Abstract
Background
Few population-based studies have examined utilization of BRCA 1/2 testing or patterns of physician recommendations for genetic testing among women diagnosed with breast cancer. The objective of the current study was to evaluate the rates and predictors of physician recommendation for BRCA 1/2 testing among breast cancer patients.
Methods
Women aged 18–64 years diagnosed with invasive breast cancer in 2007 were identified from the Pennsylvania State Cancer Registry and mailed a survey on family history of cancer, physician treatment recommendations, and BRCA 1/2 testing. Of the 4009 women who were sent surveys, 2258 responded (56%). Based on age at diagnosis and family history, women were categorized as high, moderate, or low-risk for BRCA 1/2 mutations.
Results
Nearly 25% of participants were at high risk for carrying a BRCA 1/2 mutation based on age at breast cancer diagnosis and family history of breast and/or ovarian cancer. Physician recommendations for BRCA 1/2 testing were strongly associated with risk of carrying a mutation, with 53% of high-risk women reporting a testing recommendation compared to 9% of low-risk women. In addition, physician recommendations were strongly correlated with use of testing in all risk groups. Among high-risk women, lack of a recommendation for BRCA 1/2 testing was more common among older, low income, and employed women.
Conclusions
Although BRCA 1/2 testing recommendations appear to be appropriately correlated with mutation risk, a significant proportion of breast cancer patients who meet criteria for BRCA 1/2 testing may not receive recommendations for such testing from their providers.
Introduction
Testing for mutations in BRCA1 and BRCA2 can be useful among women with a breast cancer diagnosis. Being found to carry a mutation may have treatment implications, such as eligibility for contralateral mastectomy, prophylactic oophorectomy, and experimental therapeutic agents such as PARP inhibitors. In addition, BRCA 1/2 testing can provide information for familial risk assessment. If a woman with cancer is found to have a mutation, her relatives may undergo testing for that mutation and make cancer risk reduction decisions based upon those test results.1–3 The risk of carrying a BRCA 1/2 mutation is approximately 5–10% among women diagnosed with breast cancer, and mutation risk is higher for women with early onset disease or a family history of breast and/or ovarian cancer.4, 5 Clinical guidelines suggest that breast cancer patients should receive personalized risk assessment and consider genetic counseling and testing if they have early onset disease (Age ≤45), bilateral breast cancer, triple negative disease (ER-/PR-/HER2-), Ashkenazi Jewish ancestry, a strong family history of breast and/or ovarian cancer or a combination of these characteristics.6
Currently, relatively little is known about the use of BRCA 1/2 testing among women with a breast cancer diagnosis. Although breast cancer patients are more likely to undergo genetic testing than women without breast cancer, studies suggested that rates of BRCA 1/2 testing among breast cancer patients are relatively low.7–13 Two surveys of convenience samples of breast cancer survivors found that less than 15% reported undergoing testing, with higher rates of testing among women with a family history of breast cancer, younger age at diagnosis, or Jewish ancestry.14, 15
Like breast cancer treatment decisions, the use of BRCA 1/2 testing among breast cancer patients is likely influenced by both patient preferences and the recommendations of their health care providers.15–18 Although there has been a growing interest in the use of BRCA 1/2 testing at the time of diagnosis among surgeons and oncologists, the degree to which these providers are recommending BRCA 1/2 testing, particularly to patients at high risk of carrying a mutation, is currently unknown. Thus, we conducted a retrospective cohort study of breast cancer patients in the state of Pennsylvania to examine provider recommendations for genetic testing, receipt of BRCA 1/2 testing, and factors associated with not receiving a recommendation for testing among women at high risk for carrying a mutation.
Patients and Methods
Study design & Participants
Study participants were identified through the Pennsylvania State Cancer Registry (PCR), which has achieved NAACCR Gold certification for the accuracy and completeness of data. The institutional review boards of the University of Pennsylvania and the PCR approved the study protocol. Women diagnosed with invasive breast cancer at age 18–64 in Pennsylvania between January 1 and December 31, 2007 (N=4920) were mailed an introductory letter explaining the study, followed by a second mailing with a consent form, study questionnaire, prepaid return envelope, and an unconditional incentive of five dollars. Non-respondents were sent two additional mailings. Women were excluded if they were deceased (N=252), had invalid addresses (N=645), or were otherwise ineligible (reported not having cancer and/or not able to read/speak English N=14). Of the 4009 women eligible for the study with valid addresses, 2258 women returned the questionnaire (56%).
