Abstract
Mercury (Hg) is neurotoxic, and children may be particularly susceptible to this effect. A current major challenge is the identification of children who may be uniquely susceptible to Hg toxicity because of genetic disposition. We examined the hypothesis that genetic variants of metallothionein (MT) that are reported to affect Hg toxicokinetics in adults would modify the neurotoxic effects of Hg in children. Five hundred seven children, 8–12 years of age at baseline, participated in a clinical trial to evaluate the neurobehavioral effects of Hg from dental amalgam tooth fillings. Subjects were evaluated at baseline and at 7 subsequent annual intervals for neurobehavioral performance and urinary Hg levels. Following the completion of the clinical trial, we performed genotyping assays for variants of MT isoforms MT1M (rs2270837) and MT2A (rs10636) on biological samples provided by 330 of the trial participants. Regression modeling strategies were employed to evaluate associations between allelic status, Hg exposure, and neurobehavioral test outcomes. Among girls, few significant interactions or independent main effects for Hg exposure and either of the MT gene variants were observed. In contrast, among boys, numerous significant interaction effects between variants of MT1M and MT2A, alone and combined, with Hg exposure were observed spanning multiple domains of neurobehavioral function. All dose-response associations between Hg exposure and test performance were restricted to boys and were in the direction of impaired performance. These findings suggest increased susceptibility to the adverse neurobehavioral effects of Hg among children with relatively common genetic variants of MT, and may have important public health implications for future strategies aimed at protecting children and adolescents from the potential health risks associated with Hg exposure. We note that because urinary Hg reflects a composite exposure index that cannot be attributed to a specific source, these findings do not support an association between Hg in dental amalgams specifically and the adverse neurobehavioral outcomes observed.
Keywords: mercury, behavior, neurotoxicity, genetic polymorphism, metallothionein, children
1. Introduction
Children are recognized as having heightened susceptibility to the adverse effects of environmental chemicals, compared to adults with similar exposures (Faustman et al. 2000; Landrigan and Goldman 2011; Makri et al. 2004). Of particular concern in this respect are possible neurological deficits associated with mercury (Hg) exposure, including impairment of the developing central nervous system (CNS) along with attendant personality, cognitive function and behavioral disorders (Counter and Buchanan 2004; Levy et al. 2004). A current major challenge is the identification of those children who may be uniquely susceptible to Hg-mediated neurological deficits because of genetic predisposition.
Among the biological variants likely to be associated with altered susceptibility to Hg toxicity are those affecting the synthesis and function of proteins involved in the distribution, excretion and body burden of Hg and Hg compounds. Notable among these are the metallothioneins (MTs), a multigene family of low molecular weight (6–7 kDa) proteins having approximately 30% cysteine residues that confer unique metal-binding properties and high redox capabilities. The mammalian MTs are found in four classes (MT1–MT4), with multiple isoforms of MT1 and MT2 present in humans (Aschner et al. 2006). MT1 and MT2, in particular, are ubiquitously expressed and play a crucial role in the dispersal and storage of metals such as Hg in the body. MT1/2 have important functions in the CNS including neuroprotection, regeneration and maintenance of cognitive processes (Aschner et al. 2006; Stankovic et al. 2007; West et al. 2008). Recent studies demonstrate learning and memory deficits in animals in which MT1/2 genes are lacking (Levin et al., 2006; West et al., 2008; Yoshida et al., 2004), as well as exacerbation of these deficits in MT1/2 null mice by inorganic Hg exposure (Eddins et al. 2008).
Studies in human subjects (Wang et al., 2012; Schläwicke Engström et al., 2008; Gundacker et al. 2007, 2009; Goodrich et al. 2011) have identified several common variants of genes encoding MTs that modify urinary Hg concentrations associated with Hg exposure from various sources, potentially underlying altered susceptibility to Hg toxicity. In this regard, Wang et al. (2012) evaluated dental professionals with personal and/or occupational exposure to elemental Hg (Hg0) and reported significantly reduced urinary Hg concentrations among subjects having single nucleotide polymorphisms (SNPs) of MT1M (rs2270837) or MT2A (rs10636) compared with subjects who were wildtype for these genes. Similarly, Gundacker et al. (2009) reported significant associations between polymorphisms of MT genes and measures of Hg exposure among Austrian medical students.
In the present study, we extended these findings to examine the hypothesis that the genetic variants of MT1M (rs2270837) and MT2A (rs10636) that have been reported to alter Hg toxicokinetics in adults (Wang et al. 2012) would modify the adverse neurobehavioral effects of Hg exposure in children. Subjects were children and adolescents who participated in a recently completed prospective randomized dental amalgam clinical trial between ages 8–18 and for whom longitudinal (annual) neurobehavioral assessments and quantitative measures of Hg exposure over 7 years of follow-up were available. For these assessments, we modified the clinical trial approach in 2 essential ways. First, rather than using the assignment to Hg amalgam or composite treatment groups, we employed a cumulative measure of urinary Hg concentrations (HgU) measured annually for all participants as our measure of Hg exposure. This allowed us to capture the effects of all Hg exposure, whether or not related to dental amalgam. This decision was based on the fact that assignment group accounted for, at most, 17% of the variation in HgU among boys (r2=0.171) and 15% among girls (r2=0.154), both occurring in year 2 of the clinical trial, and indicating considerable background Hg exposure unrelated to dental amalgam. Secondly, we evaluated the potential modifying effects of MT1M and MT2A genotype variants on the dose-response effects of Hg exposures, as represented by urinary Hg levels, on neurobehavioral performance. In addition to neurobehavioral assessments, we evaluated the potential modifying effects of MT1M and MT2A variants on urinary Hg levels to assess the possible correlation of urinary Hg with neurobehavioral effects by genotype. Because previous studies (Woods et al. 2007) suggested possible sex-related differences in Hg handling and susceptibility to Hg toxicity, we made these assessments independently in boys and girls.
2. Methods
2.1. The Study Population
The current study included 330 subjects who participated as children in the Casa Pia Dental Amalgam Clinical Trial (DeRouen et al. 2002; 2006) conducted between 1996 and 2006. Participants in the clinical trial included 279 boys and 228 girls, aged 8–12 yrs at baseline, who were students of the Casa Pia school system in Lisbon, Portugal. Children were initially randomized to Hg amalgam (treatment) or composite resin (control) dental treatment groups. Subjects were evaluated at baseline and at 7 subsequent annual intervals following initial dental treatment using an extensive battery of neurobehavioral assessments (Slade et al. 2008; Townes et al. 2008a, 2008b). Follow-up data were obtained on a similar number of subjects in each treatment group. Baseline urinary Hg concentrations were 1.5 ± 1.2 (0.1–7.7) and 1.4 ± 1.1 (0.0–8.6) μg/L for amalgam and composite groups, respectively. Mean urinary Hg concentrations by treatment group and by gender for each year of the clinical trial have been previously described (Woods et al. 2007).
2.2. Neurobehavioral tests employed
A comprehensive neurobehavioral test battery was used in this analysis, including measures from the Rey Auditory Verbal Learning Test (RAVLT), subtests from the Wide Range Assessment of Visual Motor Abilities (WRAVMA), the Wechsler Adult Intelligence Scale-III (WAIS-III), the Wechsler Memory Scale for Adults-III (WMS-III), Standard Reaction Time, Finger Tapping, Trailmaking A and B, and the Stroop word, color and word-color tests. The validity and rationale underlying the use of these tests in the clinical trial as well as the baseline neuropsychological performance of all subjects have been described (Martins et al. 2005; Townes et al. 2008a, b).
Table 1 lists the 23 neurobehavioral tests that were assessed and presents their means and standard deviations at their last year of administration (Year 7). Tests are organized within the behavioral domains that were evaluated in the clinical trial (DeRouen et al. 2006). Arrows depict whether the test score increases or decreases in magnitude with improved performance. Diminished or adversely affected performance associated with Hg exposure or gene variant status is described as occurring in the direction of impaired performance, whereas enhanced or beneficially affected performance associated with either of these variables is described as occurring in the direction of improved performance. The Comprehensive Test Of Nonverbal Intelligence (CTONI) (Portuguese translation) was given to each child at the beginning of the clinical trial to obtain a measure of IQ at baseline.
