Abstract
Vesicular monoamine transporter-2 (VMAT2) inhibitors reduce methamphetamine (METH) reward in rats. The current study determined the effects of VMAT2 inhibitors lobeline (LOB; 1 or 3 mg/kg) and N-(1,2R-dihydroxylpropyl)-2,6-cis-di(4-methoxyphenethyl)piperidine hydrochloride (GZ-793A; 15 or 30 mg/kg) on METH-induced (0.5 mg/kg, SC) changes in extracellular dopamine (DA) and its metabolite dihydroxyphenylacetic acid (DOPAC) in the reward-relevant nucleus accumbens (NAc) shell using in vivo microdialysis. The effect of GZ-793A (15 mg/kg) on DA synthesis in tissue also was investigated in NAc, striatum (STR), medial prefrontal cortex (mPFC) and orbitofrontal cortex (OFC). In NAc shell, METH produced a time-dependent increase in extracellular DA and decrease in DOPAC. Neither LOB nor GZ-793A alone altered extracellular DA; however, both drugs increased extracellular DOPAC. In combination with METH, LOB did not alter the effects of METH on DA; however, GZ-793A, which has greater selectivity than LOB for inhibiting VMAT2, reduced the duration of the METH-induced increase in extracellular DA. Both LOB and GZ-793A enhanced the duration of the METH-induced decrease in extracellular DOPAC. METH also increased tissue DA synthesis in NAc and STR, whereas GZ-793A decreased synthesis; no effect of METH or GZ-793A on DA synthesis was found in mPFC or OFC. These results suggest that selective inhibition of VMAT2 produces a time-dependent decrease in DA release in NAc shell as a result of alterations in tyrosine hydroxylase activity, which may play a role in the ability of GZ-793A to decrease METH reward.
Keywords: Methamphetamine, Dopamine, VMAT2, Microdialysis, Lobeline, GZ-793A
Introduction
While there is currently no widely effective treatment for methamphetamine (METH) addiction, potential therapeutic targets include those associated with its pharmacological actions thought to underlie its addictive properties (Vocci & Appel, 2007). METH has multiple mechanisms of action that increase levels of extracellular dopamine (DA), including: (1) up-regulation of tyrosine hydroxylase, the rate-limiting step in DA synthesis; (2) augmentation of cytosolic DA concentrations via direct blockade of DA uptake at the vesicular monoamine transporter-2 (VMAT2), as well as reversal of the proton-amine exchanger; (3) inhibition of monoamine oxidase (MAO), the primary enzyme for metabolizing cytosolic DA; and (4) reversal of the plasmalemma DA transporter (DAT), thereby increasing extracellular DA (Brown et al., 2000, 2001; Fleckenstein et al., 2007; Guillot & Miller, 2009; Larsen et al., 2002; Mantle et al., 1976; Sulzer et al., 1995, 2005). The increase in extracellular DA in mesocorticolimbic terminal fields contributes to METH reward (Vollm et al., 2004).
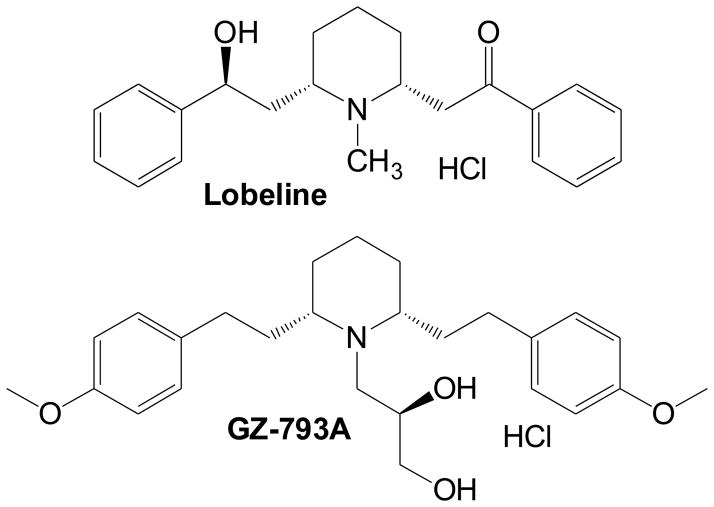
Among these neurochemical mechanisms, VMAT2 may be especially useful as a target for the treatment of METH abuse (Dwoskin & Crooks, 2002; Zheng et al., 2006). Lobeline (LOB), a pharmacologically active alkaloid found in Indian tobacco (Lobelia inflata), interacts with the tetrabenazine binding site at the N-terminus of VMAT2 and inhibits vesicular [3H]DA uptake in vitro with a Ki of 1–2 μM (Horton et al., 2011; Teng et al., 1997, 1998; see Fig. 1). LOB also has been shown to inhibit d-amphetamine-evoked endogenous dopamine overflow, METH-induced hyperactivity in rodents and METH self-administration (Harrod et al., 2001, 2003; Miller et al., 2001). In addition to VMAT2 inhibition, however, LOB acts as a DAT inhibitor, a mu opioid receptor antagonist and a nicotinic receptor antagonist (Miller et al., 2000, 2007; Wilhelm et al., 2008; Zheng et al., 2005). Since both heteromeric β2-containing and homomeric α7 nicotinic receptors are located within the midbrain DA cell body region (Besson et al., 2012; Champtiaux et al., 2003), nicotinic receptor antagonism by LOB alone may alter DA function, independently of any effect on VMAT2 in the terminal regions.
Figure 1.
Chemical structures for LOB and GZ-793A.
Recent work indicates that a novel N-dihydroxypropyl analog of lobelane, N-(1,2R-dihydroxylpropyl)-2,6-cis-di(4-methoxyphenethyl)piperidine hydrochloride (GZ-793A; see Fig 1) exhibits high affinity (Ki=0.026 μM) and selectivity at inhibiting VMAT2 in vitro (Horton et al., 2011), with negligible activity at nicotinic receptors (Ki>100 μM; unpublished observations). GZ-793A decreases METH-evoked striatal dopamine release in vitro, METH self-administration, cue- and METH-induced reinstatement and METH-induced conditioned place preference (CPP; Alvers et al., 2012; Beckmann et al., 2012; Horton et al., 2011). These results provide further preclinical support for the development of VMAT2 inhibitors as therapeutic agents for the treatment of METH abuse.
While the pharmacological actions of LOB and GZ-793A on VMAT2 and DA function have been assessed in vitro, it remains to be determined whether systemic administration of these compounds alters DA function in vivo. Given the importance of DA signaling in motivated behaviors such as drug-induced CPP and drug self-administration (Di Chiara 1995), elucidating the mechanisms by which these ligands alter DA function in vivo may further inform therapeutic approaches for the treatment of METH addiction. Thus, the present experiments evaluated the effects of LOB and GZ-793A, given alone or in combination with METH, on extracellular levels of DA and its metabolite 3,4-dihydroxypheylacetic acid (DOPAC) in the nucleus accumbens (NAc) shell of freely moving animals using microdialysis. Since GZ-793A, but not LOB, was found to reduce the time-dependent increase in METH-induced extracellular DA concentrations, a separate experiment determined if GZ-793A alters DA synthesis. In this latter experiment, tyrosine hydroxylase activity was estimated by administering an inhibitor of dihydroxyphenylanine (DOPA) decarboxylase and measuring the accumulation of DOPA in tissue from NAc. Since the striatum (STR), medial prefrontal cortex (mPFC) and orbitofrontal cortex (OFC) also have been implicated in METH abuse (Rocha and Kalivas, 2010; Vollm et al., 2004), these regions were included in the analysis for comparison to NAc.
