Abstract
Oxidative stress is one causative factor of the pathogenesis and aggressiveness of most of the cancer types, including prostate cancer (CaP). A moderate increase in reactive oxygen species (ROS) induces cell proliferation whereas excessive amounts of ROS promote apoptosis. In this study, we explored the pro-oxidant property of 3, 9-dihydroxy-2-prenylcoumestan [psoralidin (pso)], a dietary agent, on CaP (PC-3 and C4-2B) cells. Pso greatly induced ROS expression (more than 20-fold) that resulted in the growth inhibition of CaP cells. Overexpression of anti-oxidant enzymes superoxide dismutase 1 (SOD1), SOD2, and catalase, or pretreatment with the pharmacological inhibitor N-acetylcysteine (NAC) significantly attenuated both pso-mediated ROS generation and pso-mediated growth inhibition in CaP cells. Furthermore, pso administration significantly inhibited the migratory and invasive property of CaP cells by decreasing the transcription of β-catenin, snail, and slug, which promote epithelial mesenchymal transition (EMT), and by concurrently inducing E-cadherin expression in CaP cells. Pso-induced ROS generation in CaP cells resulted in loss of mitochondrial membrane potential, cytochrome-c release, and activation of caspase-3 and -9 and poly (ADP-ribose) polymerase (PARP), which led to apoptosis. On the other hand, overexpression of anti-oxidants rescued pso-mediated effects on CaP cells. These findings suggest that increasing the threshold of intracellular ROS could prevent or treat CaP growth and metastasis.
Keywords: Dietary agents, Invasion, Migration, Apoptosis
Introduction
Reactive oxygen species (ROS) generation activates intracellular signal transduction pathways that regulate multiple events, such as inflammation, cell cycle progression, apoptosis, migration, and invasion, in cancer [1]. ROS participate in the pathogenesis of prostate cancer (CaP) by altering the pro-survival machinery, which, in turn, initiates the progression of disease [2]. Advances in early detection and effective treatment strategies have led to decreased CaP-mediated mortality; however, CaP remains the third leading cause of cancer-related death in men [3]. Most patients with castration-resistant CaP (CRPC) are resistant to current therapeutic interventions; hence, identification of new therapeutic targets or agents that selectively target CaP cells is crucial.
Several studies on cancer treatment have focused on inhibiting ROS (by using anti-oxidants); however, the results from clinical trials were not encouraging [4]. On the other hand, excessive ROS expression triggers pro-apoptotic signaling pathways and induces apoptosis in many preclinical models of cancer [5]. Thus, the therapeutic use of pro-oxidants to target CaP cells is gaining momentum in cancer research [6]. A better preventive or therapeutic strategy is needed to overcome these differences in the redox status between cancer and normal cells [7,8] and, specifically, to increase the amount of ROS in cancer cells [9]. Compounds that are capable of inducing ROS specifically in cancer cells warrant further evaluation in the field of cancer prevention and therapy.
Emerging evidence also suggests that the epithelial-mesenchymal transition (EMT) is the first step by which cancer cells invade and metastasize to other organs. This event not only facilitates the aggressiveness of the disease but also promotes resistance to current treatments [10]. Elevated amounts of ROS contribute to several cellular migratory processes such as EMT, angiogenesis, and metastasis in different cancer types, including CaP [11]. A concomitant decrease of E-cadherin expression and increase of β-catenin expression are the hallmarks for the initial step of EMT [12]. Additionally, many reports suggest that cytoskeleton molecules such as Rho GTPase–family proteins [13], focal contact–forming proteins [14], and matrix metalloproteinase (MMPs) [15] contribute in the invasiveness of the diseases. Other effector proteins (i.e., Twist, Zeb, Snail, Slug [16] integrins [17], and MMPs [18]) alter the architecture of epithelial cell connections [19]. Hence, selectively inhibiting the EMT process results in reduced tumor invasion and metastasis, suggesting that EMT is an important therapeutic target for cancer therapy, especially in aggressive CaP [20,21].
Several natural compounds and dietary agents induce the expression and activity of anti-oxidant enzymes (e.g., glutathione transferase, quinone reductase, and phase-2 drugmetabolizing enzymes) [22,23]. In this study, we investigated one such natural compound, 3,9- dihydroxy-2-prenylcoumestan [psoralidin (pso)], for targeted prevention of or therapy for CRPC. Several groups have extensively studied phytochemicals, demonstrating that these agents have chemopreventive and chemotherapeutic properties against CaP [24,25]. Indeed, chemoprevention and chemotherapeutic intervention by phytochemicals provides new dimensions for the management of CRPC [26,27]. Reports have found that pso exerts cytotoxic effects in several cell culture models; therefore, we explored whether pso induces ROS and inhibits EMT in CRPC cells.
In the present study, we have delineated the oxidative stress property of pso by using two androgen-refractory (C4-2B: functional AR, and PC-3: AR null) CaP cell lines. Our results suggest that pso generates excessive amount of ROS that inhibit EMT and proliferation in CaP cells.
Materials and Methods
Cells and reagents
Human prostate carcinoma cell lines PC-3 and PzHPV-7 were obtained from American Type Cell Culture (ATCC, Manassas, VA) and cultured according to the guidelines of ATCC. C4-2B cells were obtained from ViroMed Laboratories (Minneapolis, MN). The MitoTracker Red and Molecular probe kits were purchased from (Life Technologies, Grand Island, NY) to track ROS production. L-N-acetylcysteine (NAC), 3-(4–5 dimethylthiazol-2-yl) was procured from Sigma-Aldrich (St. Louis, MO). Annexin-FITC kit was purchased from BD Biosciences (San Diego, CA). Psoralidin was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Mammalian expression plasmids for superoxide dismutase 1 (SOD1), SOD2, and catalase were obtained from Addgene (Cambridge, MA).