Table 1.
Neurobehavioral tests assessed with mean scores for Year 7 (final year of clinical trial)
| Test/Domain | Test Abbreviation | Measurea | Boys N = 120 Mean (SD) | Girls N = 119 Mean (SD) |
|---|---|---|---|---|
| Attention (6 tests) | ||||
| Stroop Test – Color | Stroop-Color | # correct ↑ | 66.16 (11.97) | 69.25 (10.39) |
| Word | Stroop-Word | # correct ↑ | 89.93 (15.16) | 91.54 (15.19) |
| Color-Word | Stroop-ColWd | # correct ↑ | 41.74 (9.76) | 43.93 (8.73) |
| WAIS-III – Digit Span | Digit Span | # correct ↑ | 14.30 (3.67) | 14.14 (2.76) |
| WAIS- III – Spatial Span | Spatial Span | # correct ↑ | 15.83 (3.03) | 15.61 (3.12) |
| Adult Trails A | Trails A | Time (sec) ↓ | 26.43 (10.63) | 30.25 (11.42) |
| Visual-Spatial (3 tests) | ||||
| Standard Reaction Time | SRT | Time (sec) ↓ | 0.74 (0.15) | 0.77 (0.13) |
| WAIS III – Digit Symbol | Digit Symbol | # correct ↑ | 72.02 (16.48) | 76.58 (13.85) |
| Symbol Search | Symbol Search | # correct ↑ | 32.99 (8.82) | 34.59 (8.01) |
| Executive Functioning (2 tests) | ||||
| Wisconsin Card Sort – Categories Completed | Card Sort-Cat | # categories ↑ | 3.05 (1.38) | 3.09 (1.47) |
| Adult Trails B | Trails B | Time (sec) ↓ | 65.97 (26.94) | 63.10 (23.80) |
| Learning & Memory (8 tests) | ||||
| RAVLT Trial 1 – List A | RAVALT 1 | # correct ↑ | 5.62 (1.49) | 6.12 (1.86) |
| Trial 5 – List A (fifth repetition) | RAVALT 5 | # correct ↑ | 11.23 (2.20) | 11.55 (2.23) |
| Trial 6 – List B | RAVALT 6 | # correct ↑ | 4.73 (1.38) | 5.28 (1.56) |
| Trial 7 – List A/Post B | RAVALT 7 | # correct ↑ | 9.85 (2.57) | 10.23 (2.48) |
| Trial 8 – List A after 20′ | RAVALT 8 | # correct ↑ | 9.29 (2.73) | 10.08 (2.76) |
| WMS-III – Visual Reproductions – Immediate | VisRep-Imm | # correct ↑ | 34.69 (4.66) | 36.62 (2.97) |
| Delayed | VisRep-Del | # correct ↑ | 32.01 (6.98) | 34.93 (4.02) |
| CVMT d-Prime | CVMT | Score ↑ | 1.50 (.94) | 1.61 (.87) |
| Motor (4 tests) | ||||
| WRAVMA – Pegs – Dominant Hand | Pegs-Dom | # Pegs ↑ | 47.35 (8.51) | 49.92 (6.28) |
| Non Dominant Hand | Pegs-NonDom | # Pegs ↑ | 44.37 (7.59) | 45.03 (6.10) |
| Finger Tapping – Dominant Hand | FT-Dom | # Taps ↑ | 52.66 (5.53) | 48.55 (5.83) |
| Non Dominant Hand | FT-NonDom | # Taps ↑ | 46.54 (5.79) | 42.53 (5.78) |
Arrows show direction of improved performance.
2.3. Genotyping assays
Genotyping was performed on DNA extracted from buccal cell samples that were obtained from study subjects following completion of the clinical trial (n=199) or from blood samples that were acquired at baseline for blood lead assessments (n=152). Genotyping was performed by the Functional Genomics & Proteomics Laboratory of the NIEHS Center for Ecogenetics and Environmental Health at the University of Washington, using commercially available TaqMan Detection System-based genotyping assays (Applied Biosystems, Inc., Hercules, CA) to characterize the MT1M (rs2270837) and MT2A (rs10636) alleles. MT1M (rs2270837) is defined as an A>G transition in the 3′-untranslated region (3′-UTR) of the MT1M gene, whereas MT2A (rs10636) is defined as a G>C transition in the 3′-UTR of the MT2A gene. Each subject was evaluated for MT1M (rs2270837) and categorized as wildtype (AA), heterozygous (AG) or homozygous mutant (GG) and for MT2A (rs10636) and categorized as wildtype (G/G), heterozygous (G/C) or homozygous mutant (CC). Relatively few subjects were genotyped as full homozygous mutant for either MT1M or MT2A. Therefore, for most analyses, we dichotomized allelic status as either wildtype (WT) or the combination of heterozygous or homozygous mutant into a single group referred to herein as HetMut. SNP descriptions are found at http://www.ncbi.nlm.nih.gov/snp/?term=rs2270837 or http://www.ncbi.nlm.nih.gov/snp/?term=rs10636 for MT1M (rs2270837) or MT2A (rs10636), respectively (National Center for Biotechnology Information 2013).
2.4. Human subjects considerations
All parents or guardians of children who participated in the clinical trial gave written consent, and all children provided signed assent, for the treatments and assessments made during the course of the trial, including collection of blood samples. Written consent was also obtained from all participants who provided buccal cell samples for genotyping subsequent to completion of the clinical trial. The study protocols for both the clinical trial and the present genotyping study were approved by the institutional review boards at the University of Lisbon and the University of Washington.
2.5. Urinary mercury analysis
A urine sample was collected from each child at baseline of the clinical trial and at each subsequent annual visit to the University of Lisbon School of Dental Medicine for dental, neurologic, and neurobehavioral evaluations. Strictly maintained sterile conditions and handling procedures throughout collection, shipping and analysis precluded contamination of urine samples by Hg or any other substance. Analysis of total mercury (Hg) was performed by continuous flow, cold vapor spectrofluorometry, as previously described (Pingree et al. 2001). Urinary creatinine concentrations were measured using a standard colorimetric procedure (Sigma #555-A; Sigma-Aldrich, St. Louis, MO, USA). Urinary Hg concentrations (HgU) were calculated as micrograms per gram creatinine (μg/g creatinine).
2.6. Assessments of Hg Exposure
As described in the Introduction, we employed urinary Hg concentrations (HgU) measured at baseline and each annual behavioral test session as the measure of Hg exposure instead of the dichotomous treatment group assignment to amalgam or composite resin as used in the clinical trial. Treatment assignment from the clinical trial was evenly split among boys (81 from the composite group and 83 from the amalgam group), whereas girls came slightly more frequently from the amalgam group (74 composite and 92 amalgam). However, treatment assignment accounted for not more than 17% of variation in HgU among either boys or girls, suggesting considerable background Hg exposure unrelated to dental amalgam treatment that is more fully captured by measures of cumulative HgU.
2.7. Study Design
This study evaluated whether MT1M (rs2270837) and/or MT2A (rs10636) gene status, alone or in combination, affected the relationship between urinary Hg concentration and tests of neurobehavioral functions among children who were evaluated annually from baseline through 7 years of follow-up after initial placement of dental amalgam or composite resin tooth fillings. The wide range in ages of subjects at the beginning of the clinical trial (8–12 years), the duration of the study which included passage through puberty for most subjects, and the associated change in specific tests administered to subjects based upon their age group during the course of the trial (e.g., child versus adult versions of some tests) introduced significant complexity into the interpretation of repeated measures analyses for this study. We, therefore, chose to evaluate the effects of Hg exposure using a cumulative measure of HgU over the entire study period with respect to performance outcomes during the last year of the study (Year 7). Cumulative HgU was calculated as the natural log of the sum of HgU from baseline and each year of follow-up adjusted by 1 (ln[(ΣHgU)+1]). The natural log best accommodates how exposures are distributed biologically, whereas adding 1 minimizes the influence of very small changes in HgU at very low levels (which otherwise could have dominated the analyses).
