Abstract
Eukaryotic gene expression involves numerous biochemical steps that are dependent on RNA structure and ribonucleoprotein (RNP) complex formation. The DEAD-box class of RNA helicases plays fundamental roles in formation of RNA and RNP structure in every aspect of RNA metabolism. In an effort to explore the diversity of biological roles for DEAD-box proteins, our laboratory recently demonstrated that the DEAD-box protein Dbp2 associates with actively transcribing genes and is required for normal gene expression in Saccharomyces cerevisiae. We now provide evidence that Dbp2 interacts genetically and physically with the mRNA export factor Yra1. In addition, we find that Dbp2 is required for in vivo assembly of mRNA-binding proteins Yra1, Nab2 and Mex67 onto poly(A)+ RNA. Strikingly, we also show that Dbp2 is an efficient RNA helicase in vitro and that Yra1 decreases the efficiency of ATP-dependent duplex unwinding. We provide a model whereby mRNP assembly requires Dbp2 unwinding activity and once the mRNP is properly assembled, inhibition by Yra1 prevents further rearrangements. Both Yra1 and Dbp2 are conserved in multicellular eukaryotes suggesting that this constitutes a broadly conserved mechanism for stepwise assembly of mature mRNPs in the nucleus.
Keywords: RNA-unwinding, RNA-annealing, gene expression, mRNP remodeling, co-transcriptional
INTRODUCTION
Over the last several decades, major advances have been made in our understanding of RNA structures and the parameters for RNA folding in vivo and in vitro 1; 2 Unlike DNA, cellular RNAs have a high propensity to form intramolecular helices and tertiary contacts that are central to the functionality of the given RNA molecule 1; 3; 4; 5. Proper folding is not only critical for small ribozymes to form active sites but also to enable highly efficient catalysis 1; 3; 5. This is also the case for more complex RNAs, such as the 18 and 28S ribosomal (r)RNAs, which also assemble with RNA-binding proteins to form a fully functional translational apparatus 6; 7.
Strikingly, while it is now common knowledge that cellular RNAs such as rRNAs, transfer RNAs (t)RNAs and spliceosomal (sn)RNAs are all highly structured and intrinsically dynamic, our knowledge regarding messenger RNA (mRNA) structure has lagged behind 8. One possible explanation for this discrepancy is that, unlike other RNAs, mRNAs are highly heterogeneous in sequence, length and assembly with RNA-binding proteins. Moreover, both the structure and composition of a given messenger ribonucleoprotein complex (mRNP) changes at different steps during synthesis, maturation and translation 9; 10. Computational predictions and genome-wide in vivo analyses demonstrate that mRNAs have significant secondary structure and this characteristic is likely a critical aspect of gene regulation 2; 11; 2. However, key mechanistic questions regarding the factors that are required for proper folding of mRNAs and subsequent assembly of the mRNA into an mRNP have not been fully addressed.
One class of enzymes that controls cellular RNA structures is the DEAD-box RNA helicase family. DEAD-box helicases are the largest class of enzymes within the RNA helicase superfamily, functioning in all aspects of RNA metabolism from transcription to translation 13; 14. DEAD-box RNA helicases are unique among other helicase enzyme families in that they are non-directional and non-processive, with maximal unwinding on duplexes that are one to one and a half turns of an A-form RNA helix. This activity makes DEAD-box proteins well suited for cellular RNAs, which rarely contain helices longer than 12 base pairs in length 14. Furthermore, DEAD-box proteins exhibit a wide array of biochemical activities including duplex unwinding, RNA-binding protein displacement from single stranded RNA, and RNA strand annealing 13; 14. Thus, although classically defined as helicases, these enzymes are more likely to function as cellular RNA chaperones that conduct a variety of biochemically distinct steps to properly assemble RNPs in vivo.
Three DEAD-box proteins, namely Sub2, Dbp5 and Dbp2, have been implicated in nuclear gene expression steps in the budding yeast Saccharomyces cerevisiae 14. The least well understood DEAD-box protein, however, is Dbp2. In multicellular eukaryotes, the Dbp2 ortholog p68 functions in multiple gene expression steps including pre-mRNA splicing, microRNA processing, and regulation of transcription initiation 15; 16; 17. This factor has also recently been linked to nuclear mRNA export and RNA quality control in yeast and metazoan cells 18; 19; 20. Moreover, recent studies from our laboratory determined that Dbp2 is directly associated with transcriptionally active chromatin 18. This suggests that Dbp2 may function as a co-transcriptional mRNA chaperone by facilitating proper mRNA folding, and likely messenger ribonucleoprotein (mRNP) formation, in the nucleus.
To shed light on the mechanisms governing mRNP structure and assembly, we focused on the biological and biochemical mechanism of Dbp2. Our results now show that Dbp2 is an efficient RNA helicase that promotes assembly of the RNA-binding proteins Yra1, Nab2 and the export receptor Mex67 onto newly synthesized mRNA. We also demonstrate that Dbp2 interacts directly with Yra1 and that Yra1 inhibits the duplex unwinding activity of Dbp2. We speculate that this may be a common mode of regulation for other DEAD-box RNA helicases and provide a model whereby Dbp2 duplex unwinding and subsequent enzymatic inhibition is necessary to properly assemble mRNPs.
RESULTS
Dbp2 Catalyzes RNA Duplex Unwinding on Blunt End and Single-Strand Overhang Substrates
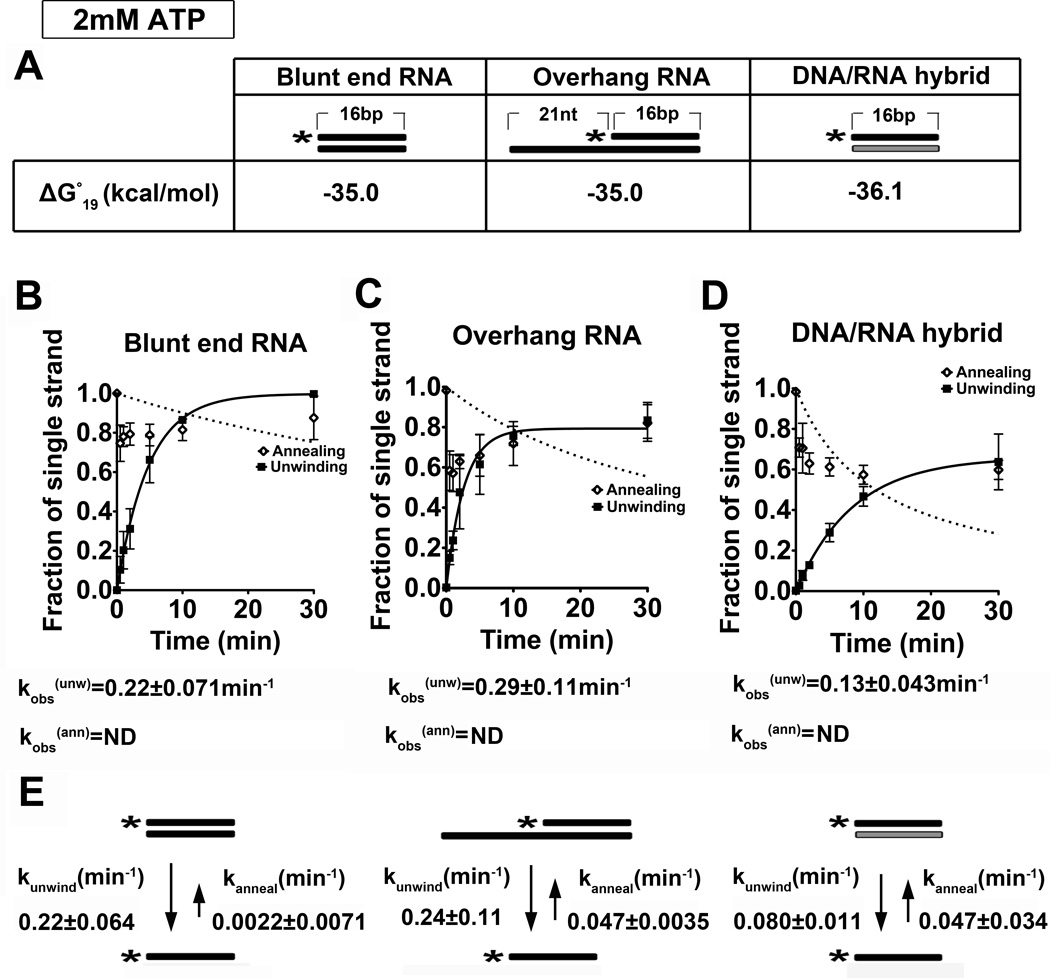
Previous studies from our laboratory established that Dbp2 is an enzymatically active ATPase associated with transcribing genes 18. Moreover, we found that loss of DBP2 in budding yeast results in RNA quality control and termination defects, suggesting that Dbp2 may function in proper assembly of mRNPs in the nucleus. To shed light on the role of Dbp2 in gene expression, we first asked if Dbp2 is a bona fide RNA helicase in vitro and if this enzyme shows any preference for specific RNA duplex substrates. It is well established that DEAD-box proteins, with the exception of DbpA, show no sequence-specific association with RNA 13. However, individual members display preferences for pure RNA duplexes and/or RNA-binding ‘platforms’ for duplex unwinding 21; 22; 23; 24. To this end, we conducted an analysis of in vitro strand unwinding under pre-steady state conditions with three different nucleic acid substrates and 2 mM ATP:Mg2+ in the presence of recombinant, purified Dbp2 (Fig. 1 and Fig. S1). These substrates include a 16 base pair (bp) blunt ended RNA duplex, a 16-bp duplex of identical sequence with a 21 nucleotide (nt) single-stranded overhang and a 16-bp RNA-DNA duplex with a different sequence but similar stability (Fig. 1A). The latter was chosen to account for the fact that RNA-DNA duplexes are less stable than their RNA-RNA counterparts and that the ability of DEAD-box RNA helicases to unwind a given substrate is inversely proportional to duplex stability 25. The unwinding assays were then conducted with 600 nM of Dbp2 and preformed duplexes over a 30 min time frame (Fig. 1 and Fig. S2).
Figure 1. Dbp2 displays ATP-dependent duplex unwinding on multiple RNA substrates at 2 mM ATP:Mg2+.
(A) Schematic representation and thermodynamic stability of RNA duplex substrates. All RNA substrates were designed with similar stability, which was calculated using the Nearest-Neighbor Database and converting to ΔG°19 using ΔG°= ΔH°-T ΔS° 79; 80. Black or gray coloring denotes RNA or DNA strands, respectively, whereas asterisks mark the position of the 32P-radiolabeled 5’ end. (B) Graphical representations of unwinding and annealing assays using radiolabeled 16-bp blunt end RNA duplexes, (C) 21-nt overhang that is 3’ to the 16-bp RNA duplexes and (D) 16-bp blunt end DNA/RNA hybrids. Reactions were performed at 19 °C with 2 mM ATP:Mg2+, 0.1 nM radiolabeled duplex, and 600 nM recombinant, purified Dbp2. The fraction of the single stranded substrate at each time point is plotted as the average of three independent reactions with standard deviations from the mean. The data was fitted to the integrated form of a homogenous first order rate law to determine the kobs(unw). kobs(ann) was determined using the integrated rate law for the bimolecular annealing reaction as previously described 26. N.D. = not determined. Representative non-denaturing gels are shown in Fig S2. (E) Kinetic parameters for Dbp2 unwinding and annealing at 2 mM ATP. The rate constants for Dbp2 unwinding and annealing were calculated as previously described26. This reveals that Dbp2 preferentially unwinds RNA-RNA duplexes irrespective of the presence of a single-stranded overhang region. Dbp2 exhibits RNA-DNA duplex unwinding but with a lower activity than RNA-RNA substrates of similar overall stability.