Intracellular Localization of ROS
ROS generation was determined by flow cytometric analysis using cell permeable fluorescent chemiluminescent probes 2′–7′-Dichlorodihydrofluorescein diacetate (H2DCFDA) and Cell ROX kits were used by confocal microscopy (Molecular Probes, Life Technologies, Grand Island, NY). Briefly, the CaP cells were grown on either cover slips or 12-well plates and either treated with different concentrations of pso, NAC, or both, and cellular ROS were measured.
Analysis of SOD and Catalase Activity
CaP cells were treated with pso, NAC, or both for the indicated time, were trypsinized, and were tested for SOD1, SOD2, and catalase activity by using specific kits (STA-340 and STA-341 OxiSelect Kits, Cell Biolabs, San Diego, CA) as per the manufacturer's specifications.
Invasion Assay
Invasion assay were performed and evaluated as described in details by BD Biosciences by employing Boyden chambers equipped with polyethylene terephthalate (PET) membranes with 8-µm pores. Transwell chambers containing 8-µm pores (BD Falcon) were coated with Matrigel (BD Scientific), and extracellular matrix proteins were layered on top of the transwell membrane. CaP cells were trypsinized, resuspended in incomplete media in the presence or absence of pso and NAC, and allowed to migrate in the Boyden chamber. Cells that remained in the upper chamber were removed with a cotton swab, and the Boyden chamber with PET membrane was stained with crystal violet. Migrated cells were counted using a Zeiss Axiovert 200M light microscope.
Migration Assay
PC3 cells were plated in six-well plates until confluent to study the effect of pso on tumor-cell migration. A linear wound was gently created in monolayers using a sterile pipette tip, followed by extensive washing with the growth medium to remove the cellular debris and to create a cell-free linear zone in each well. Next, the cells were treated with pso, NAC, or both; at different time points, the plates were photographed using Biostation CT (Nikon instruments Inc., Melville, NY), and the location of cells on the dish was noted. The photographs at different time points were superimposed on the first photo set to measure the migration of the cells. The percentage–wound closure was determined and was subjected to statistical analysis.
Western Blot Analysis
Total-protein extracts from control and pso- or anti-oxidant-treated CaP cells were analyzed by Western blot analysis, as described previously [28], using antibodies specific for AKT, phosphorylated AKT (pAKT), Slug, vimentin, β-catenin, proteolytically cleaved poly (ADP-ribose) polymerase (PARP), and proteolytically cleaved caspase-3, cleaved caspase 9 (Cell Signaling) and β-actin, SOD1, SOD2, catalase, BCL-2, and BAX (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Proteins were detected using the chemiluminescence method.
Quantitative Real-Time Reverse Transcription-PCR
Total RNA was isolated using the RNeasy Micro kit (Qiagen, Valencia, CA), and cDNA was synthesized by using the Applied Biosystem cDNA synthesis kit (AB Biosciences, Foster City, CA)). PCR was performed with SYBR green supermix (Qiagen, GmBH, Hilden) on the AB multicolor real-time PCR detection system (AB Biosciences). Cycle threshold values of SOD1, SOD2 and SOD3 were normalized to amplification measured for β-actin. Amplification was performed using the following cycling conditions: 95°C for 5 min, followed by 35 amplification cycles at 94°C for 30 s, and at 55°C for 30 s, with a final extension at 72°C for 7 min.
Measurement of Mitochondrial Membrane Potential, Cytochrome c Release and Caspase activation
CaP cells were either treated with pso or left untreated at indicated time points. Subsequently, the cells were permeabilized and washed with PBS, followed by incubation with the blocking solution. Mitochondria were labeled using MitoTracker Red (5 nM) (Invitrogen, Molecular Probes, Eugene, Oregon), as per the manufacturer's instructions. Permeabilized cells were then incubated with cytochrome c–specific antibody labeled with Alexa Fluor® 488, (BD Pharmingen, San Diego, CA), Pharmingen, San Diego, CA) and nuclei were stained with 4′,6-diamidino-2- phenylindole (DAPI) (Invitrogen, Eugene, Oregon) Cytochrome c, Mitotracker Red, and DAPI-mediated immunofluorescence of CaP cells were observed by confocal-laser scanning microscopy.
Apoptosis Analysis
Apoptotic assays were performed as described [28] to measure the pso-mediated effect on CaP cells. The Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences, San Diego, CA) was used for this assay.
Statistical Analysis
Unpaired T- test was adopted for statistical evaluation of the results. Significant differences were established at P < 0.05. All statistical analyses were performed in Prism Software (Graph Pad Prism 6, La Jolla, CA).
Results
Pso Induces ROS Generation and Inhibits the Growth of CaP Cells
To determine whether pso induces excessive amounts of ROS, we treated PC-3 and C4-2B cells with pso at different concentrations and at different time intervals. ROS generation was measured by flow cytometry and contrast fluorescent microscopy. Pso administration increased ROS generation at 15 minutes post treatment onwards in both PC-3 and C4-2B cells (Figure 1A). Cellular ROS expression increased in as low as 5 µM pso, and the induction was more than 50-fold as compared to control cells in both CaP cell lines (Figure 1B). Similar results were observed by confocal microscope (data not shown). In our experiments, we used H2O2 as a positive control for the detection of ROS in CaP cells (Data not shown). Pso inhibited PC-3 and C4-2B cell viability in a dose-dependent manner (Figure 1C). To determine whether pso specifically induces ROS generation in CaP cells, we used a normal prostate epithelial cell line, PzHPV-7, and found no significant induction of ROS generation, suggesting that pso may specifically target cancer cells (Figure 1D). These results suggest pso induces ROS generation, which, in turn, inhibits cell proliferation in CaP cells.
