Abstract
Neurocognitive impairment (NCI) remains prevalent in HIV-infection. Randomized trials have shown that physical exercise improves NCI in non HIV-infected adults, but data on HIV-infected populations is limited. Community-dwelling HIV-infected participants (n=335) completed a comprehensive neurocognitive battery that was utilized to define both global and domain-specific NCI. Participants were divided into “Exercise” (n=83) and “No Exercise” (n=252) groups based on whether they self-reported engaging in any activity that increased heart rate in the last 72 hours or not. We also measured and evaluated a series of potential confounding factors, including demographics, HIV-disease characteristics, substance use and psychiatric comorbidities, and physical functioning. Lower rates of global NCI were observed among the Exercise group (15.7%) as compared to those in the No Exercise group (31.0%; p<.01). A multivariable logistic regression controlling for potential confounds (i.e., education, AIDS status, current CD4+ lymphocyte count, self-reported physical function, current depression) showed that being in the Exercise group remained significantly associated with lower global NCI (OR=2.63, p<.05). Similar models of domain-specific NCI showed that Exercise was associated with reduced impairment in working memory (p<.05) and speed of information processing (p<.05). The present findings suggest that HIV-infected adults who exercise are approximately half as likely to show NCI as compared to those who do not. Future longitudinal studies might be best suited to address causality and intervention trials in HIV-infected individuals will determine whether exercise can prevent or ameliorate NCI in this population.
Keywords: HIV/AIDS, Mild Neurocognitive Impairment, Physical Exercise, Cognition, Lifestyle, Neuropsychology
Background
Neurocognitive impairment (NCI) continues to be highly prevalent among persons living with Human Immunodeficiency Virus (HIV) even with significant advances in antiretroviral treatment (ART). A recent study of 1,555 community dwelling HIV-infected (HIV+) adults showed that nearly 50% had some form of HIV-Associated Neurocognitive Disorder (HAND; Heaton et al. 2010). HAND ranges from asymptomatic neurocognitive impairment to more pronounced deficits that interfere with daily functioning, such as problems with financial management, driving, and medication adherence (Antinori et al. 2007; Heaton et al. 2004; Marcotte et al. 1999; Thames et al. 2012). Given the prevalence and impact of NCI on HIV+ individuals, it is important to find strategies to reduce HAND. Modifiable lifestyle factors (e.g., physical activity, education, social engagement, cognitive stimulation, diet) may provide a fruitful target for intervention. A growing body of literature indicates that physical activity may be of particular relevance in reducing HAND (Fillipas et al. 2006; Honn et al. 1999; O'Brien et al. 2010).
There is ample evidence showing that exercise may improve neurocognitive function in those not infected with HIV (Colcombe and Kramer 2003; Heyn et al. 2004). Heyn and colleagues examined 30 randomized trials involving any type of exercise with older non-HIV infected (HIV-) individuals with cognitive deficits (2004). Participants in these trials had baseline mini-mental state exam scores lower than 26 or a diagnosis of cognitive impairment or dementia. They found that increased exercise consistently improved not only physical fitness and function, but also cognitive performance, typically with moderate to large effect sizes. Additionally, Colcombe and Kramer examined 18 longitudinal supervised exercise interventions in older adults generally characterized as normal and found that exercise interventions increased cognitive performance on tasks of executive function, processing speed, and visuospatial abilities (2003).
The mechanisms underlying the association between exercise and neurocognitive function are likely to be multifactorial. However, the positive effect of exercise on cardiovascular fitness and reduced cerebrovascular disease is well established and are thought to be mediators in the relationship between exercise and cognition. Cerebrovascular disease and risk factors are prominent in HIV+ adults and have been associated with HAND (Fabbiani et al. 2012; Foley et al. 2010; McCutchan et al. 2012; Wright et al. 2010). Moreover, ART has negative side effects including cerebrovascular changes (Aboud et al. 2007; Grinspoon and Carr 2005). Among those with HIV, exercise has been found to be a safe and effective method to reduce the impact of cerebrovascular risk factors, increase general fitness, elevate well-being, and improve body image (Hand et al. 2009; O'Brien et al. 2010). Specifically, O'Brien and colleagues examined 14 randomized controlled exercise trials in HIV+ individuals, including at least 20 minutes of aerobic exercise three times per week (2010). They found that exercise caused improvements in body composition, lowered BMI, reduced waist circumference, and improved lipid levels. Neurocognitive functioning was not examined.