A small number of subjects (28) who were followed for the full 7 years of the clinical trial had missed one or more intermediate annual evaluations. Subjects missing 3 or more evaluations (n=2, both female) were eliminated from the exposure analyses. Those missing 1 (n=22, 9 female) or 2 (n=4, 3 female) annual evaluations were included in the Hg exposure analyses with their mean HgU concentrations substituted for the missing years. Hg exposure analyses conducted with and without these subjects did not significantly differ.
2.8. Statistical Analyses
The effect of a gene variant on the dose-response association between Hg exposure and neurobehavioral performance was the principal focus of this study. Thus, our analytical protocol focused on Hg-gene interactions, independently evaluating the impact of allelic status of the two MT genes separately and together on performance on each behavioral test. In all cases, boys and girls were evaluated independently. Statistical analyses were performed using SPSS Version 19 (IBM®SPSS®, Chicago, IL, USA).
Initial analysis was conducted using a full model consisting of Hg exposure (as defined above), dichotomous allelic status for each gene (WT or HetMut), and their interaction terms. Covariates in this model included age at assessment, race, and non-verbal IQ (determined at baseline). These covariates were selected because of their potential to bias neurobehavioral test performance in relation to Hg exposure (Echeverria et al. 1995) or other stressors (Krieg et al. 2001) and because data pertinent to these specific variables were available from the clinical trial from which subjects in the present study were acquired (Martins et al. 2005; Townes et al. 2003). Other factors potentially affecting behavioral test performance in relation to Hg exposure, including home environment, parent’s socioeconomic status, or medical histories, were comparable among essentially all subjects (DeRouen et al., 2002) and were, therefore, not included as covariates. Similarly, eligibility criteria for the clinical trial precluded admission of children with evidence of any preexisting psychological, behavioral, neurodevelopmental, immunosuppressive, or renal disorders (DeRouen et al. 2002), making consideration of these factors also unnecessary for inclusion as covariates in the analysis. All behavioral tests with significant interaction terms were re-evaluated for dose-response associations between Hg exposure and test performance independently for subjects with WT or HetMut allelic status for each gene. In these analyses, p<0.05 was established as the measure of significance. This strategy provides a clear description of Hg dose-response relationships within each genotypic group.
For those behavioral tests where the full model indicated no significant interaction terms, we dropped the interaction term from the model, and the main effects of Hg exposure and allelic status were evaluated independently for the full cohort of boys or girls.
Prior to fitting the regression models, we examined the assumptions of the model by scrutinizing the distributions and variances of all cognitive tests and ln(HgU). Most distributions had no significant deviation from normality or inflated variance. After fitting each model, we examined the standardized residuals for statistical outliers. Only 1 boy was an outlier in 2 cognitive measures and 1 girl was an outlier in one cognitive measure. We ran each model with and without outliers and found the results the same in each case. We also evaluated models with significant results for influential observations (Cook and Weisberg 1982). Since the outliers did not statistically affect the slope or r2 of the regression in any case, they were not considered influential points (http://stattrek.com/regression/influential-points.aspx).
3. Results
The study cohort consisted of 330 children (164 boys and 166 girls) for whom MT1M (rs 2270837) and MT2A (rs10636) gene status were available from among 507 total subjects enrolled at the start of the clinical trial. Excluded subjects either did not provide a blood sample at the initiation of the study, or were lost to follow-up for acquisition of a buccal cell sample following completion of the trial. Table 2 presents the characteristics of the cohort at Entry as well as at Years 2 and 7 of the clinical trial. Both boys and girls averaged 10.1 years of age, and most were in the 4th grade at entry into the study. At Entry, approximately 74% of boys and 71% girls were Caucasian, and each had an average non-verbal IQ score of 86. Table 2 also displays for both boys and girls the mean, standard deviation (SD), and range for urinary Hg concentrations unadjusted for creatinine and the natural log calculations for the creatinine-adjusted urinary Hg (HgU) at three time points: Entry, Year 2 and Year 7 of the clinical trial, as well as the calculated cumulative Hg exposure measure at Year 7. The change in number of total subjects between Entry and Year 7 reflects the overall 14% loss to follow-up over the course of the clinical trial. The frequencies of the homozygous common (wildtype, WT), heterozygous (Het), and homozygous mutant (Mut) alleles for boys and girls for MT1M (rs2270837) and MT2A (rs10636) are also presented.
Table 2.
Study population characteristics for participants at Entry (baseline) and in Year 2 and Year 7
| Characteristic | BOYS | GIRLS | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| ENTRY | YEAR 2 | YEAR 7 | ENTRY | YEAR 2 | YEAR 7 | |
|
| ||||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
|
| ||||||
| Age | 10.17 (.82) | 12.20 (.84) | 17.15 (.85) | 10.10 (.92) | 12.16 (.95) | 17.06 (1.03) |
|
| ||||||
| School Year | 4.05 (1.05) | 5.78 (1.2) | 9.40 (2.05) | 4.15 (1.07) | 5.92 (1.16) | 9.86 (1.51) |
|
| ||||||
| Non-Verbal IQ (at entry only) | 85.96 (9.96) | --- | --- | 85.54 (10.23) | --- | --- |
|
| ||||||
| Urinary Mercury Concentrations | ||||||
|
| ||||||
| Raw HgUa | 1.68 (1.28) | 2.18 (2.04) | 1.26 (3.01) | 1.97 (2.39) | 2.86 (2.63) | 1.76 (2.26) |
| Range | (0.14, 7.61) | (0.05, 11.5) | (.005, 31.7) | (0.11, 23.5) | (0.01, 15.6) | (.006, 15.3) |
| Calculated Ln HgUb | 0.89 (0.42) | 1.02 (0.49) | 0.62 (0.48) | 0.94 (0.48) | 1.18 (0.57) | 0.83 (0.56) |
| Range | (0.13, 2.15) | (0.04, 2.53) | (.005, 3.49) | (0.10, 3.20) | (.007, 2.81) | (.006, 2.79) |
| Calculated Maximumb | --- | --- | 1.46 (0.52) | --- | --- | 1.69 (0.54) |
| Range | --- | --- | (0.53, 3.49) | --- | --- | (0.61, 3.20) |
| Calculated Cumulativec | --- | --- | 2.47 (0.50) | --- | --- | 2.77 (0.55) |
| Range | --- | --- | (1.42, 4.03) | --- | --- | (1.44, 4.13) |
|
| ||||||
| Distribution | % (N) | % (N) | % (N) | % (N) | % (N) | % (N) |
|
| ||||||
| Total Subjects (N) | 163 | 158 | 120 | 167 | 151 | 119 |
|
| ||||||
| Caucasian - % (N) | 74.2% (121) | 73.0% (119) | 71.7% (86) | 71.3% (119) | 68.9% (104) | 69.7% (83) |
|
| ||||||
| MT1M (rs2270837) | ||||||
| Wildtype (A/A) | 66.9% (109) | 67.1% (106) | 68.3% (82) | 56.9% (95) | 57.0% (86) | 57.1% (68) |
| Heterozygous (A/G) | 31.3% (51) | 31.0% (49) | 29.2% (35) | 37.7% (63) | 37.7% (57) | 37.0% (44) |
| Homozygous Mutant (G/G) | 1.8% (3) | 1.9% (3) | 2.5% (3) | 5.4% (9) | 5.3% (8) | 5.9% (7) |
|
| ||||||
| MT2A (rs10636) | ||||||
| Wildtype (G/G) | 61.3% (100) | 60.8% (96) | 61.7% (74) | 59.9% (100) | 59.6% (90) | 58.8% (70) |
| Heterozygous (G/C) | 30.7% (50) | 31.0% (49) | 30.0% (36) | 33.5% (56) | 34.4% (52) | 34.4% (41) |
| Homozygous Mutant (C/C) | 8.0% (13) | 8.2% (13) | 8.3% (10) | 6.6% (11) | 6.0% (9) | 6.7% (8) |
μg/g creatinine;
ln(μg/g creatinine+1);
ln[(Σμg/g creatinine) +1]
3.1. Effects of MT Genotype on Urinary Hg Concentrations
The effects of MT1M and MT2A allelic status on urinary Hg concentrations were evaluated. As presented in Table 3, non-significant trends for decreased HgU appeared among boys genotyped as MT1M HetMut, compared with those as WT, when evaluated in terms of Hg exposure at Year 2 and at Year 7. In contrast, no comparable trends for decreasing HgU were apparent among boys genotyped as MT2A HetMut compared with those as WT. Additionally, no trends in urinary Hg concentrations were observed among girls when evaluated by allelic status in association with either gene (data not shown).