Figure 1.
Psoralidin (pso)-treated cells generate high levels of ROS. A. PC-3 and C4-2B cells were treated with pso or left untreated at different concentrations of pso for 1 hour and labeled with CM-H2DCFDA. ROS generation was measured by flow cytometery. B. Time-dependent kinetics of ROS generation after treatment of pso (20 µM). ROS levels were measured. C. Cell viability was determined by trypan blue dye exclusion assay. D. Pso-induced ROS generation were detected in PzHPV-7, PC-3, C4-2B cell lines with pso treatment (20 µM). Results are expressed as mean ± SEM of three independent experiments. Student’s t-test was used to calculate statistical significance. * Denotes P<0.05, ** denotes P<0.01, and *** denotes P<0.001.
The Role of Pso on Anti-oxidant Mechanisms in CaP Cells
In normal physiological conditions, excess ROS generation is neutralized by the endogenous anti-oxidant defense system. We sought to determine whether pso-induced ROS generation arises from inhibition of anti-oxidant enzymes such as SOD and catalase. We performed realtime PCR and Western blot analysis in CaP cells to observe whether pso alters transcriptional or translational expression of SOD and catalase. Our results suggest that no significant down-regulation of SOD1, SOD2, and catalase in pso-treated CaP cells (Figures 2A), and similar results were found in Western blot analyses for translational expression (Figure 2B). Next, we tested the ability of pso to inhibit the function of anti-oxidative enzymes. We found that SOD and catalase activities are not compromised by pso-treatment in CaP cells (Figure 2, C and D), suggesting that pso specifically induced ROS generation without altering either the anti-oxidants’ expression or the enzymes’ activation in CaP cells.
Figure 2.
Effect of pso on superoxide dismutase (SOD) and catalase activity. PC-3 and C4-2B cells were treated with pso (20 µM) and SOD1, SOD2, and catalase expression were detected by (A) real-time PCR and (B) Western blot analysis. C. SOD and (D) catalase activation were measured by calorimetric analysis. Student’s t-test was used to calculate statistical significance. * Denotes P<0.05, ** denotes P<0.01, and *** denotes P<0.001.
Overexpression of Anti-oxidants Partially Rescues Pso-Induced Anticancer Effect on CaP Cells
Next, we studied whether overexpression of anti-oxidants could block the anticancer effect of pso in CaP cells. We either transiently transfected CaP cells with SOD1-, SOD2-, or catalase-expressing plasmids or administered pharmacological agents (NAC) to examine pso’s function in the presence of anti-oxidants. Intriguingly, the pharmacological inhibitor NAC also abrogates pso-induced ROS production to 40.7 % in PC-3 cells and 46.57 % in C4-2B cells (Figure 3A), However fails to restore the ROS levels to control levels. Pso-induced cell viability was significantly abrogated by NAC both in PC-3 and in C4-2B cells (Figure 3B). As expected, pso-induced ROS production was inhibited by NAC in CaP cells (Figure 3C–D). Next these results were confirmed by transient transfection of antioxidants, SOD1, SOD2 and Catalase in CaP cells; Overexpression of SOD1 (P=0.003), SOD2 (P=0.003), or catalase (P=0.0009) in PC-3 cells significantly rescued pso-mediated growth inhibition (Figure 4, A–C). Further, we confirmed whether overexpression of transfectants suppress endogenous as well as pso-induced ROS production in PC-3 cells. As expected, the cell viability results were correlated with ROS production (Figure 4 D) and the overexpression of antioxidants (Figure 4,E–G) was confirmed by Western blot analysis. These results clearly suggest that pso exerts its anticancer effect by generating ROS and that alteration of intracellular ROS concentrations may be an effective strategy for the treatment of CaP.
Figure 3.
Effect of NAC on pso-mediated ROS generation and cell viability. A. PC-3 and C4-2B cells were either treated with pso (20 µM), NAC (10 mM), or both, and ROS levels were measured by CM-H2DCFDA using flow cytometry. B. Cell viability was assayed using trypan blue exclusion assay. C & D. Confocal microscopy images with CellROX Red fluorescence in PC-3 and C4-2B cells treated with pso or NAC or pso+NAC. Results are expressed as mean ± SEM of three independent experiments. Student’s t-test was used to calculate statistical significance. * Denotes P<0.05, ** denotes P<0.01, and *** denotes P<0.001.
Figure 4.
Overexpression of anti-oxidants SOD and catalase overcomes the ROS levels. A. PC-3 and C4-2B cells were transiently transfected either with SOD-1, SOD-2, or catalase expression plasmids and treated with pso (20 µM) for 24 h. Cell viability (A, B and C) were measured by trypan blue staining. ROS levels were determined by flowcyotometer analysis (D) and protein expressions were detected by Western blot analysis. (G) SOD1-, (H) SOD2- or (I) catalase-transfected CaP cells. Results are expressed as mean ± SEM of three independent experiments. Student’s t-test was used to calculate statistical significance. * Denotes P<0.05, ** denotes P<0.01, and *** denotes P<0.001.
