Abstract
Plasma membrane expression (PME) of the human GnRHR (hGnRHR) is regulated by a primate-specific Lys191 which destabilizes a Cys14-Cys200 bridge required by the cellular quality control system (QCS). A 4-amino, non-contiguous “motif” (Leu112, Gln208, Leu300, Asp302) is required for this effect. The hGnRHR sequence, with or without Lys191, decreases PME and inositol phosphate (IP) production when co-expressed with calnexin, a QCS chaperone. WT rat GnRHR, decreases PME and IP production, when co-expressed with calnexin, but to a lesser degree than hGnRH. When the human sequence contains the rat motif, IP production is closer to that of rat GnRHR. When Lys191 is deleted from hGnRHR and co-expressed with calnexin, IP production is similar to the rat sequence. When rat GnRHR containing Lys191 and the human motif is co-expressed with calnexin, IP production is similar to cells expressing the hGnRHR. The motif sequence appears to be a determinant of calnexin recognition.
Keywords: GPCR, ER protein retention, GnRH receptor, calnexin, protein trafficking
1. INTRODUCTION
Proteins are synthesized in the endoplasmic reticulum (ER) and passed through a cellular quality control system (QCS) (Rutkevich and Williams, 2011). Calnexin is among the chaperone proteins that bind to other newly synthesized proteins and aid in the folding of the process (Kleizen and Braakman, 2004; Schrag et al., 2003), allowing correctly folded molecules to reach their site of biological activity and retaining misfolded molecules in the ER (Hebert et al., 1996). Previous work in our laboratory demonstrated that there were differences in how the human and rat gonadotropin-releasing hormone receptors (GnRHRs) interacted with calnexin (Brothers et al., 2006). There is high conservation among calnexins, an observation which suggests an important role for these molecules in cellular function (Tjoelker et al., 1994).
Calnexin is localized near the ER and it interacts with nascent chains of N-glycosylated proteins (Daniels et al., 2003). Most of the G-protein coupled receptors (GPCRs) contain one or more N-glycosylation consensus sites (Asn-X-Ser/Thr) in the extracellular domain (Kornfeld and Kornfeld, 1985). The human GnRHR (hGnRHR) has two such extracellular N-glycosylation sites, Asn18 and Asn102. The rat and mouse GnRHR have an additional glycosylation site, Asn4 (Davidson et al., 1995).
Calnexin is regulated by phosphorylation which occurs in its cytosolic domain (Chevet et al., 2010; Wong et al., 1998). Several kinases participate in calnexin phosphorylation. Among these are CK2 (Ou et al., 1992; Wong et al., 1998), ERK1, MAPK and protein kinase C (PKC) (Cameron et al., 2009). Carboxyl terminal Ser504 and Ser583 were reported to reside in consensus phosphorylation sites for PKC calnexin (Roderick et al., 2000).
The GnRHR is a member of the GPCR super family (Millar et al., 2004; Sealfon et al., 1997) and is stabilized by the presence of two disulfide bridges located between residues Cys114-Cys196; similarly positioned bridges are common in other GPCRs (Janovick et al., 2006). The second disulfide bridge, Cys14-Cys200, lacks orthologs in other GPCRs. The hGnRHR sequence requires both of these bridges to be formed for passage through the QCS and arrival at the plasma membrane expression (PME). The mouse and rat GnRHRs only require the Cys114-Cys196 bridge for proper trafficking (Davidson et al., 1997; Janovick et al., 2006; Janovick et al., 2011; Millar et al., 2004).
The hGnRHR is 328 amino acids long, compared with rat/mouse sequences which contain only 327 amino acids. This difference is due to the presence of an additional amino acid, Lys191 in the human sequence but absent in rat/mouse (Castro-Fernandez et al., 2005; Chi et al., 1993; Conn et al., 2002; Janovick et al., 2006; Janovick et al., 2002; Kaiser et al., 1992; Tsutsumi et al., 1992; Ulloa-Aguirre et al., 2004). Lys191 appears to be involved in hGnRHR folding and inhibits the formation of the Cys14-Cys200 bridge in the hGnRHR. Deletion of Lys191 increases the PME (Brothers et al., 2006; Maya-Nunez et al., 2000).
A 4-amino acid motif of the hGnRHR participates in the function and in the destabilization of the Cys14-Cys200 bridge (Conn et al., 2007; Janovick et al., 2007). When this motif is replaced with rat GnRHR ortholog, the Cys14-Cys200 association is stabilized. Mutations in the hGnRHR sequence at 300 and 302 increased the PME, in contrast with rat sequence that decreased the PME (Janovick et al., 2006).
Another important species difference in GnRHRs is at residue 217 in humans (the orthologous position is 216 in rats and mice, due to the absence of Lys191). This residue is Ser217 (in human) and Ser216 (in rat) but Gly216 is present in the in mouse sequence. In hGnRHR, Ser217 stabilizes a bend in the peptide backbone that helps to align the amino acids necessary to form the Cys14-Cys200 bridge (Knollman et al., 2005). In rodents, position 216 is associated with differences in trafficking of the GnRHR. When rat GnRHR is mutated to Ser216Gly, and the mouse sequence contains the mutation Gly216Ser, and both mutants contain (the primate-specific) Lys191, IP production decreases, suggesting a relation between amino acids 191 and 216 (Janovick et al., 2006; Knollman et al., 2005). The human motif is required for this action of Ser217 to stabilize the relation of the Cys14 and Cys200 needed for bridge formation (Janovick et al., 2007) (Conn et al., 2007).
The present study was performed in order to identify amino acids of the human GnRHR and rat GnRHR which are important for the effect of calnexin on PME and IP production.
2. MATERIAL AND METHODS
The GnRH analog, D-tert-butyl-Ser6-des-Gly10-Pro9-ethylamide-GnRH (Buserelin, Hoechst-Roussel Pharmaceuticals, Somerville, NJ), myo-[2-3H(N)]-inositol and Na[125I] (Perkin Elmer, Boston, MA; NET-114A and NEZ-033L), competent cells (Promega, Madison, WI), PCR primers, DMEM, OPTI-MEM, lipofectamine, phosphate buffered saline, and pcDNA3.1 (Invitrogen, San Diego, CA), endofree maxi-prep kits (Qiagen, Valencia, CA), rat calnexin (a kind gift from Dr. Larry Tjoelker; (Tjoelker et al., 1994)) and human calnexin, cDNA (Open Biosystems, Huntsville, AL, MHS1011-60083). Other reagents were obtained from commercial sources and were of the highest degree of purity available. GnRHR and calnexin cDNA were prepared using site directed mutagenesis, as reported (Brothers et al., 2004). The identity of all cDNA mutants and the correctness of all PCR-derived coding sequences were verified by Dye Terminator Cycle Sequencing, according to manufacturer's instructions (Perkin Elmer, Foster City, CA).
