Abstract
Impaired memory may result from synaptic glutamatergic dysregulation related to chronic neuroinflammation. GLT1 is the primary excitatory amino acid transporter responsible for regulating extracellular glutamate levels in the hippocampus. We tested the hypothesis that if impaired spatial memory results from increased extracellular glutamate due to age or experimentally induced chronic neuroinflammation in the hippocampus, then pharmacological augmentation of the glutamate transporter GLT1 will attenuate deficits in a hippocampal-dependent spatial memory task. The profile of inflammation-related genes and proteins associated with normal aging, or chronic neuroinflammation experimentally-induced via a four-week LPS infusion into the IVth ventricle, were correlated with performance in the Morris water maze following treatment with Riluzole, a drug that can enhance glutamate clearance by increasing GLT1 expression. Age-associated inflammation was qualitatively different from LPS-induced neuroinflammation in young rats. LPS produced a pro-inflammatory phenotype characterized by increased IL-1β expression in the hippocampus, whereas aging was not associated with a strong central pro-inflammatory response but with a mixed peripheral immune phenotype. Riluzole attenuated the spatial memory impairment, the elevation of serum cytokines and the decrease in GLT1 gene expression in Aged rats, but had no effect on young rats infused with LPS. Our findings highlight the therapeutic potential of reducing glutamatergic function upon memory impairment in neurodegenerative diseases associated with aging.
Keywords: neuroinflammation, glutamate, Riluzole, rats, animal models
Introduction
Neuroinflammation may contribute to impaired learning and memory and, ultimately, neurodegeneration (Akiyama et al., 2000) via dysregulation of synaptic glutamatergic signaling, (Rosi et al., 2004, 2005). Indirect evidence from our previous studies suggests that extracellular glutamate is elevated in the brain with age and under conditions of chronic neuroinflammation, and that this dysregulation underlies impaired learning and memory (Rosi et al., 2004, 2005). Compounds that attenuate excess glutamatergic signaling through pre- and post-synaptic mechanisms can ameliorate the spatial memory deficit and attenuate microglia activation in LPS-infused young rats (Brothers et al. 2010; Rosi et al., 2006, 2009) as well as in aged rats (Bardou et al., 2012). These studies are consistent with the hypothesis that attenuation of increased glutamatergic signaling is sufficient to prevent cognitive deficits associated with natural aging and those due to inflammation.
Glutamate transporter 1 (GLT1; excitatory amino-acid transporter-type 2) is the primary excitatory amino acid transporter responsible for the termination of glutamate signaling (Lehre et al., 1995), and predominantly located on astrocytes (Milton et al., 1997), a cell population that changes dramatically with age and after central LPS infusion (Cerbai et al., 2012). Riluzole (Ril), currently used to treat amyotrophic lateral sclerosis, can significantly enhance glutamate clearance via an increase GLT1 expression and reduction in pre-synaptic release (Yoshizumi et al., 2012). The current study investigated whether Ril could prevent cognitive deficits associated with aging or experimentally-induced chronic neuroinflammation produced by infusion of LPS into the IVth ventricle and also restore the balance of pro- and anti-inflammatory genes and proteins.
Methods
Experimental groups
Young (3 mo) Fisher F-344 (NIA) rats, infused with either LPS or artificial cerebral spinal fluid (aCSF), and aged (24 mo) rats were treated with Ril or vehicle, generating six experimental groups: aCSF (n = 7), aCSF Ril (n = 8), LPS (n = 7), LPS Ril (n = 8), Aged (n = 8) and Aged Ril (n = 10). Experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996).
Surgery
Neuroinflammation was induced in young rats by continuous intracerebroventricular (i.c.v.) infusion over 3 weeks of LPS (0.25 µg/hr., 1 mg/ml dissolved in aCSF; E. coli, serotype 055:B5, TCA extraction; Sigma, St. Louis, MO), and controls were infused with aCSF (140 mM NaCl, 3.0 mM KCl, 2.5 mM CaCl2, 1.0 mM MgCl2, and 1.2 mM Na2HPO4 adjusted to pH 7.4). A cannula was implanted into the IVth ventricle and attached to an osmotic minipump (0.25µl/hr., Alzet model #2004; Durect Corp., Cupertino, CA) as previously described (Hauss-Wegrzyniak et al., 1998). Treatment with Ril or vehicle began the day after surgery in young rats, or one week after arrival in aged rats, and continued daily for 3 weeks. Ril (Selleck Chemicals, Houston, TX) 4 mg/kg/day intraperitoneal (i.p.) was dissolved at 8 mg/ml into the vehicle 50% polyethylene. Spatial Memory: Spatial learning and memory was tested in the Morris water maze, a task sensitive to both hippocampal damage and LPS-induced neuroinflammation, as previously described (Hauss-Wegrzyniak et al., 1998). Latency to find the platform, distance traveled, speed, thigmotaxis (time spent in the pool perimeter) and other variables were tracked and recorded (Noldus EthoVision 3.1, Noldus, Leesburg, VA).