Pso-Induced ROS Inhibits Migration and Invasion of CaP Cells
Induction or inhibition of ROS is known to block the invasion and migration of cancer cells [29]. To determine the anti-migratory and anti-invasive property of pso, we performed wound healing and migration assays on PC-3 and C4-2B cells. As seen in Figure 5A, untreated PC-3 cells showed an increase in migration (100%) at 24 h and pso-treated PC-3 cells exhibited 60% and C4-2B cells exhibited 25 % inhibition of migration. Pre-treatment with NAC partially rescued the pso-mediated inhibition of migration in PC-3 cells. To confirm further that ROS induction is responsible for blocking the migratory capacity of CaP cells, we either pre-treated PC-3 cells with NAC, pso, or both. Migration of CaP cells was abrogated by pso treatment, but in the presence of NAC migratory inhibition mediated by pso was mitigated (Figure 5A). Similarly, we investigated whether expression of ROS affects the invasive property of CaP cells. When CaP cells were treated with pso, invasion by CaP cells was significantly inhibited (Figure 5B). We observed PC-3 cells potently invaded through matrigel when compared to C4-2B cells and the results are consistent with published data [30–33]. Furthermore, we confirmed that pso treatment decreased the expression of slug, vimentin, and β-catenin in PC-3 cells by Western blot analysis (Figure 5C). Therefore, we analyzed the localization of E-cadherin and β-catenin expression in PC-3 cells. Confocal microscopic analysis suggests that in untreated PC-3 cells β- catenin expression was localized in the cell membrane whereas E-cadherin was scattered in cytosol (Figure 5D). After pso-treatment, E-cadherin was expressed highly on the cell membrane and in cellular junctions, whereas a decrease in β-catenin was observed in PC-3 cells (Figure 5D). These results suggest the localization of β-catenin and E-cadherin may determine the cell motility and migration capability of the cells and these cellular behaviors may not always correlate with expression of these proteins. Altogether, the results imply that pso-mediated excessive induction of ROS led to the altered expression of EMT markers, resulting in the inhibition of cell motility and of invasion activity.
Figure 5.
Pso inhibits migration and invasiveness of CaP cells. A. PC-3 and C4-2B cells were plated and a linear wound across the confluent monolayers was created and treated with pso or left untreated. At every 6 h, the cells were photographed by using Nikon, Biostation CT programmed for 24 h. Distance of the wound was measured in µM using NIS-Elements AR software (Nikon). B. Transwell invasion assay for CaP cells were carried out using Boyden chambers. The cells either treated with pso or left untreated and were allowed to migrate towards the lower chamber for 24 h. Invasive cells were stained with crystal violet and counted. C. Western blots analysis was performed to check the expression of EMT markers in CaP cells. Actin was used as loading control. D.β -Catenin and E-Cadherin expression and localization were visualized by confocal microscopy in PC-3 cells untreated and/or treated with pso.
Pso-Induced Alteration of Mitochondrial Membrane Potential and Cytochrome c Release in CaP Cells
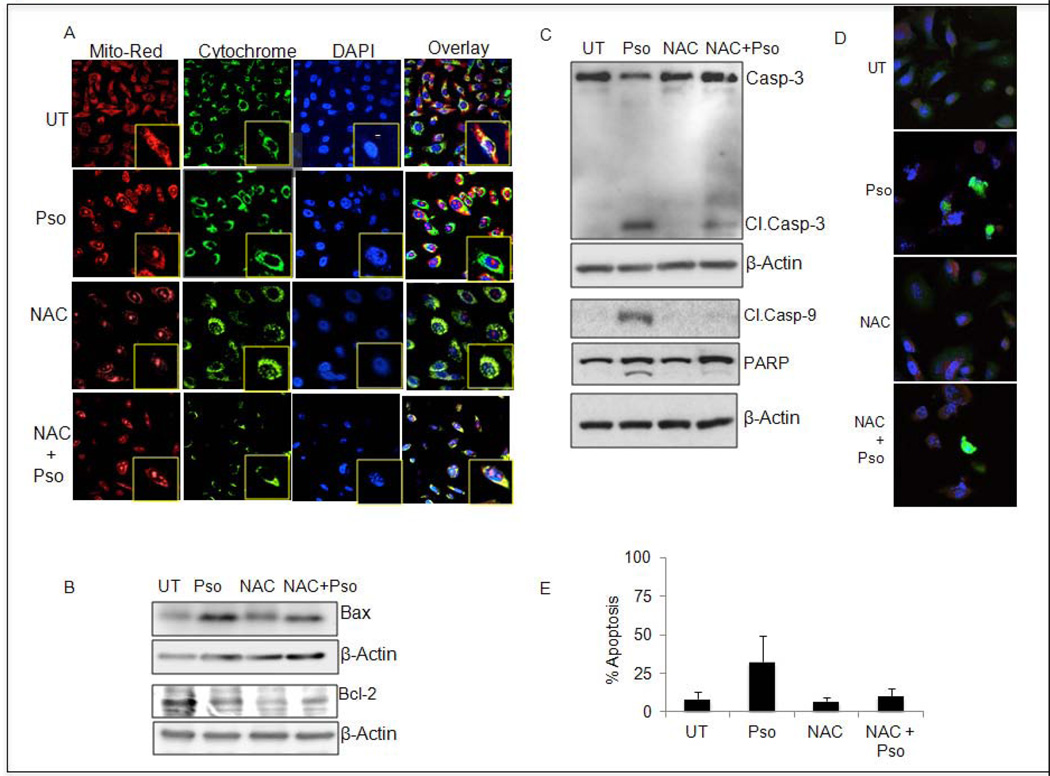
Generation of ROS by pso compelled us to further study the integrity of mitochondrial membranes by using MitoTracker Red staining in CaP cells. Notably, pso treatment showed a lessened intensity of fluorescence, suggesting a loss of mitochondrial membrane potential in CaP cells (Figure 6A). However, control or NAC-treated cells exhibited a brighter intensity of MitoTracker Red staining, representing an intact mitochondrial membrane in CaP cells (Figure 6A). As expected, combined treatment with pso and NAC reduced the intensity of brightness as compared to that observed with pso-treated CaP cells (Figure 6A). Next, we analyzed whether disruption of mitochondrial membrane potential had caused the release of cytochrome c into the cytoplasm. Immunofluorescence results revealed the presence of cytochrome c in cytosol in pso-treated cells but not in untreated or NAC-treated CaP cells (Figure 6A). This observation suggests that mitochondrial membrane potential is lost, leading to cytochrome c release from mitochondria into the cytoplasm.