2.1 Transient Transfection and Co-Transfection
COS-7 cells were cultured, plated and transfected as previously reported (Brothers et al., 2004). Cells were transfected with 25 ng (unless indicated) human or rat WT GnRHR, or mutant GnRHR and pcDNA3.1 without insert (“empty vector”) or WT calnexin cDNA (75 ng/well), and 1 μl lipofectamine in 0.125 ml OPTI-MEM (room temperature), according to manufacturer's instructions. 100 ng/well is a concentration of cDNA that does not interfere with the transfection efficiency (Brothers et al., 2004; Janovick et al., 2003). Five h after transfection, 0.125 ml DMEM with 20% fetal bovine serum and 20 μg/ml gentamicin was added to the wells.
2.2 Inositol Phosphate (IP) Assays
Twenty-seven h after transfection, cells were washed, then “pre-loaded” for 18 h with 4 μCi myo-[2-3H(N)]-inositol in 1 ml DMEM (prepared without inositol; (Brothers et al., 2004)). The cells were washed twice with 0.3 ml DMEM containing 5 mM LiCl (without inositol) and treated for 2 h in 0.25 ml of the indicated Buserelin concentration in the same medium (LiCl prevents inositol phosphate (IP) degradation). Total IPs were determined as previously described (Brothers et al., 2004; Huckle and Conn, 1987). EC50 and maximal IP production were calculated using Sigma Plot 8.02 (Jandel Scientific Software, Chicago, IL).
2.3 Saturation Binding Assays
Cells were cultured and plated in growth medium as described above, except that 105 cells in 0.5 ml growth medium were added to 24 well Costar cell culture plates (cell transfection and medium volumes were doubled accordingly). Twenty-three h after transfection, the medium was removed and replaced with 0.5 ml fresh growth medium. Twenty-seven h after transfection, cells were washed twice with 0.5 ml DMEM containing 0.1% BSA and 20 μg/ml gentamicin then 0.5 ml of DMEM was added. After 18 h, cells were washed twice with 0.5 ml DMEM/0.1% BSA/10 mM HEPES, and then 2 × 106 cpm/ml of [125I]-Buserelin (0.5 ml of the same medium) was added to the cells and allowed to incubate at room temperature for 90 minutes (Brothers et al., 2002). After 90 minutes, the media was removed and radioactivity was measured as previously described (Brothers et al., 2003). To determine non-specific binding, the same concentrations of radioligand were added to similarly transfected cells in the presence of 5 μM unlabeled GnRH.
2.4 Statistics
Each experiment was repeated a minimum of three times. Replicates of at least 4 data points for each treatment group within an experiment were analyzed with one-way ANOVA, followed by paired Student's t-test for individual comparisons (SigmaStat 3.0, Jandel Scientific Software, Chicago, IL), P < 0.05 was considered significant.
3. RESULTS
3.1 Comparisons between species: IP production of human and rat GnRHR and co-transfection with calnexin
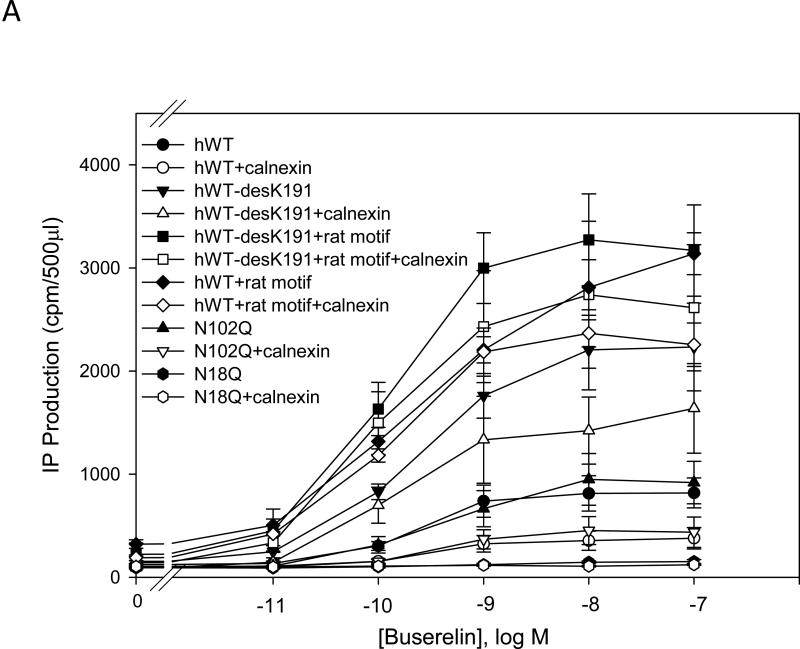
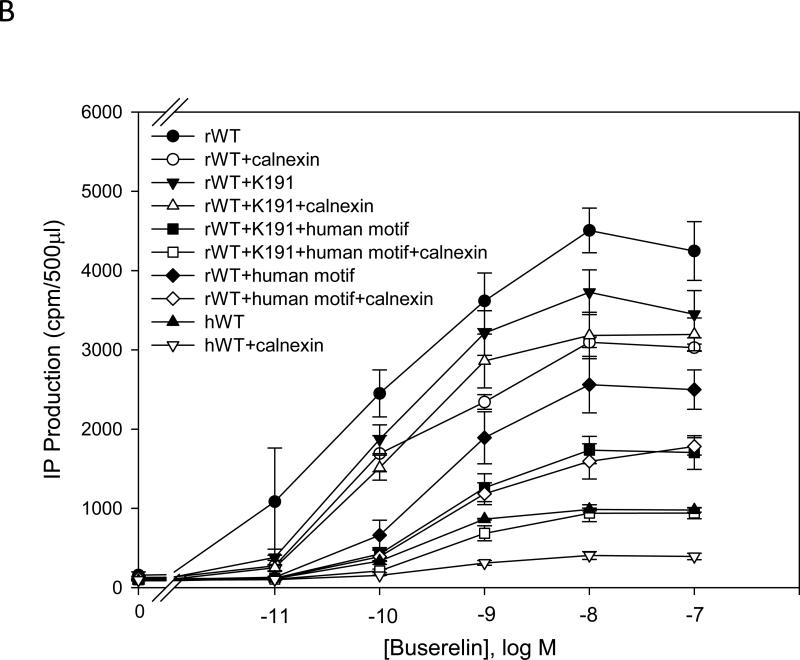
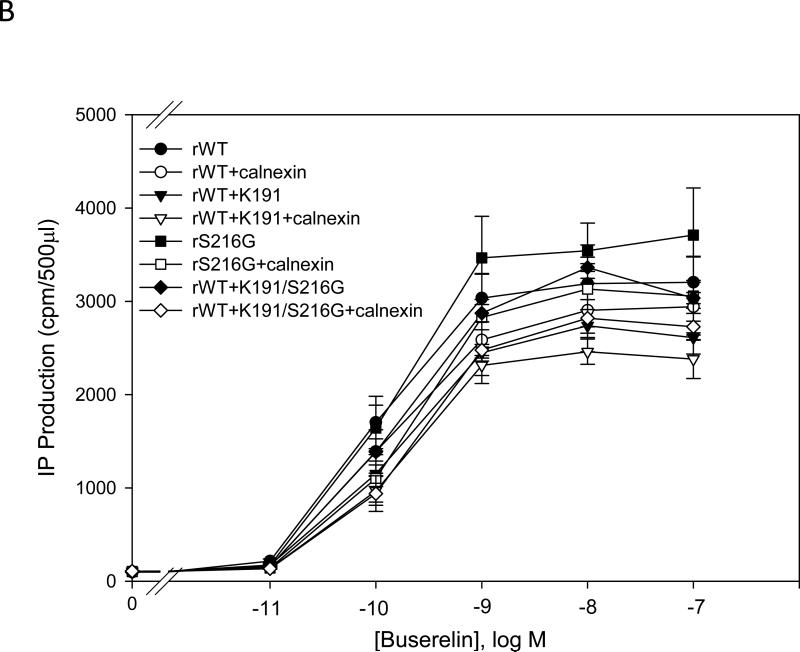
IP production was determined for the human and mutant GnRHR in the presence or absence of calnexin. There was a significant (p < 0.05) decrease in the maximal IP production comparing human WT and rat WT receptor without calnexin (Fig. 1A and Fig. 1B). The EC50 of the hGnRHR increased from 212 ± 30 pM to 318 ± 45 pM (p < 0.05), and the maximal IP production decrease from 823 ± 68 cpm to 359 ± 40 cpm (~60%) when human calnexin was co-transfected (Table 1). The EC50 of the WT rat GnRHR increased from 84 ± 18 pM to 92 ± 18 pM (p > 0.05), and the maximal response decreased from 4095 ± 252 cpm to 3066 ± 264 cpm (~30%) when rat calnexin was co-expressed (Table 2). When Lys191 was deleted from the hGnRHR the response increased approximately 2.5 fold, compared with WT receptor. This mutation decreased maximal IP production from 2234 to 1674 cpm (~25%) with human calnexin (Fig. 1A and Table 1). The maximal IP production of the hGnRHR increased even more when Lys191 was eliminated and replaced with the 4-amino acid rat motif (Phe112, Glu207, Val299, Glu301) compared with WT hGnRHR (Fig. 1A and Table 1). When hGnRHR contained the rat motif, the maximal IP production is 2944 compared with WT hGnRHR (823 cpm,Table 1). But this response decreased to 2391 cpm (~25%) and WT hGnRHR decreased to 360 cpm (~60%) when cells were transfected with human calnexin (Table 1). This is a similar level to that observed with the rat WT; in the presence of calnexin it decreased by ~30% (Table 2).
