Abstract
The nearly ubiquitous development of chemoresistant disease remains a major obstacle against improving outcomes for ovarian cancer patients. In this investigation we evaluated the preclinical activity of MLN4924, an investigational inhibitor of the NEDD8-activating enzyme, in ovarian cancer cells. Efficacy of MLN4924 both alone and in combination with platinum was assessed. Overall, single agent MLN4924 exhibited moderate activity in ovarian cancer cell lines. However, the combination of MLN4924 with cisplatin or carboplatin produced synergistic effects in SKOV3 and ES2 cells, as well as, in primary ovarian cancer cell lines established from high grade serous, clear cell, and serous borderline ovarian tumors. The efficacy of cisplatin plus MLN4924 was also evident in several in vitro models of platinum resistant ovarian cancer. Mechanistically, the combination of cisplatin and MLN4924 was not associated with DNA re-replication, altered platinum-DNA adduct formation, abrogation of FANCD2 monoubiquitination, or CHK1 phosphorylation. An siRNA screen was used to investigate the contribution of each member of the Cullin RING-Ligase (CRL) family of E3 ubiquitin ligases, the best characterized downstream mediators of MLN4924’s biological effects . Cisplatin-induced cytotoxicity was augmented by depletion of CUL3, and antagonized by siCUL1 in both ES2 and SKOV3 ovarian cancer cells. This investigation identifies inhibition of Neddylation as a novel mechanism for overcoming platinum resistance in vitro, and provides a strong rationale for clinical investigations of platinum and MLN4924 combinations in ovarian cancer.
Keywords: MLN4924, cisplatin, ovarian cancer, neddylation, cullin ring ligase, platinum resistance
Introduction
Epithelial ovarian cancer is the most lethal female reproductive malignancy, resulting in over 22,000 new cases and 15,000 deaths in the U.S. each year (1). Approximately 85% of ovarian cancers have already metastasized beyond the ovary at the time of diagnosis and require adjuvant chemotherapy following surgical excision. Compared to other solid tumors, ovarian cancer is a relatively chemo-responsive disease, and the vast majority of advanced stage epithelial ovarian cancers initially respond to adjuvant platinum-based chemotherapy. However, the inevitable development of chemoresistant disease in the vast majority of patients remains a major obstacle against improving outcomes for ovarian cancer patients. Thus, improving response and overcoming resistance to chemotherapy are of paramount importance in this disease.
NEDD8 (Neural precursor cell Expressed, Developmentally Down-regulated 8) is a small ubiquitin-like molecule, involved in regulatory post-translational modification of proteins. A new class of antineoplastic agents targeting the NEDD8 conjugation pathway (also referred to as neddylation) has been recently discovered (2). The first in class compound MLN4924 is a small molecule inhibitor that selectively inactivates NEDD8 activating enzyme (NAE), the E1 involved in this pathway (3). Neddylation is a key regulatory mechanism required for the activation of the cullin RING ligase (CRL) family of E3 ubiquitin ligases. Hence, by inhibiting neddylation, MLN4924 antagonizes the ubiquitylation activity of the downstream CRLs and results in the abnormal accumulation of their substrates. These substrates include CDK inhibitors (p21 and p27), DNA replication licensing factor CDT1 (4), as well as many other proteins (5).
The objective of our investigation was to evaluate the activity of MLN4924 against chemoresistant and chemo-sensitive ovarian cancer cells. We found that MLN4924 as a single agent exhibits moderate activity against different histological subtypes of ovarian cancer. However, the combination of MLN4924 with cisplatin or carboplatin resulted in synergistic cytotoxicity. The mechanisms for this synergy do not involve increased DNA re-replication or platinum-DNA adduct formation. Inhibition of CUL3 (but not other cullins) also had a platinum sensitizing effect suggesting this pathway may be implicated in platinum resistant ovarian cancer.
Materials & Methods
Established and Primary Cell Lines
Following IRB approval, de-identified primary human ovarian cancer cell lines of various histological subtypes were established as described previously (6). SKOV3 and ES2 cell lines were purchased from a vendor that verifies their identity using genomic fingerprinting (ATCC) and gown in McCoy’s 5A media with 10% FBS. The platinum resistant sublines, SKOV3-PR and ES2-PR, were created by sequentially treating the respective parental cell lines with escalating does of cisplatin and allowing recovery between treatments (4h treatment followed by 2-3 weeks of recovery to simulated clinical chemotherapy administration). The SKOV3-PR25 and ES2-PR20 lines were subsequently exposed to 25 and 20 uM cisplatin every 2 months, respectively, to ensure the chemoresistant phenotype is maintained. Cell lines were tested at least once a year and found to be negative for mycoplasma infection.
Short and Long Term Cell Survival Assays
Short term viability was assayed using the water soluble tetrazolium assay (WST-1 Roche) according to the manufacturer’s recommended protocol. Cells were assay 24 or 72h following start of treatment. Combination index was calculated using CalcuSyn software (Biosoft.com) according to the methods of Chou and Talalay (7). Long term effects on cell survival were investigated using a focus formation assay. Preliminary experiments were performed to optimize cell density and drug concentration. Duration of drug exposure was kept constant at 24h after which cell were washed with PBS and incubated in complete media. Vehicle treated cells were plated at lower density to avoid over-confluence. Media was changed every 2-3 days until cells were judged to be ready for staining (usually 10-12 days after plating). Foci were fixed and stained using a solution of 20% formaldehyde, 80% methanol, and 0.25% crystal violet, and rinsed with deionized water. Cell staining was captured with a high resolution CCD camera (FluorChem, Alpha Innotech) and quantified using densitometry software (AlphaEaseFC, Alpha Innotech). An empty well from each plate was used for background subtraction. Comparison of single and combination drug effects was made using the student T-test and Two-Way ANOVA with P<0.05 considered significant.