Biochemical Analysis
Each rat was deeply anesthetized, rapidly decapitated and serum was collected and stored (−80 °C). The hippocampus from one hemisphere of each rat was stored (−80 °C) and later homogenized for RNA extraction and the hippocampus from other hemisphere was immediately homogenized and stored (−80 °C) for later protein analysis. Gene and protein expression of inflammatory markers were used to approximate inflammatory phenotypes. Hippocampi from both hemispheres of a separate group of young rats infused with aCSF or LPS were homogenized immediately for flow cytometry.
Quantitative polymerase chain reaction (qPCR) determined (Bio-Rad, model CFX96, C1000 Thermal Cycler) expression of the following genes: toll-like receptor 4 (TLR4, the microglial receptor for LPS); the pro-inflammatory cytokines interleukin-1β (IL-1β), IL-1α and tumor necrosis factor-α (TNFα); the anti-inflammatory cytokines transforming growth factor-β (TGFβ), fractalkine (CX3CL1) and its receptor CX3CR1; brain-derived neurotrophic factor (BDNF); the glutamate transporter GLT1 and the glutamate-cystine anti-porter (xCT); and the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Protein expression in hippocampal tissue and serum were evaluated in each sample simultaneously using a bead-based immunoassay (MAGPIX system, Bio-Rad) for their content of granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFNγ), IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, and TNFα.
Microglia surface expression of the major histocompatibility complex-type II (MHCII), TLR4, CX3CR1 and GLT1 was examined by flow cytometry in a group of young aCSF- and LPS- infused rats (Wynne et al., 2009). Flow cytometric data were analyzed using FlowJo software (Tree Star, San Carlos, CA).
Statistics
SigmaStat (Jandel) and SPSS (IBM) software were used to compare groups by analysis of variance (ANOVA) and repeated measures ANOVA with Fisher LSD post-hoc tests, to perform paired t-tests, and to perform Pearson correlation coefficients.
Results
Spatial memory
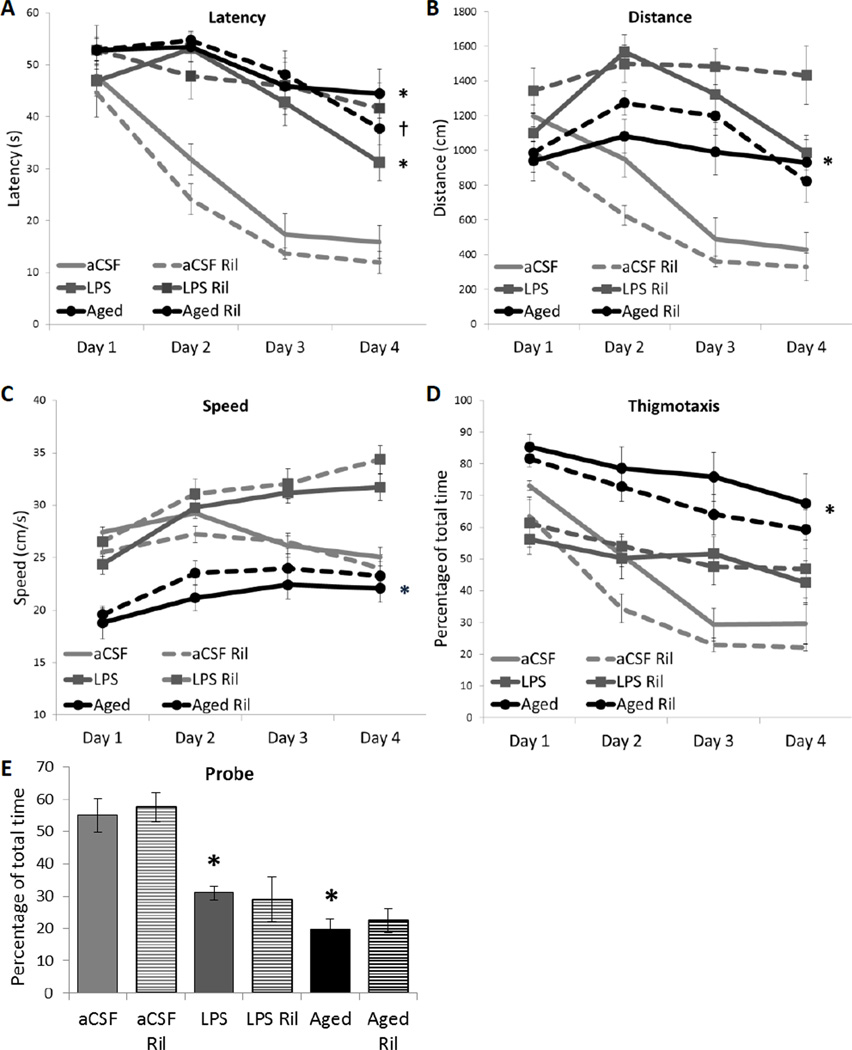
Reduced latency to find the hidden platform in the Morris water maze is used as an indicator of spatial learning and memory (Figure 1A). A 3-way repeated measures ANOVA revealed a main between-subjects effect of inflammation group (aCSF, LPS or Aged; F2, 56 = 76.005, p < 0.001), a main within-subjects effect of trial day (F3, 168 = 67.852, p < 0.001), and an interaction between inflammation group and trial day (F6, 168 = 10.416, p < 0.001). Post-hoc analysis indicates that aCSF controls found the platform in less time than LPS controls and Aged controls (*p < 0.001). Paired t-tests were conducted between the first and last trial day within each group. Generally, all groups improved across days except Aged rats, unless treated with Ril (†t = 2.602, p = 0.035).