Only a few studies have directly examined the relationship between exercise and NCI among HIV+ adults (e.g, Fillipas et al. 2006; Honn et al. 1999). Honn and colleagues examined the association between self-reported exercise (at the time of the study and prior to HIV diagnosis) and performance on a comprehensive neurocognitive battery in a group of 139 asymptomatic HIV+ men (1999). They found limited significant associations between exercise and neurocognitive function. Namely, exercise prior to HIV infection was associated with better performance on a speeded fine motor test (i.e., Grooved Pegboard), and current exercise was associated with performance on a speeded test of complex attention/working memory (i.e., Paced Auditory Serial Addition Test). Fillipas and colleagues investigated the effects of an exercise intervention (2 hours of supervised aerobic and resistance training per week) in 20 HIV+ men (2006). They found a significant improvement in self-reported cognitive problems on the Medical Outcomes Study HIV Health Survey (MOS-HIV), which served as a secondary outcome measure; however, objective neurocognitive functioning was not assessed. Overall, the scant data indicates that exercise may improve neurocognition in HIV+ populations, but the relationship between the two requires further elucidation.
The main purpose of the present study was to examine the association between self-reported participation in physical exercise and NCI in a well-characterized large cohort of HIV+ adults using a comprehensive neurocognitive battery that allowed examination of global and domain-specific neurocognitive functioning. We hypothesized that those who engaged in recent physical exercise would exhibit lower rates of NCI as compared to those who did not recently participate in these activities.
Method
Participants
The present study included 335 community-dwelling HIV+ adults who were recruited by the HIV Neurobehavioral Research Center (HNRC). Details on the HNRC study are described elsewhere (e.g, Heaton et al. 1995). Briefly, the focus of the HNRC is to examine the prevalence, features, course, etiology, and pathogenesis of HIV in the central nervous system. At the initial assessment, persons with a history of non-HIV related neurologic disorders or any other conditions known to alter neurocognitive performance (e.g., seizure disorder, head trauma, learning disabilities, psychotic disorders, current substance abuse) were excluded. The current study included participants within the HNRC cohort with a positive HIV status and with valid data for self-reported exercise and global neurocognition. Participants were evaluated between 2007 and 2011. The participants ranged from 20 to 79 years old (age: M = 47.7, SD = 10.5), were mostly male (74.3%), and had, on average, one year of college education (education: M = 13.0 SD = 3.2). Half of the participants (51.3%) described their ethnicity as non-Hispanic white. The majority were on ART (82.2%), were diagnosed with AIDS (64.7%). Duration of HIV infection from self-reported date of first HIV positive test ranged from under a month to slightly over 28 years.
Exercise Assessment
Participants were queried about their exercise practices using a staff-administered questionnaire. The measure was initially added to estimate the metabolic rate of participants over the last three days. For the purpose of the present study, we used a question that asked participants to estimate how much time (minutes) they spent exercising in the last 72 hours. Exercise was specifically described as any activity in which the heart beats rapidly and specific examples were provided (i.e., running, jogging, lifting heavy weights, aerobics, hockey, football, soccer, squash, basketball, cross country, judo, roller blading/skating, vigorous swimming, vigorous long distance bicycling).
Neurocognitive Assessment
Participants completed a standardized neurocognitive test battery that covers seven cognitive domains commonly affected by HIV including verbal fluency, working memory, speed of information processing, learning, recall, executive function, and motor function (see Table 2 for a list of specific tests by domain; Blackstone et al. 2012). The test scores were adjusted to control for age, gender, education, race/ethnicity and repeated testing. Each domain score was converted to a standard T-score then assigned a domain deficit score based on a 5-point scale (0 = no impairment to 5 = severe impairment; Blackstone et al. 2012). Individuals with a domain deficit score higher than 0.5 were considered impaired in that domain (Heaton et al. 1995). The average of the domain deficit scores was used to derive a global deficit score. Participants with a global deficit score greater than or equal to 0.5 were considered to have global NCI, as it has been found to provide the optimal sensitivity and specificity (Carey et al. 2004).
Table 2. Neurocognitive Testing Battery.