SiRNA Experiments
Preliminary experiments were performed to determine optimal knockdown conditions. Cells were transfected 24h after plating with15nM oligonucleotides (Dharmacon On Target Plus SmartPool, sequences available upon request) using RNAiMAX reagent (Invitrogen) in serum-free media. On the day after transfection equal volume of media + 20%FBS was added to each well. Drug treatment was performed 48-56h following siRNA. Duration of drug exposure was 24h after which cells were washed with PBS and fed with complete media. Cell survival assays were then conducted as described above.
Cell Cycle Analysis
Cells were trypsinized and fixed with 70% ethanol overnight at 4°C. Following fixation, cells were stained in propidium iodide solution (0.05% NP-40, 50 ng of propidium iodide per ml, and 10 μg of RNase A per ml). The labeled cells were analyzed by a FACScalibur flow cytometer (Becton Dickinson) and Flow Jo software (Tree Star). Experiments were performed in triplicate and repeated at least once. Statistical comparisons were made using the student T-Test.
Measurement of Cisplatin Induced Adducts by ELISA
The competitive ELISA was performed as previously described (8, 9) with some modifications. ES2 and SKOV3 cells were treated with 2uM of cisplatin with or without1uM of MLN4924 for 24h, following washing, cells were further incubated for 48h to allow DNA damage repair. Cells were washed twice with PBS and genomic DNA (test DNA) was isolated using Qiagen DNeasy Kit. Highly cisplatin-modified DNA (sink DNA) was prepared by incubating salmon sperm DNA with 1mM of cisplatin in vitro and used for coating plate. The highly cisplatin modified sink DNA was denatured by boiling for 5min and quick chilling on ice before equal amounts of DNA were coated in the wells of a 96-well ELISA plate in Pierce DNA Coating Solution. ELISA plates were blocked with 2% rabbit serum in PBS for 30min at 37°C. Test (or standard) DNA was boiled and diluted (50mM NaCl, 50mM NaP, pH7.4). Sixty ul of rat anti- cisplatin-modified DNA antibody (CP9/19, Abcam) diluted at 1:400,000 (0.2% BSA, 0.2% Tween-20, 90mM NaCl in PBS) was mixed with 500ng of test DNA in 60ul. After pre-incubation for 30min at 37°C, 80ul of the antibody-DNA mixture was transferred to ELISA plates precoated with cisplatin-modified sink DNA and incubated for 1h at 37°C. Plates were then washed with PBST, and anti-rat HRP conjugated antibody (1:2000 in 1% BSA, 0.2% Tween-20 in PBS) was added to measure the amount of anti-cisplatin-DNA antibody that bound to the sink DNA in each well. In this assay the amount antibody bound to the well, is inversely proportional to the quantity of cisplatin-adducts present in the test DNA used in the pre-incubation reaction. Following a 30 min incubation at 37°C and PBST washes, Ultra TMB (3,3′,5,5′-tetramentylbenzidine, Pierce) was added and absorbance at 450nm was measured. For quantification, serially diluted cisplatin-modified DNA were used to make a standard curve to calculate the relative amount of cisplatin-adducts in the test DNA. All samples were assayed in triplicate and repeated at least twice.
Immunoblotting
Protein extraction and immunoblotting were performed as described previously (6). The following primary antibodies were used: p-CHK1, CUL4A, UBE2M, GAPDH (Cell Signaling), CHK1 (Novus Biologicals), CDT1, FANCD2 (Thermo-Fisher), CUL1 (Santa Cruz), CUL2 (Invitrogen), CUL3, CUL5, and CUL7 (Bethyl), beta actin (Sigma), CUL4B (Epitomics). Where indicated quantitation of western blots was performed using QuantiScan software version 3.0 (Biosoft.com).
Results
MLN4924 exhibits a moderate anti-proliferative effect on ovarian cancer cells
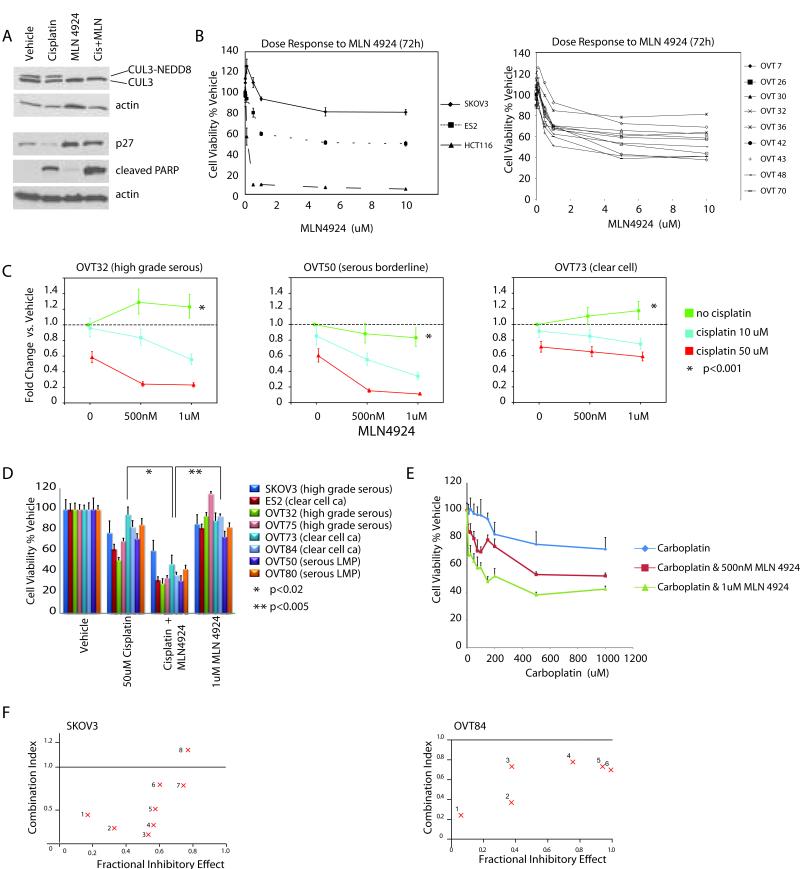
Given the paucity of data on the effects of MLN4924 on ovarian cancer, we began by investigating the effect of MLN4924 as a single agent in both established and primary ovarian cancer cell lines. As previously shown, treatment with MLN4924 led to loss of NEDD8-conjugated (neddylated) cullins and inhibition of their ubiquitin ligase activity with resulting accumulation of cullin substrates (Figure 1A). The SKOV3 and ES2 ovarian cancer cell lines were found to be much less sensitive to MLN4924 than some previously investigated cell lines such as HCT116 colon cancer cells (Figure 1B). We also tested a panel of nine primary high grade serous ovarian carcinoma cell lines and found good agreement between dose response curves of the primary and established ovarian cancer cell lines (Figure 1B).