Methods
Animals
Male Sprague-Dawley rats (225–300 g) were obtained from Harlan Industries (Indianapolis, IN, USA) and were housed individually with ad libitum access to food (2018 Teklad Global 18% Protein Rodent Diet, Harlan; Madison, WI) and water in their home cage. The colony room was maintained on a 12:12-h light/dark cycle (lights on at 0700 h) and controlled for temperature and humidity. Rats were handled and acclimated to the colony room for at least 1 week prior to the start of each experiment. Experiments were conducted during the light phase. All experimental protocols were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Drugs
D-Methamphetamine HCl and m-hydroxybenzylhydrazine (NSD-1015) were purchased from Sigma (St. Louis, MO). LOB hemisulfate was purchased from ICN (Costa Mesa, CA). N-(1,2R-Dihydroxylpropyl)-2,6-cis-di-(4-methoxyphenethyl)-piperdine HCl (GZ-793A) was synthesized according to reported methods (Horton et al., 2011). Ketamine and diazepam were purchased from N.L.S. Animal Health (Pittsburgh, PA). Drugs were dissolved in 0.9% NaCl (saline; SAL) and administered in 1 ml/kg using doses based on the salt weight, unless noted otherwise. LOB and GZ-793A were administered subcutaneous (SC), while NSD-1015, ketamine, and diazepam were administered intraperitoneal (IP). For the LOB experiments, METH was administered IP; for the GZ-793A experiments, METH was administered SC.
Apparatus
Microdialysis experiments were conducted in Plexiglas chambers (25 × 44 × 38 cm) with pine bedding. A swivel and tether system (BAS, Indianapolis, IN) was attached to the side of the chamber and connected to a microsyringe pump (KD Scientific, Model KDS250).
Surgery
For microdialysis experiments, animals were anesthetized with ketamine (80 mg/kg IP) and diazepam (5 mg/kg IP) and placed in a stereotaxic apparatus (Stoelting, Wood Dale, IL). A guide cannula (MD-2251, 22 gauge, BAS, Indianapolis, IN; secured with dental acrylic) was implanted unilaterally, aimed at the NAc shell using the following coordinates relative to bregma: AP +1.6 mm, L +0.8 mm, and D/V -5.8 mm (Paxinos & Watson, 1998). To minimize discomfort and pain, rats were given the non-opioid analgesic carprofen (5 mg/kg, SC, once daily) for 3 days after the surgical procedure. Following surgery, rats were allowed to recover for at least 3 days prior to the commencement of microdialysis.
General Procedures for Microdialysis
On the day prior to the microdialysis session, rats were fitted with a plastic neck collar. The next day, they were weighed and habituated to a Plexiglas chamber for at least 30 min. The microdialysis probe was connected to a microsyringe pump via PE10 tubing that perfused (1.2 μl/min) artificial cerebral spinal fluid (aCSF; consisting of 145 mM NaCl, 2.7mM KCl, 1 mM MgCl2, 1.2 CaCl2 and 2 mM Na2HPO4 pH adjusted to 7.4). The probe was inserted into the guide cannula and the animal was connected to the swivel system by attaching a flexible leash to the neck collar. Rats then were habituated to the Plexiglas chamber for at least 3 hr prior to collection of the baseline dialysate samples. Baseline samples were collected into polyethylene microfuge tubes containing 5 μl of 0.1 N perchloric acid every 20 min for 60 min, prior to administration of test drug.
Following each microdialysis experiment, brains were removed and flash frozen in Chromasolv® (Sigma). Brains were sectioned into 40 μm coronal slices, mounted onto slides and stained with cresyl violet. Microdialysis probe placement in NAc shell was confirmed as indicated by Paxinos and Watson (1998) and only data from rats with confirmed probe placements were included in the data analysis.
Analysis of DA, DOPAC and DOPA using HPLC-EC
For the LOB microdialysis experiment, samples were thawed and analyzed immediately for DA and DOPAC using high performance liquid chromatography with electrochemical detection (HPLC-EC). The computer-controlled HPLC-EC system consisted of a solvent delivery pump (ESA model 582), a Coulochem III 5200A electrochemical detector, and a manual injector equipped with an ESA 5011 analytical cell and 5020 guard cell. The guard cell was set at 225 mV, the reference electrode at −150 mV, and the working electrode at 225 mV. Gain was set to 1 μA and changed to 10 nA at 4.5 min. The mobile phase consisted of 75 mM NaH2PO4, 1.7 nM 1-octanesulfonic acid, 25 μM EDTA, 100 μl/l triethylamine and 10 % acetonitrile; pH 3.0, adjusted with phosphoric acid; flow rate was 0.65 ml/min. Samples were loaded into a 20 μl sample loop and manually injected onto an analytical column (BetaBasic-18 column, 150 mm × 3mm; Keystone Scientific, PA, USA).
Analysis of DA, DOPAC and DOPA in the GZ-793A microdialysis and synthesis experiments utilized the same HPLC-EC system, but employed an ESA 542 HPLC autosampler and a 5014B analytical cell and 5020 guard cell. The guard cell was set at +350 mV, electrode 1 at −150 mV, and electrode 2 at +220 mV. The mobile phase consisted of 90 mM NaH2PO4 H2O, 50 mM citric acid, 1.7 mM 1-octanesulfonic acid, 50 μM EDTA, and 10% acetonitrile (pH 3.0 adjusted with phosphoric acid; flow rate was 0.6 ml/min). Samples (20 μl) were auto-injected onto an analytical column (ESA MD 150 × 3, 150 mm × 3.2 mm) and peaks were compared with external standards using an ESA Chromatography Data System (EZChrom Elite, ESA Chelmsford, MA).
LOB Microdialysis Experiment
In Experiment 1, rats were assigned randomly to one of six groups: SAL+SAL, n=4; SAL+METH (0.5 mg/kg), n=6; LOB1 (1.0 mg/kg LOB)+SAL, n=6; LOB1 (1.0 mg/kg LOB)+METH (0.5 mg/kg), n=7; LOB3 (3.0 mg/kg LOB)+SAL, n=6; or LOB3 (3.0 mg/kg LOB)+METH (0.5 mg/kg), n=7. These LOB doses were shown previously to decrease METH self-administration (Harrod et al., 2001). After collection of 3 baseline samples, each rat was injected with either SAL or LOB (1 or 3 mg/kg, SC) and 5 min later, they were injected with SAL or METH (0.5 mg/kg, IP). Dialysis samples were collected every 20 min for an additional 3 hr after the second injection. Samples were frozen immediately on dry ice and stored at −80 °C.
GZ-793A Microdialysis Experiment
In Experiment 2, rats were assigned randomly to one of five different groups: SAL+METH (0.5 mg/kg), n=5; GZ15 (15.0 mg/kg GZ-793A)+SAL, n=5; GZ30 (30.0 mg/kg GZ-793A)+SAL, n=5; GZ15 (15.0 mg/kg GZ-793A)+METH (0.5 mg/kg), n=5; or GZ30 (30.0 mg/kg GZ-793A)+METH (0.5 mg/kg), n=6. These GZ-793A doses were shown previously to decrease METH self-administration (Beckmann et al., 2012). Since the between-group control (SAL+SAL) in the LOB experiment described above showed no change in extracellular DA or DOPAC following saline, a within-group design was used in this experiment in which the baseline for each rat served as control. This allowed the assignment of all rats into drug treatment groups providing greater power to analyze treatment effects; both between- and within-group designs are used commonly with microdialysis (e.g., Devoto et al., 2012; Garces-Ramirez et al., 2011). After collection of 3 baseline samples, all experimental groups were administered SAL (SC), and samples were collected every 20 min for an additional 60 min in order to determine whether any group differences in catecholamine signaling were observed following the injection procedure. After the last SAL sample was collected, rats were administered either SAL or GZ-793A (15 mg/kg in single volume or 30 mg/kg in double volume, SC), and 5 min later injected with SAL or METH (0.5 mg/kg, SC). Dialysis samples were collected every 20 min for an additional 3 hr after the second injection. Samples were frozen immediately on dry ice and stored at −80°C.
