Abstract
Purpose
Individuals who stutter show sensorimotor deficiencies in speech and nonspeech movements. For the mandibular system, we dissociated the sense of kinesthesia from the efferent control component to examine whether kinesthetic integrity itself is compromised in stuttering or whether deficiencies occur only when generating motor commands.
Method
We investigated 11 stuttering and 11 nonstuttering adults’ kinesthetic sensitivity threshold and kinesthetic accuracy for passive jaw movements as well as their minimal displacement threshold and positioning accuracy for active jaw movements. We also investigated the correlation with an anatomical index of jaw size.
Results
The groups showed no statistically significant differences on sensory measures for passive jaw movements. Although some stuttering individuals performed more poorly than any nonstuttering participants on the active movement tasks, between-group differences for active movements were also not statistically significant. Unlike fluent speakers, however, the stuttering group showed a statistically significant correlation between mandibular size and performance in the active and passive near-threshold tasks.
Conclusions
Previously reported minimal movement differences were not replicated. Instead, stuttering individuals’ performance varied with anatomical properties. These correlational results are consistent with the hypothesis that stuttering participants generate and perceive movements based on less accurate internal models of the involved neuromechanical systems.
Keywords: stuttering, sensorimotor control, kinesthesia, movement planning, internal model
Afferent and Efferent Aspects of Mandibular Sensorimotor Control in Adults who Stutter Converging evidence from various lines of research provides compelling support for the hypothesis that stuttering may be related to deficiencies in fundamental sensorimotor processes. For example, neuroimaging data have revealed structural and functional differences between stuttering and nonstuttering individuals in various sensory and motor regions involved in speech production (e.g., Beal, Gracco, Lafaille, & De Nil, 2007; Chang, Kenney, Loucks, & Ludlow, 2009; Ingham, Grafton, Bothe, & Ingham, 2012; Watkins, Smith, Davis, & Howell, 2007). In addition, a large body of behavioral data provides a window into potentially relevant deficiencies at the level of movement control. Based on acoustic analyses of perceptually fluent speech, numerous research groups have demonstrated longer voice onset times (e.g., Hillman & Gilbert, 1977; Howell, Sackin, & Rustin, 1995; Max & Gracco, 2005), stop gap durations (e.g., Borden, Kim, & Spiegler, 1987; Prins & Hubbard, 1990), and vowel durations (e.g., Adams, 1987; Healey & Adams, 1981) in stuttering individuals versus nonstuttering individuals. Similarly, kinematic analyses of speech movements have confirmed longer movement durations and longer temporal intervals between articulatory and phonatory events (e.g., Caruso, Abbs, & Gracco, 1988; Max, Caruso, & Gracco, 2003; Zimmermann, 1980). In fact, longer movement durations for stuttering individuals have been demonstrated even in orofacial nonspeech movements and finger movements (e.g., Howell et al., 1995; Max et al., 2003; Riley & Riley, 1986).
Taken together, these findings indicate a generalized movement slowness in people who stutter. Suggestions that this slowness may be an artifact of coping strategies or techniques learned in speech therapy (McClean, Kroll, & Loftus, 1990) seem inconsistent with the observation that similar differences are present outside of the speech domain (Howell et al., 1995; Max et al., 2003; Riley & Riley, 1986). Also, movement slowness was present across both speech and nonspeech tasks in stuttering individuals who had not received treatment for at least 8 years prior to the testing (Max et al., 2003).
One possible explanation for the observed movement slowness is that stuttering individuals may have a basic deficiency that affects one or multiple sensory modalities themselves or the integration of multi-modal sensory information (see, for example, Feng, Gracco, & Max, 2011, regarding the integration of auditory and kinesthetic feedback during speech production). A movement strategy with increased durations may then provide extra time for online feedback-based corrections (Max, 2004; Max, Guenther, Gracco, Ghosh, & Wallace, 2004). The work reported here was initially motivated by others’ suggestion that individuals who stutter may have a deficit specifically within the somatosensory modality, in particular the kinesthetic sense (for an overview of work regarding the possibility of deficits in the auditory modality, see Max, Maruthy, Cronin, Cann, & Musiek, 2013). To date, this suggestion has been supported mostly by a series of experiments reported by De Nil and colleagues (Archibald & De Nil, 1999; De Nil & Abbs, 1991; Loucks & De Nil, 2006a, 2006b) although related work has been undertaken by others (Howell et al., 1995).
First, De Nil and Abbs (1991) asked 6 stuttering and 6 nonstuttering participants to generate the smallest possible upward or downward movements (measured from baseline to a steady-state position) with the jaw, lower lip, tongue, and index finger. The tasks were completed once with and once without visual feedback. In the condition without visual feedback (i.e., only kinesthetic feedback was available), the stuttering individuals’ minimal movements of the orofacial structures were statistically significantly larger than those of the nonstuttering individuals (the between-group difference being largest for jaw movements). There was no statistically significant between-group difference for finger movements. De Nil and Abbs (1991) concluded that stuttering individuals likely have a deficiency in orosensory processing.
In a subsequent study, Archibald and De Nil (1999) used the same paradigm and the same movement transducers to examine a possible relationship with stuttering severity. Unfortunately, they did not report inferential statistics for the actual minimal displacement data for their groups of nonstuttering, very mildly stuttering, and moderately/severely stuttering participants (instead, statistical analyses focused only on the change in performance from the visual to the non-visual condition). That is, they did not report if the minimal displacement data were statistically significantly different among the three groups. Their graphically presented data, however, indicate that the differences in group means for baseline to steady-state measurements in the non-visual condition were approximately .4 mm for moderate/severe stuttering vs. control and only .1 mm for very mild stuttering vs. control whereas the between-group difference had been more than 1 mm for all stuttering vs. control in De Nil and Abbs (1991). Moreover, the data also suggest large inter-individual variability for this task given that the nonstuttering participants’ baseline to steady-state minimal movements in the non-visual condition were more than twice as large as those in the earlier study by De Nil and Abbs (> 1 mm vs. < .5 mm). This inconsistency in the results from these two studies suggests that participant characteristics such as orofacial anatomy may need to be taken into account in future studies given that it has been demonstrated that kinematic features of oral movements may indeed be influenced by anatomical factors (Earnest & Max, 2001; Kuehn & Moll, 1976).
With a second, closely related, paradigm, Loucks and De Nil (2006b) further investigated the kinesthetic deficiency hypothesis by asking 17 stuttering and 17 nonstuttering individuals to make accurate jaw opening movements from a baseline to a target 6 mm from the baseline, again including separate conditions with or without visual feedback. In addition, there were separate time pressure and no time pressure conditions for which participants were instructed to initiate the movement as fast as possible or at a self-selected time, respectively. Results showed that the stuttering individuals’ jaw movements were less accurate and more variable when visual feedback was not provided, especially under time pressure. Unfortunately, no post-hoc tests were performed to further analyze the between-group differences within each condition separately (e.g., no time pressure without visual feedback).
The above results could indeed be consistent with a deficiency within the kinesthetic system, as suggested by De Nil and colleagues (Archibald & De Nil, 1999; De Nil & Abbs, 1991; Loucks & De Nil, 2006b). However, the results cannot rule out a deficiency within the efferent control system or with the integration of kinesthetic information into the planning of the efferent control signals given that all tasks involved the generation of motor commands for active movements (Loucks & De Nil, 2006b; Namasivayam, Lieshout, & De Nil, 2008). As a first step toward differentiating between specific afferent versus efferent deficiencies, Loucks and De Nil (2006a) used a tendon vibration paradigm. Neurophysiological and behavioral studies on limb sensorimotor control have consistently shown that sustained tendon vibration effectively modulates the kinesthetic information from muscle spindles, and leads to movement inaccuracies (e.g., movement undershoot due to an increased sensation of stretch in a vibrated antagonist muscle) as well as movement illusions (again due to an increased signaling of stretch in the vibrated muscle) (Cody, Schwartz, & Smit, 1990; Goodwin, McCloskey, & Matthews, 1972; Roll & Vedel, 1982; Roll, Vedel, & Ribot, 1989). Applying tendon vibration unilaterally to the masseter muscle during jaw opening movements, Loucks and De Nil (2006a) found that the magnitude of undershoot was smaller for stuttering individuals than for nonstuttering individuals. This finding demonstrates that the stuttering participants were less affected when altered kinesthetic information was introduced. At the same time, this result merely suggests that, in the presence of vibration, stuttering individuals’ central nervous system may assign less weight to kinesthetic information, and it does not clarify whether or not there is a fundamental problem with the accuracy of unaltered information within this sensory modality. In fact, one could even argue that the stuttering individuals must have been better at using the unaltered kinesthetic information that was available from masseter spindles on the non-vibrated side of the jaw.