Fig. 1. Dose response curves of IP production by WT and mutant GnRH receptors in response to Buserelin.
IP production was assessed in response to the indicated doses of Buserelin. A) COS-7 cells were transiently transfected with 25 ng of human WT GnRH receptor or mutant receptor plus 75 ng of empty vector (closed symbols) or human calnexin (open symbols). B) COS-7 cells were transiently transfected with 25 ng of rat WT GnRH receptor or mutant receptor plus 75 ng of empty vector (closed symbols) or rat calnexin (open symbols) as described in Materials and Methods. Means ± S.E.M.s are shown for three independent experiments, each performed in replicates of four.
Table 1.
EC50 and maximal Buserelin stimulated inositol phosphate production of the WT and mutants hGnRHRs (mean ±SEM from 3 independents experiments).
| EC 50 (pM) | Maximal IP production (cpm/500μl) | |
|---|---|---|
| hWT | 212 ± 30 | 823 ± 68 |
| hWT+calnexin | 318 ± 45 | 359 ± 40 |
| hWT-desK191 | 146 ± 22 | 2234 ± 179 |
| hWT-desK191+calnexin | 210 ± 31 | 1674 ± 114 |
| hWT-desK191+rat motif | 110 ± 24 | 3091 ± 198 |
| hWT-desK191+rat motif+calnexin | 86 ± 39 | 2662 ± 284 |
| hWT+rat motif | 249 ± 15 | 2944 ± 123 |
| hWT+rat motif+calnexin | 110 ± 14 | 2391 ± 100 |
Table 2.
EC50 and maximal Buserelin stimulated inositol phosphate production of the WT and mutants rat GnRHRs (mean ±SEM from 3 independents experiments).
| EC 50 (pM) | Maximal IP production (cpm/500μl) | |
|---|---|---|
| rWT | 84 ± 18 | 4095 ± 252 |
| rWT+calnexin | 92 ± 18 | 3066 ± 264 |
| rWT+K191 | 97 ± 16 | 3531 ± 172 |
| rWT+K191+calnexin | 117 ± 17 | 3191 ± 172 |
| rWT+K191+human motif | 404 ± 11 | 1571 ± 58 |
| rWT+K191+human motif+calnexin | 500 ± 60 | 880 ± 17 |
| rWT+human motif | 351 ± 86 | 2274 ± 65 |
| rWT+human motif+calnexin | 511 ± 15 | 1617 ± 85 |
Lys191 is not normally present in the rat sequence but found in the human sequence. When the Lys191 was inserted into the rat GnRHR, the maximal IP response was 3531 cpm, compared with WT (4095 cpm, Table 2). When this mutant was co-transfected with rat calnexin, it showed only a ~10% decrease of IP production (Fig. 1B and Table 2). When the rat WT receptor contained both the human motif and Lys191, the maximal IP production decreased from 4088 cpm (WT rat GnRHR) to 1571 cpm (Fig. 1A and Fig. 1B). When this mutant was coexpressed with calnexin, the IP production decreased ~ 50%, to a similar level as that observed with WT hGnRHR (Fig. 1B) For the glycosylation site, Asn102Gln, there was no significant difference between human WT and the mutant (Fig. 1A); for Asn18 there was no response in IP production (Fig. 1A).
Table 1 shows the efficacy and potency of hGnRHR and its mutants when these were co-transfected with human calnexin. The WT hGnRHR and hWT-desK191 decreased the maximal IP production and the potency when co-transfected with calnexin. However, the hWT-desK191+rat motif and hWT + rat motif increased their potency and decreased the maximal IP production when they were co-transfected with calnexin.
Table 2 shows the efficacy and the potency of the rat GnRHR and mutants when these were co-transfected with calnexin. The efficacy, potency and maximal IP production decreased with calnexin for the mutants. The rat WT GnRHR did not show decreased potency when it was co-expressed with calnexin. The potency and the maximal response of the rat WTGnRHR decreased significantly when Lys191 and/or replaced by the human motif.