| Cognitive Domain | Test | Reference |
|---|---|---|
| Verbal Fluency | ||
| Letter fluency | Artiola et al. 1999 | |
| Noun Fluency (animals) | Gladsjo et al. 1999 | |
| Verb Fluency (actions) | Heaton et al. 2003 | |
| Working Memory | ||
| Paced Auditory Serial Addition Test | Diehr et al. 1998 | |
| WMS-III Spatial Span | Wechsler 1997 | |
| Speed of Information Processing | ||
| WAIS-R/III Digit Symbol | Heaton et al. 2003 | |
| WAIS-III Symbol Search | Heaton et al. 2003 | |
| Trail Making Test Part A | Heaton et al. 1991 | |
| Stroop Color and Word Test (Color trial) | Golden 1978 | |
| Learning & Recall | ||
| Hopkins Verbal Learning Test-Revised | Benedict et al. 1998 | |
| Brief Visuospatial Memory Test-Revised | Benedict 1997 | |
| Executive Function | ||
| Wisconsin Card Sorting Test | Kongs et al. 2000 | |
| Category Test | Heaton et al. 1991 | |
| Trail Making Test Part B | Heaton et al. 1991 | |
| Stroop Color and Word Test (interference score) | Golden 1978 | |
| Motor Function | ||
| Grooved Pegboard Test | Heaton et al. 1991 | |
Note: WMS= Wechsler Memory Scale, WAIS=Wechsler Adult Intelligence Scale
Covariates
Additionally, we investigated co-factors that may impact the relationship between exercise and NCI. A broad range of variables were considered, including demographic factors (e.g., gender, education), HIV disease characteristics (e.g., CD4+ lymphocyte count, AIDS status, estimated duration of HIV infection, months exposed to ART), hepatitis-C infection, lifetime and current substance use disorder, physical functioning, mental health status, body mass index, lifetime and current major depressive disorder diagnosis, and current mood. The complete list of co-factors examined is available in Table 1 and some variables are detailed here. Nadir CD4+ was self-reported unless a study lab value was lower. AIDS diagnosis was made using CDC classification of 3 or C (Castro et al. 1992). Plasma HIV viral loads were deemed “undetectable” below 48 copies/mL. Current mood state was evaluated using the Beck Depression Inventory II (BDI-II; Beck et al. 1996b). Substance use disorders (i.e., alcohol, amphetamine, cannabis, cocaine, hallucinogen, inhalant, sedative, opioid, PCP) and major depressive disorders were assessed through the computer-assisted Composite International Diagnostic Interview (CIDI), version 2.1 (Wittchen 1994). Current and past substance use disorder included any diagnosis of substance abuse and/or substance dependence also derived from the CIDI. Physical functioning and mental health status were evaluated using the physical health summary (PHS) and mental health summary (MHS) scores, (Revicki et al. 1998) from the MOS-HIV (Wu et al. 1997).
Table 1. Demographics, HIV disease characteristics, and psychiatric and physical health status by group (Exercise and No Exercise).
| Variable | Group | ||
|---|---|---|---|
|
| |||
| Exercise n=83 | Exercise n=252 | pa | |
| Demographics | |||
| Age (years) | 46.7 (10.0) | 48 (10.6) | 0.32 |
| Education (years)* | 14.2 (2.9) | 12.6 (3.2) | <.01 |
| Male | 81.9% | 71.8% | 0.06 |
| Ethnicity | 0.40 | ||
| Non-Hispanic White | 57.8% | 49.2% | |
| Hispanic | 20.5% | 29.37% | |
| Non-Hispanic Black | 18.1% | 16.7% | |
| HIV Disease Characteristics | |||
| Duration of Infection (months) | 163.8 (71.6-243.4) | 174.4 (113.8-229.8) | 0.41 |
| Nadir CD4 | 197 (61-303) | 150 (50-279) | 0.08 |
| Current CD4* | 578 (416-865) | 528 (338-767) | 0.04 |
| Plasma Viral load (detectable)b | 22.0% | 28.0% | 0.31 |
| AIDS diagnosis* | 54.2% | 68.1% | 0.02 |
| ART Prescribed | 86.4% | 86.4% | 1.00 |
| Months exposed to ARTb | 80.1 (37.4-135.5) | 83.8 (40.6-139.7) | 0.53 |
| Hepatitis-C Virus Infection | 3.6% | 5.2% | 0.56 |
| Mental and Physical Health | |||
| Beck Depression Inventory-II | 8 (3-15) | 10(4-17) | 0.22 |
| Mental Health Summary Scorec | 53.0 (43.1-59.1) | 50.1 (42.1-57.2) | 0.63 |
| Physical Health Summary Scorec* | 54.4 (43.2-60.0) | 47.4 (37.2-56.3) | <.01 |
| Substance Use Disorder Lifetime | 73.8% | 63.9% | 0.10 |
| Substance Use Disorder Current | 2.5% | 4.4% | 0.44 |
| Major Depressive Disorder Lifetime | 55.0% | 58.2% | 0.61 |
| Major Depressive Disorder Current* | 2.5% | 12.5% | 0.01 |
| Body Mass Indexd | 25.9 (24.4-29.4) | 26.0 (23.0-29.6) | 0.38 |
Notes: Mean (SD), Median (LQ-HQ), or %.
Results from independent sample t-test and Chi-Square tests.
Missing cases
27(5 from exercise group)
45 (0 from exercise group)
11 (1 from exercise group). If less than 10 cases missing in a given variable, this is not noted.
p< 0.05
Statistical Analyses
Given the highly skewed distribution of time spent engaging in exercise, we divided our sample into those participants that reported any amount of time exercising (i.e., Exercise), as per our question, over the last 72 hours (n= 83; Time in exercise: Median = 120 minutes, IQR=60-180) and those that reported no time exercising (n=252) in the last 72 hours (i.e., No Exercise).