Figure 1. Spatial memory performance.
A) Latency to find the hidden platform. LPS-infused controls and Aged controls were impaired (*p < 0.001) compared to young aCSF-infused rats. The performance of Aged rats improved across days only when treated with Ril (†t = 0.035). B) Distance. LPS controls swam a greater distance than aCSF controls and Aged controls (*p ≤ 0.036). C) Swim Speed. Aged rats swam the most slowly (*p ≤ 0.001). D) Thigmotaxis. Aged controls spent the greatest percentage of time within the pool perimeter (*p < 0.001). E) Probe Trial. Both LPS and Aged groups spent less time in the vicinity of the missing platform than aCSF-infused rats (*p ≤ 0.001).
Reduced path length is another indicator of improved memory performance. A 3-way repeated measures ANOVA of total distance swam (Figure 1B) revealed a main between-subjects effect of inflammation condition (F2, 56 = 54.450, p < 0.001) and a main within-subjects effect of trial day (F3, 168 = 27.811, p < 0.001) as well as interactions between inflammation group and drug treatment (F4, 56 = 3.203, p = 0.019), inflammation group and trial day (F6, 168 = 15.209, p < 0.001), and an 3-way interaction between inflammation condition, drug treatment and trial day (F6, 168 = 1.825, p = 0.048). Post hoc analysis revealed that LPS controls swim more overall distance than aCSF controls and Aged controls (*p ≤ 0.036).
Swim speed was compared between groups because differences in velocity confound the interpretation of differences in latency and distance (Figure 1C). A 3-way repeated measures ANOVA revealed a main between-subjects effect of inflammation condition (F2, 56 = 88.367, p < 0.001), a main within-subjects effect of trial day (F3, 168 = 48.155, p < 0.001), and an interaction between inflammation condition and trial day (F6, 168 = 21.980, p < 0.001). Post hoc analysis indicates that Aged controls swim with significantly less velocity than young, aCSF- or LPS-infused controls (*p < 0.001).
Thigmotaxis is time spent on a poor strategy (Figure 1D). A 3-way repeated measures ANOVA revealed a main between-subjects effect of inflammation condition (F2, 56 = 54.553, p < 0.001), a main within-subjects effect of trial day (F3, 168 = 89.923, p < 0.001), and an interaction between inflammation condition and trial day (F6, 168 = 8.013, p < 0.001). Post hoc analysis indicates that Aged controls spend a greater percentage of trial time in the pool perimeter than young, aCSF- or LPS-infused controls (*p ≤ 0.001).
A probe trial was conducted after the completion of 6 trials on the fourth day (Figure 1E). There was a main effect within inflammation group (F2, 64 = 51.82, p < 0.001). Young aCSF rats spent more time in the vicinity of the missing platform than LPS or Aged controls (*p < 0.001), which both performed similarly at chance levels. No drug treatment had an effect on the amount of time spent near the missing platform.