Table 3.
Urinary Hg (HgU) measures by MT1M and MT2A allelic status in boys
| Year | Hg Measure | Gene Status | MT1M | MT2A | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N | Mean (SD) | t (p) | N | Mean (SD) | t (p) | |||
|
| ||||||||
| 2 | Unadjusted Urinary Mercurya | WT | 106 | 2.26 (2.22) | 96 | 2.17 (2.15) | ||
| HetMut | 52 | 1.98 (1.98) | .787 (.43) | 62 | 2.16 (2.16) | .033 (.97) | ||
|
| ||||||||
| 2 | HgU (Creatinine Corrected)b | WT | 106 | 2.22 (2.01) | 96 | 2.22 (1.97) | ||
| HetMut | 52 | 2.10 (2.12) | .350 (.73) | 62 | 2.13 (2.15) | .278 (.78) | ||
|
| ||||||||
| 2 | Ln (HgU)c | WT | 106 | 1.04 (.49) | 96 | 1.03 (.49) | ||
| HetMut | 52 | .98 (.50) | .624 (.53) | 62 | 1.00 (.49) | .461 (.65) | ||
|
| ||||||||
| 7 | Unadjusted Urinary Mercurya | WT | 82 | 1.98 (6.39) | 74 | 1.26 (1.25) | ||
| HetMut | 38 | 1.59 (1.60) | .368 (.71) | 46 | 2.82 (8.46) | −1.24 (.22) | ||
|
| ||||||||
| 7 | HgU (Creatinine Corrected)b | WT | 82 | 1.27 (3.49) | 74 | 0.81 (.76) | ||
| HetMut | 38 | 1.24 (1.55) | .040 (.97) | 46 | 1.98 (4.70) | −1.68 (.10) | ||
|
| ||||||||
| 7 | Chronicd | WT | 82 | 2.51 (.49) | 74 | 2.43 (.50) | ||
| HetMut | 38 | 2.46 (.52) | .460 (.65) | 46 | 2.60 (.48) | −1.83 (.07) | ||
μg Hg/L;
μg Hg/g creatinine;
ln(μg Hg/g creatinine+1);
ln[(Σμg Hg/g creatinine) +1]
3.2. Effect of MT Genotype on the Association between Hg Exposure and Behavior
3.2.1. Hg Dose-Response associations by Allelic Status
Full model analysis of MT1M and MT2A allelic status and measures of Hg exposure on neurobehavioral test performance produced significant interaction terms on several tests, predominantly among boys. When analyzed independently by allelic status (Table 4), all significant Hg dose-response associations with behavioral performance were in the direction of impaired performance and were limited to boys genotyped as HetMut for either the MT1M or MT2A gene. Among those genotyped as MT1M HetMut allelic status, Hg dose-response effects were significant for the RAVLT Trial 5 and Trial 8 tests of Learning and Memory. Among boys genotyped as having MT2M allelic status, Hg dose-response effects were significant for the Digit Symbol and Symbol Search tests (Visual Spatial) and for the RAVLT Trial 8 and WMS-III visual reproductions-delayed tests (Learning & Memory). Of note, the RAVLT Trial 8 (Learning and Memory) was the only test with significant results among boys genotyped as having both MT1M and MT2A HetMut allelic status.
Table 4.
Hg Dose-Response Effects in Year 7 among boys with and without the MT1M or MT2A HetMut Variant
| Gene | MT1M | MT2A | ||||||
|---|---|---|---|---|---|---|---|---|
| Behavioral Test | WT | Het or Mut | WT | Het or Mut | ||||
| Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | |
| VISUALSPATIAL | ||||||||
| Digit Symbol | −1.39 (3.60) | −.05 (.70) | −12.9 (4.77) | −.39 (.01) | ||||
| Symbol Search | .05 (1.88) | .00 (.98) | −8.21 (2.62) | −.44 (.003) | ||||
| LEARNING & MEMORY | ||||||||
| RAVLT 5 | .51 (.55) | .11 (.35) | −1.56 (.53) | −.46 (.006) | ||||
| RAVLT 8 | .63 (.64) | .11 (.33) | −2.11 (.74) | −.45 (.007) | .70 (.60) | .14 (.25) | −1.96 (.86) | −.34 (.03) |
| Visual Rep - Delayed | −.74 (1.36) | .07 (.58) | −6.49 (2.27) | −.41 (.007) | ||||
Values in Bold signify p≤0.05. Age at assessment, race, and non-verbal IQ were used as covariates in analyses described in this table.
Among girls, the association between Hg exposure and impaired performance on the RAVLT Trial 1 test (Learning & Memory) was of borderline significance (p=0.05) among those genotyped as MT2A WT (data not shown).
3.2.2. Main Effects associated with either Allelic Status or Hg exposure
Among boys, no significant (p<0.05) adverse main effects were observed for MT1M HetMut allelic status, whereas main effects for MT2A HetMut allelic status were observed only for the RAVLT Trial 7 test (Learning & Memory) (not shown). Among girls, there were no significant main effects associated with either MT1M or MT2A HetMut allelic status (not shown).
Adverse main effects for Hg exposure while controlling for MT1M allelic status were observed among boys for all 3 Stroop tests (Attention), Digit Symbol and Symbol Search tests (Visual Spatial), Visual Reproductions – both immediate and delayed (Learning and Memory), and the Pegs – dominant hand (Motor Function) tests. No main effects for Hg exposures were observed among girls.
3.3. MT1M/MT2A Combined Analyses
Combined MT1M/MT2A analyses were conducted using dichotomous categories of gene status resulting in a 4-way analysis (2 genes by 2 allelic categories - WT vs. HetMut) and were restricted to boys, because the majority of single gene effects were observed among boys only. Analysis of the effects of the double variant gene status (MT1M/HetMut & MT2A/HetMut) on neurobehavioral performance associated with Hg exposure showed numerous significant interaction terms, with all other allelic combinations having only a few significant interactions. This observation was true despite there being only 10 boys with this double variant.
We took into account the small number of boys with double variant status by defining “strong” associations as those that had a partial correlation >0.50, noting that any correlation this strong would have been highly significant with the number of subjects in any of the other 4-way categories used in these analyses. Table 5 presents the estimates and partial correlations for these analyses only for boys with either double WT or double HetMut gene status. However, as noted above, the remaining mixed status groups had very few significant results.
Table 5.