Consistent with other DEAD-box proteins, Dbp2 is able to unwind all three nucleic acid substrates with a preference for an RNA-RNA duplex (Fig. 1B–D). Importantly, we observed no ATP-independent unwinding activity, as evidenced from a lack of duplex destabilization after a 30 min incubation (Fig. S2A–C, lane 10). Unlike Ded1, which exhibits unwinding rate constants of ~0.1 min−1 or ~3.8 min−1 on blunt end or single strand overhang duplexes, respectively21, Dbp2 shows no preferential unwinding of a duplexed RNA with a single stranded region. This is evidenced by observed unwinding rates that are dependent upon the presence of Dbp2 and ATP (Fig. 1B–D, bottom; Fig. S2).
DEAD-box proteins also exhibit RNA-strand annealing activity in vitro 13. To measure annealing activity, we conducted the same assay as above but with the single strand components for each substrate. This showed that the substrates have no spontaneous annealing activity whereas Dbp2 exhibits some annealing activity on all three substrates at 2 mM ATP (Fig. 1B–D, Fig. S2D–F). To determine the precise biochemical mechanism of Dbp2, we subsequently calculated rate constants for both unwinding an annealing because the observed unwinding rate does not distinguish between these two parameters. Initial attempts to measure observed annealing rates at 2mM ATP were complicated due to substantial unwinding activity. This results in a poor curve fit for annealing (open circles, Fig. 1B–D, Fig. S2), However, when this RNA-strand annealing activity is taken into account according to the steady-state equation in 26, both the unwinding and annealing rate constants were accurately determined (Fig. 1E). The similar unwinding rate constants of ~0.2 min−1 for both blunt end and overhang substrates further demonstrate that Dbp2 does not require a single stranded overhang for duplex destabilization (Fig. 1E). This is consistent with our previous studies demonstrating dsRNA-directed ATPase activity 18, and is similar to another DEAD-box protein, Mss116, whose activity is not enhanced by the presence of a single-stranded region within the RNA substrate 22. This suggests that Dbp2 recognizes duplexed RNAs directly.
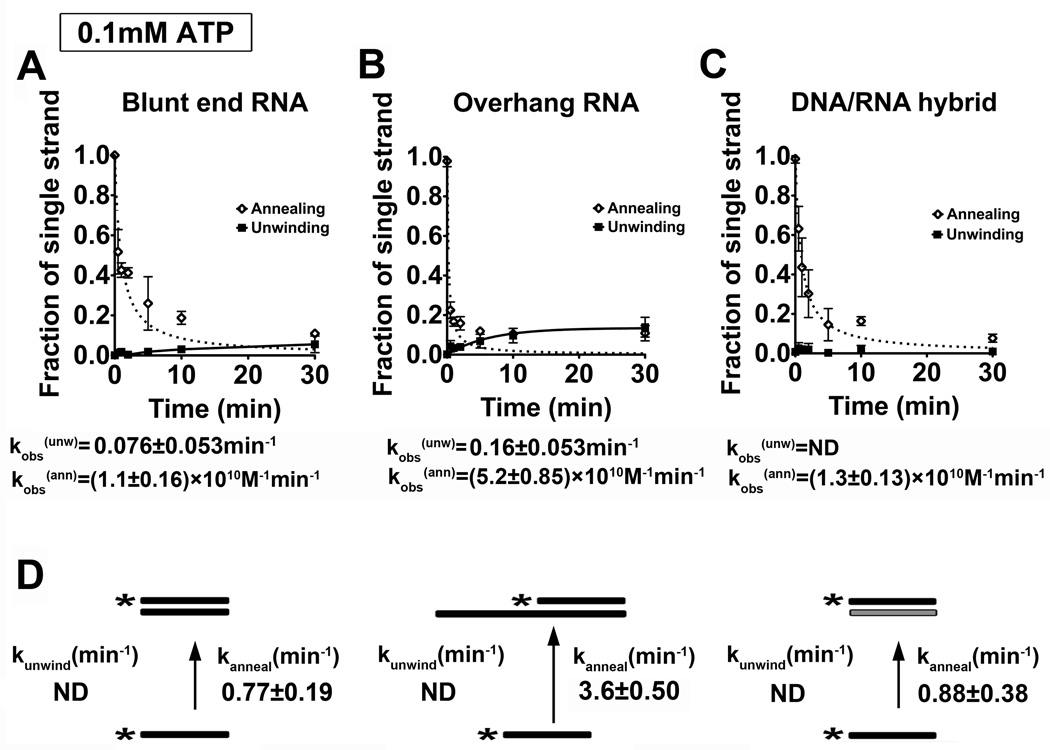
Dbp2 Preferentially Anneals RNA Duplexes with Single-stranded Regions at Low ATP Concentrations
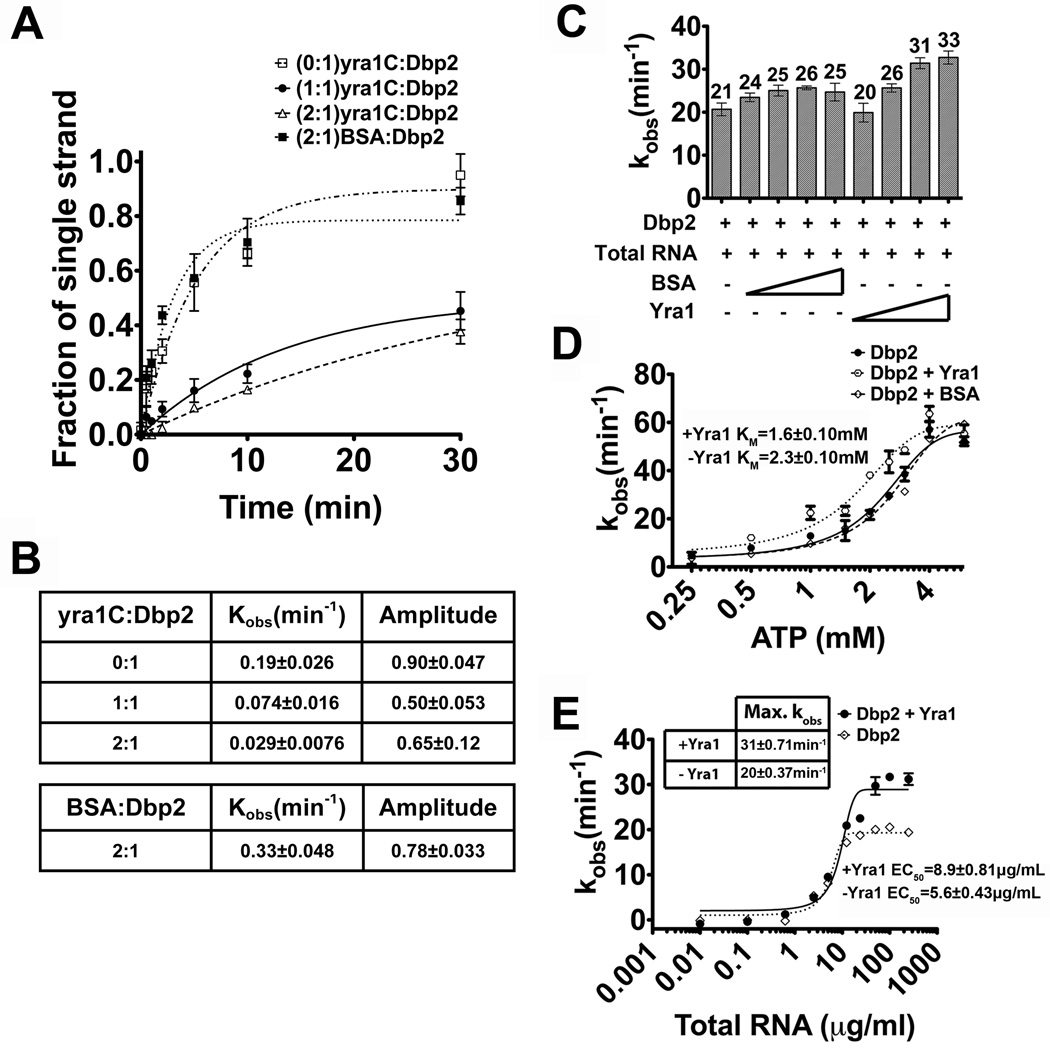
Studies of the human ortholog of Dbp2, termed p68, have shown that this enzyme promotes efficient annealing under ATP-limiting conditions 27. As mentioned above, DEAD-box proteins also facilitate strand annealing and, in some cases, this activity is biologically relevant 22; 26; 28; 29; 30. To determine if the annealing activity of Dbp2 is enhanced by reduced ATP concentrations, we conducted our unwinding and annealing assays again but with 20-fold less ATP (0.1 mM ATP). Consistent with previous studies of Ded1 and Mss116 22; 26, Dbp2 efficiently annealed all three nucleic acid substrates at low ATP concentrations with little to no detectible unwinding activity (Fig. 2A–C and Fig. S3). Moreover, Dbp2 can anneal overhang and blunt end RNA substrates in the absence of ATP (data not shown). In contrast to other DEAD-box proteins, however, Dbp2 exhibits a strong annealing preference for RNA substrates with a single stranded overhang, resulting in a kobs(ann) of 3.60 ± 0.50 min−1 (Fig. 2D). This is approximately four-fold higher than the 0.8 min−1 rate observed for the blunt end RNA-RNA and RNA-DNA duplexes. To the best of our knowledge, this preference has not been observed for any other DEAD-box protein to date, suggesting that Dbp2 has a unique ability to preferentially anneal structured RNAs with single stranded regions. In general, this is the type of secondary structure we expect to find in mRNAs, sporadic regions of duplex RNA flanked by single stranded regions. We would therefore speculate that this activity might make Dbp2 a more effective chaperone for secondary structure formation of cellular mRNAs under specific growth conditions with limited ATP (see Discussion).
Figure 2. Dbp2 exhibits a preference for strand annealing with single stranded overhang RNA substrates at low ATP concentration.
(A–C) Graphical representation of unwinding and annealing assays with 0.1 mM ATP using (A) the blunt end RNA duplex, (B) the RNA duplex with 3’ single strand overhang or (C) the blunt end RNA-DNA hybrid. Unwinding and annealing assays were conducted as above but with 0.1 mM ATP and 2 mM MgCl2. Data from the unwinding and annealing assays were fitted as above. Representative non-denaturing gels are shown in Fig S3. (D) Kinetic parameters for Dbp2 unwinding at 0.1 mM ATP. Since there is little or no observable unwinding, the unwinding data cannot be fitted with the steady state equation as mentioned above and are listed as ND (not determined). Therefore, we assumed the kobs(ann) is the same as kanneal and converted the reported kobs(ann) to the first-order rate constant as described26. This reveals that Dbp2 exhibits higher annealing on RNA duplexes with single stranded overhangs at low ATP.