Immune phenotype
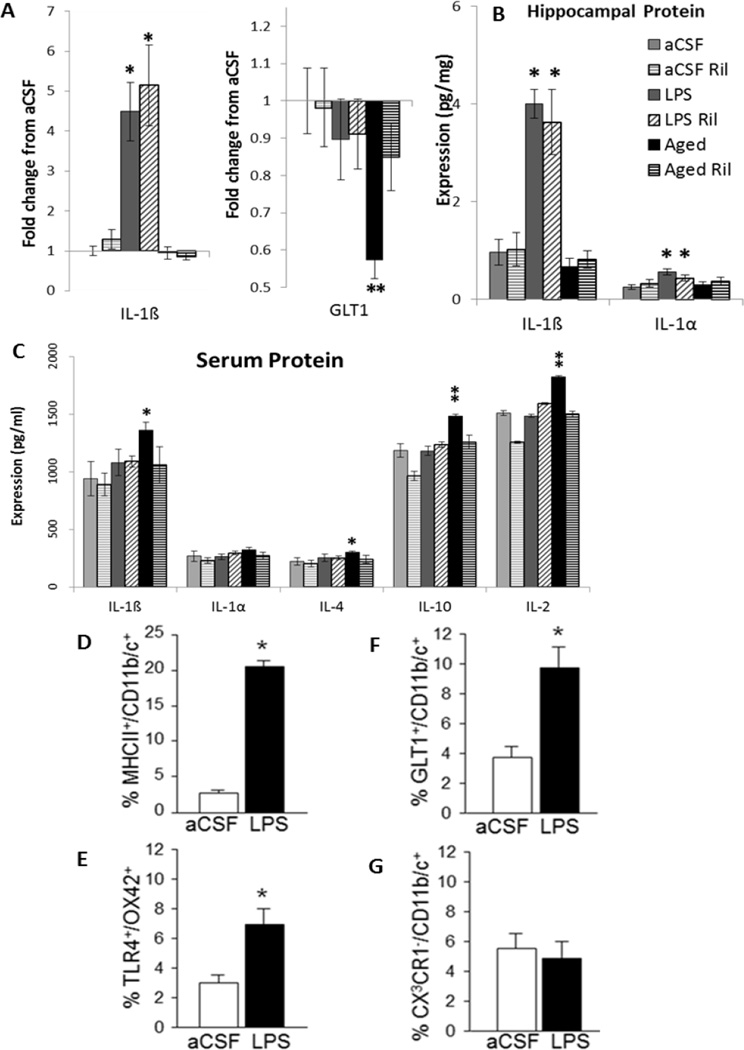
IL-1β increased in all LPS-infused rats compared to aCSF-infused and Aged (*p < 0.001, Figure 2A). Glutamate transport and glutamate-cystine exchange were evaluated by investigating GLT1 (Figure 2A) and xCT gene expression in hippocampus. There was a main effect of inflammation group (F2, 67 = 6.4, p = 0.003) in which Aged controls express less GLT1 gene than both aCSF and LPS controls (**p ≤ 0.014), unless treated with Ril (§p = 0.028) indicating that Ril restores glutamate transport in aged rats. There were no differences in expression of xCT gene. LPS controls expressed more IL-1α and IL-1β than aCSF or Aged controls (*p ≤ 0.003, Figure 2B); treatment with Ril had no significant effect. Serum cytokine protein expression (Figure 2C) was not elevated after four weeks of LPS infusion. There were, however, main effects inflammation group (p ≤ 0.031) in which pro-inflammatory cytokines IL-1β (*p = 0.007) and IL-2 (**p ≤ 0.042) and anti-inflammatory cytokines IL-4 (*p = 0.017) and IL-10 (**p ≤ 0.037) were elevated in Aged controls, indicating a mixed peripheral immune phenotype.
Figure 2. Blood and brain biochemistry.
A) Hippocampal gene expression. IL-1β total gene expression relative to aCSF controls was elevated in LPS infused rats as compared to aCSF and Aged rats (*p < 0.001). GLT1 total gene expression relative to aCSF controls was reduced in Aged rats, as compared to aCSF and LPS controls (**p ≤ 0.014), and reversed by Ril treatment (§p = 0.028). B) Hippocampal protein expression. IL-1β and IL-1α protein levels were elevated in LPS infused rats, as compared to aCSF and Aged rats (*p ≤ 0.003). C) Serum protein expression levels. IL-1β and IL-4 were elevated in Aged rats as compared to aCSF rats (*p = 0.017) and IL-10 and Il-2 were elevated in Aged rats as compared to both aCSF and LPS rats (**p ≤ 0.037). D–F) Flow cytometry analysis of CD11b/c-IR microglia in hippocampus. Four weeks of LPS infusion into the IVth ventricle of young rats induced an increase in MHCII (D), TLR4 (E) and GLT1 (F) on a subset of the total microglia population relative to aCSF-infused rats (*p < 0.05). Fractalkine receptor (CX3CR1) levels on the microglia (G) were unchanged.
Microglia activation state was determined in young aCSF- and LPS-infused rats by flow cytometry. Hippocampal microglia (CD11b/c+ cells) increased surface expression of the LPS receptor TLR4 and the pro-inflammatory markers MHCII and GLT1, but not the anti-inflammatory CX3CR1 (*p < 0.05; Figure 2D–G). MHCII is classically used to identify microglial activation, although is still present in an alternative activation state; while GLT1 on microglia is consistent with an M1-type pro-inflammatory profile (Colton and Wilcock 2010; Colton, 2009; Persson et al. 2005).