Overall, the reviewed literature suggests that stuttering may be associated with a deficiency within the kinesthetic system and/or with a deficiency within the efferent control system, but, at the same time, that individual subject characteristics such as stuttering severity or orofacial anatomy may play an important role. In light of these ambiguities in the interpretation of previous results, we designed a methodological approach with novel experimental tasks that dissociate the afferent and efferent components of sensorimotor control. Specifically, the approach allowed us to investigate, in an independent manner, adult participants’ kinesthetic sensitivity and kinesthetic accuracy for passive jaw movements (dependent variables quantifying purely afferent processes) as well as their minimal displacement and positioning accuracy for active jaw movements (dependent variables quantifying the combined effect of afferent and efferent processes). If stuttering is associated with a fundamental kinesthetic deficit, stuttering individuals should perform more poorly than nonstuttering individuals on both the passive and the active movement tasks. If, on the other hand, stuttering is associated only with deficits in the generation of efferent motor commands, stuttering individuals should perform similar to nonstuttering individuals on the passive movement tasks and more poorly than nonstuttering individuals on the active movement tasks. We also examined whether participants’ performance on these four tasks correlated with stuttering severity or an index of mandibular size.
Method
Participants
Eleven stuttering adults (9 males, 2 females; age: 20–49 years, M = 28.90 years, SD = 8.31) and eleven normally fluent adults (9 males, 2 females; age: 22–47 years, M = 29.08 years, SD = 7.17) participated after providing informed consent. All participants were naive as to the purpose of the study.
Eligibility criteria for all participants included (a) self-reported absence of psychological, neurological, or communication disorders (other than stuttering in the stuttering group), (b) not taking any medications with a possible effect on sensorimotor functioning, (c) being a native speaker of American English, and (d) no dental modifications (e.g., dentures, bridges, caps, braces, or permanent retainers) that would pose a risk for the experimental procedures described below. Additional eligibility criteria for the stuttering participants included (a) confirmation of the diagnosis of stuttering by an ASHA-certified speech-language pathologist, and (b) self-reported stuttering onset during childhood. Nonstuttering participants were individually matched with the stuttering participants based on age (± 3 years), gender, and self-reported handedness. Using the Stuttering Severity Instrument 4rd edition (SSI-4; Riley, 2008), each stuttering participant’s severity was determined by an ASHA-certified speech-language pathologist or a graduate student in speech-language pathology with training and experience in the evaluation of stuttering. Table 1 lists age, gender, handedness, overall SSI-4 score, stuttering severity classification, frequency of stuttering averaged across the SSI-4 speaking and reading tasks, and speech therapy history for each stuttering participant. To facilitate linking of these participant characteristics to the individual participant results presented below, entries in both this table and all individual participant figures are rank-ordered from lowest to highest stuttering frequency.
Table 1.
Stuttering individuals’ age (in years), gender, handedness, overall SSI-4 score (Riley, 2008), SSI-4 stuttering severity classification, average frequency of stuttering across the SSI-4 speaking and reading tasks (in percent syllables stuttered), and time of most recent stuttering treatment. Participants are rank-ordered based on stuttering frequency.
| Participant | Age | Gender | Handedness | SSI score | SSI severity | Stuttering frequency | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 26 | female | right | 13 | very mild | 2.4 | 17 years prior |
| 2 | 31 | male | right | 15 | very mild | 2.5 | no treatment |
| 3 | 30 | male | right | 27 | moderate | 5.4 | 18 years prior |
| 4 | 20 | female | right | 20 | mild | 5.5 | 12 years prior |
| 5 | 29 | male | right | 17 | very mild | 5.6 | 20 years prior |
| 6 | 27 | male | right | 30 | moderate | 6.1 | 5 years prior |
| 7 | 49 | male | right | 15 | very mild | 6.5 | 31 years prior |
| 8 | 33 | male | right | 40 | very severe | 7.8 | 16 years prior |
| 9 | 27 | male | right | 23 | mild | 9.0 | 5 years prior |
| 10 | 20 | male | right | 22 | moderate | 9.0 | 8 years prior |
| 11 | 24 | male | left | 46 | very severe | 14.5 | 6 years prior |
Procedure and Instrumentation
The study involved four experimental tasks with a common experimental set-up. A robotic device (Phantom Premium 1.0, Sensable Technologies, Wilmington, MA) implemented passive jaw movements or recorded active jaw movements. In the active movement tasks, participants moved their jaw either (a) with the smallest possible extent, or (b) to a remembered kinesthetic target. In the corresponding passive movement tasks, participants manually operated a joystick (Attack 3, Logitech, Fremont, CA) either (a) to indicate whether or not they felt a small jaw displacement that was implemented by the robot, or (b) to report the estimated jaw position after larger displacements implemented by the robot.
Throughout the recording session, participants were seated on a height-adjustable chair while wearing a head-mounted display system (eMagin Z800 3DVisor, eMagin Corporation, Bellevue, WA) that presented instructions and, for some portions of the tasks, jaw movement feedback. The robot was coupled to the jaw by means of a custom-made mandibular dental appliance (individually made for each participant) and a 6-degrees-of-freedom rotary joint (Shiller, Laboissiere, & Ostry, 2002; Tremblay, Shiller, & Ostry, 2003). To immobilize the head, participants rested the back of their head against a rigid support system, and a second individually-fitted dental appliance was secured to the maxillary teeth and held in place by two metal arms. Both the lower and the upper dental appliance were secured to the buccal surface of the teeth with a dental adhesive (Iso-Dent, Ellman International, Oceanside, NY).
The total recording session consisted of a pre-test and four experimental tasks. For each of the experimental tasks, participants were given five practice trials to familiarize themselves with the task, and the sequence of events within each trial. Prior to each task, participants were instructed to first close their jaw and to then open their jaw to a position halfway between the closed and maximum open position. These two positions (i.e., closed and open) were recorded to estimate the individual’s major axis of jaw movement. All subsequent recordings of jaw position in the robot’s 3D coordinate system were transformed to single-dimension values by projecting onto this major axis. The jaw closed position was used as the origin of the axis. For all tasks, the movement signals were digitized at a sampling frequency of 1000 Hz and digitally low-pass filtered at 10 Hz using a fourth-order Butterworth filter. Custom MATLAB (MathWorks, Natick, MA) routines were used for data processing, extraction, and analysis.
Pre-test
After the dental appliances were inserted and the robot was connected, each participant was asked to read out loud a short passage (“The rainbow passage,” Fairbanks, 1960). Jaw displacement during the reading was recorded by the robot. The extent of these speech movements was used later to verify that the range of jaw displacements in experimental tasks I through IV fell within the typical range of jaw motion for speech production.
Task I – active movements: minimal movement threshold
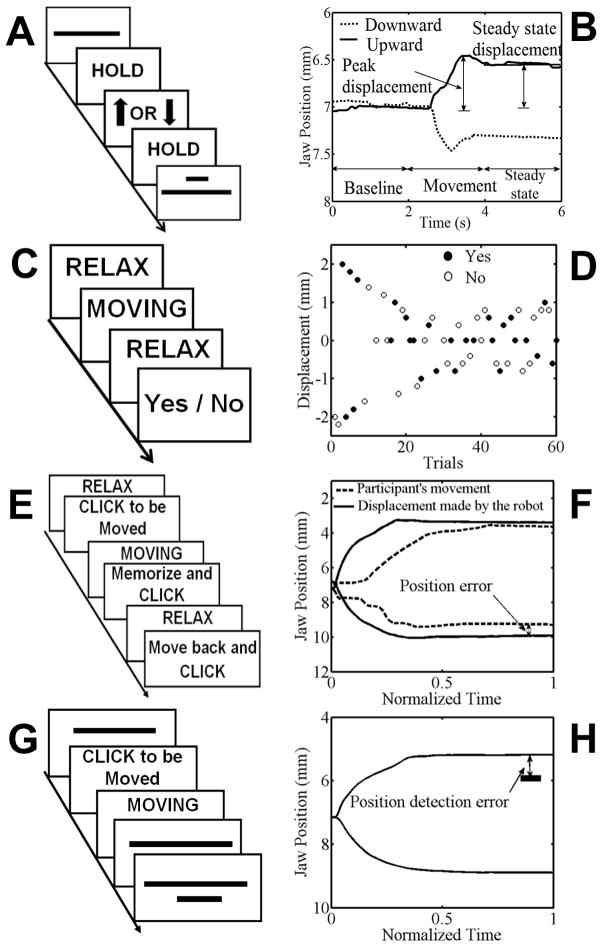
Task I was a minimal movement task in which participants were asked to actively move their jaw up or down with the smallest possible displacement. Figure 1 (panel A) illustrates the sequence of events within one trial. Each trial started with visual feedback about the jaw position (shown as a horizontal line) together with a visual representation of the range of acceptable start positions (shown as a narrow horizontal rectangle). The 0.4 mm range of acceptable start positions was centered on a position 7 mm below the fully closed jaw position, selected to be near the middle of the typical workspace for the jaw during speech (Löfqvist & Gracco, 1997; Loucks & De Nil, 2006b). To achieve a uniform mapping between visual and jaw space, 1 mm of vertical jaw displacement always corresponded to 40 pixels of vertical displacement of the horizontal line (jaw cursor), with the total display height comprising 560 pixels. Thus, a total range of 14 mm (7 mm below and 7 mm above the starting point) was shown on the visual display.
