Abstract
Endoglin (Eng), an ancillary receptor of the TGFβ signaling pathway superfamily, has been well recognized for its important function in vascular development and angiogenesis since its discovery more than a decade ago. Recent studies show that this receptor is also critical for the emergence of blood during embryonic development, and that at E7.5, endoglin together with Flk-1 identifies early mesoderm progenitors that are endowed with hematopoietic and endothelial potential. These two lineages emerge in very close association during embryogenesis, and because they share the expression of the same surface markers, it has been difficult to distinguish the earliest hematopoietic from endothelial cells. Here we evaluated the function of endoglin in hematopoiesis as development progresses past E7.5, and found that the hematopoietic and endothelial progenitors can be distinguished by the levels of endoglin in E9.5 yolk sacs. Whereas endothelial cells are Engbright, hematopoietic activity is primarily restricted to a subset of cells that display dim expression of endoglin (Engdim). Molecular characterization of these sub-fractions showed that endoglin-mediated induction of hematopoiesis occurs in concert with BMP2/BMP4 signaling. This pathway is highly active in Engdim cells but significantly down-regulated in the Eng knockout. Taken together, our findings show an important function for endoglin in mediating BMP2/BMP4 signaling during yolk sac hematopoietic development and suggest that the levels of this receptor modulate TGFβ versus BMP signaling.
Keywords: Endoglin, Flk-1, yolk sac, early hematopoiesis, TGFβ/BMP signaling, Smad1/5/8
INTRODUCTION
Endoglin (Eng or CD105) is a glycoprotein that acts as an ancillary receptor for various members of the Transforming Growth Factor Beta (TGFβ) superfamily [1, 2]. In endothelial cells, where Eng is abundantly expressed and has been mostly studied, this receptor plays an important role in the TGF-β dependent responses by balancing activating and inhibitory signals [1, 3]. Upon binding of TGF-β to the TGF-β type II receptor (Tgfbr2), TGF-β type I receptor (Tgfbr1), also known as activin receptor-like kinase 5 (Alk5), is recruited and phosphorylated. The activated Alk5 phosphorylates and activates downstream effector Smads [4]. In addition to Alk5, a second TGF-β type I receptor has been described, known as Alk1, whose expression is more restricted to endothelial cells [5]. Several studies support the premise that endoglin is necessary for efficient TGF-β/Alk1 signaling [3, 6, 7], and thus acts as a modulator of TGFβ-dependent activation of Alk1 (stimulatory signal through Smad1/5) versus Alk5 (inhibitory signal through Smad2/3) [3, 8]. Endoglin has also been shown to interact with Bone Morphogenetic Proteins (BMPs), including BMP2 and BMP7, through their respective ligand binding receptor kinases Alk3, Alk6, or Alk2, and BMPRII [2] but has not, to date, been shown to interact with BMP4.
Members of the TGFβ signaling pathway have been implicated in several critical processes of embryonic development. For instance, BMP signaling has been shown to be required for proper mesodermal formation and patterning [9–12] whereas TGF-β signaling is important for vasculogenesis [13–16]. Interestingly several of these studies also documented impaired primitive erythropoiesis in the yolk sac (YS) of knockout embryos, such as for TGFβ1 [13], TGFβRII [16], and BMP4 [9]. Endoglin-deficient (Eng−/−) mice die around E10.5 due primarily to vascular and cardiac defects [17, 18]. Even though it had been noted for more than a decade that yolk sacs from Eng−/− mice display a pale appearance12, this had been attributed to be an indirect result of insufficient blood flow. Initial experimental evidence for a role for endoglin in hematopoietic development came from studies using Eng-deficient differentiating embryonic stem cells [19, 20], however a hematopoietic role for endoglin in vivo was proven only recently: we have shown that endoglin is required for the emergence of blood progenitors from mesoderm during early embryonic development [21]. We have also demonstrated that in E7.5 wild-type embryos, hematopoietic progenitors are enriched in the Eng+Flk-1+ sub-fraction, and that this fraction has a dual hematopoietic/endothelial molecular signature [21]. This finding is consistent with and explains why the blood and endothelial lineages emerge in very close association during early embryonic development. Blood and endothelial cells are evident in the yolk sac of the murine embryo at about 8 days post-fertilization as blood islands: endothelial sacs encapsulating clusters of hematopoietic cells [22–24]. The two lineages also share the expression of several regulatory genes [25], antigens [26], and signaling receptors, such Flk-1 and Eng [20, 21, 27]. Thus it has been challenging to distinguish hematopoietic from endothelial cells during yolk sac development.
Although Eng has been detected in various hematopoietic cell types [28], including the long-term repopulating hematopoietic stem cell [29, 30], it remains unknown whether this receptor and consequently the TGFβ signaling pathway plays a role in hematopoietic ontogeny beyond primitive hematopoiesis. Here we demonstrate that hematopoietic and endothelial cells from the YS are temporally distinguishable by the levels of Eng. At E9.5, dim expression of Eng (EngdimFlk-1neg) identifies hematopoietic progenitors within the YS, whereas Engbright marks endothelial cells (EngbrightFlk-1+). Importantly, these phenotypic changes correlate with differential expression of components of the TGFβ/BMP pathway, revealing a crucial role for this signaling pathway in definitive YS hematopoiesis, and proving insight on its molecular regulation.
MATERIAL AND METHODS
Mouse Models
All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, and all animal work was approved by the University of Minnesota Institutional Animal Care and Use Committee. Eng-deficient embryos were generated by timed mating Eng+/− heterozygotes (kindly provided by Dr. Michele Letarte, University of Toronto); the morning of a positive plug was considered 0.5 dpc. Genotyping was performed as previously described [17]. Time-pregnant wild-type CD1 mice were purchased from Charles River Laboratories International, Inc.