Figure 6.
Pso-initiates mitochondrial membrane depolarization mediated apoptosis in CaP cells. A. PC-3 cells were treated as indicated above and incubated either with MitoTracker Orange dye or cytochrome c–specific antibody conjugated with FITC and mounted using mounting media containing DAPI. The fluorescence was visualized using a confocal microscope. B and C. Western blot was performed with indicated antibodies and reprobed with β-actin-specific antibody. D. Cleaved caspase activation was confirmed by immunofluorescence analysis. E. Induction of apoptosis was determined using Annexin V-FITC/ PI staining..Results are expressed as mean ± SEM of three independent experiments. Student’s t-test was used to calculate statistical significance. * Denotes P<0.05, ** denotes P<0.01, and *** denotes P<0.001.
The BCL-2 family proteins are implicated in the regulation of apoptosis by functioning as promoters (e.g., BAX) or inhibitors (e.g., BCL-2) of cell death. The treatment of CaP cells with pso resulted in decreased BCL-2 and increased BAX expression by Western blot analysis (Figure 6B). Pretreatment with NAC abrogates pso-mediated BAX induction and BCL-2 inhibition in PC-3 cells (Figure 6B). Next, we analyzed proteolytically cleaved caspase-9, cleaved caspase-3 and PARP in pso-treated PC-3 cells (Figure 6C). In addition, we also performed Immunofluorescence analysis to confirm caspase-3 activation in PC-3 cells (Figure 6D). Pso-induced apoptotic signaling was blocked in the presence of NAC in PC-3 cells (Figure 6E). Finally, our apoptotic assays using Annexin V-FITC/PI staining confirmed that pso-induced apoptotic signaling promoted the induction of apoptosis (82%) and NAC treatment ameliorated pso-induced apoptosis significantly in PC-3 cells (Figure 6D). These results clearly suggested that the induction of ROS triggered pro-apoptotic signaling and induced apoptosis in CaP cells.
Discussion
New therapeutic approaches are required to overcome the resistance of cancer cells to current treatments and to promote the induction of apoptosis that may lead to growth arrest of tumor cells. In this regard, many phytochemicals show promising anti-cancer responses for the prevention or therapeutic intervention of CaP [34]. Low levels of prolonged ROS generation are known to initiate cell proliferation in many cell systems, whereas at high concentrations, ROS promote apoptosis or necrosis [35]. Hence, ROS serve as critical signaling molecules either for cell proliferation or for apoptosis promotion [36]. ROS also regulate a variety of cellular processes including inflammation, cell cycle progression, and aging [1]. Oxidative stress is increased in prostate cancer samples as compared with that in non-malignant, benign prostate epithelial hyperplasia (BPH) samples [37]. Similarly, CaP cell lines have increased amounts of ROS as compared with that found in normal prostate epithelial cells [38,39]. Hence, increasing the ROS threshold slightly in CaP cells may facilitate the induction of apoptosis. In this study, we have used a natural agent, pso, which specifically increases ROS expression in CaP cells, which resulted in their growth arrest.
Our results revealed that pso induces ROS generation in androgen-independent CaP cells, leading to inhibition of cell proliferation. Our results demonstrated that pso induced ROS generation is independent of their androgen receptor (AR) status. Induction of ROS could arise from decreased efficiency of anti-oxidant molecules, such as SOD1, SOD2, and catalase in CaP cells [37,40]. Thus, we analyzed the activities of SODs or catalases, but no significant changes, either transcriptionally or transnationally, were observed in pso-treated CaP cells. In addition, either overexpression of anti-oxidant enzymes or administration of pharmacological inhibitors (NAC) reverted the pso-mediated anticancer effect, suggesting the induction of ROS could be the mechanism by which pso exerts anticancer effect against CaP cells. Indeed, no significant induction of ROS was seen in normal prostate epithelial cells (PzHPV-7). It is well established that dietary agents like green tea [41,42], resveratrol [43], garlic [44,45], and pomegranate [46,47] are non-toxic to normal cells. Our results also suggest pso may be one such compound that specifically target cancer cells and non-toxic to normal cells.
In the past few decades, scientists have focused on developing small anti-oxidant molecules as a treatment strategy for various cancers, including CaP [48]. Several dietary agents such as lycopene [49], selenium [50], vitamin E [51], genistein [52], and green tea [39] exhibit potent anti-oxidant properties and inhibit growth in preclinical models of CaP [53]. Although, the initial preclinical studies were encouraging and suggested that these molecules may decrease the prostate cancer risk, the results from recent clinical trials on anti-oxidants against prostate cancer patients have not been encouraging [54]. Therefore, we believe increasing ROS amounts in cancer cells could be an ideal and attractive strategy for prevention and treatment of CaP, especially at its terminal stage.