Figure 1.
Short-term antiproliferative effects of MLN4924 alone and in combination with cisplatin in ovarian cancer cells. Cell viability was assessed using WST-1 assays A. ES2 clear cell ovarian carcinoma cells were treated with DMSO, MLN4924 (1uM), cisplatin (25uM) or the combination for 24h. MLN4924 inhibits cullin neddylation resulting in stabilization of CRL substrates such as p27. The combination treated cells reveal increased apoptosis as evidenced by PARP cleavage. B. Left Panel: Dose response to single agent MLN4924 in SKOV3 (serous carcinoma) and ES2 (clear cell) ovarian carcinoma cells compared to that in HCT116 colon cancer cells. Error bars represent sd. Right Panel: Dose response in 9 primary (patient-derived) high grade serous ovarian cancer cell lines. C. MLN4924 sensitizes ovarian cancer cells to cisplatin cytotoxicity. OVT32 (platinum resistant high grade serous carcinoma), OVT50 (serous borderline tumor), and OVT73 (clear cell carcinoma) were treated as indicated and cell survival was assayed after 24h of treatment by WST1 assays. Cell viability is presented as fold change compared to vehicle treated cells. Error bars represent sd. Significance of interaction between the two drugs was tested using two-way ANOVA with P<0.001 for all three cell lines D. Cell viability following single and combination treatment in additional primary ovarian cancer cell lines. Asterisks note p values between treatments for each cell line. Error bars represent sd. E. Dose response of ES2 cells to 24h treatment with carboplatin alone or in combination with MLN4924 (using WST-1 assay). Error bars represent + sd. F. Combination indices between MLN4924 and cisplatin in SKOV3 cells and OVT84 ovarian clear cell carcinoma primary cell line. Each cell line was treated with a constant dose of MLN4924 (SKOV3-1.0uM and OVT84-0.5uM) in combination with a range of cisplatin concentrations (SKOV3: 1-5uM, 2-10uM, 3-20uM, 4-50uM, 5-100uM, 6-200uM, 7-500uM, and 8-1mM; OVT84: 1-10uM, 2-25uM, 3-50uM, 4-75uM, 5-100uM, 6-150uM). Combination indices of 1.0, less than1.0, and greater than 1.0 indicate additive, synergistic, and antagonistic effects, respectively. Fractional inhibitory effect of 1.0 signifies 100% cell death.
MLN4924 sensitizes several subtypes of ovarian cancer to cisplatin
MLN4924 has been shown to induce DNA re-replication and DNA damage by inhibiting the CRL4-CDT2 ubiquitin ligase and stabilizing the DNA replication licensing factor, CDT1 (10). Since inhibition of CRL4-CDT2 is toxic to ovarian cancer cells (data not shown) we hypothesized that combining MLN4924 with platinum drugs (most active chemotherapy against ovarian cancer) may result in additive efficacy. We began by testing a panel of ovarian cancer cell lines, including several primary lines established in our lab. This panel included high grade serous and clear cell carcinoma lines as these represent the two most common histological subtypes of ovarian cancer and are characterized by distinct molecular and clinical features (11). In contradistinction to these cancer lines, we also investigated two primary serous borderline tumor (also known as low malignant potential or LMP) cell lines. Serous borderline tumors are indolent, slow growing ovarian neoplasms that are thought to represent the precursors for low grade serous carcinomas (12). From a molecular standpoint, borderline and low grade tumors are characterized with rare p53 mutations (which are ubiquitous in high grade serous carcinomas), and approximately 50% harbor KRAS or BRAF mutations (12). High grade serous ovarian cancers typically start out chemosensitive, but develop resistance over time. In contrast, low grade serous cancers are much less chemosensitive (13), and serous borderline tumors which can metastasize and recur more than a decade after diagnosis are not considered to respond to chemotherapy.
Short term cell viability was evaluated using the WST1 (water soluble tetrazolium) cell viability assay. Compared to either drug alone, the combination of MLN4924 and cisplatin resulted in significantly lower viability in serous, clear cell, and borderline ovarian cancer cell lines (Figure 1C and D). Similar results were obtained with the combination of carboplatin and MLN4924 (Figure 1E). These results led us to perform drug interaction studies according to the methods of Chou and Talalay (7). In this analysis, a combination index of 1.0 signifies additive effect, while values less than and greater than 1.0 indicate synergy and antagonism, respectively. MLN4924 and cisplatin showed significant synergy in both SKOV3 cells with combination indices ranging between 0.2 and 0.8 for the majority of drug ratios tested (Figure 1F). Synergy was also observed in OVT84 a primary clear cell ovarian carcinoma cell lines (Figure 1F) and ES2 cells (data not shown).