DBP2 Genetically Interacts with mRNA Export Factors YRA1 and MEX67
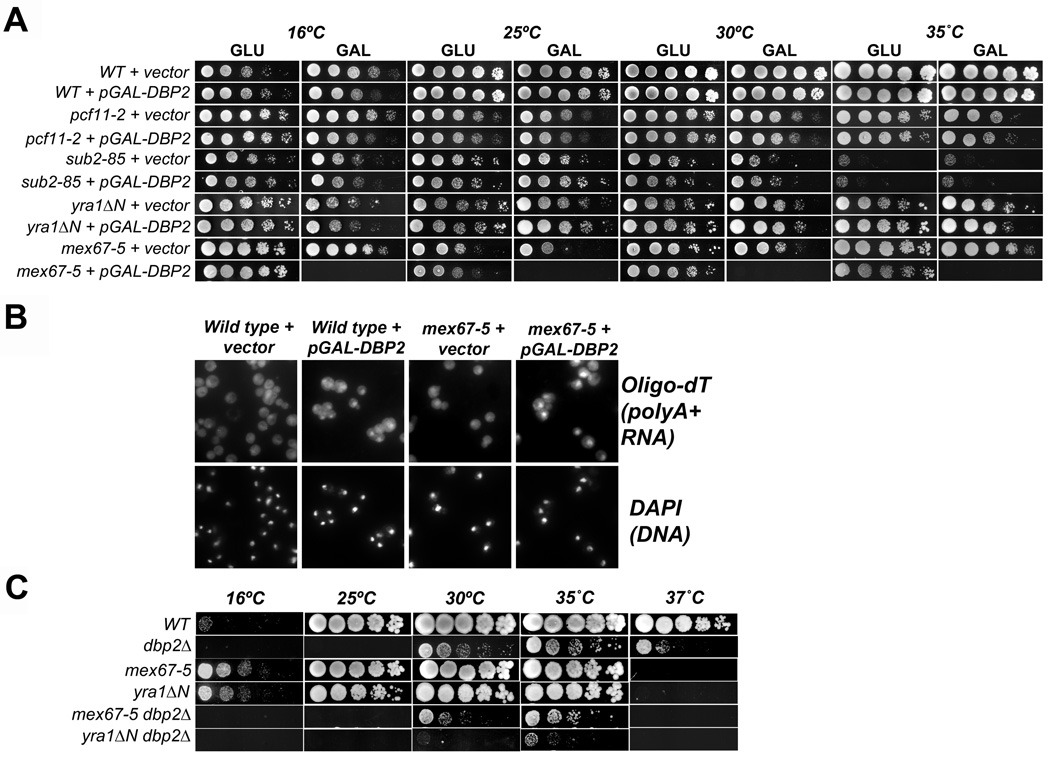
Given the biochemical activity of Dbp2, we would speculate that Dbp2 functions as an RNA chaperone for newly synthesized mRNA. Previous studies from our laboratory have provided evidence that Dbp2 is required for early gene expression steps including termination and RNA quality control 18, two processes intimately connected to mRNP assembly and export 10; 31; 32; 33. To pinpoint the precise biological role of Dbp2, we first conducted a series of genetic studies with a plasmid that overexpresses DBP2 via a galactose-inducible promoter (pGAL-DBP2), and strains harboring mutations in genes linked to 3' end formation and/or mRNA export. To this end, we selected yeast strains with mutations in the polyadenylation/cleavage factor PCF11 34; 35, the pre-mRNA splicing and export factor SUB2 36; 37, the RNA-binding protein gene YRA1 38, and the mRNA export receptor MEX67 39, with the idea that overexpression of DBP2 might either rescue or enhance the growth defects of specific mutant strains. Yeast strains were transformed with either vector alone or with a pGAL-DBP2 high copy, overexpression vector and then plated as five-fold serial dilutions onto either transcriptionally-repressive (GLU) or inducing (GAL) media at multiple temperatures. Strikingly, whereas wild type, pcf11-2, sub2–85 and yra1ΔN mutant strains displayed no obvious growth differences, overexpression of DBP2 was lethal in mex67-5 cells at all temperatures (Fig.3 A, bottom).
Figure 3. DBP2 displays genetic interactions with mRNA export factor mutants mex67-5 and yra1ΔN.
(A) Overexpression of DBP2 is lethal in mex67-5 strains. Indicated strains were transformed with empty vector or galactose-inducible pGAL-DBP2. Resulting transformants were then spotted in 5-fold serial dilutions onto transcriptionally repressive (glucose) or inducing (galactose) media and subsequently grown at the indicated temperatures from 25–35 °C. (B) Overexpression of DBP2 in the mex67-5 strain induces a mRNA export defect at the mex67-5 permissive temperature. Briefly, yeast strains were grown at 25 ˚C to mid log phase in selective media and then shifted to galactose-containing media for 1 hour to induce DBP2 overexpression. Cells were then harvested and in situ hybridization was conducted with oligodT30 to visualize accumulation of total poly(A)+ RNA. DAPI staining of DNA shows the position of the nucleus. (C) Loss of DBP2 in yra1ΔN strain results in a synthetic sick growth defect. The indicated double mutant strains were constructed using standard methods and were analyzed for growth defects as above by serial dilution analysis onto rich media. The dbp2Δ displays a cold sensitive phenotype as previously described 18.
Because Mex67 is required for mRNA exit from the nucleus, we then asked if DBP2 overexpression results in a perturbation of mRNA transport. This was addressed by conducting in situ hybridization assays to visualize the cellular localization of poly(A)+ RNAs by indirect immunofluorescence in wild type or mex67-5 cells with vector only or overexpressed DBP2. Importantly, these experiments were conducted at the permissive temperature for mex67-5, which does not typically result in accumulation of poly(A)+ RNAs in nucleus 39. Whereas both wild type and mex67-5 cells showed diffuse, whole cell staining in the presence of vector alone, mex67-5 cells with overexpressed DBP2 exhibited a striking accumulation of poly(A)+ RNA in the nucleus (Fig. 3B). We also observed a detectible accumulation of mRNA in the nucleus of wild type cells upon overexpression of DBP2 (Fig. 3B), even though we did not previously observe any growth defects in wild type cells (Fig. 3A). It is of note that this nuclear poly(A)+ RNA accumulation is not as great as when the mex67-5 cells are grown at the non-permissive temperature of 37°C (39 and data not shown) suggesting that the export block is modest or is a result of a secondary effect. Consistent with the latter, we observed no mRNA transport defects in a dbp2Δ strain (data not shown). Thus, DBP2 overexpression induces a slight mRNA export defect in mex67-5 cells, suggesting a role for this enzyme during or immediately prior to mRNA transport.
Mex67 is recruited to nascent mRNPs during transcription through protein-protein interactions with RNA-binding proteins Npl3, Yra1 and Nab2 40; 41; 42. Interestingly, recent studies have documented an interaction between p68 and Aly, the human ortholog of Yra1 19. This suggests that Dbp2 may be functionally connected to Mex67 recruitment through Yra1. To test this, we asked if loss of DBP2 results in synthetic genetic interactions with mex67-5 or yra1ΔN alleles by constructing double mutant strains and analyzing growth defects as above. Both the mex67-5 and yra1ΔN strains failed to grow at 37°C whereas the dbp2Δ exhibits a previously documented cold sensitive growth at 25°C and below 18; 39; 43. However, the yra1ΔN dbp2Δ strain displayed severely retarded growth at the permissive temperature for both single mutants alone (30°C), suggesting that DBP2 and YRA1 are functionally linked (Fig. 3C). Loss of DBP2 also results in a synthetic growth defect with mex67-5, albeit much weaker than with yra1ΔN (Fig. 3C). This suggests that Dbp2 and Yra1 function in a similar pathway and that Dbp2 is not directly required for mRNA export.
DBP2 is Required for Efficient Association of Yra1, Nab2 and Mex67 with Poly(A)+ RNA
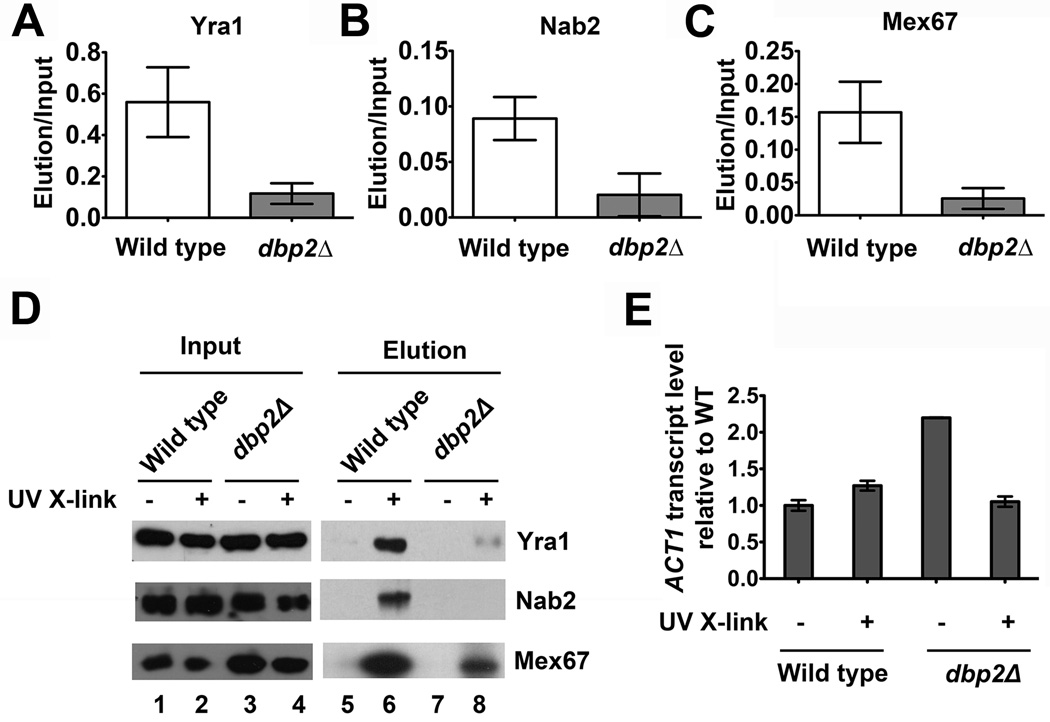
Messenger RNA is assembled with 12–30 different RNA-binding proteins to form co-transcriptionally assembled mRNPs 44. Given the genetic interactions between DBP2, YRA1 and MEX67 above, we asked if DBP2 is required for efficient association of these RNA-binding proteins with mRNA. To test this, we conducted in vivo UV crosslinking and subsequently isolated poly(A)+RNA from wild type or dbp2Δ cells. We then analyzed the association of Yra1 and Mex67 by western blotting the isolated fractions. We also analyzed Nab2, a nuclear poly(A)-RNA-binding protein that interacts directly with both Yra1 and Mex67 41; 45. Strikingly, this analysis revealed that all three proteins, Yra1, Nab2 and Mex67, exhibit reduced association with poly(A)+ RNA in dbp2Δ cells (Fig. 4A–C). This decrease is not due to differences in UV-independent, nonspecific binding, as evidenced by a representative western blot (Fig. 4D). Furthermore, analysis of ACT1 transcript abundance by reverse transcription-quantitative PCR (RT-qPCR) revealed that this reduction in dbp2Δ cells is not due to mRNA isolation efficiency (Fig. 4E). Thus, Dbp2 is required for efficient association of Yra1, Nab2 and Mex67 with poly(A)+ RNA, consistent with a role in nuclear mRNP assembly.
Figure 4. Loss of DBP2 results in reduced association of Yra1, Nab2 and Mex67 with poly(A)+ RNA.
In vivo UV crosslinking reveals reduced association of (A) Yra1, (B) Nab2 and (C) Mex67 with poly(A)+ RNA in dbp2Δ cells. Wild type and dbp2Δ cells were subjected to UV crosslinking followed by poly(A)+ RNA isolation as previously described 55. The eluted fraction of wild type and dbp2Δ cells were normalized to equal RNA concentration using equivalent A260nm absorbance units. Proteins from the eluted fractions were detected by Western blotting. The relative quantity of poly(A)+ RNA-bound proteins was determined following quantification of the resulting isolated proteins from three independent biological replicates and is reported as the amount of isolated protein relative to total (input). (D) Representative western blot of in vivo UV crosslinking. The total protein abundance (input) is shown along with the amount of isolated proteins with and without UV crosslinking. The latter serves as a background control to show that proteins isolated following UV crosslinking are not due to non-specific interactions. (E) Reverse-transcriptase, quantitative PCR (RT-qPCR) shows efficient isolation of ACT1 mRNA from both wild type and dbp2Δ cells following oligo-dT selection. Equal fractions of eluted RNA were reverse transcribed and subjected to qPCR with ACT1-specific primers as previously described 18. Transcript levels were normalized by setting the wild type elution without UV crosslinking to 1 and are a result of three technical replicates from one biological sample per strain.