Hg dose-response effects among boys having both MT1M & MT2A WT alleles or HetMut variants
| Behavioral Test | MT1M = WT MT2A = WT N = 46 | MT1M = H/M MT2A = H/M N = 10 | ||
|---|---|---|---|---|
| Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | |
| ATTENTION | ||||
| Trails A | .48 (3.04) | .02 (.88) | −11.38 (6.29) | .63 (.13) |
| Stroop Color | −1.90 (3.65) | −.08 (.60) | −13.41 (3.68) | −.85 (.02) |
| VISUAL SPATIAL | ||||
| SRT Mean | .03 (.04) | .10 (.53) | .19 (.10) | .64 (.12) |
| Digit Symbol | −2.76 (4.85) | −.09 (.57) | −24.19 (9.36) | −.77 (.05) |
| Symbol Search | −.99 (2.47) | −.06 (.69) | −10.08 (4.30) | −.72 (.07) |
| LEARNING AND MEMORY | ||||
| RAVLT 5 | .94 (.68) | .21 (.18) | −2.75 (1.55) | −.62 (.14) |
| RAVLT 7 | 1.05 (.71) | .23 (.15) | −2.30 (2.24) | −.42 (.35) |
| RAVLT 8 | 2.27 (.74) | .44 (.004) | −3.14 (1.75) | −.62 (.13) |
| Vis Rep – Delayed | −2.43 (2.08) | −.18 (.25) | −14.60 (6.87) | −.69 (.09) |
| CVMT dPrime | −.16 (.28) | −.09 (.57) | −1.14 (.94) | −.48 (.28) |
| MOTOR | ||||
| Pegs Non-Dom | −2.00 (2.19) | −.14 (.37) | −13.63 (9.25) | −.55 (.20) |
Values in Bold indicate either statistical significance or partial correlations > 0.50. One result that significantly improved with Hg exposure (in the unexpected direction) is also italicized. Age at assessment, race, and non-verbal IQ were used as covariates in analyses described in this table.
Among boys with double HetMut gene status, only two tests, Stroop–Color (Attention) and Digit Symbol (Visual Spatial), had significant (p<0.05) partial correlations with Hg exposure. However, 11 of the 23 behavioral tests evaluated had “strong” partial correlations with Hg exposure. In contrast, among boys with double WT gene status (n=46), only the RAVLT Trial 8 had significant results (partial correlations <0.05), and this was in the direction of improved performance with increasing Hg exposure.
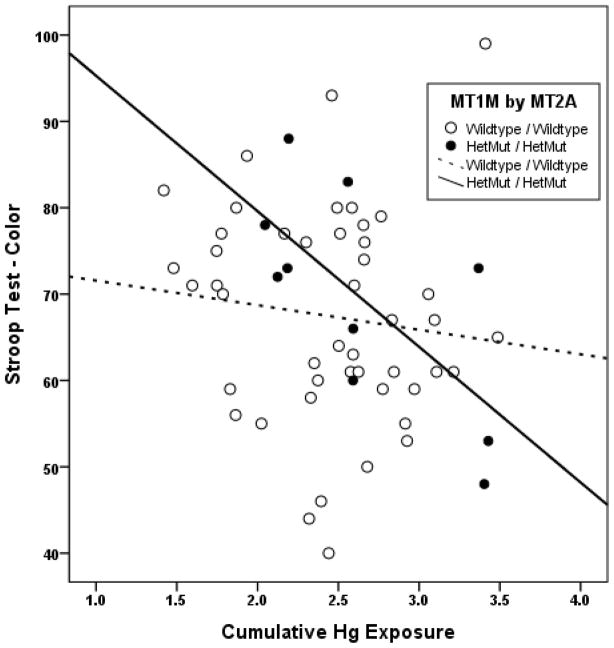
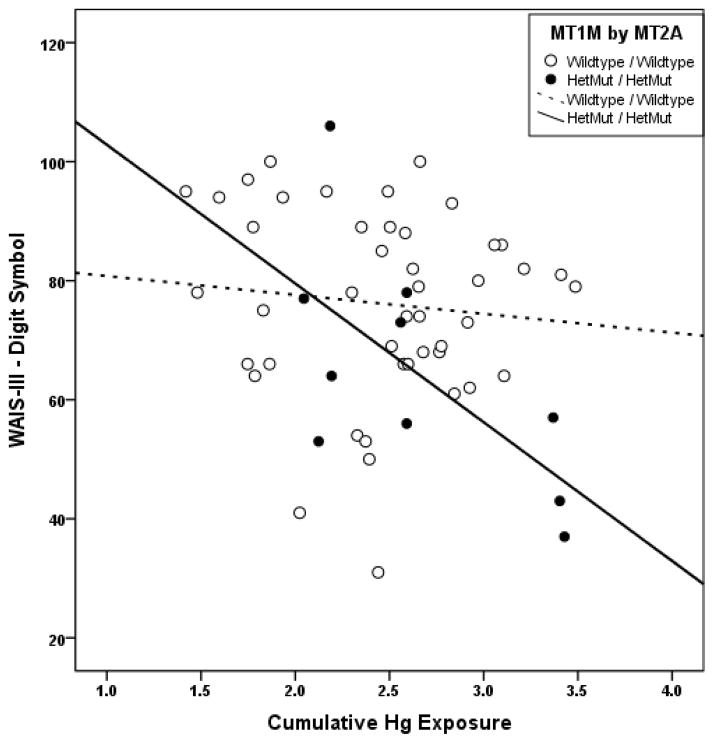
Figures 1 and 2 show performance on the Stroop-Color and Digit Symbol tests, respectively, plotted against Hg exposure among boys with double HetMut allelic status. Scatterplots distinguish between the WT/WT vs. the HetMut/HetMut subjects by the use of open vs. closed markers and dotted vs. solid lines respectively. These plots clearly demonstrate that boys with either double WT or double HetMut MT gene status respond very differently to Hg exposure in terms of their performance on the behavioral tests evaluated. For either test, Hg exposure accounted for less than 2% of performance variance among boys with double WT gene status but for 47% (Stroop-Color) or 42% (Digit Symbol) of performance variance among boys with double HetMut gene status (based upon regression r2).
Figure 1.
Associations between performance on the Stroop Color test and Hg exposure among boys. Scatter plots and simple linear regression fit lines of Stroop Color test scores by cumulative Hg exposure (ln[(ΣHgU)+1]) are plotted to distinguish boys with double wildtype (WT) (open dots, dotted line) or double HetMut allelic status (closed dots, solid line) for the two MT genes.
Figure 2.
Associations between performance on the Digit Symbol test and Hg exposure among boys. Scatter plots and simple linear regression fit lines of the Digit Symbol test scores by cumulative Hg exposure (ln[(ΣHgU)+1]) are plotted to distinguish boys with double wildtype (WT) (open dots, dotted line) or double HetMut allelic status (closed dots, solid line) for the two MT genes.
3.4. Summary of Results
MT1M and MT2A HetMut genotypes, evaluated both individually and concomitantly, significantly modify the adverse effects of Hg exposure on a number of neurobehavioral performance test results among boys but have little or no impact among comparably genotyped girls. The modification of the association between behavioral performance and Hg exposure by variant alleles of MT1M and MT2A genes occurred primarily within the domains of Visual Spatial acuity and Learning & Memory, with some additional impacts on Attention and Motor Function. Most all of these effects were in the direction of impaired performance, and many of the observed associations were significant at p ≤ 0.01. These observed associations, affecting 7 of the 23 neurobehavioral tests evaluated when assessing MT1M and MT2A independently, increased to include 11 of the 23 behavioral tests when assessing combined gene effects. The strength of associations between performance and Hg exposure was much greater among boys with HetMut genotypes for both MT genes than for either gene independently. This is true even when one of the two genes appeared to have no significant impact on the association, thus indicating a potentiation of the effect of one gene by the other.