MLN4924 sensitizes chemoresistant ovarian cancer cells to cisplatin
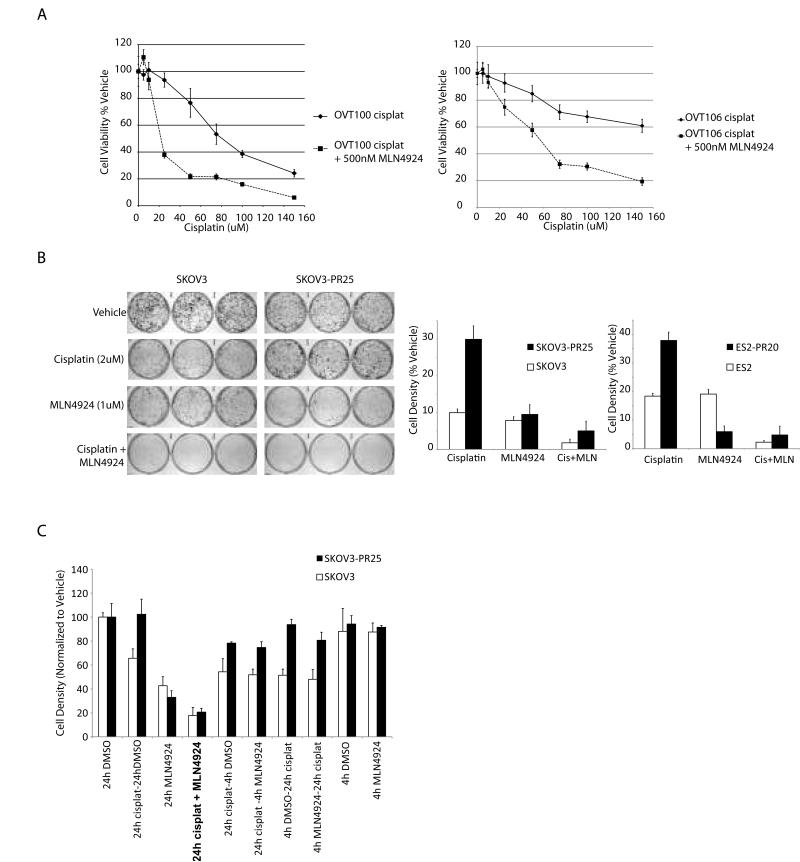
The fact that the synergistic effect of platinum and MLN4924 was observed not only in the high grade serous and clear cell ovarian cancer lines but also in the slow growing serous borderline tumors (OVT50 and OVT80 in Figure 1C and D) was intriguing. This suggested that the synergistic action of MLN4924 and cisplatin was not dependent on growth rate or p53 mutations. As noted above, serous borderline tumors are also inherently much more chemoresistant than high grade serous carcinomas. Their response led us to hypothesize that the combination of MLN4924 and platinum may also demonstrate efficacy against ovarian cancer cell lines with acquired chemoresistance. We tested this hypothesis in two in vitro models of chemoresistant ovarian cancer. First, we used primary high grade serous ovarian cancer cell lines derived from patients who had persistent tumor after 4 cycles of neoadjuvant carboplatin and paclitaxel chemotherapy. Chemotherapy produces logarithmic killing of chemosensitive cancer cells, thus, residual tumor following chemotherapy is comprised of a chemoresistant cancer cell population. Figure 2A shows the results of dose response studies in two chemoresistant primary ovarian cancer cell lines, OVT100 and OVT106. Addition of MLN4924 to cisplatin resulted in the marked sensitization of both cell lines to the cytotoxic effects of cisplatin. Similar results were observed when carboplatin was used instead of cisplatin (data not shown).
Figure 2.
MLN4924 sensitizes chemoresistant ovarian cancer cells to cisplatin. A. Dose response to cisplatin alone and in combination with MLN4924 in OVT100 and OVT106 high grade serous ovarian cancer cell lines established from patients with residual disease following neoadjuvant carboplatin and taxol chemotherapy. Error bars represent sd. B. Platinum resistant SKOV3 and ES2 cells were generated by repeated short-term exposure to escalating concentrations of cisplatin. Long-term drug induced toxicity was assayed using focus formation assays following a single 24h exposure to vehicle or the drugs as indicated (representative experiment shown and quantitated on the right; vehicle treated cells were plated at lower density). Error bars represent sd. C. The effects of duration and timing of MLN4924 (1uM) alone and in combination with cisplatin (2uM) on long term ovarian cancer cell survival. Cell viability was assayed using focus formation. The order of drugs listed on the X-axis labels corresponds to the sequence of experimental drug treatment, except for the MLN4924 + cisplatin 24h co-treated group which appears in bold font. Error bars represent sd.
