Duran S, et al. Sleep and neurofunctions throughout child development: lasting effects of early iron deficiency. J Pediatr Gastroenterol Nutr. 2009;48:S8–S15. doi: 10.1097/MPG.0b013e31819773b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shafir T, Angulo-Barroso R, Calatroni A, Jimenez E, Lozoff B. Effects of iron deficiency on patterns of motor development over time. Hum Mov Sci. 2006;25:821–838. doi: 10.1016/j.humov.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 12.Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, Kaciroti N, et al. Iron deficiency in infancy and neurocognitive functioning at 19 years: Evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci. 2010;13:54–70. doi: 10.1179/147683010X12611460763689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency and low socio-economic status: a longitudinal analysis of cognitive test scores to 19 years. Arch Pediatr Adolesc Med. 2006;160:1108–1113. doi: 10.1001/archpedi.160.11.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron deficiency anemia. Child Dev. 1998;69:24–36. [PubMed] [Google Scholar]
- 15.Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, Jimenez E, et al. Iron deficiency anemia and iron therapy: effects on infant developmental test performance. Pediatrics. 1987;79:981–995. [PubMed] [Google Scholar]
- 16.Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. New Eng J Med. 1991;325:687–694. doi: 10.1056/NEJM199109053251004. [DOI] [PubMed] [Google Scholar]
- 17.Masten AS, Burt KB, Roisman GI, Obradovic J, Long JD, Tellegen A. Resources and resilience in the transition to adulthood: continuity and change. Dev Psychopathol. 2004;16:1071–1094. doi: 10.1017/s0954579404040143. [DOI] [PubMed] [Google Scholar]
- 18.Schulenberg JE, Bryant AL, O'Malley PM. Taking hold of some kind of life: how developmental tasks relate to trajectories of well-being during the transition to adulthood. Dev Psychopathol. 2004;16:1119–1140. doi: 10.1017/s0954579404040167. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez M, Muzzo S, Ivanovic D. Escala para la medicion del nivel socioeconomico en el area de la salud. Rev Med Chil. 1985;113:243–249. [PubMed] [Google Scholar]
- 20.Roisman GI, Aguilar B, Egeland B. Antisocial behavior in the transition to adulthood: the independent and interactive roles of developmental history and emerging developmental tasks. Dev Psychopathol. 2004;16:857–871. doi: 10.1017/s0954579404040040. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA, Brown GK. Beck Depression inventory -- II. San Antonio: The Psych Corp; 1996. [Google Scholar]
- 22.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 23.Holmes TH, Rahe RH. The Social Readjustment Rating Scale. J Psychosom Med. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 24.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 25.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 26.Achenbach TM, Edelbrock C. Manual for the Child Behavior Checklist and Revised Child Behavior Profile. Berlington,VT: University of Vermont; 1983. [Google Scholar]
- 27.Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment (Revised Edition) Little Rock: University of Arkansas; 1984. [Google Scholar]
- 28.Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J Nutr. 2011;141:740S–746S. doi: 10.3945/jn.110.131169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozoff B, Black M. Impact of micronutrient deficiencies on behavior and development. In: Pettifor J, Zlotkin SH, editors. Nutrition-Micronutrient Deficiencies during the Weaning Period and the First Years of Life. Basel: Karger; 2003. [Google Scholar]
- 30.Iannotti LL, Tielsch JM, Black MM, Black RE. Iron supplementation in early childhood: health benefits and risks. Am J Clin Nutr. 2006;84:1261–1276. doi: 10.1093/ajcn/84.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]