DA Synthesis Experiment
In Experiment 3, rats were assigned randomly to one of four different groups: SAL+SAL, n=8; SAL+METH (0.5 mg/kg) n=8; GZ-793A (15.0 mg/kg)+SAL, n=8; or GZ-793A (15.0 mg/kg)+METH (0.5 mg/kg), n=8. On the day of testing, rats were injected with either SAL or GZ-793A (15 mg/kg, SC), and 5 min later were injected with either SAL or METH (0.5 mg/kg, SC). Fifteen min after the second injection, all rats were injected with the DOPA decarboxylase inhibitor NSD-1015 (100 mg/kg, IP). Following NSD-1015, rats were placed in their home cages and 30 min later will killed by rapid decapitation. NAc, striatum (STR), medial prefrontal cortex (mPFC), and orbitofrontal cortex (OFC) were dissected on an ice-cold dissection plate. Each brain region was weighed and placed in 0.1 N perchloric acid (100 mg tissue/ml for STR, mPFC and OFC; 50 mg tissue/ml for NAc), and frozen at −80° C. For analysis of DOPA, samples were thawed, centrifuged at 20,000 × g and the supernatant was analyzed by HPLC-EC as described previously.
Data Analysis
For the microdialysis experiments, baseline DA and DOPAC levels were calculated by fitting peak heights of samples taken at −40, −20, and 0 min to standard curves. Following drug administration, data was recorded as peak height for DA and DOPAC for each sample collected across the time course of the experiment. These data were expressed as a percent of baseline (average of the first 3 samples) and analyzed using a mixed-factor repeated measures ANOVA (Group × Time). Subsequently, the time course data were analyzed for each group using separate one-factor ANOVAs. If these subsequent analyses resulted in a significant effect, Dunnett’s tests were used to compare each time point following drug administration with the control (averaged baseline value). In addition, to determine the effects of LOB and GZ-793A on METH-induced changes in DA and DOPAC, two-tailed t-test comparisons were conducted between the SAL+METH group (control) and each of the LOB1+METH, LOB3+METH, GZ15+METH and GZ30+METH groups at each time point. Also, area under the curve (AUC) was calculated using the trapezoidal rule (GraphPad Prizm 5.0 Software Inc, Sand Diego, CA) for extracellular DA and DOPAC data expressed as % change from baseline following the METH injection until DA and DOPAC levels returned to baseline. AUC values were analyzed by one-factor ANOVA (Group) followed by post hoc t-tests.
For the DA synthesis experiment, data (μg/g tissue) for each brain region were analyzed by a separate two-factor ANOVA (Pretreatment × Treatment). Post hoc t-tests also were conducted. For all data analyses, a value of p<0.05 indicated a statistically significant difference.
Results
Microdialysis Probe Placements
Histological analysis of the probe tracks from the LOB and GZ-793A microdialysis experiments are depicted in Fig. 2A and 2B.
Figure 2. Placement of Microdialysis Probes.
Each vertical bar represents a probe placement for each animal that was included in the analysis of the microdialysis experiments. Panel A: Probe placements for the LOB microdialysis experiment. Panel B: Probe placements for the GZ-793A microdialysis experiment.
LOB Microdialysis Experiment
Extracellular DA
Time course data for extracellular DA collected in the microdialysate are presented in Fig. 3. The mean (±SEM) baseline DA concentration was 2.28 ± 0.17 pg/20 μL. An overall analysis of DA levels using a 6 (group) × 12 (time) ANOVA indicated significant main effects of group (F(5,30)=4.49; p<0.01) and time (F(11,330)=21.0; p<0.001), as well as a significant group × time interaction (F(55,330)=4.24; p<0.001; Fig. 3, left panel). Separate one-way ANOVAs conducted on the data from each group revealed significant effects of time for SAL+METH (F(11,55)=11.0; p<0.001), LOB1+METH (F(11,66)=11.3; p<0.001) and LOB3+METH (F(11,66)=8.61; p<0.001). Post hoc analyses indicated that the SAL+METH group had increased DA at 40, 60, 80 and 100 min relative to the pretreatment baseline. Relative to baseline, LOB1+METH and LOB3+METH groups also showed a significant increase in DA concentration at these same times, with the LOB1+METH group also showing a significant increase at 120 min (p<0.05; not denoted by symbol in figure). Between-group t-test comparisons revealed no significant differences between the SAL+METH group and either the LOB1+METH or LOB3+METH groups at any time point, indicating that LOB did not alter the effect of METH to increase extracellular DA concentration in NAc shell.
Figure 3. Extracellular DA Concentrations in NAc Shell Following LOB and/or METH.
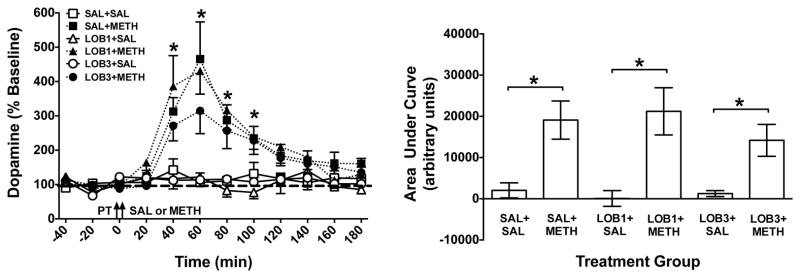
Left Panel: Mean (± SEM) DA concentration in dialysate as a percent of baseline for each time point following administration of saline (SAL), lobeline (LOB; 1 or 3 mg/kg) and/or methamphetamine (METH; 0.5 mg/kg). Arrows indicate time of pretreatment (PT) and treatment (SAL or METH). Thick dashed line represents baseline, expressed as 100%. *Indicates significant within-group change in DA levels in the SAL+METH group relative to baseline (p<0.05), n = 4 – 7 rats per group. Right Panel: Area under the curve (AUC) for each group. *Indicates significant difference between groups (p<0.05), n = 4 – 7 rats per group.
ANOVA on DA levels expressed as AUC revealed a significant main effect of group (F(5,30)=5.95; p<0.01; Fig. 3, right panel). Post hoc t-tests revealed significant differences between the SAL+SAL and SAL+METH groups, the LOB1+SAL and LOB1+METH groups, and the LOB3+SAL and LOB3+METH groups (p<0.05). No other between-group comparisons were significant, indicating that LOB did not alter the METH-induced increase in extracellular DA concentration.
Extracellular DOPAC
Time course data for extracellular DOPAC collected in the microdialysate are presented in Fig. 4. The mean (±SEM) baseline DOPAC concentration was 1.59 ± 0.08 ng/20 μL. An overall analysis of DOPAC concentration using a 6 (group) × 12 (time) ANOVA indicated significant main effects of group (F(5,30)=8.44; p<0.001) and time (F(11,330)=6.71; p<0.001), as well as a significant group × time interaction (F(55,330)=6.64; p<0.001). Separate one-way ANOVAs conducted on the data from each group revealed significant effects of time for SAL+METH (F(11,55)=3.52; p<0.01), LOB1+SAL (F(11,55)=4.67; p<0.001), LOB3+SAL (F(11,55)=3.52; p<0.01), LOB1+METH (F(11,66)=12.8; p<0.001) and LOB3+METH (F(11,66)=13.7; p<0.001) groups. Post hoc analyses indicated the SAL+METH group showed decreased DOPAC concentration at 60 and 80 min relative to baseline (p<0.05; Fig. 4, left panel). In contrast, the LOB1+SAL group had increased DOPAC concentration at 40 and 60 min relative to baseline (p<0.05) and the LOB3+SAL group had an increased DOPAC concentration at 60 and 80 min relative to baseline (p<0.05). Between-group comparisons at each time point revealed that, relative to the SAL+METH group, DOPAC concentration for the LOB1+METH group was decreased further at 60 min and for the LOB3+METH group was decreased further at 60–140 min (p<0.05), indicating that LOB enhanced the METH-induced decrease in DOPAC concentration.