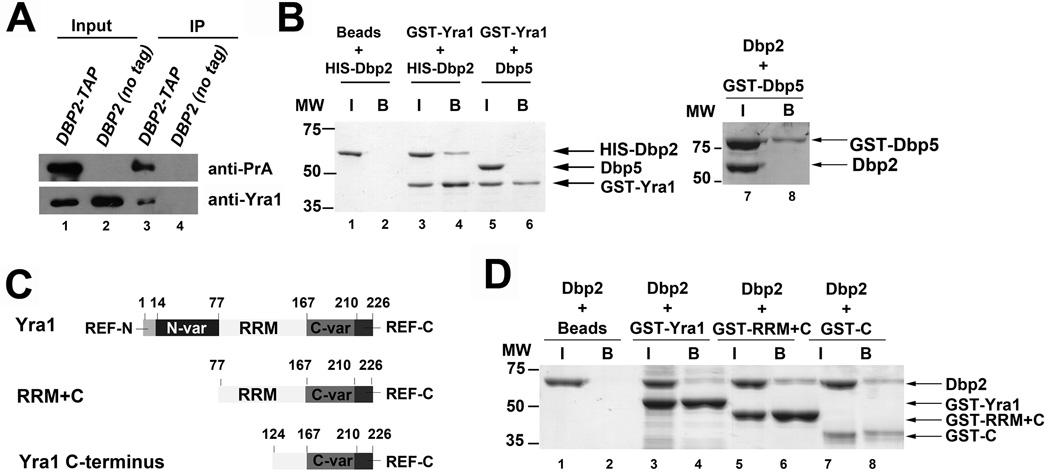
Dbp2 physically interacts with Yra1 in vivo and in vitro
Many DEAD-box proteins associate with protein co-factors that either regulate the enzymatic activity or direct the biological role of a given DEAD-box enzyme 46. Two independent studies have identified Dbp2 as a component of Yra1-bound protein complexes, suggesting that Dbp2 may interact directly with Yra1 47; 48. To test this, we first confirmed the previous interaction by asking if Yra1 copurifies with a genomically-encoded, TAP-tagged Dbp2 in yeast cells, which consists of two IgG-binding units of Protein A, a TEV cleavage site and the calmodulin-binding peptide that is fused to Dbp2 (Fig. 5A). An untagged wild type strain was utilized as a negative control for background association of Yra1 with the IgG-bound magnetic beads. Consistent with the previous studies, selection of Dbp2-TAP results in co-purification of Yra1 (Fig. 5A). No Yra1 was detected in our background control, indicating that the interaction is Dbp2-dependent. Next, we asked if the association between Dbp2 and Yra1 is direct by conducting protein pull downs with recombinant, purified proteins expressed in E. coli. Dbp2 and Yra1 were expressed as N-terminal HIS-tag or GST-tag fusion proteins, respectively, and then purified to homogeneity by standard affinity chromatography methods. The proteins were then incubated together, selected on glutathione resin selection, resolved by SDS-PAGE electrophoresis and visualized by Coomassie staining (Fig. 5B). Dbp5 is another DEAD-box protein was used as negative control for non-specific interactions. Whereas Dbp2 does not interact beads alone or with Dbp5 (Fig. 5B, lanes 2 and 8), Dbp2 was copurified with GST-tagged Yra1 (Fig. 5B, lane 4). Dbp5, on the other hand, did not copurify with GST-Yra1 (Fig. 5B, lane 6), further demonstrating the specificity of the interaction with Dbp2. Thus, Dbp2 interacts directly with Yra1.
Figure 5. Dbp2 physically interacts with Yra1 in vivo and in vitro.
(A) Yra1 co-immunoprecipitates with Dbp2. Immunoprecipitation assays were performed from wild type (DBP2 no tag) and DBP2-TAP strains using IgG-conjugated dynabeads. 10% lysate was used as input. Proteins from the input and immunoprecipitated fractions were resolved by SDS-PAGE and detected by Western blotting analysis. (B) Dbp2 interacts directly with Yra1. In vitro pull down assays were performed with recombinant, purified 6XHIS-tagged Dbp2 and GST-tagged Yra1. Briefly, recombinant, purified proteins were incubated together, 20% of the protein mix was removed as input (‘I’) and interacting proteins were selected on glutathione sepharose resin (bound ‘B’ proteins). Proteins were resolved by SDS-PAGE electrophoresis and visualized by Coomassie staining. Neither GST-Yra1 nor Dbp2 co-elute with an unrelated DEAD-box protein Dbp5 (lane 6 and 8), demonstrating that this interaction is specific. (C) Schematic representation of the primary sequence of Yra1, functional motifs and truncation mutants. Yra1 is composed of evolutionarily conserved RNA Export Factor (REF) domains at the N and C terminus separated by variable regions 38; 40; 43; 49. Yra1 also contains a central RNA recognition motif (RRM) that does not appear to harbor RNA binding activity 43 (D) The C-terminal half of Yra1 (aa 124–226) is sufficient to interact with Dbp2. GST-tagged Yra1 and truncation mutants were purified as recombinant proteins from E. coli and subjected to in vitro pull downs as above.
Yra1 is an evolutionarily conserved RNA-binding protein and export factor (REF) 38. Like other members of the REF protein family, Yra1 contains a central RNA recognition motif (RRM), two variable spacer regions, and highly conserved N- and C-termini (REF-N and REF-C, respectively)(38, Fig. 5C). Previous studies have shown that Mex67 interacts with the N-terminus (a.a. 1–77) and C-variable spacer region (a.a. 167– 210) of Yra1, whereas the N-variable spacer region (a.a. 14–77) and C-variable spacer region (a.a. 167–210) of this protein are each sufficient to interact with RNA 43. To determine what region of Yra1 is necessary for Dbp2 binding, we obtained bacterial expression plasmids for expression of two GST-tagged Yra1 truncation mutations that express either the RRM and C-terminal region (RRM+C) or the C-terminal region alone (yra1C) (Fig. 5C, 49). We then purified the truncation mutants and conducted pull down assays as above. Interestingly, Dbp2 interacted with all three proteins, full length Yra1, Yra1 RRM+C and the C-terminus alone (Fig. 5D, lanes 6 and 8), suggesting that the C-terminus constitutes the Dbp2-binding domain. We then attempted to determine if the C-terminus is necessary for this interaction, however, we were unable to express the GST-yra1ΔC mutant in bacteria. Regardless, these studies suggest that Dbp2 interacts with the C-terminus of Yra1.
Yra1 Inhibits the Helicase Activity of Dbp2
Many DEAD-box protein-binding factors also regulate the enzymatic activity of their respective RNA helicase. This includes the translation initiation factor eIF4A, whose helicase activity is activated by eIF4B, 4H and 4F, and eIF4AIII, whose ATPase activity is inhibited by Y14 and MAGOH 50; 51; 52; 53. Thus, we asked if Yra1 modulates the helicase activity of Dbp2. To test this, we first conducted in vitro unwinding assays with Dbp2 in the presence of full length Yra1. However, we were unable to accurately measure the unwinding activity of Dbp2 due to the previously documented strand annealing activity of Yra1 (54 and data not shown). To resolve this problem, we then analyzed the annealing activity of the minimal Dbp2-interacting domain, yra1C, which has previously been shown to have severely impaired RNA-binding activity in vitro43. Importantly, this revealed that the yra1C protein has no intrinsic annealing activity in vitro at the tested concentrations (Fig. S4E–F). To test the effect of yra1C on the unwinding activity of Dbp2, we conducted unwinding assays as above with the blunt end RNA duplex either with Dbp2 alone or with equimolar or two-fold excess of Yra1 (Fig. 6). Strikingly, we found that yra1C decreased both the unwinding rate (kobs(unw) ) and the amplitude of duplex unwinding by Dbp2 (Fig. 6A–B; Fig. S4A–C). In fact, the decreased unwinding rate is almost a full order of magnitude lower with yra1C (Fig. 6B). We also tested the unwinding activity of Dbp2 in the presence of two-fold molar excess of BSA to show that the unwinding inhibition effect is specific to Yra1. Interestingly, this revealed a slight increase in the kobs for unwinding most likely due to molecular crowding (Fig. 6A–B; Fig. S4D). This suggests that the inhibition of Dbp2 is specific to Yra1.
Figure 6. Yra1 modulates the enzymatic activity of Dbp2.
(A) Graphical representation of Dbp2 duplex unwinding with yra1C. Unwinding assays were conducted with the blunt end RNA duplex and either Dbp2 alone (600 nM) or with yra1C (600 nM, 1200 nM) or BSA (1200 nM). Representative non-denaturing gels are shown in Fig S4 and demonstrate that yra1C and BSA do not have intrinsic annealing activity. (B) The kobs(unw) and the amplitude of the unwinding reaction. The kobs(unw) and the amplitude are determined using the integrated rate law for a homogeneous first-order reaction as previously described26(C) Full length Yra1 moderately enhances ATP hydrolysis activity of Dbp2. In vitro ATPase assays were conducted with 200 nM of recombinant, purified Dbp2 and 250 µg/ml of total yeast RNA using a PK/lactate dehydrogenase enzyme-coupled absorbance based detection method as previously described 18. Recombinant, purified Yra1 was included where indicated at final concentrations from 100–600 nM. Equal concentrations of BSA were also tested to account for non-specific interactions. The ATPase activity of Dbp2 alone is similar to previous publications and has already been characterized 18(D) Yra1 moderately enhances the ATP binding affinity of Dbp2. In vitro ATPase assays were conducted as above with constant amounts of Dbp2, Yra1, total RNA (10 µg/mL), increasing amounts of ATP and constant MgCl2 (2 mM). Assays were also conducted with BSA in place of Yra1 to account for non-specific effects. The KM is the indicative of the ATP binding affinity of Dbp2. (E) Yra1 slightly increases the amount of RNA necessary for activation of ATP hydrolysis. In vitro ATPase assays were conducted as above with 200 nM Dbp2, 400 nM Yra1 and increasing amounts of total yeast RNA. The amount of RNA necessary for 50% stimulation of maximum ATPase activity (EC50) is reflective of the RNA binding affinity of Dbp2.
To elucidate the mechanism of inhibition, we then asked if Yra1 alters the ATPase activity of Dbp2 by conducting in vitro ATP hydrolysis assays with increasing concentrations of full length Yra1 or BSA (Fig. 6C). Consistent with our previous studies, Dbp2 exhibited an observed ATP hydrolysis rate (kobs) of 21 min−1 with saturating RNA (250 µg/ml of total yeast RNA) and 1 mM ATP (Fig. 6C). Whereas addition of BSA resulted in a slight enhancement of the kobs from 21 to 25 min−1, Yra1 gave a greater stimulation at each tested concentration. Thus, Yra1 slightly enhances the ATPase activity of Dbp2.
To determine if Yra1 enhances the ATPase rate by increasing the ATP-binding affinity of Dbp2, we measured the KM for ATP with or without a two-fold excess of Yra1 or BSA (Fig. 6D). This revealed that Yra1 reduces the KM for ATP by ~30%, from 2.3 to 1.6 mM. This modest effect is similar to the observed increase in ATPase rate. Although moderate, this increase is specific for Yra1 as addition of BSA resulted in an ATPase curve that was superimposable with Dbp2 alone. This suggests that Yra1 stimulates the ATPase activity of Dbp2 through increasing the affinity for ATP. We suggest that this decrease in the KM is biologically relevant because it occurs within the physiological range of cellular ATP concentrations.