Embryo dissection
Embryos were removed from decidua and Reichert’s membrane, and dissected in PBS supplemented with 10% fetal bovine serum (FBS). Embryos were staged according to the somite pair counting (6–12 sp for E8.5, and 18–25sp for E9.5). All Eng−/− embryos analyzed presented the same somite pair counting as the littermate controls (18–23 sp).
Flow Cytometry and Cell Sorting
E8.5–9.5 YSs were dissociated in trypsin 0.25% for 3 minutes at 37°C, and analyzed by flow cytometry using monoclonal antibodies described in Table S1. YS cells were sorted based on Flk-1, and/or Eng expression on a FACS Aria (Becton–Dickinson) after addition of propidium iodide (Pharmingen) to exclude dead cells. Data were analyzed using FlowJo software (Tree Star Inc.).
Hematopoietic progenitor assay
To determine the myelo-erythroid potential of each population, sorted cells were directly plated in hematopoietic medium for colony activity (M3434; StemCell Technologies). Plated cells were cultured in a humidified incubator (5% CO2) at 37°C. Ery-P and definitive hematopoietic colonies were scored 6 and 10 days after plating, respectively. Alternatively, sorted cells were co-cultured on OP9 stromal cells that had been pre-plated in 6-well plates containing α-MEM medium (Gibco) supplemented with 10% fetal bovine serum (Gemini), mSCF (100 ng/mL, Peprotech), mIL3 (1.17 ng/mL, Peprotech), granulocyte colony-stimulating factor (mG-CSF, 100ng/mL, Peprotech), vascular endothelial growth factor (mVEGF, 10 ng/mL, Peprotech), erythropoietin (hEpo, 2U/mL, StemCell Technologies), angiopoietin-1 (hAng-1, 100 ng/mL, R&D), 5 × 10−5 M 2-mercaptoethanol, and 1% penicillin/streptomycin. Plated cells were cultured in a humidified incubator at 37°C in an environment of 5% CO2.
Histological analysis
Dissected embryos were immediately fixed in 2% PFA for 30 minutes, incubated in 5%, and 15% sucrose solutions until the embryos decanted, and frozen in 7.5% Gelatin. Cryosections were incubated with primary antibodies, described in Table S1, overnight at 4°C. Reactions were detected following 1 hour incubation with appropriate secondary antibodies (Table S1). Staining was visualized by laser confocal scanning (Zeiss LM510).
Molecular analysis
Total RNA of E+F+ and E+F− sorted cells from E9.5 WT yolk sacs (pool of ~120 yolk sacs) were isolated using RNAqueous®-Micro kit (Ambion Inc.). For transcription analysis of E9.5 Eng−/−, Eng+/−, and Eng+/+ embryos, isolated yolk sacs were submitted to RNA extraction using TRIzol reagent (Invitrogen). qPCR was performed using TaqMan® probes (Applied Biosystems). For Western Blot analysis of WT mice, three independent E9.5 pooled YS (~180 embryos per pool) were sorted for E−F−, E+F+, and E+F− cells, which were then lysated by adding RIPA Buffer (ThermoScientific) and anti-proteolytics (Roche). For Western Blot analysis of Eng−/−, Eng+/−, and Eng+/+ embryos, isolated whole yolk sacs were directly lysated in RIPA Buffer, containing anti-proteolytics. Protein lysates (30μg for WT YS sorted cells and 15μg for Eng−/−, Eng+/−, and Eng+/+ YS) were loaded in each lane, resolved in 8% SDS-PAGE, and transferred to membranes (Immobilon-P; Millipore), which were incubated overnight at 4°C with primary antibodies (Tables S1). After incubation with HRP-conjugated secondary antibodies (Tables S1), membranes were incubated with SuperSignal West Pico Chemiluminescent Substrate or Pierce ECL Western Blot Substrate (ThermoScientific) and exposed to film. The Western Blots were quantified using ImageJ (http://imagej.nih.gov/ij/index.html).
Statistical analysis
Differences between Eng−/−, Eng+/−, and Eng+/+ E9.5 samples or E−F−, E+F+, and E+F− sorted cells were assessed by ANOVA.
RESULTS
Hematopoietic Precursor Activity Resides in Distinct Eng+ sub-populations during different stages of YS development
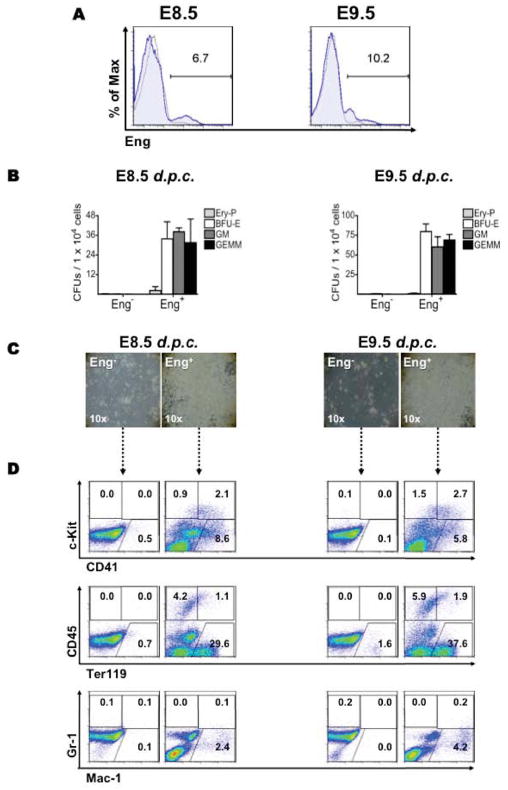
To determine whether endoglin continues to play an important role in hematopoiesis beyond its emergence at E7.5 [21], we began by analyzing the expression of this receptor in E8.5 and E9.5 yolk sacs. We observed similar levels of Eng expression at both developmental stages (between 7% and 10%; Figure 1A) and found that YS hematopoietic precursor activity was restricted to the Eng+ sub-population at E8.5 and 9.5 (Figure 1B). No hematopoietic colonies were detected when Eng− cells were directly plated into methylcellulose-based medium containing hematopoietic cytokines (Figure 1B), even when 10-fold more cells were plated (data not shown). Similar results were obtained when these two cell fractions were co-cultured on OP9 stromal cells in the presence of hematopoietic cytokines (Figure 1C). After five days in culture, typical hematopoietic cell clusters were detected only in the Eng+ fraction (Figure 1C). FACS analysis confirmed the hematopoietic nature of cell outgrowths, as evidenced by the expression of hematopoietic markers, in particular CD41, CD45, Ter119, and Mac-1 (Figure 1D) as well as their ability to produce significant numbers of hematopoietic colonies, which consisted mostly of macrophages (Figure S2). On the other hand, Eng− cells displayed very limited capacity to generate hematopoietic progeny under these culture conditions (Figure 1C–D and Figure S2).