Figure 4. Extracellular DOPAC Concentrations in NAc Shell Following LOB and/or METH.
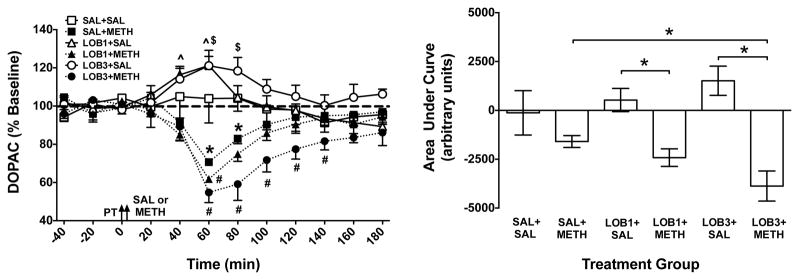
Left Panel: Mean (± SEM) extracellular DOPAC concentration in dialysate as a percent of baseline for each time point following administration of saline (SAL), lobeline (LOB; 1 or 3 mg/kg) and/or methamphetamine (METH; 0.5 mg/kg). Arrows indicate time of pretreatment (PT) and treatment (SAL or METH). The thick dashed line represents baseline, expressed as 100%. *Indicates significant within-group change in DOPAC in the SAL+METH group relative to baseline (p<0.05); ^ indicates significant within-group change in DOPAC in LOB1+SAL group relative to baseline (p<0.05); $ indicates significant within-group change in DOPAC in LOB3+SAL group relative to baseline (p<0.05); and # indicates a significant between-group difference from the SAL+METH group at the same time point (p<0.05), n = 4–7 rats per group. Right Panel: Area under the curve (AUC) following treatment for each group. *Indicates significant difference between groups (p<0.05), n = 4–7 rats per group.
Analysis of AUC also indicated a significant between-group difference in DOPAC concentration (F(5,30)=9.72; p<0.001; Fig. 4, right panel). Post-hoc t-tests revealed no significant differences between the SAL+SAL group and either the SAL+METH, LOB1+SAL or LOB3+SAL groups. However, the LOB1+METH and LOB3+METH groups showed a significant decrease in DOPAC concentration relative to the LOB1+SAL and LOB3+SAL groups respectively (p<0.05). There was also a significant decrease in the LOB3+METH group compared to SAL+METH group (p<0.05), indicating that LOB enhanced the overall effect of METH to decrease extracellular DOPAC concentrations.
GZ-793A Microdialysis Experiment
Extracellular DA
Time course data for extracellular DA collected in the microdialysate are presented in Fig. 5. The mean (±SEM) baseline DA concentration was 3.74 ± 0.68 pg/20 μL. An overall analysis of DA concentrations using a 5 (group) × 15 (time) ANOVA indicated significant main effects of group (F(4,21)=5.70; p<0.01) and time (F(14,294)=13.8; p<0.001), as well as a significant group × time interaction (F(56,280)=3.24; p<0.001). Separate ANOVAs conducted on the data from each group revealed significant effects of time for the SAL+METH (F(14,56)=6.71; p<0.001), GZ15+SAL (F(14,56)=2.18; p<0.05), GZ30+SAL (F(14,56)=3.64; p<0.001), GZ15+METH (F(14,56)=7.00; p<0.001) and GZ30+METH (F(14,70)=4.47; p<0.001) groups. To assess the effects of METH or GZ-793A alone, post hoc analyses revealed that the SAL+METH group showed a significant increase in DA concentration at 100–160 min compared to baseline (p<0.05). In contrast, GZ15+SAL and GZ30+SAL groups showed a small, but reliable decrease in DA across the sample collection period (p<0.05; not denoted by symbol in figure). Between-group t-tests also revealed that both the GZ15+METH and GZ30+METH groups had decreased DA concentrations at 160 and 200 min relative to the SAL+METH group (p<0.05). Further, the GZ15+METH group had decreased DA at 220 and 240 min relative to the SAL+METH group (p<0.05), indicating that GZ-793A reduced the effect of METH to increase extracellular DA concentrations during the latter portion of the sample collection period.
Figure 5. Extracellular DA Concentrations in NAc Shell Following GZ-793A and/or METH.
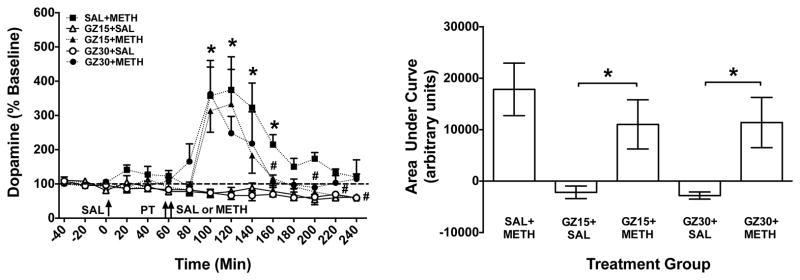
Left Panel: Mean (± SEM) DOPAC concentration in dialysate as a percent of baseline for each time point following administration of saline (SAL), GZ-793A (GZ; 15 or 30 mg/kg) and/or methamphetamine (METH; 0.5 mg/kg). Arrows indicate time of injections; saline (SAL), pretreatment (PT) and treatment (SAL or METH). Thick dashed line represents the baseline, expressed as 100%. *Indicates significant within-group change in DA levels in the SAL+METH group relative to baseline (p<0.05); # Indicates significant between-group difference from SAL+METH group, with the # at 160 and 200 min representing a difference for both GZ15+METH and GZ30+METH groups and the # at 220 and 240 min representing a difference for the GZ15+METH group (p<0.05), n = 5 – 6 rats per group. Right Panel: Area under the curve (AUC) following treatment for each group. *Indicates significant difference between groups (p<0.05), n = 5 – 6 rats per group.
Analysis of AUC also revealed a significant between-group difference (F(4,21)=5.12; p<0.01; Fig. 5, right panel). Post hoc t-tests revealed significant differences between the GZ15+SAL and GZ15+METH groups and between the GZ30+SAL and GZ30+METH groups (p<0.05); however, there were no significant differences between the SAL+METH group compared to either the GZ15+METH or the GZ30+METH groups, indicating that when the data are expressed as AUC, GZ-793A did not alter the overall effect of METH to increase extracellular DA concentrations.
Extracellular DOPAC
Time course data for extracellular DOPAC collected in the microdialysate are presented in Fig. 6. The mean (±SEM) baseline DOPAC concentration was 1.60 ± 0.14 ng/20 μL. An overall analysis of DOPAC concentrations using a 5 (group) × 15 (time) ANOVA indicated significant main effects of group (F(4,21)=3.18; p<0.05) and time (F(14,294)=28.0; p<0.001), as well as a significant group × time interaction (F(56,294)=3.91; p<0.001). Separate ANOVAs conducted on the data for each group revealed significant effects of time for the SAL+METH (F(14,56)=7.36; p<0.001), GZ15+SAL (F(14,56)=3.76; p<0.001), GZ30+SAL (F(14,56)=4.18; p<0.001), GZ15+METH (F(14,56)=12.0; p<0.001) and GZ30+METH (F(14,70)=22.4; p<0.001) groups. Post hoc analyses indicated that the SAL+METH group had decreased DOPAC concentration at 120 and 140 min relative to baseline (p<0.05; Fig. 6, left panel). In contrast, the GZ30+SAL group had increased DOPAC concentration at 120 min relative to baseline (p<0.05; Fig 6, left panel). Between-group t-test comparisons also revealed that relative to the SAL+METH group, the GZ15+METH group had decreased DOPAC concentrations at 180, 200 and 240 min, and the GZ30+METH group had decreased DOPAC at 160–240 min (p<0.05), indicating that GZ-793A enhanced the METH-induced decrease in DOPAC concentrations.