To assess the relation between exercise and NCI we first ran a series of Chi-Square tests on global and domain NCI by group. To adjust for potential confounds, we then compared our groups (Exercise vs. no Exercise) on a number of potential covariates using independent sample t-tests for continuous variables and Chi-Square tests for categorical variables. The covariate variables that were significantly different (p < 0.05) between the groups were entered into a series of multivariable logistic regression models on global and domain NCI, along with exercise. Odds ratios with 95% confidence intervals were computed to determine the strength of the relationship between exercise and global or domain NCI. All reported p-values were based on two-sided tests with significance determined at p values at or below 0.05.
Results
Table 1 shows the demographic, HIV disease, and health status characteristics by group (i.e. Exercise and No Exercise). The Exercise group reported significantly more formal education, lower prevalence of AIDS, higher current CD4+ count, less current major depressive disorder, and a higher PHS (better self-reported physical functioning).
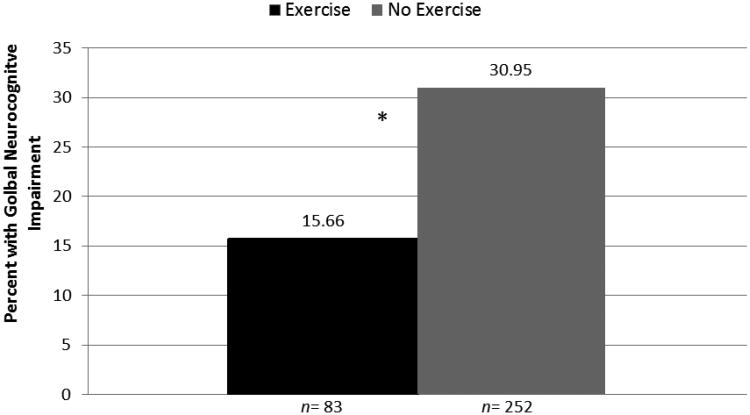
As shown in Figure 1, the Exercise group had significantly lower rates of global NCI than the No Exercise group and the rate of cognitive impairment was approximately doubled in the No Exercise group (df = 1, χ2 = 7.99, p < 0.01, OR = 2.28, CI = 1.30 - 4.80). A comparable analysis in our subset of participants with undetectable viral load (n=233) showed similar findings (χ2 = 4.02, p < 0.05, OR = 2.08, CI = 1.01-4.31). In our overall sample, a multivariable logistic regression model on global NCI was significant (χ2= 13.61, p =0.03) and showed that exercise continued to be significantly associated with global NCI (χ2 =6.02, p < 0.01, OR =2.63) after adjusting for potential confounding factors that differed between groups.
Fig. 1.
HIV-infected persons who have not exercised in last 72 hours have double the neurocognitive impairment rate of those who have exercised. Note: Statistical significance (*p<0.05) derived from multivariable logistic regressions modeling impairment in each domain by group and adjusting for potential confounds
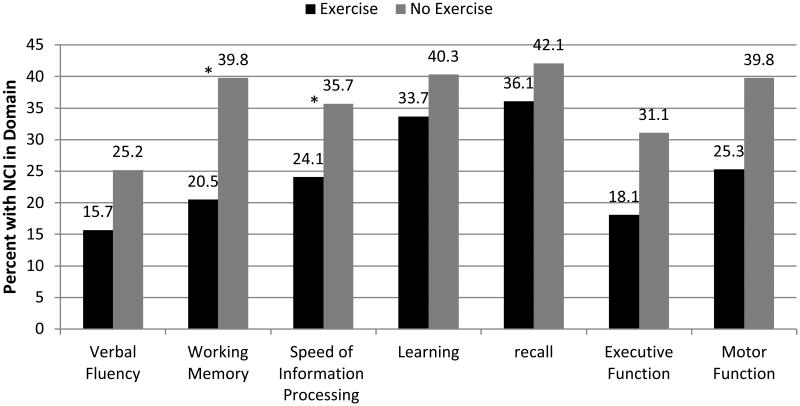
Figure 2 shows the rate of NCI in each domain by group. Unadjusted analyses showed that the Exercise group had significantly lower rates (p < 0.05) of impairment in working memory, speed of information processing, executive function, and motor function. Adjusted models showed that exercise was significantly associated with lower impairment rates (p < 0.05) in working memory and speed of information processing (Figure 2), but not in the other cognitive domains.
Fig 2.