Correlations
TLR4 gene expression correlated with increased latency to find the hidden platform (r = 0.321, p = 0.010) and increased distance traveled (r = 0.358, p = 0.003) among all comparisons; these relationships were stronger between aCSF and LPS. TLR4 expression correlated with thigmotaxis when only aCSF and Aged are analyzed (r = 0.319, p = 0.037). IL-1β and IL-1α expression in the hippocampus correlated with poor performance on the last testing day in the Morris water maze when only aCSF and LPS groups are compared. Il-1β gene expression, IL-1β protein expression and IL-1α protein expression correlated with increased time to find the hidden platform, increased swim path, and reduced time spent in the vicinity of the missing platform during the probe trial (p ≤ 0.05), and Il-1β protein expression correlated with increased time spent circling the pool perimeter (r = 0.352, p = 0.030). GLT1 gene expression correlated with reduced latency to find the hidden platform (r = −0.295, p = 0.019) and increased time spent in the vicinity of the missing probe when all data points were compared (r = 0.323, p = 0.010). These correlations were stronger when only aCSF and Aged groups were compared, and strongest when drug treated rats were not included. Time spent in the perimeter of the pool (thigmotaxis) is negatively correlated with GLT1 gene expression (r = −.0538, p = 0.0473), and positively correlated with xCT gene expression (r = 0.518, p = 0.048) when aCSF and Aged controls were examined.
Discussion
We found that LPS-induced neuroinflammation in young rats was qualitatively different from age-associated inflammation. LPS induced a central pro-inflammatory phenotype, whereas aging was not associated with a strong central pro-inflammatory response but was associated with a mixed immune phenotype. The LPS-induced memory impairment correlated with the presence of the pro-inflammatory cytokines IL-1α and IL-1β as well as the presence of the LPS binding site, TLR4 on microglia. The age-associated memory impairment, in contrast, did not correlate with the presence of inflammation markers, other than TLR4, but did correlate with a decrease in GLT1 gene expression. Furthermore, Ril treatment in aged rats restored GLT1 gene expression levels and allowed aged rats to improve their performance across trial days in a spatial memory task. In the brains of patients with Alzheimer’s disease (AD) the amyloid-β peptide significantly reduces GLT1 surface expression, leading to increased levels of synaptic glutamate (Scimemi et al., 2013). These data highlight the potential clinical use of Ril in age-associated memory decline and neurodegenerative diseases, such as AD.
Inflammation generated by continuous LPS infusion was characterized by elevations in IL-1α and IL-1β protein in the hippocampus, as well as increased microglial surface expression of MHCII, GLT1 and TLR4. IL-1β gene expression was dramatically increased after LPS infusion, but LPS-induced changes in expression of other genes were not observed. Flow cytometry confirmed that TLR4 surface expression on microglial increased after LPS infusion in young rats, suggesting that microglia were still able to respond to LPS. Elevated pro-inflammatory markers (IL-1α, IL-1β, MHCII and GLT1) in the absence of changes in anti-inflammatory markers (IL-4, IL-10, TGFβ, CX3CL1, CX3CR1) suggest that after three weeks of continuous LPS infusion the hippocampus remained in a pro-inflammatory state.
LPS-induced memory impairment correlated with TLR4 gene expression and the level of pro-inflammatory cytokines IL-1α and IL-1β. TLR4 gene expression correlated with poor performance in the water maze task, although TLR4 gene expression was not significantly elevated. This correlation was expected given that activation of TLR4 receptors by LPS is the first step in the pro-inflammatory cascade. The relationship between TLR4 gene expression and cognitive performance was more interesting in the aged rats in which there was no experimental activation of TLR4 by LPS. In aged rats, more TLR4 could reflect a recent immune challenge; this was supported by the elevation of both pro-inflammatory (IL-1β and IL-2) and anti-inflammatory cytokines (IL-4 and IL-10) in the serum of aged rats that were not elevated in young aCSF- or LPS-infused rats.
Although LPS-induced neuroinflammation may drive memory impairment through glutamatergic dysregulation, and our previous studies indicate that reducing pre- and post-synaptic glutamatergic function attenuates this impairment (Brothers et al., 2010; Rosi et al., 2006, 2009), Ril treatment was ineffective against the LPS-induced memory impairment in the current study. In young rats, Ril treatment did not change GLT1 gene expression or effect water maze performance. This may be partially explained by the ability of elevated IL-1β to increase the velocity of cystine-glutamate exchange by xCT, which elevates extracellular glutamate levels (Fogal et al., 2007), and to reduce GLT1 on astrocytes, which decreases glutamate uptake (Prow and Irani, 2008) leading to a hyperglutamatergic state (Kamikawa et al., 1998; Mascarucci et al., 1998); we speculate that these processes counteracted any benefit that was produced by the Ril treatment in young rats.