Figure 1.
The endoglin-expressing cell fraction comprises all hematopoietic precursors in YS. (A) Flow cytometric analyses for endoglin in WT E8.5 (left) and E9.5 (right) yolk sacs. Histogram plots show isotype control staining profile (gray line) versus Eng specific antibody staining profile (blue line). Gates represent the fraction of cells that expresses Eng and which was sorted for in vitro characterization. (B–C) Hematopoietic activity of Eng+ and Eng− cell fractions purified from E8.5 and E9.5 yolk sacs (gates shown in A). When freshly sorted Eng+ and Eng− cells were plated directly in methylcellulose medium enriched with hematopoietic cytokines, at both stages, hematopoietic colonies were detected only in the Eng+ fraction. No hematopoietic colony formation was observed in Eng− cells. Error bars indicate SEM from at least 3 independent experiments. (C) Hematopoietic activity was also assessed by plating Eng+ and Eng− cells onto OP9 co-cultures in the presence of hematopoietic cocktail of growth factors. After 5 days in culture, large numbers of hematopoietic cells were detected only in cultures originating from Eng+ sorted cells (right panels), at both E8.5 and E9.5 stages of yolk sac development. No obvious growth was observed in Eng− co-cultures (left panels). Pictures are representative of at least 3 independent experiments. (D) The hematopoietic nature of cell outgrowths (showed in C) was confirmed by FACS analyses following a 5-day in vitro expansion. Representative plots are shown for c-Kit and CD41 (upper panel), CD45 and Ter119 (middle panel), and Gr-1 and Mac-1 (lower panel). Fluorescence intensity for c-Kit, CD45, or Gr-1 is indicated on the y axis whereas CD41, Ter119, or Mac-1 is indicated on the x axis. Plots are representative of at least 3 independent experiments from E8.5 (left columns) and E9.5 (right columns) YS-derived cell outgrowths.
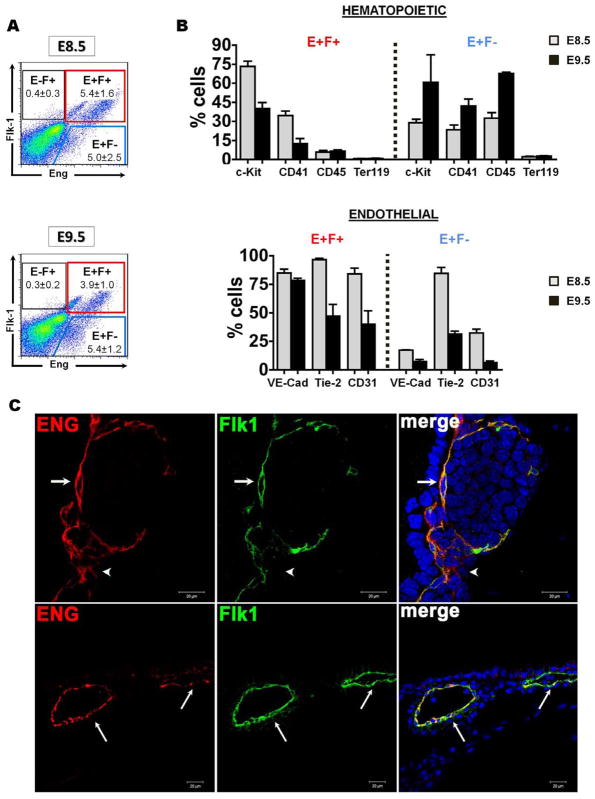
Since we have previously shown that Eng, in conjunction with Flk-1, identifies virtually all hematopoietic precursors at E7.5 [21], next we assessed whether this was also the case at later developmental stages (Figure 2A). FACS analyses demonstrated that about half of Eng+ cells (between 4% and 5%) express Flk-1 (E+F+) at both E8.5 and E9.5 yolk sacs (Figure 2A). Interestingly upon this sub-fractionation we found differential intensity levels of endoglin, such that high levels of endoglin (Engbright) were restricted to E+F+ cells, whereas dim expression of endoglin coincided with the Eng+Flk-1− (E+F−) sub-fraction (Figure S3A). This was further confirmed by immunofluorescence staining using confocal microscopy in staged embryos obtained from CD1 WT mice (Figure 2C). In both E8.5 and 9.5 yolk sacs, Eng+ cells were found abundantly in the endothelium, co-localized with Flk-1 (Figure 2C). Because endoglin expression was found to be dim in Eng+Flk-1− cells, this fraction was more difficult to detect by immunofluorescence staining (Figure 2C, arrowhead in upper panel). These and the Eng− sub-fractions were then further characterized for the expression of the hematopoietic markers c-Kit, CD41, CD45, and Ter119 [31–33] as well as endothelial markers, including VE-Cadherin, CD31, and Tie-2 [34]. At E8.5, most hematopoietic and endothelial markers were found preferentially expressed in E+F+ cells (Figure 2B and Figure S3B). These results are somewhat similar to those at E7.5 [21], however at E8.5 the expression levels for CD41 and Tie2 are basically equivalent between E+F+ and E+F− cell fractions (Figure 2B). Remarkably, at E9.5 we observed a change in this pattern as hematopoietic markers were found preferentially expressed in the E+F− fraction, while the E+F+ fraction was enriched for endothelial markers (Figure 2B). The highest level of the pan-hematopoietic marker CD45 was found at E9.5 in the E+F− cell fraction whereas E+F+ cells displayed similar low levels of this antigen at both time points (Figure 2B). Almost no Eng+ cells were found to express the erythroid marker Ter119 in both E8.5 and E9.5 (Figure 2B).