3.2 Role of the amino acid Lys191 in the hGnRHR when co-expressed with calnexin
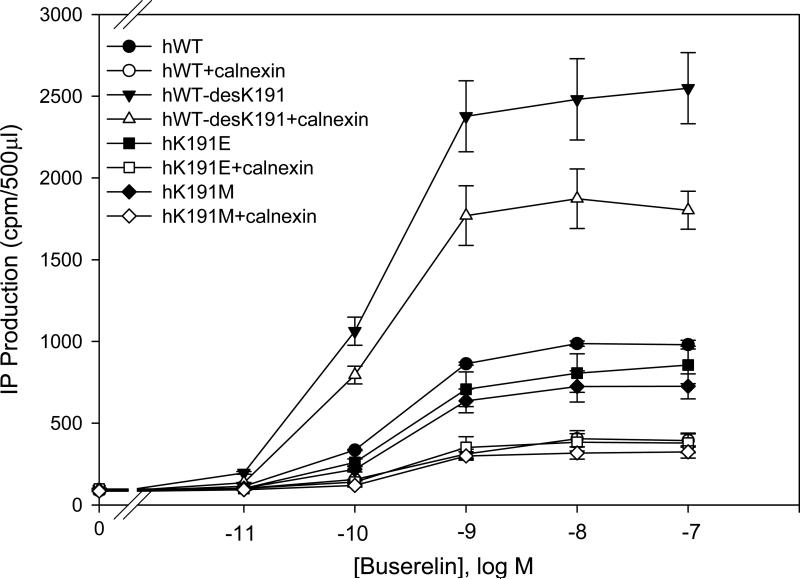
To evaluate the importance of the amino acid Lys191 in its effect with human calnexin, we replaced Lys191 with different amino acids (Lys191Glu and Lys191Met). There were no significant differences between WT hGnRHR containing mutations Lys191Glu and Lys191Met in IP production. Those mutations decreased IP production at similar levels when co-expressed with human calnexin (Fig. 2). This observation suggests that these substitutions act by a steric, not charge effect. When Lys191 was deleted in the hGnRHR there was an increase in the maximal response (approximately 2.5 fold) and this response decreased with human calnexin (Fig. 2). These results demonstrate that only when Lys191 was absent, IP production was affected in the hGnRHR.
Fig. 2. Dose response curves of IP production by human WT and mutant GnRH receptors in response to Buserelin.
IP production was assessed in response to the indicated doses of Buserelin. COS-7 cells were transiently transfected with 25 ng of WT or mutant receptors plus 75 ng of empty vector (closed symbols) or human calnexin (open symbols) as described in Materials and Methods. Means ± S.E.M.s are shown for three independent experiments, each performed in replicates of four.
3.3 The role of Ser217 (human), Ser216 (rat) or Gly216 (mouse) on GnRHRs interaction with calnexin
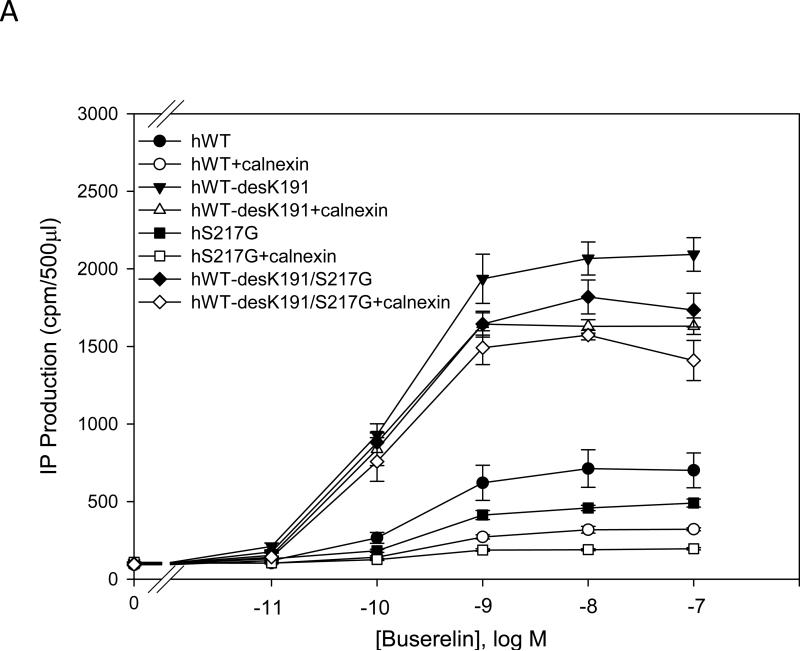
We analyzed mutants of the human GnRHR (Ser217Gly), the rat receptor (Ser216Gly) or the mouse receptor (Gly216Ser). These residues have been implicated in stabilization of the Cys14-Cys200 bridge and trafficking of the receptor to the plasma membrane. We observed that when we deleted Lys191 from hGnRHR with or without the additional substitution Ser217Gly, IP production increased approximately 4 fold, compared with WT hGnRHR (Fig. 3A). The mutant lacking Lys191 showed decreased IP production when co-transfected with calnexin, compared with when calnexin was absent. However, deletion of Lys191 and the substitution Ser217Gly together did not significantly decrease IP production (Fig. 3A). Nevertheless, when Ser217Gly was substituted in the hGnRHR, IP production was similar to the WT hGnRHR (Fig. 3A). The WT hGnRHR and the mutation Ser217Gly decreased IP production by approximately 50% when co-expressed with calnexin (Fig. 3A).
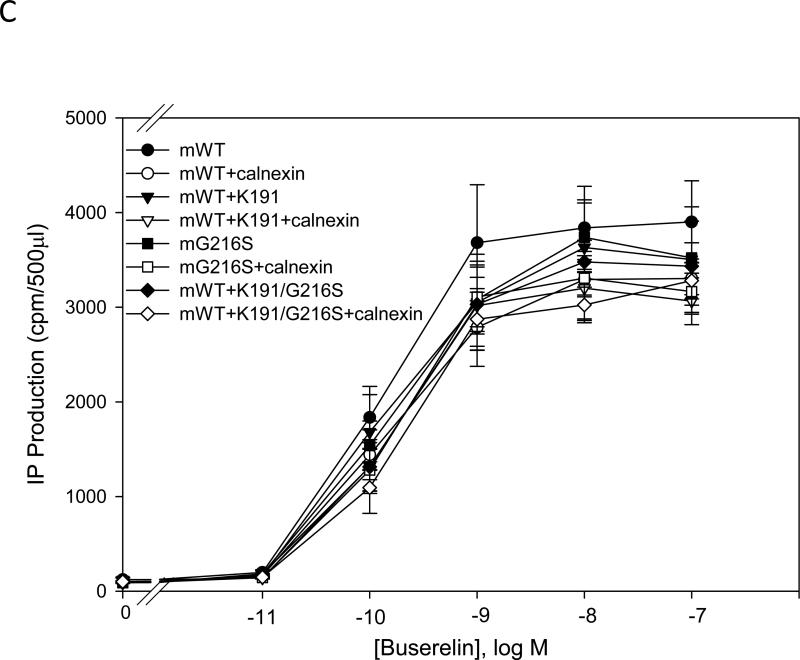
Fig. 3. Dose response curves of IP production by WT and mutant GnRH receptors in response to Buserelin.
IP production was assessed in response to the indicated doses of Buserelin. COS-7 cells were transiently transfected with 25 ng of WT or mutant plus 75 ng of empty vector (closed symbols) or human and rat calnexin (open symbols) as described in Materials and Methods. Panel A) hGnRHR, B) rat GnRHR and C) mouse GnRHR. Means ± S.E.M.s are shown for three independent experiments, each performed in replicates of four.