Figure 1.
Time sequence of events for one trial in each of four tasks: minimal movement threshold (A), kinesthetic detection threshold (C), movement accuracy (E), and kinesthetic acuity (G). Examples of single trial performance are shown in panels B, D, F and H, respectively. See text for details.
When the jaw was held within the range of acceptable start positions for 2 seconds, the trial started and visual feedback about jaw position disappeared. The visual display showed for the next 2 seconds the word “HOLD,” and recording of the jaw position was automatically initiated. Participants were instructed to maintain the same jaw position during this interval (baseline interval). Subsequently, an upward or downward arrow (“Go” signal) appeared for 2 seconds as the cue to initiate the smallest possible jaw movement up or down (movement interval). For the next 2 seconds, the visual display showed the word “HOLD” again, this time serving as the instruction to maintain the new jaw position (steady-state interval). After completion of the trial, the initial display appeared again with the range of acceptable start positions and the current jaw position. Each participant completed a total of 30 trials (15 upward and 15 downward movements) in randomized order.
For this task, two dependent variables were extracted offline (Figure 1, panel B). First, we calculated for each trial the difference between the average jaw position in the second half of the baseline interval and the average jaw position in the second half of the steady-state interval. Hereafter, this measure is referred to as the steady state displacement. Second, we calculated for each trial the difference between the average jaw position in the second half of the baseline interval and the peak displacement in the movement interval—a measure hereafter referred to as the peak displacement. The rationale for including the latter variable was that the initial displacement prior to any corrections may be more directly related to the initially-generated efferent signals whereas the steady state displacement may already reflect corrections based on afferent input. The average latency of this peak displacement for the stuttering (3.35 s) and nonstuttering (3.20 s) groups was confirmed to be within the movement period and well before the steady-state interval. Overall duration of the completed trials was not statistically different between the two groups.
Individual trials were excluded from analysis in the following cases: (1) the movement started before the Go signal appeared, (2) the movement returned to the start position before the end of the steady-state interval, or (3) the peak displacement during the trial was less than 0.15 mm. The latter criterion, included to eliminate trials during which the participant did not actually make a detectable voluntary movement, was based on a probability plot demonstrating that the small set of trials in the range from −0.15 mm to 0.15 mm was clearly discontinuous from the distribution of all other trials (with no similar discontinuities occurring below −0.15 mm or above 0.15 mm). Using the combination of these three criteria, only approximately 5% of all trials were excluded from analysis.
Task II – passive movements: kinesthetic1 detection threshold
Task II examined participants’ detection threshold for passive jaw movements implemented by the robot. Figure 1 shows the procedure (panel C) and an example of single-trial data (panel D). First, the robot moved the participant’s jaw to the range of acceptable start positions (defined as above). The robot then implemented a passive ramp and hold jaw movement. The ramp (i.e., actual displacement) was always 500 ms in duration. The task included a total of 60 trials, with each trial randomly selected (based on a uniform distribution) to involve upward movement, downward movement, or no movement. After each trial, participants were asked to indicate, by clicking different buttons on the joystick, whether or not they felt a passive jaw movement. In each trial, the visual display reminded participants to keep their jaw muscles as relaxed as possible. No-movement “catch” trials only served to increase response reliability (participants were instructed that trials without movement would be included, and that they should only click “yes” if they did in fact feel their jaw move).
The first trial started with an intended displacement of 2 mm up or down (note that due to inertia and stiffness of the jaw, the actual displacements achieved by the robot were slightly smaller than the intended displacements; importantly, statistical analysis showed no significant between-group difference in the extent to which the actually achieved movements deviated from the intended ones; hence, the two groups did not differ in the extent to which jaw stiffness resisted the robot’s movements). The participant’s response (Yes or No) determined the extent of displacement for the next trial. For example, if in the first trial the robot moved the jaw in the upward direction by 2 mm, and the participant pressed “Yes,” then the intended extent of displacement for the next upward trial decreased by 0.2 mm (i.e., 1.8 mm). On the other hand, if the participant pressed “No” for a trial during which the robot moved upward, then the extent of displacement for the next upward trial increased by 0.2 mm (i.e., 2.2 mm). Responses to the catch trials were ignored by the control software.
Upward and downward detection thresholds were determined after separating a participant’s trials for the two directions. For each displacement in a given direction, custom MATLAB code calculated the participant’s cumulative number of “Yes” answers for that displacement and all smaller displacements as well as the number of “No” answers for that displacement and all larger displacements. The detection threshold was defined as the displacement for which the absolute value of the difference between the number of “Yes” and “No” answers reached the lowest value (or the smallest displacement that met this criterion if two or more displacements were associated with the same minimum difference).
Task III – active movements: movement accuracy
Task III was a movement accuracy task in which the participants attempted to move their jaw back to a remembered kinesthetic target within the range of jaw motion for speech. At the beginning of each trial, the display again reminded participants to keep their jaw muscles as relaxed as possible. The robot then moved the jaw to the range of acceptable start positions (the same as in Task I), and participants indicated, by clicking a button on the joystick, that they were ready for the trial to start. The robot then implemented a ramp (500 ms) and hold movement to a position 1, 2, 3, or 4 mm above or below the start position (importantly, there was again no statistically significant between-group difference in the extent to which the two groups resisted this passive movement). At the end of the passive movement, participants were asked to memorize the new jaw position so that they would be able to return to the same position. Participants were allowed to take as much time as necessary to memorize the jaw position, and they clicked a button on the joystick when they were ready for the robot to move their jaw back to the start position. Subsequently, participants performed an active jaw movement to the remembered kinesthetic target, clicking a button on the joystick when they believed that the jaw had reached the target position. This sequence of events for one trial is illustrated graphically in Figure 1 (panel E). The task included a total of 40 trials (20 up, 20 down) in randomized order. Overall trial durations were not statistically significantly different between the stuttering and control group. As illustrated in panel F of Figure1, the absolute value of the difference between the target position (at the end of the passive movement) and the actually achieved position (at the end of the active movement) was calculated as the dependent variable for this task (position error).
Task IV – passive movements: kinesthetic acuity
Task IV was a passive jaw movement task assessing kinesthetic acuity. The robot moved the jaw various distances within the range of motion for speech, and participants manually moved the joystick to report the perceived jaw position. First, the robot moved the jaw to the range of acceptable start positions (the same as in Task I). The participants saw visual feedback of this passive movement from the end of the previous trial back to the start position, and manually followed with a joystick-controlled cursor the jaw position indicator to “calibrate” their joystick movements to the amount of jaw displacement (Figure 1, panel G). The range of motion of the joystick was mapped to the 560 pixels of the visual display, which corresponded to 14 mm of jaw displacement (see Task I). Thus, for all participants, 1 mm of jaw displacement always corresponded to 40 pixels of vertical displacement of the jaw cursor.
Next, visual feedback about the jaw position disappeared – only the joystick cursor remained visible – and the robot implemented a ramp and hold jaw displacement to a position 1, 2, 3, or 4 mm above or below the start position (again there was no statistically significant difference between the groups in resisting the intended displacements of the robot). The duration of the ramp phase was always 500 ms. Participants then moved the joystick cursor to the perceived jaw position, and they clicked a button on the joystick to indicate when they had reached that position. The joystick position at the time of the button click was recorded as the perceived jaw position, and the dependent variable for this task consisted of the difference between this perceived position and the actual position (position detection error). After clicking the joystick button, jaw position visual feedback returned, and, thus, participants were able to see the difference between the actual and perceived jaw positions. The task included 40 trials (20 up, 20 down) in randomized order. An individual trial is illustrated in Figure 1 (panel H).
Statistical analyses
All statistical analyses were conducted using IBM SPSS Statistics 19 (IBM, Armonk, NY) with significance levels set at p < 0.05. Data were analyzed by using analysis of variance for repeated measures (ANOVA) with group as a between-subjects variable. Given the known physiological differences between muscles for jaw closing (movements in the upward direction) and jaw opening (movements in the downward direction) (Hannam & McMillan, 1994), the direction of jaw movement was used as one repeated measures variable for all four tasks. Movement distance was used as another repeated measures variable for the two non-threshold tasks (movement accuracy in Task III and kinesthetic acuity in Task IV). In addition, based on the suggestion that stuttering individuals may show different motor practice effects than nonstuttering individuals (Smits-Bandstra, 2010), successive blocks of trials were considered as another repeated measures variable where possible – namely, for the minimal movement trials in Task I (the 30 trials were grouped into three blocks of 10 trials; 5 up and 5 down), the movement accuracy trials in Task III (the 40 trials were grouped into five blocks of 8 trials; one trial per distance-direction combination), and the kinesthetic acuity trials in Task IV (the 40 trials were grouped into five blocks of 8 trials; one trial per distance-direction combination). For the repeated measures variables with more than two levels, degrees of freedom were adjusted using the Greenhouse-Geisser correction to account for potential violations of the sphericity assumption (see Max & Onghena 1999). For all results, we report the F and p values of non-significant tests only for the group main effect and interactions involving the group factor (i.e., not for the Direction and Distance variables).