ROS play an integral role in promoting migration and invasion of cancer cells [55]. As stated above a moderate induction of ROS promotes EMT in cancer cells [56,57], however in our results a higher induction of ROS (more than 20 fold) inhibits EMT in CaP cells. EMT is a genetic program whereby epithelial cells lose their polarity and adopt mesenchymal properties by disrupting cell–cell contact. In clinical settings, the transition from adenocarcinoma to CaP metastasis is correlated with EMT status [58]. On the other hand, inhibiting the expression of EMT molecules has resulted in the inhibition of metastatic potential of CaP cells [18]. In our studies, transcriptional repression of β-catenin, snail, and slug and induction of E-cadherin expression were observed with pso-treatment, confirming the potential role of this agent on CaP cells [59]. Increased expression of EMT-inducing transcriptional factors and decreased expression of E-cadherin correlated with metastasis of CaP in clinical samples [60]. Hence, reversing the EMT process is an important strategy for the prevention of metastasis; pso is one such anticancer agent, inducing ROS activation and inhibiting migration and invasion by CaP cells.
We also observed that induction of ROS generation triggered apoptosis in CaP cells [61]. In general, there are two signal transduction pathways that regulate the induction of apoptosis, the intrinsic pathways involved with mitochondrial dysfunction, and the extrinsic pathway stimulated by death receptors located on the cell membrane [62]. Mitochondria are the major source of ROS generation; the reduced mitochondrial membrane potential is correlated with increased generation of ROS and apoptosis [63]. In this study, we demonstrated that pso disrupted the function of mitochondria and release of cytochrome c into the cytosol, which initiated caspase cascades in CaP cells. Many anticancer agents trigger cytochrome c release by decreasing the expression of BCL-2/BCL-xL, and simultaneously activating pro-apoptotic signaling like BAX, caspase and PARP function, or both, in cancer cells [64]. Pso-treatment resulted in an increase in pro-apoptotic BAX expression and a decrease in anti-apoptotic BCL-xL expression, which resulted in caspase and PARP activation [65].
In conclusion, our results suggest that the dietary agent pso attenuates the proliferation of CaP cells by increasing ROS expression. At molecular levels, up-regulating E-cadherin expression and concomitantly down-regulating mesenchymal markers resulted in invasion and migration of aggressive cell lines of CaP. Thus, specifically increasing ROS generation in cancer cells could be an alternative strategy for the prevention or treatment of CaP. In particular, reversing the EMT phenomena in CaP cells may suggest that similar strategies might be effectively applied to other cancers.
Acknowledgments
This study was supported 5R01AT002890-02 awarded by the NCCAM.
Abbreviations
- CaP
Prostate cancer
- ROS
Reactive oxygen species
- SOD
superoxide dismutase
- NAC
N-acetylcysteine
- H2DCFDA
2′–7′-Dichlorodihydrofluorescein diacetate
- EMT
epithelial mesenchymal transition
- EMT
epithelial mesenchymal transition;
- PARP
poly (ADP-ribose) polymerase
- CRPC
castration-resistant CaP
- MMPs
matrix metalloproteinase
- Pso
psoralidin
- AR
androgen receptor
References
- 1.Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25(4):695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 2.Lim SD, Sun C, Lambeth JD et al. Increased Nox1 and hydrogen peroxide in prostate cancer. The Prostate. 2005;62(2):200–207. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics,2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2009;301(1):52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 6.Kachadourian R, Day BJ. Flavonoid-induced glutathione depletion: potential implications for cancer treatment. Free Radic Biol Med. 2006;41(1):65–76. doi: 10.1016/j.freeradbiomed.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Husbeck B, Peehl DM, Knox SJ. Redox modulation of human prostate carcinoma cells by selenite increases radiation-induced cell killing. Free Radic Biol Med. 2005;38(1):50–57. doi: 10.1016/j.freeradbiomed.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.McKenzie S, Kyprianou N. Apoptosis evasion: the role of survival pathways in prostate cancer progression and therapeutic resistance. J Cell Biochem. 2006;97(1):18–32. doi: 10.1002/jcb.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnett P, Arnold RS, Mezencev R, Chung LW, Zayzafoon M, Odero-Marah V. Snail-mediated regulation of reactive oxygen species in ARCaP human prostate cancer cells. Biochem Biophys Res Commun. 2011;404(1):34–39. doi: 10.1016/j.bbrc.2010.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobar N, Caceres M, Santibanez JF, Smith PC, Martinez J. RAC1 activity and intracellular ROS modulate the migratory potential of MCF 7 cells through a NADPH oxidase and NFkappaB-dependent mechanism. Cancer Lett. 2008;267(1):125–132. doi: 10.1016/j.canlet.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Zhao JH, Luo Y, Jiang YG, He DL, Wu CT. Knockdown of beta-Catenin through shRNA cause a reversal of EMT and metastatic phenotypes induced by HIF 1alpha. Cancer Invest. 2011;29(6):377–382. doi: 10.3109/07357907.2010.512595. [DOI] [PubMed] [Google Scholar]
- 13.Pang X, Zhang L, Lai L, et al. 1'-Acetoxychavicol acetate suppresses angiogenesismediated human prostate tumor growth by targeting VEGF-mediated Src-FAK-Rho GTPase-signaling pathway. Carcinogenesis. 2011;32(6):904–912. doi: 10.1093/carcin/bgr052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desiniotis A, Kyprianou N. Significance of talin in cancer progression and metastasis. Int Rev Cell Mol Biol. 2011;289:117–147. doi: 10.1016/B978-0-12-386039-2.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svineng G, Ravuri C, Rikardsen O, Huseby NE, Winberg JO. The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function. Connect Tissue Res. 2008;49(3):197–202. doi: 10.1080/03008200802143166. [DOI] [PubMed] [Google Scholar]