Figure 2.
Characterization of YS cell sub-populations based on Eng and Flk-1 expression. (A) Representative FACS profiles for endoglin (x axis) and Flk-1 (y axis) are shown for each developmental stage. (B) E+F+ (red gate) and E+F− (blue gate) cell fractions gated in (A) were then analyzed for the presence of hematopoietic (upper panels) and endothelial (lower panels) markers. Grey and back bars represent average percentage of cells expressing a given surface marker at E8.5 and E9.5, respectively. Error bars represent SEM from at least 2 independent experiments. (C) Confocal images of E8.5 (upper panel) and 9.5 (lower panel) yolk sacs show staining for Eng (red) and Flk-1 (green), and merge for Eng and Flk-1 (yellow) (40x objective). DAPI is shown in blue. Endoglin together with Flk-1 is strongly expressed in all endothelial cells (arrows) present in the blood island. Some Eng+Flk-1− cells are also observed in the blood island (arrowhead in E8.5).
Accordingly, colony activity of E8.5 and E9.5 yolk sacs based on endoglin and Flk-1 expression (E−F−, E−F+, E+F+, and E+F−) demonstrated that at E8.5, the majority of the definitive colony-forming cells (CFCs) were present in the E+F+ cell fraction (Figure 3, left panel) whereas at E9.5, almost all hematopoietic activity was restricted to the E+F− cell fraction (Figure 3, right panel). These data suggest that Eng identifies distinct cell populations as development progresses and that in E9.5 yolk sacs, E+F+ and E+F− cell sub-fractions represent the endothelial and hematopoietic populations, respectively.
Figure 3.
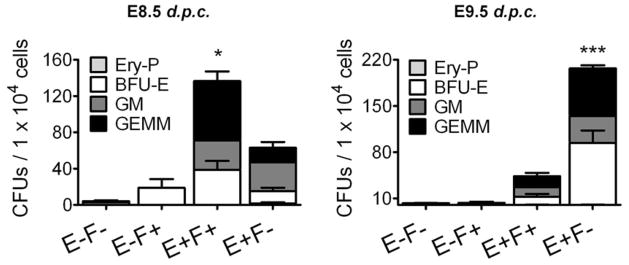
Hematopoietic precursor activity resides in distinct Eng+ sub-populations at different stages of development. Frequency of hematopoietic progenitors in E−F−, E−F+, E+F+, and E+F− cells sorted from E8.5 and 9.5 yolk sacs (representative gates are shown in Figure 2A). Error bars indicate SEM from at least 4 independent experiments. *p<0.05 and **p<0.001 for comparisons between E+F+ and E+F− cells
Molecular analysis of Eng+Flk-1− and Eng+Flk-1+ cells
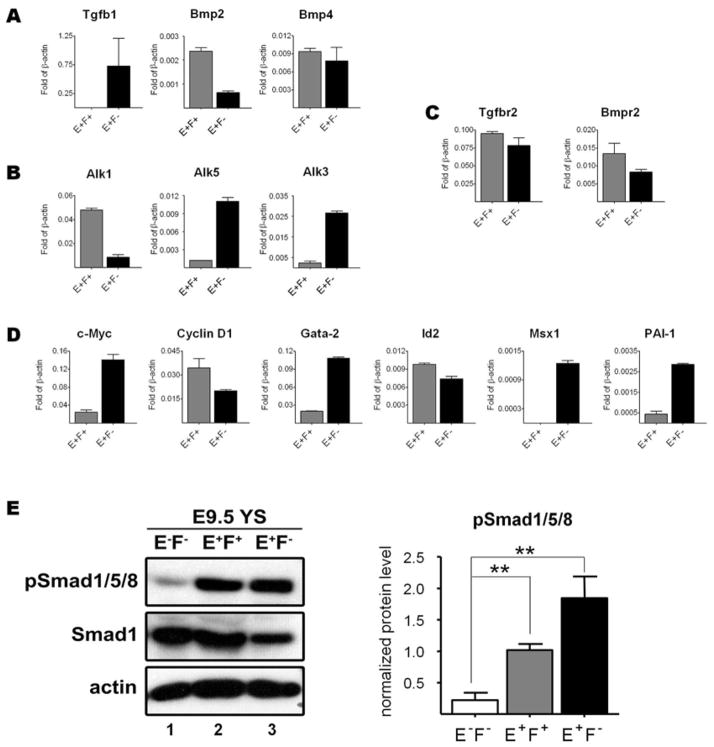
Based on the distinct characteristics of E9.5 YS E+F− and E+F+ cell sub-fractions, we next determined whether differences could be observed in terms of expression of components of the TGFβ superfamily. Real time PCR analyses demonstrated that E+F− cells, which encompass most of the hematopoietic progenitors at E9.5 YS, expressed distinctively high levels of Alk3, the type I receptor for BMP2/4 [11], as well as target genes of BMP signaling, such as Gata2 and Msx1 for BMP4 [36], and c-Myc for BMP2 [37] (Figure 4B, D). Since this was not observed in E+F+ cells, these data suggest that the BMP pathway is more active in the E+F− hematopoietic fraction. Regarding the TGFβ1 pathway, while Tgfbr2 was detected in both E+F− and E+F+ cell fractions, Alk1 was highly expressed in E+F+ cells, whereas Alk5 and its downstream target PAI-1 [8] were selectively expressed in E+F− cells (Figure 4B–D). These findings make sense as Alk-1 is well known to be primarily expressed in endothelial cells and at specific sites of epithelial-mesenchymal interactions, whereas Alk5 is ubiquitously expressed in most tissues [5].