It has been suggested previously that certain aspects of orofacial anatomy such as mandibular size may be correlated with kinematics features of speech movements (Earnest & Max, 2001; Kuehn & Moll, 1976). Therefore, we also used the dental appliances that had been individually made based on each participant’s dental impressions to calculate an index of mandibular size. Specifically, we first made 6 anatomical measurements for each participant: (a) in a coronal plane, the distance between the two canine teeth, (b) in a coronal plane, the distance between the two second premolars, (c) in a coronal plane, the distance between the two second molars, (d) in the mid-sagittal plane, the distance between the central incisors and a line connecting the two canines, (e) in the mid-sagittal plane, the distance between the central incisors and a line connecting the two second premolars, (f) in the mid-sagittal plane, the distance between the central incisors and a line connecting the two second molars. Using these measurements and standard trigonometry, we calculated an estimate of mandibular area (in the horizontal plane) as the index of mandibular size. To examine the possible influence of mandibular anatomical variation, we then determined – for each group and movement direction separately – the Pearson correlation coefficient for the relationship between this index of mandibular size and the dependent variables measured for Tasks I through IV. Lastly, we also examined whether participants’ performance on any of these four tasks was correlated with their clinical measures of stuttering frequency.
Results
Task I – Active Movements: Minimal Movement Threshold
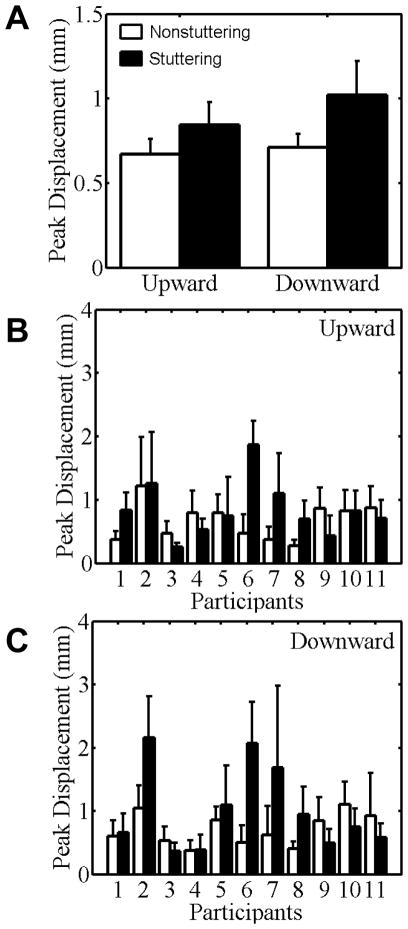
Although both the steady state displacement measure and the peak displacement measure indicated descriptively larger movement amplitudes in the downward direction than in the upward direction, the Direction main effect was not statistically significant. Similarly, although the group mean displacements were descriptively larger for the stuttering group than for the nonstuttering group (see Figure 2, panel A, for the peak displacement data), the Group main effect was also not statistically significant for either the steady state displacement measure, F(1, 20) = 2.69, p = .12, or the peak displacement measure, F(1, 20) = 1.75, p = .20. Furthermore, the Block main effect and Block × Direction interactions were not statistically significant for either measure. In addition, no statistically significant Direction × Group interactions were found for the steady state displacement measure, F(1, 20) = 1.80, p = .20, or the peak displacement measure, F(1, 20) = 1.20, p = .29. There also was no significant Block × Group interaction for either the steady state displacement measure, F(2.33,46.70) =1.92, p = .15, or peak displacement measure, F(2.60, 52.04) = 1.73, p = .18. Lastly, no statistically significant Block × Direction × Group interactions were found for either the steady state displacement measure, F(2.54,50.88) = 1.06, p = .37, or the peak displacement measure, F(2.69,53.79) = .86, p = .46.
Figure 2.
Peak displacement data for stuttering (black bars) and nonstuttering (white bars) participants’ minimal jaw movements in Task I. Group mean data for the upward and downward directions are shown in panel A. Individual participant data for the upward and downward directions are shown in panels B and C, respectively. Participants are rank-ordered from lowest to highest stuttering frequency. Error bars indicate standard errors.
Given that the group mean minimal movement data were descriptively larger for the stuttering group but in the absence of a statistically significant Group effect, Figure 2 (panels B and C) presents the individual participant peak displacement data for both groups and both directions of movement. It is clear from the figure that most stuttering participants made movements that were similarly small as those of the matched nonstuttering participants, but that some stuttering participants made movements that were substantially larger than those of the matched participant (e.g., stuttering participants #6 and #7 regardless of movement direction and stuttering participant #2 for downward movements).
Task II – Passive Movements: Kinesthetic Detection Threshold
With regard to participants’ detection threshold for passive movements (Figure 3), there was no statistically significant Direction effect. Neither was there a statistically significant Group main effect, F(1, 20) = .25, p = .62, or a Direction × Group interaction, F(1,20) = .27, p = .61. Unlike the active minimal movements in Task I, separation of the two groups’ mean passive movement detection thresholds was within one standard error for each of the two movement directions, and an examination of the individual participant data (Figure 3, B–C) did not indicate atypically poor performance in any of the stuttering participants. That is, the number of stuttering participants who had higher thresholds than their matched control participant was similar to the number of stuttering participants who had lower thresholds, and the size of these threshold differences within pairs of participants was also similar.
Figure 3.
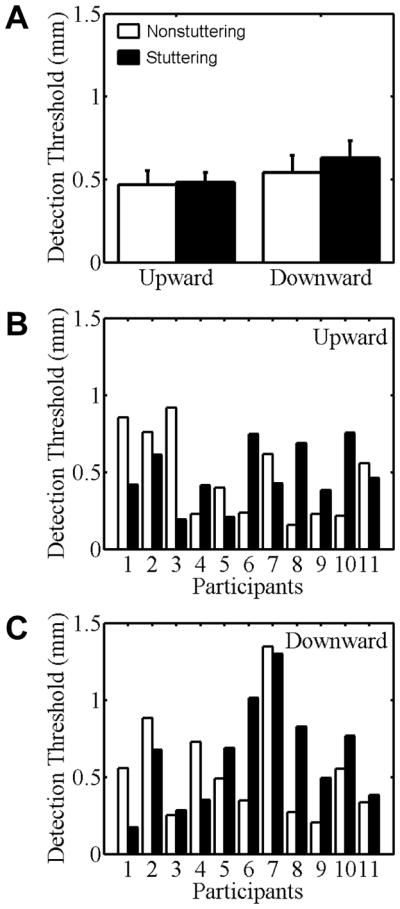
Kinesthetic detection threshold of stuttering (black bars) and nonstuttering participants (white bars) for passive jaw movements implemented by a robotic device in Task II. Group mean data for the upward and downward directions are shown in panel A. Error bars indicate standard errors. Individual participant data for the upward and downward directions are shown in panels B and C, respectively. Participants are rank-ordered from lowest to highest stuttering frequency.
Task III – Active Movements: Movement Accuracy
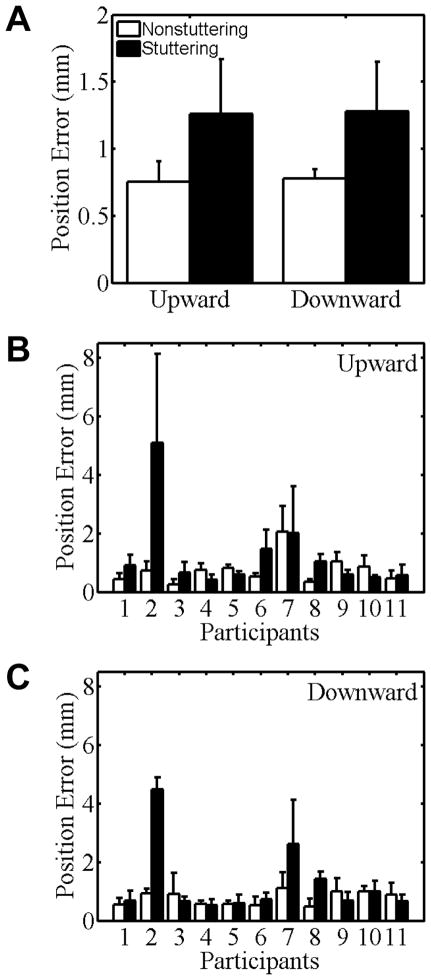
Group and individual participant data for active jaw movements toward memorized kinesthetic targets are shown in Figure 4 (panels A and B/C, respectively). No statistically significant main effects of Direction or Block were found. Both groups made larger position errors as movement distance increased, and this Distance main effect was statistically significant, F(1.32, 26.47) = 4.06, p = .04. For ease of comparison with all other figures, however, data in Figure 4 are collapsed across all distances.