Finally, we asked if Yra1 alters the effective RNA-binding activity of Dbp2. Thus, we measured the ATPase activity of Dbp2 as above but with a range of RNA concentrations from 1 ng/ml to 1 mg/ml (Fig. 6E). It is of note that the EC50 of Dbp2 alone is lower than our previous studies 18, due to a more refined purification method for enzymatically-active Dbp2 that increases its specific activity. Interestingly, inclusion of Yra1 increased the amount of RNA necessary for ATP hydrolysis by Dbp2 by ~50% (Fig. 6E). This suggests that Yra1 slightly reduces the RNA-binding affinity of Dbp2, while increasing the ATP binding and hydrolysis rate. We suggest that these subtle changes on the enzymatic parameters of Dbp2 result in release of Dbp2 from RNA, thereby inhibiting helicase activity in vitro. It is also possible that inhibition could also be due to Yra1 blocking initial association of Dbp2 with RNA. However, if this were the case, we would expect that Yra1 would reduce the RNA-dependent ATPase activity (Fig. 6C). Because we do not observe a decrease in RNA-dependent ATPase activity, this suggests that Yra1 inhibits duplex unwinding of Dbp2 through an as-of-yet uncharacterized mode distinct from other DEAD-box RNA helicase-interacting proteins.
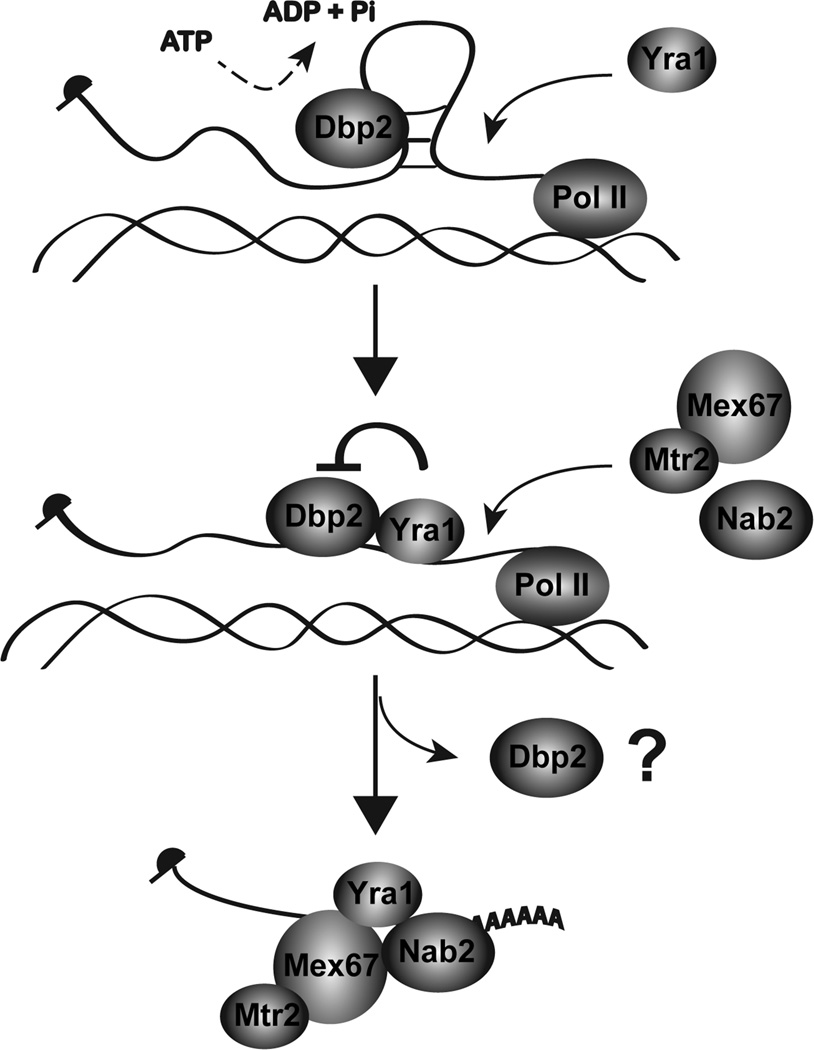
Taken together, we provide a model whereby Yra1 controls the enzymatic activity of Dbp2 to promote proper mRNP formation in gene expression (Fig. 7). During transcription, Dbp2 unwinds aberrant structures on the nascent transcript that are refractory to RNA-binding protein assembly. This facilitates the loading of Yra1, Mex67 and Nab2 and likely other RNA-binding proteins onto the mRNA. The interaction of Yra1 with Dbp2 then inhibits duplex unwinding and possibly also promotes Dbp2 release. Alternatively, Dbp2 may remain bound to the mRNA as part of a Yra1-Dbp2 complex. If this were the case, Dbp2 would function similarly to eIF4AIII, which acts as an RNA clamp for an ribonucleoprotein complex 52. With either scenario, we predict that inhibition of Dbp2 helicase activity by Yra1 prevents further remodeling of the properly assembled mRNP, as DEAD-box proteins can also efficiently remodel ribonucleoprotein complexes 55; 56; 57. This constitutes a previously unknown mechanism for regulation of RNA helicases as well as the first biochemical mechanism for co-transcriptional assembly of a mRNP complex.
Figure 7. Model for Dbp2-dependent loading of RNA-binding proteins onto mRNA.
Yra1 is recruited to the actively transcribing loci through interacting with Sub2 or Pcf11 on the C-terminal domain of the RNA polymerase II 49; 81. However, structures of the nascent mRNA prevent association with Yra1. Dbp2 unwinds these structures co-transcriptionally in an ATP-dependent manner. This promotes mRNP assembly by facilitating loading of Yra1, Nab2, and Mex67 onto nascent mRNA. Mex67 is shown interacting with its heterodimerization partner, Mtr2 82. Yra1 then inhibits the helicase activity of Dbp2 to prevent further remodeling of the assembled mRNP and may also promote release of Dbp2 from the RNA. This constitutes a biochemical mechanism of RNA helicase unwinding and subsequent inhibition during co-transcriptional assembly of mRNAs in the nucleus.
DISCUSSION
Proper nuclear mRNP assembly is crucial for co-transcriptional and post-transcriptional processing steps including removal of introns by splicing, 3’ end cleavage and polyadenylation, as well as formation of a translationally competent mRNA 10; 58. During each of these steps, the evolving mRNP must assemble with a complement of RNA-binding proteins to direct the next step in the gene expression process. Our studies now provide evidence that the DEAD-box RNA helicase, Dbp2, plays a critical role in mRNP assembly in the nucleus. The human ortholog of Dbp2, termed p68, has been implicated in numerous transcriptional and post-transcriptional events, including transcriptional regulation, alternative splicing and microRNA processing 15; 17; 59; 60. The fact that ectopic expression of human p68 in yeast fully complements the growth defects of dbp2Δ cells suggests that these roles are evolutionarily conserved 61. Given the multifaceted role, it is likely that p68 and Dbp2 are major players in the structural assembly of the transcriptome in all eukaryotes.
Our studies establish that Dbp2 is a bona fide RNA helicase, with efficient duplex unwinding activity on blunt ended duplexes. This suggests that Dbp2 recognizes secondary structure directly, without the need for a single stranded region for initial “loading” of the enzyme. This activity is consistent with a subset of DEAD-box family members with highly efficient duplex unwinding, such as CYT-19 and Mss116 22; 62; 63; 64. Moreover, it is consistent with our previous observation that Dbp2 displays dsRNA-directed ATPase activity 18. Interestingly, whereas the core sequence is conserved among all DEAD-box protein family members, these three enzymes also contain a C-terminal RGG extension. In fact, a recent biochemical and structural analysis of CYT-19 demonstrated that the RGG motif of this enzyme functions as a ‘tether” to enable multiple rounds of duplex unwinding 65.
Several DEAD-box proteins have been shown to utilize protein cofactors to trigger duplex unwinding by increasing the ATP binding or RNA-binding affinities of an inefficient DEAD-box enzyme 51; 66; 67; 68. Given the high duplex unwinding activity of Dbp2, however, inhibition may be the more important mode of regulation. In support of this, we find that Yra1 inhibits the helicase activity of Dbp2. The human ortholog of Dbp2, p68, was recently shown to interact with Aly, the human counterpart to Yra1 19, suggesting that this regulation is conserved in higher eukaryotes. We speculate that in vivo the modulation of Dbp2 helicase activity by Yra1 is utilized to prevent further remodeling of the assembled mRNP. If this is the case, this would constitute a previously unrecognized mechanism for temporal regulation of DEAD-box enzymes in vivo. Although we do not know the mechanism for inhibition of duplex unwinding by Yra1, a recent study of Mss116 revealed that DEAD-box proteins are modular enzymes 69. In fact, the C-terminal domain provides direct recognition of double-stranded RNA duplexes whereas the N-terminal domain interacts with ATP 69. The ability to couple ATP hydrolysis with duplex unwinding lies in the formation of a closed helicase with juxtaposed N and C-terminal domains 69. Because our studies suggest that Yra1 uncouples ATP hydrolysis from duplex unwinding, it will be interesting to determine the precise mechanism for Yra1-dependent inhibition of Dbp2 given this insight.
Our studies show that Dbp2 is required for assembly of Yra1, Nab2 and Mex67 onto Poly(A)+ RNA. It is well established that proper termination and 3’ end formation is required for mRNA export, as defects in these processes result in impaired recruitment of Mex67 to newly synthesized mRNAs and RNA decay 33; 70; 71 The fact that loss of DBP2 results in reduced association of Mex67 as well as the poly(A)+ RNA-binding protein Nab2, suggests that Dbp2 functions concert with termination and 3’ end formation. In support of this, loss of DBP2 results in transcription of a bicistronic GAL10-GAL7 mRNA, a characteristic phenotype of termination defects 18. This idea is also consistent with our genetic analysis and the fact that DBP2 overexpression resulted in lethality of mex67-5 strains but not sub2–85 or pcf11-2 strains. This suggests that Dbp2 functions upstream of Mex67 but downstream or independent of Sub2 and Pcf11. Interestingly, Yra1 also interacts directly with all three of these proteins 36; 40; 49, indicating that this small protein acts as a coupling factor for multiple co-transcriptional processing and assembly steps. Furthermore, recent studies from the Bentley laboratory have demonstrated that Pcf11 is required for recruitment of Yra1 to chromatin, which then functions to modulate poly(A) site selection 49; 72. Thus, the order of events for this process and role of Dbp2 in termination is an intriguing question for future studies.
In addition to canonical duplex unwinding, our studies also show that Dbp2 displays strong RNA strand-annealing activity. This is not unprecedented as the DEAD-box protein Mss116 utilizes both annealing and duplex unwinding activities to promote folding of the ai5γ group II intron in mitochondria 28; 29; 30. This would suggest that Dbp2 could function similarly, however, in contrast to Mss116, Dbp2 only displays significant annealing under ATP-limiting conditions. Interestingly, recent work from the Parker laboratory revealed that, under conditions of glucose starvation, the sub-cellular localization of numerous RNA-binding proteins is drastically altered 73. This suggests that cellular ribonucleoprotein complexes undergo dynamic alterations in nutrient-limited conditions when cellular ATP concentrations are low. Thus, it will be interesting to determine the function of Dbp2 under specific physiological growth conditions, which may promote strand annealing.
Our studies now add Dbp2 to the complement of DEAD-box proteins that function in nuclear mRNP assembly in S. cerevisiae. This includes Sub2, which functions in both splicing and formation of an export competent mRNP, and Dbp5, which promotes nuclear release of exporting transcripts 14. When considering that rRNA biogenesis requires 21 of the 25 DEAD-box proteins in budding yeast 14; 74, one might ask why there aren’t more DEAD-box RNA helicases associated with mRNP biogenesis. Unlike other cellular RNAs such as snRNAs, tRNAs and rRNAs, mRNAs stand out as distinct as tertiary structure does not appear to play a large role in the functionality of these RNAs in eukaryotes. Given the propensity for RNAs to fold and misfold in solution 1, the prevailing model is that co-transcriptional association of RNA-binding proteins maintains primarily linear structure of a nascent transcript 10. Although the average length of an mRNA is 1kb, pre-mRNA transcripts can range from a 3 Kb to ~2.5 Mb, making it likely that DEAD-box helicases function as key structural modulators of the transcriptome. The challenge then will be defining the precise molecular rearrangements that require p68/Dbp2 or other members of the DEAD-box protein family given the highly coupled nature of nuclear gene expression steps. With the advancement in RNA sequence and target identification coupled with structural studies of mRNAs 75, these questions can be addressed in the very near future.