Figure 4.
Eng+ cells express components of TGFβ/BMP signaling. (A–D) Gene expression analyses for components of the TGFβ superfamily in E+F+ and E+F− cells sorted from E9.5 wild-type YS cells, including ligands (A), type I receptors (B), type II receptors (C), and target genes (D), such as Cyclin D1, c-Myc, PAI-1, Id2, Gata2, and Msx1. Transcripts are normalized to β-actin. Error bars indicate SEM from triplicate experiments obtained with ~120 pooled yolk sacs per experiment. (E) Western Blot analyses for pSmad1/5/8 and Smad1 in protein lysates of E−F− (lane 1), E+F+ (lane 2), and E+F− (lane 3) cells sorted from E9.5 YS. Levels of Smad1 and pSmad1/5/8 were normalized to β-actin. The ratio of intensity of pSmad1/Smad1 signals is graphically represented on the right panel. Error bars represent SEM of 3 independent experiments obtained with ~180 pooled yolk sacs per experiment. **p<0.01
To confirm the activation of the TGFβ/BMP signaling pathways in these particular cell sub-populations, we evaluated the phosphorylation level of Smad1/5/8 in cell fractions sorted from E9.5 yolk sacs by Western Blot (Figure 4E) and immunofluorescence staining (Figure S3). This revealed that Smad1 is highly phosphorylated in Eng+ cells (both E+F+ and E+F− fractions) when compared with cells that do not express endoglin (E−F−) (Figure 4E–F). This was an expected outcome since Smad1/5 are downstream effectors of BMP/Alk3 and TGFβ1/Alk1 [4], which were differentially expressed in E+F− and E+F+ fractions, respectively.
Endoglin is required for BMP2/4 and TGFβ1 signaling
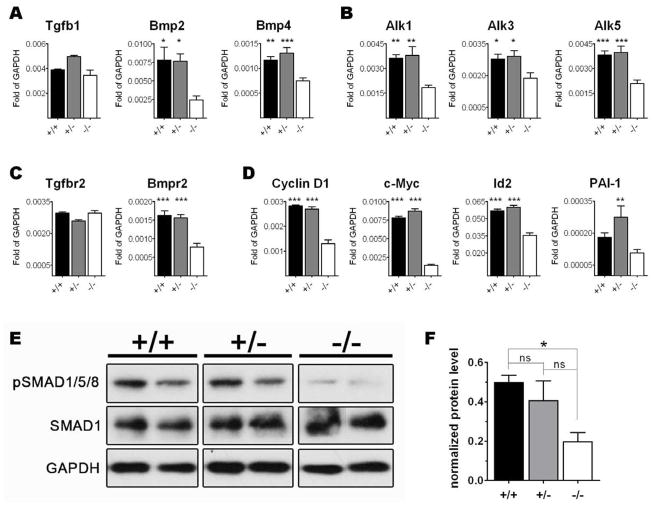
Next we assessed the expression levels of components of the TGFβ1/BMP signaling pathways in whole yolk sacs of Eng+/+, Eng+/−, and Eng−/− E9.5 embryos by qPCR. The ligands Bmp2 and Bmp4 were found down-regulated whereas Tgfβ1 levels were unaffected in the absence of endoglin (Figure 5A). Bmpr2 and Alk3 (Figure 5B–C), common receptors for BMP2/4, and their target genes Cyclin D1, c-Myc, Gata-2, and Msx1 (Figure 5D; for the last two see [21]) were significantly reduced in knockout yolk sacs when compared to WT and heterozygous embryos. Whereas no changes were observed in the expression levels of Tgfbr2 (Figure 5C), type I Tgfβ receptors Alk1 and Alk5 were significantly decreased. This was also the case for PAI-1, target gene for the Alk5 pathway. Id2, target gene for both BMP4 and TGFβ1, was also reduced in Eng-deficient mice.
Figure 5.
Lack of endoglin results in impaired activation of the TGFβ1/BMP signaling pathway. (A–D) E9.5 YSs obtained from Eng+/− litters were analyzed by qPCR for selected TGFβ superfamily members, including ligands (A), type I receptors (B), type II receptors (C), and target genes (D), such as c-Myc for and Cyclin D1 for BMP2, PAI-1 for TGFβ1, and Id2 for BMP4. Transcripts are normalized to GAPDH. Error bars indicate SEM from three independent samples constituted of 2 YS per sample for each genotype (total of 6 independent YS per genotype). (E) Levels of SMAD1 and its phosphorylated form (pSMAD1/5/8) in protein lysates of 2 representative YSs for each genotype analyzed by Western Blot. GAPDH was used as a loading control. (F) Graphic representation of the ratio pSMAD/SMAD1 intensity signal. Levels of SMAD1 and pSMAD1/5/8 were normalized to GAPDH. Error bars represent SEM of 4 independent YSs for each genotype. *p<0.05, **p<0.01, and ***p<0.001 refer to differences found between Eng−/− and both Eng+/− and Eng+/+ genotypes. §§p<0.01 refers to difference detected only between Eng−/− and the Eng+/− genotype.