Figure 6. Extracellular DOPAC Concentrations in NAc Shell Following GZ-793A and/or METH.
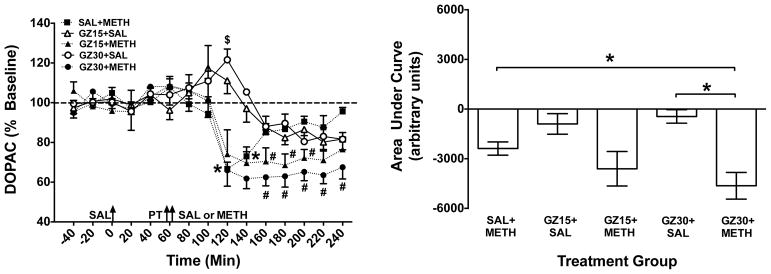
Left Panel: Mean (± SEM) DOPAC concentrations in dialysate as a percent of baseline for each time point following administration of saline (SAL), GZ-793A (GZ; 15 or 30 mg/kg) and/or methamphetamine (METH; 0.5 mg/kg). Arrows indicate time of injections; saline (SAL), pretreatment (PT) and treatment (SAL or METH). Thick dashed line represents the baseline, expressed as 100%. * Indicates significant within-group change in DOPAC in the SAL+METH group relative to baseline (p<0.05); $ indicates significant within-group change in DOPAC in GZ30+SAL group relative to baseline (p<0.05); and # indicates a significant between-group difference relative to the SAL+METH group at the same time point (p<0.05), n = 5 – 6 rats per group. Right Panel: Area under the curve (AUC) following treatment for each group. *Indicates significant difference between groups (p<0.05), n = 5 – 6 rats per group.
Analysis of AUC indicated a significant group effect (F(4,21)=6.38; p<0.01; Fig. 6, right panel). Post-hoc t-tests revealed a significant difference between the GZ30+METH and GZ30+SAL groups (p<0.05). In addition, the GZ30+METH group had less DOPAC compared to the SAL+METH group (p<0.05), indicating that GZ-793A augmented the overall effect of METH to decrease extracellular DOPAC concentrations.
GZ-793A DA Synthesis Experiment
Regional tissue DOPA levels are presented in Fig. 7. In NAc, ANOVA revealed significant main effects of GZ-793A pretreatment (F(1,28)=11.5; p<0.01) and METH treatment (F(1,28)=35.1; p<0.001) on DOPA levels, but there was no significant interaction. Similarly, in STR, ANOVA revealed significant main effects of GZ-793A pretreatment (F(1,28)=15.4; p<0.01) and METH treatment (F(1,28)=119; p<0.001) on DOPA levels, but no significant interaction. In both NAc and STR, post hoc t-tests revealed a significant difference between the SAL+SAL and SAL+METH groups (p<0.01), a significant difference between the GZ15+SAL and GZ15+METH groups, (p<0.01), and a significant difference between the SAL+METH and GZ15+METH groups (p<0.01); the decrease was ~24% in NAc and ~20% in STR. In mPFC and OFC, ANOVAs revealed no significant main effects or interactions (data not shown). Thus, GZ-793A alone produced an overall decrease in DA synthesis and attenuated the METH-induced increase in DA synthesis in a regionally specific manner.
Figure 7. Regional Accumulation of DOPA Following GZ-793A and/or METH.
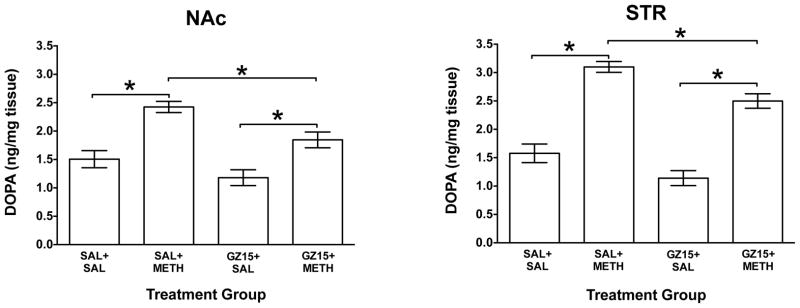
Mean (± SEM) DOPA concentrations in tissue from nucleus accumbens (NAc) and striatum (STR) following administration of GZ-793A (GZ15; 15 mg/kg) and/or methamphetamine (METH; 0.5 mg/kg). *Indicates significant difference between groups (p<0.05), n = 8 ratsv per group.
Discussion
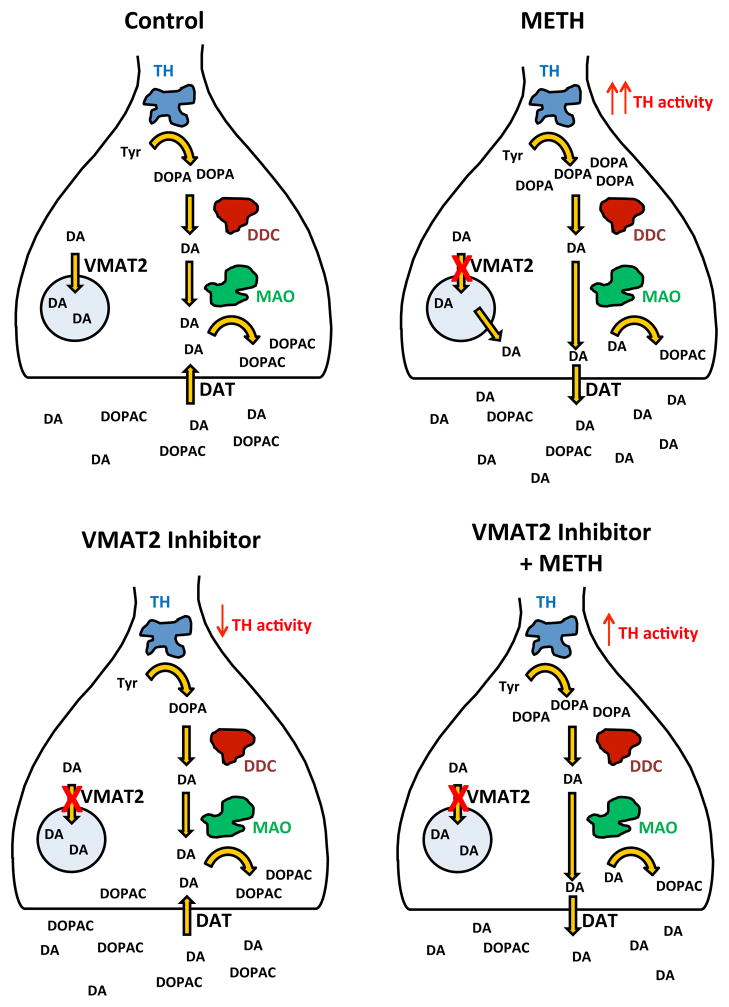
Previous research has suggested that that inhibition of VMAT2 is a potential therapeutic target for the treatment of METH addiction (Alvers et al., 2012; Beckmann et al., 2012; Dwoskin and Crooks, 2002; Harrod et al., 2001; 2003; Horton et al., 2011; Miller et al., 2001). The current results extend these findings by showing that VMAT2 inhibition in vivo by LOB and GZ-793A alters METH-induced changes in extracellular DA and DOPAC in NAc, as well as tissue DOPA, when a DOPA decarboxylase inhibitor is present. When given alone, neither LOB nor GZ-793A altered extracellular DA, however both LOB and GZ-793A increased extracellular DOPAC concentrations. The more selective VMAT2 inhibitor, GZ-793A, significantly attenuated the METH-induced increase in extracellular DA, a transient effect that was observed late in the collection period. In contrast, both LOB and GZ-793A augmented the METH-induced decrease in extracellular DOPAC. GZ-793A also attenuated the METH-induced increase in tissue DOPA, suggesting that the ability of GZ-793A to attenuate the METH-induced increase in extracellular DA may be related, at least in part, to a decrease in DA synthesis via tyrosine hydroxylase activity. A summary of these basic findings is illustrated schematically in Fig. 8.