Data collection
The study questionnaire elicited socio-demographic characteristics, detailed family history of breast and ovarian cancer, and tumor characteristics. Women were asked to list their treatment recommendations, including whether BRACA Analysis® or BRCA 1/2 testing was recommended. Women were asked about recommendations for genetic testing rather than genetic counseling, based upon prior work and pilot studies indicating that women reported referral for counseling as referral for testing and were confused when asked about recommendation for genetic counseling. Women were also asked if they had undergone BRCA 1/2 testing, and the approximate date of the test. Because of privacy concerns given the mailed questionnaire, results of genetic testing were not ascertained. Participants’ responses were linked to tumor characteristics from the PCR.
Women were asked whether their provider recommended BRCA 1/2 testing, and possible responses included “no”, “yes”, or “don’t know.” Ten percent of respondents indicated “don’t know,” and 37% of respondents left this item unanswered. We combined those who responded “no”, “don’t know”, and non-responses into a “no recommendation” group for the main analysis. Sensitivity analyses limiting the study population to those who answered “yes” or “no” to testing recommendations were performed. Women were categorized into three groups based on contact with a medical oncologist and treatment: saw an oncologist and received chemotherapy, saw an oncologist and did not receive chemotherapy, did not see an oncologist and did not receive chemotherapy. Based on cancer registry data, women were categorized as estrogen receptor (ER) or progesterone receptor (PR) positive, negative, or unknown. Because collection of HER2/neu status was not required by the PCR until 2010, self-reported HER2/neu status was used in this analysis.
BRCA 1/2 mutation risk categories
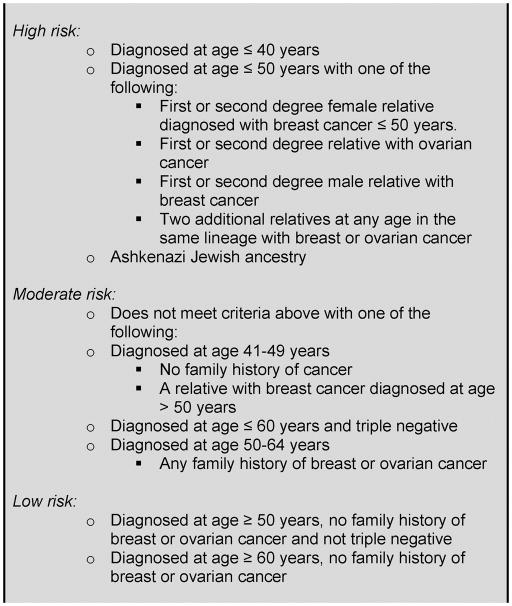
Established guidelines and other data regarding mutation prevalence4, 6, 19 were used to categorize patients into three levels of risk for BRCA 1/2 mutations based upon their age at diagnosis and family history (Figure 1). We were conservative in our definition of high-risk so as to capture women who would have been clear candidates for BRCA 1/2 testing in 2007, as well as anticipated to have insurance coverage for genetic testing and therefore should have received a recommendation from their provider. High-risk women were those diagnosed with breast cancer at age 40 or younger, women with Ashkenazi Jewish heritage, or women diagnosed at age 50 or younger who met one of the following criteria: first or second degree female relative diagnosed with breast cancer at age ≤50, first or second degree relative diagnosed with ovarian cancer, first or second degree male relative with breast cancer, or two relatives on the same side of the family diagnosed with breast or ovarian cancer. Moderate risk women were those age 41–49 not meeting the high risk criteria, women diagnosed at 50 or older with a family history of breast or ovarian cancer, and women younger than 60 with triple negative disease. Low risk women were those diagnosed 50 and older who did not have triple negative disease with no family history, or women aged 60–64 with no family history regardless of tumor biology.
Figure 1.
Categories of risk for BRCA 1/2 mutation
Statistical analysis
Patient characteristics, rates of testing recommendations, and rates of testing were compared across risk groups using t-tests and chi square tests. Agreement between recommendations and BRCA 1/2 testing and between physician recommendations was assessed using kappa statistics. Differences in the proportion of women with recommendations for BRCA1/2 testing who reporting undergoing testing were compared using chi square tests. Multivariable logistic regression was performed to estimate the odds of having a provider recommendation for BRCA1/2 testing by various patient and tumor characteristics. In addition, among high risk women we estimated the odds of lacking test recommendation by patient and tumor characteristics. All statistical tests were two-sided with alpha of 0.05.
Results
Of the 4009 eligible women with valid addresses, 2258 women returned the questionnaire (56%). The mean age of respondents (52.1 years) was the same as that of the full PA registry population of women diagnosed with breast cancer before age 65 (Table 1). Respondents were slightly more likely to be white, have ER/PR positive disease, and earlier stage at diagnosis than the full registry population.
Table 1.