To confirm that lack of Eng leads to reduced activity of TGFβ1/BMP2/4 signaling, we performed Western Blot analysis of pSMAD1/5/8 in isolated Eng+/+, Eng+/−, and Eng−/− YSs from E9.5 embryos (4 YSs each genotype). As observed in Figure 5E–F, the phosphorylation levels of SMAD1 were drastically decreased in knockout embryos when compared to the WT (about 2.5 fold) and heterozygotes (about 2 fold). These results demonstrate that Eng is required for proper activation of TGFβ1/BMP2/4 signaling, and consequently expression of important hematopoietic genes regulated by this pathway.
DISCUSSION
The importance of TGFβ signaling during embryonic development, in particular for vasculogenesis and mesodermal patterning, has been the focus of attention of many studies. For instance, BMP4 is considered the most important member of the TGFβ superfamily for mesoderm formation [9] and subsequent commitment towards hematopoietic fate in gastrulating embryos [36]. BMP4 [38] and TGFβ1 [13] have also been described as important regulators of YS vasculogenesis in E9.5 embryos. However the role of members of TGFβ signaling in mid-gestational hematopoiesis has been far less explored, and therefore poorly understood.
Here we demonstrated that endothelial cells (E+F+) and hematopoietic progenitors (E+F−) of the YS express BMP4 which binds to Alk3 and Bmpr2, phosphorylating the downstream effector Smad1/5, and ultimately activating the hematopoietic gene expression program within hematopoietic progenitor cells (E+F−) (Figures 3, 4, 5, and 6). These observations indicate that BMP4 is not only important for the specification of hematopoietic cells in early embryogenesis, but that it is also necessary for proper hematopoiesis within the YS of mid-gestational embryos. TGFβ1 may also act on E+F− cells but through Alk5/Smad2, as these cells express high levels of Alk5 and PAI-1, a target gene for Smad2 (Figures 3A–D and 5A–D,G). However we did not observe differences in Smad2/3 activation among E+F+, E+F−, and E−F− cell fractions (data not shown). This agrees with previous in vitro data as Eng-deficient differentiating ES cells showed no changes in the levels of pSmad2 whereas levels of pSmad1/5 were significantly reduced [39]. This has been confirmed here in vivo since E9.5 Eng−/− yolk sacs display a 2.5-fold reduction in the levels of pSmad1/5/8 (Figure 5E–F), which corroborate the premise that endoglin-mediated function in hematopoiesis occurs through BMP signaling, and not through TGF-β. The endothelial (E+F+) fraction at E9.5 (Figure 2C, right panel) is also characterized by high levels of pSMAD1/5/8, most likely resulting from TGFβ1/Alk1 but also possibly from BMP4/Alk3 signaling (Figure 5G).
Figure 6.
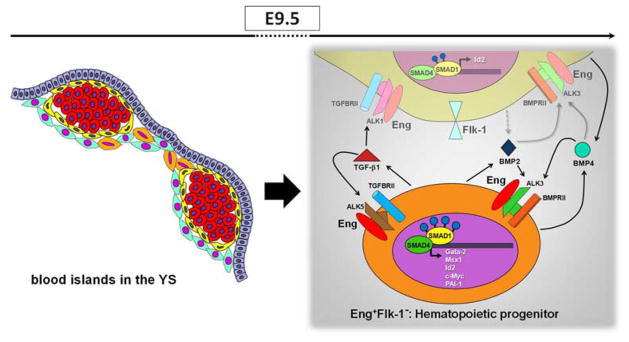
A model to illustrate how Eng and TGFβ1/BMP2/BMP4 may mediate induction of yolk sac hematopoiesis. Schematic representation (left panel) shows the localization of Eng+ cells in E9.5 blood islands. E+F+ and E+F− cells are represented in yellow and orange, respectively. Red round cells represent primitive erythrocytes. E+F+ cells represent endothelial cells whereas E+F− cells represent hematopoietic progenitors. The later secret high levels of TGFβ1 (right panel, represented in orange), which may signal in both hematopoietic (E+F−) and endothelial (E+F+) cells since both fractions contain the TGFβ receptor complex. In E+F− cells, TGFβ1 may bind to ALK5/TGFβRII/Eng, which results in high levels of PAI-1, a process mediated by SMAD2/3 (not shown). Nevertheless, the highest levels of pSMAD1/5 are observed within this fraction, and may result from BMP2/4 signaling, as E+F− cells display ALK3/BMPRII receptors, and high levels of corresponding target genes, including c-Myc, Gata2, Msx1 and Id2. More important, all target genes are drastically reduce in YS from Eng−/− embryos. E+F+ cells also exhibit significant phosphorylation of SMAD1/5, which may result from TGFβ1/ALK1 and BMP2/4 signaling. The important role played by endoglin in the YS, is possibly the modulation of the activation of TGFβ1 and BMP2/4 signaling pathways, and consequently the activation of important genes involved in the hematopoiesis. Grey dashed arrows represent possible secreted molecules, and grey solid arrows represent potential signaling pathways.
Interestingly, lack of Eng in the YS leads to decreases of 3.1- and 1.6-fold in BMP2 and BMP4 expression, respectively. This effect was correlated with the down-regulation of BMP2/4 target genes (Figure 5A–D), and decreased frequency of hematopoietic progenitors within E9.5 YSs [21]. These data lead us to think that Eng signaling might regulate the expression of BMP2/4 and other members of the TGFβ signaling pathway in YS cells.