Figure 4.
Movement accuracy (position error relative to target) for stuttering (black bars) and nonstuttering participants’ (white bars) jaw movements to a memorized kinesthetic target in Task III. Group means for the upward and downward directions are shown in panel A. Individual data for the upward and downward directions are shown in panels B and C, respectively. Participants are rank-ordered from lowest to highest stuttering frequency. Error bars indicate standard errors.
Despite the fact that the mean position error was approximately .5 mm larger for the stuttering group than for the nonstuttering group, the Group effect was not statistically significant, F(1, 20) = 1.76, p = .20. Inspection of the individual participant data in Figure 4 makes it clear that (a) for downward movements, stuttering participants #2, #7, and #8 showed larger errors than did any of the control participants, and (b) for upward movements, stuttering participants #2, #6, and #7 showed the largest errors among the stuttering group, but control participant #7 made errors of similar size as those of stuttering participant #7.
Tests of the interactions were not statistically significant for Direction × Group, F(1, 20) =.10, p = .76; Distance × Group, F(1.32, 26.47) =.31, p = .65; Block × Group, F(1.61, 32.16) =1.92, p = .17; Direction × Distance; Direction × Distance × Group, F(2.32, 46.37) =.27, p = .79; Direction × Block; Direction × Block × Group, F(2.35, 46.94) =.99, p = .39; Distance × Block; Distance × Block × Group, F(2.24, 44.79) =.87, p = .44; Direction × Distance × Block; or Direction × Distance × Block × Group, F(3.52, 70.49) =.81, p = .51.
Task IV – Passive Movements: Kinesthetic Acuity
When manually reporting the perceived jaw position after passive movements implemented by the robot, both groups made larger errors as movement distance increased. This Distance main effect was statistically significant, F(2.27, 45.33) = 11.74, p < .01. Nevertheless, for ease of comparison, Figure 5 (group data in panel A) again shows all data collapsed across distances.
Figure 5.
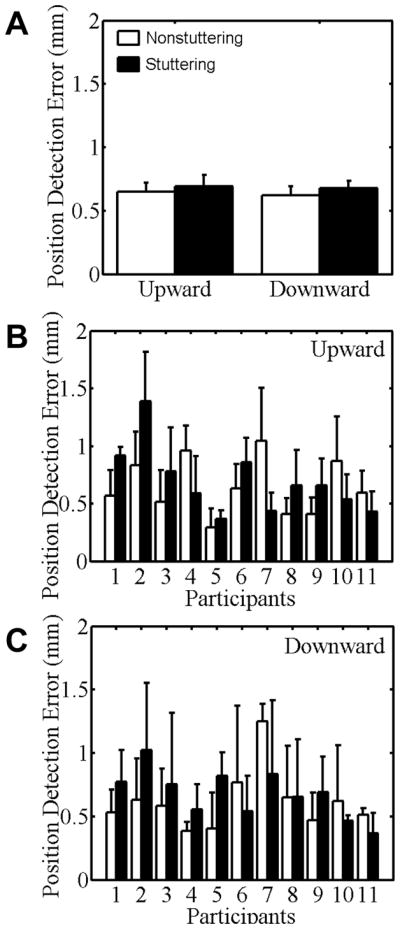
Position detection error for stuttering (black bars) and nonstuttering participants (white bars) when manually indicating the perceived jaw position after passive movements in Task IV. Group means for the upward and downward directions are shown in panel A. Individual data for the upward and downward directions are shown in panels B and C, respectively. Participants are rank-ordered from lowest to highest stuttering frequency. Error bars indicate standard errors.
There were no statistically significant main effects for Direction, Block, or Group, F(1, 20) = .35, p = .56. As was the case for the other task isolating sensory aspects (Task II), the number of stuttering participants who made larger errors than the matched control participant was approximately equal to the number of stuttering participants who made smaller errors, and the size of the error differences in either direction (i.e., larger for the stuttering or nonstuttering participant) was similar (Figure 5, B–C).
For this kinesthetic acuity task, there was a statistically significant Block × Group interaction, F(3.00,59.97) = 3.70, p = .02. Post-hoc testing indicated that only the stuttering group showed a statistically significant performance improvement from the first to the last block, t(10) = 5.21, p < .001. For the nonstuttering group, the position judgments also showed a gradual trend of improvement over the first four blocks, but then returned back to the original level in the last block.
Statistically significant interactions were also found for Block × Direction, F(3.51, 70.18) = 3.47, p = .02, and Distance × Direction, F(2.14, 42.78) = 3.19, p < .05. The Block × Direction interaction did not show any consistent pattern in the manner in which the difference between upward and downward movements varied across the blocks of trials (in some blocks upward movements were associated with larger errors whereas in other blocks downward movements showed larger errors). With regard to the Distance × Direction interaction, the deterioration of jaw position judgments with increasing distance of the passive movements was greater for downward movements than for upward movements (t(21) = −2.48, p = .02). There was also a statistically significant four-way interaction Block × Direction× Distance × Group, F(6.85, 137.29) = 2.18, p = .04. We did not find statistically significant interactions for Block × Distance; Block × Direction × Distance; Direction × Group, F(1, 20) = .02, p = .88; Distance × Group, F(2.27, 45.33) = 1.21, p = .31; Block × Direction × Group, F(3.51, 70.18) = 1.42, p = .24; Block × Distance × Group, F(6.28, 125.51) = .63, p = .71; or Direction × Distance × Group, F(2.14, 42.78) = .93, p = .41.
Correlation analyses
In Figures 2–5, individual participant data are rank-ordered based on the stuttering individuals’ average stuttering frequency during the evaluation prior to testing. The figures show that performance on the four experimental tasks did not correlate with stuttering frequency, and this was confirmed by the associated statistical analyses (Task I, r = −.35, p = .28; Task II, r = .08, p = .79; Task III, r = −.41, p = .21; Task IV, r = −.10, p = .76).
Table 2 includes the results from the correlation analyses conducted to examine the relationship between the dependent variables from Tasks I through IV and the index of mandibular size. Importantly, this index of mandibular size itself was not statistically significantly different between the stuttering and nonstuttering groups, t(20) = .41, p = .68. Within the stuttering group, the correlation between mandibular size and stuttering frequency was also not statistically significant (r = −.16, p = .65).
Table 2.
Correlation coefficients (and associated p values) for the relationship between an index of mandibular size and the dependent variables from all four tasks, for upward and downward movements separately.
| Task | Dependent variable | Stuttering group | Nonstuttering group | ||
|---|---|---|---|---|---|
|
| |||||
| Up | Down | Up | Down | ||
| Task I | minimal displacement (peak) | .64 (.03) | .53 (.09) | .08 (.82) | .39 (.24) |
| Task II | kinesthetic detection threshold | .66 (.03) | .63 (.04) | −.03 (.93) | .01 (.97) |
| Task III | position error | .12 (.73) | .08 (.82) | −.02 (.96) | −.14 (.67) |
| Task IV | position detection error | .02 (.95) | −.13 (.70) | −.11 (.74) | .09 (.78) |
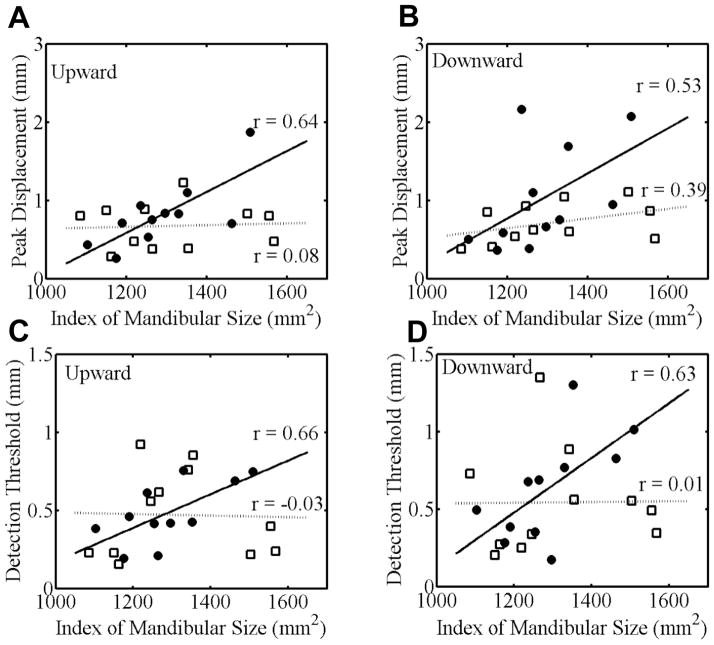
For the nonstuttering group, there was no statistically significant correlation between the index of mandibular size and minimal movement extent for either the upward or downward direction. For the stuttering group, however, there was a statistically significant positive correlation between mandibular size and minimal movement extent for the upward direction, r = .64, p =.03, as well as a positive correlation that approached statistical significance for the downward direction, r = .53, p = .09. Thus, stuttering participants with a larger jaw size tended to make larger minimal movements. These findings for the correlation between mandibular size and performance in the minimal movement task (Task I) are illustrated in Figure 6, panels A (upward movements) and B (downward movements).