Comparison of Characteristics of Women Aged 18–64 Diagnosed with Invasive Breast Cancer from the Pennsylvania Cancer Registry in 2007 and Survey Respondents
| 2007 Registry Population (N=4920) | Respondents (N=2258) | |
|---|---|---|
| Age at diagnosis (Mean ± SD) | 52.1 ± 8.2 | 52.1 ± 8.1 |
| ER/PR status, N (%) | ||
| Positive | 3728 (76) | 1779 (79)* |
| Negative | 940 (19) | 411 (18) |
| Unknown | 252 (5) | 67 (3) |
| Stage at Diagnosis, N (%) | ||
| Local | 3005 (61) | 1460 (65)* |
| Regional | 1591 (32) | 719 (32) |
| Distant | 225 (5) | 50 (2) |
| Unstaged/Unknown | 96 (2) | 29 (1) |
| Race | ||
| White | 4273 (87) | 2026 (90)* |
| Black | 494 (10) | 145 (6) |
| Hispanic | 82 (2) | 34 (2) |
| Other/Unknown | 68 (1) | 31 (1) |
p<0.05, one sample t-test
Demographic and tumor characteristics and report of BRCA 1/2 test recommendations for the 2258 respondents are listed in Table 2. Ninety percent of participants reported having health insurance, 95% identified having a medical oncologist, and 97% identified their surgeon (data not shown). Most women were diagnosed with localized disease (65%) and received chemotherapy (61%). Ninety percent of respondents were white, over 60% reported post-secondary education, and 38% reported annual household income greater than $70,000.
Table 2.
Characteristics of Survey Respondents Aged 18–65 Diagnosed with Invasive Breast Cancer in Pennsylvania in 2007, stratified by risk of BRCA 1/2 mutation (N=2258)
| Total (N=2258) | High risk (N=546) | Moderate risk (N=852) | Low risk (N=860) | p-value* | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||
| Age at Diagnosis | |||||||||
| 40 and under | 203 | 9% | 203 | 37% | --- | --- | --- | --- | <0.001 |
| 41 to 50 | 696 | 31% | 264 | 48% | 379 | 44% | 53 | 6% | |
| 51 to 60 | 973 | 43% | 47 | 9% | 349 | 41% | 577 | 68% | |
| 61 to 64 | 386 | 17% | 32 | 6% | 132 | 15% | 222 | 26% | |
|
| |||||||||
| ER/PR positive | 1780 | 79% | 417 | 76% | 648 | 75% | 715 | 84% | <0.001 |
|
| |||||||||
| HER2/neu positive | 387 | 17% | 107 | 20% | 140 | 16% | 140 | 16% | <0.001 |
|
| |||||||||
| Stage at Diagnosis | |||||||||
| Local | 1460 | 65% | 329 | 60% | 587 | 60% | 544 | 64% | 0.051 |
| Regional | 719 | 32% | 198 | 36% | 246 | 29% | 275 | 32% | |
| Distant | 50 | 2% | 13 | 2% | 14 | 2% | 23 | 3% | |
| Unstaged/Unknown | 29 | 1% | 6 | 1% | 13 | 2% | 10 | 1% | |
|
| |||||||||
| Medical oncologist care | |||||||||
| Saw an oncologist and received chemotherapy | 1383 | 61% | 406 | 74% | 529 | 62% | 448 | 53% | <0.001 |
| Saw an oncologist, did not receive chemotherapy | 753 | 33% | 125 | 23% | 285 | 33% | 343 | 40% | |
| Did not see an oncologist, did not receive chemotherapy | 122 | 5% | 15 | 3% | 46 | 5% | 61 | 7% | |
|
| |||||||||
| White | 2026 | 90% | 497 | 91% | 779 | 91% | 771 | 90% | 0.975 |
|
| |||||||||
| Employed | 1243 | 55% | 358 | 66% | 501 | 58% | 384 | 45% | <0.001 |
|
| |||||||||
| Jewish | 134 | 6% | 122 | 22% | 9 | 1% | 3 | 0% | <0.001 |
|
| |||||||||
| Married | 1560 | 69% | 397 | 73% | 580 | 67% | 583 | 68% | 0.099 |
|
| |||||||||
| Educational attainment | |||||||||
| High School or Less | 813 | 36% | 130 | 24% | 342 | 40% | 341 | 40% | <0.001 |
| College (2 or 4 year) | 960 | 43% | 249 | 46% | 373 | 43% | 338 | 40% | |
| Graduate School | 464 | 21% | 164 | 30% | 139 | 16% | 161 | 19% | |
| Missing | 21 | 1% | 3 | 1% | 6 | 1% | 12 | 1% | |
|
| |||||||||
| Annual household income | |||||||||
| <$ 30,000 | 468 | 21% | 83 | 15% | 182 | 21% | 203 | 24% | <0.001 |
| $ 30 001 to $ 70 000 | 776 | 34% | 177 | 32% | 318 | 37% | 281 | 33% | |
| > $ 70 000 | 867 | 38% | 262 | 48% | 316 | 37% | 289 | 34% | |
| Missing | 147 | 7% | 24 | 4% | 44 | 5% | 79 | 9% | |