- 16.Slabakova E, Pernicova Z, Slavickova E, Starsichova A, Kozubik A, Soucek K. TGFbeta1- induced EMT of non-transformed prostate hyperplasia cells is characterized by early induction of SNAI2/Slug. The Prostate. 2011;71(12):1332–1343. doi: 10.1002/pros.21350. [DOI] [PubMed] [Google Scholar]
- 17.van der Horst G, van den Hoogen C, Buijs JT. Targeting of alpha(v)-integrins in stem/progenitor cells and supportive microenvironment impairs bone metastasis in human prostate cancer. Neoplasia. 2011;13(6):516–525. doi: 10.1593/neo.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin CY, Tsai PH, Kandaswami CC. Matrix metalloproteinase 9 cooperates with transcription factor Snail to induce epithelial-mesenchymal transition. Cancer Sci. 2011;102(4):815–827. doi: 10.1111/j.1349-7006.2011.01861.x. [DOI] [PubMed] [Google Scholar]
- 19.Mulholland DJ, Kobayashi N, Ruscetti M, et al. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer research. 2012;72(7):1878–1889. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byles V, Zhu L, Lovaas JD, et al. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene. 2012 doi: 10.1038/onc.2011.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawata H, Shimada N, Kamiakito T, et al. RhoC and guanine nucleotide exchange factor Net1 in androgen-unresponsive mouse mammary carcinoma SC 4 cells and human prostate cancer after short-term endocrine therapy. The Prostate. 2012;72(10):1071–1079. doi: 10.1002/pros.21511. [DOI] [PubMed] [Google Scholar]
- 22.Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22(47):7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiegand H, Wagner AE, Boesch-Saadatmandi C, Kruse HP, Kulling S, Rimbach G. Effect of dietary genistein on Phase II and antioxidant enzymes in rat liver. Cancer Genomics Proteomics. 2009;6(2):85–92. [PubMed] [Google Scholar]
- 24.Choi S, Singh SV. Bax and Bak are required for apoptosis induction by sulforaphane, a cruciferous vegetable-derived cancer chemopreventive agent. Cancer research. 2005;65(5):2035–2043. doi: 10.1158/0008-5472.CAN-04-3616. [DOI] [PubMed] [Google Scholar]
- 25.Dhanalakshmi S, Agarwal C, Singh RP, Agarwal R. Silibinin up-regulates DNA-protein kinase-dependent p53 activation to enhance UVB-induced apoptosis in mouse epithelial JB6 cells. J Biol Chem. 2005;280(21):20375–20383. doi: 10.1074/jbc.M414640200. [DOI] [PubMed] [Google Scholar]
- 26.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 27.Shiota M, Yokomizo A, Naito S. Pro-survival and anti-apoptotic properties of androgen receptor signaling by oxidative stress promote treatment resistance in prostate cancer. Endocr Relat Cancer. 2012 doi: 10.1530/ERC-12-0232. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D. Par 4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer research. 2007;67(1):246–253. doi: 10.1158/0008-5472.CAN-06-2430. [DOI] [PubMed] [Google Scholar]
- 29.Thomas R, Sharifi N. SOD mimetics: a novel class of androgen receptor inhibitors that suppresses castration-resistant growth of prostate cancer. Mol Cancer Ther. 2012;11(1):87–97. doi: 10.1158/1535-7163.MCT-11-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baniwal SK, Khalid O, Gabet Y, et al. Runx2 transcriptome of prostate cancer cells: insights into invasiveness and bone metastasis. Mol Cancer. 2010;9:258. doi: 10.1186/1476-4598-9-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferguson BW, Datta S. Role of heparan sulfate 2-o-sulfotransferase in prostate cancer cell proliferation, invasion, and growth factor signaling. Prostate cancer. 2011;2011:893208. doi: 10.1155/2011/893208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yee DS, Tang Y, Li X, et al. The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition. Molecular cancer. 2010;9:162. doi: 10.1186/1476-4598-9-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabbota AL, Kim HR, Zhe X, Fridman R, Bonfil RD, Cher ML. Shedding of RANKL by tumor-associated MT1-MMP activates Src-dependent prostate cancer cell migration. Cancer research. 2010;70(13):5558–5566. doi: 10.1158/0008-5472.CAN-09-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth JA. Targeting lung cancer genes: a novel approach to cancer prevention and therapy. Semin Thorac Cardiovasc Surg. 1993;5(3):178–183. [PubMed] [Google Scholar]
- 35.Wochna A, Niemczyk E, Kurono C, et al. A possible role of oxidative stress in the switch mechanism of the cell death mode from apoptosis to necrosis--studies on rho0 cells. Mitochondrion. 2007;7(1–2):119–124. doi: 10.1016/j.mito.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aydin A, Arsova-Sarafinovska Z, Sayal A, et al. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin Biochem. 2006;39(2):176–179. doi: 10.1016/j.clinbiochem.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Chowdhury SK, Raha S, Tarnopolsky MA, Singh G. Increased expression of mitochondrial glycerophosphate dehydrogenase and antioxidant enzymes in prostate cancer cell lines/cancer. Free Radic Res. 2007;41(10):1116–1124. doi: 10.1080/10715760701579314. [DOI] [PubMed] [Google Scholar]