We evaluated the substitution Ser216Gly in the rat GnRHR. This mutant showed a slight increase in the IP production compared with WT rat GnRHR and did not decrease significantly with calnexin (Fig. 3B). When Lys191 was inserted into the rat GnRHR, IP production was slightly decreased compared with WT rat receptor (Fig. 3B). The rat GnRHR containing the mutation Ser216Gly or the mutant containing both Lys191 and Ser216Gly did not show decreased IP production compared with WT rat GnRHR, when co-expressed with calnexin (Fig. 3B). We evaluated the mutation Gly216Ser to observe whether or not this change would alter IP production. We observed that the mutation Gly216Ser showed a slight decrease in IP production compared with mouse WT GnRHR. For the mouse GnRHR, when Lys191 was inserted, or both Lys191 and Gly216Ser, were substituted in the sequence, IP production was similar to the level measured when this sequence was co-expressed with rat calnexin (Fig. 3C).
3.4 Effect of calnexin on plasma membrane expression of the human and rat GnRHRs
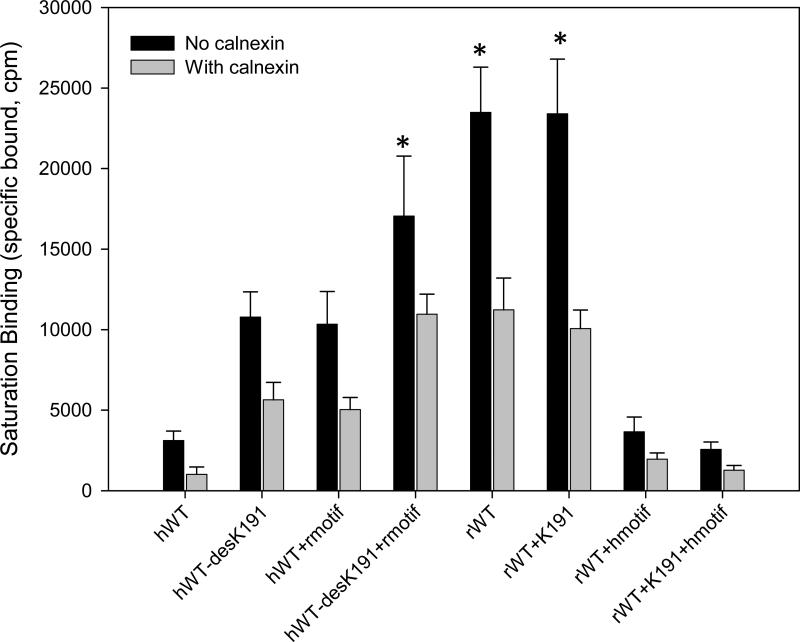
To confirm that calnexin decreased the receptors in the PME, we used radioligand binding to evaluate binding under conditions of saturating ligand (Fig. 4). The rat WT GnRHR increased specific binding of the receptors, in the plasma membrane (PM) approximately 5 fold more than hGnRHR; that expression decreased almost 60% when co-expressed with rat calnexin. The hGnRHR decreased in the PM expression almost 70% when co-transfected with human calnexin. When we deleted Lys191 from the hGnRH and substituted the rat motif, the receptors increased in the PM approximately 3-fold. These mutations decreased almost 40% when they were co-expressed with human calnexin. When the rat GnRHR contained Lys191, the expression of the receptor in the PM was similar to the rat WT receptor with or without rat calnexin. When we eliminated Lys191 and interchanged the human motif for the rat motif in the hGnRHR, the receptors at the PM increased almost 3-fold from the WT hGnRHR. The co-expression with human calnexin decreased approximately 40% of the receptors at the PM. The rat receptor with Lys191 and the human motif decreased the receptors at the PME at similar levels from the WT hGnRHR and this expression decreased almost 60% when co-expressed with rat calnexin.
Fig. 4. Binding analysis using 125I-Buserelin in COS-7 cells transfected with human and rat GnRHR and calnexin.
The cells were transfected with 25 ng of human and rat WT or mutant receptors plus 75 ng of empty vector (solid bar) or human and rat calnexin (gray bar). Means ± S.E.M.s are shown for three independent experiments, each performed in replicates of four. *p < 0.05 vs same groups with calnexin and all groups no calnexin.
3.5 Role of the phosphorylation sites of calnexin
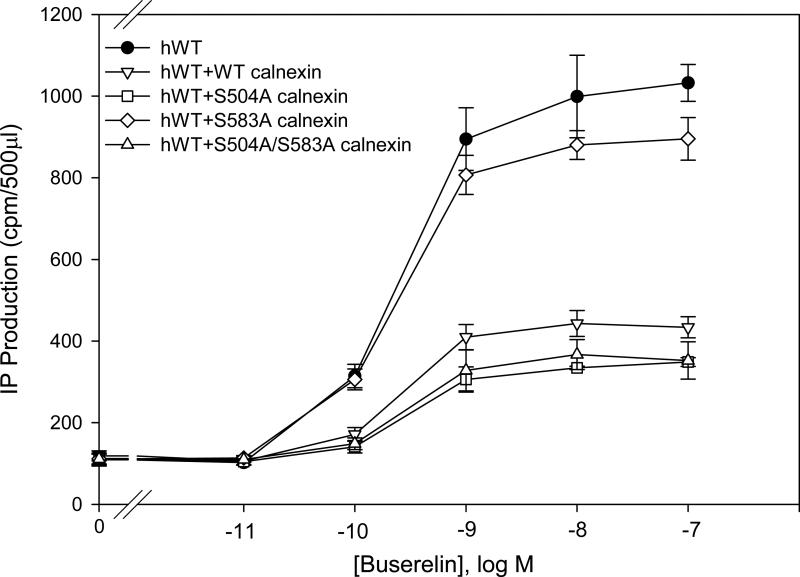
In order to demonstrate whether or not phosphorylation of calnexin was involved in its function, we analyzed two PKC phosphorylation sites, Ser504 and Ser583. The hGnRHR showed decreased IP production when co-expressed with WT calnexin (Fig. 5). When the hGnRHR was co-transfected with the mutant Ser504Ala (which blocks phosphorylation) or the double mutant Ser504Ala/Ser583Ala, the IP production decreased significantly. However, the mutation Ser583Ala did not result in a decrease of the IP production when it was co-expressed with hGnRHR. This demonstrated that Ser583 was important for the function of calnexin.
Fig. 5. Dose response curves of IP production by WT hGnRHR with phosphorylation mutant calnexin in response to Buserelin.
IP production was assessed in response to the indicated doses of Buserelin. COS-7 cells were transiently transfected with 25 ng of WT hGnRHR plus 75 ng of empty vector (closed symbols) human calnexin mutant (open symbols) as described in Materials and Methods. Means ± S.E.M.s are shown for three independent experiments, each performed in replicates of four.
4. DISCUSSION
Calnexin is a component of the QCS and has been shown to be involved in trafficking of the hGnRHR to the PM. Approximately half of newly synthesized WT hGnRHR is retained in the ER and never arrives at the plasma membrane (Conn and Janovick, 2009; Conn et al., 2006; Leanos-Miranda et al., 2002). In this study we examined particular amino acid differences in human, rat and mouse GnRHRs that participate in the stabilization and destabilization of the Cys14-Cys200 bridge. The results showed that the insertion of Lys191 and the replacement with the human motif in the rat WT GnRHR, decreases IP production comparably to WT hGnRHR levels when co-expressed with calnexin. Our data support the view that the human motif is involved in the participation of the GnRHR with the QCS.