|
| |||||||||
| Recommendation for BRCA 1/2 testing | |||||||||
| No Recommendation | 1673 | 74% | 255 | 47% | 643 | 75% | 775 | 91% | <0.001 |
| Total Recommended | 585 | 26% | 291 | 53% | 217 | 25% | 77 | 9% | |
| Medical oncologist only† | 186 | 32% | 86 | 30% | 71 | 33% | 29 | 38% | |
| Surgeon only† | 134 | 23% | 64 | 22% | 50 | 23% | 20 | 26% | |
| Both providers† | 265 | 45% | 141 | 49% | 96 | 44% | 28 | 36% | |
|
| |||||||||
| Underwent BRCA 1/2 Testing | 587 | 26% | 290 | 53% | 214 | 25% | 83 | 10% | <0.001 |
p-value from chi square test comparing risk groups,
Percentage of total recommended
In total, 26% of patients reported a recommendation for genetic testing, and a similar percentage reported undergoing BRCA 1/2 testing. We validated self-report of BRCA 1/2 testing by reviewing medical records of the 55 respondents who received breast cancer treatment at the University of Pennsylvania, of whom 40 reported BRCA 1/2 testing and 15 reported no testing. BRCA 1/2 testing was confirmed in 37 out of 40 women who reported testing (Positive Predictive Value=93%). Evidence of BRCA 1/2 testing was found in one of the 15 women who reported not being tested (Negative Predictive Value =93%).
Of the 2258 respondents, 24% met our definition of high-risk for carrying BRCA 1/2 mutation (Table 2, N=546), 38% were moderate risk (N=860), and 38% were low risk (N=852). We chose a conservative high risk definition in order to identify breast cancer patients who should have been clear candidates for genetic testing based on 2007 guidelines. In addition to the criteria for high risk listed in Figure 1, current National Comprehensive Cancer Network criteria for BRCA 1/2 testing include all women diagnosed at age ≤45, women age ≤60 with triple negative disease, and women with two or more affected relatives in the same lineage regardless of age at diagnosis.6 When these three additional guidelines were applied, 43% of the study population met these testing guidelines.
Women in the high risk group were younger (by definition), had higher education and household income and were more likely to see an oncologist and receive chemotherapy than women in the moderate or low risk groups (p<0.001). High-risk women were less likely to have ER/PR positive disease and more likely to have HER2/neu positive disease than women in the low risk group (p<0.001). Fifty-three percent of high risk women, 25% of moderate risk women, and 9% of low risk women reported receiving a recommendation for BRCA 1/2 testing from a provider. Among high risk women who received a recommendation, 30% reported recommendation from a medical oncologist only, 22% reported a recommendation from a surgeon only, and 49% reported a recommendation from both providers. There was fair agreement between medical oncologists’ and surgeons’ recommendations for BRCA 1/2 testing among high risk women (72% agreement, Kappa=0.43), and the characteristics of patients referred by each type of provider were similar (data not shown).
Provider recommendations and undergoing BRCA 1/2 testing were strongly correlated (91% agreement, kappa = 0.77). The percentage of women who underwent BRCA 1/2 testing by testing recommendation is shown in Table 3. A high percentage of women who reported a provider recommendation underwent testing (83%), particularly among the high risk group (89%), and few women without a provider recommendation reporting receiving BRCA 1/2 testing (6%), regardless of risk group (3–12%). Very few women reported BRCA 1/2 testing prior to 2007 (<1%, data not shown), and therefore the majority of BRCA 1/2 tests occurred after breast cancer diagnosis.
Table 3.