MATERIALS AND METHODS
Yeast strains, yeast plasmids and bacterial plasmids
Listed in Table 1 and Table 2.
Table 1.
Yeast and bacterial plasmids
| Name | Description | Source/Reference |
|---|---|---|
| BTP13 | pET28a-DBP2 | 29 |
| pCEN/URA3 | pRS316 | 38 |
| pGAL1-GAL10-GAL7 | pYGPM11714 | Open Biosystems (Genomic Tiling) |
| BTP22 | pMAL-TEV-Dbp2 | This study |
| BTP27 | GST-TEV-Yra1 | This study |
| pSW3319 | GST-Dbp5 | 39 |
| pRS426 | p(URA3/2µ | 38 |
| PGAL-DBP2 | pGAL-DBP2/2µ/URA3 | Open Biosystems |
| GST-Yra1 C | pET21GST-Yra1 C | 36 |
| GST-Yra1 RRM+C | pET21GST-Yra1 RRM+C | 36 |
| psub2-85 | psub2-85/CEN/TRP1 | 40 |
Table 2.
Yeast strains
| Strain | Genotype | Source |
|---|---|---|
| Wild type (BY4741) | MATahis3Δ Ieu2Δ0 met15Δ0 ura3Δ0 |
Open Biosystems |
| dbp2Δ(BTY115) | MATadbp2∷KanMx ura3Δ0 Ieu2Δ0 his3Δ0 TRP1 met- lysΔ |
29 |
| DBP2-TAP | MATaDBP2-TAP:HIS3 his3Δ Ieu2Δ0 met15Δ0 ura3Δ0 |
Open Biosystems |
| mex67-5 | MATamex67∷HIS3 ura3 ade2 his3 leu2 trpl pTRP/CEN/mex67-5 |
41 |
| Wild type (W303) | MATaura3-1 ade2-1 his3- 11, 15leu2-1 trp1-1 can1- 100 |
R. Rothstein |
| yra1ΔN + Yra1 | MATayra1∷HIS3 ura3 ade2 ade3 Ieu2-1 trp1 pRS314-yra1ΔN +pHT4467Δ-YRA1 (with intron) |
40 |
| pcf11-2 | MATaura3-1 trpΔade2-1 leu2-3, 112his3–11, 15 pcf11-2 |
42 |
| SUB2 shuffle | MATasub2∷HIS3 ade2 Ieu2, ura3, trpl pCG788 |
43 |
Recombinant Protein Expression and Purification
Expression of pMAL MBP-TEV-DBP2 in BL21 E. coli (DE3) cells (New England Bio Labs) was induced with 1 mM isopropyl 1-thio-β-D-galactopyranoside (IPTG) at 37 °C for 3 hours and was subsequently purified from the soluble fraction using amylose resin according to the manufacturer’s instructions (New England Bio Labs). The MBP tag was then cleaved with 50 U of TEV protease (Invitrogen) overnight at 16 °C. The cleaved Dbp2 was then subjected to cation exchange chromatography with SP sepharose (Sigma). Dbp2 was eluted in 50 mM Tris-HCl at pH 8 with 600 mM NaCl, and 20% glycerol and stored at –80 °C until usage. Expression of pET28a His6-DBP2 in Rosetta E. coli (DE3) cells (Novagen) was induced by 0.2 mM IPTG at 16 °C and purified as previously described 18. Two consecutive TEV sites were inserted between the GST-tag and the coding sequence of Yra1 by PCR using pFS1853 GST-Yra1 as a template, a set of primer pairs that contain the TEV sites coding sequence flanked next to the GST-tag and Yra1 coding sequence. Forward primer: 5’-GAAAACCTGTACTTCCAGGGAATGTCTGCTAACTTAGATAAATCCTTAGAC-3’ and a reverse primer: 5’-TCCCTGGAAGTACAGGTTTTCCTCGAGATGGTCGCCACCACCAAACGTGGC-3’. Expression of the GST-TEV-YRA1 in Rosetta E. coli (DE3) cells (Novagen) was induced by 0.2 mM IPTG overnight at 16 °C and was subsequently purified from the soluble fraction using glutathione sepharose according to the manufacturer’s instructions (GE healthcare). The GST tag was then cleaved with 50 U of TEV protease (Invitrogen) overnight at 16 °C. The cleaved purified recombinant proteins were subsequently subjected to SP sepharose (Sigma). Yra1 were eluted in 50 mM Tris-HCl at pH 8 with 600 mM NaCl, and 20% glycerol and stored at –80 °C until usage. Expression of the pET21 GST-Yra1C and pET21 GST-Yra1 RRM+C in Rosetta E. coli (DE3) cells (Novagen) was induced by 0.2 mM IPTG overnight at 16 °C and was subsequently purified from the soluble fraction using glutathione sepharose according to the manufacturer’s instructions (GE healthcare). The purified proteins were eluted with 20mM glutathione, 150 mM NaCl, 20% glycerol and 20 mM HEPES at pH 7.5 and stored at –80 °C until usage.
Unwinding Assays
RNA oligonucleotides were purchased from Integrated DNA Technologies (IDT), and duplex substrates were prepared as previously described 26; 76. The blunt end RNA duplex sequences are: (top strand) 5’-AGCACCGUAAAGACGC-3’ + (bottom strand) 5’-GCGUCUUUACGGUGCU-3’. The overhang RNA duplex sequences are: (top strand) 5’-AGCACCGUAAAGACGC-3’ + (bottom strand) 5’-GCGUCUUUACGGUGCUUAAAACAAAACAAAACAAAAC-3’. The blunt end RNA/DNA duplex sequences are: (top strand) 5’-GGCACGGUGGGGACCG-3’ + (bottom strand) 5’-CGGTCCCCACCGTGCC-3’. The top strand of the RNA duplex was 5’ end-labeled with [Y32P]-ATP using T4 polynucleotide kinase according to standard methods. In vitro unwinding assays were conducted as previously described 26 except for using 0.1 nM 32P labeled duplex in a 30 µl reaction mixture containing 40 mM TrisHCl (pH 8), 50 mM NaCl, 2.5 mM MgCl2, 2 mM DTT, 60 U Superase-in (Life Technologies) and 600 nM Dbp2 and 600 nM or 1200 nM of Yra1 where indicated. The reaction mixture was incubated in a 19 °C water bath for 10 min prior to the reaction. All reactions were performed at 19 °C. Unwinding reactions were initiated by adding ATP (2 mM or 0.1 mM as indicated). At the times indicated, 3 µl aliquots were removed and the reaction was stopped with a buffer containing 1% SDS, 50 mM EDTA, 0.1% xylene cyanol, 0.1% bromophenol blue and 20% glycerol. Aliquots were subsequently resolved on a 10% nondenaturing PAGE. The gels were dried and radiolabeled RNAs were quantified using ImageQuant software. The data from each time point was calculated using the formula: fraction of single stranded = (single stranded RNA / total RNA). The data was then fitted to the integrated form of a homogenous first order rate law to determine the kobs(unw). The rate constants for unwinding kunw and kann were determined using Frac ss =kunw(kunw + kann)–1(1 – exp(-(kunw + kann)t)) as described in 26.
Annealing assays
In vitro annealing assays were performed in the presence of 2 mM or 0.1 mM ATP with the same reaction mixture as unwinding assays without the 10 min pre-incubation. The RNA duplex was denatured at 95 °C for 2 min to generate single-stranded RNAs. All the reactions were conducted in a 19 °C water bath and were initiated by addition of 0.1 nM of the denatured substrate strands. Aliquots of the reactions were treated as described in the unwinding assays. The data from each time point was calculated as described in the unwinding assays. The data was then fitted to the integrated rate law for the bimolecular annealing reaction to determine the kobs(ann).
Cellular Microscopy
In situ hybridization was performed on cells that were grown to mid-log phase at the permissive temperature (25 °C) with −URA+2% glucose and then shifted to −URA+2% galactose for a 1-hour induction of DBP2 overexpression. Cells were subsequently harvested, fixed with formaldehyde and mounted on glass slides. Poly(A)+ RNA was then visualized by microscopy following hybridization with digoxygenin-conjugated oligodT50 and detection with FITC-conjugated anti-digoxygenin secondary antibody (Roche) as previously described 55. DAPI staining was utilized to visualize DNA 55. Images were collected using an Olympus BX51 microscope using Metamorph software.
TAP-tag immunoprecipitation
Cells expressing genomically encoded Dbp2-TAP or untagged Dbp2 (BY4741) were grown in YPD at 30 °C to an OD600 of 0.6. Harvested cells were pelleted and injected into liquid nitrogen. The frozen cells were then lysed in the solid phase by milling, using a planetary ball mill. The lysed cells were subsequently resuspended in 15ml of extraction buffer (20mM HEPES at pH7.4, 110 mM KoAc, 0.5% Triton X-100, 0.1% Tween-20 and 70 mM NaCl) in the presence of 1X protease inhibitor cocktail tablets (Roche) followed by centrifugation at 4700 RPM at 4 °C for 15 min as previously described 77. The soluble fraction of the lysate was incubated with IgG-conjugated dynabeads at 4 °C for 30 min. The unbound protein was washed away with extraction buffer. The bound protein was eluted with 10 U of AcTEV protease (Life Technologies) followed by TCA precipitation. The proteins were then resolved by SDS-PAGE and detected by Western blotting analysis. Western blotting analysis was conducted with standard molecular biology techniques rabbit anti-Yra1 49, rabbit anti-Protein A and horseradish peroxidase conjugated goat anti-rabbit antibody (Sigma).
In vitro binding (pull down) assays
20 µg of recombinant, purified GST-Yra1, GST-Yra1 RRM+C, GST- Yra1 C, GST-Dbp5, His-Dbp2, Dbp5 or Dbp2 were incubated with the glutathione sepharose in 20 mM HEPES pH 7.5, 150 mM NaCl and 20% glycerol at room temperature for 10min as indicated following removal of 20% of the protein mixture for input. Bound proteins were eluted with 50 mM reduced glutathione in 20 mM HEPES pH 7.5, 150 mM NaCl and 20% glycerol and were resolved by SDS-PAGE followed by Coomassie staining.
In vitro ATPase assays
In vitro ATP hydrolysis assays were performed using a PK/lactate dehydrogenase enzyme-coupled absorbance assay as previously described 18, but with 200 nM Dbp2 and increasing amounts of recombinant purified Yra1, total yeast RNA (Sigma) or ATP as indicated. Presented data is the average of three independent experiments and the error bars represent the standard deviation.
In vivo UV cross-linking assays
Wild type and dbp2Δ yeast cells were grown in YPD at 30 °C. Mid-log phase cells were harvested and resuspended into 50 ml of 10 mM Tris-HCl at pH 7.5, 500 mM NaCl and 1 mM EDTA. The resuspended cells were then subjected to UV light with 180,000 µJ/cm2 on ice for 2.5 minutes using UV Stratalinker 1800. The UV treatment was conducted twice with 45-second pause in between each treatment. The cells were then centrifuged at 4000 rpm for 10 minutes at 4 °C. The pelleted cells were resuspended into 10 ml of 10 mM Tris-HCl at pH 7.5, 500 mM NaCl, 1 mM EDTA, 500 U of Superase-in (Life Technologies), 1 mM PMSF and 0.5 X of protease inhibitor cocktail tablets (Roche). The cells were then lysed by bead beating, cleared by centrifugation and then subjected to poly(A)+ RNA pull down using oligo dT cellulose (Life Technologies). The RNA concentration from the eluted fraction was determined by measuring the absorbance at 260 nm. RNase treatment and TCA precipitation were then performed to recover bound proteins. Fractions were then resolved by SDS-PAGE and proteins were detected by western blotting with rabbit anti-Nab2 55, rabbit anti-Mex67 78, rabbit anti-Yra1 49 and horseradish peroxidase conjugated goat anti-rabbit antibodies (Promega).