In contrast to young rats, Ril treatment reversed the age-associated decrease in GLT1 gene expression and decreased serum cytokine levels. Ril had a positive effect on the spatial learning and memory performance of aged rats in the water maze task, i.e. aged rats given Ril found the hidden platform more quickly on the last trial day as compared to the first trial day. In contrast, untreated aged controls did not improve across testing days. Reduced latency to find the hidden platform across testing days also corresponded with slightly increased swim speed and reduced time spent in the pool perimeter.
Unlike LPS-infused young rats, the inflammatory phenotype in aged rats did not explain spatial memory impairment. Age-related elevations in cytokines were only observed in the serum and included the pro-inflammatory cytokines IL-1β and IL-2 in addition to the anti-inflammatory cytokines IL-4 and IL-10; indicating a mixed peripheral immune phenotype. Age-associated neuroinflammation is characterized by an increased number of hippocampal microglia expressing the MHCII surface marker, albeit fewer than in LPS-infused young rats (Barrientos et al., 2010; Bardou et al., 2012; Rosi et al., 2005;). The number of MHC II immunoreactive microglia in the hippocampus of aged rats does not correlate with cognitive impairment (Hauss-Wegrzyniak et al., 1999; Van Guilder et al., 2011). The hippocampal cytokine profile of the aged rats in this study did not differ from young aCSF-infused rats, suggesting that the increased presence of MHCII immunoreactive microglia in the hippocampus does not necessarily indicate a pro-inflammatory environment.
We speculate that the age-associated memory impairment was due to dysregulation of glutamatergic homeostasis. GLT1 gene expression was reduced significantly in aged rats compared with young aCSF-infused rats; Ril treatment significantly increased GLT1 gene expression. The reduction in GLT1 in our aged F-344 rats was consistent with both an observed decrease of GLT1 in aged Sprague-Dawley rats that was associated with reduced glutamate uptake, and in aged humans (Lehre et al., 1995; Milton et al., 1997; Potier et al. 2010). GLT1 expression is reduced in early stages of AD in the hippocampus but not in the cerebellum, a region that is spared from pathology (Jacob et al. 2007). GLT1 increases in parallel with astrogliosis in brain regions with the greatest pathology in AD (Masliah et al. 1996) but is reduced globally in autopsied AD brains as compared to non-AD controls (Scott et al., 2011). Increased astrogliosis and GLT1 expression in regions with AD pathology may be interpreted as a compensatory response in order to clear more glutamate from the synapse. Glutamate transport in patients with AD pathology tested in fibroblasts was decreased by 60% compared to controls (Begni et al. 2004), and splice variants that decrease glutamate transport are increased with regional variability in autopsied AD tissue (Scott et al., 2011). Furthermore, GLT1 interacts with the predominant AD pathology, i.e. it interacts with phosphorylated tau and is colocalized with neurofibrillary tangles in AD patients (Sasaki et al. 2009; Woltjer et al. 2010). GLT1 is also colocalized with Aβ in AD tissue and is oxidized by Aβ to a detergent insoluble form that is associated with progression of AD cognitive impairment (Lauderback et al. 2001; Li et al. 1997; Woltjer et al. 2010). Decreases in GLT1 and glutamate clearance in aged rats, in healthy aged humans and in AD suggest the involvement of glutamatergic dysregulation in age-associated memory loss and neurodegenerative disease.
GLT1 and xCT were the only markers examined, in addition to TLR4, which correlated with poor spatial memory in aged rats. Higher GLT1 levels correlated with successful water maze performance (decreased latency and thigmotaxis, increased time spent in the area of the missing platform), while xCT inversely correlated with successful water maze performance (increased thigmotaxis) when young aCSF-infused and aged rats were compared. The opposing functions of GLT1 and xCT on synaptic glutamate may explain their opposite relationships with cognitive performance. Less GLT1 in aged rats could result in a retardation and/or reduction in glutamate clearance, and potentially prolong the post-synaptic response to pre-synaptic glutamate release. Furthermore, reduced clearance by GLT1 in the presence of glutamate export by xCT may amplify accumulation of glutamate in the synapse.