Proportion of Women with Breast Cancer Undergoing BRCA 1/2 Testing by Provider Recommendation (N=2258)
| Testing Recommendation | Percent Reporting BRCA 1/2 testing | p-value* | |||
|---|---|---|---|---|---|
| Total | High risk | Moderate Risk | Low Risk | ||
| No Recommendation | 6% | 12% | 7% | 3% | <0.001 |
| Any Recommendation | 83% | 89% | 77% | 75% | <0.001 |
p-value from chi square test of percent reporting BRCA 1/2 test by risk groups
After adjusting for age at diagnosis, tumor characteristics, demographics, and socioeconomic factors (Table 4), receiving a recommendation for BRCA 1/2 testing was strongly associated with risk category; women at high risk had six times the odds (OR=5.84, 95% CI 4.03–8.46, p<0.001) and women at moderate risk had three times the odds (OR=2.98 95% CI 2.19–4.04, p<0.001) of a recommendation compared to low risk women. Receiving a BRCA 1/2 test recommendation was inversely associated with age at diagnosis (p<0.001) and was less common among women who did not see an oncologist or receive chemotherapy (OR=0.48, 95% CI 0.25–0.91, p=0.018). Education and annual household income were strongly associated with test recommendation. (OR=1.75 95% CI 1.21–2.54, p=0.007 for income >$70,000 compared to <$30,000 and OR=1.54, 95% CI 1.19–1.99, p=0.001 for any college vs. high school or less).
Table 4.
Predictors of Recommendation for BRCA 1/2 testing, N=2258*
| Any BRCA 1/2 test recommendation | |||
|---|---|---|---|
| OR | 95% CI | p-value | |
| Risk Group | |||
| Low Risk | 1.00 | Reference | |
| Moderate Risk | 2.98 | 2.19–4.04 | <0.001 |
| High Risk | 5.84 | 4.03–8.46 | <0.001 |
|
| |||
| Age at Diagnosis | |||
| 40 and under | 1.00 | Reference | |
| 41 to 50 | 0.41 | 0.28–0.60 | <0.001 |
| 51 to 60 | 0.30 | 0.19–0.47 | <0.001 |
| 61 to 64 | 0.29 | 0.17–0.47 | <0.001 |
|
| |||
| ER/PR status | |||
| ER/PR Positive/Unknown | 1.00 | Reference | |
| ER/PR Negative | 1.09 | 0.83–1.43 | 0.525 |
|
| |||
| HER2 status | |||
| HER2 Negative/Unknown | 1.00 | Reference | |
| HER2 Positive | 1.13 | 0.86–1.49 | 0.372 |
|
| |||
| Medical Oncologist Care | |||
| Saw oncologist, received chemotherapy | 1.00 | Reference | |
| Saw oncologist, did not receive chemotherapy | 0.88 | 0.68–1.15 | 0.145 |
| Did not see oncologist, did not receive chemotherapy | 0.48 | 0.25–0.91 | 0.018 |
|
| |||
| Race | |||
| White | 1.00 | Reference | |
| Non-white | 0.81 | 0.55–1.19 | 0.375 |
|
| |||
| Employment status | |||
| Not employed | 1.00 | Reference | |
| Employed | 0.87 | 0.69–1.10 | 0.264 |
|
| |||
| Education | |||
| High School or less | 1.00 | Reference | |
| College (2–4 year) | 1.54 | 1.19–1.99 | 0.001 |
| Graduate School | 1.58 | 1.15–2.18 | 0.005 |
| Missing | 2.08 | 0.63–6.84 | 0.229 |
|
| |||
| Annual Household Income | |||
| <$30,000 | 1.00 | Reference | |
| $30,001–70,000 | 1.56 | 1.10–2.20 | 0.018 |
| >$70,000 | 1.75 | 1.21–2.54 | 0.007 |
| Missing | 1.33 | 0.76–2.30 | 0.288 |
Logistic regression adjusted for all factors in the model, stage at diagnosis, and marital status
Among high risk women, we examined the risk factors for not receiving a BRCA 1/2 test recommendation from a provider (Table 5). Age at diagnosis was the strongest predictor of not receiving a recommendation for testing. Compared to women age 40 and younger, women who were age 41–50 at diagnosis had over twice the odds of no test recommendation (OR=2.20, 95% CI 1.47–3.30, p<0.001), and women 51 to 64 had over five times the odds of no test recommendation (OR=5.55, 95% CI 2.40–12.8, p<0.001). Low income women (<$30,000) were more than twice the odds of lacking test recommendation as women in the highest income group (OR=2.36, 95% CI 1.24–4.51, p=0.009). In addition, women who were employed were more likely to lack testing recommendation compared to unemployed women (OR=1.57, 95% CI 1.04–2.36, p=0.031). We repeated the logistic regression analyses among women who definitively answered “yes” or “no” as to whether their physician had recommended BRCA 1/2 testing, excluding those who answered “don’t know” or did not respond, and the results were similar to the full analyses.
Table 5.