4. Discussion
Numerous studies (Braun et al. 2006; Gundacker et al. 2010; Engström et al. 2012; Schläwicke Engström et al. 2008; Suk and Collman 1998) have proposed a component of genetic susceptibility to neurobehavioral deficits associated with Hg and other xenobiotic exposures, although the modifying effects of commonly expressed genetic variants on these associations are just beginning to be defined. In this regard, we recently described a genetic polymorphism of the gene encoding the heme biosynthetic pathway enzyme, coproporphyrinogen oxidase, (CPOX4 rs1131857), which modifies the adverse effects of Hg exposure on a wide variety of neurobehavioral functions in children (Woods et al. 2012). In the present studies, we provide further evidence of genetic susceptibility to Hg toxicity in children in describing significant modification of Hg effects on multiple neurobehavioral functions, principally among boys with variants of MT1M (rs2270837) and MT2A (rs10636) genes. Although we observed only suggestive or no trends of decreasing urinary Hg concentrations among boys genotyped with MT1/2 variants in this study, the present neurobehavioral findings support the view that these SNPs modify the distribution and/or actions of Hg in such a way as to exacerbate Hg effects on neurological functions in children. These findings are consistent with those derived from animal studies demonstrating that MT1/2 are involved in spatial learning and memory functions (Levin et al. 2006) and, moreover, that deletion of MT1/2 genes exacerbates learning and memory impairments associated with Hg exposure (Eddins et al. 2008). This report is the first to our knowledge to describe significant modification of the adverse effects of chronic Hg exposure on comparable neurobehavioral functions by genetic variants of MT in children and adolescents, as well as potentiation of such effects associated with multiple variant status.
Participants in this study differed from U.S. populations in several ways. One is the somewhat lower IQ assessment, as determined by the Comprehensive Test of Nonverbal Intelligence (CTONI) (Hammill et al. 1997) administered to subjects at the beginning of the clinical trial. This differential is not unexpected, inasmuch as scores on the CTONI have been found to vary by one standard deviation or so below the U.S. test norms when evaluated among non-U.S.-born subjects of similar educational level in numerous cross-cultural contexts (Martins et al. 2005). Because of this “test bias”, a lower IQ boundary was chosen as an inclusion criterion for the clinical trial from which subjects for the present study were drawn. This does not in any way, however, imply impaired intelligence of study subjects. Another difference between subjects in this study and those of U.S. populations is the relatively higher urinary Hg concentrations observed at baseline, notably common to both boys and girls, implying higher pre-trial background Hg exposure levels. Extensive assessment of potential sources of Hg exposure prior to initiation of the clinical trial in 1996 confidently excluded Hg from household, medicinal or ceremonial products, flooring materials or laboratory use. In contrast, general exposures associated with fossil fuel combustion for multiple uses within the local urban environment could have constituted a source of Hg exposure contributing to the baseline urinary Hg concentrations observed. Additionally, because urine may include inorganic Hg derived from demethylation of ingested MeHg (Sherman et al. 2013), fish consumption could constitute a source of elevated background urinary Hg observed among subjects in this study. In this respect, Portugal has Europe’s highest fish consumption per capita and is among the top 4 countries in the world for this indicator. Foremost among species of fish consumed is cod, which is reported to contain mean Hg concentrations of 0.111 ± 0.152 μg/g (United States Food and Drug Administration, 2011). Of note, 61% of parents or caregivers reported that their children in the present study consumed fish on a weekly basis (Evens et al., 2001).
The effects of MT1M and MT2A variants on the association of neurobehavioral test performance and Hg exposure among boys were observed predominantly within the domains of Visual Spatial acuity and Learning and Memory, suggesting decrements of verbal learning and memory as well as of perceptual cognition as principal consequences of this interaction. Test performance within the domains of Attention and Executive Function were also adversely affected among boys genotyped as HetMut, particularly among those carrying allelic variants of both MT1M and MT2A. These observations suggest possible impairment of attentional vitality and flexibility, e.g., ability to sustain attention or to shift between 2 sequences held in working memory, as a consequence of Hg exposure. These findings may have important public health implications, inasmuch as mean urinary Hg levels among boys in this study ranged from 1.4 (1.3–1.6) μg/g creatinine at baseline to a maximum of 2.2 (1.8–2.5) μg/g creatinine at Year 2 of follow-up in the dental amalgam clinical trial. By comparison, mean urinary Hg levels measured among a nationally representative sample of children 12–19 years of age acquired as part of the 2003–2004 U.S. National Health and Nutrition Examination Survey (Centers for Disease Control and Prevention 2007) were 0.358 (0.313–0.408) μg/g creatinine. Although this value is substantially lower than those measured in the present study, the mean urinary Hg concentration in the 90th percentile of that sample was 1.59 (1.13–2.52) μg/g creatinine, comparable to the range of Hg concentrations at which adverse neurologic effects of Hg were observed herein among boys with variants of MT1M or MT2A. These observations suggest potential adverse neurobehavioral effects of Hg among boys with either or both MT1/2 variant(s) who fall within the top 10% of subjects sampled within that survey for Hg exposure.
The mechanisms by which genetic variants of MT1M and MT2A modify behavior and/or exacerbate the adverse effects of Hg on these functions remain to be delineated. Numerous studies have demonstrated a neuroprotective role for MTs via regulation and maintenance of essential metals such as Zn and Cu in areas of the CNS associated with cognition, memory and behavior (West et al. 2008). Such findings suggest that alterations in MT function per se could affect susceptibility to neurological dysfunction through deregulation of metal roles in these processes. Although we observed very few main effects of MT1M or MT2A variants on neurobehavioral test performance here, studies demonstrating protection against the adverse effects of Zn deregulation on multi-organ functions by MT1/2 (Kelly et al. 1996) and, conversely, that Zn regulates MT gene expression in various tissues (Lin et al. 2009) support this role.
Alternatively, changes in the processing of neurotransmitters that regulate behavioral functions, such as serotonin and dopamine, could underlie adverse effects associated with genetically-based alterations in MT function. Dopamine, in particular, plays an important role in the regulation of cognitive processes in the prefrontal cortex, and an increase in dopamine concentrations in response to environmental or psychological stressors has been linked to improved performance on prefrontally-dependent cognitive tasks, particularly those that require working memory and response inhibition (Diamond 2007; Diamond et al. 2004). In contrast, reducing or blocking dopamine produces deficits on tasks that are dependent on this region. Although no main effects of MT1/2 null genotype on frontal cortical dopamine concentrations have been reported, Hg treatment has been found to differentially increase frontal dopamine levels in WT but not in MT1/2 null mice (Eddins et al. 2008). This Hg-induced increase in frontal cortical dopamine levels in WT mice may be relevant to protection against memory impairment, since MT1/2 null mice, which did not show this Hg-induced dopamine increase, had significant deficits in spatial learning and memory tasks. These observations support the idea that MT1/2 are in some manner involved in the processing of neurotransmitters that mediate learning and memory and that deletion of MT1/2 genes exacerbates impairments in these functions caused by Hg exposure by mitigating the increase in dopamine that otherwise protects against this effect. The present findings that MT1M and MT2A variants, individually or together, modify effects of Hg on Learning & Memory and Visual Spatial acuity in children lend support to this view.
From a functional perspective, MT proteins encoded by variants of MT1M and MT2A genes may modify the adverse effects of Hg in target tissues by being less effective as compared with proteins encoded by wildtype genes in terms of metal binding capabilities, redox properties or other characteristics affecting metal functions. Notably, both MT1M (rs2270837) and MT2A (rs10636) SNPs are located in the 3′-UTRs of their respective genes, regions known to be important for the regulation of mRNA stability, translation and protein localization within the cell (Hesketh 2004). Since binding of heavy metals varies depending on the molecular structures and subcellular localization of specific MT proteins (Krezel and Maret 2007), disruption of these post-transcriptional control mechanisms for MT synthesis, stability and distribution caused by SNPs in their respective 3′-UTRs could lead to alterations in Hg-binding capacity and/or tissue levels, subsequently influencing Hg neurotoxicity. In this respect, an intronic variant of MT1M (rs2270836) was found to be largely ineffective in modifying the effects of Hg on neurobehavioral functions among children in the present study (data not shown), consistent with this view. While these ideas provide scientific rationale for the Hg-impaired neurobehavioral performance observed here among boys with genetic variants of MT1M and MT2A, further studies are required to define the specific mechanisms underlying these effects.