Excessive extracellular glutamate may impair spatial memory directly, as discussed above. Furthermore, elevated extracellular glutamate may also depolarize the post-synaptic membrane and engage extrasynaptic NMDARs that express more NR2B subunits, which are more likely to lead to cell death then synaptically located NDMARs with the predominant 2A subunit (Hardingham and Bading 2010; Potier et al. 2010). Therefore, elevating glutamate clearance by GLT1 not only has therapeutic potential as a mediator of memory function, but may also be disease-modifying in neurodegenerative diseases associated with memory loss.
The correlations between GLT1 and xCT expression with spatial learning and memory in aged rats suggest that the pharmacological enhancement of GLT1 and modulation of the glutamatergic system in general may lead to disease-modifying treatments for AD. Already, drugs that modify glutamatergic function including Memantine, a non-competitive NMDAR antagonist, and the anti-epileptic Levetiracetam (Keppra) are currently being used or tested for use, respectively, in AD (Wenk et al., 2006; Sanchez et al. 2012). Ril and other drugs that might enhance glutamate clearance are currently in clinical trials for use in AD, amyotrophic lateral sclerosis and multiple sclerosis; highlighting the therapeutic potential of enhancing glutamate transport in diseases characterized by neuroinflammation and neurodegeneration. Enhancing glutamate uptake within the tripartite synapse is likely to counteract the effects of neuroinflammation as well as improve cognition in AD, and will be a beneficial part of a multifactorial treatment approach to AD.
Acknowledgements
Dr. Glenn Lin for sharing control tissue for GLT1 staining. Supported by U.S. Public Health Service, RO1 AG030331, RO1 AG037320 to GLW and The Ohio State University Women and Philanthropy Program.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Akiyama H, Barger S, Barnum S, et al. Inflammation in Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardou I, DiPatrizio N, Brothers HM, Kaercher RM, Baranger K, Mitchem M, Hopp SC, Wenk GL, Marchalant Y. Pharmacological manipulation of cannabinoid neurotransmission reduces neuroinflammation associated with normal aging. Health. 2012;4:679–684. [Google Scholar]
- Barrientos RM, Frank MG, Watkins LR, Maier SF. Memory impairments in healthy aging: Role of aging-induced microglial sensitization. Aging Dis. 2010;1:212–231. [PMC free article] [PubMed] [Google Scholar]
- Begni B, Brighina L, Sirtori E, et al. Oxidative stress impairs glutamate uptake in fibroblasts from patients with Alzheimer’s disease. Free Radical Biol Med. 2004;37:892–901. doi: 10.1016/j.freeradbiomed.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Brothers HM, Marchalant Y, Wenk GL. Caffeine attenuates lipopolysaccharide-induced neuroinflammation. Neurosci Lett. 2010;480:97–100. doi: 10.1016/j.neulet.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerbai F, Lana D, Nosi D, Petkova-Kirova P, Zecchi S, Brothers H, Wenk GL, Giovannini MG. The neuron-astrocyte-microglia triad in normal brain aging and a model of neuroinflammation in the rat hippocampus. PlosOne. 2012;7:e45250. doi: 10.1371/journal.pone.0045250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Wilcock DM. Assessing Activation States in Microglia. CNS & Neurol Disord Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Fogal B, Li J, Lobner D, McCullough JD, Hewett SF. System x(c)- activity and astrocytes are necessary for interleukin-1 beta-mediated hypoxic neuronal injury. J Neurosci. 2007;27:10094–10105. doi: 10.1523/JNEUROSCI.2459-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AD, Wyttenbach A, Perry VH, Teeling JL. Age related changes in microglial phenotype vary between CNS regions: grey versus white matter differences. Brain, Behav Immun. 2012;26:754–765. doi: 10.1016/j.bbi.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Vraniak P, Wenk GL. The effects of a novel NSAID upon chronic neuroinflammation are age dependent. Neurobiol Aging. 1999;20:305–313. doi: 10.1016/s0197-4580(99)00028-7. [DOI] [PubMed] [Google Scholar]
- Jacob CP, Koutsilieri E, Bartl J, et al. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. J Alz Dis. 2007;11:97–116. doi: 10.3233/jad-2007-11113. [DOI] [PubMed] [Google Scholar]
- Kamikawa H, Hori T, Nakane H, Aou S, Tashiro N. IL-1beta increases norepinephrine level in rat frontal cortex: involvement of prostanoids, NO, and glutamate. Am J Physiol. 1998;275:R803–R810. doi: 10.1152/ajpregu.1998.275.3.R803. [DOI] [PubMed] [Google Scholar]