To test the potential platinum-sensitizing effect of MLN4924 in an isogenic model of chemoresistant cells, we generated platinum resistant sublines of SKOV3 and ES2 cells. These resistant cell lines were designated as SKOV3-PR and ES2-PR, respectively, followed by the highest concentration (uM) of cisplatin the cells were able to tolerate (e.g. SKOV3-PR25). The cytotoxicity of cisplatin and MLN4924 both alone and in combination was assessed using a focus formation cell survival assays (Figure 2B). As expected, SKOV3-PR25 and ES2-PR20 cells showed 2-3 fold enhanced survival following cisplatin treatment. However, the combination of cisplatin with MLN4924 sensitized these cells, confirming the synergistic effect observed in the parental cell lines and the chemoresistant primary ovarian cancer cells. Thus, in both models of chemoresistant ovarian cancer the addition of MLN4924 to platinum was able to sensitize the cells and result in significant cytotoxicity. The longer term focus formation assays also revealed that single agent MLN4924 had a significant cytotoxic effect by itself which was not apparent in the short-term cell viability assay (WST-1) results (Figures 2B & C vs. Figures 1C & D), suggesting delayed cytotoxic effects (see the discussion of time course below). Furthermore, the ES2-PR20 cells were more sensitive to the effects of MLN4924 by itself compared to the parental ES2 cells (Figure 2B), suggesting that cullins or other neddylation-sensitive pathways may be implicated in the development of platinum resistance in this model.
Short-term pre- or post-cisplatin treatment with MLN4924 is not as effective as co-administration
The kinetics of NEDD8 activating enzyme inhibition by MLN4924 are rapid, and accumulation of CRL substrates is readily detectable at 1-4h post treatment (3). We therefore investigated whether shorter exposure to MLN4924 either alone or prior, during, or after cisplatin treatment may be equally as efficacious as co-administration with cisplatin for 24h. As shown in Figure 2C, short-term (4h) treatment with MLN4924, was no different than the vehicle control, when used as a single agent. Furthermore, neither pre- nor post-treatment with 4 hours of MLN4924 appreciably altered the cytotoxicity of cisplatin (Figure 2C). Thus long term (24h) treatment with MLN4924 is required for both single agent effect and synergy with cisplatin, at least in this model system.
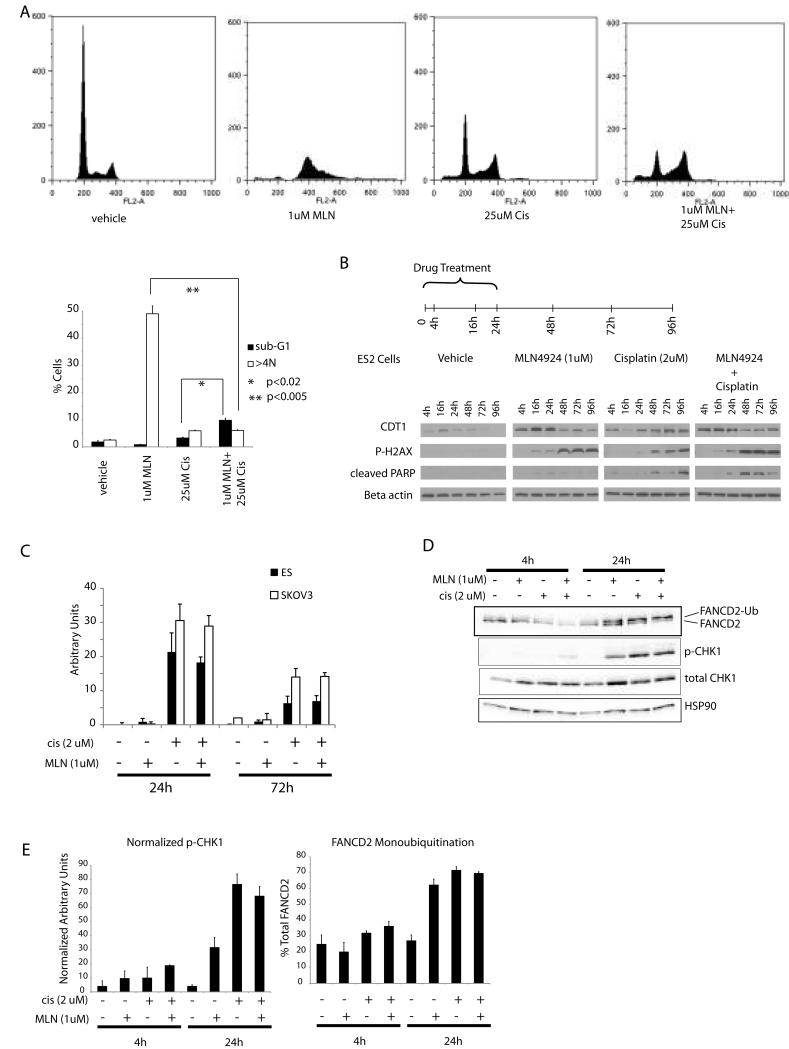
MLN4924 enhances cisplatin induced DNA damage and apoptosis without DNA re-replication
Published work by our group and other investigators had revealed that in HCT116 colon cancer cells the toxicity of MLN4924 is due in large part to induction of inappropriate DNA replication (DNA re-replication) (3, 10). One possible explanation for the enhanced cytotoxicity of the MLN4924-platinum combination would be if this combination increased the DNA re-replication induced by MLN4924 alone. Consistent with previous reports, MLN4924 induced DNA re-replication (as evidenced by >4N DNA content) by 72h post treatment in SKOV3 cells (Figure 3A). Cisplatin treatment resulted in a G1/early S phase block which was also the dominant effect in the combination group (Figure 3A). Similar findings were observed in the ES2 cells (data not shown). Thus, the synergistic action of MLN4924 and cisplatin is not due to enhanced DNA re-replication.
Figure 3.