Figure 6.
Top row: Correlation between mandibular size and the peak displacement measure from the minimal movement task (Task I) in which stuttering (black circles) and nonstuttering (white squares) individuals performed the smallest possible upward (A) and downward (B) jaw movements. Bottom row: Correlation between mandibular size and the detection threshold from the passive movement detection task (Task II) in which stuttering (black circles) and nonstuttering (white squares) individuals reported whether or not they detected upward (C) or downward (D) passive movements implemented by a robotic device.
With regard to the relationship between mandibular size and the kinesthetic detection threshold measured in Task II, correlation coefficients for the nonstuttering group were again not statistically significant whereas those for the stuttering group were statistically significant for both the upward direction, r = .66, p = .03, and the downward direction, r = .63, p = .04. Thus, stuttering participants with a larger jaw size had a higher kinesthetic detection threshold. This presence versus absence of a statistically significant correlation for the stuttering and nonstuttering groups, respectively, is illustrated in the bottom two panels (C, D) of Figure 6.
The accuracy-related dependent variables extracted for active jaw movements toward memorized kinesthetic targets (Task III) or for the detection of jaw position after passive movements (Task IV) did not show any statistically significant correlations with mandibular size for either the stuttering or the nonstuttering group. This was the case for upward as well as downward movements, and for error measures averaged across blocks and movement distances (the values included in Table 2) as well as for error measures separated by block and movement distance.
Discussion
The purpose of this study was to investigate the involvement of afferent versus efferent deficits in stuttering. Toward this goal, we investigated adult stuttering participants’ kinesthetic sensitivity (Task II) and kinesthetic acuity (Task IV) for passive jaw movements (i.e., dependent variables reflecting afferent processes only) separately from their minimal movement ability (Task I) and spatial accuracy (Task III) for active jaw movements (i.e., dependent variables quantifying the combined effect of afferent and efferent processes). In addition, we investigated whether performance on these tasks correlated with stuttering frequency or an index of mandibular size.
Statistically, the stuttering participants did not differ from the nonstuttering participants on any of the four tasks. Descriptively, at both the group and individual level, performance of the stuttering participants was most similar to that of the nonstuttering participants in those tasks where the afferent system was studied in isolation (i.e., in the absence of a requirement to generate motor commands): the kinesthetic detection threshold task (Task II) and the kinesthetic acuity task (Task IV). That is, when the jaw was moved passively by a robotic device, stuttering participants performed similarly to nonstuttering participants in detecting whether or not a small passive jaw movement had occurred and in manually reporting to which position the jaw had been moved over a larger distance. On the other hand, when it was necessary to generate motor commands for active jaw movements over a minimal distance (Task I) or to reach a memorized kinesthetic target (Task III), a few of the stuttering participants performed more poorly than all nonstuttering participants. When the jaw had to be actively moved the smallest possible distance, these stuttering participants moved further than the other participants, and when the jaw had to be actively moved back to a previously experienced position, these participants made larger positioning errors.
At first sight, our finding of statistically nonsignificant between-group differences in the minimal movement task may seem in contrast with previous studies on minimal movements in stuttering individuals (Archibald & De Nil, 1999; De Nil & Abbs, 1991). However, with regard to the study by Archibald and De Nil (1999), it should be noted that those authors did not report whether or not there was a statistically significant between-group difference in movement amplitude (inferential statistics were reported only for the change in movement amplitude between conditions with and without visual feedback), and that no individual participant data were included. In addition, for their non-visual condition (i.e., the condition most similar to the present study), Archibald and De Nil (1999) graphically reported a large group mean peak displacement of approximately 1.2 mm even for their nonstuttering participants. As can be seen in Figure 2 (panel A) of the present paper, a mean peak displacement of 1.2 mm would correspond to a performance level worse than what we obtained for either our nonstuttering or stuttering groups. Unfortunately, it cannot be determined at this time whether such inconsistencies between the studies are related to methodological differences (e.g., instructions or recording instrumentation) or whether they reflect a very high level of inter-individual variability in performance on minimal movement tasks. In this context, the results from our correlation analyses (discussed in more detail below) clearly demonstrate an important need to use anatomical measures to control for one source of variation among participants: within our stuttering group, minimal movement extent was positively correlated with mandible size (thus, among the stuttering participants, larger minimal movements were recorded from those participants with a larger jaw). In the present study, the stuttering and nonstuttering groups showed no statistically significant difference in terms of mandible size. However, no information is available to verify whether this was also the case in the previous studies cited above, and, thus, anatomical differences may have contributed to the reported performance differences.
With regard to the non-visual condition in the study by De Nil and Abbs (1991), 4 out of 6 stuttering individuals (67%) made considerably larger minimal jaw movements than the nonstuttering individuals whereas the performance of the remaining 2 stuttering individuals was very similar to that of the nonstuttering individuals. Overall, steady-state jaw displacement for the nonstuttering group in the De Nil and Abbs (1991) study was similar to our own nonstuttering group’s steady state displacement. In our study, however, only 3 out of 11 stuttering individuals (27%) made minimal jaw movements that were outside the range of the nonstuttering individuals (for the downward direction). This finding suggests that, as is the case for numerous behavioral and neural characteristics associated with stuttering (Bloodstein & Bernstein Ratner, 2008), stuttering individuals’ performance on minimal movement tasks may be associated with substantial inter-individual variability. As mentioned above, variability in jaw anatomy was found to be one factor contributing to this variability. In addition, methodological differences may have influenced the results of our study as compared with the results of De Nil and Abbs (1991). For example, in our minimal movement task, participants had 6 seconds to finish each trial whereas in the study by De Nil and Abbs (1991) participants had only 3 seconds to finish the trial. In other words, De Nil and Abbs’s participants experienced more time pressure, and this may have differentially affected the stuttering vs. nonstuttering groups.
Our finding that only a small subset of the stuttering participants performed more poorly than the nonstuttering participants on the second active movement task – that is, the jaw positioning task that involved moving the jaw back to a kinesthetic target (Task III) -- may also seem at odds with the work by Loucks and De Nil (2006b). Those authors reported a statistically significant between-group difference in jaw positioning accuracy when moving the jaw to a visual target. Once again, however, this different outcome is difficult to interpret in light of many methodological differences. First, Loucks and De Nil’s (2006b) study involved response time (self-selected vs. reaction time) and feedback (visual vs. non-visual) as within-subjects variables. Findings included statistically significant group and response time main effects as well as statistically significant interactions of group × response time, group × feedback, and group × response time × feedback. However, no post-hoc results were reported regarding the significance of between-group differences in each condition separately. Therefore, it is not clear whether or not the performance of the two groups in the self-selected non-visual condition (i.e., the condition most similar to the present study) was statistically significantly different. Second, given that Loucks and De Nil (2006b) did not report individual participant data, it is not possible to compare inter-individual variability across the two studies. Third, Loucks and De Nil’s (2006b) task involved jaw opening movements from a baseline position (jaw closed) to a visual target (shown on a monitor) that was always 6 mm from the baseline. In contrast, the present movement accuracy task involved jaw opening and closing movements from a baseline position (7 mm below the closed position) to previously experienced and memorized kinesthetic targets at smaller, varying distances from the baseline (±1, ±2, ±3, ±4 mm).
Most interestingly, our correlation analyses yielded different results for the stuttering and nonstuttering groups. For nonstuttering participants, there was no statistically significant correlation between the calculated index of mandibular size and the dependent variables from any of the four tasks. Thus, the performance of the nonstuttering participants did not vary with biomechanical factors related to mandibular size (e.g., mass, distance from lower incisors to the rotational axis at the temperomandibular joint, etc.). For the stuttering group, there also was no statistically significant correlation between mandibular size and the dependent variables extracted for the movement accuracy and kinesthetic acuity tasks in which participants made or perceived above-threshold jaw movements (Tasks III and IV). However, the stuttering group did show strong positive and statistically significant correlations between mandibular size and the dependent variables extracted for the minimal movement threshold task and the kinesthetic detection threshold task in which participants made or perceived near-threshold jaw movements (Tasks I and II). This finding suggests that the manner in which biomechanical factors are taken into account during movement planning and perception may differ in the central nervous system (CNS) of stuttering versus nonstuttering individuals.