- 39.Kanwal R, Pandey M, Bhaskaran N, et al. Protection against oxidative DNA damage and stress in human prostate by glutathione S-transferase P1. Mol Carcinog. 2012 doi: 10.1002/mc.21939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arsova-Sarafinovska Z, Eken A, Matevska N, et al. Increased oxidative/nitrosative stress and decreased antioxidant enzyme activities in prostate cancer. Clin Biochem. 2009;42(12):1228–1235. doi: 10.1016/j.clinbiochem.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Ozten-Kandas N, Bosland MC. Chemoprevention of prostate cancer: Natural compounds, antiandrogens, and antioxidants - In vivo evidence. Journal of carcinogenesis. 2011;10:27. doi: 10.4103/1477-3163.90438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connors SK, Chornokur G, Kumar NB. New insights into the mechanisms of green tea catechins in the chemoprevention of prostate cancer. Nutrition and cancer. 2012;64(1):4–22. doi: 10.1080/01635581.2012.630158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klink JC, Tewari AK, Masko EM, et al. Resveratrol worsens survival in SCID mice with prostate cancer xenografts in a cell-line specific manner, through paradoxical effects on oncogenic pathways. The Prostate. 2012 doi: 10.1002/pros.22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao D, Herman-Antosiewicz A, Antosiewicz J, et al. Diallyl trisulfide-induced G(2)-M phase cell cycle arrest in human prostate cancer cells is caused by reactive oxygen species-dependent destruction and hyperphosphorylation of Cdc 25 C. Oncogene. 2005;24(41):6256–6268. doi: 10.1038/sj.onc.1208759. [DOI] [PubMed] [Google Scholar]
- 45.Antosiewicz J, Herman-Antosiewicz A, Marynowski SW, Singh SV. c-Jun NH(2)-terminal kinase signaling axis regulates diallyl trisulfide-induced generation of reactive oxygen species and cell cycle arrest in human prostate cancer cells. Cancer research. 2006;66(10):5379–5386. doi: 10.1158/0008-5472.CAN-06-0356. [DOI] [PubMed] [Google Scholar]
- 46.Adhami VM, Khan N, Mukhtar H. Cancer chemoprevention by pomegranate: laboratory and clinical evidence. Nutrition and cancer. 2009;61(6):811–815. doi: 10.1080/01635580903285064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koyama S, Cobb LJ, Mehta HH, et al. Pomegranate extract induces apoptosis in human prostate cancer cells by modulation of the IGF-IGFBP axis. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society. 2010;20(1):55–62. doi: 10.1016/j.ghir.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung KA, Kwak MK. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15(10):7266–7291. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ford NA, Elsen AC, Zuniga K, Lindshield BL, Erdman JW., Jr Lycopene and apo 12'- lycopenal reduce cell proliferation and alter cell cycle progression in human prostate cancer cells. Nutrition and cancer. 2011;63(2):256–263. doi: 10.1080/01635581.2011.523494. [DOI] [PubMed] [Google Scholar]
- 50.Richman EL, Chan JM. Selenium and prostate cancer: the puzzle isn't finished yet. Am J Clin Nutr. 2012;96(1):1–2. doi: 10.3945/ajcn.112.042119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walsh PC. Re: vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Urol. 2012;187(5):1640–1641. doi: 10.1016/j.juro.2012.01.108. [DOI] [PubMed] [Google Scholar]
- 52.Ullah MF, Ahmad A, Zubair H, et al. Soy isoflavone genistein induces cell death in breast cancer cells through mobilization of endogenous copper ions and generation of reactive oxygen species. Mol Nutr Food Res. 2011;55(4):553–559. doi: 10.1002/mnfr.201000329. [DOI] [PubMed] [Google Scholar]
- 53.Davalli P, Rizzi F, Caporali A, et al. Anticancer activity of green tea polyphenols in prostate gland. Oxid Med Cell Longev. 2012;2012:984219. doi: 10.1155/2012/984219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akaza H, Ikemoto I, Namiki M, et al. Efficacy of S 1 in patients with castration-resistant prostate cancer: a phase II study. Oncology. 2010;78(5–6):323–328. doi: 10.1159/000319939. [DOI] [PubMed] [Google Scholar]
- 55.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen HL, Zucker S, Zarrabi K, Kadam P, Schmidt C, Cao J. Oxidative stress and prostate cancer progression are elicited by membrane-type 1 matrix metalloproteinase. Mol Cancer Res. 2011;9(10):1305–1318. doi: 10.1158/1541-7786.MCR-11-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer research. 2008;68(6):1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 58.Mehta HH, Gao Q, Galet C, et al. IGFBP 3 is a metastasis suppression gene in prostate cancer. Cancer research. 2011;71(15):5154–5163. doi: 10.1158/0008-5472.CAN-10-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jaggi M, Johansson SL, Baker JJ, Smith LM, Galich A, Balaji KC. Aberrant expression of E-cadherin and beta-catenin in human prostate cancer. Urol Oncol. 2005;23(6):402–406. doi: 10.1016/j.urolonc.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 60.Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin 1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4(6):499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 61.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5(5):415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 62.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87(1):99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 63.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95(10):957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 64.Tang Y, Parmakhtiar B, Simoneau AR. Lycopene enhances docetaxel's effect in castration-resistant prostate cancer associated with insulin-like growth factor I receptor levels. Neoplasia. 2011;13(2):108–119. doi: 10.1593/neo.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu J, Shen HM, Ong CN. Role of intracellular thiol depletion, mitochondrial dysfunction and reactive oxygen species in Salvia miltiorrhiza-induced apoptosis in human hepatoma HepG2 cells. Life Sci. 2001;69(16):1833–1850. doi: 10.1016/s0024-3205(01)01267-x. [DOI] [PubMed] [Google Scholar]