Predictors of Lack of Recommendation for BRCA 1/2 testing among High Risk Women (N=546)
|
BRCA 1/2 test recommendation
|
Lack of BRCA 1/2 test Recommendation* | ||||||
|---|---|---|---|---|---|---|---|
| No N=255 |
Yes N=291 |
||||||
|
| |||||||
| N | % | N | % | Odds Ratio | 95% CI | p-value | |
| Age at Diagnosis | |||||||
| 40 and under | 66 | 26 | 137 | 47 | 1.00 | Reference | |
| 41 to 50 | 134 | 53 | 130 | 45 | 2.20 | 1.47–3.30 | <0.001 |
| 51 to 64 | 55 | 22 | 24 | 8 | 5.55 | 2.40–12.8 | <0.001 |
|
| |||||||
| ER or PR Positive | |||||||
| ER/PR Positive/Unknown | 208 | 82 | 225 | 77 | 1.00 | Reference | |
| ER/PR Negative | 47 | 18 | 66 | 23 | 0.83 | 0.52–1.32 | 0.431 |
|
| |||||||
| HER2 positive | |||||||
| HER2 Negative/Unknown | 207 | 81 | 232 | 80 | 1.00 | Reference | |
| HER2 Positive | 48 | 19 | 59 | 20 | 1.12 | 0.70–1.78 | 0.644 |
|
| |||||||
| Medical oncologist care | |||||||
| Saw an oncologist and received chemotherapy | 175 | 69 | 231 | 79 | 1.00 | Reference | |
| Saw an oncologist, did not receive chemotherapy | 69 | 13 | 56 | 19 | 1.24 | 0.77–1.99 | 0.381 |
| Did not see an oncologist, did not receive chemotherapy | 11 | 4 | 4 | 1 | --- | --- | --- |
|
| |||||||
| Race | |||||||
| White | 228 | 89 | 262 | 90 | 1.00 | Reference | |
| Non-White | 27 | 11 | 29 | 10 | 1.37 | 0.74–2.53 | 0.320 |
|
| |||||||
| Employed | |||||||
| Not employed | 84 | 33 | 104 | 36 | 1.00 | Reference | |
| Employed | 171 | 67 | 187 | 64 | 1.57 | 1.04–2.36 | 0.031 |
|
| |||||||
| Educational attainment | |||||||
| High School or Less | 73 | 29 | 57 | 20 | 1.00 | Reference | |
| College (2 or 4 year) | 113 | 44 | 136 | 47 | 0.71 | 0.45–1.13 | 0.150 |
| Graduate School | 68 | 27 | 96 | 33 | 0.59 | 0.33–1.04 | 0.069 |
| Missing | 1 | 0 | 2 | 1 | --- | --- | --- |
|
| |||||||
| Jewish Ancestry | |||||||
| No | 183 | 72 | 241 | 83 | 1.00 | Reference | |
| Yes | 72 | 28 | 50 | 17 | 1.10 | 0.57–2.11 | 0.785 |
|
| |||||||
| Annual household income | |||||||
| >$ 70,000 | 107 | 42 | 155 | 53 | 1.00 | Reference | |
| $30,001 to $ 70,000 | 88 | 35 | 89 | 31 | 1.51 | 0.96–2.36 | 0.073 |
| <$30,000 | 49 | 19 | 34 | 12 | 2.36 | 1.24–4.51 | 0.009 |
| Missing | 11 | 4 | 13 | 4 | 1.11 | 0.44–2.75 | 0.828 |
Logistic regression, odds of lack of recommendation for BRCA 1/2 testing, adjusted for all factors in the table, stage at diagnosis and marital status
Discussion
To our knowledge, this is the first population based study to examine the rates of physician recommendation for BRCA 1/2 testing in breast cancer patients. Our results confirm that physician recommendations are a critical determinant of the use of genetic testing among all risk groups; few patients received genetic testing without first receiving a physician recommendation. Testing recommendations among breast cancer patients appear to be largely and appropriately driven by patient risk factors for carrying a mutation (i.e. risk group), with 53% of high risk women reporting a physician recommendation for genetic testing compared to 9% of women at low risk of carrying a mutation. Nearly 25% of breast cancer patients diagnosed before age 65 met our conservative definition of high mutation risk, and over 40% met current NCCN guidelines.6 While testing guidelines vary somewhat across professional organizations and change over time, patients categorized as high-risk in our study should have been clear candidates for referral to genetic counseling and testing at the time of breast cancer diagnosis. Our results suggest that a significant proportion of women with high risk of carrying a BRCA1/2 mutation may not receive a testing recommendation from their provider. In addition to risk group, factors independently associated with testing recommendation included age at diagnosis, ER/PR status, and higher socioeconomic status.