We showed that deletion of Lys191 from the hGnRHR increased both PME and IP production. However, when this mutant was co-expressed with calnexin, we observed a decrease in the IP production of about 25%. This decrease is similar to that observed for the rat WT GnRHR (~30%). These results show that when the Cys14-Cys200 bridge is stabilized in the mutant hGnRHR, the participation of the calnexin decreased.
Calnexin retains the hGnRHR in the ER more effectively that the rat GnRHR (Brothers et al., 2006). The Lys191 residue, found in the hGnRHR, but not the rat counterpart, is known to play a role in trafficking of the hGnRHR to the plasma membrane. It does this by decreasing the probability of formation of the Cys14-Cys200 bridge during synthesis (Janovick et al., 2006; Maya-Nunez et al., 2000). This bridge is required for the hGnRHR to pass through the QCS. The rat GnRHR, which has no residue orthologous to the Lys191 in the human sequence, appears to be more efficiently trafficked to the PM than the hGnRHR (Brothers et al., 2006; Janovick et al., 2006; Knollman et al., 2005). The deletion of Lys191 in hGnRHR increases PME and IP production (Leanos-Miranda et al., 2002; Maya-Nunez et al., 2000). This suggests that Lys191 itself does not participate in retention of the hGnRHR in the ER when co-expressed with calnexin.
The 4-amino-acid motif supports the role of the Lys191 in destabilization of the formation of the disulfide bridge (Janovick et al., 2006). When the hGnRHR motif was replaced by the rat motif and co-expressed with calnexin, the IP production and the PME were closer to the IP response observed for rat GnRHR. When the rat WT GnRHR has Lys191 inserted and interchanged by the human motif, the potency decreased from 84.4 pM to 447.1 pM. Both potencies decrease when co-expressed with calnexin (Table 2). A previous study showed that when the Cys bridge was broken, in the Cys14Ala mutant this decreased expression of the human, but not rat sequence. The rat motif appeared to stabilize the association of positions 14 and 200 in hGnRHR and obviate the need for the covalent bridge (Janovick et al., 2006).
Our results are consistent with the observation that Lys191, together with the human motif, destabilizes the disulfide bridge in the rat GnRHR when these substitutions are made in that molecule. The destabilization of the Cys14-Cys200 bridge increases the ER retention of the GnRHR. In contrast when Lys191 is deleted from the hGnRHR, and human motif replaced by the rat counterpart, this results in enhanced stabilization of the disulfide bridge.
We evaluated the participation of the amino acids Ser217/216 (human and rat) and Gly216 (mouse) of the GnRHR in IP production when they were co-expressed with calnexin. Our results demonstrated that the mutation Ser217Gly in hGnRHR together with the deletion of Lys191 did not decrease IP production when co-expressed with calnexin. Calnexin did not decrease IP production when the rat receptor contained both the Ser216Gly and Lys191. When the mouse GnRHR was replaced by Gly216Ser, and Lys191 added, the IP production did not decrease with calnexin, suggesting that the residues do not participate in the recognition of calnexin. The rat and mouse sequences did not contain orthologous Lys191 and the Cys bridge was not required to pass QCS (Janovick et al., 2007; Janovick et al., 2006). The residue Gly216 in mouse GnRHR increased the trafficking of the GnRHR to the PM. (Janovick et al., 2006).
In the saturation binding, we showed that the WT rat GnRHR, containing Lys191, was expressed more highly at the plasma membrane than the WT hGnRHR but at levels that were similar to rat WT receptor expression. In contrast, the receptors decreased significantly in the PME when the rat GnRHR contained human motif alone and together with Lys191. These results were similar to IP production measured for the same mutants. These results demonstrate that the human motif and Lys191, expressed together with human motif, destabilizes the Cys14-Cys200 bridge. Previous work showed that the rat GnRHR had a higher level of expression at the PM than the hGnRHR (Brothers et al., 2006; Janovick et al., 2006). Another study demonstrated that when Lys191 was deleted from the hGnRHR, the expression was higher than observed for the WT hGnRHR (Janovick et al., 2007). The PME of the hGnRHR without Lys191, when it contained rat motif, increased, compared with WT hGnRHR. Calnexin decreased the expression of all mutant receptors at the PM approximately 50%. Previous work showed that the calnexin decreased the PME of the human and rat GnRHR by about 40%, but not the IP production in rat GnRHR (Brothers et al., 2006). Other GPCRs, like α1D adrenoreceptor (Uberti et al., 2005) and luteinizing hormone receptor (Pietila et al., 2005) are also expressed inefficiently at the PM.
Other laboratories have demonstrated that the opioid-receptor-bound calnexin at its disulfide bridge, affects the plasma membrane expression of the receptor (Ge et al., 2009). Using the angiotensin type I receptor (AT1), it was possible to show co-precipitation with calnexin and thus decreased the number of the receptors at the plasma membrane (Lanctot et al., 2006). In the present studies calnexin decreased the PME for WT rat GnRHR, hGnRHR both without Lys191 and containing the rat motif. The effect of cotransfecting calnexin on Buserelin-stimulated IP production by WT human GnRH (~60%) far exceeded that for WT rat GnRHR (~30%). The hGnRHR is a spare receptor system (Blum and Conn, 1982) which could explain why, for some mutants, the IP production did not decrease while the PME (binding) increased (Brothers et al., 2006).
Proteins are synthesized in the ER and the majority of them are glycosylated on Asn residues (Rutkevich and Williams, 2011). Two glycosylated residues have been identified in the hGnRHR, Asn18 and Asn102 (Davidson et al., 1995). Here we showed that the mutation Asn102Gln did not modify IP production when co-expressed with calnexin. Other work demonstrated that elimination of mannose residues had no influence on the effect of calnexin on the angiotensin type 1 receptor AT1 (Lanctot et al., 2006). When we substituted Asn18Gln we observed no response to the agonist. Some possible explanations for this observation are that the mutant receptor did not traffic to the membrane or did not bind the agonist (Wheatley and Hawtin, 1999).
The single mutation Ser583Ala in calnexin resulted in substantial loss of the ability to decrease IP production by hGnRHR in response to Buserelin. There was evidence to suggest that calnexin phosphorylation was required for its activity (Chevet et al., 2010). Calnexin was phosphorylated by ERK1 at Ser563; this phosphorylation regulated the ER quality control (Cameron et al., 2009). Calnexin is phosphorylated in residues Ser534 and Ser544 by the kinase CK2, this phosphorylation resulted in a re-distribution of calnexin from peripheral ER to juxtanuclear ER (Myhill et al., 2008). Another study showed indirect evidence that the PKC may be phosphorylated to calnexin through the regulation of PR calcium concentration (Roderick et al., 2000).
The specificity of the affect we report here appears to reside in the GnRHR since rat or human GnRHR, when co-expressed with either rat or human calnexin, shows similar effects (Supplementary Figure 1).