RT-qPCR analysis
RNA was isolated from oligo dT-purified RNPs (see UV crosslinking) by standard acid phenol purification. Equal fractions from the elution were then reverse transcribed into complementary DNA (cDNA) (Qiagen) and the quantity of ACT1 RNA was measured by quantitative PCR using BioRad CFX96 system. The sequences for ACT1 primers were: (Forward primer) 5’-TGGATTCCGGTGATGGTGTT3’ and (Reverse primer) 5’-TCAAAATGGCGTGAGGTAGAGA-3’. The fold change of ACT1 transcript abundance was calculated by normalizing the signal from each sample to the signal obtained from wild type without UV treatment and are reported as the average of three technical repeats with standard error from the mean (S.E.).
Serial dilution growth assay
Indicated strains were grown in −URA+2% glucose or YPD liquid cultures and then harvested at mid-log phase. Cells were then spotted in 5-fold serial dilutions onto URA+2% glucose, −URA+2% galactose, or YPD plates and incubated at temperatures ranging from 16°C-37°C as indicated.
Supplementary Material
HIGHLIGHTS.
The DEAD-box RNA helicase Dbp2 from S. cerevisiae efficiently unwinds RNA duplexes.
Loss of DBP2 in yeast cells results in defective assembly of nuclear mRNPs.
Loading of Yra1, Nab2 and Mex67 into mRNPs requires DBP2.
Yra1 interacts directly with Dbp2 and also inhibits RNA-duplex unwinding.
A model for unwinding of structured, nascent mRNA prior to mRNP assembly is shown.
ACKNOWLEDGEMENTS
We thank Barb Golden constructive criticism regarding this manuscript. We also thank other members of the Tran laboratory for thoughtful discussions.
ABBREVIATIONS
- Pre-mRNA
precursor messenger RNA
- mRNA
messenger RNA
- mRNP
messenger ribonucleoprotein complex
- ATP
adenosine triphosphate
- Poly(A)
polyadenosine
- UV
ultraviolet
- TAP
tandem affinity purification
- 6XHIS
hexahistadine
- GST
glutathione-S-transferase
- S. cerevisiae
Saccharomyces cerevisiae
- RT-qPCR
reverse transcription-quantitative PCR
- bp
base pair
- nt
nucleotide
- Kb
kilobases
- Mb
megabases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Zemora G, Waldsich C. RNA folding in living cells. RNA Biol. 2010;7:634–641. doi: 10.4161/rna.7.6.13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan Y, Qu K, Ouyang Z, Kertesz M, Li J, Tibshirani R, Makino DL, Nutter RC, Segal E, Chang HY. Genome-wide measurement of RNA folding energies. Mol Cell. 2012;48:169–181. doi: 10.1016/j.molcel.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodson SA. Compact intermediates in RNA folding. Annu Rev Biophys. 2010;39:61–77. doi: 10.1146/annurev.biophys.093008.131334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treiber DK, Williamson JR. Exposing the kinetic traps in RNA folding. Curr Opin Struct Biol. 1999;9:339–345. doi: 10.1016/S0959-440X(99)80045-1. [DOI] [PubMed] [Google Scholar]
- 5.Wilson TJ, Nahas M, Ha T, Lilley DM. Folding and catalysis of the hairpin ribozyme. Biochem Soc Trans. 2005;33:461–465. doi: 10.1042/BST0330461. [DOI] [PubMed] [Google Scholar]
- 6.Woodson SA. RNA folding and ribosome assembly. Curr Opin Chem Biol. 2008;12:667–673. doi: 10.1016/j.cbpa.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern S, Powers T, Changchien LM, Noller HF. RNA-protein interactions in 30S ribosomal subunits: folding and function of 16S rRNA. Science. 1989;244:783–790. doi: 10.1126/science.2658053. [DOI] [PubMed] [Google Scholar]
- 8.Rajkowitsch L, Chen D, Stampfl S, Semrad K, Waldsich C, Mayer O, Jantsch MF, Konrat R, Blasi U, Schroeder R. RNA chaperones, RNA annealers and RNA helicases. RNA Biol. 2007;4:118–130. doi: 10.4161/rna.4.3.5445. [DOI] [PubMed] [Google Scholar]
- 9.Moore MJ. From birth to death: The complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 10.Schmid M, Jensen TH. Nuclear quality control of RNA polymerase II transcripts. Wiley interdisciplinary reviews RNA. 2010;1:474–485. doi: 10.1002/wrna.24. [DOI] [PubMed] [Google Scholar]
- 11.Gaspar P, Moura G, Santos MA, Oliveira JL. mRNA secondary structure optimization using a correlated stem-loop prediction. Nucleic Acids Res. 2013:41. doi: 10.1093/nar/gks1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kertesz M, Wan Y, Mazor E, Rinn JL, Nutter RC, Chang HY, Segal E. Genome-wide measurement of RNA secondary structure in yeast. Nature. 2010;467:103–107. doi: 10.1038/nature09322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putnam AA, Jankowsky E. DEAD-box helicases as integrators of RNA, nucleotide and protein binding. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbagrm.2013.02.002. http://dx.doi.org/10.1016/j.bbagrm.2013.02.002. [DOI] [PMC free article] [PubMed]
- 14.Linder P, Jankowsky E. From unwinding to clamping - the DEAD box RNA helicase family. Nature reviews Molecular cell biology. 2011;12:505–516. doi: 10.1038/nrm3154. [DOI] [PubMed] [Google Scholar]
- 15.Janknecht R. Multi-talented DEAD-box proteins and potential tumor promoters: p68 RNA helicase (DDX5) and its paralog, p72 RNA helicase (DDX17) American journal of translational research. 2010;2:223–234. [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuda T, Yamagata K, Fujiyama S, Matsumoto T, Koshida I, Yoshimura K, Mihara M, Naitou M, Endoh H, Nakamura T, Akimoto C, Yamamoto Y, Katagiri T, Foulds C, Takezawa S, Kitagawa H, Takeyama K, O’Malley BW, Kato S. DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nat Cell Biol. 2007;9:604–611. doi: 10.1038/ncb1577. [DOI] [PubMed] [Google Scholar]
- 17.Caretti G, Schiltz RL, Dilworth FJ, Di Padova M, Zhao P, Ogryzko V, Fuller-Pace FV, Hoffman EP, Tapscott SJ, Sartorelli V. The RNA helicases p68/p72 and the noncoding RNA SRA are coregulators of MyoD and skeletal muscle differentiation. Dev Cell. 2006;11:547–560. doi: 10.1016/j.devcel.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Cloutier SC, Ma WK, Nguyen LT, Tran EJ. The DEAD-box RNA Helicase Dbp2 Connects RNA Quality Control with Repression of Aberrant Transcription. The Journal of biological chemistry. 2012 doi: 10.1074/jbc.M112.383075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zonta E, Bittencourt D, Samaan S, Germann S, Dutertre M, Auboeuf D. The RNA helicase DDX5/p68 is a key factor promoting c-fos expression at different levels from transcription to mRNA export. Nucleic Acids Res. 2013;41:554–564. doi: 10.1093/nar/gks1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buszczak M, Spradling AC. The Drosophila P68 RNA helicase regulates transcriptional deactivation by promoting RNA release from chromatin. Genes Dev. 2006;20:977–989. doi: 10.1101/gad.1396306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Q, Del Campo M, Lambowitz AM, Jankowsky E. DEAD-box proteins unwind duplexes by local strand separation. Molecular Cell. 2007;28:253–263. doi: 10.1016/j.molcel.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Halls C, Mohr S, Del Campo M, Yang Q, Jankowsky E, Lambowitz AM. Involvement of DEAD-box proteins in group I and group II intron splicing. Biochemical characterization of Mss116p, ATP hydrolysis-dependent and independent mechanisms, and general RNA chaperone activity. J Mol Biol. 2007;365:835–855. doi: 10.1016/j.jmb.2006.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tijerina P, Bhaskaran H, Russell R. Nonspecific binding to structured RNA and preferential unwinding of an exposed helix by the CYT-19 protein, a DEAD-box RNA chaperone. Proc Natl Acad Sci U S A. 2006;103:16698–16703. doi: 10.1073/pnas.0603127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner AM, Love CF, Alexander RW, Jones PG. Mutational analysis of the Escherichia coli DEAD box protein CsdA. J Bacteriol. 2007;189:2769–2776. doi: 10.1128/JB.01509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stampfl S, Doetsch M, Beich-Frandsen M, Schroeder R. Characterization of the kinetics of RNA annealing and strand displacement activities of the E. coli DEAD-box helicase CsdA. RNA Biol. 2013;10:149–156. doi: 10.4161/rna.23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Q, nkowsky E. ATP- and ADP-dependent modulation of RNA unwinding and strand annealing activities by the DEAD-box protein DED1. Biochemistry. 2005;44:13591–13601. doi: 10.1021/bi0508946. [DOI] [PubMed] [Google Scholar]
- 27.Rossler OG, Straka A, Stahl H. Rearrangement of structured RNA via branch migration structures catalysed by the highly related DEAD-box proteins p68 and p72. Nucleic Acids Res. 2001;29:2088–2096. doi: 10.1093/nar/29.10.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zingler N, Solem A, Pyle AM. Dual roles for the Mss116 cofactor during splicing of the ai5gamma group II intron. Nucleic Acids Res. 2010;38:6602–6609. doi: 10.1093/nar/gkq530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fedorova O, Solem A, Pyle AM. Protein-facilitated folding of group II intron ribozymes. J Mol Biol. 2010;397:799–813. doi: 10.1016/j.jmb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liebeg A, Mayer O, Waldsich C. DEAD-box protein facilitated RNA folding in vivo. RNA Biol. 2010;7:803–811. doi: 10.4161/rna.7.6.13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore MJ, Proudfoot NJ. Pre-mRNA processing reaches back to transcription and ahead to translation. Cell. 2009;136:688–700. doi: 10.1016/j.cell.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Fasken MB, Corbett AH. Mechanisms of nuclear mRNA quality control. RNA Biol. 2009;6:237–241. doi: 10.4161/rna.6.3.8330. [DOI] [PubMed] [Google Scholar]
- 33.Qu X, Lykke-Andersen S, Nasser T, Saguez C, Bertrand E, Jensen TH, Moore C. Assembly of an export-competent mRNP is needed for efficient release of the 3'-end processing complex after polyadenylation. Mol Cell Biol. 2009;29:5327–5338. doi: 10.1128/MCB.00468-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birse CE, Minvielle-Sebastia L, Lee BA, Keller W, Proudfoot NJ. Coupling termination of transcription to messenger RNA maturation in yeast. Science. 1998;280:298–301. doi: 10.1126/science.280.5361.298. [DOI] [PubMed] [Google Scholar]
- 35.Amrani N, Minet M, Wyers F, Dufour ME, Aggerbeck LP, Lacroute F. PCF11 encodes a third protein component of yeast cleavage and polyadenylation factor I. Mol Cell Biol. 1997;17:1102–1109. doi: 10.1128/mcb.17.3.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strasser K, Hurt E. Splicing factor Sub2p is required for nuclear mRNA export through its interaction with Yra1p. Nature. 2001;413:648–652. doi: 10.1038/35098113. [DOI] [PubMed] [Google Scholar]