Indeed, a large body of recent empirical and theoretical work suggests that the CNS takes the physical properties of end effectors and the environment into account during movement planning. Specifically, the CNS uses internal models of the body and environment so that – taking account of those physical properties – it can accurately determine the motor commands that will achieve a desired movement goal and predict their sensory consequences (see, for example, Desmurget & Grafton, 2000; Flanagan & Lolley, 2001; Kawato, 1999; Shadmehr & Mussa-Ivaldi, 1994; Volkinshtein & Meir 2011; Wagner & Smith 2008; Wolpert, Ghahramani, & Flanagan, 2001). In this framework, internal models are conceptualized as continually updated inverse and forward neural representations of the mapping between central motor commands and their sensory consequences (Wolpert, Diedrichsen, & Flanagan, 2011). Such motor-to-sensory transformations are effector-specific, dynamic, and complex given the time- and environment-dependent influence of neuromuscular factors (e.g., size principle of neural recruitment; contraction response of muscle fibers to different neural firing rates; muscle force-length and force-velocity relationships) as well as biomechanical factors (e.g., effector size and mass; gravity, interaction forces/torques).
In the present study, the nonstuttering group’s lack of a correlation between mandibular properties related to size (and, thus, presumably mass) and measures of movement performance or detection suggests that the CNS of these participants did indeed take account of mandibular anatomy during movement planning and detection (such that observable performance was not affected by structural properties). In contrast, our finding that the Task I (active movement threshold) and Task II (passive movement detection threshold) performance of the stuttering group was in fact correlated with mandibular size suggests that the CNS of these stuttering participants (a) did not rely to the same extent on internal models of mandibular geometry, or (b) relied on internal models that were less accurate. This interesting finding is consistent with our laboratory’s previously formulated proposal that stuttering may be related to the CNS’s use of inaccurate or insufficiently activated internal models of the complex transformations from neuromuscular activation to vocal tract configurations and from those articulatory movements and postures to the acoustic speech output (for a detailed description of how inaccurate internal models may lead to stuttered speech dysfluencies, see Max, 2004; Max et al., 2004; Max, Gracco, Guenther, Ghosh, & Wallace, 2004; Neilson & Neilson, 1987). The fact that participants in the present study completed only single-articulator nonspeech tasks without sound production – that is, tasks with sensorimotor transformations that were substantially less complex than those involved in the control of speech movements – may be one reason why the study lacked sufficient sensitivity to detect statistically significant between-group differences in the non-correlational analyses.
A related question that deserves to be explored in future studies is why the stuttering group’s performance on the minimal movement task (Task I) and the kinesthetic detection threshold task (Task II) reflected the influence of mandibular anatomy whereas this was not the case for their performance on the two tasks that involved larger movements. If it is indeed the case that, in stuttering speakers, smaller orofacial movements are more likely to be affected by not appropriately taking account of biomechanical factors during movement planning, this finding may have theoretical as well as clinical implications. Future work may be especially informative if appropriate methods can be developed to investigate this hypothesis in both adults and children who stutter.
Lastly, one might ask why inaccurate internal models would affect the correlational analyses for both the active movements performed in Task I and the kinesthetic detection threshold investigated in Task II. Upon closer inspection of the literature, however, this finding is not surprising. Multiple recent studies have yielded ample evidence revealing close links between action performance and perception, including direct demonstrations that the learning or updating of sensorimotor mappings is accompanied by parallel changes in auditory perception in the case of speech (Nasir & Ostry, 2009; Shiller, Sato, Gracco, & Baum, 2009) and changes in upper limb kinesthesia in the case of reaching movements (Ostry, Darainy, Mattar, Wong, & Gribble, 2010). In addition, it has been suggested that internal models play a direct role in the processing and integration of sensory inputs (Merfeld, Zupan, & Peterka, 1999; Snyder, 1999; Zupan, Park, & Merfeld, 2004). Consequently, it is not only plausible but likely that the impact of a limitation in shared neural mechanisms would be observed in both action performance and perception, as was the case here for the correlational analyses of both near-threshold tasks.
In conclusion, (a) the descriptive data from the present study indicate that some of the stuttering individuals had difficulties with generating precise motor commands rather than with the processing of kinesthetic information, and (b) the correlational analyses are consistent with the hypothesis that the CNS of stuttering individuals may generate motor commands on the basis of less accurate internal models of the orofacial effector system. It appears worthwhile for future studies to investigate this hypothesis more directly for orofacial articulatory movements performed during speech production.
Acknowledgments
This research was supported in part by grants R01DC007603 and P30DC004661 from the National Institute on Deafness and Other Communication Disorders and grant DF05009 from The Patrick and Catherine Weldon Donaghue Medical Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders, the National Institutes of Health, or the Donaghue Foundation.
Footnotes
Although kinesthesia is the primary sensory modality for detecting passive movement, we acknowledge that – given the need to move the jaw with a robotic device that was coupled to the mandibular teeth by means of an acrylic appliance – additional sensory input may have been provided by periodontal mechanoreceptors. Contributions from other sources of sensory information (e.g., auditory) are less likely. We verified with a sound level meter (Brüel & Kjær Type 2250) that in neither the active nor the passive tasks the robot generated any noise that exceeded the background noise level of the laboratory (which was ~30 dB SPL).
Contributor Information
Ayoub Daliri, Department of Speech and Hearing Sciences, University of Washington, Seattle, WA.
Roman A. Prokopenko, Russian Academy of Sciences, Moscow, Russia
Ludo Max, Department of Speech and Hearing Sciences, University of Washington, Seattle, WA, and Haskins Laboratories, New Haven, CT.
References
- Adams MR. Voice onsets and segment durations of normal speakers and beginning stutterers. Journal of Fluency Disorders. 1987;12(2):133–139. doi: 10.1016/0094-730x(87)90019-2. [DOI] [Google Scholar]
- Archibald L, De Nil LF. The relationship between stuttering severity and kinesthetic acuity for jaw movements in adults who stutter. Journal of Fluency Disorders. 1999;24(1):25–42. [Google Scholar]
- Beal DS, Gracco VL, Lafaille SJ, De Nil LF. Voxel-based morphometry of auditory and speech-related cortex in stutterers. NeuroReport. 2007;18(12):1257–1260. doi: 10.1097/WNR.0b013e3282202c4d. [DOI] [PubMed] [Google Scholar]
- Bloodstein O, Bernstein Ratner N. A handbook on stuttering. 6. Clifton Park, NY: Delmar; 2008. [Google Scholar]
- Borden GJ, Kim DH, Spiegler K. Acoustics of stop consonant vowel relationships during fluent and stuttered utterances. Journal of Fluency Disorders. 1987;12(3):175–184. doi: 10.1016/0094-730x(87)90024-6. [DOI] [Google Scholar]
- Caruso AJ, Abbs JH, Gracco VL. Kinematic analysis of multiple movement coordination during speech in stutterers. Brain. 1988;111:439–455. doi: 10.1093/brain/111.2.439. [DOI] [PubMed] [Google Scholar]
- Chang SE, Kenney MK, Loucks TMJ, Ludlow CL. Brain activation abnormalities during speech and non-speech in stuttering speakers. Neuroimage. 2009;46(1):201–212. doi: 10.1016/j.neuroimage.2009.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody FWJ, Schwartz MP, Smit GP. Proprioceptive guidance of human voluntary wrist movements studied using muscle vibration. Journal of Physiology-London. 1990;427:455–470. doi: 10.1113/jphysiol.1990.sp018181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nil LF, Abbs JH. Kinesthetic acuity of stutterers and non-stutterers for oral and non-oral movements. Brain. 1991;114(5):2145–2158. doi: 10.1093/brain/114.5.2145. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends in Cognitive Sciences. 2000;4(11):423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- Earnest MM, Max L. Maxillary and mandibular morphometry and the relation to articulatory kinematics. In: Maassen B, Hulstijn W, Kent R, Peters HFM, van Lieshout PHHM, editors. Speech motor control in normal and disordered speech. Nijmegen, The Netherlands: Vantilt; 2001. pp. 134–137. [Google Scholar]
- Fairbanks G. Voice and articulation drillbook. 2. New York: Harper & Row; 1960. [Google Scholar]