Working memory and speed of information processing are significantly impaired in the No Exercise group as compared to the Exercise group. Note: Statistical significance (*p<0.05) derived from multivariable logistic regressions modeling impairment in each domain by group and adjusting for potential confounds
Follow-up analysis
In order to more stringently adjust for the potential effect of demographic variables on NCI, we selected a subgroup of participants from the No Exercise group (n= 83; Age: M=47.5, SD=8.7; Education: M=14.2, SD=2.8; 79.5% male; 59.0% non-Hispanic White) that were matched on demographic characteristics (i.e. age, years of education, gender, and ethnicity) to the Exercise group (n = 83). This subgroup of participants in the No Exercise group continued to have significantly lower CD4 counts (Median =485, IQR=233-702), higher rates of current major depressive disorder (15.7%), and higher instances of AIDS (79.3%) than the Exercise group (ps<.05), with no other significant group differences on covariates. The rate of global NCI in the No Exercise group (28.9%) continued to be double that of the Exercise group (χ2 = 4.21, p = 0.04, OR = 2.19, CI = 1.03-4.68) and exercise remained a significant predictor of global impairment after adjusting for potential confounding factors that differed between groups (i.e., current CD4, current major depressive disorder, AIDS status).
Discussion
The present study is among the first to examine the direct relationship between exercise and neurocognition among HIV+ individuals. We found that self-reported recent engagement in exercise was significantly associated with lower rates of global NCI in a large cohort of HIV+ individuals. Further, this association continued to be significant even after examining a number of potential confounds, including demographic factors, HIV disease characteristics, substance use disorders, past and current depression, mental health status and physical functioning. Among the cognitive domains examined, lower rates of impairment in working memory and speed of information processing were significantly associated with exercise. These findings support exercise as a modifiable lifestyle behavior that may reduce or potentially prevent NCI in HIV+ persons. It is also relevant to note that our findings correspond to a growing body of studies that support the hypothesis that exercise has a positive effect on neurocognition (e.g., Heyn et al. 2004; Lautenschlager et al. 2008; Rosenberg et al. 2012).
Our results are consistent with prior findings indicating an association between exercise and cognition among persons living with HIV, and extend the current literature by showing that this association is present in a diverse, large group of HIV+ patients who have completed a comprehensive neurocognitive assessment (Fillipas et al. 2006; Honn et al. 1999). The only intervention study on this topic among HIV+ adults was limited by a small sample size (i.e., 17-18 participants per group) and the use of a self-report measure of cognitive symptoms, which might be impacted by responding bias and poor insight (Fillipas et al. 2006). A prior observational study found only a limited association between exercise and neurocognition in a group of young (M=33 years), medically-asymptomatic HIV+ adult males (Honn et al. 1999). Although it included a comprehensive neurocognitive battery, the authors did not report data on cognitive domains, but rather individual tests. However, similar to our present findings, this study reported that current exercise was associated with performance on a cognitive test of working memory that requires speeded information processing.
Our analyses, when adjusted for potential confounds, showed that exercise was significantly associated with lower impairment in working memory and speed of information processing domains. Similar associations have been reported in randomized control exercise interventions of HIV-uninfected persons (Smith et al. 2010). These cognitive domains are thought to be mediated by frontal and subcortical brain systems which are typically most affected by cerebrovascular disease, (DeCarli et al. 1995; Kennedy and Raz 2009; Raz et al. 2003), suggesting that this factor could potentially mediate the relationship between exercise and improved cognition in HIV infection. The major benefit of exercise to the brain may be reduced neurocognitive risk factors, such as high blood pressure and hyperlipidemia. Metabolic syndrome associated with ART use is also associated with increased risk for developing diabetes, hypertension, obesity, and dyslipidemia (Aboud et al. 2007; Grinspoon and Carr 2005). All of these phenomena are cerebrovascular risk factors that may in turn cause NCI (Gorelick et al. 2011). Studies have shown that aerobic exercise can improve body composition, reduce waist circumference and weight, and improve lipid levels in those with HIV infection (O'Brien et al. 2010). Exercise interventions would likely reduce waist circumference and BMI, factors that our group has previously shown to be associated with NCI (McCutchan et al. 2012). In summary, our findings are consistent with the notion of cerebrovascular disease as a possible mechanism underlying the association between exercise and neurocognition.
It is worth noting, however, that exercise has direct impacts on the brain as well. Studies have also shown that exercise reduces oxidative stress and inflammatory markers, and increases neurogenesis, angiogenesis, and synaptogenesis in the brain (Lista and Sorrentino 2010; Ahlskog et al. 2011). Further, it is possible that pain, neuropathy, or some other factor we did not examine may both limit exercise and impair cognition. Alternatively, our results could be interpreted to mean that lower neurocognitive functioning may be a barrier to participating in exercise. The relationship may be a complex and bidirectional in that exercise could influence NCI, and in turn NCI could influence one's ability to engage in exercise.