Figure 8. Schematic Illustration Summarizing the Results.
Upper Left Panel: Figure shows baseline control levels of extracellular DA, extracellular DOPAC and tissue DOPA. Upper Right Panel: Figure shows changes in extracellular DA, extracellular DOPAC and tissue DOPA following METH alone. The red X denotes blockade of VMAT2 activity due to METH serving as substrate that enters vesicle, thus releasing DA from vesicle; the red denotes enhancement of TH activity due to DA release into the extracellular space, thus removing end-product inhibition on TH. Also note that METH reverses DAT and that newly synthesized DA is most subject to this reversal. Lower Left Panel: The figure shows levels of extracellular DA, extracellular DOPAC and tissue DOPA following VMAT2 inhibitor alone. The red X denotes a direct inhibition of VMAT2, thus increasing cytosolic DA levels available for end product inhibition of TH and enhanced metabolism to DOPAC via MAO. Lower Right Panel: Figure shows METH-induced changes in extracellular DA, extracellular DOPAC and tissue DOPA following VMAT2 inhibitor and METH combination. The red X denotes blockade of VMAT2 activity, thus increasing cytosolic DA; the red denotes enhancement of TH activity due to DA release into extracellular space, thus removing end-product inhibition on TH. Note that reversal of DAT by METH normally produces enhanced DA synthesis by removal of end-product inhibition on TH; however, due to the presence of the VMAT2 inhibitor, there is continued end product inhibition and the effect of METH on DA synthesis is blunted (upper right vs. lower right panel). Thus, the decrease in TH activity leads to a reduction in METH-induced extracellular DA and also leads to a further decrease in DOPAC. Abbreviations: TH, tyrosine hydroxylase; tyr, tyrosine; DOPA, dihydroxyphenylalanine; DDC, dihydroxyphenylalanine decarboxylase; DA, dopamine; DOPAC, dihydroxyphenylacetic acid; MAO, monoamine oxidase; DAT, dopamine transporter; VMAT2, vesicular monoamine transporter 2.
The METH-induced increase in extracellular DA and decrease in extracellular DOPAC in NAc shell observed in the current study is consistent with previous reports (Narita et al., 2003; Shoblock et al., 2003). As indicated previously, multiple neuronal mechanisms underlie METH-induced alterations in extracellular DA and DOPAC, including inhibition of VMAT2 uptake, reversal of DAT function, inhibition of MAO activity and enhanced tyrosine hydroxylase activity (Brown et al., 2000, 2001; Fleckenstein et al., 2007; Guillot & Miller, 2009; Larsen et al., 2002; Mantle et al., 1976; Sulzer et al., 1995; 2005). One possible mechanism for the METH-induced increase in extracellular DA and decrease in extracellular DOPAC involves the inhibition of MAO activity, resulting in decreased metabolism of DA to DOPAC and greater levels of cytosolic DA available for reverse transport by DAT (Brown 2001; Eisenhofer et al., 2004; Mantle et al., 1976; Sulzer et al., 2005). However, since the relatively low dose of METH (0.5 mg/kg, SC) used in the current study may not inhibit MAO (Rothman, 1999), a more likely explanation is that the METH-induced increase in extracellular DA reflects inhibition of VMAT2 uptake, perhaps via disruption of the amine-proton gradient that maintains vesicular stores (Fleckenstein et al., 2007). This action would enhance cytosolic DA available for release into the extracellular space by reversal of DAT. Newly synthesized DA also is thought to be preferentially released by reverse transport in the presence of METH, reducing availability of DA for MAO degradation (Shimosato et al., 2003; Zetterstrom et al., 1988). As cytosolic DA levels decrease due to the METH-induced reversal of DAT, DA-mediated inhibition of tyrosine hydroxylase activity is reduced (Karobath, 1971; Kumar and Vrana, 1996; Gordon et al., 2008) and thus, DA synthesis is enhanced. The net result of these actions is an increase in extracellular DA, and a decrease in extracellular DOPAC (see Fig 8, upper left vs. upper right panels).
In the present study, behaviorally relevant doses of either LOB (1 or 3 mg/kg) or GZ-793A (15 or 30 mg/kg) alone did not alter extracellular DA, but increased extracellular DOPAC; GZ-793A also decreased tissue DOPA in the presence of a DOPA decarboxylase inhibitor. VMAT2 inhibition by LOB and GZ-793A would be expected to increase cytosolic DA, thus promoting intracellular metabolism of DA by MAO and leading to greater extracellular DOPAC concentrations (Dwoskin and Crooks, 2002; Teng et al., 1998). The increase in cytosolic DA would be accompanied by an overall GZ-793A-induced decrease in dopamine synthesis, likely resulting from end-product inhibition of tyrosine hydroxylase activity (Karobath, 1971). The net result of these actions is an increase in extracellular DOPAC and a decrease in tissue DOPA in the presence of a DOPA decarboxylase inhibitor as in the current study (see Fig 8, upper left vs. lower left panels).
Current results from the LOB experiment are consistent with previous reports demonstrating that LOB (10 mg/kg) does not alter extracellular DA levels in NAc core or STR using in vivo microdialysis in rats (Benwell & Balfour, 1998; Eyerman & Yamamoto, 2005) and extends these findings to GZ-793A, a more selective VMAT2 inhibitor. In contrast to the LOB-induced increase in extracellular DOPAC concentrations reported here, previous studies have shown that LOB does not alter extracellular DOPAC levels in NAc core (Benwell & Balfour, 1998). This discrepancy may be due to differences in the LOB doses used (1 and 3 mg/kg used in Benwell and Balfour vs. 10 mg/kg used here) or the brain regions evaluated (NAc shell vs. NAc core). However, the current in vivo results are consistent with earlier in vitro results showing that LOB alone (1 μM) does not alter extracellular DA concentrations, but increases extracellular DOPAC concentrations in STR (Miller et al., 2001; Teng et al., 1997). Some caution is needed in comparing previous in vitro results with the current in vivo results, as LOB and GZ-793A concentrations in brain following systemic administration were not determined. In addition, it is possible that altered impulse flow from DA cell bodies, which is absent in the in vitro assays, may play a role in the effects of LOB and GZ-793A on DOPAC levels. While there are no reported in vivo electrophysiology studies to address this issue directly, it is known that heteromeric nicotinic receptors, composed of α4, α6 and β2 subunits, mediate nicotine-evoked DA impulse flow in the nigrostriatal and mesolimbic pathways (Champtiaux et al., 2003; Dani et al., 2011; Keath et al., 2007). However, even though nicotinic receptors play a role in the ability of LOB to alter DA function (Miller, et al., 2000), GZ-793A has negligible activity at nicotinic receptors (unpublished observations).
The current results obtained from the combined treatment with LOB and METH indicate that LOB does not alter the METH-induced increase in extracellular DA in NAc in vivo. These results are consistent with another in vivo study indicating that LOB (10 mg/kg) does not alter extracellular DA levels in STR following METH (10 mg/kg; Eyerman and Yamamoto, 2005). However, these in vivo findings contrast with in vitro results showing that LOB decreases amphetamine- and METH-evoked DA release in STR (Miller et al., 2001). Another study using cultured human embryonic kidney cells expressing both DAT and VMAT2 found that LOB (100 μM) decreases METH-evoked DA release (Wilhelm et al., 2008). Thus, as mentioned previously, these results suggest that either impulse flow from DA cell bodies, and/or differences between concentrations reached following in vivo administration versus in vitro exposure, may play a role in the differential ability of LOB to reduce the METH-induced increase in extracellular DA.