As in previous studies (Woods et al. 2012), we found notable differences between boys and girls in the effects of Hg exposure and the MT1M/MT2A genetic variants on neurobehavioral test performance in this study. Although Hg exposure from dental amalgam was comparable among boys and girls participating in the clinical trial (DeRouen et al. 2006), sex-related differences in Hg toxicokinetics that may afford greater Hg excretion and, consequently, lesser likelihood of Hg retention and accumulation in girls than boys may contribute to this effect (Woods et al. 2007). Notably, however, we found no significant differential effects of gender on urinary Hg levels when assessed by genotype in the present study. Numerous other factors that include genetic and hormonal differences affecting brain development, structure and function between boys and girls might also to contribute to the gender differences observed here and in other studies (Calabrese 1985; Gochfeld 2007; Hines et al. 2010; Vahter et al. 2007a, b; Valentino et al. 2012; Weiss 2002). The sexually divergent responses to Hg exposure and genetic disposition observed in the present study highlight the importance of considering such differences in development of strategies aimed at risk assessment and prevention, especially in children.
Strengths and Limitations
A principal limitation of the present study is the relatively small population of subjects among whom we sought to assess potential altered susceptibility to the neurobehavioral effects of Hg associated with specific genetic variants of MT, particularly when considering potential gene-gene interactions. Despite this shortcoming, subject participation was sufficient to demonstrate significant effect modification of Hg neurotoxicity by variants of both MT1M and MT2A, individually and together, in association with Hg exposure. However, the substantial effects on cognitive functions observed among boys with both MT SNP variants should be interpreted with caution until confirmed in a larger population study. Additionally, because the participants of the present study were Portuguese children, concerns may arise regarding the generalizibility of study findings. In this respect, the principal question of this study was whether MT genotype altered the adverse effects of Hg on neurobehavioral performance among children, not whether test performance was representative of children in the United States or any other country. Notably, the results of this study demonstrated genetic modification of neurobehavioral test outcomes in relation to Hg exposure but not by genotype per se. If results were not generalizable, it would imply that genotype differentially affects children in different cultures, which seems unlikely notwithstanding possible variances in genotype prevalence.
In conclusion, the present studies demonstrate significant adverse effects of two relatively common metallothionein gene variants on neurobehavioral functions associated with Hg exposure among boys. These findings extend previous observations describing altered genetic susceptibility to the adverse neurobehavioral effects of Hg in children, and may have important public health implications for future strategies aimed at protecting children and adolescents from the potential health risks of associated with Hg exposure.
Highlights.
Common genetic variants of metallothionein (MT) increase susceptibility of children to Hg neurotoxicity.
Dose-response associations between Hg exposure and adverse neurobehavioral test performance are restricted predominantly to boys.
Adverse behavioral performance with Hg exposure is substantially heightened among boys with two MT variants, indicating potentiation of gene effects.
Acknowledgments
This research was funded by grants P42ES04696, P30ES07033 and R21ES019632 to the University of Washington from the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health. Additional funding was provided by the Wallace Research Foundation. We thank Ms. Jasmine Wilkerson and Mr. Jesse M. Tsai, Functional Genomics Laboratory, University of Washington, for excellent technical assistance in the conduct of this study.
Abbreviations
- Hg
mercury
- SNP
single nucleotide polymorphism
- IQ
intelligence quotient
- MT
metallothionein
- MT1M
metallothionein class1, isoform M
- MT2A
metallothionein class 2, isoform A
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aschner M, Syversen T, Souza DO, Rocha JBT. Metallothioneins: Mercury species-specific induction and their potential role in attenuating neurotoxicity. Exp Biol Med. 2006;231:1468–1473. doi: 10.1177/153537020623100904. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ Health Perspect. 2006;114:1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ. Toxic Susceptibility: Male/Female Differences. Wiley-Interscience; New York: 1985. [Google Scholar]
- Centers for Disease Control and Prevention. [accessed 7 March 2013];National Health and Nutrition Examination Survey. 2007 Available: http://www.cdc.gov/nchs/nhanes.htm.
- Cook RD, Weisberg S. Residuals and Influence in Regression (Monograph on Statistics and Applied Probability) New York: Chapman & Hall; 1982. [Google Scholar]
- Counter SA, Buchanan LH. Mercury exposure in children: a review. Toxicol Appl Pharmacol. 2004;198:209–230. doi: 10.1016/j.taap.2003.11.032. [DOI] [PubMed] [Google Scholar]
- DeRouen TA, Leroux BG, Martin MD, Townes BD, Woods JS, Leitão J, et al. Issues in the design and analysis of a randomized clinical trial to assess the safety of dental amalgam restorations in children. Contr Clin Trials. 2002;23:301–320. doi: 10.1016/s0197-2456(01)00206-9. [DOI] [PubMed] [Google Scholar]
- DeRouen TA, Martin MD, Leroux BG, Townes BD, Woods JS, Leitão J, et al. Neurobehavioral effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295(15):1784–1792. doi: 10.1001/jama.295.15.1784. [DOI] [PubMed] [Google Scholar]
- Diamond A. Consequences of variations in genes that affect dopamine in prefrontal cortex. Cerebral Cortex. 2007;17:i161–i170. doi: 10.1093/cercor/bhm082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. Am J Psychiat. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Echeverria D, Heyer NJ, Martin MD, Naleway CA, Woods JS, Bittner AC., Jr Behavioral effects of low-level exposure to Hg0 among dentists. Neurotoxicol Teratol. 1995;17:161–168. doi: 10.1016/0892-0362(94)00049-j. [DOI] [PubMed] [Google Scholar]
- Eddins D, Petro A, Pollard N, Freedman JH, Levin ED. Mercury-induced cognitive impairment in metallothionein-1/2 null mice. Neurotoxicol Teratol. 2008;30:88–95. doi: 10.1016/j.ntt.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström K, Ameer S, Bernaudat L, Drash G, Baeuml J, Skeerfving S, et al. Polymorphisms in genes encoding potential mercury transporters and urine mercury concentrations in populations exposed to mercury vapor from gold mining. Environ Health Perspect. 2012;121:85–91. doi: 10.1289/ehp.1204951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evens CC, Martin MD, Woods JS, Soares HL, Bernardo M, Leitão J, et al. Examination of dietary methylmercury exposure in the Casa Pia study of the health effects of dental amalgams in children. J Toxicol Environ Health. 2001;64:521–530. doi: 10.1080/15287390152627219. [DOI] [PubMed] [Google Scholar]
- Faustman EM, Silbernagel SM, Fenske RA, Burbacher TM, Ponce RA. Mechanisms underlying children’s susceptibility to environmental toxicants. Environ Health Perspect. 2000;108 (Suppl 1):13–21. doi: 10.1289/ehp.00108s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochfeld M. Framework for gender differences in human and animal toxicology. Environ Res. 2007;104:4–21. doi: 10.1016/j.envres.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Goodrich JM, Wing Y, Gillespie B, Werner R, Franzblau A, Basu N. Glutathione enzyme and selenoprotein polymorphisms associate with mercury biomarker levels in Michigan dental professional. Toxicol Appl Pharmacol. 2011;257:301–308. doi: 10.1016/j.taap.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundacker C, Gencik M, Hengstschläger M. The relevance of individual genetic background for the toxicokinetics of two significant neurodevelopmental toxicants: mercury and lead. Mut Res. 2010;705:130–140. doi: 10.1016/j.mrrev.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Gundacker C, Komarnick G, Jagiello P, Gencikova A, Dahmen N, Wittmann KJ, et al. Glutathioine-S-transferase polymorphism, metallothionein expression, and mercury levels among students in Austria. Sci Tot Environ. 2007;385:37–47. doi: 10.1016/j.scitotenv.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Gundaker C, Wittmann KJ, Kukuckova M, Komarnicki G, Hikkel I, Gencik G. Genetic background of lead and mercury metabolism in a group of students in Austria. Environ Res. 2009;109:786–796. doi: 10.1016/j.envres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Hammill DD, Pearson NA, Wiederholt JL. Comprehensive Test of Nonverbal Intelligence – Manual. Pro-Ed; Austin TX: 1997. [Google Scholar]