Several studies show that calnexin interacts with other GPCRs. Among these are the human pituitary vasopressin receptor (V3) which contains a hydrophobic sequence FN(X)2LL(X)3L in the proximal carboxyl terminus, important for ER-retention and for interaction with calnexin (Robert et al., 2005b). The human pituitary V3 receptor mutant associated with calnexin in the ER and led to degradation by the ubiquitin-proteosomal machinery (Robert et al., 2005a). The vasopressin V2 receptor termination mutant, R337X, which lacks part of the carboxyl tail, interacted with calnexin more effectively than the WT V2 vasopressin receptor (Morello et al., 2001). The follicle-stimulating hormone (FSH) and luteinizing hormone (LH) receptors are also associated with calnexin. This association facilitates the proper folding of these receptors (Rozell et al., 1998). These studies demonstrate the importance of the QCS in GPCRs signaling.
Here we showed that the calnexin decreased PME and IP production of the hGnRHR potentially by a direct interaction between these molecules. This is consistent with the observations that calnexin co-precipitates with the GnRHR and siRNA knockdown studies result in increased hGnRH signaling (Brothers et al., 2006).
5. CONCLUSIONS
The motif participates in recognition of ER retention of the GnRHR by calnexin. Residues Lys191, Ser217, Ser216 and Gly216 that are important for trafficking do not appear to participate in ER retention of the rat, mouse and human GnRHR by calnexin. The results showed the importance of the Cys14-Cys200 bridge in the effect of the calnexin in the IP production and PME of GnRHR.
Supplementary Material
Supplementary Fig. 1. Dose response curves of IP production by WT human or rat GnRHR with human or rat calnexin in response to Buserelin. IP production was assessed in response to the indicated doses of Buserelin. COS-7 cells were transiently transfected with 25 ng of WT human and rat GnRHR plus 75 ng of empty vector (closed symbols) calnexin (open symbols) as described in Materials and Methods. Means ± S.E.M.s are shown for replicates of four.
HIGHLIGHTS SEE REVISED MATERIAL SENT SEPARATELY.
We evaluated the difference between rat and human GnRHR in terms of IP production and plasma membrane expression (PME) when cells were co-transfected with calnexin.
IP production and PME showed that a four-amino-acid “motif” was involved in the effect of calnexin on human and rat GnRHR.
The Cys14-Cys200 bridge participated in the effect of calnexin on IP production and PME of human and rat GnRHR.
The primate-specific Lys191 was not involved in the effect of calnexin on IP production and PME of human and rat GnRHR.
HIGHLIGHTS.
A 4-residue “motif” is involved in the effect of calnexin on human and rat GnRHR.
The C14-C200 bridge participates in the effect of calnexin on IP production and PME of the GnRHR.
The primate-specific K191 is not involved in these effects of calnexin on the GnRHR.
ACKNOWLEDGEMENTS
We thank Jo Ann Binkerd for formatting the manuscript and Guadalupe Maya-Núñez for comments on the manuscript. This work was supported by National Institutes of Health Grants TW/HD-00668 and OD 011092-53
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Blum JJ, Conn PM. Gonadotropin-releasing hormone stimulation of luteinizing hormone release: A ligand-receptor-effector model. Proc Natl Acad Sci U S A. 1982;79:7307–11. doi: 10.1073/pnas.79.23.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers SP, et al. Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol Endocrinol. 2004;18:1787–97. doi: 10.1210/me.2004-0091. [DOI] [PubMed] [Google Scholar]
- Brothers SP, et al. Unexpected effects of epitope and chimeric tags on gonadotropin-releasing hormone receptors: implications for understanding the molecular etiology of hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2003;88:6107–12. doi: 10.1210/jc.2003-031047. [DOI] [PubMed] [Google Scholar]
- Brothers SP, et al. Calnexin regulated gonadotropin-releasing hormone receptor plasma membrane expression. J Mol Endocrinol. 2006;37:479–88. doi: 10.1677/jme.1.02142. [DOI] [PubMed] [Google Scholar]
- Brothers SP, et al. Conserved mammalian gonadotropin-releasing hormone receptor carboxyl terminal amino acids regulate ligand binding, effector coupling and internalization. Mol Cell Endocrinol. 2002;190:19–27. doi: 10.1016/s0303-7207(02)00040-0. [DOI] [PubMed] [Google Scholar]
- Cameron PH, et al. Calnexin phosphorylation attenuates the release of partially misfolded alpha1-antitrypsin to the secretory pathway. J Biol Chem. 2009;284:34570–9. doi: 10.1074/jbc.M109.053165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Fernandez C, et al. Beyond the signal sequence: protein routing in health and disease. Endocr Rev. 2005;26:479–503. doi: 10.1210/er.2004-0010. [DOI] [PubMed] [Google Scholar]
- Chevet E, et al. Calnexin phosphorylation: linking cytoplasmic signalling to endoplasmic reticulum lumenal functions. Semin Cell Dev Biol. 2010;21:486–90. doi: 10.1016/j.semcdb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Chi L, et al. Cloning and characterization of the human GnRH receptor. Mol Cell Endocrinol. 1993;91:R1–6. doi: 10.1016/0303-7207(93)90278-r. [DOI] [PubMed] [Google Scholar]
- Conn PM, Janovick JA. Drug development and the cellular quality control system. Trends Pharmacol Sci. 2009;30:228–33. doi: 10.1016/j.tips.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Conn PM, et al. ‘Effective inefficiency’: cellular control of protein trafficking as a mechanism of post-translational regulation. J Endocrinol. 2006;190:13–6. doi: 10.1677/joe.1.06771. [DOI] [PubMed] [Google Scholar]
- Conn PM, et al. Protein origami: therapeutic rescue of misfolded gene products. Mol Interv. 2002;2:308–16. doi: 10.1124/mi.2.5.308. [DOI] [PubMed] [Google Scholar]
- Conn PM, et al. G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev. 2007;59:225–50. doi: 10.1124/pr.59.3.2. [DOI] [PubMed] [Google Scholar]
- Daniels R, et al. N-linked glycans direct the cotranslational folding pathway of influenza hemagglutinin. Mol Cell. 2003;11:79–90. doi: 10.1016/s1097-2765(02)00821-3. [DOI] [PubMed] [Google Scholar]
- Davidson JS, et al. Irreversible activation of the gonadotropin-releasing hormone receptor by photoaffinity cross-linking: localization of attachment site to Cys residue in N-terminal segment. Biochemistry. 1997;36:12881–9. doi: 10.1021/bi971377t. [DOI] [PubMed] [Google Scholar]
- Davidson JS, et al. Identification of N-glycosylation sites in the gonadotropin-releasing hormone receptor: role in receptor expression but not ligand binding. Mol Cell Endocrinol. 1995;107:241–5. doi: 10.1016/0303-7207(94)03449-4. [DOI] [PubMed] [Google Scholar]
- Ge X, et al. mu-Opioid receptor cell surface expression is regulated by its direct interaction with Ribophorin I. Mol Pharmacol. 2009;75:1307–16. doi: 10.1124/mol.108.054064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert TE, et al. A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem. 1996;271:16384–92. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- Huckle WR, Conn PM. Use of lithium ion in measurement of stimulated pituitary inositol phospholipid turnover. Methods Enzymol. 1987;141:149–55. doi: 10.1016/0076-6879(87)41063-x. [DOI] [PubMed] [Google Scholar]