Our study has several limitations. We measured exercise by self-report, which might be subjected to bias. Our questionnaire was short and did not specify the frequency or quantity of exercise in various categories (e.g., leisure vs. work-related exercise). Moreover, we did not quantify exercise over a long time span. However, studies across many domains have suggested that shorter recall periods may lead to more accurate self-report of various behaviors (Jerant et al. 2008; Napper et al. 2010). Therefore, estimates of exercise time over a relatively short and recent epoch (the previous 72 hours) may be more precise compared to longer periods. We cannot infer causality due to the cross sectional nature of these data, and an interventional study is needed to fully demonstrate the impact of exercise on neurocognition. However, the influence of exercise on NCI is supported in the literature by prospective randomized controlled trials of exercise interventions that have been shown to improve cognition among HIV-uninfected individuals (Heyn et al. 2004). Differences in demographics and systemic function between participants in the Exercise and No Exercise groups might confound the relationship between exercise and neurocognition. In order to adjust for these potential confounds we examined many covariates and included any that significantly differed between groups in our models. Further, we re-ran our core models in a subgroup of participants matched on demographic characteristics. The strength of this study includes the assessment of a large, well-characterized cohort and the extensive and well-validated neurocognitive battery (Heaton et al. 1995).
Although we have shown that self-reported exercise is associated with better cognitive function, additional research is needed to determine the intensity and frequency of exercise needed to achieve the best neurocognitive outcomes. A better understanding of the relationship between exercise and NCI may be possible if patients are followed longitudinally or if objective measurements of exercise are used, such as pedometers, accelerometers, or supervised exercise. Future studies should also examine the ability of exercise interventions to improve cognitive function in the HIV+ population. In addition to objectively measuring exercise, it has been shown that pedometers can motivate an increase in daily physical activity across all age groups and even in older, sedentary individuals (Kang et al. 2009). Recent studies have also demonstrated significant benefits from using mobile phone interventions to influence exercise behavior. Hurling and colleagues used a text messaging intervention in adults and were able to increase moderate exercise by two hours per week, and reduce body fat percent in nine weeks (2007). Text messaging interventions have even been shown to modify behavior in cognitively impaired schizophrenia patients (Pijnenborg et al. 2010). Thus, using techniques that include pedometer-motivated exercise or a text-messaging interface may increase exercise engagement and lead to improved neurocognitive performance in HIV+ individuals.
In summary, NCI still affects nearly half of the HIV+ population. Our results suggest that exercise is associated with less NCI among HIV infected persons, and may have specific impact on working memory and speed of information processing. Future intervention studies would help better determine whether exercise is an effective tool to address the neurocognitive deficits associated with this disease.
Acknowledgments
Funding/Support: The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH.
Biography
The San Diego HIV Neurobehavioral Research Program group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Terry Alexander, R.N., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Terry Jernigan, Ph.D., Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D., Ian Everall, FRCPsych., FRCPath., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S., Christi Kao, M.S.
Footnotes
Financial Disclosure: Nothing to disclose.
Publisher's Disclaimer: Disclaimer: The views expressed herein are those of the authors and do not reflect the official policy or position of the US government.
Contributor Information
Ms Catherine A. Dufour, Email: cadufour@ucsd.edu.
Dr Maria J. Marquine, Email: mmarquine@ucsd.edu.
Dr Pariya L. Fazeli, Email: plfazeli@ucsd.edu.
Dr Brook L. Henry, Email: blhenry@ucsd.edu.
Dr Ronald J. Ellis, Email: roellis@ucsd.edu.
Dr Igor Grant, Email: igrant@ucsd.edu.