- Hesketh J. 3′-Untranslated regions are important for mRNA localization and translation: lessons from selenium and metallothionein. Biochem Soc Trans. 2004;32:990–993. doi: 10.1042/BST0320990. [DOI] [PubMed] [Google Scholar]
- Hines RN, Sargent D, Autrup H, Birnbaum LS, Brent RL, Doerrer NG, et al. Approaches for assessing risk to sensitive populations: Lessons learned from evaluating risk in the pediatric population. Toxicol Sci. 2010;113:4–26. doi: 10.1093/toxsci/kfp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly EJ, Quaife CJ, Froelick GJ, Palmiter RD. Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J Nutr. 1996;126:1782–1790. doi: 10.1093/jn/126.7.1782. [DOI] [PubMed] [Google Scholar]
- Krezel A, Maret W. Dual nanomolar and picomolar Zn(II) binding properties of metallothionein. J Am Chem Soc. 2007;129:10911–10921. doi: 10.1021/ja071979s. [DOI] [PubMed] [Google Scholar]
- Krieg EF, Jr, Chrislip DW, Letz RE, Otto DA, Crespo CJ, Brightwell WS, et al. Neurobehavioral test performance in the third National Health a Nutrition Examination Survery. Neurotox Teratol. 2001;23:569–589. doi: 10.1016/s0892-0362(01)00177-5. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Goldman LR. Children’s vulnerability to toxic chemicals: A challenge and opportunity to strengthen health and environmental policy. Health Affairs. 2011;30:842–850. doi: 10.1377/hlthaff.2011.0151. [DOI] [PubMed] [Google Scholar]
- Levin ED, Perraut C, Pollard N, Freedman JH. Metallothionein expression and neurocognitive functions in mice. Physiol Behav. 2006;87:513–518. doi: 10.1016/j.physbeh.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Levy M, Schwartz S, Dijak M, Weber J-P, Tardif R, Rouah F. Childhood urine mercury excretion: dental amalgam and fish consumption as exposure factors. Environ Res. 2004;94:283–290. doi: 10.1016/j.envres.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Lin S-F, Hua W, Madder D, Franklin RB, Feng P. Profiling of zinc-altered gene expression in human prostate normal vs cancer cells: a time course study. J Nutr Biochem. 2009;20:1000–1012. doi: 10.1016/j.jnutbio.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makri, Goveia M, Balbus J, Parkin R. Children’s susceptibility to chemicals: A review by developmental stage. J Toxicol Environ Health, Pt B. 2004;7:417–435. doi: 10.1080/10937400490512465. [DOI] [PubMed] [Google Scholar]
- Martins IP, Castro-Caldas A, Townes B, Ferreira G, Rodrigues P, Marques S, et al. Age and sex difference in neurobehavioral performance: a study of Portuguese elementary school children. Int J Neurosci. 2005;115:1687–1709. doi: 10.1080/00207450590958556. [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. [accessed 7 May 2013];SNP linked to Gene MT1M(rs2270837) or Gene MT2A(rs10636) Via Contig Annotation. 2013 Available: at http://www.ncbi.nlm.nih.gov/snp/?term=rs2270837 or http://www.ncbi.nlm.nih.gov/snp/?term=rs10636.
- Pingree SD, Simmonds PL, Woods JS. Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on tissue and urine mercury levels following prolonged methylmercury exposure in rats. Toxicol Sci. 2001;61:224–233. doi: 10.1093/toxsci/61.2.224. [DOI] [PubMed] [Google Scholar]
- Schläwicke Engström KS, Strömberg U, Lundh T, Jonhansson I, Vessby B, Hallmans G, et al. Genetic variation in glutathione-related genes and body burden of methylmercury. Environ Health Perspect. 2008;116:734–739. doi: 10.1289/ehp.10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LS, Blum JD, Franzblau A, Basu N. New insight into biomarkers of human mercury exposure using naturally occurring mercury isotopes. Environ Sci Technol. 2013;47:3403–3409. doi: 10.1021/es305250z. [DOI] [PubMed] [Google Scholar]
- Slade PD, Townes BD, Rosenbaum G, Martins IP, Luis H, Bernardo M, et al. The serial use of child neurocognitive tests: development versus practice effects. Psychol Assess. 2008;20:361–369. doi: 10.1037/a0012950. [DOI] [PubMed] [Google Scholar]
- Stankovic RK, Chung RS, Penkowa M. Metallothioneins I and II: Neuroprotective significance during CNS pathology. Int J Biochem Cell Biol. 2007;39:484–489. doi: 10.1016/j.biocel.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Suk WA, Collman GW. Genes and the environment: Their impact on children’s health. Environ Health Perspect. 1998;106(suppl 3):817–820. doi: 10.1289/ehp.98106817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townes BD, Rosenbaum JG, Martins IP, Castro-Caldas A. Neurobehavioral assessment in children: A cross-cultural perspective. Psychologica. 2003;34:177–185. [Google Scholar]
- Townes BD, Rosenbaum G, Martin MD, Martins IP, Luis H, Bernardo M. A longitudinal factor analytic study of children’s neurocognitive abilities. Int J Neurosci. 2008a;118(7):1009–1023. doi: 10.1080/00207450701768895. [DOI] [PubMed] [Google Scholar]
- Townes BD, Martins IP, Castro-Caldas A, Rosenbaum G, DeRouen T. Repeat test scores on neurobehavioral measures over an eight-year period in a sample of Portuguese children. Int J Neurosci. 2008b;118(1):79–93. doi: 10.1080/00207450601042102. [DOI] [PubMed] [Google Scholar]
- United States Food and Drug Administration. Mercury levels in Commercial Fish and Shellfish (1990–2010) 2011 http://www.fda.gov/Food/FoodborneIllnessContaminants/Metals/ucm115644.htm.
- Vahter M, Åkesson A, Lidén C, Ceccatelli S, Berglund M. Genetic differences in the disposition and toxicity of metals. Environ Res. 2007a;104:85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vahter M, Gochfeld M, Casati B, Thiruchelvam M, Falk-Filippson A, Kavlock R, et al. Implications of gender differences for human health risk assessment and toxicology. Environ Res. 2007b;104:70–84. doi: 10.1016/j.envres.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Reyes B, Bockstaele EV, Bangasser D. Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacol. 2012;62:13–20. doi: 10.1016/j.neuropharm.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Goodrich JM, Gillespie B, Werner R, Basu N, Franzblau A. An investigation of modifying effects of metallothionein single-nucleotide polymorphisms on the association between mercury exposure and biomarker levels. Environ Health Perspect. 2012;120:530–534. doi: 10.1289/ehp.1104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Sexually dimorphic nonreproductive behaviors as indicators of endocrine disruption. Environ Health Perspect. 2002;110(suppl 3):387–391. doi: 10.1289/ehp.02110s3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AK, Hidalgo J, Eddins D, Levin LD, Aschner M. Metallothionein in the central nervous system: Roles in protection, regeneration and cognition. NeuroToxicol. 2008;29:489–503. doi: 10.1016/j.neuro.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JS, Heyer HJ, Echeverria D, Russo JE, Martin MD, Bernardo MF, et al. Modification of neurobehavioral effects of mercury by a genetic polymorphism of coproporphyrinogen oxidase in children. Neurotoxicol Teratol. 2012;34:513–521. doi: 10.1016/j.ntt.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JS, Martin MD, Leroux BG, DeRouen TA, Leitão JG, Bernardo M, et al. The contribution of dental amalgam to urinary mercury excretion in children. Environ Health Perspect. 2007;115:527–1531. doi: 10.1289/ehp.10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Watanabe C, Satoh M, Yasutake A, Sawada M, Ohtsuka Y, et al. Susceptibility of metallothionein-null mice to the behavioral alterations caused by exposure to mercury vapor at human-relevant concentration. Toxicol Sci. 2004;80:69–73. doi: 10.1093/toxsci/kfh138. [DOI] [PubMed] [Google Scholar]