Among high risk women, risk factors for failure to receive a testing recommendation included relatively late age at diagnosis, lower household income, and employment outside of the home. While it is appropriate that early age at diagnosis was used to target genetic testing recommendations among cancer patients, these results suggest that some strong candidates for genetic testing may be more likely to be overlooked by providers if they are older. This underscores the importance of detailed family history assessment for breast cancer patients regardless of age at diagnosis. The strong association of household income with testing recommendation among high risk women was surprising given that the vast majority of our study population reported having health insurance, and most health insurers cover BRCA 1/2 testing in high risk populations. However, coverage criteria and copays may continue to represent substantial barriers to testing among patients with financial concerns or inadequate insurance coverage, or may be perceived as creating such barriers by providers.20 In addition, among women in the high risk group, those who were employed were more likely to lack recommendation for testing. This could be due to concerns about discrimination or loss of insurance coverage21 given that women in the study were diagnosed with cancer prior to passage of the Genetic Information Nondiscrimination Act (GINA) in 2008,22 or due to time constraints limiting women’s ability to participate in pre-test genetic counseling.
Providers appear to be successful at limiting testing among women at low risk of carrying a mutation. The small number of women who received a recommendation for testing in the low risk group (9%) limited our ability to identify the determinants of testing recommendations in that group. However, in studies that included women with and without breast cancer, physician recommendation for testing was associated with family history, age at diagnosis, education, and income, which have been previously identified as predictors of both BRCA 1/2 test recommendation15 and uptake of genetic testing9, 11, 12, 14, 23. Minority race was not significantly associated with testing recommendation in this sample; however the small number of minority women limited the power to identify an association.
The main strength of our study is the population-based design with recruitment from the PCR, which provided a large sample of women with cancer diagnosed prior to age 65. Many studies of genetic testing have relied on convenience samples or patients referred to genetic testing clinics, and such sampling techniques are subject to bias.7, 11, 23–25 Recruiting from the cancer registry provides a well-defined cohort and allows us to better assess the generalizability of our results. In addition, our study elicited detailed information on family history, allowing us to categorize individuals’ mutation risk level relative to NCCN guidelines.6 We chose a conservative definition of high-risk of mutation in order to focus on those who should have been clear candidates for referral for genetic testing in 2007.
Several limitations of the data should be considered. First, we relied on self-report of physician recommendations and BRCA 1/2 testing. We found good agreement between self-reported BRCA 1/2 testing and medical records in the small subset of women treated at our institution; however, we cannot validate physician recommendations for testing. Patients may not have remembered a recommendation for genetic testing, and therefore our rates of physician recommendation may be underestimated.26 Underreporting of physician recommendations is also of concern, particularly in the high-risk group, since it suggests lack of effective communication between patients and providers. Recommendations for genetic testing should ideally be accompanied by genetic counseling. It is likely that women who participated in genetic counseling and who underwent genetic testing would be more likely to recall a physician’s recommendation for BRCA 1/2 testing, and this may partly explain the high concordance between physician recommendation and BRCA 1/2 testing rates. The response rate to the mailed questionnaire was modest, and minority women and women with later stage disease were slightly underrepresented, limiting our ability to evaluate genetic testing utilization in these groups. Finally, our sample was drawn only from Pennsylvania and may not be generalizable to other areas of the country.
In summary, our results from a population-based study of breast cancer patients diagnosed before age 65 suggest that a significant number of women who meet established testing criteria may not receive recommendations for BRCA 1/2 testing. Women at high risk for mutations who were older at diagnosis, had lower incomes, or were employed were less likely to report a physician recommendation for genetic testing. Interventions aimed at standardizing the use of familial risk assessment, increasing physician referrals for genetic counseling and testing for high risk women, and facilitating provider-patient communication regarding genetic testing may be warranted to help identify breast cancer patients who are most likely to benefit from BRCA 1/2 testing.
Acknowledgments
Grant Support: This work was supported by an NIH grant from the National Cancer Institute (5-R01-CA133004-3).
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22:1055–1062. doi: 10.1200/JCO.2004.04.188. [DOI] [PubMed] [Google Scholar]
- 2.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. Journal of the National Cancer Institute. 2009;101:80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA: the journal of the American Medical Association. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20:1480–1490. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. British journal of cancer. 2008;98:1457–1466. doi: 10.1038/sj.bjc.6604305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly MB, Axilbund JE, Buys S, et al. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. [accessed 2012/09/07];Genetic/familial high-risk assessment: breast and ovarian Version 1.2012. Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf.