References
- Aboud M, Elgalib A, Kulasegaram R, Peters B. Insulin Resistance and Hiv Infection: A Review. Int J Clin Pract. 2007;61:463–72. doi: 10.1111/j.1742-1241.2006.01267.x. [DOI] [PubMed] [Google Scholar]
- Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical Exercise as a Preventive or Disease-Modifying Treatment of Dementia and Brain Aging. Mayo Clin Proc. 2011;86:876–84. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated Research Nosology for Hiv-Associated Neurocognitive Disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artiola L, Hermosillo D, Heaton R, Pardee R. Manual De Normas Y Procedimientos Para La Bateria Neuropsicologica En Espanol. Tucson: mPress; 1999. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-Ii. San Antonio, TX: Psychological Corporation; 1996b. pp. 1–82. [Google Scholar]
- Benedict R. Brief Visuospatial Memory Test-Revised. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test Revised: Normative Data and Analysis of Inter-Form and Test-Retest Reliability. Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Blackstone K, Moore DJ, Heaton RK, Franklin DR, Jr, Woods SP, Clifford DB, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, Rivera-Mindt M, Deutsch R, Ellis RJ, Hampton Atkinson J, Grant I. Diagnosing Symptomatic Hiv-Associated Neurocognitive Disorders: Self-Report Versus Performance-Based Assessment of Everyday Functioning. J Int Neuropsychol Soc. 2012;18:79–88. doi: 10.1017/S135561771100141X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive Validity of Global Deficit Scores in Detecting Neuropsychological Impairment in Hiv Infection. Journal of Clinical and Experimental Neuropsychology. 2004;26:307–19. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Castro KG, Ward JW, Slutsker L, Buehler JW, Jaffe HW, Berkelman RL. 1993 Revised Classification System for Hiv Infection and Expanded Surveillance Case Definition for Aids among Adolescents and Adults. US Department of Health and Human Services; 1992. [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study. Psychol Sci. 2003;14:125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, Salerno JA, Gonzales-Aviles A, Horwitz B, Rapoport SI, et al. The Effect of White Matter Hyperintensity Volume on Brain Structure, Cognitive Performance, and Cerebral Metabolism of Glucose in 51 Healthy Adults. Neurology. 1995;45:2077–84. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- Diehr MC, Heaton RK, Miller W, Grant I. The Paced Auditory Serial Addition Task (Pasat): Norms for Age, Education, and Ethnicity. Assessment. 1998;5:375–87. doi: 10.1177/107319119800500407. [DOI] [PubMed] [Google Scholar]
- Fabbiani M, Ciccarelli N, Tana M, Farina S, Baldonero E, Di Cristo V, Colafigli M, Tamburrini E, Cauda R, Silveri M, Grima P, Di Giambenedetto S. Cardiovascular Risk Factors and Carotid Intima-Media Thickness Are Associated with Lower Cognitive Performance in Hiv-Infected Patients. HIV Med. 2012 doi: 10.1111/j.1468-1293.2012.01044.x. [DOI] [PubMed] [Google Scholar]
- Fillipas S, Oldmeadow LB, Bailey MJ, Cherry CL. A Six-Month, Supervised, Aerobic and Resistance Exercise Program Improves Self-Efficacy in People with Human Immunodeficiency Virus: A Randomised Controlled Trial. Aust J Physiother. 2006;52:185–90. doi: 10.1016/s0004-9514(06)70027-7. [DOI] [PubMed] [Google Scholar]
- Foley J, Ettenhofer M, Wright MJ, Siddiqi I, Choi M, Thames AD, Mason K, Castellon S, Hinkin CH. Neurocognitive Functioning in Hiv-1 Infection: Effects of Cerebrovascular Risk Factors and Age. Clin Neuropsychol. 2010;24:265–85. doi: 10.1080/13854040903482830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for Letter and Category Fluency: Demographic Corrections for Age, Education, and Ethnicity. Assessment. 1999;6:147. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test 1978 [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular Contributions to Cognitive Impairment and Dementia: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke; a journal of cerebral circulation. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon S, Carr A. Cardiovascular Risk and Body-Fat Abnormalities in Hiv-Infected Adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- Hand GA, Lyerly GW, Jaggers JR, Dudgeon WD. Impact of Aerobic and Resistance Exercise on the Health of Hiv-Infected Persons. American Journal of Lifestyle Medicine. 2009;3:489–499. doi: 10.1177/1559827609342198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. Hiv-Associated Neurocognitive Disorders Persist in the Era of Potent Antiretroviral Therapy: Charter Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, et al. The Hnrc 500--Neuropsychology of Hiv Infection at Different Disease Stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:231–51. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I. The Impact of Hiv-Associated Neuropsychological Impairment on Everyday Functioning. Journal of the International Neuropsychological Society : JINS. 2004;10:317–31. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Taylor MJ, Manly J. Wms-Iii. Clinical interpretation of the WAIS-III and WMS-III. 2003:181. [Google Scholar]
- Heyn P, Abreu BC, Ottenbacher KJ. The Effects of Exercise Training on Elderly Persons with Cognitive Impairment and Dementia: A Meta-Analysis. Arch Phys Med Rehabil. 2004;85:1694–704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Honn VJ, Para MF, Whitacre CC, Bornstein RA. Effect of Exercise on Neuropsychological Performance in Asymptomatic Hiv Infection. AIDS and Behavior. 1999;3:67–74. [Google Scholar]