Cell cycle profile following treatment with MLN4924 and cisplatin. A. Top panel: reresentative propidium iodide FACS histograms of SKOV3 ovarian cancer cells treated with vehicle only, cisplatin, MLN4924, or the combination. Following 24h of drug treatment cells were washed with PBS and incubated with fresh media, and harvested 48h later for cell cycle analysis. Bottom panel: bar graph represents the proportion of cells with subG1 and >4N profiles. Error bars represent sd. B. Time course of CDT1 accumulation, DNA damage, and apoptosis following treatment with MLN4924 and cisplatin. Duration of drug treatment was 24h except for the 4 and 16h time points. For the later time points cells were washed with PBS and incubated with fresh media after 24h of drug treatment. C. Cisplatin-DNA adductlevels as determined by an ELISA in SKOV3 and ES2 cells treated with cisplatin for 24h with or without MLN4924. Error bars represent sd. D. FANCD2 monoubiquitination and p-CHK1 (Ser 345) in ES2 cells 4 and 24h after initiation of treatment. E. Bar graphs represent densitometric quantitation of the mono-ubiquitinated form of FANCD2 (as percentage of total FANCD2), and relative abundance of phosphorylated CHK1 (Ser345) (normalized to HSP-90 used as a loading control). Error bars represent sd.
The FACS analysis also revealed sub-G1 peaks in the both the single agent cisplatin and MLN4924, and the combination treated cells consistent with apoptosis that was detectable 72h after treatment initiation. To better understand the nature and timing of both DNA re-replication and apoptosis induced by MLN4924 and cisplatin we investigated the protein levels of CDT1 (marker for MLN4924 effect and abnormal replication), γ-H2AX (marker for DNA damage), and cleaved PARP (marker for apoptosis) over a 96h period. Treatment with MLN4924 both alone and in combination with cisplatin resulted in early abnormal CDT1 accumulation during the first 24h after treatment initiation (Figure 3B and Supplementary Figure 1). This was followed by DNA damage as evidenced by γ-H2AX accumulation, with a notable increase between 24 and 48h. Apoptosis was most evident 48-96h post-treatment initiation in both the MLN4924 and cisplatin alone groups. Furthermore, the combination treatment resulted in enhancement of DNA damage and accelerated apoptosis (Figure 3B and supplementary Figure 2).
DNA-platinum adduct formation and repair are not affected by MLN4924
The principal mechanism of platinum induced cytotoxicity is the formation of DNA-platinum adducts resulting in intra- and interstrand crosslinks. The cell’s proficiency at repairing these crosslinks determines whether cell cycle is restored or apoptosis is induced (14). Thus, increased apoptosis in the MLN4924-platinum combination group may result from increased DNA-platinum adduct formation or interference with DNA adduct repair processes. In order to test this possibility we measured DNA-platinum adduct levels using a competitive ELISA in SKOV3 and ES2 cells treated with cisplatin and MLN4924 alone or in combination (Figure 3C). As expected, platinum-DNA adduct levels in MLN4924 only treated cells were at background levels. Cisplatin treatment for 24h resulted in the robust induction of adducts in both cell lines. Forty-eight hours after cisplatin had been removed (72h after start of treatment) adduct levels had decreased by approximately 50% indicating DNA damage repair. Addition of MLN4924 had little effect on the maximal quantity or the decrease in platinum-DNA adducts (Figure 3C). These results suggest that MLN4924’s effect on cisplatin toxicity is not mediated through increased cisplatin-DNA adduct formation or impaired DNA adduct repair.
Given the lack of detectable differences in DNA adduct formation between cisplatin alone and cisplatin plus MLN4924 treated cell, we investigated possible differences in downstream checkpoint activation. In addition, the Fanconi anemia pathway is instrumental to DNA crosslink repair (15), and MLN4924 was recently reported to suppress FANCD2 monoubiquitination induced by cisplatin (16). We, therefore, also investigated FANCD2 monoubiquitination, and CHK1 activation at 4 and 24h time points in our system. In both SKOV3 and ES2 cells FANCD2 monoubiquitination increased between 4 and 24h time points in all treatment groups (Figure 3D and E). Checkpoint activation, as detected by phosphorylated CHK1 (Ser345), was detectable following both MLN4924 and cisplatin treatment in ES2 (Figure 3D and E) and SKOV3 cells (data not shown). Thus, MLN4924 did not appreciably interfere with FANCD2 mono-ubiquitination or checkpoint activation under treatment conditions where it significantly augmented cisplatin-induced DNA damage and apoptosis.