We also observed that hematopoietic and endothelial cells from the YS are temporally distinguishable by the levels of Eng. At E8.5, E+F+ cell fraction comprises approximately two thirds of the hematopoietic progenitors within the YS, in particular the most uncommitted precursor GEMM, while the E+F− fraction contains the remaining third (Figure 3, left panel). As development progresses, hematopoietic activity transits to the E+F− population (Figure 3, right panel). At E9.5, dim expression of Eng identifies hematopoietic progenitors within the YS, whereas bright endoglin expression marks endothelial cells (Figure 2 and Figure S3). EngdimFlk-1− cells were found in clusters adjacent to endothelial cells of the blood islands (Figure 2C). The endothelial cells of the BI were EnghighFlk-1+ while the differentiated blood cells themselves were E−F−. Consistent with this, CD41 expression was enriched in the E+F− at E9.5 when compared to the E+F+ cell fraction (42.0 ± 14.6% vs. 12.6 ± 10.2%). These results are supported by a previous study showing that CD41+ cells present within the BI are enriched for hematopoietic progenitors [31]. Interestingly the localization of CD41+ cells within the YS found in that study resembles the position found here for Eng+ cells. It is important to note that at E9.5, E+F+ cells still contained hematopoietic activity but to a lesser extent. These results suggest that levels of Eng in the plasma membrane should be reduced as hematopoietic commitment occurs, while Flk-1 expression is completely abolished. Others have demonstrated that decreasing VEGF signaling through Flk-1 down-regulation is necessary for the development of definitive hematopoiesis [40, 41]. However, this is the first time that such regulation has been described for the TGFβ signaling pathway during embryogenesis.
Altogether, our data demonstrate that endoglin plays a crucial role in regulating the activation of members of the TGFβ superfamily in yolk sac of mid-gestational embryos. This regulation is also associated with a differential expression of TGFβ members within diverse cell types of the YS, which ultimately controls hematopoiesis.
Supplementary Material
Figure S1. Isotype control staining. Single cell suspensions of E8.5 and E9.5 YSs were stained with isotype control antibodies, IgG2aκ-APC (y axis) and IgG2aκ-PECy7 (x axis). FACS plot represents a typical YS staining using these isotype antibodies
Figure S2. Hematopoietic colony activity of Eng+ and Eng− cells following OP9 co-culture. Frequency of hematopoietic colonies obtained from Eng+- and Eng−-derived outgrowths at E8.5 (left panel) and E9.5 (right panel) YSs, following a 5-day co-culture on OP9 stromal cells. GM colonies were composed of granulocytes (G) and/or macrophage (M) cells. The vast majority contained macrophages only due possibly to the co-culture conditions.
Figure S3. Characterization of sub-fractions based on Eng and Flk-1 expression. (A) E+F+ (red) and E+F− (blue) cells were gated and ploted as a histogram. Overlays of Eng fluorescence intensity show that Eng expression in E+F+ cells is brighter than in E+F− cells. (B) Additional FACS characterization of Eng+ cells at different stages of development. WT E8.5–9.5 YS cells (left and right panels, respectively) were evaluated by FACS for endoglin expression as well as hematopoietic and endothelial surface markers. Representative plots for Flk-1, c-Kit, CD41, VE-Cadherin, CD31, or Tie-2 (y axis) and endoglin (x axis) are shown for each stage. Graphics represent cell percentage for each gated fraction. Error bars represent standard errors of at least 3 independent experiments. A subset of Eng+ cells expresses hematopoietic and endothelial cell markers in all assessed stages.
Figure S4. Immunofluorescence staining for pSmad1/5. Immunostaining of E8.5 and E9.5 yolk sacs show that pSMAD1/5 is highly expressed in a subset of endothelial cells in the blood island (square area shown in E9.5 depicts enlarged figure of the arrowhead region).
Acknowledgments
This project was supported by NIH grants R01 HL085840-01 and U01 HL100407.
Footnotes
AUTHORSHIP CONTRIBUTIONS
L.B. designed and conducted experiments, analyzed and interpreted the data, and wrote the paper. M.I. designed, conducted experiments, and analyzed the data. N.K. conducted experiments. D.J.G. and M.K. interpreted the data. R.C.R.P. supervised the overall project, designed experiments, analyzed the data and wrote the paper.
References
- 1.Cheifetz S, Bellon T, Cales C, et al. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 2.Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem. 1999;274:584–594. doi: 10.1074/jbc.274.2.584. [DOI] [PubMed] [Google Scholar]
- 3.Lebrin F, Goumans MJ, Jonker L, et al. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. The EMBO Journal. 2004;23:4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 5.Roelen BA, van Rooijen MA, Mummery CL. Expression of ALK-1, a type 1 serine/threonine kinase receptor, coincides with sites of vasculogenesis and angiogenesis in early mouse development. Dev Dyn. 1997;209:418–430. doi: 10.1002/(SICI)1097-0177(199708)209:4<418::AID-AJA9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Lux A, Attisano L, Marchuk DA. Assignment of transforming growth factor beta1 and beta3 and a third new ligand to the type I receptor ALK-1. J Biol Chem. 1999;274:9984–9992. doi: 10.1074/jbc.274.15.9984. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen LK, Brooke BS, Li DY, et al. Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGFbeta coreceptor. Dev Biol. 2003;261:235–250. doi: 10.1016/s0012-1606(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 8.Goumans MJ, Valdimarsdottir G, Itoh S, et al. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winnier G, Blessing M, Labosky PA, et al. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 11.Mishina Y, Suzuki A, Ueno N, et al. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 12.Beppu H, Kawabata M, Hamamoto T, et al. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221:249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- 13.Dickson MC, Martin JS, Cousins FM, et al. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 14.Larsson J, Goumans MJ, Sjostrand LJ, et al. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh SP, Seki T, Goss KA, et al. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshima M, Oshima H, Taketo MM. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179:297–302. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- 17.Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest. 1999;104:1343–1351. doi: 10.1172/JCI8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arthur HM, Ure J, Smith AJ, et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217:42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- 19.Cho SK, Bourdeau A, Letarte M, et al. Expression and function of CD105 during the onset of hematopoiesis from Flk1(+) precursors. Blood. 2001;98:3635–3642. doi: 10.1182/blood.v98.13.3635. [DOI] [PubMed] [Google Scholar]