- Lauderback CM, Hackett JM, Juang FF, et al. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Aβ1–42. J Neurochem. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Mallory M, Alford M, Tanaka S, Masliah E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J Neuropathol Exp Neurol. 1997;56:901–911. doi: 10.1097/00005072-199708000-00008. [DOI] [PubMed] [Google Scholar]
- Mascarucci P, Perego C, Terrazzino S, De Simoni MG. Glutamate release in the nucleus tractus solitarius induced by peripheral lipopolysaccharide and interleukin-1 beta. Neurosci. 1998;86:1285–1290. doi: 10.1016/s0306-4522(98)00105-5. [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- Milton ID, Banner SJ, Ince PG, et al. Expression of the glial glutamate transporter EAAT2 in the human CNS: an immuno-histochemical study. Brain Res Molec Brain Res. 1997;52:17–31. doi: 10.1016/s0169-328x(97)00233-7. [DOI] [PubMed] [Google Scholar]
- Persson M, Brantefjord M, Hansson E, Ronnback L. Lipopolysaccharide increases microglial GLT-1 expression and glutamate uptake capacity in vitro by a mechanism dependent on TNF-alpha. Glia. 2005;51:111–120. doi: 10.1002/glia.20191. [DOI] [PubMed] [Google Scholar]
- Potier B, Billard JM, Riviere S, Sinet PM, Denis I, Champeil-Potokar G, Grintal B, Jouvenceau A, Kollen M, Dutar P. Reduction in glutamate uptake is associated with extrasynaptic NMDA and metabotropic glutamate receptor activation at the hippocampal CA1 synapse of aged rats. Aging Cell. 2010;33:722–735. doi: 10.1111/j.1474-9726.2010.00593.x. [DOI] [PubMed] [Google Scholar]
- Prow NA, Irani DN. The inflammatory cytokine, interleukin-1 beta, mediates loss of astroglial glutamate transport and drives excitotoxic motor neuron injury in the spinal cord during acute viral encephalomyelitis. J Neurochem. 2008;105:1276–1286. doi: 10.1111/j.1471-4159.2008.05230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Vazdarjanova A, Ramirez-Amaya V, Worley PF, Barnes CA, Wenk GL. Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat. Neuroscience. 2006;142(4):1303–1315. doi: 10.1016/j.neuroscience.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Hauss-Wegrzyniak B, Wenk GL. Chronic brain inflammation leads to a decline in hippocampal NMDA R1 receptors. J Neuroinflamm. 2004;1:12–18. doi: 10.1186/1742-2094-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Esperanza E, Larkin P, Fike JR, Wenk GL, Barnes CA. Accuracy of hippocampal network is disrupted by neuroinflammation: rescue by memantine. Brain. 2009;132:2464–2486. doi: 10.1093/brain/awp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci. 2005;25:723–731. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR, Devidze N, Ho K, Yu GQ, Palop JJ, Mucke L. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. PNAS. 2012;109:2895–2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Shimura H, Itaya M, et al. Excitatory amino acid transporter 2 associates with phosphorylated tau and is localized in neurofibrillary tangles of tauopathic brains. FEBS Lett. 2009;583:2194–2200. doi: 10.1016/j.febslet.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Meabon JS, Woltjer RL, Sullivan JM, Diamond JS, Cook DG. Amyloid-β1– 42 slows clearance of synaptically released glutamate by mislocalizing astrocytic GLT-1. J Neurosci. 2013;33:5312–5318. doi: 10.1523/JNEUROSCI.5274-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H, Gebhardt FM, Mitrovic AD, Vanderberg RJ, Dodd PR. Glutamate transporter variants reduce glutamate uptake in Alzheimer’s disease. Neurobiol Aging. 2011;32:553.e1–553.e11. doi: 10.1016/j.neurobiolaging.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Van Guilder HD, Bixler GV, Brucklacher RM, Farley JA, Yan H, Warrington JP, Sonntag WE, Freeman WM. Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. J Neuroinflamm. 2011;8:138. doi: 10.1186/1742-2094-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL, Parsons CG, Danysz W. Potential role of N-methyl-D-aspartate receptors as executors of neurodegeneration resulting from diverse insults: focus on memantine. Behav Pharmacol. 2006;17:411–424. doi: 10.1097/00008877-200609000-00007. [DOI] [PubMed] [Google Scholar]
- Woltjer RL, Duerson K, Fullmer JM, et al. Aberrant detergent-insoluble excitatory amino acid transporter 2 accumulates in Alzheimer disease. J Neuropathol Exp Neurol. 2010;69:667–676. doi: 10.1097/NEN.0b013e3181e24adb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Godbout JP. Immune and behavioral consequences of microglial reactivity in the aged brain. Integr Comp Biol. 2009;49:254–266. doi: 10.1093/icb/icp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizumi M, Eisenach JC, Hayashida K. Riluzole and gabapentinoids activate glutamate transporters to facilitate glutamate-induced glutamate release from cultured astrocytes. Eur J Pharmacol. 2012;677:87–92. doi: 10.1016/j.ejphar.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]