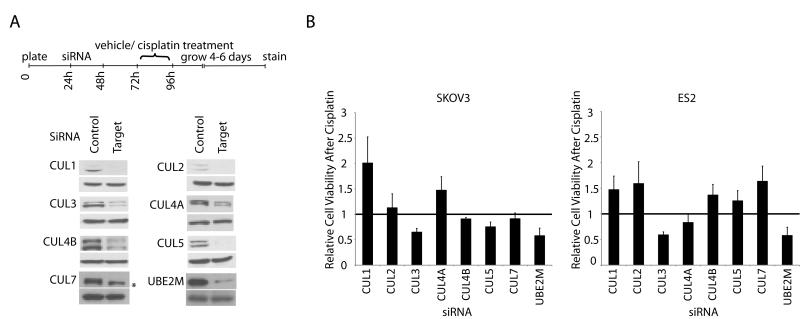
Contribution of CRLs to MLN4924-platinum toxicity
Cullin neddylation is a key activating modification of the CRL class of E3 ubiquitin ligases. As such, CRLs are the best known downstream effectors of MLN4924, and many of the effects of MLN4924 have been shown to be mediated by the inhibition of specific CRLs. We hypothesized that the synergy between MLN4924 and platinum may be mediated, at least in part, through one or more of the CRLs. The cullin family consists of 7 major members: CUL1, CUL2, CUL3, CUL4A, CUL4B, CUL5, and CUL7 (17). All have been shown to undergo neddylation except for CUL7 (18). Using an siRNA screening approach, we investigate whether the inhibition of cullins 1-7 affected cisplatin-induced cytotoxicity in SKOV3 and ES2 cells. Cell viability following treatment with cisplatin and cullin-targeting siRNA was tested using focus formation assays and normalized to the effect of each cullin’s siRNA alone (Figure 4A and B). Knockdown of CUL3 produced the greatest enhancement of cisplatin toxicity, reducing cell survival by approximately 35-45%. The knockdown of CUL5 was associated with a more modest 25% decrease in SKOV3 cells but not in ES2 cells. In contrast, depletion of CUL1 and several other cullins showed an antagonistic effect on cisplatin induced cell death (Figure 4B). Of note, knockdown of CUL7, which is not neddylated and therefore not subject to MLN4924 inhibition, did not appreciably alter cisplatin-induced toxicity is SKOV3 cells and antagonized cisplatin effect in ES2 cells (Figure 4B). Finally, to investigate the possibility that MLN4924’s enhancement of cisplatin cytotoxicity reflects the cumulative inhibition of other CRLs in addition to CRL3 and CRL5, we also performed siRNA against UBE2M (aka UBC12). UBE2M is the E2 enzyme in the NEDD8 conjugation pathway and transfers the activated NEDD8 to cullins 1-4 (19, 20). As expected, knockdown of UBE2M phenocopied MLN4924, resulting in approximately 45% reduction in cell viability following cisplatin treatment (Figure 4B). Notably this enhancement of cisplatin toxicity is in the same range as that observed with siCUL3 (35-40%). Together these results support a model in which CRL3 inhibition may represent a major contributing mechanism underlying the synergy between MLN4924 and cisplatin.
Figure 4.
Effect of cullin knockdown on cell survival following cisplatin treatment. Cell viability was assayed using focus formation following control or target directed siRNA, and in the presence or absence of cisplatin treatment. A. Timeline of the experiment and representative protein levels of siRNA targets assessed by Western blotting at the time of cisplatin/vehicle treatment. The bottom panel represents loading control (GAPDH). Asterix represents a cross reacting band B. Incremental effect on cisplatin-induced cell viability was calculated by dividing the change in cell survival in siRNA+cisplatin treated cells by that of the siRNA+vehicle cells (survival in both conditions were first normalized to that of control siRNA). A ratio of 1.0 indicated no incremental effect on cisplatin toxicity. Ratios less and more than 1.0 indicated enhancement and antagonism of cisplatin effect, respectively. Data shown are average of 4 independent experiments. Error bars represent SEM.
Discussion
Our investigation highlights a synergic cytotoxic effect between cisplatin and MLN4924 in both platinum sensitive and platinum resistant ovarian cancer cells. These findings are consistent with recent reports of MLN4924 sensitizing cells to interstrand cross-linking agents (16), and ionizing radiation (21, 22). In contrast to a previous report (16), however, we did not observe any significant inhibition of FANCD2 mono-ubiquitination or abrogation of CHK1 Ser345 phosphorylation under experimental conditions that resulted in significant DNA damage and cooperative cell death. This discrepancy may be related to differences in cell models, time course, or doses of the drugs used.
Investigations of the mechanisms of anti-neoplastic action of MLN4924 have implicated a number of CRL substrates in mediating the cytotoxic effects of this drug (23). However, given that inhibition of CRL pathways by MLN4924 can result in the alteration of as many as 20% of all proteasome-regulated proteins (3), we focused our mechanistic studies more upstream on the CRLs themselves. This approach was instructive in that we observed that inhibition of CRL3 enhanced the cytotoxic effects of cisplatin in both SKOV3 and ES2 cells. Interestingly, we also uncovered that knockdown of CUL1 and other cullins can result in resistance to platinum-induced cell death (in a cell-specific manner), which to our knowledge has not been previously reported. This highlights the fact that while the net effect of MLN4924 in our preclinical investigation is the enhancement of cisplatin toxicity, the biological contribution of the different CRL pathways and the role of their respective substrates can be variable and at times oppositional.
The implication of CRL3, in particular, is interesting because of links to several pathways that could influence cell survival. CUL3 interacts with Kelch-like erythroid cell-associated protein-1 (KEAP1) and the RING domain protein RBX1 to form the CRL3-KEAP1 ubiquitin ligase. Substrates for CRL3-KEAP1 include NRF2, a key transactivator of the antioxidant response; IKKβ, a positive regulator of NF-κB; and the BCL2 and BCL-XL anti-apoptotic proteins (24). While it is tempting to speculate how alterations in these or other substrates may regulate sensitivity to cisplatin, as noted for the inhibition of individual cullins, substrate stabilization alone may be correlative rather than causative, and different substrates may have opposing effects. It is also important to note that identification of CRL substrates is an active area of research, and the above list of potential substrates and corresponding pathways is far from complete.
Finally, we showed a synergistic effect between MLN4924 and cisplatin is several in vitro models of platinum/chemoresistant ovarian cancer. Naturally, this leads us to hypothesize that the addition of MLN4924 to platinum-based chemotherapy may increase its efficacy and/or mitigate the invariable development of platinum resistant in ovarian cancer patients. However, this hypothesis requires rigorous testing in additional preclinical studies and clinical trials.
Supplementary Material
Acknowledgements
The authors wish to thanks the members of the Division of Gynecologic Oncology and the Tissue Procurement Facility at the University of Virginia.
Grant Support
Financial support for this research was provided by the American Cancer Society Mentored Research Scholar Grant, MRSG-09-035-01-CCE, University of Virginia’s Cancer Center (Women’s Oncology Program), and Department of Obstetrics & Gynecology to A. A. Jazaeri, and a grant from the NIH, R01-CA60499 to A.Dutta.