- Janovick JA, et al. Specializations of a G-protein-coupled receptor that appear to aid with detection of frequency-modulated signals from its ligand. FASEB J. 2007;21:384–92. doi: 10.1096/fj.06-6901com. [DOI] [PubMed] [Google Scholar]
- Janovick JA, et al. Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther. 2003;305:608–14. doi: 10.1124/jpet.102.048454. [DOI] [PubMed] [Google Scholar]
- Janovick JA, et al. Regulation of G protein-coupled receptor trafficking by inefficient plasma membrane expression: molecular basis of an evolved strategy. J Biol Chem. 2006;281:8417–25. doi: 10.1074/jbc.M510601200. [DOI] [PubMed] [Google Scholar]
- Janovick JA, et al. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab. 2002;87:3255–62. doi: 10.1210/jcem.87.7.8582. [DOI] [PubMed] [Google Scholar]
- Janovick JA, et al. Salt bridges overlapping the gonadotropin-releasing hormone receptor agonist binding site reveal a coincidence detector for G protein-coupled receptor activation. J Pharmacol Exp Ther. 2011;338:430–42. doi: 10.1124/jpet.111.180869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser UB, et al. Isolation and characterization of cDNAs encoding the rat pituitary gonadotropin-releasing hormone receptor. Biochem Biophys Res Commun. 1992;189:1645–52. doi: 10.1016/0006-291x(92)90266-n. [DOI] [PubMed] [Google Scholar]
- Kleizen B, Braakman I. Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol. 2004;16:343–9. doi: 10.1016/j.ceb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Knollman PE, et al. Parallel regulation of membrane trafficking and dominant-negative effects by misrouted gonadotropin-releasing hormone receptor mutants. J Biol Chem. 2005;280:24506–14. doi: 10.1074/jbc.M501978200. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–64. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lanctot PM, et al. Role of N-glycan-dependent quality control in the cell-surface expression of the AT1 receptor. Biochem Biophys Res Commun. 2006;340:395–402. doi: 10.1016/j.bbrc.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Leanos-Miranda A, et al. Receptor-misrouting: an unexpectedly prevalent and rescuable etiology in gonadotropin-releasing hormone receptor-mediated hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:4825–8. doi: 10.1210/jc.2002-020961. [DOI] [PubMed] [Google Scholar]
- Maya-Nunez G, et al. Combined modification of intracellular and extracellular loci on human gonadotropin-releasing hormone receptor provides a mechanism for enhanced expression. Endocrine. 2000;13:401–7. doi: 10.1385/ENDO:13:3:401. [DOI] [PubMed] [Google Scholar]
- Millar RP, et al. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235–75. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- Morello JP, et al. Association of calnexin with wild type and mutant AVPR2 that causes nephrogenic diabetes insipidus. Biochemistry. 2001;40:6766–75. doi: 10.1021/bi002699r. [DOI] [PubMed] [Google Scholar]
- Myhill N, et al. The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell. 2008;19:2777–88. doi: 10.1091/mbc.E07-10-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou WJ, et al. Casein kinase II phosphorylation of signal sequence receptor alpha and the associated membrane chaperone calnexin. J Biol Chem. 1992;267:23789–96. [PubMed] [Google Scholar]
- Pietila EM, et al. Inefficient maturation of the rat luteinizing hormone receptor. A putative way to regulate receptor numbers at the cell surface. J Biol Chem. 2005;280:26622–9. doi: 10.1074/jbc.M413815200. [DOI] [PubMed] [Google Scholar]
- Robert J, et al. Mechanisms of cell-surface rerouting of an endoplasmic reticulum-retained mutant of the vasopressin V1b/V3 receptor by a pharmacological chaperone. J Biol Chem. 2005a;280:42198–206. doi: 10.1074/jbc.M510180200. [DOI] [PubMed] [Google Scholar]
- Robert J, et al. A novel C-terminal motif is necessary for the export of the vasopressin V1b/V3 receptor to the plasma membrane. J Biol Chem. 2005b;280:2300–8. doi: 10.1074/jbc.M410655200. [DOI] [PubMed] [Google Scholar]
- Roderick HL, et al. Cytosolic phosphorylation of calnexin controls intracellular Ca(2+) oscillations via an interaction with SERCA2b. J Cell Biol. 2000;149:1235–48. doi: 10.1083/jcb.149.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozell TG, et al. Association of gonadotropin receptor precursors with the protein folding chaperone calnexin. Endocrinology. 1998;139:1588–93. doi: 10.1210/endo.139.4.5881. [DOI] [PubMed] [Google Scholar]
- Rutkevich LA, Williams DB. Participation of lectin chaperones and thiol oxidoreductases in protein folding within the endoplasmic reticulum. Curr Opin Cell Biol. 2011;23:157–66. doi: 10.1016/j.ceb.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Schrag JD, et al. Lectin control of protein folding and sorting in the secretory pathway. Trends Biochem Sci. 2003;28:49–57. doi: 10.1016/s0968-0004(02)00004-x. [DOI] [PubMed] [Google Scholar]
- Sealfon SC, et al. Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr Rev. 1997;18:180–205. doi: 10.1210/edrv.18.2.0295. [DOI] [PubMed] [Google Scholar]
- Tjoelker LW, et al. Human, mouse, and rat calnexin cDNA cloning: identification of potential calcium binding motifs and gene localization to human chromosome 5. Biochemistry. 1994;33:3229–36. doi: 10.1021/bi00177a013. [DOI] [PubMed] [Google Scholar]
- Tsutsumi M, et al. Cloning and functional expression of a mouse gonadotropin-releasing hormone receptor. Mol Endocrinol. 1992;6:1163–9. doi: 10.1210/mend.6.7.1324422. [DOI] [PubMed] [Google Scholar]
- Uberti MA, et al. Heterodimerization with beta2-adrenergic receptors promotes surface expression and functional activity of alpha1D-adrenergic receptors. J Pharmacol Exp Ther. 2005;313:16–23. doi: 10.1124/jpet.104.079541. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, et al. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic. 2004;5:821–37. doi: 10.1111/j.1600-0854.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- Wheatley M, Hawtin SR. Glycosylation of G-protein-coupled receptors for hormones central to normal reproductive functioning: its occurrence and role. Hum Reprod Update. 1999;5:356–64. doi: 10.1093/humupd/5.4.356. [DOI] [PubMed] [Google Scholar]
- Wong HN, et al. Conserved in vivo phosphorylation of calnexin at casein kinase II sites as well as a protein kinase C/proline-directed kinase site. J Biol Chem. 1998;273:17227–35. doi: 10.1074/jbc.273.27.17227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Dose response curves of IP production by WT human or rat GnRHR with human or rat calnexin in response to Buserelin. IP production was assessed in response to the indicated doses of Buserelin. COS-7 cells were transiently transfected with 25 ng of WT human and rat GnRHR plus 75 ng of empty vector (closed symbols) calnexin (open symbols) as described in Materials and Methods. Means ± S.E.M.s are shown for replicates of four.