- 37.Kistler AL, Guthrie C. Deletion of MUD2, the yeast homolog of U2AF65, can bypass the requirement for sub2, an essential spliceosomal ATPase. Genes Dev. 2001;15:42–49. doi: 10.1101/gad.851301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stutz F, Bachi A, Doerks T, Braun IC, Seraphin B, Wilm M, Bork P, Izaurralde E. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA. 2000;6:638–650. doi: 10.1017/s1355838200000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segref A, Sharma K, Doye V, Hellwig A, Huber J, Luhrmann R, Hurt E. Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J. 1997;16:3256–3271. doi: 10.1093/emboj/16.11.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strasser K, Hurt E. Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export. EMBO J. 2000;19:410–420. doi: 10.1093/emboj/19.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iglesias N, Tutucci E, Gwizdek C, Vinciguerra P, Von Dach E, Corbett AH, Dargemont C, Stutz F. Ubiquitin-mediated mRNP dynamics and surveillance prior to budding yeast mRNA export. Genes Dev. 2010;24:1927–1938. doi: 10.1101/gad.583310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilbert W, Guthrie C. The Glc7p nuclear phosphatase promotes mRNA export by facilitating association of Mex67p with mRNA. Mol Cell. 2004;13:201–212. doi: 10.1016/s1097-2765(04)00030-9. [DOI] [PubMed] [Google Scholar]
- 43.Zenklusen D, Vinciguerra P, Strahm Y, Stutz F. The yeast hnRNP-Like proteins Yra1p and Yra2p participate in mRNA export through interaction with Mex67p. Mol Cell Biol. 2001;21:4219–4232. doi: 10.1128/MCB.21.13.4219-4232.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hogan DJ, Riordan DP, Gerber AP, Herschlag D, Brown PO. Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system. PLoS Biol. 2008;6:e255. doi: 10.1371/journal.pbio.0060255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson JT, Wilson SM, Datar KV, Swanson MS. NAB2: a yeast nuclear polyadenylated RNA-binding protein essential for cell viability. Mol Cell Biol. 1993;13:2730–2741. doi: 10.1128/mcb.13.5.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jankowsky E. RNA helicases at work: binding and rearranging. Trends in biochemical sciences. 2011;36:19–29. doi: 10.1016/j.tibs.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oeffinger M, Wei KE, Rogers R, DeGrasse JA, Chait BT, Aitchison JD, Rout MP. Comprehensive analysis of diverse ribonucleoprotein complexes. Nat Methods. 2007;4:951–956. doi: 10.1038/nmeth1101. [DOI] [PubMed] [Google Scholar]
- 48.Kashyap AK, Schieltz D, Yates J, Kellogg DR. Biochemical and genetic characterization of Yra1p in budding yeast. Yeast. 2005;22:43–56. doi: 10.1002/yea.1185. [DOI] [PubMed] [Google Scholar]
- 49.Johnson SA, Cubberley G, Bentley DL. Cotranscriptional recruitment of the mRNA export factor Yra1 by direct interaction with the 3'-end processing factor Pcf11. Mol Cell. 2009;33:215–226. doi: 10.1016/j.molcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oberer M, Marintchev A, Wagner G. Structural basis for the enhancement of eIF4A helicase activity by eIF4G. Genes Dev. 2005;19:2212–2223. doi: 10.1101/gad.1335305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogers GW, Jr, Richter NJ, Lima WF, Merrick WC. Modulation of the helicase activity of eIF4A by eIF4B, eIF4H, and eIF4F. J Biol Chem. 2001;276:30914–30922. doi: 10.1074/jbc.M100157200. [DOI] [PubMed] [Google Scholar]
- 52.Ballut L, Marchadier B, Baguet A, Tomasetto C, Seraphin B, Le Hir H. The exon junction core complex is locked onto RNA by inhibition of eIF4AIII ATPase activity. Nat Struct Mol Biol. 2005;12:861–869. doi: 10.1038/nsmb990. [DOI] [PubMed] [Google Scholar]
- 53.Nielsen KH, Chamieh H, Andersen CB, Fredslund F, Hamborg K, Le Hir H, Andersen GR. Mechanism of ATP turnover inhibition in the EJC. RNA. 2009;15:67–75. doi: 10.1261/rna.1283109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Portman DS, O'Connor JP, Dreyfuss G. YRA1, an essential Saccharomyces cerevisiae gene, encodes a novel nuclear protein with RNA annealing activity. RNA. 1997;3:527–537. [PMC free article] [PubMed] [Google Scholar]
- 55.Tran EJ, Zhou Y, Corbett AH, Wente SR. The DEAD-box protein Dbp5 controls mRNA export by triggering specific RNA:protein remodeling events. Mol Cell. 2007;28:850–859. doi: 10.1016/j.molcel.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 56.Jankowsky E, Gross CH, Shuman S, Pyle AM. Active disruption of an RNA-protein interactionby a DExH/D RNA helicase. Science. 2001;291:121–125. doi: 10.1126/science.291.5501.121. [DOI] [PubMed] [Google Scholar]
- 57.Fairman ME, Maroney PA, Wang W, Bowers HA, Gollnick P, Nilsen TW, Jankowsky E. Protein displacement by DExH/D “RNA helicases” without duplex unwinding. Science. 2004;304:730–734. doi: 10.1126/science.1095596. [DOI] [PubMed] [Google Scholar]
- 58.Kallehauge TB, Robert MC, Bertrand E, Jensen TH. Nuclear retention prevents premature cytoplasmic appearance of mRNA. Mol Cell. 2012;48:145–152. doi: 10.1016/j.molcel.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 59.Kar A, Fushimi K, Zhou X, Ray P, Shi C, Chen X, Liu Z, Chen S, Wu JY. RNA helicase p68 (DDX5) regulates tau exon 10 splicing by modulating a stem-loop structure at the 5' splice site. Molecular and Cellular Biology. 2011;31:1812–1821. doi: 10.1128/MCB.01149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salzman DW, Shubert-Coleman J, Furneaux H. P68 RNA helicase unwinds the human let-7 microRNA precursor duplex and is required for let-7-directed silencing of gene expression. J Biol Chem. 2007;282:32773–32779. doi: 10.1074/jbc.M705054200. [DOI] [PubMed] [Google Scholar]
- 61.Barta I, Iggo R. Autoregulation of expression of the yeast Dbp2p ‘DEAD-box’ protein is mediated by sequences in the conserved DBP2 intron. EMBO J. 1995;14:3800–3808. doi: 10.1002/j.1460-2075.1995.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y, Potratz JP, Tijerina P, Del Campo M, Lambowitz AM, Russell R. DEAD-box proteins can completely separate an RNA duplex using a single ATP. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20203–20208. doi: 10.1073/pnas.0811075106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Del Campo M, Mohr S, Jiang Y, Jia H, Jankowsky E, Lambowitz AM. Unwinding by local strand separation is critical for the function of DEAD-box proteins as RNA chaperones. J Mol Biol. 2009;389:674–693. doi: 10.1016/j.jmb.2009.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Q, Jankowsky E. The DEAD-box protein Ded1 unwinds RNA duplexes by a mode distinct from translocating helicases. Nat Struct Mol Biol. 2006;13:981–986. doi: 10.1038/nsmb1165. [DOI] [PubMed] [Google Scholar]
- 65.Mallam AL, Jarmoskaite I, Tijerina P, Del Campo M, Seifert S, Guo L, Russell R, Lambowitz AM. Solution structures of DEAD-box RNA chaperones reveal conformational changes and nucleic acid tethering by a basic tail. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12254–12259. doi: 10.1073/pnas.1109566108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Granneman S, Lin C, Champion EA, Nandineni MR, Zorca C, Baserga SJ. The nucleolar protein Esf2 interacts directly with the DExD/H box RNA helicase, Dbp8, to stimulate ATP hydrolysis. Nucleic Acids Res. 2006;34:3189–3199. doi: 10.1093/nar/gkl419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alcazar-Roman AR, Tran EJ, Guo S, Wente SR. Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export. Nat Cell Biol. 2006;8:711–716. doi: 10.1038/ncb1427. [DOI] [PubMed] [Google Scholar]
- 68.Weirich CS, Erzberger JP, Flick JS, Berger JM, Thorner J, Weis K. Activation of the DExD/H-box protein Dbp5 by the nuclear-pore protein Gle1 and its coactivator InsP6 is required for mRNA export. Nat Cell Biol. 2006;8:668–676. doi: 10.1038/ncb1424. [DOI] [PubMed] [Google Scholar]
- 69.Mallam AL, Del Campo M, Gilman B, Sidote DJ, Lambowitz AM. Structural basis for RNA-duplex recognition and unwinding by the DEAD-box helicase Mss116p. Nature. 2012;490:121–125. doi: 10.1038/nature11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmid M, Poulsen MB, Olszewski P, Pelechano V, Saguez C, Gupta I, Steinmetz LM, Moore C, Jensen TH. Rrp6p controls mRNA poly(A) tail length and its decoration with poly(A) binding proteins. Mol Cell. 2012;47:267–280. doi: 10.1016/j.molcel.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saguez C, Schmid M, Olesen JR, Ghazy MA, Qu X, Poulsen MB, Nasser T, Moore C, Jensen TH. Nuclear mRNA surveillance in THO/sub2 mutants is triggered by inefficient polyadenylation. Mol Cell. 2008;31:91–103. doi: 10.1016/j.molcel.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 72.Johnson SA, Kim H, Erickson B, Bentley DL. The export factor Yra1 modulates mRNA 3' end processing. Nat Struct Mol Biol. 2011;18:1164–1171. doi: 10.1038/nsmb.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitchell SF, Jain S, She M, Parker R. Global analysis of yeast mRNPs. Nat Struct Mol Biol. 2013;20:127–133. doi: 10.1038/nsmb.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cordin O, Banroques J, Tanner NK, Linder P. The DEAD-box protein family of RNA helicases. Gene. 2006;367:17–37. doi: 10.1016/j.gene.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 75.Wan Y, Kertesz M, Spitale RC, Segal E, Chang HY. Understanding the transcriptome through RNA structure. Nat Rev Genet. 2011;12:641–655. doi: 10.1038/nrg3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jankowsky E, Putnam A. Duplex unwinding with DEAD-box proteins. Methods in molecular biology. 2010;587:245–264. doi: 10.1007/978-1-60327-355-8_18. [DOI] [PubMed] [Google Scholar]
- 77.Carmody SR, Tran EJ, Apponi LH, Corbett AH, Wente SR. The mitogen-activated protein kinase Slt2 regulates nuclear retention of non-heat shock mRNAs during heat shock-induced stress. Mol Cell Biol. 2010;30:5168–5179. doi: 10.1128/MCB.00735-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gwizdek C, Iglesias N, Rodriguez MS, Ossareh-Nazari B, Hobeika M, Divita G, Stutz F, Dargemont C. Ubiquitin-associated domain of Mex67 synchronizes recruitment of the mRNA export machinery with transcription. Proc Natl Acad Sci U S A. 2006;103:16376–16381. doi: 10.1073/pnas.0607941103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugimoto N, Nakano S, Katoh M, Matsumura A, Nakamuta H, Ohmichi T, Yoneyama M, Sasaki M. Thermodynamic parameters to predict stability of RNA/DNA hybrid duplexes. Biochemistry. 1995;34:11211–11216. doi: 10.1021/bi00035a029. [DOI] [PubMed] [Google Scholar]
- 80.Turner DH, Mathews DH. NNDB: the nearest neighbor parameter database for predicting stability of nucleic acid secondary structure. Nucleic Acids Res. 2010;38:D280–D282. doi: 10.1093/nar/gkp892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Strasser K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, Rondon AG, Aguilera A, Struhl K, Reed R, Hurt E. TREX is a conserved complex coupling transcription with messenger RNA export. Nature. 2002;417:304–308. doi: 10.1038/nature746. [DOI] [PubMed] [Google Scholar]
- 82.Katahira J, Strasser K, Podtelejnikov A, Mann M, Jung JU, Hurt E. The Mex67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J. 1999;18:2593–2609. doi: 10.1093/emboj/18.9.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.