- 20.Perlingeiro RC. Endoglin is required for hemangioblast and early hematopoietic development. Development. 2007;134:3041–3048. doi: 10.1242/dev.002907. [DOI] [PubMed] [Google Scholar]
- 21.Borges L, Iacovino M, Mayerhofer T, et al. A critical role for endoglin in the emergence of blood during embryonic development. Blood. 2012;119:5417–5428. doi: 10.1182/blood-2011-11-391896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray PDF. The development ‘in vitro’ of blood of the early chick embryo. Strangeways Res. Labor; Cambridge: 1932. pp. 497–521. [Google Scholar]
- 23.Moore MAS, Metcalf D. Ontogeny of the haematopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 24.Palis J, Robertson S, Kennedy M, et al. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 25.Kallianpur AR, Jordan JE, Brandt SJ. The SCL/TAL-1 gene is expressed in progenitors of both the hematopoietic and vascular systems during embryogenesis. Blood. 1994;83:1200–1208. [PubMed] [Google Scholar]
- 26.Young PE, Baumhueter S, Lasky LA. The sialomucin CD34 is expressed on hematopoietic cells and blood vessels during murine development. Blood. 1995;85:96–105. [PubMed] [Google Scholar]
- 27.Yamaguchi TP, Dumont DJ, Conlon RA, et al. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- 28.Rokhlin OW, Cohen MB, Kubagawa H, et al. Differential expression of endoglin on fetal and adult hematopoietic cells in human bone marrow. J Immunol. 1995;154:4456–4465. [PubMed] [Google Scholar]
- 29.Chen CZ, Li M, de Graaf D, et al. Identification of endoglin as a functional marker that defines long-term repopulating hematopoietic stem cells. Proc Natl Acad Sci U S A. 2002;99:15468–15473. doi: 10.1073/pnas.202614899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roques M, Durand C, Gautier R, et al. Endoglin expression level discriminates long-term hematopoietic from short-term clonogenic progenitor cells in the aorta. Haematologica. 2012 doi: 10.3324/haematol.2011.046235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikkola HK, Fujiwara Y, Schlaeger TM, et al. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003;101:508–516. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- 32.Mitjavila-Garcia MT, Cailleret M, Godin I, et al. Expression of CD41 on hematopoietic progenitors derived from embryonic hematopoietic cells. Development. 2002;129:2003–2013. doi: 10.1242/dev.129.8.2003. [DOI] [PubMed] [Google Scholar]
- 33.Ferkowicz MJ, Starr M, Xie X, et al. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130:4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita J, Itoh H, Hirashima M, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 36.Sadlon TJ, Lewis ID, D’Andrea RJ. BMP4: its role in development of the hematopoietic system and potential as a hematopoietic growth factor. Stem Cells. 2004;22:457–474. doi: 10.1634/stemcells.22-4-457. [DOI] [PubMed] [Google Scholar]
- 37.de Jesus Perez VA, Alastalo TP, Wu JC, et al. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-beta-catenin and Wnt-RhoA-Rac1 pathways. J Cell Biol. 2009;184:83–99. doi: 10.1083/jcb.200806049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Astorga J, Carlsson P. Hedgehog induction of murine vasculogenesis is mediated by Foxf1 and Bmp4. Development. 2007;134:3753–3761. doi: 10.1242/dev.004432. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Magli A, Catanese J, et al. Modulation of TGF-beta signaling by endoglin in murine hemangioblast development and primitive hematopoiesis. Blood. 2011 doi: 10.1182/blood-2010-12-325019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirai H, Samokhvalov IM, Fujimoto T, et al. Involvement of Runx1 in the down-regulation of fetal liver kinase-1 expression during transition of endothelial cells to hematopoietic cells. Blood. 2005;106:1948–1955. doi: 10.1182/blood-2004-12-4872. [DOI] [PubMed] [Google Scholar]
- 41.Eichmann A, Corbel C, Nataf V, et al. Ligand-dependent development of the endothelial and hemopoietic lineages from embryonic mesodermal cells expressing vascular endothelial growth factor receptor 2. Proc Natl Acad Sci U S A. 1997;94:5141–5146. doi: 10.1073/pnas.94.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Isotype control staining. Single cell suspensions of E8.5 and E9.5 YSs were stained with isotype control antibodies, IgG2aκ-APC (y axis) and IgG2aκ-PECy7 (x axis). FACS plot represents a typical YS staining using these isotype antibodies
Figure S2. Hematopoietic colony activity of Eng+ and Eng− cells following OP9 co-culture. Frequency of hematopoietic colonies obtained from Eng+- and Eng−-derived outgrowths at E8.5 (left panel) and E9.5 (right panel) YSs, following a 5-day co-culture on OP9 stromal cells. GM colonies were composed of granulocytes (G) and/or macrophage (M) cells. The vast majority contained macrophages only due possibly to the co-culture conditions.
Figure S3. Characterization of sub-fractions based on Eng and Flk-1 expression. (A) E+F+ (red) and E+F− (blue) cells were gated and ploted as a histogram. Overlays of Eng fluorescence intensity show that Eng expression in E+F+ cells is brighter than in E+F− cells. (B) Additional FACS characterization of Eng+ cells at different stages of development. WT E8.5–9.5 YS cells (left and right panels, respectively) were evaluated by FACS for endoglin expression as well as hematopoietic and endothelial surface markers. Representative plots for Flk-1, c-Kit, CD41, VE-Cadherin, CD31, or Tie-2 (y axis) and endoglin (x axis) are shown for each stage. Graphics represent cell percentage for each gated fraction. Error bars represent standard errors of at least 3 independent experiments. A subset of Eng+ cells expresses hematopoietic and endothelial cell markers in all assessed stages.
Figure S4. Immunofluorescence staining for pSmad1/5. Immunostaining of E8.5 and E9.5 yolk sacs show that pSMAD1/5 is highly expressed in a subset of endothelial cells in the blood island (square area shown in E9.5 depicts enlarged figure of the arrowhead region).