- Feng Y, Gracco VL, Max L. Integration of auditory and somatosensory error signals in the neural control of speech movements. Journal of Neurophysiology. 2011;106(2):667–679. doi: 10.1152/jn.00638.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JR, Lolley S. The inertial anisotropy of the arm is accurately predicted during movement planning. Journal of Neuroscience. 2001;21:1361–1369. doi: 10.1523/JNEUROSCI.21-04-01361.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloske Di, Matthews PB. Contribution of muscle afferents to kinesthesia shown by vibration induced illusions of movement and by effects of paralyzing joint afferents. Brain. 1972;95:705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Hannam AG, McMillan AS. Internal organization in the human jaw muscles. Critical Reviews in Oral Biology & Medicine. 1994;5(1):55–89. doi: 10.1177/10454411940050010301. [DOI] [PubMed] [Google Scholar]
- Healey EC, Adams MR. Speech timing skills of normally fluent and stuttering children and adults. Journal of Fluency Disorders. 1981;6(3):233–246. doi: 10.1016/0094-730x(81)90004-8. [DOI] [Google Scholar]
- Hillman RE, Gilbert HR. Voice onset time for voiceless stop consonants in fluent reading of stutterers and non-stutterers. Journal of the Acoustical Society of America. 1977;61(2):610–611. doi: 10.1121/1.381308. [DOI] [PubMed] [Google Scholar]
- Howell P, Sackin S, Rustin L. Comparison of speech motor development in stutterers and fluent speakers between 7 and 12 years old. Journal of Fluency Disorders. 1995;20(3):243–255. [Google Scholar]
- Ingham RJ, Grafton ST, Bothe AK, Ingham JC. Brain activity in adults who stutter: similarities across speaking tasks and correlations with stuttering frequency and speaking rate. Brain and Language. 2012;122(1):11–24. doi: 10.1016/j.bandl.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L, Bauer A, Kaiser P, Kalveram KT. Timing and stiffness in speech motor control of stuttering and nonstuttering adults. Journal of Fluency Disorders. 1997;22(4):309–321. [Google Scholar]
- Kawato M. Internal models for motor control and trajectory planning. Current Opinion in Neurobiology. 1999;9(6):718–727. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Kuehn D, Moll KL. A cineradiographic study of VC and CV articulatory velocities. Journal of Phonetics. 1976;4:303–320. [Google Scholar]
- Löfqvist A, Gracco VL. Lip and jaw kinematics in bilabial stop consonant production. Journal of Speech Language and Hearing Research. 1997;40(4):877–893. doi: 10.1044/jslhr.4004.877. [DOI] [PubMed] [Google Scholar]
- Loucks TMJ, De Nil LF. Anomalous sensorimotor integration in adults who stutter: A tendon vibration study. Neuroscience Letters. 2006a;402(1–2):195–200. doi: 10.1016/j.neulet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Loucks TMJ, De Nil LF. Oral kinesthetic deficit in adults who stutter: A target-accuracy study. Journal of Motor Behavior. 2006b;38(3):238–246. doi: 10.3200/jmbr.38.3.238-247. [DOI] [PubMed] [Google Scholar]
- Max L, Maruthy S, Cronin KL, Cann C, Musiek FE. Are auditory processing deficits involved in stuttering? A comprehensive review of studies on central auditory pathway activation from brainstem to cortex. 2013. Manuscript submitted for publication. [Google Scholar]
- Max L. Stuttering and internal models for sensorimotor control: a theoretical perspective to generate testable hypotheses. In: Maassen B, Kent R, Peters HF, van Lieshout P, Hulstijin W, editors. Speech Motor Control in Normal and Disordered Speech. Oxford, England: Oxford University Press; 2004. pp. 357–388. [Google Scholar]
- Max L, Gracco VL. Coordination of oral and laryngeal movements in the perceptually fluent speech of adults who stutter. Journal of Speech Language and Hearing Research. 2005;48(3):524–542. doi: 10.1044/1092-4388(2005/036). [DOI] [PubMed] [Google Scholar]
- Max L, Onghena P. Some issues in the statistical analysis of completely randomized and repeated measures designs for speech, language, and hearing research. Journal of Speech, Language, and Hearing Research. 1999;42:261–270. doi: 10.1044/jslhr.4202.261. [DOI] [PubMed] [Google Scholar]
- Max L, Caruso AJ, Gracco VL. Kinematic analyses of speech, orofacial nonspeech, and finger movements in stuttering and nonstuttering adults. Journal of Speech Language and Hearing Research. 2003;46(1):215–232. doi: 10.1044/1092-4388(2003/017). [DOI] [PubMed] [Google Scholar]
- Max L, Gracco VL, Guenther FH, Ghosh SS, Wallace ME. A sensorimotor model of stuttering: Insights from the neuroscience of motor control. In: Packman A, Meltzer A, Peters HFM, editors. Proceedings of the 4th World Congress on Fluency Disorders. Nijmegen, The Netherlands: University of Nijmegen Press; 2004. pp. 353–360. [Google Scholar]
- Max L, Guenther FH, Gracco VL, Ghosh SS, Wallace ME. Unstable or insufficiently activated internal models and feedback-biased motor control as sources of dysfluency: A theoretical model of stuttering. Contemporary Issues in Communication Sciences and Disorders. 2004;31:105–122. [Google Scholar]
- McClean MD, Kroll RM, Loftus NS. Kinematic analysis of lip closure in stutterers fluent speech. Journal of Speech and Hearing Research. 1990;33(4):755–760. doi: 10.1044/jshr.3304.755. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Zupan L, Peterka RJ. Humans use internal models to estimate gravity and linear acceleration. Nature. 1999;398(6728):615–618. doi: 10.1038/19303. [DOI] [PubMed] [Google Scholar]
- Namasivayam AK, van Lieshout P, De Nil L. Bite-block perturbation in people who stutter: Immediate compensatory and delayed adaptive processes. Journal of Communication Disorders. 2008;41(4):372–394. doi: 10.1016/j.jcomdis.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Nasir SM, Ostry DJ. Auditory plasticity and speech motor learning. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(48):20470–20475. doi: 10.1073/pnas.0907032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson MD, Neilson PD. Speech motor control and stuttering: a computational model of adaptive sensory-motor processing. Speech Communications. 1987;6:325–333. doi: 10.1016/0167-6393(87)90007-0. [DOI] [Google Scholar]
- Ostry DJ, Darainy M, Mattar AAG, Wong J, Gribble PL. Somatosensory plasticity and motor learning. The Journal of Neuroscience. 2010;30(15):5384–5393. doi: 10.1523/jneurosci.4571-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins D, Hubbard CP. Acoustical durations of speech segments during stuttering adaptation. Journal of Speech and Hearing Research. 1990;33(3):494–504. doi: 10.1044/jshr.3303.494. [DOI] [PubMed] [Google Scholar]
- Riley G. Stuttering Severity Instrument. 4. San Antonio, TX: Pearson; 2008. (SSI-4) [Google Scholar]
- Riley G, Riley J. Oral motor discoordination among children who stutter. Journal of Fluency Disorders. 1986;11(4):335–344. doi: 10.1016/0094-730x(86)90021-5. [DOI] [Google Scholar]
- Roll JP, Vedel JP. Kinesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Experimental Brain Research. 1982;47(2):177–190. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP, Ribot E. Alteration of proprioceptive messages induced by tendon vibration in man - a microneurographic study. Experimental Brain Research. 1989;76(1):213–222. doi: 10.1007/BF00253639. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. Journal of Neuroscience. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiller DM, Sato M, Gracco VL, Baum SR. Perceptual recalibration of speech sounds following speech motor learning. Journal of the Acoustical Society of America. 2009;125(2):1103–1113. doi: 10.1121/1.3058638. [DOI] [PubMed] [Google Scholar]
- Smits-Bandstra S. Methodological considerations in the measurement of reaction time in persons who stutter. Journal of Fluency Disorders. 2010;35(1):19–32. doi: 10.1016/j.jfludis.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Snyder L. This way up: illusions and internal models in the vestibular system. Nature Neuroscience. 1999;2(5):396–398. doi: 10.1038/8056. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Shiller DM, Ostry DJ. Somatosensory basis of speech production. Nature. 2003;423(6942):866–869. doi: 10.1038/nature01710. [DOI] [PubMed] [Google Scholar]
- Volkinshtein D, Meir R. Delayed feedback control requires an internal forward model. Biological Cybernetics. 2011;105:41–53. doi: 10.1007/s00422-011-0450-x. [DOI] [PubMed] [Google Scholar]
- Wagner MJ, Smith MA. Shared internal models for feedforward and feedback control. Journal of Neuroscience. 2008;28:10663–10673. doi: 10.1523/JNEUROSCI.5479-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2007;131:50–59. doi: 10.1093/brain/awm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nature Reviews Neuroscience. 2011;12:739–751. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Flanagan JR. Perspectives and problems in motor learning. Trends in Cognitive Sciences. 2001;5(11):487–494. doi: 10.1016/s1364-6613(00)01773-3. [DOI] [PubMed] [Google Scholar]
- Zimmermann G. Articulatory dynamics of fluent utterances of stutterers and non-stutterers. Journal of Speech and Hearing Research. 1980;23(1):95–107. doi: 10.1044/jshr.2301.95. [DOI] [PubMed] [Google Scholar]
- Zupan LH, Park S, Merfeld DM. The nervous system uses internal models to achieve sensory integration. Conference Proceedings of the IEEE: Engineering in Medicine and Biology Society. 2004;6:4487–4490. doi: 10.1109/IEMBS.2004.1404247. [DOI] [PubMed] [Google Scholar]