In any case, the more selective VMAT2 inhibitor GZ-793A reduced the effect of METH to increase extracellular DA concentrations during the latter portion of the time course, which is consistent with previous in vitro findings (Horton et al., 2011). While the magnitude of effect of METH to increase DA release was not altered, GZ-793A reduced the duration of effect of METH. The most likely explanation for the differential effects of LOB and GZ-793A to inhibit the METH-induced increase in extracellular DA relates to the greater selectivity of GZ-793A for VMAT2. As discussed above, METH-induced dopamine release into the extracellular space via reversal of DAT enhances DA synthesis by removal of the end-product inhibition of tyrosine hydroxylase. However, due to the presence of the VMAT2 inhibitor and the consequent increase in cytosolic DA concentrations, there is continued end product inhibition and the effect of METH on DA synthesis is blunted. In addition, the present results also demonstrate that both LOB and GZ-793A potentiate the METH-induced decrease in extracellular DOPAC and that GZ-793A attenuates the METH-induced increase in DA synthesis. The GZ-793A-induced indirect decrease in tyrosine hydroxylase activity leads to a reduction in cytosolic DA available for METH-induced extracellular release, as well as to a further decrease in DOPAC. The net result of these actions is a blunting of the METH-induced increase in extracellular DA, a blunting of the METH-induced increase in tissue DOPA in the presence of a DOPA decarboxylase inhibitor and an augmentation of the METH-induced decrease in extracellular DOPAC (see Fig 8, upper right vs. lower right panels).
While the precise molecular mechanisms for VMAT2 inhibition by GZ-793A is not known, VMAT2 possesses a DA recognition and translocation site and a dihydrotetrabenazine binding site, as well as binding sites for LOB and structurally related analogs such as GZ-793A (Horton et al., 2011). The ability of METH to reverse DA uptake at VMAT2 is impeded when the LOB binding site is occupied (Horton et al., 2011; Nickel et al., 2010). This presumably would result in a reduction in cytosolic DA available for MAO metabolism to DOPAC compared to METH alone, as well as reducing tyrosine hydroxylase activity. The current results are consistent with this explanation.
From a therapeutic perspective, since both LOB and GZ-793A decrease the reward-related effects of METH (Alvers et al., 2012; Beckmann et al., 2012; Harrod et al., 2001; Miller et al., 2001), it is interesting that only GZ-793A decreased METH-induced extracellular DA release. LOB and GZ-793A may be attenuating the behavioral effects of METH via mechanisms other than extracellular DA levels in NAc shell. However, some caution is needed in comparing the current neurochemical results to previous behavioral studies, since drug self-administration was obtained using different doses of METH (0.001–0.56 mg/kg/infusion) and a different route of administration (IV) compared to the current report. Furthermore, the current in vivo study investigated the effect of acute pretreatment of LOB or GZ-793A on acute METH treatment. In the self-administration studies, rats were given repeated METH self-infusions prior to evaluating the effects of LOB or GZ-793A (Beckmann et al., 2012; Harrod et al., 2001). Repeated administration of METH is thought to enhance DA release (Davidson et al., 2005; Nakagawa et al., 2011). In this respect, the augmented DA release accompanying repeated METH may be more susceptible to inhibition by LOB and GZ-793A, providing support for the development of selective VMAT2 inhibitors as potential pharmacotherapeutics for METH abuse.
Acknowledgments
We thank Emily Denehy, Travis McCuddy, Luke Holderfield, Joshua Beckmann, Kristin Howell, and Justin Yates for technical assistance. This work was supported by National Institute on Drug Abuse grants U01 DA13519, T32 DA016176 and UL1TR000117. LOB microdialysis experiments were completed as part of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy at the University of Kentucky. The University of Kentucky holds patents on LOB, lobelane, and GZ-793A. A potential royalty stream to Dwoskin, Crooks, and Zheng may occur consistent with University of Kentucky policy.
ABBREVIATIONS
- VMAT2
vesicular monoamine transporter-2
- METH
methamphetamine
- LOB
lobeline
- GZ-793A
N-(1,2R-dihydroxylpropyl)-2,6-cis-di(4-methoxyphenethyl)piperidine hydrochloride
- DA
dopamine
- DOPAC
dihydroxyphenylacetic acid
- DOPA
dihydroxyphenylalanine
- NAc
nucleus accumbens
- STR
striatum
- mPFC
medial prefrontal cortex
- OFC
orbitofrontal cortex
- MAO
monoamine oxidase
- DAT
dopamine transporter
- CPP
conditioned place preference
- NSD-1015
m-hydroxybenzylhydrazine
- SAL
saline
- SC
subcutaneous
- IP
intraperitoneal
Footnotes
Contributors
All authors have contributed to and approved the final manuscript. Andrew Meyer and Nichole Neugebauer designed the experiments, conducted the experiments, analyzed the data, and prepared the manuscript. Guangrong Zheng contributed new reagents. Peter Crooks, Linda Dwoskin, and Michael Bardo, as PIs on the project, participated in study design and manuscript preparation.
References
- Alvers KM, Beckmann JS, Zheng G, Crooks PA, Dwoskin LP, Bardo MT. The effect of VMAT2 inhibitor GZ-793A on the reinstatement of methamphetamine-seeking in rats. Psychopharmacology (Berl) 2012;224:255–262. doi: 10.1007/s00213-012-2748-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Denehy ED, Zheng G, Crooks PA, Dwoskin LP, Bardo MT. The effect of novel VMAT2 inhibitor, GZ-793A, on methamphetamine reward in rats. Psychopharmacology. 2012;220:395–403. doi: 10.1007/s00213-011-2488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The influence of lobeline on nucleus accumbens dopamine and locomotor responses to nicotine in nicotine-pretreated rats. Br J Pharmacol. 1998;125:1115–1119. doi: 10.1038/sj.bjp.0702161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, David V, Baudonnat M, Cazala P, Guilloux JP, Reperant C, Cloez-Tayarani I, Changeux JP, Gardier AM, Granon S. Alpha7-nicotinic receptors modulate nicotine-induced reinforcement and extracellular dopamine outflow in the mesolimbic system in mice. Psychopharmacology (Berl) 2012;220:1–14. doi: 10.1007/s00213-011-2422-1. [DOI] [PubMed] [Google Scholar]
- Brown JM, Hanson GR, Fleckenstein AE. Methamphetamine rapidly decreases vesicular dopamine uptake. J Neurochem. 2000;74:2221–2223. doi: 10.1046/j.1471-4159.2000.0742221.x. [DOI] [PubMed] [Google Scholar]