- 7.Julian-Reynier C, Sobol H, Sevilla C, Nogues C, Bourret P. Uptake of hereditary breast/ovarian cancer genetic testing in a French national sample of BRCA1 families. The French Cancer Genetic Network. Psycho-oncology. 2000;9:504–510. doi: 10.1002/1099-1611(200011/12)9:6<504::aid-pon491>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Cappelli M, Surh L, Humphreys L, et al. Psychological and social determinants of women’s decisions to undergo genetic counseling and testing for breast cancer. Clinical genetics. 1999;55:419–430. doi: 10.1034/j.1399-0004.1999.550605.x. [DOI] [PubMed] [Google Scholar]
- 9.Kieran S, Loescher LJ, Lim KH. The role of financial factors in acceptance of clinical BRCA genetic testing. Genetic testing. 2007;11:101–110. doi: 10.1089/gte.2006.9999. [DOI] [PubMed] [Google Scholar]
- 10.Finlay E, Stopfer JE, Burlingame E, et al. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genetic testing. 2008;12:81–91. doi: 10.1089/gte.2007.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ropka ME, Wenzel J, Phillips EK, Siadaty M, Philbrick JT. Uptake rates for breast cancer genetic testing: a systematic review. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:840–855. doi: 10.1158/1055-9965.EPI-05-0002. [DOI] [PubMed] [Google Scholar]
- 12.Olaya W, Esquivel P, Wong JH, et al. Disparities in BRCA testing: when insurance coverage is not a barrier. American journal of surgery. 2009;198:562–565. doi: 10.1016/j.amjsurg.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Samphao S, Wheeler AJ, Rafferty E, et al. Diagnosis of breast cancer in women age 40 and younger: delays in diagnosis result from underuse of genetic testing and breast imaging. American journal of surgery. 2009;198:538–543. doi: 10.1016/j.amjsurg.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Peters N, Domchek SM, Rose A, Polis R, Stopfer J, Armstrong K. Knowledge, attitudes, and utilization of BRCA1/2 testing among women with early-onset breast cancer. Genetic testing. 2005;9:48–53. doi: 10.1089/gte.2005.9.48. [DOI] [PubMed] [Google Scholar]
- 15.Brown KL, Hutchison R, Zinberg RE, McGovern MM. Referral and experience with genetic testing among women with early onset breast cancer. Genetic testing. 2005;9:301–305. doi: 10.1089/gte.2005.9.301. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong K, Stopfer J, Calzone K, Fitzgerald G, Coyne J, Weber B. What does my doctor think? Preferences for knowing the doctor’s opinion among women considering clinical testing for BRCA1/2 mutations. Genetic testing. 2002;6:115–118. doi: 10.1089/10906570260199366. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz MD, Lerman C, Brogan B, et al. Utilization of BRCA1/BRCA2 mutation testing in newly diagnosed breast cancer patients. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14:1003–1007. doi: 10.1158/1055-9965.EPI-03-0545. [DOI] [PubMed] [Google Scholar]
- 18.Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:5526–5533. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]
- 19.Kwon JS, Daniels MS, Sun CC, Lu KH. Preventing future cancers by testing women with ovarian cancer for BRCA mutations. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:675–682. doi: 10.1200/JCO.2008.21.4684. [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Beattie MS, Ponce NA, Phillips KA. Eligibility criteria in private and public coverage policies for BRCA genetic testing and genetic counseling. Genetics in medicine: official journal of the American College of Medical Genetics. 2011;13:1045–1050. doi: 10.1097/GIM.0b013e31822a8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowstuter KJ, Sand S, Blazer KR, et al. Influence of genetic discrimination perceptions and knowledge on cancer genetics referral practice among clinicians. Genetics in medicine: official journal of the American College of Medical Genetics. 2008;10:691–698. doi: 10.1097/gim.0b013e3181837246. [DOI] [PubMed] [Google Scholar]
- 22.Genetic Information Nondiscrimination Act of 2008, 2008.
- 23.Ruddy KJ, Gelber S, Shin J, et al. Genetic testing in young women with breast cancer: results from a Web-based survey. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2010;21:741–747. doi: 10.1093/annonc/mdp355. [DOI] [PubMed] [Google Scholar]
- 24.Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: breast and ovarian. Journal of the National Comprehensive Cancer Network: JNCCN. 2010;8:562–594. doi: 10.6004/jnccn.2010.0043. [DOI] [PubMed] [Google Scholar]
- 25.Koil CE, Everett JN, Hoechstetter L, Ricer RE, Huelsman KM. Differences in physician referral practices and attitudes regarding hereditary breast cancer by clinical practice location. Genetics in medicine: official journal of the American College of Medical Genetics. 2003;5:364–369. doi: 10.1097/01.gim.0000086477.00766.c9. [DOI] [PubMed] [Google Scholar]
- 26.Bober SL, Hoke LA, Duda RB, Tung NM. Recommendation recall and satisfaction after attending breast/ovarian cancer risk counseling. Journal of genetic counseling. 2007;16:755–762. doi: 10.1007/s10897-007-9109-0. [DOI] [PubMed] [Google Scholar]