- Hurling R, Catt M, Boni MD, Fairley BW, Hurst T, Murray P, Richardson A, Sodhi JS. Using Internet and Mobile Phone Technology to Deliver an Automated Physical Activity Program: Randomized Controlled Trial. Journal of Medical Internet Research. 2007;9:e7. doi: 10.2196/jmir.9.2.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerant A, DiMatteo R, Arnsten J, Moore-Hill M, Franks P. Self-Report Adherence Measures in Chronic Illness: Retest Reliability and Predictive Validity. Medical care. 2008;46:1134–9. doi: 10.1097/MLR.0b013e31817924e4. [DOI] [PubMed] [Google Scholar]
- Kang M, Marshall SJ, Barreira TV, Lee JO. Effect of Pedometer-Based Physical Activity Interventions: A Meta-Analysis. Research Quarterly for Exercise & Sport. 2009;80:648–55. doi: 10.1080/02701367.2009.10599604. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging White Matter and Cognition: Differential Effects of Regional Variations in Diffusion Properties on Memory, Executive Functions, and Speed. Neuropsychologia. 2009;47:916–27. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test: 64-Card Version (Wcst,Äì64) Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of Physical Activity on Cognitive Function in Older Adults at Risk for Alzheimer Disease: A Randomized Trial. JAMA: the Journal of the American Medical Association. 2008;300:1027–37. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- Lista I, Sorrentino G. Biological Mechanisms of Physical Activity in Preventing Cognitive Decline. Cellular and molecular neurobiology. 2010;30:493–503. doi: 10.1007/s10571-009-9488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte TD, Heaton RK, Wolfson T, Taylor MJ, Alhassoon O, Arfaa K, Ellis RJ, Grant I. The Impact of Hiv-Related Neuropsychological Dysfunction on Driving Behavior. The Hnrc Group. Journal of the International Neuropsychological Society : JINS. 1999;5:579–92. doi: 10.1017/s1355617799577011. [DOI] [PubMed] [Google Scholar]
- McCutchan JA, Marquie-Beck JA, Fitzsimons CA, Letendre SL, Ellis RJ, Heaton RK, Wolfson T, Rosario D, Alexander TJ, Marra C, Ances BM, Grant I. Role of Obesity, Metabolic Variables, and Diabetes in Hiv-Associated Neurocognitive Disorder. Neurology. 2012;78:485–92. doi: 10.1212/WNL.0b013e3182478d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napper LE, Fisher DG, Reynolds GL, Johnson ME. Hiv Risk Behavior Self-Report Reliability at Different Recall Periods. AIDS and behavior. 2010;14:152–61. doi: 10.1007/s10461-009-9575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien K, Nixon S, Tynan AM, Glazier R. Aerobic Exercise Interventions for Adults Living with Hiv/Aids. Cochrane Database Syst Rev. 2010:CD001796. doi: 10.1002/14651858.CD001796.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenborg GH, Withaar FK, Brouwer WH, Timmerman ME, van den Bosch RJ, Evans JJ. The Efficacy of Sms Text Messages to Compensate for the Effects of Cognitive Impairments in Schizophrenia. The British Journal of Clinical Psychology/the British Psychological Society. 2010;49:259–74. doi: 10.1348/014466509X467828. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the Brain: Vulnerability of the Prefrontal Regions and Executive Functions. Behavioral Neuroscience. 2003;117:1169–80. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Revicki DA, Sorensen S, Wu AW. Reliability and Validity of Physical and Mental Health Summary Scores from the Medical Outcomes Study Hiv Health Survey. Medical care. 1998;36:126–37. doi: 10.1097/00005650-199802000-00003. [DOI] [PubMed] [Google Scholar]
- Rosenberg DE, Kerr J, Sallis JF, Norman GJ, Calfas K, Patrick K. Promoting Walking among Older Adults Living in Retirement Communities. Journal of Aging and Physical Activity. 2012;20:379–94. doi: 10.1123/japa.20.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic Exercise and Neurocognitive Performance: A Meta-Analytic Review of Randomized Controlled Trials. Psychosomatic Medicine. 2010;72:239–52. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Arentoft A, Rivera-Mindt M, Hinkin CH. Functional Disability in Medication Management and Driving among Individuals with Hiv: A 1-Year Follow-up Study. Journal of Clinical and Experimental Neuropsychology. 2012 doi: 10.1080/13803395.2012.747596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale,Äìthird Edition and Wechsler Memory Scale,Äìthird Edition Technical Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wittchen HU. Reliability and Validity Studies of the Who--Composite International Diagnostic Interview (Cidi): A Critical Review. J Psychiatr Res. 1994;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Wright EJ, Grund B, Robertson K, Brew BJ, Roediger M, Bain MP, Drummond F, Vjecha MJ, Hoy J, Miller C, Penalva de Oliveira AC, Pumpradit W, Shlay JC, El-Sadr W, Price RW. Cardiovascular Risk Factors Associated with Lower Baseline Cognitive Performance in Hiv-Positive Persons. Neurology. 2010;75:864–73. doi: 10.1212/WNL.0b013e3181f11bd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AW, Revicki DA, Jacobson D, Malitz FE. Evidence for Reliability, Validity and Usefulness of the Medical Outcomes Study Hiv Health Survey (Mos-Hiv) Quality of Life Research : an international journal of quality of life aspects of treatment, care and rehabilitation. 1997;6:481–93. doi: 10.1023/a:1018451930750. [DOI] [PubMed] [Google Scholar]