- Brown JM, Hanson GR, Fleckenstein AE. Regulation of the vesicular monoamine transporter-2: a novel mechanism for cocaine and other psychostimulants. J Pharmacol Exp Ther. 2001;296:762–767. [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybyliski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Jenson D, Broussard JI, De Biasi M. Neurophysiology of nicotine addiction. J Addict Res Ther. 2011;S1 doi: 10.4172/2155-6105.S1-001. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C, Lee TH, Ellinwood EH. Acute and chronic continuous methamphetamine have different long-term behavioral and neurochemical consequences. Neurochem Int. 2005;46:189–203. doi: 10.1016/j.neuint.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Saba P, Bini V, Gessa GL. The dopamine beta-hydroxylase inhibitor nepicastat increases dopamine release and potentiates psychostimulant-induced dopamine release in the prefrontal cortex. Addict Biol. 2013 doi: 10.1111/adb.12026. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Di Chiara GD. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA. A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol. 2002;63:89–98. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. Lobeline attenuates methamphetamine-induced changes in vesicular monoamine transporter 2 immunoreactivity and monoamine depletions in the striatum. J Pharmacol Exp Ther. 2005;312:160–169. doi: 10.1124/jpet.104.072264. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Ann Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Garces-Ramirez L, Green JL, Hiranita T, Kopajtic TA, Mereu M, Thomas AM, Mesangeau C, Narayanan S, McCurdy CR, Katz JL, Tanda G. Sigma receptor agonists: receptor binding and effects on mesolimbic dopamine neurotransmission assessed by microdialysis. Biol Psychiatry. 2011;69:208–217. doi: 10.1016/j.biopsych.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SL, Quinsey NS, Dunkley PR, Dickson PW. Tyrosine hydroxylase activity is regulated by two distinct dopamine-binding sites. J Neurochem. 2008;106:1614–1623. doi: 10.1111/j.1471-4159.2008.05509.x. [DOI] [PubMed] [Google Scholar]
- Guillot TS, Miller GW. Protective actions of vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol Neurobiol. 2009;30:149–170. doi: 10.1007/s12035-009-8059-y. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT. Lobeline attenuates d-methamphetamine self-administration in rats. J Pharmacol Exp Ther. 2001;298:172–179. [PubMed] [Google Scholar]
- Harrod SB, Dwoskin LP, Green TA, Gehrke BJ, Bardo MT. Lobeline does not serve as a reinforcer in rats. Psychopharmacology (Berl) 2003;165:397–494. doi: 10.1007/s00213-002-1289-6. [DOI] [PubMed] [Google Scholar]
- Horton DB, Siripurapu KB, Zheng G, Crooks PA, Dwoskin LP. Novel N-1,2-dihydroxypropyl analogs of lobelane inhibit vesicular monoamine transporter-2 function and methamphetamine-evoked dopamine release. J Pharmacol Exp Ther. 2011;339:286–297. doi: 10.1124/jpet.111.184770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karobath M. Catecholamines and the hydroxylation of tyrosine in synaptosomes isolated from rat brain. Proc Nat Acad Sci. 1971;68(10):2370–2373. doi: 10.1073/pnas.68.10.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keath JR, Iacoviello MP, Barrett LE, Mansvelder HD, McGehee DS. Differential modulation by nicotine of substantia nigra versus ventral tegmental area dopamine neurons. J Neurophysiol. 2007;98:3388–3396. doi: 10.1152/jn.00760.2007. [DOI] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci. 2002;22:8951–8960. doi: 10.1523/JNEUROSCI.22-20-08951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle TJ, Tipton KF, Garrett NJ. Inhibition of monoamine oxidase by amphetamine and related compounds. Biochem Pharmacol. 1976;25:2073–2077. doi: 10.1016/0006-2952(76)90432-9. [DOI] [PubMed] [Google Scholar]
- Miller DK, Crooks PA, Dwoskin LP. Lobeline inhibits nicotine-evoked [3H]dopamine overflow from rat striatal slices and nicotine-evoked (86)Rb(+) efflux from thalamic synaptosomes. Neuropharmacology. 2000;39:2654–2662. doi: 10.1016/s0028-3908(00)00140-4. [DOI] [PubMed] [Google Scholar]
- Miller DK, Crooks PA, Teng L, Witkin JM, Munzar P, Goldberg SR, Acri JB, Dwoskin LP. Lobeline inhibits the neurochemical and behavioral effects of amphetamine. J Pharmacol Exp Ther. 2001;296:1023–1034. [PubMed] [Google Scholar]
- Miller DK, Lever JR, Rodvelt KR, Baskett JA, Will MJ, Kracke GR. Lobeline, a potential pharmacotherapy for drug addiction, binds to mu opioid receptors and diminishes the effects of opioid receptor agonists. Drug Alcohol Depend. 2007;89 (2–3):282–91. doi: 10.1016/j.drugalcdep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Suzuki Y, Nagayasu K, Kitaichi M, Shirakawa H, Kaneko S. Repeated exposure to methamphetamine, cocaine or morphine induces augmentation of dopamine release in rat mesocorticolimbic slice co-cultures. PLoS One. 2011;6:e24865. doi: 10.1371/journal.pone.0024865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Takagi M, Aoki K, Kuzumaki N, Suzuki T. Implication of Rho-associated kinase in the elevation of extracellular dopamine levels and its related behaviors induced by methamphetamine in rats. J Neurochem. 2003;86:273–282. doi: 10.1046/j.1471-4159.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- Nickell JR, Krishnamurthy S, Norrholm S, Deaciuc G, Siripurapu KB, Zheng G, Crooks PA, Dwoskin LP. Lobeline inhibits methamphetamine-evoked dopamine release via inhibition of the vesicular monoamine transporter-2. J Pharmacol Exp Ther. 2010;332:612–621. doi: 10.1124/jpet.109.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press; London: 1998. [DOI] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB. Is phentramine an inhibitor of monoamine oxidase? A critical appraisal Synapse. 1999;32:141–145. doi: 10.1002/(SICI)1098-2396(199905)32:2<141::AID-SYN8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Shimosato K, Nagao N, Watanabe S, Kitayama S. Suppressive effects of trihexyphenidyl on methamphetamine-induced dopamine release as measured by in vivo microdialysis. Synapse. 2003;49:47–54. doi: 10.1002/syn.10191. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology (Berl) 2003;165:359–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonder MS, Poulsen NW, Galli A. Mechanism of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Teng L, Crooks PA, Dwoskin LP. Lobeline displaces [3H]dihydrotetrabenazine binding and releases [3H]dopamine from rat striatal synaptic vesicles: comparison with d-amphetamine. J Neurochem. 1998;71:258–265. doi: 10.1046/j.1471-4159.1998.71010258.x. [DOI] [PubMed] [Google Scholar]
- Teng L, Crooks PA, Sonsalla PK, Dwoskin LP. Lobeline and nicotine evoke [3H]overflow from rat striatal slices preloaded with [3H]dopamine: differential inhibition of synaptosomal and vesicular [3H]dopamine uptake. J Pharmacol Exp Ther. 1997;280:1432–1444. [PubMed] [Google Scholar]
- Vollm BA, de Araujo IE, Cowen PJ, Rolls ET, Kringelbach ML, Smith KA, Jezzard P, Heal RJ, Matthews PM. Methamphetamine activates reward circuitry in drug naive human subjects. Neuropsychopharmacology. 2004;29:1715–1722. doi: 10.1038/sj.npp.1300481. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;102(Suppl 1):96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Johnson RA, Eshleman AJ, Janowsky A. Lobeline effects on tonic and methamphetamine-induced dopamine release. Biochem Pharmacol. 2008;75:1411–1415. doi: 10.1016/j.bcp.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterstrom T, Sharp T, Collin AK, Ungerstedt U. In vivo measurement of extracellular dopamine and DOPAC in rat striatum after various dopamine-releasing drugs; implications for the origin of extracellular DOPAC. Eur J Pharmacol. 1988;148:327–334. doi: 10.1016/0014-2999(88)90110-0. [DOI] [PubMed] [Google Scholar]
- Zheng G, Dwoskin LP, Crooks PA. Vesicular monoamine transporter 2: role as a novel target for drug development. AAPS J. 2006;8:E682–692. doi: 10.1208/aapsj080478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Dwoskin LP, Deaciuc AG, Norrholm SD, Crooks PA. Defunctionalized lobeline analogues: structure-activity of novel ligands for the vesicular monoamine transporter. J Med Chem. 2005;25:5551–5560. doi: 10.1021/jm0501228. [DOI] [PMC free article] [PubMed] [Google Scholar]