Financial Information :
Financial support for this research was provided by the American Cancer Society Mentored Research Scholar Grant, MRSG-09-035-01-CCE, University of Virginia’s Cancer Center (Women’s Oncology Program), and Department of Obstetrics & Gynecology to A. A. Jazaeri, and a grant from the NIH, R01-CA60499 to A.Dutta.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Soucy TA, Smith PG, Rolfe M. Targeting NEDD8-activated cullin-RING ligases for the treatment of cancer. Clin Cancer Res. 2009;15:3912–6. doi: 10.1158/1078-0432.CCR-09-0343. [DOI] [PubMed] [Google Scholar]
- 3.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–6. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Zhou P. Cullins and cancer. Genes Cancer. 2010;1:690–9. doi: 10.1177/1947601910382899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emanuele MJ, Elia AE, Xu Q, Thoma CR, Izhar L, Leng Y, et al. Global identification of modular cullin-RING ligase substrates. Cell. 2011;147:459–74. doi: 10.1016/j.cell.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jazaeri AA, Bryant JL, Park H, Li H, Dahiya N, Stoler MH, et al. Molecular requirements for transformation of fallopian tube epithelial cells into serous carcinoma. Neoplasia. 2011;13:899–911. doi: 10.1593/neo.11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou TC. Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 8.Tilby MJ, Johnson C, Knox RJ, Cordell J, Roberts JJ, Dean CJ. Sensitive detection of DNA modifications induced by cisplatin and carboplatin in vitro and in vivo using a monoclonal antibody. Cancer Res. 1991;51:123–9. [PubMed] [Google Scholar]
- 9.De Silva IU, McHugh PJ, Clingen PH, Hartley JA. Defects in interstrand cross-link uncoupling do not account for the extreme sensitivity of ERCC1 and XPF cells to cisplatin. Nucleic Acids Res. 2002;30:3848–56. doi: 10.1093/nar/gkf479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin JJ, Milhollen MA, Smith PG, Narayanan U, Dutta A. NEDD8-targeting drug MLN4924 elicits DNA rereplication by stabilizing Cdt1 in S phase, triggering checkpoint activation, apoptosis, and senescence in cancer cells. Cancer Res. 2010;70:10310–20. doi: 10.1158/0008-5472.CAN-10-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Carmen MG, Birrer M, Schorge JO. Clear cell carcinoma of the ovary: A review of the literature. Gynecol Oncol. 2012;126:481–90. doi: 10.1016/j.ygyno.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 12.McCluggage WG. Morphological subtypes of ovarian carcinoma: A review with emphasis on new developments and pathogenesis. Pathology. 2011;43:420–32. doi: 10.1097/PAT.0b013e328348a6e7. [DOI] [PubMed] [Google Scholar]
- 13.Gershenson DM, Sun CC, Lu KH, Coleman RL, Sood AK, Malpica A, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006;108:361–8. doi: 10.1097/01.AOG.0000227787.24587.d1. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–20. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 15.Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat Rev Cancer. 2011;11:467–80. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kee Y, Huang M, Chang S, Moreau LA, Park E, Smith PG, et al. Inhibition of the Nedd8 system sensitizes cells to DNA interstrand cross-linking agents. Mol Cancer Res. 2012;10:369–77. doi: 10.1158/1541-7786.MCR-11-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia L, Sun Y. SCF E3 ubiquitin ligases as anticancer targets. Curr Cancer Drug Targets. 2011;11:347–56. doi: 10.2174/156800911794519734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaar JR, Florens L, Tsutsumi T, Arai T, Tron A, Swanson SK, et al. PARC and CUL7 form atypical cullin RING ligase complexes. Cancer Res. 2007;67:2006–14. doi: 10.1158/0008-5472.CAN-06-3241. [DOI] [PubMed] [Google Scholar]
- 19.Gong L, Yeh ET. Identification of the activating and conjugating enzymes of the NEDD8 conjugation pathway. J Biol Chem. 1999;274:12036–42. doi: 10.1074/jbc.274.17.12036. [DOI] [PubMed] [Google Scholar]
- 20.Huang DT, Ayrault O, Hunt HW, Taherbhoy AM, Duda DM, Scott DC, et al. E2-RING expansion of the NEDD8 cascade confers specificity to cullin modification. Mol Cell. 2009;33:483–95. doi: 10.1016/j.molcel.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei D, Li H, Yu J, Sebolt JT, Zhao L, Lawrence TS, et al. Radiosensitization of human pancreatic cancer cells by MLN4924, an investigational NEDD8-activating enzyme inhibitor. Cancer Res. 2012;72:282–93. doi: 10.1158/0008-5472.CAN-11-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang D, Tan M, Wang G, Sun Y. The p21-dependent radiosensitization of human breast cancer cells by MLN4924, an investigational inhibitor of NEDD8 activating enzyme. PLoS One. 2012;7:e34079. doi: 10.1371/journal.pone.0034079. doi: 10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nawrocki ST, Griffin P, Kelly KR, Carew JS. MLN4924 : A novel first-in-class inhibitor of NEDD8-activating enzyme for cancer therapy. Expert Opin Investig Drugs. 2012;21:1563–73. doi: 10.1517/13543784.2012.707192. [DOI] [PubMed] [Google Scholar]
- 24.Tian H, Zhang B, Di J, Jiang G, Chen F, Li H, et al. Keap1: One stone kills three birds Nrf2, IKKbeta and bcl-2/bcl-xL. Cancer Lett. 2012;325:26–34. doi: 10.1016/j.canlet.2012.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.